Summary
The trade-off between selectivity and adsorption capacity with porous materials is a major roadblock to reducing the energy footprint of gas separation technologies. To address this matter, we report herein a systematic crystal engineering study of C2H2 removal from CO2 in a family of hybrid ultramicroporous materials (HUMs). The HUMs are composed of the same organic linker ligand, 4-(3,5-dimethyl-1H-pyrazol-4-yl)pyridine, pypz, three inorganic pillar ligands, and two metal cations, thereby affording six isostructural pcu topology HUMs. All six HUMs exhibited strong binding sites for C2H2 and weaker affinity for CO2. The tuning of pore size and chemistry enabled by crystal engineering resulted in benchmark C2H2/CO2 separation performance. Fixed-bed dynamic column breakthrough experiments for an equimolar (v/v = 1:1) C2H2/CO2 binary gas mixture revealed that one sorbent, SIFSIX-21-Ni, was the first C2H2 selective sorbent that combines exceptional separation selectivity (27.7) with high adsorption capacity (4 mmol·g−1).
Keywords: crystal engineering, porous materials, physisorbent, hybrid ultramicroporous material, gas purification, gas separation, selectivity, acetylene, carbon dioxide
Graphical abstract
Highlights
-
•
Six isostructural hybrid ultramicroporous materials are prepared and characterized
-
•
Crystal engineering approach enabled fine-tuning of pore size and chemistry
-
•
Weak CO2/strong C2H2 affinity resulted in high C2H2/CO2 separation selectivities
-
•
SIFSIX-21-Ni: benchmark selectivity/uptake capacity for C2H2/CO2 separation
The bigger picture
It is generally recognized that porous solids (sorbents) with high selectivity and high adsorption capacity offer potential for energy-efficient gas separations. Unfortunately, there is generally a trade-off between capacity and selectivity, which represents a roadblock to the utility of sorbents in key industrial processes. For example, acetylene (C2H2), an important fuel and chemical intermediate, is produced with CO2 as an impurity, and the similar physicochemical properties of C2H2 and CO2 mean that most sorbents are poorly selective. Hybrid ultramicroporous materials (HUMs) are candidates for gas separations as they exhibit benchmark selectivity for several key gas pairs. Unfortunately, existing HUMs are handicapped by low capacity. We report a new HUM, SIFSIX-21-Ni, that addresses the trade-off between selectivity and capacity that has plagued sorbents, as its high uptake and high selectivity renders it the new benchmark for C2H2/CO2 separation performance.
A new family of six isostructural hybrid ultramicroporous materials that enabled high adsorption capacity as well as high experimental separation selectivity values for C2H2/CO2 separation.
Introduction
Acetylene (C2H2) is an important chemical commodity; it is used to manufacture vinyl and acrylate polymers and is a combustion fuel in oxy-acetylene torches.1,2 The latter application stems from its flammability range, the widest known (2.5%–81%), but can thereby represent an explosion hazard at >2.5% concentrations.3 Whereas the utility of C2H2 as an oxy-combustion fuel typically requires >98% purity grade for its use as a chemical feedstock, a higher purity grade is essential.4 Bulk C2H2 is produced by either oxidative coupling (partial combustion) of methane or downstream thermal cracking of hydrocarbons; CO2 is a by-product of both processes.5 C2H2 production generates CO2 as an impurity, which means that selective separation/purification of high-purity (>99% v/v) C2H2 from C2H2/CO2 mixtures is of industrial relevance.6 Three technologies are currently used to remove C2H2 from C2H2/CO2 mixtures: (1) bulk solvent extraction, resulting in solvent waste such as N,N-dimethylformamide and acetone7; (2) partial hydrogenation of C2H2 to ethylene, C2H4, with costly noble-metal catalysts such as Ag(0)8; and (3) cryogenic distillation, an energy-intensive process.9 All three processes suffer from high cost and low efficiency. Physisorbents offer potential for energy-efficient gas purification as exemplified by recent reports on physisorbents that exhibit benchmark performance for key separations such as CO2/N2,10,11 CO2/CH4,12 C2H2/C2H4,13,14 and C2H6/C2H4,15 among others.16 Nevertheless, commercially viable C2H2 capture from CO2 using physisorbents remains an unmet challenge since traditional physisorbents, such as zeolites, mesoporous silicas, and activated carbons, exhibit poor selectivity for C2H2 over CO217 thanks to their similar physicochemical properties (molecular dimensions: C2H2 = 3.32 × 3.34 × 5.7 Å3; CO2 = 3.18 × 3.33 × 5.36 Å3; kinetic diameters for both molecules = 3.3 Å; boiling points: C2H2 = 189.3 K, CO2 = 194.7 K).18,19 These physicochemical properties also practically rule out the application of molecular sieving.20
In this context, metal-organic materials (MOMs),21 also known as metal-organic frameworks (MOFs)22,23 or porous coordination polymers (PCPs),24 have emerged as sorbent candidates to serve as C2H2 selective physisorbents in C2H2/CO2 separation.25 Unlike traditional classes of sorbents, the modularity of MOMs enables fine-tuning of pore size and pore chemistry using crystal engineering design approaches.21 Nevertheless, there are >100,000 MOMs in the CSD MOF subset (2020.3 CSD release).26 To the best of our knowledge, only a few (20) have been experimentally studied for C2H2/CO2 separation under dynamic conditions (e.g., dynamic column breakthrough [DCB] experiments, see Table S1) including recently reported benchmarks set by ATC-Cu,27 FJI-H8-Me,28 and SIFSIX-Cu-TPA.29 For example, ATC-Cu was found to exhibit an ideal adsorbed solution theory (IAST) selectivity of 53.6 for 1:1 C2H2/CO2 separation.27 The need to study sorbent performances under dynamic conditions arises because calculation of separation performance from C2H2 and CO2 single-component isotherms tends to overestimate separation performance. IAST and fixed-bed simulated breakthrough calculations are, therefore, indicative rather than confirmative. Our review of the literature reveals that C2H2/CO2 separation selectivity (αAC) values for equimolar (v/v) mixtures have been experimentally measured for only 16 sorbents, most of which are classified as MOFs: SIFSIX-Cu-TPA,29 TCuI,30 TCuBr,30 TCuCl,30 JCM-1,31 NKMOF-1-Ni,32 FJU-22a,33 FJU-89a,34 HOF-3,35 FJU-6-TATB,36 SSNU-45,37 JXNU-5,38 FJU-36a,39 FeNiM′MOF,40 UTSA-74a,41 and sql-16-Cu-NO3-α'.42 Whereas these results are promising, they do not fully address the spectrum of performance parameters needed before a material can be considered for commercialization.43 In particular, in addition to selectivity, working capacity and recyclability (including the kinetics and energy of regeneration) are also key performance parameters.25,44 As revealed in Table S1, UTSA-74a is the only sorbent that offers separation selectivity ≥5 and adsorption capacity ≥3.5 mmolg−1. Unfortunately, UTSA-74a requires a high temperature for sorbent regeneration and activation (473 K under high vacuum) to generate the unsaturated metal centers (UMCs) that drive preferential binding for C2H2 over CO2.
Hybrid ultramicroporous materials (HUMs), sorbents that exhibit <0.7 nm pore diameter and are comprised of both organic and inorganic linker ligands, are an emerging subclass of MOMs.45 HUMs are outstanding candidates for physisorptive separation of small gas sorbates as they offer benchmark adsorption selectivities for C2H2 over C2H4 (SAE)14,18 and CO2 over N2 (SCN).16,19,46 This performance has been attributed to two key features that enhance selective binding: (1) narrow pore sizes and (2) pore surfaces/walls offering strong electrostatics. HUMs are also modular, which enables crystal engineering-driven control of pore size and chemistry to facilitate removal of even trace impurities. Unfortunately, narrow pore size, as seen in the prototypal pyrazine-linked HUMs, tends to limit surface area and uptake capacity. We recently reported that an HUM based on an expanded symmetrical ligand, 3,3′,5,5′-tetramethyl-1H,1′H-4,4′-bipyrazole, pzpz, offered a highly selective CO2 binding site that was also hydrophobic.11 Six members of a new HUM platform based on an unsymmetrical ligand that is related to pzpz, 4-(3,5-dimethyl-1H-pyrazol-4-yl)pyridine (pypz) are introduced herein. The six HUMs were obtained by inorganic ligand (SiF62-, SIFSIX; TiF62-, TIFSIX; and NbOF52-, NbOFFIVE) and/or metal (Ni2+ or Cu2+) substitution (Figure 1A) and offer higher gravimetric surface areas (and, therefore, potentially higher working capacity) than the corresponding pyrazine-linked HUMs.47 Herein, we report the exceptional adsorptive separation performance for C2H2 versus CO2 of these HUMs as evaluated by single-component gas sorption measurements, dynamic gas breakthrough experiments, in situ IR studies, solid-state NMR experiments, and molecular modeling.
