Abstract
Aims
Although cardiac involvement has prognostic significance in coronavirus disease 2019 (COVID-19) and is associated with severe forms, few studies have explored the prognostic role of transthoracic echocardiography (TTE). We investigated the link between TTE parameters and prognosis in COVID-19.
Methods and results
Consecutive patients with COVID-19 admitted to 24 French hospitals were retrospectively included. Comprehensive data, including clinical and biological parameters, were recorded at admission. Focused TTE was performed during hospitalization, according to clinical indication. Patients were followed for a primary composite outcome of death or transfer to intensive care unit (ICU) during hospitalization. Among 2878 patients, 445 (15%) underwent TTE. Most of these had cardiovascular risk factors, a history of cardiovascular disease, and were on cardiovascular treatments. Dilatation and dysfunction were observed in, respectively, 12% (48/412) and 23% (102/442) of patients for the left ventricle, and in 12% (47/407) and 16% (65/402) for the right ventricle (RV). Primary composite outcome occurred in 44% (n = 196) of patients [9% (n = 42) for death without ICU transfer and 35% (n = 154) for admission to ICU]. RV dilatation was the only TTE parameter associated with the primary outcome. After adjustment, male sex [hazard ratio (HR) 1.56, 95% confidence interval (CI) 1.09 − 2.25; P = 0.02], higher body mass index (HR 1.10, 95% CI 1.02 − 1.18; P = 0.01), anticoagulation (HR 0.53, 95% CI 0.33 − 0.86; P = 0.01), and RV dilatation (HR 1.66, 95% CI 1.05 − 2.64; P = 0.03) remained independently associated with the primary outcome.
Conclusion
Echocardiographic evaluation of RV dilatation could be useful for assessing risk of severe COVID-19 developing in hospitalized patients.
Keywords: COVID-19, right ventricular, echocardiography, prognosis
Introduction
Starting at the end of 2019, a global pandemic spread rapidly across the globe, caused by a new severe acute respiratory syndrome coronavirus (SARS-Cov-2), resulting in a public health crisis of unprecedented magnitude.1 The clinical picture of coronavirus disease 2019 (COVID-19) ranges from asymptomatic to rapidly developing acute respiratory distress syndrome associated with a high fatality rate.2,3 Beyond the pulmonary symptoms of COVID-19, cardiac involvement (expressed primarily as acute heart failure, acute coronary syndromes, myocardial injury, and pulmonary embolism) appears to be a significant feature of COVID-19 and is associated with the worst prognosis.4–6 Moreover, patients with pre-existing cardiovascular disease display a higher risk of developing severe disease or dying.7 In this context, early risk stratification of patients at risk of severe COVID-19 is paramount to optimize management of patient-flow and resource allocation.8
Besides markers of cardiac injury (e.g. natriuretic peptides and troponin), transthoracic echocardiography (TTE) is an accessible, non-invasive, and widely used first-line diagnostic tool9 to monitor the effects of COVID-19 on the heart. The American Society of Echocardiography and the European Association of Cardiovascular Imaging both published recommendations for TTE in the context of COVID-19.10,11 Limited data are currently available on the diagnostic contribution of TTE in COVID-19 and the link with clinical outcomes.12–14 We sought to investigate the prognostic value of TTE parameters in a multicentre cohort of patients with COVID-19, in terms of in-hospital death or transfer to intensive care (ICU).
Methods
Study population and design
The methodology and population have been described.15 This retrospective, multicentre study used data from all consecutive patients with a diagnosis of SARS-Cov-2 admitted to 24 French hospitals between 26 February and 20 April 2020. According to World Health Organization criteria, a confirmed case of SARS-Cov-2 was defined as a positive result on real-time reverse transcriptase–polymerase chain reaction of nasopharyngeal swabs or lower respiratory tract aspirates (confirmed case) or as typical imaging characteristics on chest computed tomography when laboratory testing results were inconclusive (probable case).16 Patients <18 years or who were admitted direct to ICU were excluded. Patient selection for ICU transfer was left to the discretion of the referring medical team in accordance with recommendations during the ‘first wave’.17
The study was initiated by the French Society of Cardiology (Critical COVID-19 France, NCT04344327) and declared to the French data protection committee (Commission Nationale Informatique et Liberté, CNIL, authorization MR3910090420). It was conducted in accordance with the Declaration of Helsinki and its later amendments. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and approved the manuscript as written.
