Key Points
Question
In adult patients who receive inhaled pulmonary vasodilators during lung transplant (LT), is there a difference in the rates of severe/grade 3 primary graft dysfunction (PGD-3) between patients who receive inhaled epoprostenol (iEPO) and those who receive inhaled nitric oxide (iNO)?
Findings
In this randomized clinical trial of 201 LT recipients, PGD-3 determined at 24, 48, or 72 hours after LT occurred in 46 of 103 patients (44.7%) in the iEPO group and in 39 of 98 patients (39.8%) in the iNO group. This 4.9% risk difference was included within the margin to favor equivalence.
Meaning
These findings show that PGD-3 rates after LT were similar between patients who received iEPO and iNO.
This randomized clinical trial assesses whether administration of inhaled epoprostenol results in similar rates of severe primary graft dysfunction and other outcomes compared with inhaled nitric oxide in patients undergoing lung transplant.
Abstract
Importance
Inhaled nitric oxide (iNO) is commonly administered for selectively inhaled pulmonary vasodilation and prevention of oxidative injury after lung transplant (LT). Inhaled epoprostenol (iEPO) has been introduced worldwide as a cost-saving alternative to iNO without high-grade evidence for this indication.
Objective
To investigate whether the use of iEPO will lead to similar rates of severe/grade 3 primary graft dysfunction (PGD-3) after adult LT when compared with use of iNO.
Design, Setting, and Participants
This health system–funded, randomized, blinded (to participants, clinicians, data managers, and the statistician), parallel-designed, equivalence clinical trial included 201 adult patients who underwent single or bilateral LT between May 30, 2017, and March 21, 2020. Patients were grouped into 5 strata according to key prognostic clinical features and randomized per stratum to receive either iNO or iEPO at the time of LT via 1:1 treatment allocation.
Interventions
Treatment with iNO or iEPO initiated in the operating room before lung allograft reperfusion and administered continously until cessation criteria met in the intensive care unit (ICU).
Main Outcomes and Measures
The primary outcome was PGD-3 development at 24, 48, or 72 hours after LT. The primary analysis was for equivalence using a two one-sided test (TOST) procedure (90% CI) with a margin of 19% for between-group PGD-3 risk difference. Secondary outcomes included duration of mechanical ventilation, hospital and ICU lengths of stay, incidence and severity of acute kidney injury, postoperative tracheostomy placement, and in-hospital, 30-day, and 90-day mortality rates. An intention-to-treat analysis was performed for the primary and secondary outcomes, supplemented by per-protocol analysis for the primary outcome.
Results
A total of 201 randomized patients met eligibility criteria at the time of LT (129 men [64.2%]). In the intention-to-treat population, 103 patients received iEPO and 98 received iNO. The primary outcome occurred in 46 of 103 patients (44.7%) in the iEPO group and 39 of 98 (39.8%) in the iNO group, leading to a risk difference of 4.9% (TOST 90% CI, –6.4% to 16.2%; P = .02 for equivalence). There were no significant between-group differences for secondary outcomes.
Conclusions and Relevance
Among patients undergoing LT, use of iEPO was associated with similar risks for PGD-3 development and other postoperative outcomes compared with the use of iNO.
Trial Registration
ClinicalTrials.gov identifier: NCT03081052
Introduction
Inhaled nitric oxide (iNO) is administered after lung transplant (LT) to promote lung-allograft function1,2 by improving oxygenation and lowering pulmonary vascular resistance.3,4,5,6 Consequently, iNO may help mitigate development of severe (grade 3) primary graft dysfunction (PGD-3),7 which is diagnosed within 72 hours after LT8 and is strongly associated with short- and long-term mortality.9,10 Although iNO is not approved by the US Food and Drug Administration for this indication, international guidelines support its use after LT.8 In a recent survey of 74 LT centers worldwide, the primary inhaled pulmonary vasodilator (iPVD) was iNO in 54 centers (73%), followed by aerosolized or inhaled epoprostenol (iEPO) in 9 (12%).11
The cost of iNO exceeds millions of dollars annually for large health care systems nationwide, and iNO is approximately 7-fold more expensive than iEPO.12 Accordingly, iEPO has emerged as a cost-saving iNO alternative at several institutions. Although similar antioxidative and vasodilatory properties of iEPO have been reported in LT,13,14 the evidence supporting its use is not based on robust comparisons with iNO that assessed clinically meaningful outcomes. Furthermore, available data interpretation is complicated by retrospective observational studies, differing epoprostenol formulations15 and aerosol-generating devices,16 and lack of standardized criteria for discontinuing treatment with either agent.
