Abstract
Aging listeners often experience difficulties in perceiving temporally complex acoustic cues in noisy environments. These difficulties likely have neurophysiological contributors from various levels of auditory processing. Cochlear synapses between inner hair cells and auditory nerve fibers exhibit a progressive decline with age which is not reflected in the threshold audiogram. The functional consequences of this loss for the coding of suprathreshold sound remain poorly understood. Recent studies suggest that cochlear synaptopathy results in degraded representations of temporal envelope cues at the earliest levels of the auditory pathway. Central nuclei downstream of the auditory nerve exhibit a compensatory plasticity in response to this deafferentation, in the form of altered gain. This results in a modulation frequency selective increase in the representation of envelope cues at the level of the auditory midbrain and cortex. These changes may be shaped by mechanisms such as decreased inhibitory neurotransmission occurring with age across various central auditory nuclei. Altered representations of the differing temporal components of speech due to these interactions between multiple levels of the auditory pathway may contribute to the age-related difficulties hearing speech in noisy environments.
Keywords: auditory, cochlear synaptopathy, aging, inferior colliculus, compensatory plasticity
Aging listeners often experience difficulties in processing speech and other temporally complex sounds, particularly in noisy environments. These difficulties can arise due to changes occurring at several levels of the auditory pathway from the ear to the brain, with or without threshold sensitivity losses. In this review, we consider the role of altered representations of the temporal properties of complex sounds in the peripheral and central auditory pathways, and their contributions to functional declines in hearing with age.
SUPRATHRESHOLD DECLINES IN AUDITORY TEMPORAL PROCESSING WITH AGE
In the auditory periphery, cochlear hair cell damage or loss has long been considered a hallmark of age-related sensorineural hearing loss. In particular, outer hair cell damage is often associated with poorer hearing thresholds (Dallos and Harris, 1978), assessed clinically by the behavioral pure tone audiogram. Age-related hearing loss defined as declines in threshold sensitivity is exceedingly common; estimates suggest that over 60% of individuals over 70 years of age have a hearing loss significant enough to interfere with communication (Lin et al., 2011a). Left untreated, hearing loss decreases the quality of life and has also been associated with other age-related comorbidities such as cognitive impairment, dementia or Alzheimer’s disease (Gates et al., 2011; Lin and Albert, 2014; Dawes et al., 2015; Fischer et al., 2016; Deal et al., 2017; Wei et al., 2017). Patterns of loss in hearing sensitivity have been associated with underlying cochlear pathologies due to age, both using histological studies of human temporal bones (Schuknecht and Gacek, 1993) and informed by experiments in animal models (Dubno et al., 2013). However, increasing evidence shows that the overt loss of threshold sensitivity measured by the audiogram fails to capture critical aspects of functional hearing declines that older adults experience (He et al., 1998, Hind et al., 2011, Tremblay et al., 2015). Even when matched for good audiometric thresholds, older listeners show performance declines on tasks that require the processing of timing cues in sounds (Pichora-Fuller and Souza, 2001, Frisina and Frisina, 1997).
The auditory system processes behaviorally relevant sound stimuli across multiple time scales. Word and syllabic rates captured by the fluctuations of the speech envelope are <50 Hz, the periodicity envelope which conveys speaker identity and emotion is between 50 and 500 Hz and the rapidly changing temporal fine structure required for hearing speech in noise when envelope cues are degraded occurs at rates >500 Hz to a few thousand Hz (Bregman, 1990; Rosen, 1992). Effective representations of these temporal features are critical for real world communication, which often occurs in a background of other talkers or environmental noise (Mattys et al., 2012). Older and even middle-aged adults experience the most difficulties in processing these complex spectro-temporal cues necessary for communication, especially in challenging acoustic environments (Walton, 2010; Ruggles et al., 2012; Fullgrabe et al., 2015). The presence of multiple speakers, background noise or reverberation degrade the temporal regularities found in speech, and cause deleterious effects on speech perception (Best et al., 2009, 2010; Anderson et al., 2011; Ruggles et al., 2011), even when thresholds remain well preserved (Frisina and Frisina 1997; Gordon-Salant and Fitzgibbons 2001; Goupell, et al. 2017; Jaekel, et al. 2018). Even without threshold evidence for peripheral involvement, such declines in hearing function with age must nevertheless consider neural changes in the peripheral and central auditory pathway.
PROGRESSIVE COCHLEAR DEAFFERENTATION WITH AGING
Recent studies have revealed what may be an important peripheral contributor to these temporal processing declines. Synapses between inner hair cells (IHCs) and auditory nerve fibers (ANFs) are highly vulnerable to both aging and noise exposure (Fig. 1A, B, for review see Kujawa and Liberman, 2015). Normal aging results in a progressive decline in the number of these synapses (Sergeyenko et al., 2013). Synaptic loss precedes loss of hair cells (Fig. 1C) and changes in hearing thresholds. Loss of ANFs and their cell bodies in the spiral ganglion follows with a delay, but the functional connections/communication between affected fibers and their target IHCs are lost with the synapses. Age-related cochlear synaptopathy can be exacerbated by early exposure to noise that causes synaptopathy but no permanent threshold shifts (Fernandez et al., 2015), suggesting that these two etiologies yielding synapse loss may be targeting the same neuronal subpopulations. Initially reported in mouse (Kujawa and Liberman, 2009), this cochlear deafferentation with aging and/or noise exposure is widespread in various mammalian species, including guinea pigs (Lin et al., 2011b), rats (Mohrle et al., 2016), gerbils (Gleich et al., 2016), chinchillas (Hickox et al., 2017), rhesus macaques (Valero et al., 2017) and in post-mortem human temporal bones (Viana et al., 2015; Wu et al., 2019). Since subtotal synaptopathy goes largely undetected by the threshold audiogram, it has been termed as a ‘hidden’ hearing loss (Schaette and McAlpine, 2011).
Fig. 1.

Progressive cochlear synaptopathy with aging is accompanied by degraded representation of envelope cues in the early auditory pathway. (A) Schematic cross section showing three of the ~20 auditory nerve fibers (ANFs) making synaptic contact with an IHC. Presynaptic ribbons and postsynaptic receptor patches are also schematized. The x–y–z axis shows the viewing angle for the confocal x–y projections shown for example IHCs in (B), where immunostaining reveals the juxtaposition of pre-synaptic ribbons (red) and post-synaptic receptor patches (green). Inset shows magnified example images of presynaptic ribbons (red) with apposing post-synaptic glutamate receptor patches (green). (C) Mean (±SEMs) percent survival of cochlear synapses (green line), inner hair cells (gray solid line) and outer hair cells (gray dashed line), relative to 16-week-old animals, at 30 kHz. (D) Mean (±SEM) EFR amplitudes at 1024 Hz AM measured as a function of sound level across the various age groups at 30-kHz frequency region. Dashed lines indicate responses below the noise floor. (E) Mean (±SEM) EFR amplitudes at equal sensation levels (SL) of 0 dB (threshold) to 30 dB. (F) Correlation between EFR amplitudes from panel E at 30-dB SL (dashed box) and the number of remaining synapses/IHC across all the age groups. (G) Mean (±SEM) ABR wave 5: wave 1 ratios across age at 80-dB SPL. Figure modified from Parthasarathy and Kujawa, 2018).
