Abstract
Rift Valley fever (RVF) is a complex emerging arboviral hemorrhagic disease that causes significant illness in animals and humans. Camel trade across the land borders between Nigeria and the Niger Republic occurs frequently and poses a significant risk for RVF transmission to pastoralists and traders. We carried a cross-sectional study between November 2016 and April 2017 in two northern States (Katsina and Jigawa) known for camel trade in Nigeria to investigate the seroprevalence and potential risk factors for RVFV occurrence. We collected 720 sera and administered questionnaire to pastoralists. We used the competitive enzyme-linked immunosorbent assay (c-ELISA) to determine the previous exposure to RVFV infection. We retrieved environmental information from public data sources that might explain RVFV seropositivity at the LGA level. To asses potential risk factors,we categorized LGAs with RVFV as "1" and those without a case" 0". We fitted a logistic model to the data and estimated odds ratios and 95% confidence intervals. An overall 19.9% prevalence was reported among camel herd—the highest seropositivity (33.3%) was recorded in SuleTankarkar LGA. In the multivariable model, only rain-fed croplands was significantly associated with RVFV antibodies occurrence p = 0.048 (OR = 0.87, 95% CI: 0.76–0.99). Only a minority of the respondents, 19.3% (n = 17/88), knew that RVF is zoonotic. Separation of healthy animals from the infected animals was carried out by 53.4% (47/88) pastoralists while 59.1% (52/88) pastoralists still use ethnoveterinary practices to control or mitigate disease outbreaks. Our study demonstrates the presence of RVFV antibodies among camel in Nigeria and the associated risk factors. These findings highlight the need for enhancing surveillance and control efforts and the public health education of camel pastoralists. Further investigation to unravel the zoonotic transmission potential to pastoralists and other animal species is pertinent.
Keywords: One-humped camels, Rift Valley fever virus, Pastoralists, Risk factors, Zoonosis, Nigeria
Highlights
-
•
An overall seroprevalence of 19.9% was recorded in our study.
-
•
Rain-fed croplands were significantly associated with RVFV.
-
•
There is need for public health education among pastoralists.
1. Introduction
RVF is a vector-borne arboviral hemorrhagic disease of animals (domestic and wild) and humans [1]. It is caused by the Rift Valley fever virus (RVFV), which belongs to the Genus Phlebovirus of the Family Phenuiviridae [2]. RVFV infection occurs primarily through bites of infected mosquitoes (Aedes and Culex) and other haematophagous arthropods. It also occurs through direct contact with infected animal materials and fluids due to occupational hazards [3]. RVF is endemic in Sub-Saharan Africa and has been reported in many African countries, including Kenya, Uganda, South Africa and Sudan- negatively impacting the affected communities' socio-economic livelihoods [[4], [5], [6], [7]]. There have also been outbreaks of RVF on shores of Africa and the Arabian Peninsula involving ruminants and humans [8]. Nigeria reported the first case of RVF in 1959 among Merino sheep imported from South Africa [9]. Besides, it has also isolated RVFV from Culicoides and Culex antennatus on a farm [10]. A previous study reported the potential of RVF spread through the movement of viremic animals along trade and cattle routes [11]. RVF is mainly associated with high-rise neonatal mortality, abortions in ruminants, and decreased human productivity [11].
Camels are found primarily in the semi-arid and arid regions of Northern Nigeria [12]. They provide high-value sources of meat and milk, support efficient services in agriculture, and contribute to environmental-friendly transport leisure [12,13]. They were initially assumed to be resistant to most of the diseases affecting livestock. However, current evidence has shown that they are susceptible to several infectious diseases, including those with zoonotic potential like RVF [[14], [15], [16]]. Evidence suggests RVF is associated with several ecological factors, and scientists have used several methods to generate information on habitat suitability for different species and disease occurrence. For example, a study in Tanzania has demonstrated that soil type, precipitation, livestock density and rainfall patterns were significantly associated with RVF [17]. Another survey from Kenya observed a direct relationship between RVF and soil type [18]. Their model predicted that in an inter-epizootic period (IEP), low rainfall enhanced the maintenance of RVFV in non- El- Nino climatic seasons. A study in Nigeria has demonstrated an increase in the mosquito population, water bodies and vegetation as crucial drivers of RVF In cattle herd in Niger State [20]. A good understanding of RVFV occurrence using ecological determinants is essential for designing an effective control program.
Previous studies in Nigeria have looked at the occurrence of RVFV in cattle and sheep [19,20]. A study in Niger found that individuals involved in cattle rearing are deficient in public health knowledge about RVFV, including its transmission mode and clinical signs [20]. Those studies have provided insight into the virus existence in Nigeria and associated risk factors among the sheep and cattle population. Information about the status of RVFV among the camel population is lacking, despite the regular movement of camels between land borders located in Katsina and Jigawa States by pastoralists and traders. Understanding pastoralists attitudes and knowledge about RVF and the role of ecological drivers are central to designing a robust control program.This study aimed to determine the sero-prevalence of RVFV among one-humped camels (Camelus dromedarius) and to explore risk factors driving exposure among camels pastoralist in Northern Nigeria. And to assess pastoralists attitudes, knowledge and practices towards RVF to guide public health control and surveillance strategies to mitigate risks of outbreaks.