Transthoracic echocardiography
During hospitalization, a focused TTE examination was performed by clinicians experienced in echocardiography. Optimal protective precautions were taken for sonographers or physicians during each examination, in accordance with the recommendations for cardiac imaging in COVID-19.10,11
As the goal was to perform a focused TTE examination in COVID-19 patients, the following regular TTE parameters were recorded. Left ventricular (LV) size was measured in the parasternal long-axis views: LV dilatation was defined as an end-diastolic diameter >52 mm (women) or >58 mm (men); LV hypertrophy was defined as an LV mass >95 g/m2 (women) or >115 g/m2 (men).18 LV ejection fraction (LVEF) was assessed either by visual evaluation or by Simpson’s biplane method when image quality allowed for accurate quantification. Precautions were taken for a complete analysis of the right ventricle (RV), with good delimitation of the RV-free wall, thus avoiding RV foreshortening. RV dilatation was defined when the basal diameter was >41 mm (RV focused view) or when the RV/LV ratio was >0.6 (four-chamber or subcostal view). RV dysfunction was defined as tricuspid annular plane systolic excursion <17 mm by M-mode, or a tricuspid S wave <9.5 cm/s by Doppler tissue imaging, or a fractional area change <35%.18 Pericardial effusion was also reported.
Data collection and outcomes
Data were collected by local investigators and entered into an electronic case-report form via REDCap software (Research Electronic Data Capture, Vanderbilt University, USA), hosted by a secure server from the French Institute of Health and Medical Research at the Paris Cardiovascular Research Centre. Patient baseline information included demographic characteristics, coexisting medical conditions, and chronic medications. Clinical variables and laboratory findings were recorded at admission. Data on pharmacological therapies, complications or associated diagnoses, mode of respiratory support, and final vital status were collected. Medical interventions, including anticoagulation and pharmacological treatments for COVID-19, were left to the discretion of the referring medical team.
The primary composite outcome was all-cause death or ICU transfer during hospitalization and was defined before starting data collection.
Statistical analysis
Continuous data are reported as mean ± standard deviation (SD) or median (interquartile range). Categorical data are reported as counts and percentages. Comparisons used the χ2 test or Fisher’s exact test for categorical variables and Student’s t-test or the Mann–Whitney–Wilcoxon test, as appropriate, for continuous variables. The Kaplan–Meier method was used to plot the survival curves, which were compared with the log-rank test.
Cox proportional hazard models were used to identify parameters associated with the primary outcome. Variables with a probability of <0.10 were integrated in the multivariable analysis. The final selection was based on the most favourable goodness-of-fit measures (Bayesian information criterion). A two-tailed P < 0.05 was considered statistically significant. Data were analysed using R software, version 4.0.0 (R Project for Statistical Computing).
Results
Among 2878 consecutive patients hospitalized for SARS-Cov-2 infection in 24 French centres (58% were men; mean age 65 ± 17 years, Supplementary data online, TableS1), 445 (15%) underwent TTE (Figure 1). Patient baseline characteristics according to performance of TTE are presented in Table 1. Compared with those who did not undergo TTE, patients who had TTE during hospitalization were older, predominantly male, with a higher prevalence of cardiovascular risk factors (e.g. hypertension and diabetes), history of chronic kidney disease, atrial fibrillation, chronic heart failure, ischaemic cardiomyopathy, dilated cardiomyopathy, and cardiovascular treatments. TTE was more frequently performed for signs of heart failure. Concentrations of N-terminal-pro-B-type natriuretic peptide (NT-pro-BNP), B-type natriuretic peptide (BNP), and troponin were higher among patients who underwent TTE.
Figure 1.
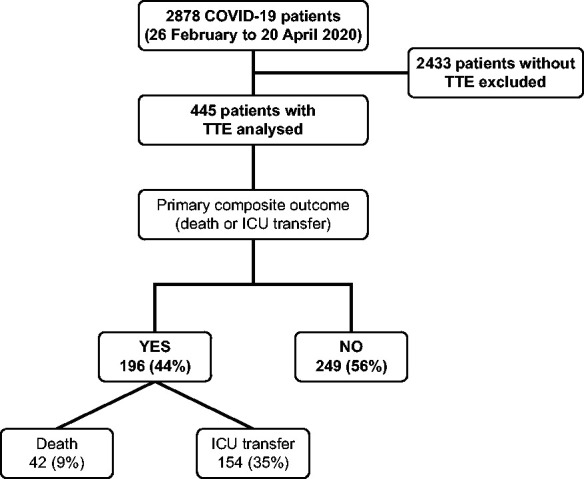
Flowchart and occurrence of primary outcome in patients with COVID-19. COVID-19, coronavirus disease-2019; ICU, intensive care unit; TTE, transthoracic echocardiography.
Table 1.
