Abstract
BACKGROUND
Delayed cerebral vasospasm is a feared complication of aneurysmal subarachnoid hemorrhage (SAH).
OBJECTIVE
To investigate the relationship of systemic inflammation, measured using the systemic immune-inflammation (SII) index, with delayed angiographic or sonographic vasospasm. We hypothesize that early elevations in SII index serve as an independent predictor of vasospasm.
METHODS
We retrospectively reviewed the medical records of 289 SAH patients for angiographic or sonographic evidence of delayed cerebral vasospasm. SII index [(neutrophils × platelets/lymphocytes)/1000] was calculated from laboratory data at admission and dichotomized based on whether or not the patient developed vasospasm. Multivariable logistic regression and receiver operating characteristic (ROC) analysis were performed to determine the ability of SII index to predict the development of vasospasm.
RESULTS
A total of 246 patients were included in our study, of which 166 (67.5%) developed angiographic or sonographic evidence of cerebral vasospasm. Admission SII index was elevated for SAH in patients with vasospasm compared to those without (P < .001). In univariate logistic regression, leukocytes, neutrophils, lymphocytes, neutrophil-lymphocyte ratio (NLR), and SII index were associated with vasospasm. After adjustment for age, aneurysm location, diabetes mellitus, hyperlipidemia, and modified Fisher scale, SII index remained an independent predictor of vasospasm (odds ratio 1.386, P = .003). ROC analysis revealed that SII index accurately distinguished between patients who develop vasospasm vs those who do not (area under the curve = 0.767, P < .001).
CONCLUSION
Early elevation in SII index can independently predict the development of delayed cerebral vasospasm in aneurysmal SAH.
Keywords: Subarachnoid hemorrhage, Inflammation, Vasospasm, Aneurysm, Systemic immune-inflammation index
ABBREVIATIONS
- ALC
absolute lymphocyte count
- ANC
absolute neutrophil count
- CNS
central nervous system
- DND
delayed neurological deterioration
- EBI
early brain injury
- NLR
neutrophil-lymphocyte ratio
- PLT
platelet count
- SII
systemic immune-inflammation
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- TCD
transcranial Doppler ultrasonography
Subarachnoid hemorrhage (SAH) is a life-threatening neurological event commonly resulting from ruptured intracranial aneurysms. SAH accounts for 5% of strokes annually with high rates of mortality and long-term disability.1 One of the feared complications of SAH is delayed cerebral vasospasm, defined as arterial narrowing of large cerebral vessels typically occurring between 3 and 14 d after SAH in up to 70% of patients.2,3 In those who survive the initial rupture, vasospasm is a major cause of morbidity and mortality. Vasospasm can be diagnosed through transcranial Doppler ultrasonography (TCD), digital subtraction angiography (DSA), or computed tomography angiography (CTA). However, despite the ability to identify vasospasm when it has already developed, we lack specific, objective biomarkers to predict this damaging consequence.
Although no longer recognized as the sole contributor to delayed neurological deterioration (DND), the prevention and treatment of vasospasm after SAH remain an important therapeutic goal.3 Recent investigations have shown an association between inflammatory responses after SAH and poor functional outcomes.4,5 This inflammatory response is most pronounced during the early brain injury (EBI) period up to 72 h following SAH.4-7 Crosstalk between the brain and immune systems during SAH may be one potential mechanism contributing to brain injury, complications, and outcome.
The systemic immune-inflammation (SII) index is a novel biomarker of systemic inflammatory response underexplored in SAH. The SII index has been described in the oncology literature as an independent predictor of poor prognosis in gastrointestinal, pancreatic, cervical, and bladder cancers.8-10 In the central nervous system (CNS), SII index helps differentiate between high- and low-grade gliomas.11,12 More recently, SII index was shown to predict poor outcome following intracerebral hemorrhage.13 While similar to other indices such as the neutrophil-lymphocyte ratio (NLR),14 the SII index also incorporates changes in platelets into a single index. Given the strong interaction between inflammation and thrombosis,3,15 the SII index may serve as a superior tool to NLR and other laboratory measures for predicting complications after SAH. Here, we hypothesized that elevated SII index at admission predicts the development of delayed angiographic or sonographic vasospasm in aneurysmal SAH (aSAH).
METHODS
This study was approved by the local Institutional Review Board (#2015-0559), which determined that informed consent was unnecessary. All recorded data were anonymized. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Patient Population
We identified cases of aSAH admitted to the University of Illinois Hospital & Health Sciences System, a large comprehensive stroke center located in Chicago, Illinois. Subjects were identified using
International Classification of Diseases (ICD)-9 (430) and ICD-10 (I60.XX) discharge codes. We included all patients admitted between January 2013 and July 2019 with aSAH above 18 yr old. SAH was confirmed by head computed tomography (CT) and/or lumbar puncture based on clinical guidelines.2 DSA or CTA was performed to confirm the presence of cerebral aneurysms.