Various lines of evidence have suggested that the vulnerable subpopulations of neurons in the auditory nerve may be those with low spontaneous rates (low-SR) of firing. Losses in these low-SR neurons with aging, noise exposure or ouabain-induced neuropathy have been studied using single-neuron recordings from the auditory nerve (Schmiedt et al., 1996; Furman et al., 2013; Bourien et al., 2014) as well as immunohistological analysis of the spatial organization of these synapses along the modiolar-pillar axis of the inner hair cell (Liberman et al., 2011, 2015; Yin et al., 2014;). While the exact mechanism by which the preferential loss of low-SR fibers may affect auditory processing is still under debate (Carney, 2018), these neurons have higher thresholds, show a greater preference for synchronized firing at moderate to high sound levels and are resistant to the effects of background noise, properties that should aid suprathreshold sound processing in real-world listening situations (Costalupes et al., 1984; Joris and Yin, 1992).
Multiple physiological measures have shown promise as non-invasive assays of cochlear synaptopathy. These include the wave 1 of the auditory brainstem response (ABR) (Kujawa and Liberman, 2009; Sergeyenko et al., 2013), the middle ear muscle reflex (MEMR) (Valero et al., 2016; Valero et al., 2018) and envelope following responses (EFRs; Shaheen et al., 2015; Parthasarathy and Kujawa, 2018). Additionally, MEMRs, which have low-SR neurons as primary drivers (Liberman and Kiang, 1984; Kobler et al., 1992), and EFRs, which use stimuli that may more effectively activate low-SR neurons (Joris and Yin, 1992), may be useful in probing functional consequences of the targeted loss of these neurons in aging and noise-exposed individuals. While these measures have been shown to be reliable indicators of synaptopathy in animal models, the results from human subject groups have been mixed, with some studies showing results that could be consistent with the presence of an underlying synaptopathy (Konrad-Martin et al., 2012; Stamper and Johnson, 2015; Liberman et al., 2016; Mehraei et al., 2016; Wojtczak et al., 2017; Mehraei et al., 2017; Bramhall et al., 2017, 2018; Valderrama et al 2018), some mixed outcomes (Grose et al., 2017) and others finding no evidence of its presence (Grinn et al., 2017, Guest et al., 2017; Prendergast et al., 2017a,b; Fulbright et al., 2017). Age-graded declines in auditory nerve fiber contacts with IHCs documented in human temporal bones (Viana et al., 2015; Wu et al., 2019) have closely paralleled the age-graded declines in mouse (Sergeyenko et al., 2013); however, the possibility that functional consequences of this loss are less robust in humans than those suggested by the animal data cannot currently be ruled out. Additional contributors to the discrepancy may include uncertainties associated with estimating lifetime noise exposure using self-report questionnaires, variability in the nature of the exposures experienced by the various studied subjects, which ranges from military arms fire to single exposures using personal music players, discrepancies in measuring ABRs and EFRs in humans due to variability in head sizes and noise floors, limitations of time which make testing multiple sound levels and frequencies impractical and, of course, the inherently greater variability in highly outbred species, like humans. The presence of outer hair cell loss is another confounding factor, especially when studying age-related cochlear synaptopathy. However, in animal models, synaptopathy due to age is progressive throughout the lifespan, and begins well before frank outer hair cell loss. Therefore, testing middle aged or older listeners with normal audiograms might be beneficial in isolating the effects of synaptopathy. Future work in optimizing stimulus parameters, recording techniques and assessment of noise exposure history are needed to help resolve these inconsistencies. Although the diagnosis of underlying synaptopathy is in itself an important question to be addressed, the next step is a greater understanding of the functional consequences of this synaptopathy on the neural coding of sounds.
ASSESSING THE FUNCTIONAL CONSEQUENCES OF AGE-RELATED SYNAPTOPATHY ON THE NEURAL CODING OF SOUNDS
Cochlear synaptopathy is hypothesized to affect sound processing through the degradation of complex temporal cues. One theory of stochastic undersampling posits that partial deafferentation would reduce the “sampling rate” with which neurons of the auditory nerve represent sound (Marmel et al., 2015). According to this view, synaptopathy would contribute to the degraded temporal resolution seen in older listeners as temporally complex stimuli would require a greater “resolution” to effectively represent all their acoustic features compared to simple stimuli (Lopez-Poveda and Barrios, 2013; Lopez-Poveda, 2014).
To test these theories in human clinical populations and in animal models requires non-invasive measures that can represent the spectro-temporal complexities present in relevant real world stimuli. It is in this context that the EFRs provide valuable, additional information. Since EFRs are a faithful representation of the stimulus envelope, they can provide a snapshot of auditory processing to temporally complex stimuli. EFRs are population responses evoked by the synchronized responses of groups of neurons along the auditory pathway and provide complementary information to the ABRs evoked by brief phasic stimuli (Parthasarathy et al., 2014). Neurons of the auditory pathway represent the temporal aspects of sounds in their firing patterns with different degrees of fidelity (Joris et al., 2004), and it is the ensemble activity of these neurons that is thought to be captured at the scalp by the EFRs. EFRs with generators in the auditory nerve may be more sensitive to noise-induced cochlear synaptopathy than the ABR wave 1 (Shaheen et al., 2015), since the low-SR fibers thought to be preferentially affected by synaptopathy contribute to the sustained neural responses to longer, temporally complex sounds (Joris and Yin, 1992). EFRs elicited to speech sounds, such as a consonant–syllable complex, or to sinusoidally amplitude-modulated tones have been used successfully to probe auditory processing in various human clinical populations, including aging listeners (Tremblay et al., 2003; Clinard et al., 2010; Krishnan and Agrawal, 2010; Anderson et al., 2012; Clinard and Tremblay, 2013; Bidelman et al., 2014; Ananthakrishnan et al., 2016), and in animal studies where they can be recorded along with more invasive measurements and correlated with underlying histopathology (Zhong et al., 2014; Shaheen et al., 2015; Herrmann et al., 2017; Parthasarathy et al., 2018). Hence the EFR shows promise in bridging the gap between human studies and animal models in understanding the temporal processing deficits caused by age-related cochlear synaptopathy.
For understanding the progression and interaction of peripheral and central changes with aging, it would be advantageous to dissociate neural generators. Within this context of spatial specificity, one limitation of the EFR is the lack of spatial resolution compared to metrics like the ABR, which have clearly defined peaks whose neural generators have been more or less localized. The longer duration of stimuli in the EFRs causes some overlap between the responses evoked from different generators with differing response latencies. However, this limitation can be minimized by a few approaches. The first is stimulus selection. The upper limit of phase-locking, or the degree to which neurons faithfully represent the temporal regularities in sound in their spike timings, decreases along the ascending auditory pathway (Joris et al., 2004). Although exact boundaries differ by species, neurons in the auditory nerve can phase lock up to a few thousand hertz, neurons in the inferior colliculus in the midbrain can only phase lock to a few hundred hertz, and neurons in the auditory cortex typically reach their limit by one hundred hertz (see Joris et al., 2004 for review). While using speech sounds to evoke EFRs provides real-world relevance, the use of sinusoidally amplitude-modulated tones can provide better localization of neural generators by fine tuning the stimulus temporal characteristics. EFRs elicited by slower AM frequencies (~40 Hz) are thought to be primarily cortical in origin (Herdman et al., 2002; Kuwada et al., 2002; Ross et al., 2003), whereas EFRs elicited by AM frequencies around 110 Hz are thought to be subcortical, reflecting thalamic, midbrain and brainstem activity (Picton et al., 2003; Parthasarathy and Bartlett, 2012). Recent studies have extended these observations using faster AM frequencies around 800–1000 Hz, with generators from the auditory nerve, as confirmed by the application of ouabain, a neurotoxin, to the round window of mice. Ouabain can be applied at doses that eliminate neural responses without affecting outer hair cell based DPOAE thresholds or amplitudes (Lang et al., 2011; Yuan et al., 2014). Ouabain application suggests that EFRs to modulation frequencies centered around 1000 Hz have putative generators in the auditory nerve (Shaheen et al., 2015; Parthasarathy and Kujawa, 2018) and that faster EFRs to ~4000 Hz AM may even be used to probe hair cell based responses to temporally modulated stimuli (Parthasarathy and Kujawa, 2018). These measurements can serve to confirm that EFRs can be obtained from the earliest neural generators of the auditory pathway.