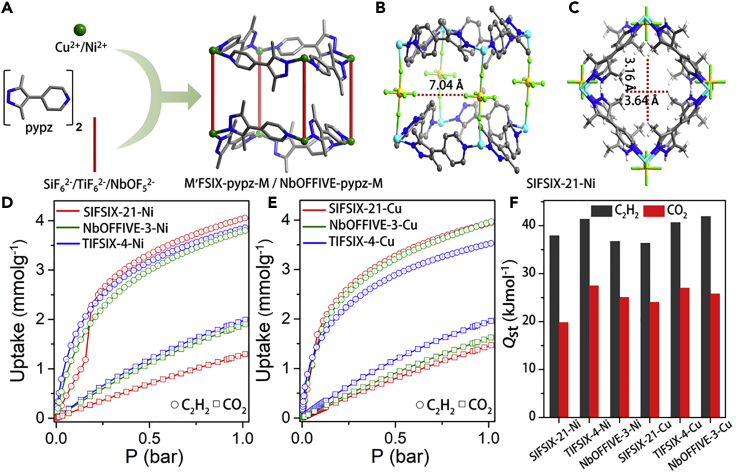
Figure 1.
The family of isostructural HUMs reported herein and their single-crystal X-ray structures, single-component adsorption isotherms, and isosteric heats of adsorption
(A) Schematic illustration of the building blocks and pcu network topology of M′FSIX-pypz-M and NbOFFIVE-pypz-M.
(B) C2H2 binding site in SIFSIX-21-Ni viewed across diagonally opposite F atoms of SiF62- pillars.
(C) The ultramicropore in SIFSIX-21-Ni viewed along the crystallographic b axis.
(D) C2H2 and CO2 isotherms of SIFSIX-21-Ni, NbOFFIVE-3-Ni, and TIFSIX-4-Ni at 298 K.
(E) C2H2 and CO2 isotherms of SIFSIX-21-Cu, NbOFFIVE-3-Cu, and TIFSIX-4-Cu at 298 K.
(F) Comparative bar diagram of isosteric heat of adsorptions (C2H2 and CO2). (Color codes in Figures 1A–1C: C, gray; N, blue; Si, yellow; F, light green; Ni, cyan.)
Results and discussion
Synthesis and structural characterization
Single crystals of SIFSIX-21-Ni, TIFSIX-4-Ni, SIFSIX-21-Cu, and TIFSIX-4-Cu, and microcrystalline samples of NbOFFIVE-3-Ni and NbOFFIVE-3-Cu were prepared as detailed in the supplemental information. Single-crystal X-ray diffraction (SCXRD) studies revealed that SIFSIX-21-Ni, TIFSIX-4-Ni, SIFSIX-21-Cu, and TIFSIX-4-Cu are isostructural and crystallize as pcu topology networks in orthorhombic space group Pnna. The crystallographic data and refinement parameters for SIFSIX-21-Ni, TIFSIX-4-Ni, SIFSIX-21-Cu, and TIFSIX-4-Cu are presented in Table S2. The unit cell parameters calculated from powder X-ray diffraction (PXRD) for NbOFFIVE-3-Cu and NbOFFIVE-3-Ni are close to those of the M’FSIX analogs (Figures S3 and S4). Unit cell volumes are as follows: TIFSIX-4-Cu (3,458.8 Å3)> SIFSIX-21-Cu (3,361.1 Å3)> NbOFFIVE-3-Cu (3,338 Å3)> TIFSIX-4-Ni (3,291.7 Å3)> NbOFFIVE-3-Ni (3,236 Å3)> SIFSIX-21-Ni (3,199.2 Å3). Solvent-accessible free volumes were calculated to be ca. 30%. Polycrystalline samples of SIFSIX-21-Ni, TIFSIX-4-Ni, SIFSIX-21-Cu, and TIFSIX-4-Cu used for physicochemical characterization and sorption studies were prepared solvothermally in methanol (see supplemental information for details). Bulk phase purity was established by comparison of experimental and calculated PXRD patterns (Figures S1 and S2). Thermogravimetric analysis and variable temperature PXRD (VT-PXRD) experiments revealed each material retained crystallinity as follows: 613 K (NbOFFIVE-3-Ni) > 573 K (TIFSIX-4-Ni) > 513 K (SIFSIX-21-Ni); 533 K (TIFSIX-4-Cu) > 513 K (NbOFFIVE-3-Cu) > 493 K (SIFSIX-21-Cu) (Figures S5–S10 and S13–S14).
Single-component gas isotherms and binding sites
To evaluate microporosity, N2 and CO2 adsorption isotherms were measured at 77 K and 195 K, respectively (Figures S15, S17, S19, S21, S23, and S25). Following the Rouquerol criteria,48 Brunauer-Emmett-Teller (BET) surface areas were experimentally determined from the N2 adsorption isotherms as follows: TIFSIX-4-Ni (931 m2g−1) > SIFSIX-21-Ni (871 m2g−1) > SIFSIX-21-Cu (839 m2g−1) > NbOFFIVE-3-Cu (805 m2g−1) > NbOFFIVE-3-Ni (761 m2g−1) > TIFSIX-4-Cu (747 m2g−1). C2H2 and CO2 single-component gas sorption isotherms were collected at 298 K and 273 K (Figures S16, S18, S20, S22, S24, and S26). All six HUMs were observed to exhibit higher affinity for C2H2 than CO2 with C2H2 uptakes >3.5 mmolg−1 and CO2 uptakes <2.0 mmolg−1 at 298 K and 1 bar (Figures 1D and 1E). SIFSIX-21-Ni was found to exhibit the highest C2H2 uptake (~4.05 mmolg−1), followed by NbOFFIVE-3-Cu (3.97 mmolg−1), SIFSIX-21-Cu (3.94 mmolg−1), TIFSIX-4-Ni (3.85 mmolg−1), NbOFFIVE-3-Ni (3.79 mmolg−1), and TIFSIX-4-Cu (3.53 mmolg−1) (Figures 1D and 1E). The stepped isotherms observed for SIFSIX-21-Ni (Figure S16) and SIFSIX-21-Cu (Figure S22) prompted us to conduct in situ PXRD measurements on these two HUMs. These measurements (Figures S11 and S12) reveal no significant change in the PXRD patterns with C2H2 loading until 1 bar indicated that only subtle structural changes occurred during C2H2 sorption. Isosteric heats of adsorption, Qst, were determined from virial fits of these experimental single-component gas isotherms. Qst values for CO2 at zero loading, Qst(CO2), were as follows: 19.8 kJ mol−1 (SIFSIX-21-Ni) < 24.0 kJ mol−1 (SIFSIX-21-Cu) < 25.1 kJ mol−1 (NbOFFIVE-3-Ni) < 25.8 kJ mol−1 (NbOFFIVE-3-Cu) < 27.0 kJ mol−1 (TIFSIX-4-Cu) < 27.5 kJ mol−1 (TIFSIX-4-Ni). Qst (C2H2) values were determined to be as follows: 36.3 kJ mol−1 (SIFSIX-21-Cu) < 36.7 kJ mol−1 (NbOFFIVE-3-Ni) < 37.9 kJ mol−1 (SIFSIX-21-Ni) < 40.6 kJ mol−1 (TIFSIX-4-Cu) < 41.3 kJ mol−1 (TIFSIX-4-Ni) < 41.9 kJ mol−1 (NbOFFIVE-3-Cu) (Figures 1F amd S27–S32; all virial fitting parameters are provided in Figures S33–S44). The differences between Qst(C2H2) and Qst(CO2), (ΔQst)AC = [Qst(C2H2) − Qst(CO2)], were as follows: 18.1 kJ mol−1 (SIFSIX-21-Ni) > 16.1 kJ mol−1 (NbOFFIVE-3-Cu) > 13.9 kJ mol−1 (TIFSIX-4-Ni) > 13.6 kJ mol−1 (TIFSIX-4-Cu) > 12.3 kJ mol−1 (SIFSIX-21-Cu) > 11.6 kJ mol−1 (NbOFFIVE-3-Ni). These Qst and ΔQst values reveal the relative thermodynamic preferences of the studied sorbents toward the competing sorbates, C2H2 and CO2. Adsorption selectivities were calculated using IAST.49 For C2H2/CO2 (v/v: 1:1 and 2:1) at 1 bar and 298 K, IAST selectivities (SAC) were calculated upon fitting the single-component isotherms to the dual-site Langmuir-Freundlich equation (see supplemental information for details; Figures S51, S52, and Table S3). SAC (1:1/2:1) values at 1 bar were thereby determined to be 10.0/9.4 (SIFSIX-21-Cu) > 9.5/9.0 (NbOFFIVE-3-Cu) > 8.3/8.1 (TIFSIX-4-Cu) > 7.8/7.8 (SIFSIX-21-Ni) > 7.6/7.4 (TIFSIX-4-Ni) > 6.0/5.9 (NbOFFIVE-3-Ni) (Figures S45–S50). As presented in Table S1, under relevant partial pressures, SAC for the six HUMs studied in this contribution were found to be comparable to leading C2H2-capture sorbents such as SIFSIX-Cu-TPA(5.3),29 MUF-17(6),50 ZJU-60a(6.7),51 FJU-22a(7.1),33 TCuBr (9.5),30 ZJUT-2a(10),52 TIFSIX-2-Cu-i(10),53 FJI-H8-Me(10.4),28 and MIL-100(Fe)(12.5).54