Study Design
Electronic medical records were reviewed and demographic, clinical, and laboratory data were collected into a secure database. The primary outcome was evidence of angiographic and/or sonographic cerebral vasospasm. Vasospasm was defined as evidence of cerebral arterial narrowing, regardless of clinical symptoms, based on DSA findings and impression of the neurointerventionalist performing the procedure as well as daily TCD performed by registered vascular technologists and interpreted by board-certified neurosonologists. TCD diagnosis of vasospasm was done using published, standardized criteria based on mean flow velocities > 120 cm/s and Lindegaard ratio > 3.2,16,17 At our center, angiographic vasospasm is documented as mild (decreased vessel caliber up to 25% decrease), moderate (25%-50%), or severe (>50%). Follow-up angiography is performed 7 d after SAH in all patients who undergo surgical clipping, and all patients who experience clinical deterioration or worsening TCD. Those who do not receive follow-up angiography are followed through daily TCD only. Any patient for which the presence or absence of vasospasm could not be confirmed was excluded. We additionally assessed for symptomatic vasospasm based on the presence of angiographic vasospasm and DND, the latter defined as new focal neurological deficits and/or decreased consciousness (persistent drop in Glasgow Coma Scale [GCS] by ≥ 2 points).17 Baseline characteristics collected were age, sex, race, ethnicity, aneurysm location (anterior or posterior circulation), and past medical history of cardiovascular disease and/or stroke. We collected clinical and radiographic scores obtained on admission including the modified Fisher scale, Hunt-Hess classification, and GCS.18-19 These scores were obtained in all patients as standard protocol by neurosurgeons blinded to SII index data. We further collected laboratory data within 24 h of admission, including total leukocyte count, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), NLR, and platelet count (PLT). SII index was calculated using the following equation: SII = [(PLT × ANC/ALC)/1000].
Statistical Analysis
Descriptive variables are presented as number of patients with percentages or median and interquartile range. Fisher's exact test or Pearson's Chi-squared test was used to compare nominal variables, while the Mann-Whitney test or Kruskal-Wallis test was used to compare continuous variables based on data distribution. Logistic regression was used to identify laboratory predictors of cerebral vasospasm. We performed univariate logistic regression for each laboratory variable. Variables with P < .10 on univariate analysis were entered into a multivariable logistic regression model, whereby each laboratory value was adjusted by covariates. The presence of multicollinearity among independent variables, defined as variance inflation factor ≥5 or tolerance of <0.20, was assessed using weighted linear regression.20 Receiver operating characteristic (ROC) analysis was performed to assess ability of SII index, modified Fisher scale, and the multivariable model for SII index to distinguish between patients who did or did not develop vasospasm. Youden's index was calculated to determine optimal test cut-offs. A P-value of < .05 was considered statistically significant unless otherwise indicated. Statistical Package for the Social Sciences (Version 27, IBM® SPSS®, Chicago, Illinois) software was used to conduct the analysis. Figures were made using GraphPad Prism (Version 9, La Jolla, California).
RESULTS
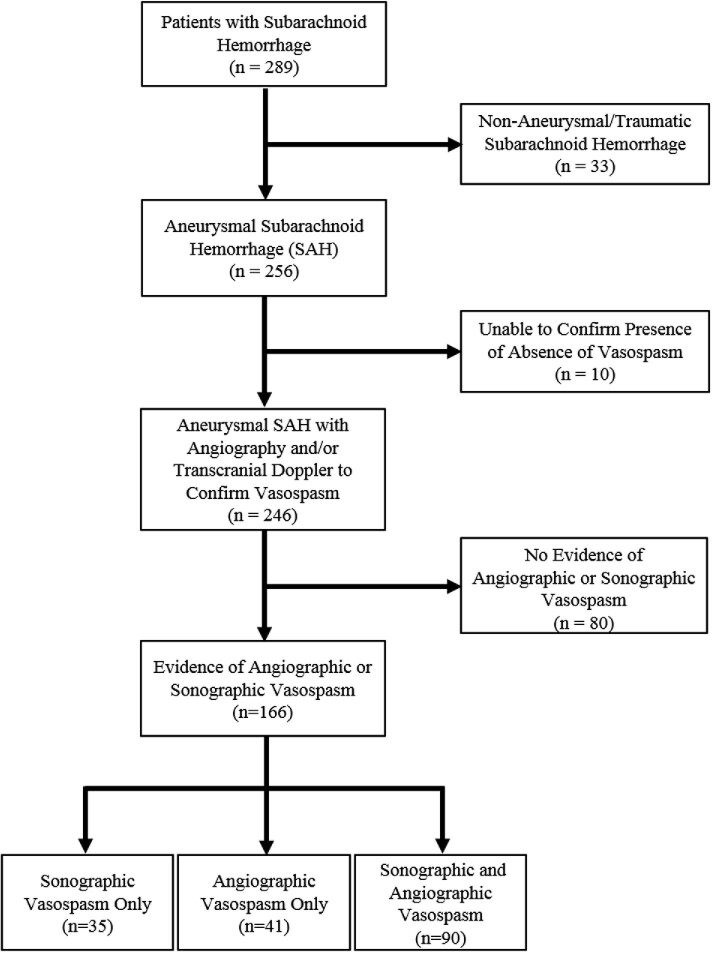
Of 289 screened SAH patients, 256 (88.6%) had aSAH. Of these, 246 (96.1%) met our inclusion criteria (Figure 1). Ten patients with aSAH were excluded from the study as vasospasm diagnosis was uncertain based on review of medical records and imaging data. Of these 10, 8 were deceased within 3 d of admission while the remaining 2 were high-grade aSAH patients of advanced age who did not receive follow-up angiography and had suboptimal TCD due to poor bone windows.
FIGURE 1.
Study design with inclusion and exclusion criteria for aSAH patients. Of 289 consecutive patients with SAH, 256 (88.6%) had an intracranial aneurysm as the confirmed source of hemorrhage. From these 256 patients, 246 (96.1%) were included in the study based on available transcranial Doppler ultrasound and angiography findings to confirm the presence or absence of delayed cerebral vasospasm. Of these, 166 (67.5%) had angiographic or sonographic evidence of vasospasm.
