Abstract
Background
Invasive pulmonary aspergillosis (IPA) is increasingly recognized as a complication of severe influenza and coronavirus disease 2019. The extent to which other respiratory viral infections (RVIs) predispose to IPA is unclear.
Methods
We performed a retrospective review of IPA occurring within 90 days of respiratory syncytial virus (RSV), parainfluenza, or adenovirus infections (noninfluenza respiratory viral infections [NI-RVIs]) in patients who underwent solid organ transplant between 1/15/2011 and 12/19/2017.
Results
At a median post-transplant follow-up of 43.4 months, 221 of 2986 patients (7.4%) developed 255 RSV, parainfluenza, or adenovirus infections. IPA complicating these NI-RVIs was exclusively observed in lung and small bowel transplant recipients, in whom incidence was 5% and 33%, respectively. Cumulative prednisone doses >140mg within 7 days and pneumonia at the time of NI-RVI were independent risk factors for IPA (odds ratio [OR], 22.6; 95% CI, 4.5–112; and OR, 7.2; 95% CI, 1.6–31.7; respectively). Mortality at 180 days following NI-RVI was 27% and 7% among patients with and without IPA, respectively (P = .04).
Conclusions
In conclusion, IPA can complicate RSV, parainfluenza, and adenovirus infection in lung and small bowel transplant recipients. Future research is needed on the epidemiology of IPA complicating various RVIs. In the interim, physicians should be aware of this complication.
Keywords: invasive pulmonary aspergillosis, respiratory syncytial virus, parainfluenza, adenovirus, solid organ transplantation
Recent studies have drawn attention to invasive pulmonary aspergillosis (IPA) as a complication of coronavirus disease 2019 (COVID-19). At a number of centers, the incidence of IPA has ranged from 5% to 30% among critically ill patients with severe COVID-19 requiring intensive care unit (ICU) care [1–6]. Before the COVID-19 pandemic, IPA was increasingly recognized as a complication of influenza in ICU patients [7, 8]. The incidence of IPA among solid organ transplant (SOT) recipients with severe influenza has been reported to be as high as 34% [7]. Noninfluenza respiratory viral infections (NI-RVIs), in particular respiratory syncytial virus (RSV), parainfluenza (PIV), and adenovirus (ADV), are now recognized as underappreciated causes of community-acquired pneumonia [9, 10]. IPA has been observed following severe RSV and PIV infections among patients with hematologic malignancies, but incidence rates have not been reported [11]. Indeed, it is unclear whether NI-RVIs predispose patients to IPA (noninfluenza RVI-associated IPA [NI-IPA]). The goal of this study was to examine the association between RSV, PIV, and ADV infections and IPA among SOT recipients.
METHODS
Study Design
We retrospectively reviewed the electronic medical records of consecutive adults (>18 years old) who underwent SOT between January 15, 2011, and December 19, 2017, at the University of Pittsburgh Medical Center (UPMC) who had laboratory-confirmed NI-RVI caused by RSV, PIV, or ADV post-transplant through June 30, 2018. We excluded (a) patients who had an RVI with viruses other than ADV, PIV or RSV, (b) patients who had a positive Aspergillus respiratory fungal culture ≥7 days before RVI, and (c) patients who died within 30 days of SOT. Epidemiologic, clinical, microbiologic, radiographic, cytopathologic, and treatment data were extracted from inpatient and outpatient records.
Patient Consent
This study was reviewed by the Institutional Review Boards of the University of Pittsburgh and qualified for “Exempt” status as defined by federal regulation.
Definitions
Over the study period, ADV, PIV, and RSV infections were identified by polymerase chain reaction (PCR)–based multiplexed Luminex xTAG RVP assay (Luminex Corporation, Austin, TX, USA) of nasopharyngenal (NP) swabs and/or bronchoalveolar lavage (BAL) specimens. We included patients at the time of the initial RVI diagnosis. Multiple RVP tests of the same patient were excluded unless they were (a) diagnostic of different NI-RVIs or (b) obtained ≥6 months apart.