Characteristics of patients according to performance of TTE (N = 2878)
| Variables | TTE |
P-value | |
|---|---|---|---|
| No (n = 2433) | Yes (n = 445) | ||
| Demographics | |||
| Age, years (n = 2873) | 66 ± 17 | 68 ± 16 | 0.018 |
| Male sex | 1370 (56) | 296 (66) | <0.001 |
| Body mass index (kg/m2, n = 2493) | 27.8 ± 6.0 | 28.0 ± 6.3 | 0.64 |
| Time from illness onset to hospitalization (days, n = 2777) | 6.8 ± 4.5 | 6.7 ± 5.6 | 0.81 |
| Cardiovascular risk factor | |||
| Smoking (n = 2810) | 310 (13) | 68 (15.8) | 0.14 |
| Hypertension (n = 2859) | 1188 (49) | 265 (60) | <0.001 |
| Diabetes (n = 2860) | 547 (23) | 130 (29) | 0.003 |
| Dyslipidaemia (n = 2859) | 662 (27) | 138 (31) | 0.11 |
| Comorbid conditions | |||
| Chronic obstructive pulmonary disease | 130 (5) | 34 (8) | 0.12 |
| Chronic kidney disease (n = 2836) | 312 (13) | 93 (21) | <0.001 |
| Stroke (n = 2837) | 213 (9) | 40 (9) | 0.95 |
| Peripheral artery disease (n = 2838) | 118 (5) | 29 (7) | 0.17 |
| Atrial fibrillation (n = 2852) | 323 (13) | 93 (21) | <0.001 |
| Chronic heart failure (n = 2831) | 183 (8) | 127 (29) | <0.001 |
| Ischaemic cardiomyopathy | 237 (10) | 76 (17) | <0.001 |
| Dilated cardiomyopathy | 25 (1) | 15 (3) | <0.001 |
| Treatment before hospitalization | |||
| Anticoagulation | 324 (13) | 94 (21) | <0.001 |
| Beta-blocker | 575 (24) | 160 (36) | <0.001 |
| Renin–angiotensin system inhibitor | 835 (34) | 219 (49) | <0.001 |
| Antiplatelet | 501 (21) | 126 (28) | <0.001 |
| Statin | 523 (22) | 130 (29) | <0.001 |
| Heart failure at admission | |||
| New York Heart Association functional class III/IV (n = 2498) | 1106 (52) | 175 (48) | 0.19 |
| Heart failure signs (n = 2824) | 133 (6) | 57 (13) | <0.001 |
| Laboratory values | |||
| NT-pro-B-type natriuretic peptide (pg/mL, n = 1092) | 2374 ± 7515 | 3905 ± 6624 | 0.002 |
| B-type natriuretic peptide (pg/mL, n = 691) | 209 ± 614 | 377 ± 783 | 0.031 |
| Troponin elevation (n = 1763) | 408 (29) | 164 (47) | <0.001 |
Data are presented as n (%) or mean ± standard deviation.
TTE, transthoracic echocardiography.
Echocardiographic findings
Echocardiographic parameters are presented in Table 2. Mean LVEF was 54 ± 13% and 102 (23%) patients had reduced LVEF (<50%). Twelve per cent of patients had LV dilatation and 25% had LV hypertrophy; 16% had RV dysfunction; and 12% had RV dilatation. Seventy-seven per cent of patients had no valvular heart disease and pericardial effusion was detected in 11%.
Table 2.
Univariable analysis to assess TTE determinants of primary composite outcomea
| Variables | Overall | Primary outcome |
HR (95% CI) | P-value | |
|---|---|---|---|---|---|
| No (n = 249) | Yes (n = 196) | ||||
| LV characteristics | |||||
| LV ejection fraction, % | (n = 369) 54 ± 13 | 54 ± 13 | 54 ± 14 | 1.00 (0.99–1.01) | 0.98 |
| Category | (n = 442) | 0.40 | |||
| ≥50% | 340 (77) | 187 (75) | 153 (79) | Reference | |
| 36 − 49% | 50 (11) | 33 (13) | 17 (9) | 0.71 (0.43–1.17) | |
| <35% | 52 (12) | 29 (12) | 23 (12) | 0.98 (0.63–1.52) | |
| Dilated LV | (n = 412) 48 (12) | 29 (12) | 19 (11) | 0.91 (0.57–1.47) | 0.72 |
| Hypertrophic LV | (n = 397) 100 (25) | 68 (29) | 32 (20) | 0.70 (0.48–1.03) | 0.07 |
| Valvular disease | n = 445 | ||||
| None | 342 (77) | 193 (78) | 149 (76) | 1.06 (0.77–1.48) | 0.76 |
| Moderate/severe AR | 25 (6) | 15 (3) | 10 (2) | 0.58 (0.33–1.03) | 0.06 |
| Moderate/severe MR | 29 (7) | 18 (4) | 11 (2) | 0.77 (0.42–1.18) | 0.20 |
| Moderate/severe TR | 12 (3) | 7 (3) | 5 (3) | 0.81 (0.33–1.96) | 0.65 |
| RV | |||||
| Dilatation | (n = 407) 47 (12) | 18 (8) | 29 (16) | 1.61 (1.08–2.40) | 0.017 |
| Dysfunction | (n = 402) 65 (16) | 29 (13) | 36 (21) | 1.33 (0.92–1.92) | 0.12 |
| Pericardial effusion | (n = 416) 46 (11) | 23 (10) | 23 (13) | 1.20 (0.77–1.86) | 0.41 |
Data are presented as n (%) or mean ± SD.