2. Materials and methods
2.1. Study area
The study was conducted in Jigawa and Katsina States (Fig. 1) in the Sudan savannah of Nigeria's North-West geopolitical zone. Jigawa State lies between latitude 11.00°N to 13.00°N and longitudes 8.00°E to 10.15°E, and Katsina State is located between latitudes 11°08′N and 13°22′N and longitudes 6°52′E and 9°20′E [21]. These states harbor's common transhumance routes that pastoralist and their livestock migrate every season. The agro-ecology of these states is strongly shaped by the proliferation of dams such as the Sabke dam in Maiadua, Jibiya dam, Dutsi and Mashi dams and rivers Hadejia Jama'are that support agricultural activities. The rivers and dams feed the fertile flood plains used for irrigation or flood recession farming and provide a suitable grazing environment for livestock across the states. The two states have international livestock markets located near their borders with the Niger Republic. And there is the continuous in-and-out movement of animals through the common porous borders without any form of disease monitoring or surveillance. Both states experience two distinct seasons; the dry season (harmattan), which begins in October and ends in May of the following year, and the wet season (rainy) runs from June to September. The two states have an average annual rainfall of 750 mm [22].
Fig. 1.
Map of Nigeria indicating Jigawa and Katsina States.
2.2. Study design and target population
We carried out a cross-sectional study between November 2016 and April 2017. The target populations were agro-pastoralists who engage in crop and livestock farming, including one-humped camels (C. dromedarius). And transhumance pastoralists that seasonally move in and out across international borders searching for livestock pasture and settle in distant areas across the states during the study.
2.3. Sample size and sampling procedure
Using the formula: n = Z2pq/d2 [23], we determine the sample size. Where, n-minimum sample size, Z-appropriate value for the normal standard deviation set 95% CI or 1.96, p- expected prevalence, and q- complementary probability. Expected prevalence was set at 50% with 4% absolute precision, and 95% confidence interval was used. We arrived at 600. However, we used 20% contingency because RVF was rare and also to increase precision, we increased the sample size to 720. We set the sample size for the questionnaire at an 11% margin of error to take care of non-response due to low literacy levels10% contingency was provided. The final sample size was 88. Sampling herds and households were purposively selected, and simple random sampling was used to select animals in each herd proportionally to each herd's size, although the average herd size was ten camels.
2.4. Sample collection and processing
First, we profiled individual camel to obtain age, sex and location; they were restrained in a crouching position after that. We then collected 5 mL of blood using a 10 mL syringe with an 18G needle through the jugular vein. The blood was gently transferred into a labelled non-anticoagulant sample bottle and allowed to stand in a slant position in cooler containers overnight and transported to the National Veterinary Research Institute (NVRI), Vom, Nigeria. At NVRI, we centrifuged at 10,000 ×g for fifteen minutes to properly separate serum from the clotted blood. We harvested the Sera using a sterile pipette into 2 mL cryovial tubes, labelled and stored at –20 °C for sample analysis.
2.5. Laboratory analysis
In this study, we used a competitive enzyme-linked immunosorbent assay (c-ELISA) to determine evidence of previous exposure to RVFV infection. We used the multi-species competitive ID Screen® RVF IgG ELISA kits (ID-Vet Innovative Diagnostics, Grabels, France) to test the serum samples for anti-RVFV IgG antibody per the manufacturer's instruction [24]. To validate the test, we calculated the mean value for the optical density at 450 nm (OD450 nm) when the positive control (OD450 nmPCc) was less than 0.3 of the negative control (OD450 nmPC) and the mean value of the OD for negative control (OD450 nmPC) was >0.7. We read the result using an ELISA reader set at λ = 450 nm. For individual sample, a competition percentage (S/N %) was calculated using S/N%=(OD450 nmSample/OD450 nmNC) × 100. Samples with competition percentage (S/N %) less than or equal to 40% were deemed ‘positive’, those between 40% and50% were counted ‘doubtful,’ i.e. marginal/inconclusive. Samples with a competition percentage > 50% were regarded as ‘negative’.
2.6. Environmental and demographic variables
We obtained an urban extent grid (v.1) showing the proportion of rural and urban areas in the Local Government Areas (LGAs) from the Global Rural-Urban Mapping Project(GRUMP v.1) [25] and population density 30 arc-second (~1 km at the equator) resolution from World Pop project site. Elevation (at 10-m resolution) and we extracted the mean temperature data was from WorldClim (v.2) (https://www.worldclim.org), and obtained land cover types from DIVA-GIS. Zonal mean values for each raster datasets were obtained at each LGA polygon using the Zonal Statistics module in the Spatial Analyst toolbox in ArcGIS software and stored in Microsoft Excel for further analysis.