We classified NI-RVIs as (a) asymptomatic, (b) upper respiratory infections (URIs), or (c) lung infections (pneumonia) based on clinical and radiographic data. Asymptomatic NI-RVI was defined by absence of symptoms or signs of infection (eg, testing of BAL specimens from lung transplant recipients obtained during routine bronchoscopies for rejection surveillance). URI was defined as the presence of upper respiratory symptoms such as sore throat, nasal congestion, or cough in the absence of radiographic findings. Pneumonia was defined by (a) presence of acute onset fever, cough, dyspnea, or hypoxia (arterial oxygen saturation <90% on room air or an increase in baseline supplementary oxygen dose) and (b) typical chest radiography or computed tomography findings consistent with lung infection.
Potential cases of NI-IPA were identified by BAL/bronchial wash or lung biopsy specimen cultures positive for Aspergillus species within 90 days after NI-RVI. Nonculture diagnostic tests such as galactomannan detection were not available at our center during the study period. We classified IPA as proven or probable according to revised European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria [12]. Presumed IPA was defined as cases with positive Aspergillus respiratory culture that did not fulfill definitions of proven or probable IPA, but that were treated with a mold-active antifungal agent. In presenting and discussing data below, we will use “NI-IPA” to refer to proven and probable NI-IPA, unless presumed IPA is specifically mentioned. Aspergillus colonization was defined as positive respiratory culture that did not fulfill criteria for proven, probable, or presumed IPA.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics, version 23 (IBM, Armonk, NY, USA). Descriptive statistics and measures of central tendency were used where appropriate. Comparisons between groups were performed by Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables. Variables significant by univariate analysis (P ≤ .05) were entered into a multivariate logistic regression model to determine independent risk factors for NI-IPA. Kaplan-Meier curves were used to estimate (a) time to NI-RVI and (b) survival among NI-RVI patients with and without NI-IPA; the log-rank test was used to compare curves between groups. Significance was defined as P ≤ .05 (2-tailed).
RESULTS
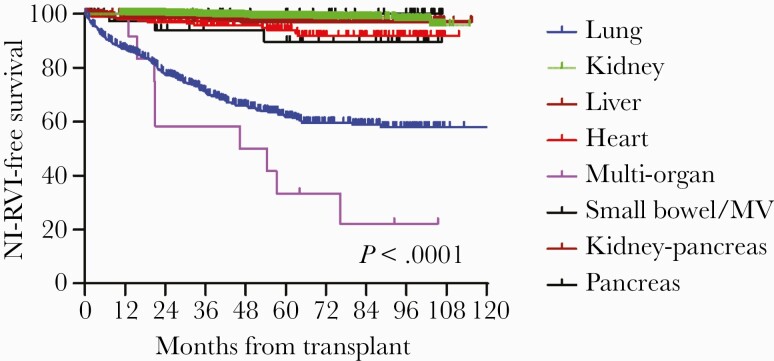
Over the study period, 2986 patients underwent SOT and fulfilled our inclusion criteria, including kidney (1254), lungs (711), liver (614), heart (233), kidney-pancreas (82), small bowel/multivisceral (42), pancreas (38), and multiple organ (12) recipients. The median length of post-SOT follow-up (range) was 43.4 (0.24–70.9) months. Two hundred twenty-one patients transplanted during the study period were diagnosed with 255 episodes of NI-RVI through June 30, 2018 (incidence, 7.4%, 221/2986). Thirty-two patients had >1 NI-RVI episode, and 4 patients were co-infected with 2 NI respiratory viruses (RVs). PIV was the most common cause of NI-RVI (44%, 113/255), followed by RSV (41%, 104/255) and ADV (15%, 38/255). Times to first episode of RVI are presented in Figure 1. Small bowel and lung transplant recipients were more likely to acquire RVI than were recipients of other organs (P < .0001).
Figure 1.