Given the serious nature of PGD-3 development and significant economic considerations of continued iNO use, clinician-investigators designed and conducted a randomized trial funded by the health system to determine whether iEPO delivery would result in similar rates of PGD-3 and other outcomes after LT compared with iNO.
Methods
Design
This parallel-designed clinical trial randomly assigned LT recipients to receive either iNO or iEPO. This study is registered as part of the Inhaled Selective Pulmonary Vasodilators for Advanced Heart Failure Therapies and Lung Transplantation Outcomes (INSPIRE-FLO) trial; the trial protocol is found in Supplement 1. Two populations undergoing surgery were evaluated under this registration with separate, independent analysis plans. Analysis for the population undergoing LT is described herein. The study was approved by the institutional review board of Duke University. All patients provided written informed consent. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Funding
Research-related activities were funded through the Duke University Health System, Durham, North Carolina. A separate process was established to ensure trial medication costs were covered by insurance providers. First, a blanket approval for trial enrollment was obtained for patients insured through the Centers for Medicare & Medicaid Services before trial commencement. For other eligible patients, an enrollment request letter was sent to private insurers. The institutional review board approved the protocol without a data safety monitoring board because both medications were on formulary and either could be used as standard care. Adverse events were reviewed each quarter by the principal investigator (K.G.) and research team while blinded to treatment assignment.
Participants
Patients with end-stage lung disease, 18 years or older, and with insurance approval for enrollment were screened for eligibility at the time of transplant listing, approached for consent, and randomized at the time of consent. Notable exclusion criteria included combined-organ transplant and the presence of extracorporeal membrane oxygenation (ECMO) before LT. Given the variable duration from randomization to potential treatment initiation during LT, participants were included in the primary analysis if they were not withdrawn, did not die, or did not develop changes to eligibility after randomization.
Randomization and Blinding
Five randomization strata were created based on primary indication and single or bilateral lung-allograft transplant. Within each strata, participants were assigned to receive either iNO or iEPO at the time of LT via 1:1 treatment allocation with block sizes of 4. Randomization sequence was generated before trial commencement using nQuery Advisor, version 7 (Statsols Inc). At the time of notification from the transplant coordinator that a participant would be undergoing LT, the research team would contact the study respiratory therapist and pharmacist. The pharmacist would access the password-protected randomization sequence list and prepare the allocated treatment.
Using an in-line system for blinding iEPO and iNO delivery adopted from Preston et al17 (eMethods in Supplement 2), blinding was preserved for all participants and clinicians involved in patient care. In addition, allocated treatment was masked in the electronic record in a separate clinical documentation platform developed for this study (Maestro Care; Epic Systems Corporation). All research team members with database access (data managers) were blinded to treatment assignment. After study completion, an independent statistician created a blinded-treatment assignment code for use during analysis, and the study statistician remained blinded to the assignment until all analyses were completed.
Intervention
A blinded 50-mL syringe solution of either 5% sodium chloride (if randomized to iNO) or epoprostenol, 30 000 ng/mL (Veletri; Actelion Pharmaceuticals) was prepared by the study pharmacist. Epoprostenol concentration in the syringe was based on standard compounding by the pharmacy department. The study respiratory therapist would obtain the syringe from pharmacy, verbally confirm the solution identity, and place the syringe in a dedicated refrigerator. Fifteen minutes before reperfusion of the first transplanted lung, the study respiratory therapist would initiate the treatment in the operating room. Participants randomized to iNO (iNOMax; Mallinckrodt Pharmaceuticals) would continuously receive 20 ppm, whereas the iEPO group would continuously receive 50 ng/kg/min (ideal body weight) delivered using a syringe pump and vibrating mesh aerosolizer (Pro-X; Aerogen). Inhaled epoprostenol dosing was derived from a dose-response study that displayed improved oxygenation from 10 to 50 ng/kg/min in acute respiratory distress syndrome,18 and this dosing range has been adopted at multiple institutions for previous studies.14,17,19,20,21,22
After LT, the study therapist accompanied the clinical care team to the intensive care unit (ICU) and ensured appropriate treatment delivery and blinding. In the ICU, a nonstudy respiratory therapist then assumed direct patient care. The study therapist remained immediately available to manage treatment delivery and was notified to wean each treatment by protocol once discontinuation criteria were identified (Supplement 1).
Standardized Care for LT
Standardized care for LT management at our institution, including intensive care, infection prophylaxis, and immunosuppression, has been reviewed.23 Relevant protocols for mechanical ventilation (Supplement 1) and ECMO management (eMethods in Supplement 2) are included.