CI, confidence interval; LV, left ventricular; AR, aortic regurgitation; MR, mitral regurgitation; TR, tricuspid regurgitation; HR, hazard ratio; RV, right ventricular; TTE, transthoracic echocardiography.
Composite of death from any cause or transfer to intensive care unit during hospitalization.
Factors associated with outcomes
The primary composite outcome occurred in 196 (44%) patients: 42 (9%) patients died before being transferred to ICU and 154 (35%) patients were admitted to the ICU (Figure 1). Overall, 65 (15%) patients died during hospitalization (23 of whom died in the ICU).
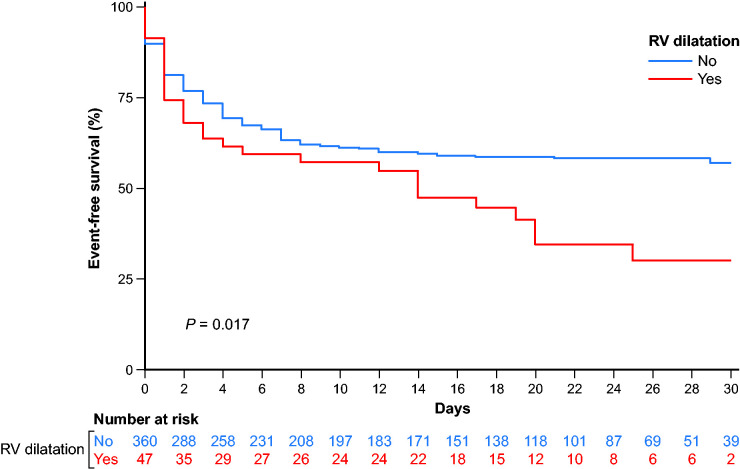
On Cox univariable analysis, occurrence of the primary outcome was associated with RV dilatation (P = 0.017, Table 2 and Figure 2), male sex (P = 0.007), higher body mass index (P = 0.001), diabetes (P = 0.023), and higher New York Heart Association class at admission (P < 0.001); conversely, atrial fibrillation (P = 0.029) and anticoagulation therapy (P = 0.013) were associated with a lower risk of the primary outcome (Table 3).
Figure 2.
Kaplan–Meier curves showing event-free survival according to presence of RV dilatation in TTE. RV, right ventricular; TTE, transthoracic echocardiography.
Table 3.
Univariable analysis to assess clinical and biological determinants of the primary composite outcomea
| Variables | Primary outcome |
HR (95% CI) | P-value | |
|---|---|---|---|---|
| No (n = 249) | Yes (n = 196) | |||
| Demographics | ||||
| Age (years) | 69 ± 17 | 68 ± 14 | 0.99 (0.99–1.00) | 0.18 |
| Male sex | 151 (61) | 144 (74) | 1.55 (1.13–2.14) | 0.007 |
| Body mass index (kg/m2) | 27.1 ± 6.3 | 29.0 ± 6.1 | 1.03 (1.01–1.06)b | 0.001 |
| Time from illness onset to hospitalization (days) | 6.4 ± 5.8 | 7.1 ± 5.3 | 1.02 (0.99–1.04)b | 0.17 |
| Cardiovascular risk factor | ||||
| Smoking | 33 (14) | 34 (18) | 1.28 (0.88–1.85) | 0.19 |
| Hypertension | 147 (59) | 118 (60) | 0.98 (0.73–1.30) | 0.90 |
| Diabetes | 62 (25) | 67 (34) | 1.40 (1.04–1.89) | 0.023 |
| Dyslipidaemia | 69 (28) | 69 (35) | 1.23 (0.91–1.65) | 0.16 |
| Comorbid conditions | ||||
| Chronic obstructive pulmonary disease | 13 (5) | 21 (11) | 1.46 (0.92–2.30) | 0.24 |
| Chronic kidney disease | 47 (19) | 46 (24) | 1.15 (0.83–1.60) | 0.38 |
| Stroke | 26 (11) | 14 (7) | 0.72 (0.42–1.25) | 0.25 |
| Peripheral artery disease | 15 (6) | 14 (7) | 1.16 (0.67–2.00) | 0.61 |
| Atrial fibrillation | 60 (24) | 33 (17) | 0.65 (0.45–0.95) | 0.029 |
| Chronic heart failure | 71 (29) | 56 (29) | 0.95 (0.70–1.30) | 0.78 |
| Ischaemic cardiomyopathy | 43 (22) | 33 (19) | 0.76 (0.52–1.11) | 0.16 |
| Dilated cardiomyopathy | 9 (5) | 6 (3) | 0.67 (0.30–1.52) | 0.34 |
| Treatment before hospitalization | ||||
| Anticoagulant | 61 (25) | 32 (16) | 0.62 (0.42–0.90) | 0.013 |
| Beta-blocker | 96 (39) | 63 (32) | 0.77 (0.57–1.03) | 0.09 |
| Renin–angiotensin–system inhibitor | 125 (50) | 91 (46) | 1.00 (0.75–1.32) | 0.997 |
| Antiplatelet | 67 (27) | 58 (30) | 1.05 (0.77–1.43) | 0.72 |
| Statin | 72 (29) | 58 (30) | 0.97 (0.71–1.32) | 0.89 |
| Heart failure at admission | ||||