Baseline characteristics of patients are shown in Table 1. Of 246 aSAH patients, 240 (97.6%) had initial DSA and 168 (70.0%) had follow-up DSA within 5 to 7 d. Of these 168, 131 (78.0%) had angiographic vasospasm. A total of 245/246 (99.6%) patients had TCD studies, and 125/245 (51.0%) had sonographic evidence of vasospasm. Of these patients, 90 had both angiographic and sonographic evidence of vasospasm, 41 had only angiographic evidence, while 35 had only sonographic evidence, due to lack of follow-up angiogram. In total, 166 (67.5%) aSAH patients developed evidence of angiographic or sonographic vasospasm. Of patients with angiographic or sonographic vasospasm, 83 (50.0%) had DND or symptomatic vasospasm. Given the smaller sample with symptomatic vasospasm, we focused on patients with angiographic or sonographic vasospasm. Average time to develop vasospasm across patients was 5.5 ± 2.8 d. Compared to those without vasospasm, patients who developed vasospasm were younger (P < .001), had anterior circulation aneurysms (P = .015), were less likely to have hyperlipidemia (P = .017), and had higher modified Fisher scales (P = .039; Table 1).
TABLE 1.
Baseline Characteristics of aSAH Patients With and Without Delayed Cerebral Vasospasm
| Variable | All, N = 246 | No vasospasm, N = 80 | Vasospasm, N = 166 | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 55.2 (46.1-63.0) | 60.8 (52.5-70.8) | 52.2 (44.1-59.2) | <.001* |
| Female sex | 158 (64.2) | 53 (66.3) | 105 (63.3) | .673§ |
| Race | .798§ | |||
| Black | 77 (31.3) | 26 (32.5) | 51 (30.7) | |
| White | 51 (20.7) | 18 (22.5) | 33 (19.9) | |
| Other | 118 (48.0) | 36 (45.0) | 82 (49.4) | |
| Hispanic ethnicity | 53 (21.5) | 21 (26.3) | 32 (19.3) | .247§ |
| Aneurysm location | .015 § | |||
| Anterior circulation | 208 (84.6) | 61 (76.3) | 147 (88.6) | |
| Posterior circulation | 38 (15.4) | 19 (23.8) | 19 (11.4) | |
| Past medical history | ||||
| Hypertension | 130 (54.6) | 48 (62.3) | 82 (50.9) | .126§ |
| Diabetes mellitus | 29 (12.2) | 14 (18.2) | 15 (9.3) | .058§ |
| Hyperlipidemia | 41 (17.2) | 20 (26.0) | 21 (13.0) | .017§ |
| Ischemic stroke | 6 (2.5) | 2 (2.6) | 4 (2.5) | >.999§ |
| SAH | 14 (5.9) | 5 (6.5) | 9 (5.6) | .774§ |
| Intracerebral hemorrhage | 2 (0.8) | 1 (1.3) | 1 (0.6) | .543§ |
| Clinical scores on admission | ||||
| Modified Fisher scale | .039 § | |||
| 0 | 3 (1.2) | 2 (2.5) | 1 (0.6) | |
| 1 | 13 (5.3) | 8 (10.0) | 5 (3.0) | |
| 2 | 28 (11.4) | 13 (16.3) | 15 (9.0) | |
| 3 | 87 (35.4) | 24 (30.0) | 63 (38.0) | |
| 4 | 109 (44.3) | 33 (41.3) | 76 (45.8) | |
| Unknown | 6 (2.4) | 0 (0.0) | 6 (3.6) | |
| Hunt and Hess classification | .193§ | |||
| 1 | 20 (8.1) | 11 (13.8) | 9 (5.4) | |
| 2 | 86 (35.0) | 30 (37.5) | 56 (33.7) | |
| 3 | 55 (22.4) | 18 (22.5) | 37 (22.3) | |
| 4 | 28 (11.4) | 6 (7.5) | 22 (13.3) | |
| 5 | 32 (13.0) | 10 (12.5) | 22 (13.3) | |
| Unknown | 25 (10.2) | 5 (6.3) | 20 (12.0) | |
| GCS | .369§ | |||
| 3-8 | 64 (26.0) | 17 (21.3) | 47 (28.3) | |
| 9-12 | 19 (7.7) | 6 (7.5) | 13 (7.8) | |
| 13-15 | 163 (66.4) | 57 (71.3) | 106 (63.9) | |
| Laboratory data on admission | ||||
| Leukocytes, 103/μL | 12.7 (10.3-16.1) | 11.7 (9.2-14.2) | 13.6 (10.6-17.1) | <.001* |
| Neutrophils, 103/μL | 10.6 (8.1-14.1) | 9.2 (6.2-12.2) | 11.8 (8.8-14.4) | <.001* |
| Lymphocytes, 103/μL | 1.2 (0.9-1.7) | 1.4 (0.9-1.9) | 1.2 (0.8-1.6) | .049* |
| NLR | 9.1 (5.4-13.8) | 7.0 (4.4-11.4) | 10.8 (6.2-15.2) | <.001* |
| Platelets, 103/μL | 236.0 (198.0-285.0) | 229 (185.3-285.0) | 240 (202.0-288.0) | .165* |
| SII index, 103/μL | 2.1 (1.3-3.4) | 1.6 (0.8-2.6) | 2.3 (1.5-3.8) | <.001* |
Values are median (interquartile range) or number of patients (%) unless otherwise indicated. Boldface type indicates statistical significance with P < .05. *Mann-Whitney test; §Chi-squared or Fisher's exact test.