Time to noninfluenza respiratory virus infection, stratified by type of solid organ transplant. Abbreviations: MV, multi-visceral organ transplant; NI-RVI, noninfluenza respiratory virus infection.
Clinical characteristics of patients with NI-RVI, stratified by virus, are presented in Table 1. Receipt of belatacept at the time of diagnosis was more likely among patients with ADV infections (10%, 4/38) than among those with PIV (2%, 2/113) or RSV (0%, 0/104; P = .003) infections. Likewise, CMV viremia within 3 months of diagnosis was more likely among patients with ADV infections (16%, 6/38) than among those infected with PIV (1%, 1/113) or RSV (10%, 10/104; P = .001). Overall, 58% (147/255) of patients with NI-RVIs were admitted to the hospital; admission rates did not differ by virus. Among patients who required admission, those infected with ADV had significantly longer lengths of stay (median, 16 days) than those infected with PIV (median, 8 days) or RSV (median, 7 days; P = .04).
Table 1.
Characteristics of Solid Organ Transplant Recipients With Noninfluenza Respiratory Virus Infections
| Patient Characteristics | ADV (n = 38) | PIV (n = 113) | RSV (n = 104) | Total (n = 255) |
|---|---|---|---|---|
| Median time (IQR) from transplant to NI-RVI, mo | 10.8 (2–10.8) | 17.8 (6.5–31.6) | 19.7 (6.3–37.3) | 18.4 (5.9–35) |
| Demographics | ||||
| Age, median (IQR), y | 56.5 (45–65) | 57 (45–65) | 58 (49–65) | 57 (46–65) |
| Men | 50 (19) | 61 (69) | 64 (67) | 61 (155) |
| Organ transplanted | ||||
| Lung | 87 (33) | 78 (88) | 75 (78) | 78 (199) |
| Kidney | 3 (1) | 5 (6) | 6 (6) | 5 (13) |
| Liver | 0 (0) | 4 (4) | 9 (9) | 5 (13) |
| Heart | 3 (1) | 5 (6) | 6 (6) | 5 (13) |
| Small bowel | 0 (0) | 2 (2) | 1 (1) | 1 (3) |
| Kidney-pancreas | 3 (1) | 0 | 1 (1) | 1 (2) |
| Multiple | 5 (2) | 6 (7) | 3 (3) | 5 (12) |
| Immunosuppression at the time of NI-RVI | ||||
| Cyclosporine | 21 (8) | 20 (23) | 23 (24) | 21 (54) |
| Tacrolimus | 74 (28) | 80 (91) | 75 (78) | 77 (197) |
| Sirolimus/everolimus | 5 (2) | 18 (21) | 21 (22) | 17 (44) |
| Azathioprine | 5 (2) | 11 (13) | 10 (11) | 10 (26) |
| Mycophenolate mofetil | 79 (30) | 65 (74) | 62 (65) | 66 (169) |
| Belatacepta | 10 (4) | 2 (2) | 0 (0) | 2 (6) |
| Steroids | 92 (35) | 92 (104) | 84 (87) | 89 (226) |
| Alemtuzumab/antithymocyte globulin/rituximab within 3 mo of NI-RVI | 16 (6) | 9 (10) | 8 (8) | 9 (24) |
| Basiliximab within 3 mo of NI-RVI | 0 | 2 (2) | 6 (6) | 3 (8) |
| Acute rejection requiring bolus steroids within 3 mo before NI-RVIb | 29 (11) | 23 (26) | 12 (12) | 19 (49) |
| Acute rejection requiring bolus steroids within 1 mo before NI-RVI | 10 (4) | 12 (13) | 10 (11) | 11 (28) |
| Clinical characteristics at the time of NI-RVI | ||||
| ESRD on RRT | 5 (2) | 7 (8) | 13 (14) | 9 (24) |
| CMV infectionc | 16 (6) | 1 (1) | 10 (10) | 7 (17) |
| Lung involvement (pneumonia) | 24 (9) | 29 (33) | 24 (25) | 26 (67) |
| Hospital admission | 66 (25) | 54 (61) | 59 (61) | 58 (147) |
| Median inpatient length of stay (range), d | 16 (2–114) | 8 (1–150) | 7 (1–371) | 8 (1–371) |
Data are presented as % (No.) unless otherwise indicated. There were only 3 patients with neutropenia in our cohort, with absolute neutrophil counts of 920, 1150, and 1320/mm3 at the time of NI-RVI diagnosis.