2.7. Statistical analysis and variable selection
We set the LGAs within Katsina and Jigawa as the spatial unit of analysis. We classified LGAs within both states based on the absence or presence of RVF confirmed by ELISA. LGA with at least one positive case was classified “1” as one while those without classified “0”. We performed univariable logistic regression to select factors associated with RVF at the LGA level in Katsina and Jigawa state. We assessed multicollinearity and removed highly collinear variable with a higher p-value. We entered any significant variables based on a conservative p-value (i.e., p < 0.25) in the univariable analysis into the multivariable model. We assessed confounder by examining remaining significant variables after removal of the potentially confounding variable. If the coefficient for one of these variables changed more than 25%, the removed variable was considered a confounder and retained in the model. A p-value of <0.05 was deemed to be significant. We used Statistical software Stata version 13.1 (Stata Corporation, College Station, TX, USA) for the data analysis.
2.8. Questionnaire design and data collection
The survey team embarked on advocacy visits a month before the commencement of the study to sensitize communities, grazing reserves and livestock routes on the significance of the research and the need for their cooperation and support. The team briefed the community leaders (Ardos/ Sarkin rakuma) and key informants on the research aim and obtained the necessary permission from the community leaders in charge of camels.
The team administered a pre-tested close-ended structured questionnaire to pastoralists selected from two herds before the administration of the real-time questionnaire, for the purpose of identifying and eliminating any problems before the actual data acquisition was conducted to ease data processing, minimize variation, and improve responses (Thrusfield, 2007). The questionnaire was in English but administered to the participants through an oral interview in “Hausa”, a predominant local language in northern parts of Nigeria. We trained four enumerators fluent in English and Hausa languages as interviewers who administered the questionnaires daily under supervision. We obtained verbal consent from the respondent and briefed them about the study's objectives.
The questionnaire assessed their knowledge, practices and mitigation measures regarding RVF. Socio-demographic information such as their age, gender, occupation, and level of education. In addition, their knowledge of RVF-associated clinical signs, including anorexia, listlessness in newborn, abortion in pregnant animals, hemorrhages, fetid diarrhea, zoonosis, and endemicity. Participants were asked about practices deployed to quell the scourge of RVF, including the use of repellent against arthropods, avoiding swampy areas during grazing, contact with aborted fetuses, separation of healthy animals from infected animals, loaning of animals and the use of ethnoveterinary practices. Finally, we assessed some environmental risk factors, such as high mosquito density, rainfall, irrigated rice fields or dams, bushy vegetation and access to the presence of water bodies. They were assured of voluntary participation, in line with the Helsinki Declaration (World Medical Association Declaration of Helsinki [26].
3. Results
3.1. Prevalence of Rift Valley fever virus in camel herds
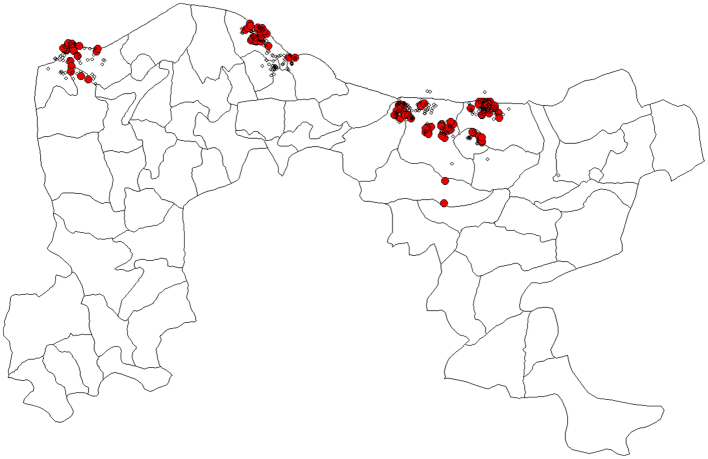
The study recorded an overall prevalence of 19.9% to RVFV among camel herds from the two states. Camels in SuleTankarkar LGA of Jigawa State recorded the highest seropositivity of 33.3%, while those from Daura LGA of Katsina State had the least prevalence (3.33%) (Table 1). The spatial distribution of RVFV antibodies (Fig. 2) with more seropositivity cases in LGAs bordering the Niger Republic.
Table 1.
Prevalence of Rift Valley fever virus antibodies in camels in the sampled LGA'sof Jigawa and Katsina States, Nigeria.
| State | Location | Total no. sampled | Positive on Ig | Percentage (%) | 95% confidence interval |
|---|---|---|---|---|---|
| Jigawa | Maigatari | 220 | 37 | 16.8 | 12.31, 22.20 |
| Babura | 100 | 18 | 18.0 | 11.38, 26.45 | |
| SuleTankarkar | 60 | 20 | 33.3 | 22.31, 45.93 | |
| Gumel | 40 | 8 | 20.0 | 9.75, 34.47 | |
| Katsina | Maiadua | 150 | 37 | 24.7 | 18.27, 32.04 |
| Jibiya | 90 | 21 | 23.3 | 15.47, 32.89 | |
| Daura | 60 | 2 | 3.3 | 0.56, 10.58 | |
| Total | 720 | 143 | 19.9 | 17.07, 22.90 |
CI - Confidence interval.