We next investigated differences in laboratory data on admission. Compared to those without vasospasm, patients who developed vasospasm had higher median total leukocytes (13.6 vs 11.7 103/μL, P < .001), ANC (11.8 vs 9.2 103/μL, P < .001), NLR (10.8 vs 7.0, P < .001), and SII index (2.3 vs 1.6 103/μL, P < .001) in addition to lower ALC (1.2 vs 1.4 103/μL, P = .049). While a statistical difference was observed in those with moderate-to-severe vasospasm compared to those without vasospasm (P = .018), no difference was observed in SII index between those with mild vasospasm and those without vasospasm (P = .276). Additionally, SII was elevated in patients with symptomatic (P = .002) and asymptomatic vasospasm (P < .001) compared to those without vasospasm; however, there was no statistical difference between those with symptomatic vs asymptomatic vasospasm (2.2 vs 2.3 103/μL, P = .354). Univariate logistic regression for laboratory variables revealed similar ability to predict development of cerebral vasospasm (Table 2). After adjusting for baseline differences in age, aneurysm location, diabetes mellitus, hyperlipidemia, and modified Fisher scale in multivariable logistic regression, total leukocyte count, ANC, NLR, and SII index predicted vasospasm (all P < .05). However, SII index had the highest odds ratio (OR) of 1.386 (95% CI 1.006-1.603, P = .003; Table 3). Three other factors that remained independent predictors of vasospasm across each model included age, anterior circulation aneurysms, and modified Fisher scale (all P < .05; Table 3). Multicollinearity was not observed between the independent variables and development of vasospasm.
TABLE 2.
Univariate Logistic Regression Analysis of Admission Laboratory Values to Predict Delayed Cerebral Vasospasm
| Unadjusted | |||
|---|---|---|---|
| Variable | Beta (SE) | OR (95% CI) | P-value |
| Leukocytes, 103/μL | 0.123 (0.035) | 1.131 (1.056-1.212) | <.001 |
| Neutrophil, 103/μL | 0.134 (0.036) | 1.143 (1.066-1.225) | <.001 |
| Lymphocytes, 103/μL | −0.377 (0.183) | 0.686 (0.479-0.983) | .040 |
| NLR | 0.089 (0.025) | 1.093 (1.041-1.148) | <.001 |
| Platelets, 103/μL | 0.002 (0.002) | 1.002 (0.998-1.006) | .259 |
| SII Index, 103/μL | 0.381 (0.103) | 1.464 (1.196-1.793) | <.001 |
SE, Standard Error.
Boldface type indicates P < .05.
TABLE 3.
Multivariable Logistic Regression Analysis of Admission Laboratory Values as Predictors of Delayed Cerebral Vasospasm
| Models | Variable | Beta (SE) | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|
| 1 | Age, yr | −0.053 (0.014) | 0.948 (0.923-0.974) | <.001 |
| Anterior aneurysm | 0.878 (0.417) | 2.407 (1.062-5.453) | .035 | |
| Diabetes mellitus | −0.364 (0.469) | 0.695 (0.277-1.743) | .437 | |
| Hyperlipidemia | −0.313 (0.422) | 0.732 (0.320-1.6710 | .458 | |
| Modified Fisher scale | 0.478 (0.184) | 1.613 (1.124-2.314) | .009 | |
| Leukocytes, 103/μL | 0.095 (0.040) | 1.100 (1.018-1.189) | .017 | |
| 2 | Age, yr | −0.052 (0.014) | 0.949 (0.924-0.974) | <.001 |
| Anterior aneurysm | 0.878 (0.418) | 2.406 (1.061-5.457) | .036 | |
| Diabetes mellitus | −0.365 (0.470) | 0.694 (0.276-1.744) | .437 | |
| Hyperlipidemia | −0.303 (0.422) | 0.738 (0.323-1.689) | .472 | |
| Modified Fisher scale | 0.451 (0.186) | 1.569 (1.091-2.258) | .015 | |
| Neutrophils, 103/μL | 0.108 (0.040) | 1.114 (1.029-1.206) | .007 | |
| 3 | Age, yr | −0.055 (0.013) | 0.947 (0.922-0.972) | <.001 |
| Anterior aneurysm | 0.955 (0.417) | 2.598 (1.146-5.888) | .022 | |
| Diabetes mellitus | −0.149 (0.466) | 0.862 (0.346-2.149) | .750 | |
| Hyperlipidemia | −0.268 (0.408) | 0.765 (0.344-1.701) | 511 | |
| Modified Fisher scale | 0.545 (0.182) | 1.724 (1.206-2.464) | .003 | |
| Lymphocytes, 103/μL | −0.387 (0.208) | 0.679 (0.452-1.021) | .063 | |
| 4 | Age, yr | −0.057 (0.014) | 0.945 (0.919-0.971) | <.001 |
| Anterior aneurysm | 1.091 (0.434) | 2.977 (1.272-6.970) | .012 | |
| Diabetes mellitus | −0.226 (0.472) | 0.798 (0.316-2.013) | .633 | |
| Hyperlipidemia | −0.178 (0.418) | 0.837 (0.369-1.900) | .670 | |
| Modified Fisher scale | 0.485 (0.186) | 1.625 (1.128-2.341) | .009 | |
| NLR | 0.085 (0.027) | 1.088 (1.032-1.147) | .002 | |
| 5 | Age, yr | −0.054 (0.014) | 0.947 (0.922-0.973) | <.001 |
| Anterior aneurysm | 1.008 (0.430) | 2.739 (1.180-6.359) | .019 | |
| Diabetes mellitus | −0.205 (0.480) | 0.815 (0.318-2.087) | .669 | |
| Hyperlipidemia | −0.229 (0.419) | 0.795 (0.350-1.809) | .585 | |
| Modified Fisher scale | 0.511 (0.185) | 1.667 (1.159-2.397) | .006 | |
| SII index, 103/μL | 0.327 (0.110) | 1.386 (1.118-1.719) | .003 |
SE, Standard Error.