| New York Heart Association functional class III–IV | 78 (38) | 97 (62) | 2.19 (1.58–3.03)c | <0.001 |
| Heart failure signs | 57 (13) | 23 (12) | 0.88 (0.52–1.24) | 0.35 |
| Laboratory values | ||||
| NT-pro-B-type natriuretic peptide (pg/mL) | 4561 ± 7401 | 3120 ± 5487 | 0.8 (0.63–1.01)d | 0.06 |
| B-type natriuretic peptide (pg/mL) | 440 ± 948 | 291 ± 465 | 0.79 (0.51–1.24)d | 0.31 |
| Troponin elevation | 94 (46) | 69 (47) | 0.98 (0.71–1.36)e | 0.93 |
Data are presented as n (%) or mean ± standard deviation.
Composite of death from any cause or transfer to intensive care unit during hospitalization.
Per unit increase.
Referent: New York Heart Association functional class ≤ II.
Referent: per 1 standard deviation increment.
Referent: normal troponin concentration.
After multivariable adjustment using complete-case Cox regression analysis in 358 patients, higher body mass index (P = 0.01), male sex (P = 0.02), anticoagulation (P = 0.01), and RV dilatation (P = 0.03) remained independently associated with the primary outcome (Figure 3).
Figure 3.
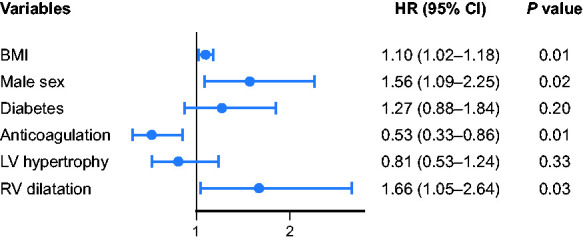
Factors independently associated with the primary composite outcome in multivariable analysis. BMI, body mass index; CI, confidence interval; HR, hazard ratio; LV, left ventricular; RV, right ventricular.
A sensitivity analysis was performed to test the robustness of the final model. When the variable atrial fibrillation was forced into the final multivariable model, it was outperformed by the anticoagulation status (Supplementary data online, TableS2).
The analysis of the primary outcome according to the death (n = 65) underlined also the prognosis value of the RV dilatation (Supplementary data online, TableS3).
RV dilatation in COVID-19 patients
Among the 47 patients with RV dilatation, 33 underwent computed tomography pulmonary angiography to search for pulmonary embolism, of whom 8 (24%) had a confirmed diagnosis. In the group without RV dilatation (n = 360), 191 underwent computed tomography pulmonary angiography, of whom 27 (14%) had pulmonary embolism. Patients with vs. without RV dilatation had a higher prevalence of RV dysfunction (43% vs. 13%; P < 0.001) and there was a collinearity between these two parameters (P < 0.001). There were no differences between patients with and without RV dilatation in terms of pulmonary embolism (P = 0.22), heart failure signs (P = 0.12), NT-pro-BNP, or BNP concentrations (P > 0.05), oxygen administration (P = 1.00), severity of lung involvement on computed tomography (P = 0.73), and d-dimer, C-reactive protein and fibrinogen concentrations (P > 0.05).
Discussion
In this retrospective study, TTE was performed in 15% of 2878 patients hospitalized for SARS-Cov-2 infection, mostly in patients with cardiovascular risk factors and for the purpose of exploring underlying cardiovascular disease. Among the 445 patients who underwent TTE, dilatation and dysfunction were, respectively, observed in 12% and 23% of patients for the LV and in 12% and 16% for the RV. Besides clinical parameters, RV dilatation was the only TTE parameter independently associated with the primary composite outcome.