Fig. 2.
Spatial distribution of seropositive camels in Katsina and Jigawa States, Nigeria with more cases in LGAs bordering Niger Republic.
3.2. Environmental determinants of Rift Valley fever virus antibodies in Jigawa and Katsina, Nigeria
Our univariable result determined that RVF occurrence within LGAs in Katsina and Jigawa were significantly associated with elevation, urban areas, Mosaic vegetation, and rain-fed croplands (Table 2). In the multivariable model, rain-fed croplands were significantly associated with RVFV antibodies occurrence, while the urban area was marginally significant (Table 3).
Table 2.
Univariable Analysis of Ecological risk factors associated with RVF in Jigawa and Katsina State, Nigeria.
| Variables | Odds ratio (95CL) | p-value |
|---|---|---|
| Elevation | 0.99 (0.98–1.00) | 0.123 |
| Urban- (proportion of urban in Jigawa and Katsina ,Nigeria) | 3.88 (0.65 23.29) | 0.137 |
| Population density | 1.31 (0.99–1.182) | 0.688 |
| Mosaic vegetation | 0.99 (1.99–1.00) | 0.101 |
| Rainfed croplands | 0.92 (0.83–1.02) | 0.096 |
| Average temperature | 0.65 (0.11–3.95) | 0.639 |
| Sparse (<15%) vegetation | 1.13 (0.72–1.75) | 0.591 |
| Grassland, savannas or lichens/mosses | 1.12 (0.912–1.38) | 0.273 |
| Shrub land | 0.90 (0.57–1.41) | 0.659 |
Table 3.
Multivariable model Analysis of Ecological risk factors associated with RVF in Jigawa and Katsina State, Nigeria.
| Variables | Odds ratio (95CL) | p-value |
|---|---|---|
| Mosaic vegetation | 1.007354 (0.9959172–1.018922) | 0.208 |
| Elevation | 0.9862615 (0.96–1.59) | 0.168 |
| Urban | 36.51 (0 0.78–1707.77) | 0.067 |
| Rainfed croplands | 0.87 (0.76–0.99) | 0.048⁎ |
P < 0.05 is significant.
3.3. Socio-demographic information
All selected 88 pastoralists participated in the study. The highest response of 27.3% (n = 24/88) was recorded from age group 40–49, and all the respondents were males. A vast majority, 65.9% (n = 58/88), had no formal education. On occupation, 56.8% (n = 50/88) of the participants were transhumance or nomadic pastoralists while 43.2% (n = 38) were resident agro-pastoralists (Table 4).
Table 4.
Socio-demographic characteristics of camel pastoralists in Jigawa and Katsina States.
| Variable | Frequency (n) | Percentage (%) |
|---|---|---|
| Age (in years) | ||
| 20–29 | 8 | 9.1 |
| 30–39 | 19 | 21.5 |
| 40–49 | 24 | 27.3 |
| 50–59 | 17 | 19.3 |
| 60–69 | 18 | 20.5 |
| 70–79 | 2 | 2.3 |
| Gender | ||
| Male | 88 | 100.0 |
| Female | 0 | 0.0 |
| Marital status | ||
| Married | 81 | 92.1 |
| Single | 7 | 7.9 |
| Occupation | ||
| Agro pastoralists | 38 | 43.2 |
| Nomadic/transhumance pastoralists | 50 | 56.8 |
| Formal education | ||
| None | 58 | 65.9 |
| Primary | 19 | 21.6 |
| Secondary | 8 | 9.1 |
| Tertiary | 3 | 3.4 |
3.4. Knowledge about Rift Valley fever
Respondents were assessed on the knowledge of their perceived clinical signs associated with RVF, and 33% (n = 29/88) of them indicated abortion in pregnant camels as their major observation. Only 19.3% (n = 17/88) mentioned that RVF has a zoonotic implication. However, 28.4% (n = 25/88) of pastoralists indicated that RVF is endemic in their environments (Table 5).
Table 5.
Pastoralists' knowledge about RVF occurrence in camel herds in Northern Nigeria.
| Variable | Yes (n) | Percentage (%) | 95% CI |
|---|---|---|---|
| Sign of RVF in camel | |||
| Anorexia | 22 | 25.0 | 16.8–34.82 |
| Listlessness in newborn calves | 12 | 13.6 | 7.61–22.03 |
| Sudden deaths in newborns | 18 | 20.5 | 13.0–29.83 |
| Abortions in pregnant animals | 29 | 33.0 | 23.75–43.26 |
| Hemorrhages | 15 | 17.1 | 10.25–25.98 |
| Fetid diarrhea | 26 | 29.6 | 20.73–39.69 |
| All of the above | 5 | 5.7 | 2.11–12.14 |
| Zoonotic nature of RVF | |||
| RVF can be transmitted from camels to humans | 17 | 19.3 | 12.07–28.56 |
| Endemic nature | |||
| RVF is endemic in the area | 25 | 28.4 | 19.74–38.48 |
CI - Confidence interval
3.5. Mitigation measures practice against Rift Valley fever
On the mitigation measures against the disease, 72% (n = 64/88) of pastoralists indicated avoiding the tradition of animal loaning, borrowing and dowry payment with camels. At the same time, 17.1% (n = 15/88) used repellents on animal against arthropods. Furthermore, 17.1% (n = 15/88) of the respondents also indicated measures such as avoiding ponds or swampy areas while grazing were the mitigation measures practised against RVF in camel settlements in the study locations. In addition, 53.4% (n = 47/88) of the pastoralists practice the biosecurity method of segregation of healthy animals from infected ones, and 59.1% (51/88) employed ethnoveterinary practices to control or mitigate disease outbreaks (Table 6).