The second approach to achieving better spatial resolution with EFRs is to use simultaneous recording from multiple channels (Galbraith et al., 2001; Galbraith et al., 2006; Parthasarathy and Bartlett, 2012; Bidelman, 2015; King et al., 2016). Using different electrode montages results in each channel primarily capturing a different electrical dipole, and the difference in the spatial geometry of the dipoles can emphasize rostral versus caudal generators along the auditory pathway (Parthasarathy and Bartlett, 2012; Parthasarathy et al., 2018). Multichannel recordings can also be leveraged to obtain better SNRs of the recordings, by modeling the various dipoles at the scalp and deconstructing them using a complex PCA (Bharadwaj and Shinn-Cunningham, 2014). These approaches can be useful especially when using speech-like stimuli, where the stimulus properties cannot be manipulated to emphasize different generators without compromising on different temporal cues essential for speech comprehension.
TEMPORAL PROCESSING DEFICITS WITH AGE MEASURED USING ENVELOPE FOLLOWING RESPONSES
Temporal processing probed using EFRs reveal widespread deficits along the auditory pathway with age. Degradations in the representation of envelope cues begin at the earliest neural generators with age (Fig. 1D). These deficits begin prior to any changes in hearing thresholds, decline progressively with age and persist at suprathreshold sound levels (Fig. 1E). EFR amplitudes are strongly correlated with the remaining number of cochlear synapses (Fig. 1F). This suggests that age-related cochlear synaptopathy is associated with decreased representation of envelope cues. Aging also results in a decreased dynamic range for representation of level and amplitude modulation depth at these early neural generators (Parthasarathy and Kujawa, 2018). The use of such high modulation frequencies is technically challenging in humans due to the interactions among filter widths, cochlear frequencies and modulation frequencies. Moreover the response amplitudes at faster modulation frequencies also show a much steeper roll off. However, studies which have measured tonal phase-locking to carrier frequencies around 1000 Hz show greater deficits in response amplitudes in aged listeners (Clinard et al., 2010; Marmel et al., 2013).
Degradations in envelope processing persist when using slower modulation frequencies (~100–500 Hz) with generators in the midbrain (Kiren et al., 1994; Chandrasekaran and Kraus, 2010; Herdman et al., 2002). Results from studies using speech tokens to evoke EFRs can also be generally interpreted to contain responses arising primarily from similar subcortical sources, since the F0 envelope periodicities used in these tokens correspond to similar modulation frequencies (Rosen, 1992; Bidelman, 2018). Multiple studies have shown that aging decreases the strength of phase-locking to envelope cues (Anderson et al., 2012; Clinard and Tremblay, 2013; Schoof and Rosen, 2016). EFRs elicited using amplitude modulation frequencies in similar ranges also show a decrease with age (Parthasarathy and Bartlett, 2012). Hence, declines in the integrity of temporal processing seen at the level of the auditory nerve can also be observed along the ascending auditory pathway with age.
However, some evidence suggests that these changes are often minimal when the envelope cues are salient, such as in quiet, with slow modulation frequencies (<50 Hz) or with large modulation depths (Parthasarathy et al., 2010; Parthasarathy and Bartlett, 2011). The wave 1:5 ratio, a marker for central compensation, increases with age (Fig. 1G, Sergeyenko et al., 2013; Parthasarathy and Kujawa, 2018). Compensating for loss of hearing thresholds by increasing sound level does not result in equal response amplitudes in both ABRs and EFRs (Lai et al., 2017). ABR and EFR amplitudes are correlated in young but not aged animals, suggesting a decoupling between the neural mechanisms producing phasic onset responses that constitute the ABR and the sustained responses that constitute the EFR (Parthasarathy et al., 2014). Matching ABR wave 1 amplitudes between young and aged animals results in increased response amplitudes of the EFRs with age at high modulation depths (Lai et al., 2017). Deficits are also exacerbated with the addition of background noise, lower modulation depths or multiple stimuli (Parthasarathy et al., 2010, 2016; Parthasarathy and Bartlett, 2011). These results suggest some form of compensatory activity emerging at the level of the brainstem or midbrain that may restore evoked potential responses in quiet and for strong envelope cues but not for complex listening conditions and degraded envelope cues.
The compensatory plasticity seen at the level of the midbrain is stronger at cortical levels, especially in quiet. EFR amplitudes for responses arising primarily from cortical generators are less affected by aging (Bidelman et al., 2014; Goossens et al., 2016). However, these compensatory mechanisms do not seem sufficient to minimize age-related declines in the presence of signal degradations such as decreased modulation depths (Dimitrijevic et al., 2016) and the presence of multiple speakers (Presacco et al., 2016a), or for adaptation to more complex stimulus statistics (Herrmann et al., 2018). There is also evidence from human literature of compensatory gain at the cortical level, and an altered interaction between the auditory nuclei of the midbrain and the cortex (Bidelman et al., 2014; Presacco et al., 2016b; Valderrama et al., 2018). This could, in part, explain why many studies find only subtle differences in temporal processing with age (Schoof and Rosen, 2016; Paraouty and Lorenzi, 2017), and even when they do find an age-related degradation of temporal processing using both behavioral and neurophysiological measures, they are often not correlated (Clinard et al., 2010; Schoof and Rosen, 2016). These results could be explained by compensatory plasticity occurring at the higher levels of the auditory pathway and causing a divergence between brainstem encoding of sound features and the eventual perception of sound.
CELLULAR MECHANISMS UNDERLYING COMPENSATORY PLASTICITY WITH AGE
The specific neural mechanisms by which the aging brain encodes the temporal regularities in sound, especially at suprathreshold sound levels, is an ongoing field of study that will be critical in understanding the neural basis for difficulties understanding speech in noise. Changes in the interaction between multiple levels of processing after the auditory nerve and the mechanisms underlying these changes are not yet fully understood. Within the cochlear nuclei, auditory nerve projections terminate onto a variety of excitatory and inhibitory cell types that each process their inputs differently and have different spectro-temporal profiles (Trussell, 1999; Yu and Young, 2000), all of which change with age (e.g. Schatteman et al., 2008; Xie, 2016; Xie and Manis, 2017). Cochlear synaptopathy and the silencing of auditory neurons that results may affect suprathreshold coding by degrading the representation of temporal cues being sent to the rest of the auditory pathway (Xie, 2016; Goodman et al., 2018; Parthasarathy and Kujawa, 2018). This would occur across cochlear nucleus neurons tuned to a wide range of frequencies due to the contributions of the low-frequency tails of higher-frequency neurons (Parthasarathy et al., 2016; Lai and Bartlett 2018). Auditory nuclei further downstream may show compensatory plasticity in an attempt to reestablish the homeo-static balance between excitation and inhibition. This compensatory plasticity has been observed in other forms of sensorineural hearing loss and after neurotoxic ouabain, where a decrease in afferent outflow from the periphery results in increased activity in the auditory midbrain and cortex (Kotak and Sanes, 2003; Kotak et al., 2003, 2005; Barsz et al., 2007; Chambers et al., 2016a, b). This helps compensate auditory responses to simple stimuli but comes at the cost of altered temporal processing to complex sounds (Chambers et al., 2016a). These compensatory changes are, in part, mediated by cortical inhibitory circuits which are thought to decrease with a decrease in peripheral inputs (Resnik and Polley, 2017). Inhibitory neurotransmitters GABA and glycine serve to shape responses to temporally complex sounds in the central auditory pathway including the cochlear nucleus (Backoff et al., 1999; Keine et al., 2016), inferior colliculus (Palombi and Caspary, 1996a; Caspary et al., 2002), auditory thalamus (Cai and Caspary, 2015) and cortex (Wang et al., 2002; Razak and Fuzessery, 2009, 2010; Gaucher et al., 2013), increasing their selectivity for acoustic features in stimuli like envelope cues, frequency modulation and preferred frequency. Hence the decrease in inhibition may serve to decrease the selectivity of neurons to the acoustic features in sounds, and thereby decrease the fidelity of neural coding.