TTE in the context of COVID-19
In accordance with recommendations10,11 to prevent contamination of cardiac imagers and avoid inappropriate use of personal protective equipment, TTE was not performed routinely in patients with COVID-19; in our study, TTE was performed in 15% of the population hospitalized for COVID-19. Compared with those who did not undergo TTE, patients who did were more likely to be male, older, have a higher prevalence of cardiovascular comorbidities (including risk factors), have more signs of heart failure, and have higher concentrations of cardiac biomarkers (i.e. NT-pro-BNP and troponin). The literature on cardiac injury and COVID-19 should prove helpful in refining the indications for TTE in the context of COVID-19.11,13,14,19
TTE findings in COVID-19
Several studies have described the role of cardiac imaging in evaluating patients with COVID-19 (Supplementary data online, TableS4).14,19–23 Our study represents one of the largest echocardiographic cohorts in such patients requiring hospitalization.
At the start of the pandemic, several case reports24–26 and reviews7,27 suggested that COVID-19 myocardial injury and cytokine storm led to LV systolic dysfunction (including myocardial infarction, myocarditis, and takotsubo cardiomyopathy). Myocardial injury was frequent, in two-thirds of COVID-19 patients, and was associated with an increased in-hospital mortality, particularly if echocardiographic abnormalities were present.19
In our study, 23% of the patients had reduced LVEF. Dweck et al.13 reported similar findings, whereas Jain et al.28 found that 39% of the 72 patients in their study had reduced LVEF. Szekely et al.,14 in a cohort of 100 individuals with COVID-19 who underwent systematic TTE within 24 h of admission, reported that 10% had systolic LV dysfunction and 16% had LV diastolic dysfunction (grade 2 or 3); their findings are in agreement with our data. Whereas RV dilatation and dysfunction were found in 12% and 16% of our patients, respectively, consistent with Dweck et al.,13 Szekely et al.14 found RV dilatation with or without dysfunction in 39% of patients; direct comparisons are, however, limited by differences in the studies.
Clinical significance of TTE parameters
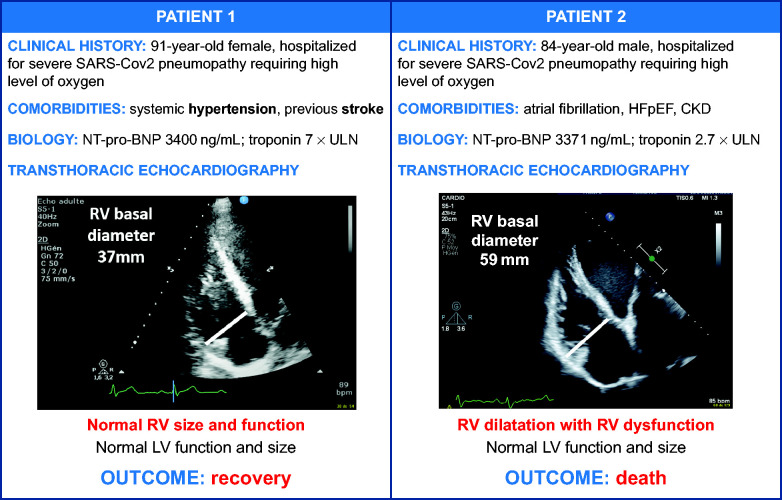
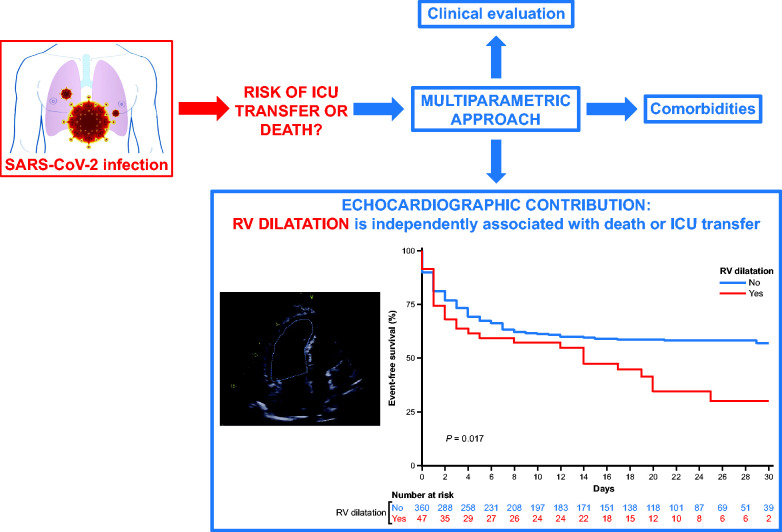
TTE appears useful for risk stratification in COVID-19, with a key role as a widely available bedside test. Figure 5 illustrates two cases where TTE provided important information on the RV. In the study by Dweck et al.,13 an immediate change in management occurred in 33% of 1216 patients who underwent TTE. As all medical interventions in our study were at the discretion of the referring team, we do not know precisely when TTE played a role in the decision to transfer to ICU. TTE results alone cannot determine patient orientation; rather, the decision is made based on a multiparametric strategy that includes clinical and comorbidities findings (Figure 4). However, echocardiography appears to be a valuable tool, not only for initiating or modifying treatments but also to facilitate decisions regarding changes in the level of patient care (i.e. ICU transfer and haemodynamic support).13
Figure 5.