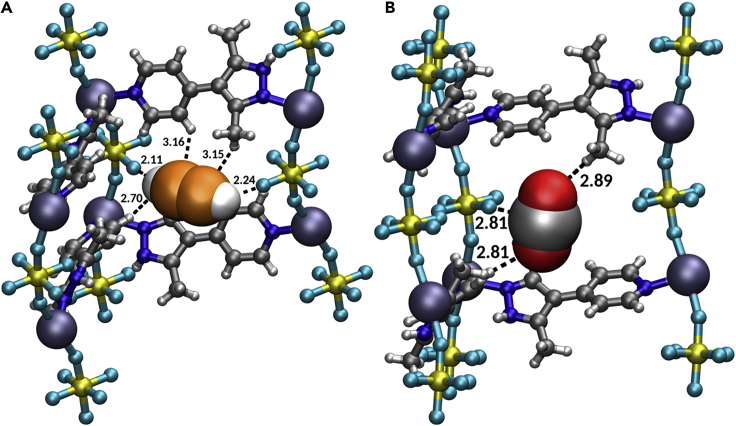
The binding sites for CO2 and C2H2 in SIFSIX-21-Ni were determined by simulated annealing calculations (Figure 2). The binding energies at 0.1 bar obtained from canonical Monte Carlo (CMC) were in agreement with the experimentally derived low loading Qst(C2H2) and Qst(CO2) obtained from single-component isotherms (Table S4). Results derived by these two simulation methods revealed that C2H2 molecules interact with a pair of diagonally opposite F atoms of the inorganic pillars (SiF62−) via two (C2H2)CHδ+···Fδ− interactions. There are also CH···C(C2H2) interactions. CO2 molecules form Cδ+···Fδ- and CH···O(CO2) interactions. To further understand these binding sites, density function theory (DFT) refinements were conducted on the strongest binding sites of SIFSIX-21-Ni as identified by CMC simulations in order to calculate the adsorption enthalpies at low loading (one adsorbate molecule per unit cell). Employing the BEEF-vdW functional,55 adsorption enthalpies of −45.3 and −30.1 kJ mol−1 were calculated for C2H2 and CO2, respectively. The adsorption enthalpy difference of 15 kJ mol−1 correlates well with experimental ΔQst values (Figure 1F). For the BEEF-vdW optimized structures (see supplemental information), the optimized binding pocket with adsorbed C2H2 has two short Fδ-···Hδ+ interactions (2.15 Å each, Figure S53A). For CO2, Fδ-···Cδ+ interactions of 3.69 Å and CH···O(CO2) interactions of 2.95 and 3.34 Å (Figure S53B) were determined. For CO2 adsorption, an alternate binding site with similar adsorption enthalpies (± 1 kJ mol−1) with shorter Fδ-···Cδ+ interactions of 3.10 Å and CH···O(CO2) interactions of 2.59 and 3.15 Å were observed (Figure S53C). Because the adsorbates maximized their interactions within the binding pocket (Figure S53), these enthalpies are higher than the Qst(C2H2) and Qst(CO2) determined from CMC simulations, the latter representing the average binding energy distribution at a certain pressure. Therefore, the loss in translational entropy was found to be higher for C2H2 than for CO2. Experimental Qst values representing a distribution averaged energy over all possible adsorption configurations of 16.1 kJmol−1 (CO2) and 40.6 kJmol−1 (C2H2) were found to be slightly lower than the adsorption enthalpies computationally predicted from the optimal binding sites. Therefore, one can conclude that the average residence time of C2H2 in its most stable binding site compared with that of CO2 is higher, suggesting that CO2 will exhibit faster kinetics. When the CO2 binding site of SIFSIX-21-Ni is compared with that of SIFSIX-3-Ni, unlike the single Cδ+···Fδ- binding interaction in SIFSIX-21-Ni, SIFSIX-3-Ni was found to exhibit four Cδ+···Fδ- binding interactions to electronegative F atoms from four independent SiF62– anions.56 Like SIFSIX-18-Ni-β, SIFSIX-21-Ni exhibits Cδ+···Fδ- and CH···O interactions with CO2, however SIFSIX-18-Ni-β was found to exhibit multiple CH···O interactions thanks to the presence of extra methyl groups in pzpz over pypz.11 Therefore, both SIFSIX-3-Ni and SIFSIX-18-Ni-β exhibit significantly higher CO2 binding energies compared with SIFSIX-21-Ni.11,56 Conversely, SIFSIX-21-Ni exhibits CHδ+···Fδ- interactions with C2H2 similar to those observed in SIFSIX-2-Cu-i and TIFSIX-2-Cu-i thanks to their “sweet spots” for C2H2 binding (F∙∙∙F distance of ca. 7 Å, see Figure 1B).13,53 In summary, the binding sites in the pypz HUMs reported herein combine key features that imply strong C2H2 affinity versus CO2: CHδ+···Fδ+ interaction-driven binding sites for C2H2; weak CO2-sorbent interactions.
Figure 2.
The binding sites in SIFSIX-21-Ni that result in strong C2H2 affinity versus CO2
(A and B) Views of (A) C2H2 binding sites and (B) CO2 binding sites in SIFSIX-21-Ni as determined from molecular simulations (C2H2 and CO2 molecules are shown in space-filling model whereas SIFSIX-21-Ni is presented in ball-and-stick model) (color codes: N, blue; Si, yellow; F, turquoise; Ni, lilac; O, red; H, white; C, gray, except in the C2H2 molecule: orange).
Adsorption kinetics
Kinetics is a key factor in determining the efficiency of gas separations.44 We, therefore, studied the pure gas adsorption kinetics for C2H2 and CO2 for each of the activated samples (SIFSIX-21-Ni, TIFSIX-4-Ni, NbOFFIVE-3-Ni, SIFSIX-21-Cu, TIFSIX-4-Cu, and NbOFFIVE-3-Cu) by exposure to a constant flow of 10 cm3min−1 of C2H2 or CO2 at 303 K and 1.0 bar (Figures S54 and S55). After 5 cycles of C2H2 sorption, the order of uptakes were as follows: SIFSIX-21-Cu (7.8%) < TIFSIX-4-Cu (9.7%) < NbOFFIVE-3-Cu (10%) for the Cu(II) HUMs; NbOFFIVE-3-Ni (4.2%) ~ TIFSIX-4-Ni (4.3%) < SIFSIX-21-Ni (5.9%) for the Ni(II) HUMs (Figures S54 and S55). Meanwhile, the corresponding order after 3 cycles of CO2 sorption were as follows: NbOFFIVE-3-Cu (0.73%) ~ SIFSIX-21-Cu (0.78%) < TIFSIX-4-Cu (1.5%) and SIFSIX-21-Ni (0.84%) ~ TIFSIX-4-Ni (0.85%) < NbOFFIVE-3-Ni (0.96%) for the Cu(II) and Ni(II) HUMs, respectively. The HUMs with the highest gravimetric uptakes, NbOFFIVE-3-Cu and SIFSIX-21-Ni, reached ca. 99% of their saturation uptakes in 54 and 35 min, respectively. For all HUMs, sorbent regeneration was conducted over five consecutive C2H2 adsorption/desorption cycles at 333 K under N2 flow in <30 min (flow rate: 20 cm3min−1; Figures S54 andS55) for C2H2.