Evidence for this compensatory plasticity with age is seen as early as the cochlear nucleus, where there is a decrease in inhibitory glycine binding sites (Wang et al., 2009). This decrease in inhibitory neurotransmission is accompanied by age-related temporal processing deficits in the cochlear nucleus; for example, decreased selectivity for AM frequencies and decreased phase-locking to AM sounds especially evident at lower modulation depths (Caspary et al., 2005; Schatteman et al., 2008). At the inferior colliculus, there is a decrease in GABAergic neurotransmission with age (Raza et al., 1994; Burianova et al., 2009; Rabang et al., 2012). This is accompanied by a shift in the temporal response properties of the neurons toward lower modulation frequencies and decreased responses to rapid modulation rates. However, differences in the responses of aged IC neurons to simple monaural and binaural stimuli and slow modulation frequencies are minimal (Willott et al., 1988a,b, 1991; Palombi and Caspary, 1996a,b; Rabang et al. 2012; Herrmann et al. 2017), and changes emerge only when using faster modulation rates or more complex stimuli like speech envelopes. Comparing local field potentials, as a marker for synaptic inputs to IC neurons (Logothetis et al., 2001), and the spiking activity of the IC neurons shows that the inputs coming in to the IC are degraded with age. However, there is a relative increase in activity in the aged IC neurons that is selective to slower modulation frequencies (Herrmann et al., 2017). Similar experiments using speech-like envelopes indicate a selective increase in representation of periodicity envelope cues, relative to the synaptic inputs, in the neurons of the aged IC (Fig. 2; Parthasarathy et al., 2018). The decreasing inhibitory neurotransmission is further observed in the auditory forebrain, with decreased GABAA receptor density and currents in the thalamus (Richardson et al., 2013b) and in the auditory cortex (Caspary et al., 2013; Stebbings et al., 2016). This is accompanied by an increase in spontaneous firing rate indicative of an altered balance between excitation and inhibition, but physiological changes with age, such as a decrease in synchronization to some amplitude-modulated sounds, are subtle under simple conditions and emerge only with degraded spectro-temporal cues (Richardson et al., 2013a; Engle and Recanzone, 2013; Overton and Recanzone, 2016; Cai et al., 2016; Aushana et al., 2018). These studies indicate that the results seen at the population level using scalp electrodes are mostly corroborated by intra-IC LFPs and partially corroborated at the single-unit level.
Fig. 2.
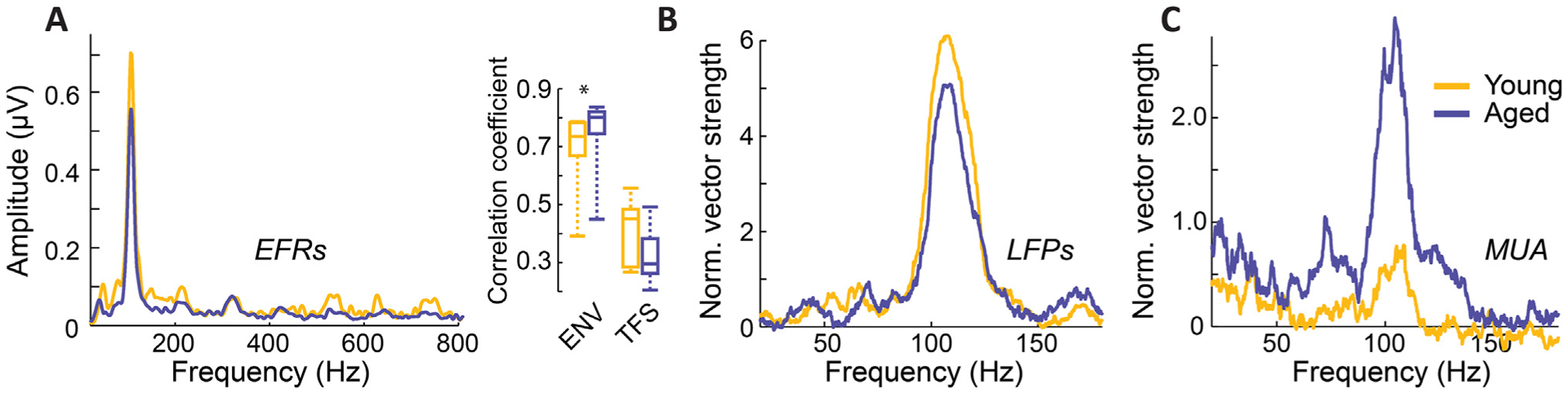
Responses from the inferior colliculus show evidence for magnified representation of envelope cues with age (A) Amplitude spectra derived from fast Fourier transforms of scalp-recorded envelope following responses to a modified speech token. Boxplots show coefficients from the cross-correlation for different frequency bands. (B) Spectrum of normalized vector strength for local field potentials (LFPs) recorded from the inferior colliculus of young and aged F-344 rats around the periodicity envelope frequency (105–115 Hz). (C) Spectrum of normalized vector strength of multi-unit activity recorded from the inferior colliculus of young and aged Fischer-344 rats. *p < 0.05. (Figure modified from Parthasarathy et al., 2018).
Understanding the neural mechanisms underlying altered temporal processing with age ultimately will require tracking the changes in identified neuronal subpopulations as a function of age, and comparing them to far-field evoked potential recordings to see how changes observed at the cellular level translate to population responses in humans and animal models. In this process, computational modeling can help to understand how the underlying cellular mechanisms translate to age-related changes in population responses that are seen at the scalp. Phenomenological models can recreate physiological responses observed in humans and animal models (Zilany et al., 2009, 2014; Verhulst et al., 2015; Saremi et al., 2016), and are especially useful in considering multiple nuclei and frequencies contributing to suprathreshold coding observed using evoked potentials (Nelson and Carney, 2004; Verhulst et al., 2018). These models can help isolate the potential contributions of the various components, such as threshold elevations, outer hair cell loss and synaptopathy, in carefully controlled ways that are harder to do in aging humans and to a lesser extent other animal models (Bharadwaj et al., 2014, Verhulst et al., 2015, 2016; Parthasarathy et al., 2016). Biophysical models can help us further understand the cellular basis for age-related changes in sound representation (Rabang and Bartlett, 2011; Rabang et al., 2012; Manis and Campagnola 2018), though optimal constraining of parameters is required when dealing with such models to avoid overfitting (Oleksiak et al., 2011; Vayrynen et al., 2016; Coventry et al., 2017). Titrating the various excitatory and inhibitory components to recreate neuronal responses can help us understand the cellular basis for the changes occurring due to aging at multiple stages of the auditory pathway.