Boldface type used for all white blood cell count multivariable logistic regression results.
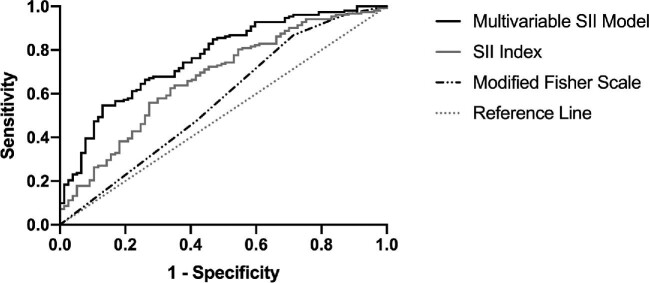
Finally, ROC analysis was performed to determine ability of SII index to distinguish between aSAH patients who did or did not develop vasospasm (Figure 2). We compared the ROC curves for SII index and the multivariable SII model to that of the modified Fisher scale, used clinically for predicting vasospasm. Modified Fisher scale showed modest ability to distinguish between patients with and without vasospasm (area under the curve [AUC] = 0.569, 95% CI 0.487-0.650, P = .089). Optimal cutoff for modified Fisher scale was 2, whereby those with a score >2 would be likely to develop vasospasm (Youden's index = 0.154, sensitivity 86.8%, specificity 28.6%). However, SII index significantly differentiated between groups (AUC = 0.672, 95% CI 0.698-0.747, P < .001). The optimal cutoff for SII level was 1.924 103/μL, whereby those with SII index above this level would be likely to develop vasospasm (Youden's index = 0.287, sensitivity 63.8%, specificity 64.9%). After covariate adjustment, the multivariable SII model demonstrated significant improvement in distinguishing between patients with or without vasospasm (AUC = 0.767, 95% CI 0.703-0.831, P < .001). The optimal predicted probability cutoff determined from the multivariable SII model was 0.764 (Youden's index = 0.416, sensitivity 58.9%, specificity 80.6%).
FIGURE 2.
ROC curve for admission SII index to identify patients with cerebral vasospasm. ROC analysis was performed to determine the association of SII index with the development of delayed cerebral vasospasm. SII index is compared to modified Fisher scale, a measure routinely used in clinical practice to predict vasospasm. Predicted probabilities from the multivariable logistic model, whereby SII was adjusted by age, aneurysm location, diabetes mellitus, hyperlipidemia, and modified Fisher scale, were also plotted. Adjusted SII identified patients at risk of vasospasm (AUC = 0.767, P < .001). The optimal predictive cut-off value for adjusted SII from the multivariable model's predicted probabilities based on Youden's index was a 0.764, corresponding to a sensitivity of 54.6% and specificity of 87.0%.
DISCUSSION
Systemic inflammation is common after aSAH and may contribute to feared complications such as delayed cerebral vasospasm. Here, we show for the first time that SII index is elevated in aSAH patients who develop vasospasm compared to those who do not. Admission SII index independently predicted development of vasospasm, which peaks around 1 wk after aSAH with an optimal cutoff of 1.924 103/μL (sensitivity 63.8%, specificity 64.9%). The predictive power of the SII index was improved when age, aneurysm location, diabetes mellitus, hyperlipidemia, and modified Fisher scale were included as covariates (Table 3 and Figure 2) with an optimal predicted probability cutoff of 0.764 (sensitivity 58.9%, specificity 80.6%). The combination of these measurements resulted in improved specificity for predicting vasospasm.
While approaches such as TCD and DSA exist to diagnose patients actively experiencing vasospasm, we lack specific, objective methods to predict its development prior to onset. The modified Fisher scale is a useful radiographic tool to stratify risk of developing vasospasm based on admission CT; however, despite sufficient sensitivity, there is modest specificity and inter-rater reliability.21-23 Thus, SII index, which integrates changes in peripheral neutrophils, lymphocytes, and platelets, is a novel, readily available, and noninvasive biomarker with added specificity to help identify patients at high risk of developing vasospasm and guide early management.
Differences in SII index observed in this study suggest a potential role for early inflammatory responses in driving delayed sequelae after aSAH.5 Our study expands upon previous studies demonstrating changes in circulating leukocytes after aSAH. Several studies have investigated the relationship between systemic inflammatory response syndrome and outcome after aSAH.24-28 Elevated total leukocytes and neutrophils were shown to be independently associated with vasospasm after adjusting for age and clinical severity.29-31 Decreased serum lymphocytes plays a role in infectious complications of aSAH, although the association with vasospasm is unclear.31,32 Elevated NLR independently predicts DND and functional outcome, although predictive ability for vasospasm has not been fully established.14,33,34 Further, evidence supports the role of platelet activation in driving poor outcomes after aSAH.3,35-39 Platelet activation and aggregation occur during the period of EBI within the cerebral microvasculature35,38 but can also be observed peripherally.36,37,39 In this study, we show that by integrating changes in neutrophils, lymphocytes, and platelets into a single measurement, the SII index is superior in predicting vasospasm compared to its individual components.
Two other variables that remained significant predictors of vasospasm in multivariable analysis included age and aneurysm location. These findings are consistent with previous literature showing increased incidence of vasospasm in patients less than 60 yr old.40 Data on aneurysm location and incidence of vasospasm are less clear, although some studies have shown increased incidence with anterior circulation aneurysms.41 Taken together, early identification of patients at higher risk of vasospasm may guide clinical management, including earlier or more frequent monitoring. Future studies should also assess the relationship of SII index to DND, neurological, and cognitive outcomes.