Dynamic column breakthrough (DCB) studies
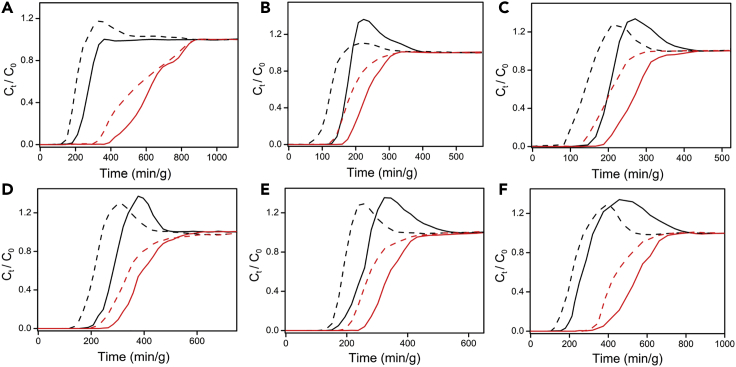
Next, we investigated the C2H2/CO2 separation performances of these HUMs through DCB experiments57 with inlet gas mixture ratios 1:1 or 2:1 (v/v) for C2H2/CO2.1 Binary gas mixtures were passed through a fixed-bed reactor (8 mm diameter) filled with ca. 0.5 g of each HUM with a total gas flow rate of 1 cm3min−1 at 1 bar and 298 K. Pre-activated samples were first heated at 333 K in a 20 cm3min−1 flow of He to remove atmospheric impurities as monitored by gas chromatographic (GC) analysis of the effluent gas stream. The sorbent beds were then cooled to room temperature under continuous He flow and subjected to DCB experiments. Eluted gaseous components were continuously monitored through GC analysis (see supplemental information for details, Figure S56). Figure 3 reveals that CO2 breakthrough occurred before that of C2H2 for each HUM and that SIFSIX-21-Ni was the best-performing sorbent, C2H2 breakthrough occurring at 363 and 298 min g−1 for the 1:1 and 2:1 gas mixtures, respectively. In contrast, the corresponding CO2 breakthrough occurred at 152 and 114 min g−1 for the 1:1 and 2:1 gas mixtures, respectively. The time lags of 211 and 184 min g−1 between C2H2 and CO2 breakthroughs for 1:1 and 2:1 gas mixtures, respectively, imply high C2H2 productivities.
Figure 3.
Experimental dynamic column breakthrough curves
(A–F) Binary C2H2/CO2 mixture-based DCB experimental curves at 298 K and 1 bar on the studied family of pypz HUM sorbents (v/v = 1:1, solid line; 2:1, dashed line; C2H2, red; CO2, black) (A), (B), and (C): SIFSIX-21-Ni, TIFSIX-4-Ni, and NbOFFIVE-3-Ni, respectively, and (D), (E), and (F): SIFSIX-21-Cu, TIFSIX-4-Cu, and NbOFFIVE-3-Cu, respectively (see details in the supplemental information).
GC data revealed that, for the 1:1 experiments, C2H2 levels in the effluent CO2 gas stream were 623, 910, 1,751, 2,368, 2,368, and, 2,718 ppm for SIFSIX-21-Ni, NbOFFIVE-3-Cu, TIFSIX-4-Cu, SIFSIX-21-Cu, TIFSIX-4-Ni, and NbOFFIVE-3-Ni, respectively. Unlike the low uptakes of CO2 registered in single-component isotherms (Figures 1D and 1E), 1:1 and 2:1 C2H2/CO2 DCB experiments (Figure 3) revealed higher adsorbed CO2 amounts indicated by coadsorption at lower partial pressures in dynamic experiments. Until C2H2 breakthrough occurred, CO2 purity values in the effluent streams were found to be as follows: >99.9% for SIFSIX-21-Ni and NbOFFIVE-3-Cu; >99.7% for TIFSIX-4-Cu, SIFSIX-21-Cu, TIFSIX-4-Ni, and NbOFFIVE-3-Ni, i.e., higher than the commercial specification for CO2 (N2.0, 99%). C2H2 uptakes calculated from the breakthrough curves for SIFSIX-21-Ni, NbOFFIVE-3-Cu, TIFSIX-4-Cu, SIFSIX-21-Cu, TIFSIX-4-Ni, and NbOFFIVE-3-Ni were observed to be 3.22, 3.08, 2.8, 3.07, 3.02, and 2.88 mmolg−1, respectively (see supplemental information for details)58,59; these values are consistent with the respective isotherm-based uptakes at 0.5 bar. The DCB experiments enabled calculation of the separation selectivities (αAC) for 1:1 and 2:1 C2H2:CO2 mixtures: SIFSIX-21-Ni (27.7/10.0) > NbOFFIVE-3-Cu (16.9/7.9) > NbOFFIVE-3-Ni (15.0/6.5) > TIFSIX-4-Cu (5.4/4.1) > SIFSIX-21-Cu (4.6/3.1) > TIFSIX-4-Ni (4.4/3.1). SIFSIX-21-Ni exceeds the separation selectivities reported for UTSA-74a (20.1), JXNU-5 (9.9), JCM-1 (4.4), FJU-89 (3), SNNU-45 (2.9), NKMOF-1-Ni (2.6), FJU-6-TATB (2.3), FJU-36a (2.1), HOF-3 (2), SIFSIX-Cu-TPA (1.97), FJU-22a (1.9), and FeNi-M’MOF (1.7) (Table S1). After full saturation in the equimolar DCB experiments, temperature programmed desorption measurements were conducted at 333 K (Figure S57). The desorption profiles revealed complete adsorbent regeneration in <120 min under a He flow of 20 cm3 min−1 for SIFSIX-21-Ni and NbOFFIVE-3-Cu.
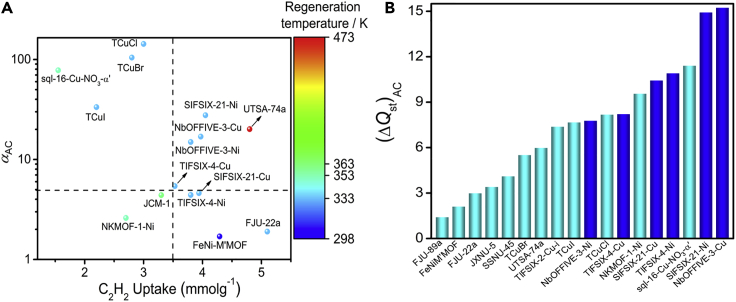
A mapping of αAC versus equilibrium single-component C2H2 uptakes at 1 bar indicates that SIFSIX-21-Ni, NbOFFIVE-3-Cu, NbOFFIVE-3-Ni, TIFSIX-4-Cu, and UTSA-74a have potential to address the trade-off between adsorption capacity and separation selectivity for C2H2/CO2 separation as they are the only sorbents that exhibit αAC ≥ 5 and adsorption capacity ≥ 3.5 mmolg−1 (see Figure 4A for a comparison of performance parameters). The UMCs in UTSA-74a require high temperature for activation (473 K under high vacuum (Figure 4A). Conversely, the HUM sorbents require heating to only 333 K for sorbent regeneration. Overall, SIFSIX-21-Ni outperforms the other HUMs reported herein like UTSA-74a and other known C2H2 selective sorbents thanks to its high separation selectivity (27.7), C2H2 uptake (4.0 mmolg−1), and low regeneration temperature. Interestingly, the high C2H2/CO2 separation selectivities for M’FSIX-pypz-M/NbOFFIVE-pypz-M can be attributed as much to weak CO2 binding as to strong C2H2 affinity as reflected in (ΔQst)AC values. SIFSIX-21-Ni exhibits a relatively low Qst(CO2) of 19.8 kJ mol−1 at low loading versus other C2H2 selective physisorbents (Table S1), resulting in a high (ΔQst)AC of 18.1 kJ mol−1. Indeed, (ΔQst)AC at low loading for SIFSIX-21-Ni is close to that of NKMOF-1-Ni (19.4) kJ mol−1 (Figure S59), which exhibits an exceptionally high Qst(C2H2) of 60.3 kJ mol−1. However, as Figure S58 reveals, the Qst(C2H2) for NKMOF-1-Ni rapidly declines to 46.0 kJ mol−1 at half loading, at which point (ΔQst)AC is reduced to 9.5 kJ mol−1 (Figure 4B). (ΔQst)AC values at zero coverage, although widely used, tend to overestimate separation performance at relevant partial pressures. In our experience, for most C2H2 selective sorbents, particularly those with high surface areas, Qst(C2H2) at half and full coverage decline from their zero loading values because of weak multilayer adsorption. Therefore, comparing (ΔQst)AC at low coverage does not always translate well to a prediction of relative binding affinity and is why we consider (ΔQst)AC at half loading to be a suitable metric to estimate relative binding for an equimolar mixture.42 Figure 4B reveals that NbOFFIVE-3-Cu and SIFSIX-21-Ni set new benchmarks of (ΔQst)AC values at half loading, 15.2 and 14.9 kJmol−1, respectively. This result is consistent with their DCB separation performances especially higher αAC relative to those of TIFSIX-4-Ni, SIFSIX-21-Cu, TIFSIX-4-Cu, and NbOFFIVE-3-Ni. Overall, (ΔQst)AC values of at least 7.5 kJmol−1 for all HUMs reported herein suggests thermodynamic preference for C2H2 over CO2 and correlates well with their high αAC values.
Figure 4.
Comparison of separation selectivity versus uptake capacity and ΔQst
(A) Comparison of C2H2/CO2 separation selectivity αAC (v/v = 1:1) and gravimetric C2H2 uptake at 1 bar in benchmark C2H2/CO2 separating adsorbents; regeneration/activation temperatures (range 298 to 473 K shown on the right side).
(B) Comparison of (ΔQst)AC for the best-performing adsorbents at half loadings.