Finally, a task as complex as speech perception is dependent on various extra-sensory processes including attention, motivation, cognitive abilities, working memory and listening effort (Clayton et al., 2016; Eckert et al., 2016; Hornsby et al., 2016; DeCaro et al., 2016). A detailed consideration of these studies is beyond the scope of this review (but see Peelle, 2018 for review, and Pichora-Fuller et al., 2016 for a framework for effortful listening). However, the effects of sensory changes, peripheral and central, on higher order executive functions, and vice versa are critical components in studying age-related hearing loss (see Peelle and Wingfield, 2016 for review). Degradations in the sensory coding of auditory stimuli with age may be compensated by increased listening effort (Ayasse et al., 2017), though this may come at the cost of mental fatigue (Hornsby et al., 2016; Moore et al., 2017). A true understanding of age-related deficits with speech communication will require a consideration of all these higher order executive functions, and their interactions with sensory processing within the auditory pathway.
The effects of age on hearing can manifest at multiple levels of the auditory pathway. Progressive cochlear synaptopathy is an important peripheral contributor to age-related declines in hearing function and is associated with decreased representation of temporal envelope cues at the earliest regions of the auditory pathway. These deficits persist at suprathreshold sound levels and are independent of changes in hearing thresholds. More central auditory regions may exhibit compensatory plasticity due to this reduced peripheral drive, mediated in part by the decrease in inhibitory neurotransmission. These compensatory changes have differential effects on the various components of speech, with aberrant enhancement of periodicity envelope cues, and a decrease in representation of temporal fine structure. A deeper understanding of these compensatory changes as well as the contributions of top-down, auditory and non-auditory processes will be required to fully understand difficulties in speech comprehension with age.
ACKNOWLEDGMENTS
Funding was provided by the National Institutes of Health (NIDCD DC-011580) to ELB and the Department of Defense (W81XWH-15-1-0103) to SGK.
Abbreviations:
- ABR
auditory brainstem response
- ANFs
auditory nerve fibers
- EFRs
envelope following responses
- IHCs
inner hair cells
- low-SR
low spontaneous rates
- MEMR
middle ear muscle reflex
REFERENCES
- Ananthakrishnan S, Krishnan A, Bartlett E (2016) Human frequency following response: neural representation of envelope and temporal fine structure in listeners with normal hearing and sensorineural hearing loss. Ear Hear 37(2):e91–e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N (2012) Aging affects neural precision of speech encoding. J Neurosci 32 (41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, Yi H-G, Kraus N (2011) A Neural basis of speech-in-noise perception in older adults. Ear Hear 32 (6):750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aushana Y, Souffi S, Edeline JM, Lorenzi C, Huetz C (2018) Robust neuronal discrimination in primary auditory cortex despite degradations of spectro-temporal acoustic details: comparison between guinea pigs with normal hearing and mild age-related hearing loss. J Assoc Res Otolaryngol 19(2):163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayasse ND, Lash A, Wingfield A (2017) Effort not speed characterizes comprehension of spoken sentences by older adults with mild hearing impairment. Front Aging Neurosci 8:329. 10.3389/fnagi.2016.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backoff PM, Palombi PS, Caspary DM (1999) gamma-Aminobutyric acidergic and glycinergic inputs shape coding of amplitude modulation in the chinchilla cochlear nucleus. Hear Res 134(1–2):77–88. [DOI] [PubMed] [Google Scholar]
- Barsz K, Wilson WW, Walton JP (2007) Reorganization of receptive fields following hearing loss in inferior colliculus neurons. Neuroscience 147(2):532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best V, Gallun FJ, Mason CR, Kidd G Jr, Shinn-Cunningham BG (2010) The impact of noise and hearing loss on the processing of simultaneous sentences. Ear Hear 31(2):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best V, Marrone N, Mason CR, Kidd G Jr, Shinn-Cunningham BG (2009) Effects of sensorineural hearing loss on visually guided attention in a multitalker environment. J Assoc Res Otolaryngol 10 (1):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG (2014) Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci 8:26. 10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Shinn-Cunningham BG (2014) Rapid acquisition of auditory subcortical steady state responses using multichannel recordings. Clin Neurophysiol 125(9):1878–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM (2015) Multichannel recordings of the human brainstem frequency-following response: scalp topography, source generators, and distinctions from the transient ABR. Hear Res 323:68–80. [DOI] [PubMed] [Google Scholar]
- Bidelman GM (2018) Subcortical sources dominate the neuroelectric auditory frequency-following response to speech. Neuroimage 175:56–69. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Villafuerte JW, Moreno S, Alain C (2014) Age-related changes in the subcortical-cortical encoding and categorical perception of speech. Neurobiol Aging 35(11):2526–2540. [DOI] [PubMed] [Google Scholar]
- Bourien J, Tang Y, Batrel C, Huet A, Lenoir M, Ladrech S, Desmadryl G, Nouvian R, Puel JL, Wang J (2014) Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol 112(5):1025–1039. [DOI] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP, Griest SE (2017) Auditory brainstem response altered in humans with noise exposure despite normal outer hair cell function. Ear Hear 38 (1):E1–E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Konrad-Martin D, McMillan GP (2018) Tinnitus and auditory perception after a history of noise exposure: relationship to auditory brainstem response measures. Ear Hear 39 (5):881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman AS (1990) Auditory scene analysis; the perceptual organization of sound. Cambridge, MA: MIT Press. [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J (2009) Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol 44(3):161–169. [DOI] [PubMed] [Google Scholar]
- Cai R, Caspary DM (2015) GABAergic inhibition shapes sam responses in rat auditory thalamus. Neuroscience 299:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai R, Richardson BD, Caspary DM (2016) Responses to predictable versus random temporally complex stimuli from single units in auditory thalamus: impact of aging and anesthesia. J Neurosci 36 (41):10696–10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney LH (2018) Supra-threshold hearing and fluctuation profiles: implications for sensorineural and hidden hearing loss. J Assoc Res Otolaryngol 19(4):331–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Ling LL (2013) Age-related GABA(A) receptor changes in rat auditory cortex. Neurobiol Aging 34 (5):1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Palombi PS, Hughes LF (2002) GABAergic inputs shape responses to amplitude modulated stimuli in the inferior colliculus. Hear Res 168(1–2). PII S0378–5955(02)00363–5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF (2005) Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci 25 (47):10952–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan YS, Whitton JP, Edge AS, Liberman MC, Polley DB (2016a) Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89 (4):867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Salazar JJ, Polley DB (2016b) Persistent thalamic sound processing despite profound cochlear denervation. Front Neural Circuits 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N (2010) The scalp-recorded brainstem response to speech: neural origins and plasticity. Psychophysiology 47(2):236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton KK, Swaminathan J, Yazdanbakhsh A, Zuk J, Patel AD, Kidd G (2016) Executive function, visual attention and the cocktail party problem in musicians and non-musicians. PLoS ONE 11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL (2013) Aging degrades the neural encoding of simple and complex sounds in the human brainstem. J Am Acad Audiol 24(7):590–599. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR (2010) Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hear Res 264(1–2):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costalupes JA, Young ED, Gibson DJ (1984) Effects of continuous noise backgrounds on rate response of auditory-nerve fibers in cat. J Neurophysiol 51(6):1326–1344. [DOI] [PubMed] [Google Scholar]
- Coventry BS, Parthasarathy A, Sommer AL, Bartlett EL (2017) Hierarchical winner-take-all particle swarm optimization social network for neural model fitting. J Comput Neurosci 42(1):71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Harris D (1978) Properties of auditory-nerve responses in absence of outer hair cells. J Neurophysiol 41(2):365–383. [DOI] [PubMed] [Google Scholar]