Illustration of the importance of the echocardiographic parameters in the natural history of patients hospitalized for COVID-19. CKD, chronic kidney disease; COVID-19, coronavirus disease-2019; HFpEF, heart failure with preserved ejection fraction; NT-pro-BNP, N-terminal-pro-B-type natriuretic peptide; RV, right ventricle; SARS-Cov-2, severe acute respiratory syndrome coronavirus; ULN, upper limit of normal.
Figure 4.
Prognostic value of RV dilatation in patients with COVID-19. COVID-19, coronavirus disease-2019; ICU, intensive care unit; RV, right ventricular; SARS-Cov-2, severe acute respiratory syndrome coronavirus.
The association between right-side TTE parameters and prognosis in patients with COVID-19 has been explored. Szekely et al.14 reported that the most frequent abnormality among patients with clinical deterioration during follow-up was RV dilatation (with or without dysfunction). In univariable analysis, they found that shorter pulmonary acceleration time was associated with clinical deterioration and that increased RV end-diastolic area was associated with mortality. Similarly, other studies12,29 showed that RV global longitudinal strain was a powerful predictor of death in patients with COVID-19. In an analysis from a US multicentre retrospective study, adverse RV remodelling conferred a >2-fold increase in mortality risk.21 In addition, our study underlined the importance of the multiparametric approach including clinical variables (body mass index, sex), comorbidities (anticoagulation), and echocardiography (RV dilatation) to improve prediction of death but also of ICU transfer.
Some studies reported that in addition to clinical and biological factors, a reduction of LVEF had also a poor prognosis in COVID-19.30,31 Rath et al.32 described the poor prognosis of COVID-19 patients with both RV and LV dysfunction. In our study, RV dilatation evaluated during a bedside echocardiography was independently associated with death or ICU transfer in patients with COVID-19.
Several studies reported an abnormally higher rate of pulmonary embolism in patients with COVID-19,33,34 associated with a poorer prognosis.4 Nevertheless, pulmonary embolism is not the only cause of RV dilatation. In our study, there was no statistically significant difference in terms of pulmonary embolism between patients with or without RV dilatation, confirming that RV dilatation reflects more than just pulmonary vascular disease. In reality, RV dilatation in patients who met the primary outcome could be explained by the extent of lung involvement, both parenchymal and vascular. Indeed, lung involvement can be characterized by both diffuse alveolar disease and pulmonary intravascular coagulopathy in the context of COVID-19.35 Nevertheless, there were no statistical differences in our study between patients with or without RV dilatation in terms of oxygen administration, severity of lung involvement on computed tomography, and biological inflammatory parameters, which also highlights the key role of TTE in stratifying a patient’s risk of death or transfer to ICU.
Limitations
Data collection was retrospective but was done in a relatively short period between the patient’s hospitalization and the gathering of data (15 days, inter-quartile range 10–20), enabling investigators to recover a large amount of relevant data. Second, in contrast to the study by Szekely et al.,14 our TTE examinations were ‘focused’ rather than ‘comprehensive’, and we cannot exclude the possibility of inter-observer variability.10,11 Third, echocardiography results were analysed in each centre and not in a central echocardiography core laboratory, which may induce a bias in the reproducibility of the measurements. However, the large number of participating centres reduces this bias, and our findings concerning RV dilatation/dysfunction are similar to other studies.13 Fourth, the precise indications of TTE and the timing of TTE use were not studied. Nevertheless, patients who underwent TTE had a higher prevalence of cardiovascular risk factors and/or cardiovascular history, but especially more cardiovascular manifestations and/or probably higher suspected complications secondary to COVID-19, which may have motivated the performance of TTE. Fifth, among patients with RV dilatation, computed tomography pulmonary angiography was not systematically performed, which does not allow us to rule out the possibility that RV dilatation is statistically linked to pulmonary embolism.