Spectroscopic studies
In situ infrared spectroscopy
To further probe the binding sites of C2H2 and CO2 in these HUMs, we carried out in situ infrared (IR) spectroscopy measurements of C2H2 and CO2 adsorption in the two best-performing adsorbents, NbOFFIVE-3-Cu and SIFSIX-21-Ni. The difference spectra for NbOFFIVE-3-Cu (Figure 5) and SIFSIX-21-Ni (Figure S60) demonstrate characteristic stretching bands for adsorbed C2H2 and CO2 molecules, i.e., νas(C2H2) at 3,309–3,209 cm−1 and νas(CO2) at 2,338 cm−1, whereas the perturbations of vibrational bands in the HUMs are characterized by the decrease in ν(CH)phenyl peak intensity and its derivative feature in the shorter region, 1,700–1,000 cm−1. The perturbed bands v(CH)phenyl, ν(CC)phenyl, δ(CH)ip, phenyl, and ν(CN)phenyl (see Figures 5, S60, and S61) indicate that C2H2 and CO2 interact with the linker ligand’s phenyl rings,60 as predicted computationally (Figure 2). Analysis of these data revealed that concomitant loading of C2H2 perturbs the vibrational bands of the HUMs more than CO2, which is also indicative of stronger sorbent-sorbate interactions for C2H2. Particularly, two distinct νas(C2H2) bands are present at 3,209 and 3,309 cm−1 in both NbOFFIVE-3-Cu and SIFSIX-21-Ni. These bands suggest two types of C2H2 binding sites with different binding strengths. This is in contrast to adsorbed CO2, which displays a single peak at 2,338 cm−1 in the corresponding IR spectrum, indicating only a single type of adsorbed CO2 molecule. The 3,209 cm−1 IR band decays slightly slower than the one at 3,309 cm−1 (Figure S63), also implying stronger C2H2 binding inside the HUMs. Higher relative intensity suggests that the stronger binding sites are more populated with C2H2. C2H2 is well-known to be relatively acidic (pKa = 25) in the context of hydrocarbons and, thus, tends to form hydrogen bonding interactions, as observed in several MOMs.56,61 Similar to the well-studied OH or N-H stretch vibrations,62 the νas(C2H2) band undergoes a downward shift with respect to the gas phase value (at 3,287 cm−1) upon forming intermolecular hydrogen bonds. The 3,209 cm−1 band represents a red-shift of 78 cm−1 and is consistent with the C2H2 binding site identified by simulation in Figure 2A. Overall, these in situ IR studies support the high adsorption selectivities for C2H2 over CO2.
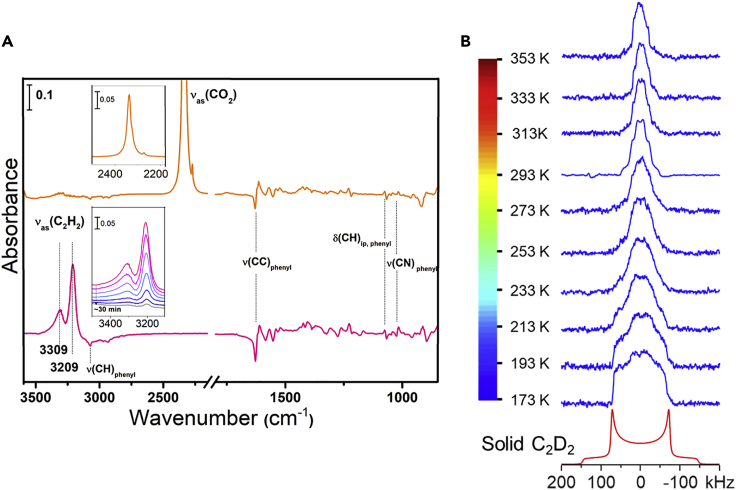
Figure 5.
In situ infrared spectra and 2H static NMR spectra
(A) Difference IR spectra showing the adsorbed CO2 (orange) and C2H2 (pink) upon loading at 298 K and 1 bar adsorbate pressure into NbOFFIVE-3-Cu and subsequent evacuation of the gas phase within 3 s, respectively. Each is referenced to the spectrum of activated HUMs. Top inset shows the νas(CO2) band and the bottom inset shows the decay of νas(C2H2) bands under vacuum. Notation and acronym: ν, stretching; δ, deformation; ip, in plane.
(B) Experimental 2H static NMR spectra of C2D2 adsorbed in NbOFFIVE-3-Cu (at a loading level of 0.4 C2D2 per Cu) as a function of temperature (blue lines) and simulated 2H spectrum of static C2D2 (red line).
Solid-state NMR spectroscopy
Solid-state NMR spectroscopy is a powerful technique for investigating the behavior of gaseous molecules adsorbed by porous materials.63, 64, 65 To better understand the adsorptive properties of the HUMs studied herein, 13C and 2H static solid-state NMR experiments were conducted to directly monitor the behavior of 13CO2 and C2D2 molecules adsorbed by NbOFFIVE-3-Cu. Figure S64 illustrates the 13C NMR spectra of NbOFFIVE-3-Cu loaded with 13C-labeled CO2 at various temperatures. At 373 K, the spectrum contains a relatively sharp, symmetric peak at 125 ppm superimposed on a very broad profile. Comparing the spectrum of the HUM loaded with CO2 with the spectrum of the empty HUM (Figure S64) indicates that the broad resonance in the spectrum of CO2-loaded HUM likely originates from the linkers in the framework. The position of the sharp signal indicates that this signal is from adsorbed CO2. The sharpness of the resonance suggests that CO2 molecules are rather mobile, implying weak interaction of CO2 with the framework. Considering that 13C enrichment of 13CO2 is 99% and that the framework carbon atoms are at natural abundance (1.1%), the relative intensity of the two signals suggests that a relatively small amount of CO2 was adsorbed by the HUM, which is consistent with the poor S/N ratio of the spectrum. Previous studies showed that for MOMs featuring good affinity toward CO2, the 13C spectra of adsorbed CO2 usually exhibited a significantly higher intensity under similar adsorption conditions.66, 67, 68 Overall, the 13C NMR results indicate that NbOFFIVE-3-Cu does not adsorb CO2 well. It is worth noting that the isotropic chemical shift of adsorbed CO2 (125 ppm) is the same as that of CO2 adsorbed in the diamagnetic MOMs,66, 67, 68 suggesting the lack of a significant paramagnetic interaction between CO2 and Cu(II). This is likely due to the distances between the carbon of CO2 and nearby paramagnetic metal ions being rather long, i.e., 8.03, 7.16, 6.03, 6.31 Å (Figure S65), and that CO2 is highly mobile. Similar situations have been observed in other Cu(II)-MOMs.69,70 Upon lowering the temperature, the sharp characteristic peak of CO2 gradually became broader and merged with the broad framework peak at 253 K. We attribute this to reduced mobility of CO2 with decreasing temperature and chemical shift anisotropy, which is largely averaged by molecular motions at higher temperatures, becoming dominant and broadening the signal.
In contrast to the 13C spectra of CO2 loaded in NbOFFIVE-3-Cu, the 2H static NMR spectra of C2D2 loaded in this HUM exhibited much stronger signals (Figure 5B), suggesting that NbOFFIVE-3-Cu has a higher affinity for C2D2 versus CO2 under the same loading conditions. The 2H static spectrum of adsorbed C2D2 at room temperature (293 K) exhibits a narrow pattern, suggesting a lack of significant paramagnetic interaction between the deuterons of C2D2 and Cu(II). This is consistent with the modeling study herein that indicates that two deuterium atoms in C2D2 are distant from nearby metal centers (5.1–6.4 Å). Inspection of the spectrum also reveals a well-defined line-shape with characteristic horns, shoulders, and “feet.” However, such a pattern cannot be simulated by a single site. Instead, analytical simulation using WSolids software71 revealed two components: a narrower component with a quadrupolar coupling constant (CQ) of 43 kHz and a non-zero asymmetry parameter (ηQ) of 0.60 and a broader component with a CQ of 65 kHz and ηQ of 0.0 (Figure S66B). The overall breadths of both patterns are markedly smaller than that of a static C2D2 (Figure 5B), which has a CQ of 198 kHz and ηQ = 0.72 This observation indicates that the first-order quadrupolar interaction is averaged by molecular motions experienced by C2D2. Seeing two separate patterns suggests that there are two types of C2D2 molecules in the unit cell and that they undergo different motions. In an attempt to identify the types of motions, dynamic simulations were performed by using the Express software package.73 Based on the simulation results, we propose that the narrower pattern results from the C2D2 molecules that simultaneously undergo two motions: (1) localized wobbling motion modeled by a C3 rotation and (2) a delocalized hopping about a C2 axis (Figure S66D). A small portion (around 10%) of C2D2 only wobbles at its absorption site (Figure S66C), yielding a broader line. The spectra between 293 and 353 K look similar, implying that the motions are in the fast exchange regime. In the temperature range 273–173 K, the pattern for each spectrum is gradually broadened and loses its characteristic discontinuities. The overall breadth of the pattern at 173 K is much broader than at 293 K, inferring that C2D2 adsorbed by the HUM becomes much less mobile. However, it is difficult to analyze the spectra at low temperature. When loading was increased, the 2H spectrum comprised a sharp peak in the middle and a broad component at the bottom (Figure S67). The sharp peak is likely due to the C2D2 molecules inside the pores undergoing fast exchange with the C2D2 outside the HUM. They do not interact with the framework strongly. The broad component is attributed to C2D2 molecules inside the pores that have strong interactions with the framework. The preference of C2H2 versus CO2 indicated by solid-state NMR spectra further supports our results from single-component sorption, DCB experiments, molecular modeling, and in situ FTIR spectroscopic studies.