Outcomes
The primary outcome was PGD-3 development based on daily grading assigned at 24, 48, or 72 hours after post-LT arrival in the ICU. Based on PGD guidelines,8 grade 3 is diagnosed based on poor systemic oxygenation (defined by ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <200 or ECMO use) and radiographic evidence of lung-allograft edema.8
Secondary outcomes included duration of mechanical ventilation measured from ICU arrival to endotracheal extubation, censored for those who underwent postoperative tracheostomy placement. Acute kidney injury was determined by the Kidney Disease: Improving Global Outcomes criteria (eMethods in Supplement 2) up to 7 days after LT based on studies that have supported iEPO24 and iNO25 for kidney protection. Other outcomes included hospital and ICU lengths of stay and early postoperative mortality (in-hospital, 30-day, and 90-day). In addition, we compared daily mean values for mean pulmonary arterial pressures between groups through postoperative day 3.
Statistical Analysis
The statistical analysis plan (Supplement 3) was prepared in accordance with journal guidelines.26 Analyses were performed using SAS, version 9.4 (SAS Institute Inc).
The trial was designed to demonstrate clinical equivalence between iEPO and iNO by a prespecified lower and upper bound around the PGD-3 outcome measure. The margin of equivalence was set to 19% with an anticipated PGD-3 incidence of 30% for the iNO group based on previous PGD-3 event rates that used the first 72 hours after LT as the primary outcome time frame.7,10,27,28,29,30 Assessment of PGD-3 was performed while participants remained in the hospital, and loss to follow-up was not factored into sample size determination. Thus, 200 participants allocated 1:1 would be sufficient to establish equivalence for the prespecified margin at 80% power. The α value was controlled at .05 for all comparisons. Two one-sided tests (TOSTs) were used for primary outcome analyses (α = .05); 2-sided hypothesis testing was used for secondary outcomes (α = .05).
An intention-to-treat analysis was planned for the primary and secondary outcomes, supplemented by per-protocol analysis for the primary outcome. Baseline characteristics were summarized for each treatment group and reported as mean (SD) or median (IQR) for continuous variables and as count (percentage) for categorical ones. Summaries were used to assess randomization performance and protocol adherence. Using the TOST procedure, the primary outcome was determined by calculating the point estimate and corresponding 90% CIs for the risk difference between iEPO and iNO. If the 90% CIs were contained inside the equivalence margin, then there would be sufficient evidence to conclude that PGD-3 rates in each group would be similar (P < .05). Relative risk estimates for PGD-3 development if treated with iNO compared with iEPO (95% CI) were reported. Baseline characteristics were used to evaluate the balance of patient factors between treatment groups. All covariates meeting P < .15 association between treatment groups were considered for variable selection to build a stepwise, multivariable regression model for PGD-3 to adjust the treatment difference for these potential confounders. Secondary outcomes were assessed for treatment differences under typical null hypothesis testing using univariable effect estimates and