Conclusion
In this multicentre, retrospective French study, bedside echocardiographic evaluation of RV dilatation could be a useful tool for assessing the risk of the severe form of COVID-19 developing in hospitalized patients.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Supplementary Material
Acknowledgements
Sophie Rushton-Smith, PhD (MedLink Healthcare Communications, London) provided editorial assistance in the preparation of the manuscript (limited to editing for style, referencing, and figure and table editing) and was funded by the French Society of Cardiology, Paris, France.
Conflict of interest: A.C. acknowledges the following, none of which are related to the current manuscript: research grant from RESICARD (research nurses); consultant and lecture fees from Amgen, AstraZeneca, Bayer Pharma, Alliance BMS-Pfizer, Novartis, and Sanofi-Aventis. The other authors have nothing to declare.
Data availability
The data in this report were obtained from patients, we are not allowed to distribute the data in accordance with the data protection rules in France, and we can therefore not make the data publicly available.
References
- 1. Carter P, Anderson M, Mossialos E.. Health system, public health, and economic implications of managing COVID-19 from a cardiovascular perspective. Eur Heart J 2020;41:2516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A. et al. ; for the COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020;323:2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N. et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 2020;41:3058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G. et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol 2020;75:2352–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A. et al. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med 2020;382:2049–55. [DOI] [PubMed] [Google Scholar]
- 9. Neskovic AN, Skinner H, Price S, Via G, De Hert S, Stankovic I. et al. ; Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Focus cardiac ultrasound core curriculum and core syllabus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2018;19:475–81. [DOI] [PubMed] [Google Scholar]
- 10. Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M.. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Coll Cardiol 2020;75:3078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo GD, Donal E. et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020;21:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Li H, Zhu S, Xie Y, Wang B, He L. et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging 2020;13:2287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR. et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging 2020;21:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I. et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation 2020;142:342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonnet G, Weizman O, Trimaille A, Pommier T, Cellier J, Geneste L. et al. Characteristics and outcomes of patients hospitalized for COVID-19 in France: the Critical COVID-19 France (CCF) study. Arch Cardiovasc Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization. Global Surveillance for COVID-19 Caused by Human Infection With COVID-19 Virus: Interim Guidance; 20 March 2020. World Health Organization. https://apps.who.int/iris/handle/10665/331506.
- 17. Phua J, Weng L, Ling L, Egi M, Lim CM, Divatia JV. et al. ; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020;8:506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 19. Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M. et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol 2020;76:2043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR. et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging 2020;21:949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim J, Volodarskiy A, Sultana R, Pollie MP, Yum B, Nambiar L. et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol 2020;76:1965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Li H, Zhu S, Xie Y, Wang B, He L. et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging 2020;13:2287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ojha V, Verma M, Pandey NN, Mani A, Malhi AS, Kumar S. et al. Cardiac magnetic resonance imaging in coronavirus disease 2019 (COVID-19): a systematic review of cardiac magnetic resonance imaging findings in 199 patients. J Thorac Imaging 2020;36:73–83. [DOI] [PubMed] [Google Scholar]
- 24. Meyer P, Degrauwe S, Van Delden C, Ghadri JR, Templin C.. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J 2020;41:1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B. et al. ST-segment elevation in patients with Covid-19—a case series. N Engl J Med 2020;382:2478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu H, Ma F, Wei X, Fang Y.. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2021;42:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng YY, Ma YT, Zhang JY, Xie X.. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020;17:259–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jain SS, Liu Q, Raikhelkar J, Fried J, Elias P, Poterucha TJ. et al. Indications for and findings on transthoracic echocardiography in COVID-19. J Am Soc Echocardiogr 2020;33:1278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bursi F, Santangelo G, Sansalone D, Valli F, Vella AM, Toriello F. et al. Prognostic utility of quantitative offline 2D-echocardiography in hospitalized patients with COVID-19 disease. Echocardiography 2020;37:2029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faridi KF, Hennessey KC, Shah N, Soufer A, Wang Y, Sugeng L. et al. Left ventricular systolic function and inpatient mortality in patients hospitalized with coronavirus disease 2019 (COVID-19). J Am Soc Echocardiogr 2020;33:1414–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soulat-Dufour L, Lang S, Ederhy S, Adavane-Scheuble S, Chauvet-Droit M, Nhan P. et al. Left ventricular ejection fraction: an additional risk marker in COVID-19. Arch Cardiovasc Dis 2020;113:760–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rath D, Petersen-Uribe A, Avdiu A, Witzel K, Jaeger P, Zdanyte M. et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol 2020;109:1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F. et al. ; Lille ICU Haemostasis COVID-19 Group. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation 2020;142:184–6. [DOI] [PubMed] [Google Scholar]
- 34. Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O. et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 2020;296:E189–E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM. et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this report were obtained from patients, we are not allowed to distribute the data in accordance with the data protection rules in France, and we can therefore not make the data publicly available.