It has become increasingly clear that SAH is a disease not exclusively limited to the CNS. SAH affects the cardiac and respiratory systems in addition to systemic inflammatory responses.6,7,42,43 Most studies investigating inflammatory responses and immune dysregulation after aSAH have suggested a prominent role of the autonomic nervous system, involving overactive sympathetic tone from the hypothalamic-pituitary-adrenal axis.44 This promotes systemic inflammation, as major reservoirs of immune cells respond to catecholamines and cortisol.7,44 Despite this, clinical studies of anti-inflammatory agents in aSAH patients have been conflicting.3 A systematic review of corticosteroids for preventing poor outcome after aSAH revealed no evidence of beneficial or adverse effects.45 However, a randomized, open-label, single-blinded study administering subcutaneous interleukin-1 receptor antagonist demonstrated efficacy in reducing inflammatory markers.46 While not powered to investigate clinical efficacy, a trend for improved functional outcome at 6 mo was observed, supporting the need for a phase III study.46 Our observations, combined with data obtained in animal models, suggest that inflammation may modulate vascular tone after aSAH, offering a potential therapeutic target for intervention. While it has been established that nonspecific immunomodulation after aSAH (through use of corticosteroids) does not offer benefit,45 future studies may use more specific immunomodulatory agents to identify targeted subpopulations who may benefit. Additionally, studies in experimental SAH have demonstrated that reducing circulating leukocytes improves microvascular function, microthrombosis, and neurological outcome.3,15,47-49
Limitations
Limitations to this study include its single-center, retrospective nature. Follow-up angiography studies were not performed in all patients and, therefore, we also relied on TCD evidence of vasospasm. Though both TCD and DSA are validated ways to assess vasospasm, DSA is the gold standard.2,16,17 Based on the results of a meta-analysis, the sensitivity and specificity of TCD for detecting vasospasm are approximately 70% and 100%, respectively.50 Most studies included in this meta-analysis used the same TCD cutoffs used in our study. Only 10 patients with aSAH were excluded as we could not confirm the presence or absence of vasospasm. We also used vasospasm regardless of symptoms as our primary endpoint. Thus, we cannot comment on the effect of SII on symptomatic vasospasm. Future prospective, multicenter studies are required to assess the prognostic value of SII index for the diagnosis of symptomatic vasospasm in aSAH patients. This would also permit incorporation of additional prospective metrics including the Hijdra sum score.51 Finally, measurement of admission SII does not account for the few patients who may present in a delayed fashion; therefore, differences observed here in SII index may be an underestimate, as acute inflammatory responses normalize over time.
CONCLUSION
In summary, early elevation in SII index after aSAH assists in the prediction of delayed cerebral vasospasm measured via angiography and TCD. Systemic inflammation is common after SAH and SII index offers a reliable, objective, specific, and noninvasive method of assessing systemic inflammatory responses that prognosticate cerebrovascular complications.
Funding
This study did not receive any funding or financial support.
Disclosures
Mr Geraghty receives grant support from the National Institute of Neurological Disorders and Stroke (grant no. 1F31NS105525-01A1). Dr Testai receives private donations from Louis and Christine Friedrich. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Notes
Portions of this paper were presented as a poster and abstract at the European Stroke Organization-World Stroke Organization (ESO-WSO) 2020 Virtual Conference, November 7-9, 2020.
Contributor Information
Joseph R Geraghty, Department of Neurology and Rehabilitation, University of Illinois College of Medicine, Chicago, Illinois, USA; Medical Scientist Training Program, University of Illinois College of Medicine, Chicago, Illinois, USA.
Tyler J Lung, Department of Neurology and Rehabilitation, University of Illinois College of Medicine, Chicago, Illinois, USA.
Yonatan Hirsch, Department of Neurology and Rehabilitation, University of Illinois College of Medicine, Chicago, Illinois, USA.
Eitan A Katz, Department of Neurology and Rehabilitation, University of Illinois College of Medicine, Chicago, Illinois, USA.
Tiffany Cheng, Department of Neurology and Rehabilitation, University of Illinois College of Medicine, Chicago, Illinois, USA.
Neil S Saini, Department of Neurology and Rehabilitation, University of Illinois College of Medicine, Chicago, Illinois, USA.
Dilip K Pandey, Department of Neurology and Rehabilitation, University of Illinois College of Medicine, Chicago, Illinois, USA.
Fernando D Testai, Department of Neurology and Rehabilitation, University of Illinois College of Medicine, Chicago, Illinois, USA.
COMMENT
In this study, the authors present a single center series of 246 patients with aneurysmal subarachnoid hemorrhage and compare admission laboratory markers with development of delayed vasospasm. The authors conclude that early elevation in the systemic immune-inflammation (SII) index is predictive of cerebral vasospasm in patients with ruptured aneurysms. This evidence is based on elevations in SII in predicting outcomes in patients with various cancers as well as glioma and intracranial hemorrhages.