corresponding 2-sided 95% CI. Binary secondary outcomes (tracheostomy, acute kidney injury, mortality) were assessed via risk differences and relative risk, whereas continuous secondary outcomes (ICU and hospital lengths of stay) were assessed via risk differences and mean ratios estimated from log-linear regression models. Kaplan-Meier point estimates (95% CI) were used to determine mechanical ventilation duration censored for postoperative tracheostomy placement. A post hoc analysis was performed to determine overall and between-group PGD-3 rates using 2 commonly reported subintervals of the 72-hour outcome time frame: 48 or 72 hours9,29 and 72 hours alone.31
Results
Population
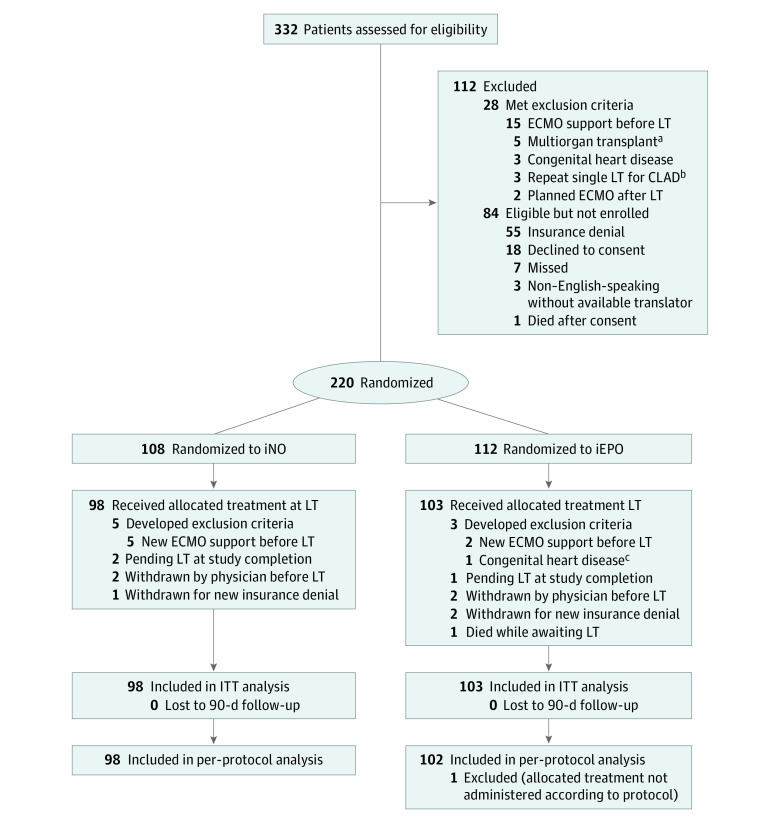
We screened 332 patients from May 30, 2017, to March 21, 2020. Of these, 112 patients did not meet eligibility criteria during screening, including 28 (8.4%) who met exclusion criteria and 84 (25.3%) who were eligible but were not enrolled for various reasons (Figure 1). Of 220 randomized patients, 19 (8.6%) developed changes to eligibility before they could receive the allocated treatment (7 needed ECMO before LT, 4 were withdrawn by physician for clinical deterioration, 3 were withdrawn for insurance denial, 3 were awaiting LT at the time of study completion, 1 had congenital heart disease, and 1 died), leaving 98 patients who were allocated to the iNO group and 103 to the iEPO group (n = 201) (129 men [64.2%] and 72 women [35.8%]). Final 90-day follow-up for mortality was performed on June 19, 2020.
Figure 1. Flow of Participants in a Study of Inhaled Pulmonary Vasodilators for Adult Lung Transplant (LT).
In all analyses, patients were analyzed according to their randomized group (inhaled nitric oxide [iNO] and inhaled epoprostenol [iEPO]). Participants were excluded from the intention-to-treat (ITT) analysis if they were withdrawn, developed exclusion criteria after randomization, or remained on the LT list and did not receive a transplant. Those who received the allocated treatment at the time of LT were included in an ITT analysis. Study enrollment was completed once the calculated sample size was achieved. None of the participants were lost to 90-day follow-up. CLAD indicates chronic lung allograft dysfunction; ECMO, extracorporeal membrane oxygenation.
aIncluded 1 patient for lung-kidney and 4 patients for lung-liver transplants.
bPatient with diagnosis that did not fit 1 of the 5 randomization strata.
cIneligibility for enrollment noted after consent and randomization but before LT.
Baseline and clinical characteristics for participants and organ donors in the intention-to-treat analysis are provided in Table 1. Common indications for LT were restrictive (126 [62.7%]) and obstructive (42 [20.9%]) lung disease, which are proportionally consistent with the 2016 LT registry report.32 A total of 173 patients (86.1%) underwent bilateral LT and 28 (13.9%) underwent single LT. Indications for single or bilateral LT were similar between treatment groups, indicating success of the stratified randomization. For donor characteristics, donor-to-recipient sex mismatch was observed in 20 participants (20.4%) in the iNO group and in 34 (33.0%) in the iEPO group, with a predominance of male donor–to–female recipient mismatch (14 [14.3%] for iNO group and 18 [17.5%] for the iEPO group).
Table 1. Baseline Characteristicsa.