Accelerated stability tests74 (313 K and 75% RH for 14 days) revealed that NbOFFIVE-3-Ni, SIFSIX-21-Cu, TIFSIX-4-Cu, and NbOFFIVE-3-Cu exhibit excellent hydrolytic stability (Figures S68C–S68F). Accelerated stability tests also revealed that both SIFSIX-21-Ni and TIFSIX-4-Ni (Figures S68A and S68B) underwent a phase change after exposure to humidity. Such phase changes have been observed in other HUMs and were attributed to inorganic pillar ligands being replaced by aqua ligands to afford corresponding sql networks.75 SIFSIX-21-Ni was regenerated by heating the humidity-exposed sample at 358 K for 10 h in MeOH (Figure S69), whereas TIFSIX-4-Ni was regenerated by heating at 393 K (Figure S70).
Conclusions
In summary, six new HUMs, SIFSIX-21-Ni, TIFSIX-4-Ni, NbOFFIVE-3-Ni, SIFSIX-21-Cu, TIFSIX-4-Cu, and NbOFFIVE-3-Cu, were studied with respect to their ability to separate C2H2 from CO2. Single-component sorption isotherms and equimolar C2H2/CO2 binary gas mixture DCB experiments revealed that four of these HUMs, SIFSIX-21-Ni, NbOFFIVE-3-Ni, TIFSIX-4-Cu, and NbOFFIVE-3-Cu, break the trade-off between high adsorption capacities ≥3.5 mmol·g−1 and high separation selectivities ≥5. SIFSIX-21-Ni outperformed all sorbents thanks to its benchmark separation selectivity (27.7) and high adsorption capacity (4 mmol·g−1). The key to the performance of SIFSIX-21-Ni is pore size and chemistry that enables relatively high surface area and strong C2H2 binding sites. This study once again highlights the ability of ultramicroporous sorbents to offer hitherto unattainable selectivity values for key binary gas separations.25
Experimental procedures
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Michael J. Zaworotko (xtal@ul.ie).
Materials availability
All materials generated in this study are available from the lead contact on request.
Data and code availability
The accession number for the crystal structures reported in this paper is Cambridge Crystallographic Data Centre, CCDC: 2052024, 2052025, 2052046, and 2052047.
Acknowledgments
M.J.Z. acknowledges the support of the Science Foundation Ireland (SFI awards 13/RP/B2549 and 16/IA/4624) and the European Research Council (award ADG 885695). T.P., K.A.F., and B.S. acknowledge the National Science Foundation (award no. DMR-1607989), including support from the Major Research Instrumentation Program (award no. CHE-1531590). P.E.K gratefully acknowledges the MacDiarmid Institute for Advanced Materials and Nanotechnology. Computational resources were made available by an XSEDE grant (no. TG-DMR090028) awarded to B.S. and by Research Computing at the University of South Florida. We thank Matthew Mostrom for his assistance with calculating the partial charges. K.T. acknowledges the U. S. Department of Energy, Office of Science, Basic Energy Sciences under award no. DE-SC0019902. Y.H. thanks the Natural Science and Engineering Research Council (NSERC) of Canada for a Discovery Grant. M.V. acknowledges the Irish Centre for High-End Computing (ICHEC) for the provision of computational facilities and support.
Author contributions
Conceptualization, N.K., S.M., and M.J.Z.; methodology, N.K. and S.M.; validation, N.K., S.M., N.C.H.-R., and A.A.B.; investigation, N.K., S.M., N.C.H.-R., A.A.B., K.T., V.M., L.M.v.W., K.O., and K.M.P.; formal analysis, M.V., T.P., and K.A.F.; resources, N.H.R. and A.K.; data curation, N.K., S.M., A.A.B., M.V., T.P., and K.A.F.; writing – original draft, N.K., S.M., N.H.R., A.A.B., K.T., V.M., M.V., T.P., Y.H., and M.J.Z; writing – review & editing, all authors; funding acquisition, K.T., L.B., B.S., Y.H., P.E.K., and M.J.Z; supervision, M.J.Z.
Declaration of interests
The authors declare no competing interests.
Published: August 10, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.chempr.2021.07.007.
Supplemental information
References
- 1.Pässler P., Hefner W., Buckl K., Meinass H., Meiswinkel A., Wernicke H.-J., Ebersberg G., Müller R., Bässler J., Behringer H., et al. Ullmann's Encyclopedia of Industrial Chemistry. Wiley; 2011. Acetylene. [Google Scholar]
- 2.Himbert G. In: Stang P.J., Diederich F., editors. Vol. 35. 1996. Book review: modern acetylene chemistry; pp. 2154–2155. (Angew. Chem. Int. Ed. Engl.). [Google Scholar]
- 3.Lower and upper explosive limits for flammable gases and vapors(LEL/UEL). https://www.chrysalisscientific.com/pg443-Lower-LEL-Upper-UEL-Explosive-Limits.pdf.
- 4.Gannon R.E., Krukonis V.J., Schoenberg T. Conversion of coal to acetylene in arc-heated hydrogen. Prod. R D. 1970;9:343–347. [Google Scholar]
- 5.Granada A., Karra S.B., Senkan S.M. Conversion of methane into acetylene and ethylene by the chlorine-catalyzed oxidative-pyrolysis (CCOP) process. 1. Oxidative pyrolysis of chloromethane. Ind. Eng. Chem. Res. 1987;26:1901–1905. [Google Scholar]
- 6.Guo C.J., Shen D., Bülow M. 18-O-03 - Kinetic separation of binary mixtures of carbon dioxide and C2 hydrocarbons on modified LTA-type zeolites. Studies in Surface Science and Catalysis. 2001;135:144. [Google Scholar]
- 7.Arpe H.-J., Weissermel K. Industrial Organic Chemistry. Fourth Edition Edition. Wiley-VCH Press; 2003. Aromatics — production and conversion; pp. 313–336. [Google Scholar]
- 8.Thanh, C.N., Didillon, B., Sarrazin, P., and Cameron, S. (1996). Selective hydrogenation catalyst and a process using that catalyst. US patent US6054409A, filed December 20, 1996, and granted April 25, 2000.