Abbreviations: ADV, adenovirus; CMV, cytomegalovirus; ESRD, end-stage renal disease; IQR, interquartile range; NI-RVIs, noninfluenza respiratory virus infections; PIV, parainfluenza virus; RRT, renal replacement therapy; RSV, respiratory syncytial virus.
P = .003; 10% (4/38) of patients with ADV vs 1% (2/217) with RVP due to PIV or RSV infections received belatacept before RVI (P = .005).
P = .02; 92% (139/151) of patients with either ADV or PIV infections had received corticosteroid at the time of NI-RVI diagnosis vs those with RSV infection (P = .04).
P = .001; 16% (6/38) of patients with ADV vs 5% (11/217) with RVP due to PIV or RSV infections had CMV infection within 30 days before NI-RVI (P = .03).
Twenty-six percent (67/255), 47% (120/255), and 27% (68/255) of NI-RVIs were lung infections, URIs, and asymptomatic, respectively. Fifty-seven percent (146/255) and 64% (164/255) of patients with an NI-RVI were treated with an antiviral agent and adjunctive corticosteroids, respectively. Sixty-four percent (67/104) of RSV-infected patients received ribavirin (63 oral, 2 inhaled, 1 intravenous, and 1 combination oral and inhaled). Sixty-seven percent (76/113) of PIV-infected patients received ribavirin (71 oral and 5 inhaled). Five percent (2/38) and 3% (1/38) of ADV-infected patients received cidofovir and brincidofovir, respectively.
NI-IPA
Aspergillus spp. were recovered from a lower respiratory culture within 90 days of NI-RVI in 17% (43/255) of patients. Eleven cases fulfilled a definition of proven (n = 3) or probable (n = 8) NI-IPA, corresponding to a disease incidence of 4% (11/255) in patients with NI-RVI. Disease manifestations were disseminated aspergillosis (including IPA, n = 1), both IPA and endobronchial infection (n = 1), and IPA (n = 9). Seven additional cases (3% incidence) were defined as presumed NI-IPA, as patients did not fulfill criteria for proven or probable NI-IPA but were treated with antifungals. In the remaining 25 cases (10% incidence), positive cultures represented Aspergillus colonization.
Ninety-one percent (10/11) of patients with proven or probable NI-IPA were lung or heart-lung transplant recipients (including a patient who underwent heart-lung and kidney transplantation); the other patient was a small bowel recipient. The incidence of NI-IPA was 5% (10/207) and 33% (1/3) among lung and small bowel recipients with NI-RVI, respectively. The median time from diagnosis of NI-RVI to diagnosis of NI-IPA (interquartile range [IQR]) was 30 (0–60) days; the median time to NI-IPA was shorter among patients with lower RVI than among those with upper RVI (17 days vs 80.5 days; P = .08). Thirty-six percent (4/11) and 64% (7/11) of proven/probable NI-IPA occurred within 8 days and >17 days after NI-RVI, respectively; cases were diagnosed at a median (range) 10.8 (1.3–53.6) months post-SOT. Causative species were A. fumigatus (n = 6) and A. flavus, A. lentulus, A. terreus, A. ustus, and A. versicolor (n = 1 each). Eighty-two percent (9/11) of patients with NI-IPA were admitted at the time of RVI diagnosis.