| Characteristic | Treatment group | |
|---|---|---|
| iNO (n = 98) | iEPO (n = 103) | |
| Patient characteristics | ||
| Age, median (IQR), y | 64 (54 to 68) | 64 (51 to 69) |
| Sex | ||
| Male | 59 (60.2) | 70 (68.0) |
| Female | 39 (39.8) | 33 (32.0) |
| Race and ethnicity | ||
| African American or Black | 12 (12.2) | 9 (8.7) |
| White | 83 (84.7) | 91 (88.3) |
| Otherb | 3 (3.1) | 3 (2.9) |
| BMI, median (IQR) | 25.0 (22.1 to 26.7) | 26.0 (22.8 to 27.3) |
| Hypertension | 48 (49.0) | 43 (41.7) |
| Pulmonary hypertension diagnosis | 42 (42.9) | 54 (52.4) |
| Severity of pulmonary hypertension | ||
| Mild | 6 (6.1) | 7 (6.8) |
| Moderate | 29 (29.5) | 33 (32.0) |
| Severe | 7 (7.1) | 14 (13.6) |
| Type 1 or 2 diabetes | 17 (17.3) | 25 (24.3) |
| COPD | 34 (34.7) | 45 (43.7) |
| Preoperative LVEF, %c | ||
| ≥50 (Normal) | 94 (95.9) | 100 (97.1) |
| 40-49 (Mild dysfunction) | 0 | 1 (1.0) |
| 30-39 (Moderate dysfunction) | 0 | 1 (1.0) |
| Previous sternotomy for cardiac surgery | 4 (4.1) | 2 (1.9) |
| Previous LT | 6 (6.1) | 4 (3.9) |
| Lung allocation score | 42.0 (36.9 to 51.9) | 42.8 (37.2 to 52.4) |
| Common indications for LT | ||
| Group A: obstructive lung disease | 21 (21.4) | 21 (20.4) |
| Group B: pulmonary vascular disease | 2 (2.0) | 1 (1.0) |
| Group C: infectious lung disease | 8 (8.2) | 15 (14.6) |
| Group D: restrictive lung disease | 63 (64.3) | 63 (61.2) |
| Otherd | 4 (4.1) | 3 (2.9) |
| Preoperative laboratory values | ||
| Estimated GFR, median (IQR), mL/min | 85 (70 to 98) | 88 (75 to 100) |
| Hemoglobin level, mean (SD), g/dL | 12.30 (1.65) | 12.57 (1.73) |
| Creatinine level, median (IQR), mg/dL | 0.9 (0.7 to 1.0) | 0.9 (0.7 to 1.0) |
| Class 1 PRA >0 | 17 (17.3) | 17 (16.5) |
| Class 1 PRA, median (IQR), % among those >0 | 17 (7 to 75) | 29 (17 to 57) |
| Class 2 PRA >0 | 13 (13.3) | 13 (12.6) |
| Class 2 PRA, median (IQR), % among those >0 | 38 (26 to 49) | 30 (22 to 40) |
| Right heart catheterization values | ||
| Cardiac index, median (IQR), L/min/m2 | 2.8 (2.5 to 3.2) | 2.9 (2.7 to 3.3) |
| Mean PAP, median (IQR), mm Hg | 22.8 (18.3 to 27.7) | 24.7 (20.0 to 29.7) |
| Procedural characteristics | ||
| Bilateral LTe | 84 (85.7) | 89 (86.4) |
| Obstructive lung disease | 26 (26.5) | 31 (30.1) |
| Restrictive lung disease | 52 (53.1) | 53 (51.5) |
| Pulmonary vascular disease | 2 (2.0) | 1 (1.0) |
| Other diagnosisf | 4 (4.1) | 4 (3.9) |
| Single LT for restrictive lung diseasee | 14 (14.3) | 14 (13.6) |
| Concurrent cardiac operation | 7 (7.1) | 7 (6.8) |
| Intraoperative CPB used | 19 (19.4) | 19 (18.4) |
| Intraoperative ECMO used | 33 (33.7) | 27 (26.2) |
| Ischemia time, single LT only, median (IQR), min | 325 (304 to 353) | 325 (261 to 340) |
| Ischemia time, second LT only, median (IQR), ming | 395 (349 to 489) | 432 (352 to 495) |
| Use of transmedics OCS/EVLPh | 4 (4.1) | 3 (2.9) |
| Donor characteristics | ||
| Age, median (IQR), y | 35 (26 to 46) | 35 (27 to 47) |
| Sex donor-to-recipient mismatch | ||
| Matched | 78 (79.6) | 69 (67.0) |
| Female donor to male recipient | 6 (6.1) | 16 (15.5) |
| Male donor to female recipient | 14 (14.3) | 18 (17.5) |
| Race and ethnicity | ||
| African American or Black | 16 (16.3) | 17 (16.5) |
| White | 72 (73.5) | 74 (71.8) |
| Otheri | 10 (10.2) | 12 (11.7) |
| BMI of donor-recipient mismatch, median (IQR), %j | –4.5 (–20.4 to 10.0) | –7.5 (–20.3 to 7.2) |
| Donor Pao2:Fio2 ratio, median (IQR) | 443 (396 to 494) | 425 (378 to 495) |
| Donor cigarette use >20 pack-years | 11 (11.2) | 10 (9.7) |
| Donation after cardiac death | 10 (10.2) | 13 (12.6) |
| Donation after brain death | 88 (89.8) | 90 (87.4) |
| Cause of brain death | ||
| Anoxia | 33 (33.7) | 30 (29.1) |
| CVA/stroke | 26 (26.5) | 29 (28.2) |
| Head trauma | 37 (37.8) | 41 (39.8) |
| CNS tumor | 1 (1.0) | 0 |
| Other | 1 (1.0) | 3 (2.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CNS, central nervous system; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; CVA, cerebrovascular accident; ECMO, extracorporeal membrane oxygenation; EVLP, ex vivo lung perfusion; GFR, glomerular filtration rate; iEPO, inhaled epoprostenol; iNO, inhaled nitric oxide; LT, lung transplant; LVEF, left ventricular ejection fraction; OCS, organ care system; Pao2:Fio2, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen; PAP, pulmonary arterial pressure; PRA, panel-reactive antibody.