- Dawes P, Emsley R, Cruickshanks KJ, Moore DR, Fortnum H, Edmondson-Jones M, McCormack A, Munro KJ (2015) Hearing loss and cognition: the role of hearing aids, social isolation and depression. PLoS ONE 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal JA, Betz J, Yaffe K, Harris T, Purchase-Helzner E, Satterfield S, Pratt S, Govil N, Simonsick EM, Lin FR, Health ABCSG (2017) Hearing impairment and incident dementia and cognitive decline in older adults: the health ABC study. J Gerontol Series A-Biol Sci Med Sci 72(5):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaro R, Peelle JE, Grossman M, Wingfield A (2016) The two sides of sensory-cognitive interactions: effects of age, hearing acuity, and working memory span on sentence comprehension. Front Psychol 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic A, Alsamri J, John MS, Purcell D, George S, Zeng FG (2016) Human envelope following responses to amplitude modulation: effects of aging and modulation depth. Ear Hear 37 (5):E322–E335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno JR, Eckert MA, Lee FS, Matthews LJ, Schmiedt RA (2013) Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol 14 (5):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Teubner-Rhodes S, Vaden KI (2016) Is listening in noise worth it? Neurobiology of speech recognition in challenging listening conditions. Ear Hear 37:101S–110S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle JR, Recanzone GH (2013) Characterizing spatial tuning functions of neurons in the auditory cortex of young and aged monkeys: a new perspective on old data. Front Aging Neurosci 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PWC, Lall K, Liberman MC, Kujawa SG (2015) Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci 35(19):7509–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ME, Cruickshanks KJ, Schubert CR, Pinto AA, Carlsson CM, Klein BEK, Klein R, Tweed TS (2016) Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc 64(10):1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD (1997) Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res 106(1–2):95–104. [DOI] [PubMed] [Google Scholar]
- Fulbright ANC, Le Prel CGI, Griffiths SK, Lobarinas E (2017) Effects of recreational noise on threshold and suprathreshold measures of auditory function. Semin Hear 38(04):298–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullgrabe C, Moore BCJ, Stone MA (2015) Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci 6:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC (2013) Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol 110(3):577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith G, Waschek J, Armstrong B, Edmond J, Lopez I, Liu WX, Kurtz I (2006) Murine auditory brainstem evoked response: putative two-channel differentiation of peripheral and central neural pathways. J Neurosci Methods 153(2):214–220. [DOI] [PubMed] [Google Scholar]
- Galbraith GC, Bagasan B, Sulahian J (2001) Brainstem frequency-following response recorded from one vertical and three horizontal electrode derivations. Percept Mot Skills 92(1):99–106. [DOI] [PubMed] [Google Scholar]
- Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB (2011) Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol-Head Neck Surg 137(4):390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher Q, Huetz C, Gourevitch B, Edeline JM (2013) Cortical Inhibition reduces information redundancy at presentation of communication sounds in the primary auditory cortex. J Neurosci 33(26). pp. 10713-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich O, Semmler P, Strutz J (2016) Behavioral auditory thresholds and loss of ribbon synapses at inner hair cells in aged gerbils. Exp Gerontol 84:61–70. [DOI] [PubMed] [Google Scholar]
- Goodman DFM, Winter IM, Leger AC, de Cheveigne A, Lorenzi C (2018) Modelling firing regularity in the ventral cochlear nucleus: mechanisms, and effects of stimulus level and synaptopathy. Hear Res 358:98–110. [DOI] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J, van Wieringen A (2016) Aging affects neural synchronization to speech-related acoustic modulations. Front Aging Neurosci 8:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ (2001) Sources of age-related recognition difficulty for time-compressed speech. J Speech Language Hear Res 44(4):709–719. [DOI] [PubMed] [Google Scholar]
- Goupell MJ, Gaskins CR, Shader MJ, Walter EP, Anderson S, Gordon-Salant S (2017) Age-related differences in the processing of temporal envelope and spectral cues in a speech segment. Ear Hear 38(6):E335–E342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinn SK, Wiseman KB, Baker JA, Le Prell CG (2017) Hidden hearing loss? No Effect of common recreational noise exposure on cochlear nerve response amplitude in humans. Front Neurosci 11:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Buss E, Hall JW (2017) Loud music exposure and cochlear synaptopathy in young adults: isolated auditory brainstem response effects but no perceptual consequences. Trends Hear 21. 2331216517737417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ (2017) Tinnitus with a normal audiogram: relation to noise exposure but no evidence for cochlear synaptopathy. Hear Res 344:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He NJ, Dubno JR, Mills JH (1998) Frequency and intensity discrimination measured in a maximum-likelihood procedure from young and aged normal-hearing subjects. J Acoust Soc Am 103(1):553–565. [DOI] [PubMed] [Google Scholar]
- Herdman AT, Picton TW, Stapells DR (2002) Place specificity of multiple auditory steady-state responses. J Acoust Soc Am 112 (4):1569–1582. [DOI] [PubMed] [Google Scholar]
- Herrmann B, Maess B, Johnsrude IS (2018) Aging affects adaptation to sound-level statistics in human auditory cortex. J Neurosci 38 (8):1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann B, Parthasarathy A, Bartlett EL (2017) Ageing affects dual encoding of periodicity and envelope shape in rat inferior colliculus neurons. Eur J Neurosci 45(2):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickox AE, Larsen E, Heinz MG, Shinobu L, Whitton JP (2017) Translational issues in cochlear synaptopathy. Hear Res 349:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind SE, Haines-Bazrafshan R, Benton CL, Brassington W, Towle B, Moore DR (2011) Prevalence of clinical referrals having hearing thresholds within normal limits. Int J Audiol 50(10):708–716. [DOI] [PubMed] [Google Scholar]
- Hornsby BWY, Naylor G, Bess FH (2016) A taxonomy of fatigue concepts and their relation to hearing loss. Ear Hear 37:136S–144S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaekel BN, Newman RS, Goupell MJ (2018) Age effects on perceptual restoration of degraded interrupted sentences. J Acoust Soc Am 143(1):84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A (2004) Neural processing of amplitude-modulated sounds. Physiol Rev 84(2):541–577. [DOI] [PubMed] [Google Scholar]
- Joris PX, Yin TCT (1992) Responses to amplitude-modulated tones in the auditory-nerve of the cat. J Acoust Soc Am 91(1):215–232. [DOI] [PubMed] [Google Scholar]
- Keine C, Rubsamen R, Englitz B (2016) Inhibition in the auditory brainstem enhances signal representation and regulates gain in complex acoustic environments. Elife 5. pii: e19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Hopkins K, Plack CJ (2016) Differential group delay of the frequency following response measured vertically and horizontally. J Assoc Res Otolaryngol 17(2):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiren T, Aoyagi M, Furuse H, Koike Y (1994) An experimental-study on the generator of amplitude-modulation following response. Acta Otolaryngol:28–33. [PubMed] [Google Scholar]
- Kobler JB, Guinan JJ, Vacher SR, Norris BE (1992) Acoustic reflex frequency-selectivity in single stapedius motoneurons of the cat. J Neurophysiol 68(3):807–817. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, Griest S, McDermott D, Fausti SA, Austin DF (2012) Age-related changes in the auditory brainstem response. J Am Acad Audiol 23(1):18–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH (2005) Hearing loss raises excitability in the auditory cortex. J Neurosci 25(15):3908–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH (2003) Gain adjustment of inhibitory synapses in the auditory system. Biol Cybern 89(5):363–370. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Ter-Mikaelian M, Lee FA, Sanes DH (2003) Deafening alters the balance between excitation and inhibition in the auditory cortex. Soc Neurosci Abstract Viewer Itinerary Planner. Abstract No. 181.2-Abstract No. 181.2. [Google Scholar]
- Krishnan A, Agrawal S (2010) Human frequency-following response to speech-like sounds: correlates of off-frequency masking. Audiol Neuro-Otol 15(4):221–228. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29(45):14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D’angelo WR (2002) Sources of the scalp-recorded amplitude-modulation following response. J. Am. Acad. Audiol 13:188–204. [PubMed] [Google Scholar]