- 9.Xu G.L.F., Yang Y., Hu Y., Zhang K., Liu W. An improved CO2 separation and purification system based on cryogenic separation and distillation. Energies. 2014;7:3484–3502. [Google Scholar]
- 10.Nugent P., Belmabkhout Y., Burd S.D., Cairns A.J., Luebke R., Forrest K., et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature. 2013;495:80–84. doi: 10.1038/nature11893. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee S., Sikdar N., O’Nolan D., Franz D.M., Gascón V., Kumar A., et al. Trace CO2 capture by an ultramicroporous physisorbent with low water affinity. Sci. Adv. 2019;5:eaax9171. doi: 10.1126/sciadv.aax9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couck S., Denayer J.F.M., Baron G.V., Rémy T., Gascon J., Kapteijn F. An amine-functionalized MIL-53 metal−organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 2009;131:6326–6327. doi: 10.1021/ja900555r. [DOI] [PubMed] [Google Scholar]
- 13.Cui X., Chen K., Xing H., Yang Q., Krishna R., Bao Z., Wu H., Zhou W., Dong X., Han Y., et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science. 2016;353:141–144. doi: 10.1126/science.aaf2458. [DOI] [PubMed] [Google Scholar]
- 14.Li B., Cui X., O'Nolan D., Wen H.-M., Jiang M., Krishna R., Wu H., Lin R.-B., Chen Y.-S., Yuan D., et al. An ideal molecular sieve for acetylene removal from ethylene with record selectivity and productivity. Adv. Mater. 2017;29:1704210. doi: 10.1002/adma.201704210. [DOI] [PubMed] [Google Scholar]
- 15.Chen C., Wei Z., Pham T., Lan P.C., Zhang L., Forrest K.A., Chen S., Al-Enizi A.M., Nafady A., Su C., et al. Nanospace engineering of metal–organic frameworks through dynamic spacer installation of multifunctionalities for efficient separation of ethane from ethane/ethylene mixtures. Angew. Chem. Int. Ed. 2021;60:9680–9685. doi: 10.1002/anie.202100114. [DOI] [PubMed] [Google Scholar]
- 16.Lin R.-B., Xiang S., Zhou W., Chen B. Microporous metal-organic framework materials for gas separation. Chem. 2020;6:337–363. [Google Scholar]
- 17.Matsuda R., Kitaura R., Kitagawa S., Kubota Y., Belosludov R.V., Kobayashi T.C., Sakamoto H., Chiba T., Takata M., Kawazoe Y., Mita Y. Highly controlled acetylene accommodation in a metal–organic microporous material. Nature. 2005;436:238–241. doi: 10.1038/nature03852. [DOI] [PubMed] [Google Scholar]
- 18.Li J.R., Kuppler R.J., Zhou H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009;38:1477–1504. doi: 10.1039/b802426j. [DOI] [PubMed] [Google Scholar]
- 19.Sircar S. Basic Research needs for design of adsorptive gas separation processes. Ind. Eng. Chem. Res. 2006;45:5435–5448. [Google Scholar]
- 20.Cui W.G., Hu T.L., Bu X.H. Metal–organic framework materials for the separation and purification of light hydrocarbons. Adv. Mater. 2020;32:e1806445. doi: 10.1002/adma.201806445. [DOI] [PubMed] [Google Scholar]
- 21.Perry J.J., Perman J.A., Zaworotko M.J. Design and synthesis of metal–organic frameworks using metal–organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009;38:1400–1417. doi: 10.1039/b807086p. [DOI] [PubMed] [Google Scholar]
- 22.MacGillivray R. John Wiley & Sons; 2010. Metal-Organic Frameworks: Design and Application. [Google Scholar]
- 23.Schröder M., Banerjee M. Springer-Verlag; 2009. Functional Metal-Organic Frameworks: Gas Storage, Separation and Catalysis. [Google Scholar]
- 24.Kitagawa S., Kitaura R., Noro S.-i. Functional porous coordination polymers. Angew. Chem. Int. Ed. Engl. 2004;43:2334–2375. doi: 10.1002/anie.200300610. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee S., Sensharma D., Chen K.J., Zaworotko M.J. Crystal engineering of porous coordination networks to enable separation of C2 hydrocarbons. Chem. Commun. (Camb) 2020;56:10419–10441. doi: 10.1039/d0cc04645k. [DOI] [PubMed] [Google Scholar]
- 26.CCDC . 2020. New data and improvements - 2020.3 CSD data release.https://www.ccdc.cam.ac.uk/Community/blog/New_Data_and_improvements_2020.3_blog/ [Google Scholar]
- 27.Niu Z., Cui X., Pham T., Verma G., Lan P.C., Shan C., et al. A MOF-based ultra-strong acetylene nano-trap for highly efficient C2H2/CO2 separation. Angew. Chem. Int. Ed. Engl. 2021;60:5283–5288. doi: 10.1002/anie.202016225. [DOI] [PubMed] [Google Scholar]
- 28.Di Z., Liu C., Pang J., Chen C., Hu F., Yuan D., et al. Cage-like porous materials with simultaneous high C2H2 storage and excellent C2H2/CO2 separation performance. Angew. Chem. Int. Ed. Engl. 2021;60:10828–10832. doi: 10.1002/anie.202101907. [DOI] [PubMed] [Google Scholar]
- 29.Li H., Liu C., Chen C., Di Z., Yuan D., Pang J., Wei W., Wu M., Hong M. An unprecedented pillar–cage fluorinated hybrid porous framework with highly efficient acetylene storage and separation. Angew. Chem. Int. Ed. Engl. 2021;60:7547–7552. doi: 10.1002/anie.202013988. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee S., He Y., Franz D., Wang S.Q., Xian W.R., Bezrukov A.A., et al. Halogen–C2H2 binding in ultramicroporous metal–organic frameworks (MOFs) for benchmark C2H2/CO2 separation selectivity. Chem. Eur. J. 2020;26:4923–4929. doi: 10.1002/chem.202000008. [DOI] [PubMed] [Google Scholar]
- 31.Lee J., Chuah C.Y., Kim J., Kim Y., Ko N., Seo Y., Kim K., Bae T.H., Lee E. Separation of acetylene from carbon dioxide and ethylene by a water-stable microporous metal–organic framework with aligned imidazolium groups inside the channels. Angew. Chem. Int. Ed. Engl. 2018;57:7869–7873. doi: 10.1002/anie.201804442. [DOI] [PubMed] [Google Scholar]
- 32.Peng Y.L., Pham T., Li P., Wang T., Chen Y., Chen K.J., Forrest K.A., Space B., Cheng P., Zaworotko M.J., Zhang Z. Robust ultramicroporous metal–organic frameworks with benchmark affinity for acetylene. Angew. Chem. Int. Ed. Engl. 2018;57:10971–10975. doi: 10.1002/anie.201806732. [DOI] [PubMed] [Google Scholar]
- 33.Yao Z., Zhang Z., Liu L., Li Z., Zhou W., Zhao Y., et al. Extraordinary separation of acetylene-containing mixtures with microporous metal–organic frameworks with open O donor sites and tunable robustness through control of the helical chain secondary building units. Chem. Eur. J. 2016;22:5676–5683. doi: 10.1002/chem.201505107. [DOI] [PubMed] [Google Scholar]
- 34.Ye Y., Chen S., Chen L., Huang J., Ma Z., Li Z., Yao Z., Zhang J., Zhang Z., Xiang S. Additive-induced supramolecular isomerism and enhancement of robustness in Co(II)-based MOFs for efficiently trapping acetylene from acetylene-containing mixtures. ACS Appl. Mater. Interfaces. 2018;10:30912–30918. doi: 10.1021/acsami.8b11999. [DOI] [PubMed] [Google Scholar]
- 35.Li P., He Y., Zhao Y., Weng L., Wang H., Krishna R., et al. A rod-packing microporous hydrogen-bonded organic framework for highly selective separation of C2H2/CO2 at room temperature. Angew. Chem. Int. Ed. Engl. 2015;54:574–577. doi: 10.1002/anie.201410077. [DOI] [PubMed] [Google Scholar]
- 36.Liu L., Yao Z., Ye Y., Yang Y., Lin Q., Zhang Z., et al. Integrating the pillared-layer strategy and pore-space partition method to construct multicomponent MOFs for C2H2/CO2 separation. J. Am. Chem. Soc. 2020;142:9258–9266. doi: 10.1021/jacs.0c00612. [DOI] [PubMed] [Google Scholar]
- 37.Li Y.P., Wang Y., Xue Y.Y., Li H.P., Zhai Q.G., Li S.N., Jiang Y.C., Hu M.C., Bu X. Ultramicroporous building units as a path to bi-microporous metal–organic frameworks with high acetylene storage and separation performance. Angew. Chem. Int. Ed. Engl. 2019;58:13590–13595. doi: 10.1002/anie.201908378. [DOI] [PubMed] [Google Scholar]
- 38.Liu R., Liu Q.Y., Krishna R., Wang W., He C.T., Wang Y.L. Water-stable europium 1,3,6,8-Tetrakis(4-carboxylphenyl)pyrene framework for efficient C2H2/CO2 separation. Inorg. Chem. 2019;58:5089–5095. doi: 10.1021/acs.inorgchem.9b00169. [DOI] [PubMed] [Google Scholar]
- 39.Liu L., Yao Z., Ye Y., Chen L., Lin Q., Yang Y., Zhang Z., Xiang S. Robustness, selective gas separation, and nitrobenzene sensing on two isomers of cadmium metal–organic frameworks containing various metal–O–metal chains. Inorg. Chem. 2018;57:12961–12968. doi: 10.1021/acs.inorgchem.8b02212. [DOI] [PubMed] [Google Scholar]
- 40.Gao J., Qian X., Lin R.-B., Krishna R., Wu H., Zhou W., et al. Mixed metal–organic framework with multiple binding sites for efficient C2H2/CO2 separation. Angew. Chem. Int. Ed. 2020;59:4396–4400. doi: 10.1002/anie.202000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo F., Yan C., Dang L., Krishna R., Zhou W., Wu H., Dong X., Han Y., Hu T.L., O’Keeffe M., et al. UTSA-74: a MOF-74 isomer with two accessible binding sites per metal center for zhighly selective gas separation. J. Am. Chem. Soc. 2016;138:5678–5684. doi: 10.1021/jacs.6b02030. [DOI] [PubMed] [Google Scholar]