Risk Factors for NI-IPA
Cumulative steroid dose within 7 days of diagnosing NI-RVI and presence of pneumonia at the time of NI-RVI were significant risk factors for NI-IPA by univariate analysis, as well as independent risk factors for NI-IPA by multivariate analysis (Table 2). By receiver operating characteristic analysis, a cumulative prednisone dose of 140mg within 7 days of NI-RVI was the factor that best differentiated between patients with and without NI-IPA. Thirty-one percent (5/16) of patients receiving >140mg of prednisone developed NI-IPA, compared with 3% (6/207) of those receiving <140mg of prednisone. Thirty-nine percent (99/255) of NI-RVI episodes were associated with antecedent antifungal utilization. There was no difference in the rate of NI-IPA among patients receiving or not receiving antifungals at the time of NI-RVI diagnosis.
Table 2.
Risk Factors for Noninfluenza Invasive Pulmonary Aspergillosis
| Risk Factor | NI-IPA (n = 11) | No IPAa (n = 213) | Univariate Analysis P Value | Multivariate Analysis P Value | Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| Median age at time of NI-RVI (IQR), y | 48 (34–63) | 57 (45–65) | .3 | ||
| Median number of months from transplant to NI-RVI (IQR) | 10.8 (8.4–42.4) | 19.0 (5.1–37.8) | .9 | ||
| Organ transplanted | |||||
| Lung or heart-lung | 82 (9) | 75 (157) | .31 | ||
| Kidney | 0 | 6 (13) | |||
| Liver | 0 | 6 (13) | |||
| Heart | 0 | 6 (13) | |||
| Small bowel/multivisceral | 10 (1) | 1 (2) | |||
| Kidney-pancreas | 0 | 1 (2) | |||
| Multi-organb | 10 (1) | 5 (10) | |||
| Immunosuppression | |||||
| Cumulative dose of prednisone equivalent within 7 d of NI-RVP, mg | 96 (35–140) | 35 (25–70) | .02 | ||
| Cumulative dose >140mg of prednisone equivalent within 7 d of NI-RVP | 45 (5) | 5 (11) | <.0001 | <.0001 | 22.6 (4.5–112) |
| Alemtuzumab, thymoglobulin, or rituximab | 20 (2) | 9 (20) | .26 | ||
| Basiliximab | 18 (2) | 3 (6) | .052 | ||
| Tacrolimus | 70 (7) | 76 (160) | .71 | ||
| Cyclosporine A | 20 (2) | 23 (48) | 1 | ||
| Sirolimus/everolimus | 10 (1) | 17 (37) | 1 | ||
| Mycophenolate | 60 (6) | 65 (137) | .74 | ||
| Azathioprine | 10 (1) | 10 (22) | 1 | ||
| Belatacept | 10 (1) | 2 (5) | .25 | ||
| Renal replacement therapy | 0 | 11 (24) | .61 | ||
| Respiratory viruses | .048 | .053 | |||
| Adenovirus | 27 (3) | 15 (32) | |||
| Parainfluenza virus | 64 (7) | 41 (87) | |||
| RSV | 9 (1) | 44 (94) | |||
| Lung involvement (pneumonia) | 64 (7) | 25 (54) | .01 | .009 | 7.2 (1.6–31.7) |
| CMV viremia | 18 (2) | 6 (13) | .16 | ||
| Steroid therapy added within 30 d after RVI diagnosis | 82 (9) | 61 (130) | .22 | ||
| Length of stay, median (IQR), d | 11 (6–22) | 8 (4–24) | .48 | ||
Data are presented as % (No.) unless otherwise indicated. There was no difference in NI-RVI manifestations (asymptomatic vs symptomatic [either URI or pneumonia]) and receipt of NI-RVI treatment between patients with or without NI-IPA.
Abbreviations: CMV, cytomegalovirus; IQR, interquartile range; IPA, invasive pulmonary aspergillosis; NI-IPA, noninfluenza invasive pulmonary aspergillosis; NI-RVIs, noninfluenza respiratory virus infections; RSV, respiratory syncytial virus; RVI, respiratory viral infection.
Thirty-one patients with presumed IPA or Aspergillus colonization were excluded from the analysis.
One patient underwent combined heart-lung and kidney transplant.