SI conversion factors: To convert creatinine to μmol/L, multiply by 88.4; hemoglobin to g/L, multiply by 10.0.
Unless otherwise indicated, data are expressed as number (%) of patients. Percentages have been rounded and may not total 100.
Includes American Indian, Asian, multiple races/ethnicities, and unknown race or ethnicity.
Available in 94 of 98 patients in the iNO group and 102 of 103 participants in the iEPO group.
Includes diagnoses that were not otherwise classifiable under groups A to D.
Randomization strata are based on single or bilateral LT and primary diagnosis for LT.
Includes bronchiolitis obliterans syndrome (n = 2), occupational fiberglass exposure (n = 1), and adult respiratory distress syndrome (n = 1) in the iNO group and bronchiolitis obliterans syndrome (n = 2) and coal worker’s pneumoconiosis (n = 2) in the iEPO group.
By convention, for bilateral LT, the ischemia time of the second lung only is reported.
Organ care system (Transmedics OCS) use during lung-allograft transport after donor harvest has shown promise in reducing rates of grade 3 primary graft dysfunction.39
Includes Asian, Hispanic, and multiple races/ethnicities.
Negative percentage indicates recipient BMI is less than donor BMI.
Intervention
Median duration from randomization to treatment was 5 (IQR, 1-20) days. Once initiated, allocated treatment durations (median, 45.7 [IQR, 33.1-102.6] hours in the iNO group vs 46.6 [IQR, 30.4-83.6] hours in the iEPO group) were similar between groups (Table 2). In addition, allogeneic packed red blood cell transfusion and ECMO support present on ICU arrival and placed within 72 hours of LT were also similar between groups (Table 2). Delayed chest closure was observed in 15 patients (15.3%) in the iNO group and in 7 (6.8%) in the iEPO group (P = .053).
Table 2. Participant Characteristics Relevant to Outcome Determination After Treatment Exposure.
| Parameter | Patient group | P value | |
|---|---|---|---|
| iNO (n = 98) | iEPO (n = 103) | ||
| Duration of treatment, median (IQR), ha | 45.7 (33.1-102.6) | 46.6 (30.4-83.6) | .43b |
| Delayed chest closure, No. (%) | 15 (15.3) | 7 (6.8) | .053c |
| ECMO present on ICU arrival, No. (%)d | 17 (17.3) | 16 (15.5) | .73c |
| ECMO placed within 72 h, No. (%)e | 7 (7.1) | 6 (5.8) | .70f |
| PRBC transfusion, median (IQR), Ug | 2 (0-4) | 2 (1-4) | .62b |
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; iEPO, inhaled epoprostenol; iNO, inhaled nitric oxide; PRBC, packed red blood cells.
In the per-protocol population, there were 102 patients in the iEPO group, with a median duration of 46.6 (IQR, 30.7-83.6) hours after lung transplant (LT) before discontinuation of iEPO.
Calculated using the Wilcoxon rank sum test.
Calculated using the χ2 test.
Placement in the operating room during LT that continued into the ICU.
Placement between ICU arrival and 72 hours after LT.
Calculated using the equal variance t test.
Transfusion data were missing for 1 patient in the iNO group.
Outcomes
In the unadjusted intention-to-treat analysis, PGD-3 incidence was 39.8% (n = 39) in the iNO group and 44.7% (n = 46) in the iEPO group for a risk difference of 4.9% (TOST 90% CI, –6.4% to 16.2%; P = .02 in support for equivalence). The results of the adjusted intention-to-treat analysis using a stepwise, data-driven, model-building approach (eTable 1 in Supplement 2) and per-protocol analysis confirmed those of the unadjusted intention-to-treat analysis (Figure 2).
Figure 2. Risk Differences and Relative Risks of Severe Primary Graft Dysfunction (PGD-3) Development Between Treatment Groups.