- Lai J, Bartlett EL (2018) Masking differentially affects envelope-following responses in young and aged animals. Neuroscience 386:150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Sommer AL, Bartlett EL (2017) Age-related changes in envelope-following responses at equalized peripheral or central activation. Neurobiol Aging 58:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Li M, Kilpatrick LA, Zhu J, Samuvel DJ, Krug EL, et al. (2011) Sox2 up-regulation and glial cell proliferation following degeneration of spiral ganglion neurons in the adult mouse inner ear. J. Assoc. Res. Otolaryngol 12:151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Suzuki J, Liberman MC (2015) Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol 16(2):205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Wang HB, Liberman MC (2011) Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J Neurosci 31(3):801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang HB, Maison SF (2016) Toward a differential diagnosis of hidden hearing loss in humans. PLoS ONE 11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Kiang NYS (1984) Single-neuron labeling and chronic cochlear pathology. 4. Stereocilia damage and alterations in rate-level and phase-level functions. Hear Res 16(1):75–90. [DOI] [PubMed] [Google Scholar]
- Lin FR, Albert M (2014) Hearing loss and dementia – who is listening? Aging Mental Health 18(6):671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L (2011a) Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol Series A-Biol Sci Med Sci 66(5):582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC (2011b) Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol 12 (5):605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412(6843):150–157. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA (2014) Why do I hear but not understand? Stochastic undersampling as a model of degraded neural encoding of speech. Front Neurosci 8:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Barrios P (2013) Perception of stochastically undersampled sound waveforms: a model of auditory deafferentation. Front Neurosci 7:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB, Campagnola L (2018) A biophysical modelling platform of the cochlear nucleus and other auditory circuits: from channels to networks. Hear Res 360:76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmel F, Linley D, Carlyon RP, Gockel HE, Hopkins K, Plack CJ (2013) Subcortical neural synchrony and absolute thresholds predict frequency discrimination independently. J Assoc Res Otolaryngol 14(5):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmel F, Rodriguez-Mendoza MA, Lopez-Poveda EA (2015) Stochastic undersampling steepens auditory threshold/duration functions: implications for understanding auditory deafferentation and aging. Front Aging Neurosci 7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattys SL, Davis MH, Bradlow AR, Scott SK (2012) Speech recognition in adverse conditions: a review. Language Cognit Processes 27(7–8):953–978. [Google Scholar]
- Mehraei G, Gallardo AP, Shinn-Cunningham BG, Dau T (2017) Auditory brainstem response latency in forward masking, a marker of sensory deficits in listeners with normal hearing thresholds. Hear Res 346:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG (2016) Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci 36(13):3755–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrle D, Ni K, Varakina K, Bing D, Lee SC, Zimmermann U, Knipper M, Ruttiger L (2016) Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain. Neurobiol Aging 44:173–184. [DOI] [PubMed] [Google Scholar]
- Moore TM, Key AP, Thelen A, Hornsby BWY (2017) Neural mechanisms of mental fatigue elicited by sustained auditory processing. Neuropsychologia 106:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC, Carney LH (2004) A phenomenological model of peripheral and central neural responses to amplitude-modulated tones. J Acoust Soc Am 116(4):2173–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksiak A, Klink PC, Postma A, van der Ham IJM, Lankheet MJ, van Wezel RJA (2011) Spatial summation in macaque parietal area 7a follows a winner-take-all rule. J Neurophysiol 105(3): 1150–1158. [DOI] [PubMed] [Google Scholar]
- Overton JA, Recanzone GH (2016) Effects of aging on the response of single neurons to amplitude-modulated noise in primary auditory cortex of rhesus macaque. J Neurophysiol 115 (6):2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM (1996a) GABA inputs control discharge rate primarily within frequency receptive fields of inferior colliculus neurons. J Neurophysiol 75(6):2211–2219. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM (1996b) Physiology of the aged Fischer 344 rat inferior colliculus: responses to contralateral monaural stimuli. J Neurophysiol 76(5):3114–3125. [DOI] [PubMed] [Google Scholar]
- Paraouty N, Lorenzi C (2017) Using individual differences to assess modulation-processing mechanisms and age effects. Hear Res 344:38–49. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett E (2012) Two-channel recording of auditory-evoked potentials to detect age-related deficits in temporal processing. Hear Res 289(1–2):52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL (2011) Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience 192:619–630. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Cunningham PA, Bartlett EL (2010) Age-related differences in auditory processing as assessed by amplitude-modulation following responses in quiet and in noise. Front Aging Neurosci 2:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Datta J, Torres JAL, Hopkins C, Bartlett EL (2014) Age-related changes in the relationship between auditory brainstem responses and envelope-following responses. J Assoc Res Otolaryngol 15(4):649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Herrmann B, Bartlett EL (2018) Aging alters envelope representations of speech-like sounds in the inferior colliculus. Neurobiol Aging. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Kujawa SG (2018) Synaptopathy in the aging cochlea: characterizing early-neural deficits in auditory temporal envelope processing. J Neurosci 38(32):7108–7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Lai J, Bartlett EL (2016) Age-related changes in processing simultaneous amplitude modulated sounds assessed using envelope following responses. J Assoc Res Otolaryngol 17 (2):119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Wingfield A (2016) The neural consequences of age-related hearing loss. Trends Neurosci 39(7):486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE (2018) Listening effort: How the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear and Hearing 39:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, John MS, Dimitrijevic A, Purcell D (2003) Human auditory steady-state responses. Int. J. Audiol 42:177–219. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BWY, Humes LE, Lemke U, Lunner T, Matthen M, Mackersie CL, Naylor G, Phillips NA, Richter M, Rudner M, Sommers MS, Tremblay KL, Wingfield A (2016) Hearing impairment and cognitive energy: the framework for understanding effortful listening (FUEL). Ear Hear 37:5S–27S. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Souza PE (2001) Effects of aging on auditory processing of speech. In: Workshop on candidature for and delivery of audiological services – special needs of older people. Eriksholm, Denmark, November: B C Decker Inc. p. S11–S16. [Google Scholar]
- Prendergast G, Guest H, Munro KJ, Kluk K, Leger A, Hall DA, Heinz MG, Plack CJ (2017a) Effects of noise exposure on young adults with normal audiograms I: electrophysiology. Hear Res 344:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G, Millman RE, Guest H, Munro KJ, Kluk K, Dewey RS, Hall DA, Heinz MG, Plack CJ (2017b) Effects of noise exposure on young adults with normal audiograms II: Behavioral measures. Hear Res 356:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S (2016a) Effect of informational content of noise on speech representation in the aging midbrain and cortex. J Neurophysiol 116(5):2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S (2016b) Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. J Neurophysiol 116(5):2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabang CF, Bartlett EL (2011) A computational model of cellular mechanisms of temporal coding in the medial geniculate body (MGB). PLoS ONE 6(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabang CF, Parthasarathy A, Venkataraman Y, Fisher ZL, Gardner SM, Bartlett EL (2012) A computational model of inferior colliculus responses to amplitude modulated sounds in young and aged rats. Front Neural Circuits 6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza A, Milbrandt JC, Arneric SP, Caspary DM (1994) Age-related-changes in brain-stem auditory neurotransmitters – measures of gaba and acetylcholine function. Hear Res 77(1–2):221–230. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM (2009) GABA shapes selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. J Neurophysiol 102(3):1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM (2010) GABA shapes a systematic map of binaural sensitivity in the auditory cortex. J Neurophysiol 104 (1):517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik J, Polley DB (2017) Fast-spiking GABA circuit dynamics in the auditory cortex predict recovery of sensory processing following peripheral nerve damage. Elife 6. pii: e21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Hancock KE, Caspary DM (2013a) Stimulus-specific adaptation in auditory thalamus of young and aged awake rats. J Neurophysiol 110(8):1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM (2013b) Reduced GABA(A) receptor-mediated tonic inhibition in aged rat auditory thalamus. J Neurosci 33(3). pp. 1218-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S (1992) Temporal information in speech – acoustic, auditory and linguistic aspects. Philos Trans Royal Soc London Series BBiol Sci 336(1278):367–373. [DOI] [PubMed] [Google Scholar]