Table 6.
Mitigation measures practiced by pastoralists against RVF in camel settlements in Northern Nigeria.
| Practice | Yes (n) | Percenatge (%) | 95% CI |
|---|---|---|---|
| Use of repellants on animalsagainst arthropods | 15 | 17.1 | 10.25–25.98 |
| Avoiding ponds and swampy areas during grazing | 15 | 17.1 | 10.25–25.98 |
| Avoiding contacts of healthycamels with aborted fetuses | 30 | 34.1 | 24.77–44.44 |
| Separation of healthy animals from infected ones | 47 | 53.4 | 42.96–63.64 |
| Avoiding culture of animal loaning, borrowing or dowry | 64 | 72.7 | 62.73–81.25 |
| Ethno-veterinary practices | 52 | 59.1 | 48.6–68.99 |
3.6. Precipitating factors that influenced camels' exposure to Rift Valley fever virus in Northern Nigeria
The precipitating factors are components or elements that trigger the onset/ occurrence of RVF given the right environmental conditions. Here, we looked at these factors in relation to nomadic and agro pastoralists animal rearing practices. Nomadic pastoralists that move from one place to another in search of pasture for their camels. In a comparison of precipitating factors that could influence the exposure of RVFV, nomadic pastoralists' respondents had a higher percentage concerning access to high mosquito density (72%), high rainfall (64%), irrigated rice field/dams (68%), bushy vegetation (74%) and availability of water bodies (78%) compared to agro-pastoralists (Table 7).
Table 7.
Precipitating factors that influence Rift Valley fever occurrence in camel settlements in Northern Nigeria.
| Factors | Poor influence (%) | Satisfactory influence (%) |
|---|---|---|
| High mosquitoes density | ||
| Agro pastoralists | 25 (65.8) | 13 (34.2) |
| Nomadic pastoralists | 14 (28.0) | 36 (72.0) |
| High rainfall | ||
| Agro pastoralists | 21 (55.3) | 17 (44.7) |
| Nomadic pastoralists | 18 (36.0) | 32 (64.0) |
| Irrigated rice fields and dams | ||
| Agro pastoralists | 27 (71.1) | 11 (28.9) |
| Nomadic pastoralists | 16 (32.0) | 34 (68.0) |
| Bushy vegetation | ||
| Agro pastoralists | 24 (63.2) | 14 (36.8) |
| Nomadic pastoralists | 13 (26.0) | 37 (74.0) |
| Presence of water bodies | ||
| Agro pastoralists | 31 (81.6) | 7 (18.4) |
| Nomadic pastoralists | 11 (22.0) | 39 (78.0) |
4. Discussion
In this study, we surveyed two states in Nigeria for evidence of RVFV antibodies and analyses ecological determinants associated with RVFV at the LGA level in two Northern Nigeria States known for camel trade. We also administered the questionnaire to camel pastoralists to assess their awareness of RVF. The overall presence of 19.9% was obtained in this study, which is higher than previous reports in Nigeria. This finding could be explained due to the increased movement of camels via transhumance from Nigeria to neighboring countries and back into Nigeria. Besides, both states share an international border with the Niger Republic, with unhindered animal movement occurring between the land borders, providing an opportunity for a disease outbreak. Besides, RVF has been previously reported [27].Consistent with the report from Egypt, where RVF was introduced through animal movement from Sudan, which suggested the need for RVF surveillance between land borders [28].
The highest seropositivity recorded in Suletankarkar LGA of Jigawa state could result from two transhumance routes that traverse the LGA to the different grazing reserves across the state. Also, adjacent to this LGA, there exist an international livestock market in Maigatari where various livestock conglomerate from other African countries and states in Nigeria for trading, thereby exposing healthy animals with sick animals whose disease can be amplified by vectors. This assertion agrees with previous studies [29,30]; where they observed that transboundary animal diseases could spread over a considerable distance if the conditions are favorable for virus survival.
The significance of rain-fed croplands could be due to the various proliferation of dams and agricultural irrigation schemes in the two states to alleviate the scourge of food insecurity challenges. These rain-fed croplands can serve as suitable breeding sites for Aedes mosquitoes which is the chief vector of RVF virus due to high humidity. This finding is in agreement with a previous study that observed that the Aedes genus is mainly associated with short term water bodies such as flooded area, temporary pond, puddles, and rice fields [31].