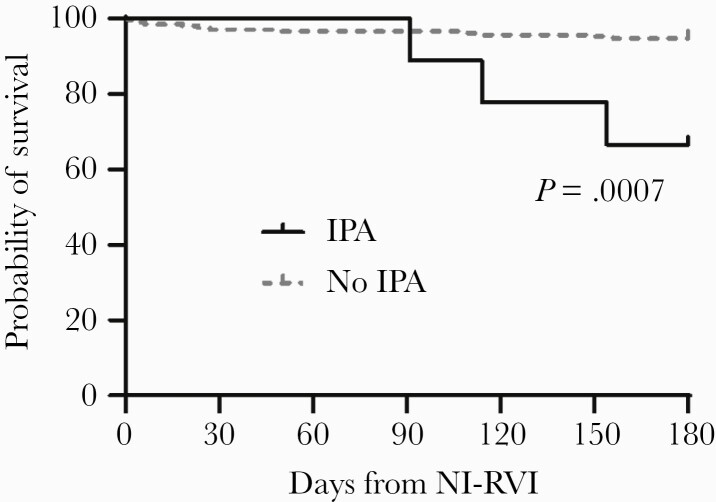
Mortality
Mortality at 180 days post-NI-RVI was significantly higher among patients with NI-IPA than among patients without NI-IPA (P = .0009) (Figure 2).
Figure 2.
Mortality of patients with noninfluenza respiratory virus infection, stratified by presence or absence of invasive pulmonary aspergillosis. Abbreviations: IPA, invasive pulmonary aspergillosis; NI-RVI, noninfluenza respiratory virus infection.
DISCUSSION
To our knowledge, this is the largest study of NI-IPA among SOT recipients. Our experience with 221 patients with NI-RVI was notable for several findings. First, NI-IPA occurred exclusively in lung and small bowel transplant recipients, with an incidence of 5% and 33%, respectively, for proven and probable cases. These findings were broadly in keeping with TRANSNET data, which showed that lung and small bowel recipients had the highest probability of invasive fungal infections among SOT recipients [13]. Second, higher cumulative steroid doses within 7 days of NI-RVI and pneumonia at the time of NI-RVI were independent risk factors for NI-IPA. Lastly, mortality among patients with NI-IPA was higher than mortality among patients with NI-RVI but no NI-IPA (P = .0009). Taken together, these data expand our knowledge of the link between RVIs and IPA and provide important insights for clinicians and researchers.
IPA as a complication of RVIs has been best studied following severe influenza (influenza-associated pulmonary aspergillosis [IAPA]). Among critically ill patients with influenza, the incidence of IAPA is 7%–19% [7, 8]. IPA has also been linked to COVID-19 (CAPA), with reported rates among critically ill patients ranging from 5% to 30% at certain centers [1–5]. Our study of NI-IPA included SOT recipients who ranged from asymptomatic outpatients diagnosed by surveillance bronchoscopy to critically ill, intubated patients with pneumonia. In contrast, studies of IAPA and CAPA have predominantly included intubated patients in the ICU. The incidence of IAPA or CAPA would be lower if all patients with influenza and COVID-19 were included, rather than simply those who were critically ill. Receipt of steroids was a common risk factor for IAPA [7, 8] and CAPA [2, 4, 6, 8]. In our patients, cumulative prednisone doses >140mg within 7 days of NI-RVI increased the odds of NI-IPA by 23-fold (95% CI, 4–112), and pneumonia at time of NI-RVI diagnosis increased the odds by 7-fold (95% CI, 2–32). Mortality at 180 days from NI-RVI of patients with and without NI-IPA was 27% (3/11) and 7% (14/213), respectively. In previous studies, 90-day mortality of IAPA was almost double that of influenza patients in the ICU without IAPA (51% vs 28%) [8]. Likewise, mortality rates of ICU patients with and without CAPA were 58% and 38%, respectively [6].