- Ross B, Draganova R, Picton TW, Pantev C (2003) Frequency specificity of 40-Hz auditory steady-state responses. Hear Res. 186:57–68. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG (2011) Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. PNAS 108(37):15516–15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG (2012) Why middle-aged listeners have trouble hearing in everyday settings. Curr Biol 22(15):1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saremi A, Beutelmann R, Dietz M, Ashida G, Kretzberg J, Verhulst S (2016) A comparative study of seven human cochlear filter models. J Acoust Soc Am 140(3):1618–1634. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D (2011) Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci 31(38):13452–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatteman TA, Hughes LF, Caspary DM (2008) Aged-related loss of temporal processing: altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience 154 (1):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA (1996) Age-related loss of activity of auditory-nerve fibers. J Neurophysiol 76(4): 2799–2803. [DOI] [PubMed] [Google Scholar]
- Schoof T, Rosen S (2016) The role of age-related declines in subcortical auditory processing in speech perception in noise. J Assoc Res Otolaryngol 17(5):441–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR (1993) Cochlear pathology in presbyacusis. Annals of Otology, Rhinology & Laryngology 102:1–16. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013) Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33(34):13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen LA, Valero MD, Liberman MC (2015) Towards a diagnosis of cochlear neuropathy with envelope following responses. J Assoc Res Otolaryngol 16(6):727–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA (2015) Auditory function in normal-hearing, noise-exposed human ears. Ear Hear 36(2):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbings KA, Choi HW, Ravindra A, Caspary DM, Turner JG, Llano DA (2016) Ageing-related changes in GABAergic inhibition in mouse auditory cortex, measured using in vitro flavoprotein autofluorescence imaging. J Physiol-London 594(1):207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Pinto A, Fischer ME, Klein BEK, Klein R, Levy S, Tweed TS, Cruickshanks KJ (2015) Self-reported hearing difficulties among adults with normal audiograms: the beaver dam offspring study. Ear Hear 36(6):E290–E299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P (2003) Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol 114(7):1332–1343. [DOI] [PubMed] [Google Scholar]
- Trussell LO (1999) Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol 61(1):477–496. [DOI] [PubMed] [Google Scholar]
- Valderrama JT, Beach EF, Yeend I, Sharma M, Van Dun B, Dillon H (2018) Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility. Hear Res 365:36–48. [DOI] [PubMed] [Google Scholar]
- Valero MD, Burton JA, Hauser SN, Hackett TA, Ramachandran R, Liberman MC (2017) Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hear Res 353:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero MD, Hancock KE, Liberman MC (2016) The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hear Res 332:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero MD, Hancock KE, Maison SF, Liberman MC (2018) Effects of cochlear synaptopathy on middle-ear muscle reflexes in unanesthetized mice. Hear Res 363:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayrynen E, Noponen K, Vipin A, Thow XY, Al-Nashash H, Kortelainen J, All A (2016) Automatic parametrization of somatosensory evoked potentials with chirp modeling. IEEE Trans Neural Syst Rehabil Eng 24(9):981–992. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Altoe A, Vasilkov V (2018) Computational modeling of the human auditory periphery: auditory-nerve responses, evoked potentials and hearing loss. Hear Res 360:55–75. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Bharadwaj HM, Mehraei G, Shera CA, Shinn-Cunningham BG (2015) Functional modeling of the human auditory brainstem response to broadband stimulation. J Acoust Soc Am 138(3):1637–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Jagadeesh A, Mauermann M, Ernst F (2016) Individual differences in auditory brainstem response wave characteristics: relations to different aspects of peripheral hearing loss. Trends Hear 20:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira C, Santos F, Merchant SN, Liberman LD, Liberman MC (2015) Cochlear neuropathy in human presbycusis: confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res 327:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP (2010) Timing is everything: temporal processing deficits in the aged auditory brainstem. Hear Res 264(1–2):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM (2009) Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience 160(1):227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JA, McFadden SL, Caspary D, Salvi R (2002) Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res 944(1–2). PII S0006–8993 (02)02926–8. [DOI] [PubMed] [Google Scholar]
- Wei JK, Hu YR, Zhang L, Hao Q, Yang RW, Lu HD, Zhang X, Chandrasekar EK (2017) Hearing impairment, mild cognitive impairment, and dementia: a meta-analysis of cohort studies. Dementia Geriatric Cognitive Disorders Extra 7(3):440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Parham K, Hunter KP (1988a) Response properties of inferior colliculus neurons in middle-aged c57bl/6j mice with presbycusis. Hear Res 37(1):15–27. [DOI] [PubMed] [Google Scholar]
- Willott JF, Parham K, Hunter KP (1988b) Response properties of inferior colliculus neurons in young and very old cba/j mice. Hear Res 37(1):1–14. [DOI] [PubMed] [Google Scholar]
- Willott JF, Parham K, Hunter KP (1991) Comparison of the auditory-sensitivity of neurons in the cochlear nucleus and inferior colliculus of young and aging c57bl/6j and cba/j mice. Hear Res 53(1):78–94. [DOI] [PubMed] [Google Scholar]
- Wojtczak M, Beim JA, Oxenham AJ (2017) Weak middle-ear-muscle reflex in humans with noise-induced tinnitus and normal hearing may reflect cochlear synaptopathy. Eneuro 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, Liberman MC (2019) Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience 407:2–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie RL (2016) Transmission of auditory sensory information decreases in rate and temporal precision at the endbulb of Held synapse during age-related hearing loss. J Neurophysiol 116 (6):2695–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie RL, Manis PB (2017) Synaptic transmission at the endbulb of Held deteriorates during age-related hearing loss. J Physiol-London 595(3):919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin YB, Liberman LD, Maison SF, Liberman MC (2014) Olivocochlear innervation maintains the normal modiolar-pillar and habenularcuticular gradients in cochlear synaptic morphology. J Assoc Res Otolaryngol 15(4):571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJ, Young ED (2000) Linear and nonlinear pathways of spectral information transmission in the cochlear nucleus. PNAS 97 (22):11780–11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Shi F, Yin Y, Tong M, Lang H, Polley DB, Liberman MC, Edge ASB (2014) Ouabain-induced cochlear nerve degeneration: synaptic loss and plasticity in a mouse model of auditory neuropathy. J Assoc Res Otolaryngol 15:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong ZW, Henry KS, Heinz MG (2014) Sensorineural hearing loss amplifies neural coding of envelope information in the central auditory system of chinchillas. Hear Res 309:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilany MSA, Bruce IC, Carney LH (2014) Updated parameters and expanded simulation options for a model of the auditory periphery. J Acoust Soc Am 135(1):283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilany MSA, Bruce IC, Nelson PC, Carney LH (2009) A phenomenological model of the synapse between the inner hair cell and auditory nerve: Long-term adaptation with power-law dynamics. J Acoust Soc Am 126(5):2390–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]