This study was the first to evaluate pastoralists' knowledge and practices regarding RVF in camels in Nigeria. Most of the surveyed pastoralists lack formal education from our research, which makes the dissemination of information about the disease pattern through symposia herculean task. Formal and informal education should utilize to expand risks awareness for RVF in vulnerable communities in Nigeria. Pastoralists in the age group of 40–49 years and above possessed requisite facts and consciousness about RVF due to longer close contact relationships during animal husbandry than those in the lower age categories. Unexpected abortion in pregnant camels was the predominant clinical manifestation stated by most respondents; we observed a low knowledge level regarding other RVF clinical signs reported in Tanzania among herders [5]. The observed low proportion of pastoralists with awareness regarding RVF could result from an absence of informed programs earmarked for educating herders on emerging and re-emerging infectious diseases. A previous study in Nigeria has reported limited surveillance efforts across the Nigerian States [32]. Limited surveillance for emerging and neglected tropical diseases in parts of Nigeria can result in sporadic cases going unreported, which hinders overall public health responses. The essential element in designing a RVF control strategy for surveillance and implementing mitigation measures in animals and humans is fundamental to “One Health” Approach [33]. This approach will be collaborative strategy between various stakeholders such as virologists, livestock economists, medical professionals, veterinarians, environmental scientists, entomologists, risk communicators, anthropologists, pastoralists and policy makers to map out an effective public health preventive and control measures of RFV. This can be done through public health education, surveillance, monitoring, reporting and sharing information between member countries. Low pastoralists knowledge about RVF zoonosis may be attributed to a low level of formal education, making them susceptible to potential risks of infection. And indeed a farmer from Northern Nigeria died due to preventable RVF zoonotic disease [1].
Knowledge of the mode of transmission of RVFV plays a vital role in guiding effective action plans focused on disease mitigation. We found the use of repellents on livestock against arthropods to be low among the respondents. This may be due to a lack of proper dissemination of information by extension workers on best practices on preventing vector-borne diseases like RVF [34,35], which is chiefly transmitted by Aedes and amplified by other arthropods. There is a need for enlightenment in view of mitigating the occurrence and spread of RVF. Our study found that participants responded poorly to RVF questions via exposure to aborted fetuses and segregation of healthy animals from infected ones. These precarious traditions increase the likelihood of the pastoralists to zoonotic diseases such as RVF.
Traditionally, nomadic pastoralists migrate seasonally in search of pasture and water as their essential requirement. This makes both nomads and livestock vulnerable to infectious pathogens. As a result of this movement, healthy animals contact infected animals, wildlife and arthropods, most often reservoirs of zoonosis agents such as RVFV [[36], [37], [38]]. Precipitating risk factors in this study had the highest respondents among nomadic pastoralists. They move strategically to different countries, searching for ecologically viable resources as a source of feed for their animals. The findings of this study should be interpreted in light of some limitations. Due to limited responses from pastoralists on knowledge, attitude and practices, we could not carry out a quantitative analysis. As future direction to this study, sampling non-transhumance pastoralists that live closely with camels will shed more light on the status of RVF in the human- animal interface.
5. Conclusion
This study has found a 19.9% prevalence of RVFV antibodies among one-humped camel in Northern Nigeria and identified risk factors for its occurrence. We also observed a low level of awareness among Camel pastoralists. There is a need for targeted public health intervention by the government and other stakeholders in Nigeria and entire West African regions to improve public health awareness against this zoonotic disease, which will mitigate possible outbreak and enhance food security.
Funding
None.
Ethics statement
The Ahmadu Bello University Ethical Committee Animal Care Unit approved this study (ref ABUCAUC/2020/67). Approval was also granted by Directors of Veterinary Services in both States (Jigawa and Katsina states). The study conforms to the Declaration of Helsinki.
Authors contribution
AAM, AL, SKBA, ANB,BEO and SAY conceived and designed the study and interpreted the results. AAM, PPM, M-MS,ISI, AAA, NWD,GBS and OA conducted data collection and analysis. BOE, PPM and DAA performed data interpretation and edited the manuscript. AAM and SAY edited and wrote the final manuscript. All authors gave consent to the final version of the manuscript.
Declaration of Competing Interest
The authors declare no conflict of interests regarding the publication of this study.
Acknowledgements
We are grateful to the Directors of Veterinary Services in Jigawa and Katsina States for their cooperation. Special thanks to Habila Iliyasu and Salisu Usman,Sanusi Faby, Zaradeen Adamu, and Sani Abdulmumuni for their support during the sampling.