The pathophysiology of NI-IPA is multifactorial. The human respiratory tract is a reservoir of diverse commensals, which can act as pathogens (pathobionts) under suitable circumstances [14]. Colonization is a crucial step in the pathogenesis of IPA. The cumulative incidence of airway colonization by molds ranges from 20% to 50% among lung transplant recipients [15, 16], with Aspergillus spp. predominating. In our study, 17% of SOT recipients and 20% of lung transplant recipients with NI-RVI had respiratory cultures that grew Aspergillus within 90 days after an NI-RVI diagnosis. However, most Aspergillus-positive cultures here represented colonization (60%) rather than proven, probable (26%), or presumed (14%) NI-IPA. RVs can damage the epithelial layer, leading to denudation, basement membrane exposure, and subsequent pathobiont adherence and invasion [14]. Likewise, RV damage of ciliated cells can impair pathobiont clearance. Steroids, particularly at higher doses, can compromise alveolar macrophage phagocytosis, predisposing to invasion by Aspergillus [17]. Steroids and other immunosuppressive drugs also impact adaptive immunity. Specific RV strains may differ in the intensity by which they influence the above factors, and in their relative virulence. We found that ADV and PIV were associated with a higher rate of NI-IPA than RSV (9%, 7%, and 1%, respectively; P = .048) by univariate analysis, but this observation was not confirmed by multivariate analysis (P = .053). Nevertheless, this trend was noteworthy and should be specifically addressed in future studies. ADV infection, unlike other RVs, can be caused by reactivation of latent infection in SOT recipients. This difference might explain why ADV infection was more common among patients receiving betalacept, a fusion protein that selectively inhibits T-cell activation.
We demonstrated that time to diagnosis of NI-IPA after NI-RVI followed a biphasic pattern. Thirty-six percent of patients with NI-RVI had Aspergillus recovered within 8 days of NI-RVI diagnosis (early onset). The remaining 64% patients had Aspergillus recovered between days 17 and 90 (late onset). The timeline of early-onset NI-IPA is similar to that of IAPA, in which Aspergillus infection typically occurs within 5 days of influenza diagnosis [8]. Short timelines are likely associated with high viral loads and significant airway damage. Later-onset NI-IPA more closely resembles CAPA, which is diagnosed at a median of 15 days following diagnosis of COVID-19 [4]. Later onset of IPA after COVID-19 and NI-RFI might stem from effects of corticosteroids, which are often used to treat both types of viral infection. Steroid augmentation is commonly employed after a diagnosis of NI-RVI in lung transplant recipients at our institution and many other centers to reduce risk of RV-induced chronic allograft dysfunction [18, 19].
The higher incidence of NI-RVI among lung transplant recipients is likely attributable, in part, to more aggressive immunosuppressive regimens than used in other SOT recipients and to direct exposure of the allograft to the external environment. It is also plausible that lung transplant recipients have a lower threshold to seek care and get tested for RVI because respiratory symptoms involve their transplanted organ. Detection of RVIs is more likely at lung transplant programs like ours, where BAL samples obtained during post-transplant surveillance bronchoscopies are routinely screened for RVs.
This study has several limitations. First, it is retrospective and subject to potential selection and confounding biases. Second, we did not include other RVs such as influenza virus, human metapneumovirus, rhinovirus, human coronavirus, etc. Third, culturing of respiratory samples was not standardized, but rather dependent upon the discretion of individual physicians. The incidence of NI-IPA might have been higher than reported here if we only included patients with more severe NI-RVI requiring hospitalization or ICU admission or if fungal biomarkers were incorporated into diagnoses. Finally, the relative small number of patients included here limited our ability to examine other predisposing factors for NI-IPA.
In conclusion, the incidence of proven or probable NI-IPA among SOT recipients with NI-RVI at our center was 4%. NI-IPA was diagnosed exclusively among lung and small bowel recipients. Independent risk factors for NI-IPA were receipt of >140mg of prednisone within 7 days of RVI diagnosis and presence of viral pneumonia. If our findings are confirmed by other studies, trials of antifungal prophylaxis would be warranted in a subset of lung and small bowel transplant recipients with NI-RVI. NI-IPA had features that were distinct from those of other types of viral-associated aspergillosis, including IAPA and CAPA, as well as features that were shared with these diseases. RVIs should be recognized as nonclassical risk factors for invasive mold infections.