In the study, the authors carefully note that statistically significant differences in SII were present between those with moderate and severe vasospasm and those without vasospasm, yet no difference was found between those with mild and those without vasospasm. Furthermore, regarding clinical vasospasm, there were differences between symptomatic and asymptomatic vasospasm compared to patients without vasospasm, while no difference was observed between patients with or without clinical symptoms. This suggests a possible association with systemic inflammation and vascular spasm, but the SII cannot be used to determine clinical consequences of this spasm. Furthermore, as the authors note, other signs of inflammation can be used to predict the same consequence (radiographic spasm as noted on transcranial doppler or angiogram, with or without symptoms), such as total leukocyte count, neurotrophil count (ANC), and neutrophil-lymphocyte ratio (NLR). Given these considerations, SII is likely somewhat limited for application to this purpose. While the SII might be used to help indicate possible spasm, the inability to distinguish between symptomatic and asymptomatic vasospasm may lead to unnecessary treatment or invasive monitoring.
Kurt Yaeger
J Mocco
New York, NY, USA
REFERENCES
- 1. Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354(4):387-396. [DOI] [PubMed] [Google Scholar]
- 2. Connolly ES Jr, Rabinstein AA, Carhuapoma JRet al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43(6):1711-1737. [DOI] [PubMed] [Google Scholar]
- 3. Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19(12):50. [DOI] [PubMed] [Google Scholar]
- 4. Geraghty JR, Davis JL, Testai FD. Neuroinflammation and microvascular dysfunction after experimental subarachnoid hemorrhage: emerging components of early brain injury related to outcome. Neurocrit Care. 2019;31(2):373-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirsch Y, Geraghty JR, Katz EA, Testai FD. Inflammasome caspase-1 activity is elevated in cerebrospinal fluid after aneurysmal subarachnoid hemorrhage and predicts functional outcome. Neurocrit Care. 2021;34(3):889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Savarraj J, Parsha K, Hergenroeder Get al. Early brain injury associated with systemic inflammation after subarachnoid hemorrhage. Neurocrit Care. 2018;28(2):203-211. [DOI] [PubMed] [Google Scholar]
- 7. Saand AR, Yu F, Chen J, Chou SH. Systemic inflammation in hemorrhagic strokes—a novel neurological sign and therapeutic target? J Cereb Blood Flow Metab. 2019;39(6):959-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aziz MH, Sideras K, Aziz NAet al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a retrospective multicenter cohort study. Ann Surg. 2019;270(1):139-146. [DOI] [PubMed] [Google Scholar]
- 9. Huang H, Liu Q, Zhu Let al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9(1):3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W, Wang R, Ma Wet al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Transl Med. 2019;7(18):431-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang R, Chen N, Li M, Wang X, Mao Q, Liu Y. Significance of systemic immune-inflammation index in the differential diagnosis of high- and low-grade gliomas. Clin Neurol Neurosurg. 2018;164:50-52. [DOI] [PubMed] [Google Scholar]
- 12. Liang R, Li J, Tang X, Liu Y. The prognostic role of preoperative systemic immune-inflammation index and albumin/globulin ratio in patients with newly diagnosed high-grade glioma. Clin Neurol Neurosurg. 2019;184:105397. [DOI] [PubMed] [Google Scholar]
- 13. Trifan G, Testai FD. Systemic immune-inflammation (SII) index predicts poor outcome after spontaneous supratentorial intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(9):105057. [DOI] [PubMed] [Google Scholar]
- 14. Giede-Jeppe A, Reichl J, Sprügel MIet al. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2019;132(2):400-407. [DOI] [PubMed] [Google Scholar]
- 15. McBride DW, Blackburn SL, Peeyush KT, Matsumura K, Zhang JH. The role of thromboinflammation in delayed cerebral ischemia after subarachnoid hemorrhage. Front Neurol. 2017;8:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marshall SA, Nyquist P, Ziai WC. The role of transcranial Doppler ultrasonography in the diagnosis and management of vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21(2):291-303. [DOI] [PubMed] [Google Scholar]
- 17. Vergouwen MDI, Vermeulen M, Van Gijn Jet al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391-2395. [DOI] [PubMed] [Google Scholar]
- 18. Jaja BN, Cusimano MD, Etminan Net al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: a systematic review. Neurocrit Care. 2013;18(1):143-153. [DOI] [PubMed] [Google Scholar]
- 19. Lantigua H, Ortega-Gutierrez S, Schmidt JMet al. Subarachnoid hemorrhage: who dies, and why? Crit Care. 2015;19(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geraghty JR, Lara-Angulo MN, Spegar M, Reeh J, Testai FD. Severe cognitive impairment in aneurysmal subarachnoid hemorrhage: predictors and relationship to functional outcome. J Stroke Cerebrovasc Dis. 2020;29(9):105027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1-9. [DOI] [PubMed] [Google Scholar]
- 22. Frontera JA, Claassen J, Schmidt JMet al. Prediction of sympatomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21-27. [DOI] [PubMed] [Google Scholar]
- 23. Melinosky C, Kincaid H, Claassen Jet al. The modified fisher scale lacks interrater reliability. Neurocrit Care. 2021;35(1):72-78. [DOI] [PubMed] [Google Scholar]
- 24. Muroi C, Hugelshofer M, Seule Met al. Correlation among systemic inflammatory parameter, occurrence of delayed neurological deficits, and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2013;72(3):367-375. [DOI] [PubMed] [Google Scholar]