References
- 1.Davies F.G., Martin V. Recognising Rift Valley fever. Vet. Ital. 2006;42(1):31–53. [PubMed] [Google Scholar]
- 2.Adams M.J., Lefkowitz E.J., King A.M., Harrach B., Harrison R.L., Knowles N.J., Kropinski A.M., Krupovic M., Kuhn J.H., Mushegian A.R., Nibert M. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017) Arch. Virol. 2017;162(8):2505–2538. doi: 10.1007/s00705-017-3358-5. [DOI] [PubMed] [Google Scholar]
- 3.Pepin M., Bouloy M., Bird B.H., Kemp A., Paweska J. Rift Valley fever virus (Bunyaviridae:Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet. Res. 2010 Nov 1;41(6):61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rich K.M., Wanyoike F. An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya. Am. J. Trop. Med. Hygiene. 2010 August 5;83(2_Suppl):52–57. doi: 10.4269/ajtmh.2010.09-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chengula A.A., Mdegela R.H., Kasanga C.J. Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania. Springerplus. 2013 Dec 1;2(1):549. doi: 10.1186/2193-1801-2-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansfield K.L., Banyard A.C., McElhinney L., Johnson N., Horton D.L., Hernández-Triana L.M., Fooks A.R. Rift Valley fever virus: a review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015 Oct 13;33(42):5520–5531. doi: 10.1016/j.vaccine.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Jansen van Vuren P., Kgaladi J., Patharoo V., Ohaebosim P., Msimang V., Nyokong B., Paweska J.T. Human cases of Rift Valley fever in South Africa, 2018. Vector Borne Zoonot. Dis. 2018 Nov 28;18(12):713–715. doi: 10.1089/vbz.2018.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balkhy H.H., Memish Z.A. Rift Valley fever: an uninvited zoonosis in the Arabian peninsula. Int. J. Antimicrob. Agents. 2003 Feb 1;21(2):153–157. doi: 10.1016/s0924-8579(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson W. Identification of Rift Valley fever in Nigeria. Bull. Epizoot. Dis. Afr. 1959;7:317–318. [Google Scholar]
- 10.Lee V.H. Isolation of viruses from field populations of Culicoides (Diptera: Ceratopogonidae) in Nigeria. J. Med. Entomol. 1979 Sep 12;16(1):76. doi: 10.1093/jmedent/16.1.76. [DOI] [PubMed] [Google Scholar]
- 11.Hoogstraal H., Meegan J.M., Khalil G.M., Adham F.K. The Rift Valley fever epizootic in Egypt 1977–1978 2. Ecological and entomological studies. Trans. R. Soc. Trop. Med. Hyg. 1979;73(6):624–629. doi: 10.1016/0035-9203(79)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed A.K., Sackey A.K.B., Tekdek L.B., Gefu J.O. Common health problems of the one humped camel (Camelusdromedarius) introduced into the sub-humid climate in Zaria. Res. J. Anim. Sci. 2007;1:1–5. [Google Scholar]
- 13.Abdussamad A.M., Holtz W., Gauly M., Suleiman M.S., Bello M.B. Reproduction and breeding in dromedary camels: insights from pastoralists in some selected villages of the Nigeria-Niger corridor. Livestock Res. Rural Dev. 2011;23(8):2011. http://www.lrrd.org/lrrd23/8/abdu23178.htm Available at: (Accessed on 6 July 2020) [Google Scholar]
- 14.Mohammed I. CuvillierVerlag; Goettingen: 2000. Study of the Integration of the Dromedary in the Smallholder Crop Livestock Production System in Northwestern Nigeria; p. 228. [Google Scholar]
- 15.Chafe U.M., Musa A., Dogara B. Studies of some health aspects of traditional camel management in Northwestern Nigeria. Livest. Res. Rural. Dev. 2008;20(2):2008. http://www.lrrd.org/lrrd20/2/chaf20031.htm Available at: (Accessed on 6 July 2020) [Google Scholar]
- 16.El Harrak M., Faye B., Bengoum M. Conf. OIE. 2011. Main pathologies of camels, breeding of camels, constraints, benefits and perspectives; pp. 1–6. [Google Scholar]
- 17.Swai E.S., Sindato C. Seroprevalence of Rift Valley fever virus infection in camels (dromedaries) in northern Tanzania. Trop. Anim. Health Prod. 2015 Feb 1;47(2):347–352. doi: 10.1007/s11250-014-0726-y. [DOI] [PubMed] [Google Scholar]
- 18.Njenga M.K., Bett B. Rift Valley fever virus—how and where virus is maintained during inter-epidemic periods. Curr. Clin. Microbiol. Rep. 2019 Mar 15;6(1):18–24. [Google Scholar]
- 19.Adamu A.M., Enem S.I., Ngbede E.O., Owolodun O.A., Dzikwi A.A., Ajagbe O.A.…Simon A.Y. Serosurvey on sheep unravel circulation of Rift Valley fever virus in Nigeria. EcoHealth. 2020;17(3):393–397. doi: 10.1007/s10393-020-01490-z. [DOI] [PubMed] [Google Scholar]