Acknowledgments
The authors thank Lloyd Clarke, who assisted with data extraction for this study.
Financial support. This study was supported in part by an award from the National Institutes of Health (R21AI153575 to M.H.N.).
Potential conflicts of interest. C.J.C. has been awarded investigator-initiated research grants from Astellas, Merck, Melinta, and Cidara for studies unrelated to this project, served on advisory boards or consulted for Astellas, Merck, the Medicines Company, Cidara, Scynexis, Shionogi, Qpex, and Needham & Company, and spoken at symposia sponsored by Merck and T2Biosystems. M.H.N. has been awarded investigator-initiated research grants from Astellas, Merck, Pulmocide, and Scynexis for studies unrelated to this project and served on advisory boards or consulted for Astellas, Pulmocide, and Scynexis.
Patient consent. The design of this study was approved by the UPMC Institutional Review Board.
References
- 1. Apostolopoulou A, Esquer Garrigos Z, Vijayvargiya P, Lerner AH, Farmakiotis D.. Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: a systematic review of the literature. Diagnostics (Basel). 2020; 10:807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartoletti M, Pascale R, Cricca M, et al. . Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study [published online ahead of print July 28, 2020]. Clin Infect Dis. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoenigl M. Invasive fungal disease complicating COVID-19: when it rains it pours. Clin Infect Dis. 2021; 73:e1645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Permpalung N, Chiang TP, Massie AB, et al. . COVID-19 associated pulmonary aspergillosis in mechanically ventilated patients [published online ahead of print March 9, 2021]. Clin Infect Dis. doi: 10.1093/cid/ciab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salmanton-Garcia J, Sprute R, Stemler J, et al. . COVID-19-associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis 2021; 27:1077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White PL, Dhillon R, Cordey A, et al. . A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2021; 73:e1634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. ; Dutch-Belgian Mycosis study group . Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 2018; 6:782–92. [DOI] [PubMed] [Google Scholar]
- 8. Verweij PE, Rijnders BJA, Brüggemann RJM, et al. . Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med 2020; 46:1524–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nichols WG, Peck Campbell AJ, Boeckh M.. Respiratory viruses other than influenza virus: impact and therapeutic advances. Clin Microbiol Rev 2008; 21:274–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marr KA, Carter RA, Boeckh M, et al. . Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100:4358–66. [DOI] [PubMed] [Google Scholar]
- 11. Magira EE, Chemaly RF, Jiang Y, et al. . Outcomes in invasive pulmonary aspergillosis infections complicated by respiratory viral infections in patients with hematologic malignancies: a case-control study. Open Forum Infect Dis 2019; 6:ofz247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donnelly JP, Chen SC, Kauffman CA, et al. . Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pappas PG, Alexander BD, Andes DR, et al. . Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50:1101–11. [DOI] [PubMed] [Google Scholar]
- 14. Bosch AA, Biesbroek G, Trzcinski K, et al. . Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog 2013; 9:e1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am 2003; 17:113–34, viii. [DOI] [PubMed] [Google Scholar]
- 16. Chang A, Musk M, Lavender M, et al. . Epidemiology of invasive fungal infections in lung transplant recipients in Western Australia. Transpl Infect Dis 2019; 21:e13085. [DOI] [PubMed] [Google Scholar]
- 17. Philippe B, Ibrahim-Granet O, Prévost MC, et al. . Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun 2003; 71:3034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bharat A, Kuo E, Saini D, et al. . Respiratory virus-induced dysregulation of T-regulatory cells leads to chronic rejection. Ann Thorac Surg 2010; 90:1637–44; discussion 1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisher CE, Preiksaitis CM, Lease ED, et al. . Symptomatic respiratory virus infection and chronic lung allograft dysfunction. Clin Infect Dis 2016; 62:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]