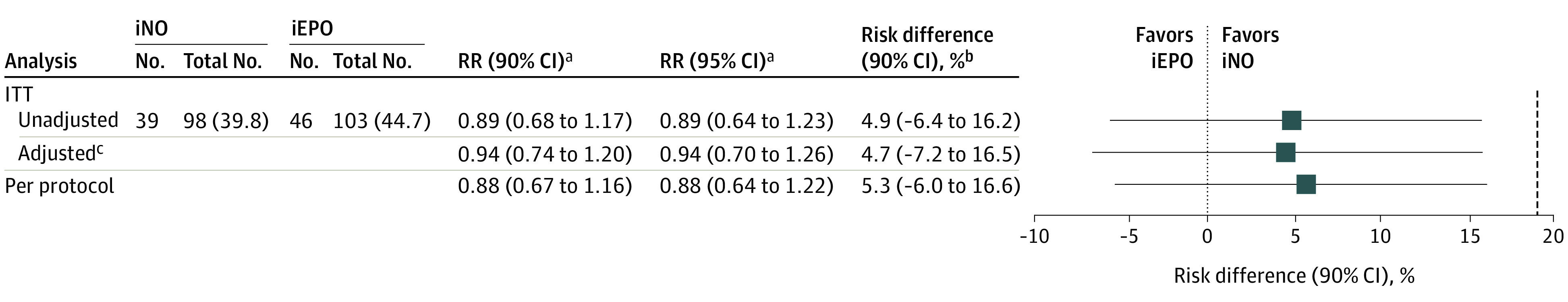
To determine the presence of clinical equivalence between inhaled nitric oxide (iNO) and inhaled epoprostenol (iEPO), lower and upper bounds of −19% and 19% were prespecified. Risk difference and relative risk (RR) are derived from the multivariable logistic regression model. Differences between adjusted and unadjusted risk difference and RR are owing to the difference in comparing 2 patients in the adjusted analysis with the same-sex mismatch and chest closure status. However, number of events and their distribution between the unadjusted and adjusted analyses remain the same.
aIndicates the risk of developing PGD-3 if treated with iNO compared with iEPO.
bIndicates the absolute difference between grade 3 primary graft dysfunction (PGD-3) rates between groups and is determined by the two one-sided test (TOST) procedure. Setting α at .05 and testing the upper and lower bounds separately, equivalence is concluded only if both test results are significant. To transform this procedure into a single CI, 1 − 2α (90%) is used and the TOST CI becomes the intersection of the TOST CIs. A more conservative 95% CI (1 − α) was determined and also demonstrated exclusion of the lower and upper bounds of the margin in support of equivalence for the unadjusted intention to treat (ITT) (−8.6% to 18.3%), adjusted ITT (−9.5% to 18.8%), and per-protocol (−8.2% to 18.8%) analyses.
cMultivariable logistic regression adjusted for delayed chest closure and donor-recipient sex mismatch from the selected model (eTable 1 in Supplement 2).
For secondary outcomes (Table 3), there were no significant between-group differences for mortality, acute kidney injury, tracheostomy placement, lengths of stay, or duration of mechanical ventilation censored for tracheostomy placement (eFigure 1 in Supplement 2). No important differences were found in adverse events (eTable 2 in Supplement 2), daily mean pulmonary arterial pressures (eFigure 2 in Supplement 2), or PGD-3 outcome measures according to randomization strata (eTable 3 in Supplement 2).
Table 3. Secondary Outcomes.
| Outcome | Patient group | Risk difference (95% CI), % | Relative risk (95% CI)a | P value | |
|---|---|---|---|---|---|
| iNO (n = 98) | iEPO (n = 103) | ||||
| Mortality, No. (%) | |||||
| 30-d | 2 (2.0) | 1 (1.0) | –1.0 (–4.0 to 2.0) | 2.10 (0.19 to 22.81) | .61 |
| 90-d | 4 (4.1) | 4 (3.9) | 0.2 (–6.0 to 5.0) | 1.05 (0.27 to 4.09) | .94 |
| In-hospital | 5 (5.1) | 7 (6.8) | 1.7 (–5.0 to 8.0) | 0.75 (0.25 to 2.29) | .61 |
| Tracheostomy, No. (%) | 22 (22.4) | 29 (28.2) | 5.8 (–6.0 to 18.0) | 0.80 (0.49 to 1.29) | .35 |
| AKI, No. (%)b | |||||
| Any stage | 72 (73.5) | 67 (65.0) | –8.5 (–20.0 to 5.0) | 1.12 (0.93 to 1.34) | .23 |
| Stages 2 or 3 | 29 (29.6) | 24 (23.3) | –6.3 (–18.0 to 6.0) | 1.26 (0.79 to 2.00) | .33 |
| ICU LOS, median (IQR), d | 4 (2 to 10) | 4 (2 to 10) | 0 (–1 to 1)c | 1.19 (0.76 to 1.87)d | .45 |
| Hospital LOS, median (IQR), d | 23 (16 to 38) | 23 (15 to 38) | 0 (–3 to 3)c | 1.03 (0.75 to 1.41)d | .86 |
| Duration of mechanical ventilation, Kaplan-Meier estimate, median (95% CI), he | 19 (15 to 24) | 22 (17 to 36) | NA | NA | .75f |
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; iEPO, inhaled epoprostenol; iNO, inhaled nitric oxide; LOS, length of stay; NA, not applicable.