- 25. Wessell AP, Kole MJ, Cannarsa Get al. A sustained systemic inflammatory response syndrome is associated with shunt-dependent hydrocephalus after aneurysmal subarachnoid hemorrhage [published online ahead of print: June 1, 2018]. J Neurosurg. doi:10.3171/2018.1.JNS172925. [DOI] [PubMed] [Google Scholar]
- 26. Yoshimoto Y, Tanaka Y, Hoya K. Acute systemic inflammatory response syndrome in subarachnoid hemorrhage. Stroke. 2001;32(9):1989-1993. [DOI] [PubMed] [Google Scholar]
- 27. Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care. 2008;8(3):404-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tam AKH, Ilodigwe D, Mocco Jet al. Impact of systemic inflammatory response syndrome on vasospasm, cerebral infarction, and outcome after subarachnoid hemorrhage: exploratory analysis of CONSCIOUS-1 database. Neurocrit Care. 2010;13(2):182-189. [DOI] [PubMed] [Google Scholar]
- 29. Chou SHY, Feske SK, Simmons SLet al. Elevated peripheral neutrophils and matrix metalloproteinase 9 as biomarkers of functional outcome following subarachnoid hemorrhage. Transl Stroke Res. 2011;2(4):600-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGirt MJ, Mavropoulous JC, McGirt LYet al. Leukocytosis as an independent risk factor for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;98(6):1222-1226. [DOI] [PubMed] [Google Scholar]
- 31. Sarrafzadeh A, Schlenk F, Meisel Aet al. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42(1):53-58. [DOI] [PubMed] [Google Scholar]
- 32. Attanasio L, Grimaldi D, Ramiz RAet al. Early lymphopenia and infections in nontraumatic subarachnoid hemorrhage patients [published online ahead of print: November 17, 2020]. J Neurosurg Anesthesiol. doi:10.1097/ANA.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 33. Tao C, Wang J, Hu Xet al. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017;26(3):393-401. [DOI] [PubMed] [Google Scholar]
- 34. Al-Mufti F, Amuluru K, Damodara Net al. Admission neutrophil-lymphocyte ratio predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J NeuroIntervent Surg. 2019;11(11):1135-1140. [DOI] [PubMed] [Google Scholar]
- 35. Vergouwen MD, Vermeulen M, Coert BA, Stroes ESG, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28(11):1761-1770. [DOI] [PubMed] [Google Scholar]
- 36. Frontera JA, Provencio JJ, Sehba FAet al. The role of platelet activation and inflammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care. 2017;26(1):48-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perez P, Lukszewicz A, Lenck Set al. Platelet activation and aggregation after aneurysmal subarachnoid hemorrhage. BMC Neurol. 2018;18(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarke JV, Suggs JM, Diwan Det al. Microvascular platelet aggregation and thrombosis after subarachnoid hemorrhage: a review and synthesis. J Cereb Blood Flow Metab. 2020;40(8):1565-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rzepliński R, Kostyra K, Skadorwa T, Sługocki M, Kostkiewicz B. Acute platelet response to aneurysmal subarachnoid hemorrhage depends on severity and distribution of bleeding: an observational cohort study [published online ahead of print: November 25, 2020]. Neurosurg Rev. doi:10.1007/s10143-020-01444-7. [DOI] [PubMed] [Google Scholar]
- 40. Wachter D, Hans F, Kreitschmann-Andermahr I, Rohde V. Lower incidence of transcranial Doppler and symptomatic vasospasm after aneurysmal subarachnoid hemorrhage and aneurysm clipping in the elderly patient? Neurosurgery. 2011;69(2):261-267. [DOI] [PubMed] [Google Scholar]
- 41. Rumalla K, Lin M, Ding Let al. Risk factors for cerebral vasospasm in aneurysmal subarachnoid hemorrhage: a population-based study of 8346 patients. World Neurosurg. 2021;145:e233-e241. [DOI] [PubMed] [Google Scholar]
- 42. Tung P, Kopelnik A, Banki Net al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35(2):548-551. [DOI] [PubMed] [Google Scholar]
- 43. Wartenberg KE, Schmidt JM, Claassen Jet al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34(3):617-623. [DOI] [PubMed] [Google Scholar]
- 44. Naredi S, Lambert G, Edén Eet al. Increased sympathetic nervous activity in patients with nontraumatic subarachnoid hemorrhage. Stroke. 2000;31(4):901-906. [DOI] [PubMed] [Google Scholar]
- 45. Feigin VL, Anderson N, Rinkel GJEet al. Corticosteroids for aneurysmal subarachnoid haemorrhage and primary intracerebral haemorrhage. Cochrane Database Syst Rev. 2005;(3):CD004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Galea J, Ogungbenro K, Hulme Set al. Reduction of inflammation after administration of interleukin-1 receptor antagonist following aneurysmal subarachnoid hemorrhage: results of the subcutaneous interleukin-1ra in SAH (SCIL-SAH) study. J Neurosurg. 2018;128(2):515-523. [DOI] [PubMed] [Google Scholar]
- 47. Xu H, Testai FD, Valyi-Nagy Tet al. VAP-1 blockade prevents subarachnoid hemorrhage-associated cerebrovascular dilating dysfunction via repression of neutrophil recruitment-related mechanism. Brain Res. 2015;1603:141-149. [DOI] [PubMed] [Google Scholar]
- 48. Xu H, Pelligrino DA, Paisansathan C, Testai FD. Protective role of fingolimod (FTY720) in rats subjected to subarachnoid hemorrhage. J Neuroinflammation. 2015;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. De Oliveira Manoel AL, Macdonald RL. Neuroinflammation as a target for intervention in subarachnoid hemorrhage. Front Neurol. 2018;9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lysakowski C, Walder B, Costanza MC, Tramèr MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke. 2001;32(10):2292-2298. [DOI] [PubMed] [Google Scholar]
- 51. Dupont SA, Wijdicks EFM, Manno EM, Lanzino G, Rabinstein AA. Prediction of angiographic vasospasm after aneurysmal subarachnoid hemorrhage: value of the Hijdra sum scoring system. Neurocrit Care. 2009;11(2):172-176. [DOI] [PubMed] [Google Scholar]