- 42.Kumar N., Mukherjee S., Bezrukov A.A., Vandichel M., Shivanna M., Sensharma D., et al. A square lattice topology coordination network that exhibits highly selective C2H2/CO2 separation performance. SmartMat. 2020;1 [Google Scholar]
- 43.Mukherjee S., Kumar A., Zaworotko M.J. In: Metal-Organic Frameworks (MOFs) for Environmental Applications. Ghosh S.K., editor. Elsevier; 2019. 2 - Metal-organic framework based carbon capture and purification technologies for clean environment; pp. 5–61. [Google Scholar]
- 44.Oschatz M., Antonietti M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018;11:57–70. [Google Scholar]
- 45.Scott H.S., Bajpai A., Chen K.-J., Pham T., Space B., Perry J.J., et al. Novel mode of 2-fold interpenetration observed in a primitive cubic network of formula [Ni(1,2-bis(4-pyridyl)acetylene)2(Cr2O7)]n. Chem. Commun. 2015;51:14832–14835. doi: 10.1039/c5cc05866j. [DOI] [PubMed] [Google Scholar]
- 46.Bhatt P.M., Belmabkhout Y., Cadiau A., Adil K., Shekhah O., Shkurenko A., et al. A fine-tuned fluorinated MOF addresses the needs for trace CO2 removal and air capture using physisorption. J. Am. Chem. Soc. 2016;138:9301–9307. doi: 10.1021/jacs.6b05345. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee S., Zaworotko M.J. Crystal engineering of hybrid coordination networks: From form to function. Trends in Chemistry. 2020;2:506–518. [Google Scholar]
- 48.Howarth A.J., Peters A.W., Vermeulen N.A., Wang T.C., Hupp J.T., Farha O.K. Best practices for the synthesis, activation, and characterization of metal–organic frameworks. Chem. Mater. 2017;29:26–39. [Google Scholar]
- 49.Myers A.L., Prausnitz J.M. Thermodynamics of mixed-gas adsorption. AIChE J. 1965;11:121–127. [Google Scholar]
- 50.Qazvini O.T., Babarao R., Telfer S.G. Multipurpose metal–organic framework for the adsorption of acetylene: ethylene purification and carbon dioxide removal. Chem. Mater. 2019;31:4919–4926. [Google Scholar]
- 51.Duan X., Zhang Q., Cai J., Yang Y., Cui Y., He Y., Wu C., Krishna R., Chen B., Qian G. A new metal–organic framework with potential for adsorptive separation of methane from carbon dioxide, acetylene, ethylene, and ethane established by simulated breakthrough experiments. J. Mater. Chem. A. 2014;2:2628–2633. [Google Scholar]
- 52.Wen H.M., Liao C., Li L., Yang L., Wang J., Huang L., et al. Reversing C2H2–CO2 adsorption selectivity in an ultramicroporous metal–organic framework platform. Chem. Commun. (Camb) 2019;55:11354–11357. doi: 10.1039/c9cc05997k. [DOI] [PubMed] [Google Scholar]
- 53.Chen K.-J., Scott H.S., Madden D.G., Pham T., Kumar A., Bajpai A., et al. Benchmark C2H2/CO2 and CO2/C2H2 separation by two closely related hybrid ultramicroporous materials. Chem. 2016;1:753–765. [Google Scholar]
- 54.Yoon J.W., Lee J.S., Lee S., Cho K.H., Hwang Y.K., Daturi M., et al. Adsorptive separation of acetylene from light hydrocarbons by mesoporous iron Trimesate MIL-100(Fe) Chem. Eur. J. 2015;21:18431–18438. doi: 10.1002/chem.201502893. [DOI] [PubMed] [Google Scholar]
- 55.Wellendorff J., Lundgaard K.T., Møgelhøj A., Petzold V., Landis D.D., Nørskov J.K., Bligaard T., Jacobsen K.W. Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys. Rev. B. 2012;85:235149. [Google Scholar]
- 56.Chen K.J., Madden D.G., Mukherjee S., Pham T., Forrest K.A., Kumar A., Space B., Kong J., Zhang Q.Y., Zaworotko M.J. Synergistic sorbent separation for one-step ethylene purification from a four-component mixture. Science. 2019;366:241–246. doi: 10.1126/science.aax8666. [DOI] [PubMed] [Google Scholar]
- 57.Rajendran A., Kariwala V., Farooq S. Correction procedures for extra-column effects in dynamic column breakthrough experiments. Chem. Eng. Sci. 2008;63:2696–2706. [Google Scholar]
- 58.Shen J., He X., Ke T., Krishna R., van Baten J.M.V., Chen R., Bao Z., Xing H., Dincǎ M., Zhang Z., et al. Simultaneous interlayer and intralayer space control in two-dimensional metal−organic frameworks for acetylene/ethylene separation. Nat. Commun. 2020;11:6259. doi: 10.1038/s41467-020-20101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z., Tan B., Wang P., Cui X., Xing H. Highly efficient separation of linear and branched C4 isomers with a tailor-made metal–organic framework. AIChE J. 2020;66:e16236. [Google Scholar]
- 60.Swoboda A.R., Kunze G.W. Infrared study of pyridine adsorbed on montmorillonite surfaces. Clays Clay Miner. 1964;13:277–288. [Google Scholar]
- 61.Nijem N., Wu H.H., Canepa P., Marti A., Balkus K.J., Thonhauser T., Li J., Chabal Y.J. Tuning the gate opening pressure of metal-organic frameworks (MOFs) for the selective separation of hydrocarbons. J. Am. Chem. Soc. 2012;134:15201–15204. doi: 10.1021/ja305754f. [DOI] [PubMed] [Google Scholar]
- 62.Marechal Y. Elsevier Science; 2007. The Hydrogen Bond and the Water Molecule: the Physics and Chemistry of Water, Aqueous and Bio-Media. [Google Scholar]
- 63.Wong Y.T.A., Martins V., Lucier B.E.G., Huang Y. Solid-state NMR spectroscopy: a powerful technique to directly study small gas molecules adsorbed in metal-organic frameworks. Chem. Eur. J. 2019;25:1848–1853. doi: 10.1002/chem.201803866. [DOI] [PubMed] [Google Scholar]
- 64.Bertmer M. Solid-state NMR of small molecule adsorption in metal–organic frameworks (MOFs) Annu. Rep. NMR Spectrosc. 2020;101:1–64. [Google Scholar]
- 65.Witherspoon V.J., Xu J., Reimer J.A. Solid-state NMR investigations of carbon dioxide gas in metal–organic frameworks: insights into molecular motion and adsorptive behavior. Chem. Rev. 2018;118:10033–10048. doi: 10.1021/acs.chemrev.7b00695. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y., Lucier B.E.G., Huang Y. Deducing CO2 motion, adsorption locations and binding strengths in a flexible metal–organic framework without open metal sites. Phys. Chem. Chem. Phys. 2016;18:8327–8341. doi: 10.1039/c5cp04984a. [DOI] [PubMed] [Google Scholar]
- 67.Lu Y., Lucier B.E.G., Zhang Y., Ren P., Zheng A., Huang Y. Sizable dynamics in small pores: CO2 location and motion in the α-Mg formate metal–organic framework. Phys. Chem. Chem. Phys. 2017;19:6130–6141. doi: 10.1039/c7cp00199a. [DOI] [PubMed] [Google Scholar]
- 68.Wu B., Wong Y.T.A., Lucier B.E.G., Boyle P.D., Huang Y. Exploring host-guest interactions in the α-Zn3(HCOO)6 metal-organic framework. ACS Omega. 2019;4:4000–4011. doi: 10.1021/acsomega.8b03623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen M., Chen S., Chen W., Lucier B.E.G., Zhang Y., Zheng A., Huang Y. Analyzing gas adsorption in an amide-functionalized metal organic framework: are the carbonyl or amine groups responsible? Chem. Mater. 2018;30:3613–3617. [Google Scholar]
- 70.Gul-E-Noor F., Mendt M., Michel D., Pöppl A., Krautscheid H., Haase J., et al. Adsorption of Small Molecules on Cu3(btc)2 and Cu3–xZnx(btc)2 Metal–Organic Frameworks (MOF) As Studied by Solid-State NMR. J. Phys. Chem. C. 2013;117:7703–7712. [Google Scholar]
- 71.Eichele K. Universität Tübingen; Tübingen, Germany: 2011. WSolids1, Version 1.20.15.http://anorganik.uni-tuebingen.de/klaus/soft/wsolids1/wsolids1.pdf [Google Scholar]
- 72.Millett F.S., Dailey B.P. NMR determination of some deuterium quadrupole coupling constants in nematic solutions. J. Chem. Phys. 1972;56:3249–3256. [Google Scholar]
- 73.Vold R.L., Hoatson G.L. Effects of jump dynamics on solid state nuclear magnetic resonance line shapes and spin relaxation times. J. Magn. Reson. 2009;198:57–72. doi: 10.1016/j.jmr.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Waterman K.C. In: Handbook of Stability Testing in Pharmaceutical Development: Regulations, Methodologies, and Best Practices. Huynh-Ba K., editor. Springer; 2009. Understanding and predicting pharmaceutical product shelf-life; pp. 115–135. [Google Scholar]
- 75.O’Nolan D., Kumar A., Zaworotko M.J. Water vapor sorption in hybrid pillared square grid materials. J. Am. Chem. Soc. 2017;139:8508–8513. doi: 10.1021/jacs.7b01682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the crystal structures reported in this paper is Cambridge Crystallographic Data Centre, CCDC: 2052024, 2052025, 2052046, and 2052047.