- 20.Alhaji N.B., Aminu J., Lawan M.K., Babalobi O.O., Ghali-Mohammed I., Odetokun A.I. Seropositivity and associated intrinsic and extrinsic factors for Rift Valley fever virus occurrence in pastoral herds of Nigeria: a cross sectional survey. BMC Vet. Res. 2020;16:243–250. doi: 10.1186/s12917-020-02455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Bureau of Statistics Demographic Statistics Bulletin. 2017. https://nigerianstat.gov.ng/download/775 (Retrieved September 2019)
- 22.NiMET Nigerian Meteorological Agency (NiMeT) 2019 Seasonal Rainfall Prediction. 2019. https://fscluster.org/nigeria/document/nigerian-meteorological-agency-nimet Retrieved via. (September, 2019)
- 23.Thrusfield M.V. 3rd edition. Blackwell Science Ltd; Oxford, UK: 2007. Veterinary Epidemiology; pp. 228–242. [Google Scholar]
- 24.Kortekaas J., Kant J., Vloet R., Cêtre-Sossah C., Marianneau P., Lacote S., Banyard A.C., Jeffries C., Eiden M., Groschup M., Jäckel S. European ring trial to evaluate ELISAs for the diagnosis of infection with Rift Valley fever virus. J. Virol. Methods. 2013 Jan 1;187(1):177–181. doi: 10.1016/j.jviromet.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Audu S.W., et al. Two fatal cases of rabies in humans who did not receive rabies postexposure prophylaxis in Nigeria. Clin. Case Rep. 2019;7(4):749–752. doi: 10.1002/ccr3.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WMADH . Vol. 79. Bulletin of World Health Organization; 2001. World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects; pp. 373–374. [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Rift Valley Fever in Niger. 2016. http://www.who.int/csr/don/29-september-2016-rift-valley-fever-niger/en/ (Retrieved on 8 Novemeber 2017)
- 28.Napp S., Chevalier V., Busquets N., Calistri P., Casal J., Attia M., Elbassal R., Hosni H., Farrag H., Hassan N., Tawfik R. Understanding the legal trade of cattle and camels and the derived risk of Rift Valley fever introduction into and transmission within Egypt. PLoS Neglect. Trop. Dis. 2018 Jan 19;12(1) doi: 10.1371/journal.pntd.0006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin V., Chevalier V., Ceccato P., Anyamba A., De Simone L., Lubroth J., de La Rocque S., Domenech J. The impact of climate change on the epidemiology and control of Rift Valley fever. Revue Scientifiqueet Technique-Office international des épizooties. 2008;27(2):413–426. [PubMed] [Google Scholar]
- 30.Di Nardo A., Rossi D., Saleh S.M., Lejlifa S.M., Hamdi S.J., Di Gennaro A., Savini G., Thrusfield M.V. Evidence of Rift Valley fever seroprevalence in the Sahrawi semi-nomadic pastoralist system, Western Sahara. BMC Vet. Res. 2014 Dec;10(1):92. doi: 10.1186/1746-6148-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran A., Trevennec C., Lutwama J., Sserugga J., Gely M., Pittiglio C.…Chevalier V. Development and assessment of a geographic knowledge-based model for mapping suitable areas for Rift Valley fever transmission in Eastern Africa. PLoS Negl. Trop. Dis. 2016;10(9) doi: 10.1371/journal.pntd.0004999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mshelbwala P.P., Weese J.S. 2017. Rabies in the Developing World: Challenges & Prospects. (Cliniciansbrief. com) [Google Scholar]
- 33.Hassan O.A., Ahlm C., Sang R., Evander M. The 2007 Rift Valley fever outbreak in Sudan. PLoS Negl. Trop. Dis. 2011;5(9) doi: 10.1371/journal.pntd.0001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shabani S.S., Ezekiel M.J., Mohamed M., Moshiro C.S. Knowledge, attitudes and practices on Rift Valley fever among agro pastoral communities in Kongwa and Kilombero districts, Tanzania. BMC Infect. Dis. 2015;15(1):1–9. doi: 10.1186/s12879-015-1099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alhaji N.B., Babalobi O.O., Isola T.O. A quantitative exploration of nomadic pastoralists’ knowledge and practices towards Rift Valley fever in Niger State, North-central Nigeria: the associated socio-cultural drivers. One Health. 2018;6:16–22. doi: 10.1016/j.onehlt.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aagaard-Hansen J., Nombela N., Alvar J. Population movement: a key factor in the epidemiology of neglected tropical diseases. Tropical Med. Int. Health. 2010;15(11):1281–1288. doi: 10.1111/j.1365-3156.2010.02629.x. [DOI] [PubMed] [Google Scholar]
- 37.Tempia S., Braidotti F., Aden H.H., Abdulle M.H., Costagli R., Otieno F.T. Mapping cattle trade routes in southern Somalia: a method for mobile livestock keeping systems. Revue scientifiqueet technique. 2010;29(3):485. doi: 10.20506/rst.29.3.1997. [DOI] [PubMed] [Google Scholar]
- 38.Muga G.O., Onyango-Ouma W., Sang R., Affognon H. Sociocultural and economic dimensions of Rift Valley fever. Am. J. Trop. Med. Hygiene. 2015;92(4):730–738. doi: 10.4269/ajtmh.14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]