Indicates relative risk of developing the outcome if participants receive iNO compared with iEPO.
Kidney Disease: Improving Global Outcomes AKI grading includes stages 1, 2, or 3 in ascending order of severity. AKI stages 2 and 3 are more commonly associated with poor outcomes after lung transplant (LT), and AKI incidence is independent of PGD-3 occurrence.40
Measured using the Hodges-Lehmann nonnormal difference estimator.
Measured as mean ratio with P values from log-linear models.
Measured from 197 patients (4 patients had tracheostomy before LT). For those who received postoperative tracheostomy, time to extubation was censored at the time of tracheostomy placement to avoid underestimating the distribution of time to end of mechanical ventilation.
Log-rank P value.
Post Hoc Analysis
Using the intention-to-treat population, the PGD-3 incidence at 48 or 72 hours was 26.5% (n = 26) in the iNO group and 28.2% (n = 29) in the iEPO group, for a risk difference of 1.6% (90% CI, –8.8% to 12.0% for equivalence). The PGD-3 incidence at the 72-hour mark was 16.3% (n = 16) in the iNO group and 21.3% (n = 22) in the iEPO group for a risk difference of 5.0% (90% CI, –4.5% to 14.6% for equivalence).
Discussion
In this trial of adult patients undergoing LT who prophylactically received iPVD to promote lung-allograft function, iEPO was associated with similar PGD-3 development as seen with iNO. Furthermore, no significant between-group differences were observed in durations of mechanical ventilation, lengths of stay, tracheostomy, incidence of acute kidney injury, or mortality to 90 days.
The impetus to financially support this study originated from the large economic impact of iNO use in this population on the health system. Although specific contract pricing for iNO cannot be disclosed, the 2017 noncontracted price for iNOmax was $220.26/h compared with $6.52/h for iEPO delivery in a 70-kg adult.12 Although contract pricing may lower the hourly cost, the contract cost per hour of iNO remained 7-fold more than that of iEPO at our institution. In another study, McGinn and Reichert33 reviewed 98 patients undergoing cardiothoracic surgery and found the median iEPO cost-per-patient was also 7-fold higher than that for iNO ($364 [IQR, $226-$865] vs $2563 [IQR, $1875-$8625]; P < .01). In 2017, the annual estimated expenditures for health systems that used iEPO could range from $200 000 to $1 000 000, whereas expenditures for iNO could range from $3 000 000 to $8 000 000.12
To our knowledge, this is the largest randomized clinical trial comparing iEPO and iNO after adult LT using the PGD-3 outcome, which is diagnosed within an established time frame after LT. Severe primary graft dysfunction may be modified through lowering of the pulmonary vascular resistance and limiting oxidative injury of the lung allograft. Development of PGD-3 can be devastating for long-term functional status with increased risk for a repeated LT due to chronic lung-allograft dysfunction.10 The benefits of iNO for PGD-3 prevention through prophylactic use were reported in a placebo-controlled trial7 in which iNO treatment was initiated before lung-allograft reperfusion and discontinued within 48 hours and was associated with lower PGD biomarkers and 2-fold lower PGD-3 incidence compared with placebo (45% vs 17%; P < .035).7 The investigators defined PGD-3 development within 72 hours after LT using radiographic evidence of allograft edema, ratio of partial pressure of arterial oxygen to fraction of inspired oxygen of less than 200, and no other cause for allograft dysfunction (ie, anastomotic venous obstruction, infection, or cardiogenic allograft edema).7 Whereas our study incorporated clinical protocols to wean the allocated iPVD when indicated, the previous study used a fixed 48-hour duration. Interestingly, we found similar between-group median durations for postoperative iPVD use that approximated 48 hours (Table 2).
Equivalence testing was chosen, rather than noninferiority alone, because both medications are used in LT centers worldwide, and guidelines support use of either medication to promote allograft function.34 The choice of the margin was based on the potential loss of relative efficacy that was acceptable with iNO in return for nonefficacy advantages with iEPO. Thus, considerations encompassed risk differences of previous studies, powering for an important outcome and feasibility in accomplishing the study based on annual operations. In addition, the 2016 US Food and Drug Administration guidance document for noninferiority trials was reviewed, which endorsed using a margin that was less than the risk difference of best-available evidence between active control (ie, iNO) and placebo.35 US Food and Drug Administration guidance was also reviewed for equivalence testing using the TOST procedure and allowable margin selection up to 20%.36 Thus, the prespecified equivalence margin for this study satisfied these considerations.
Bias was minimized in this study through concealed allocation, analysis by randomized assignment, and medication blinding to patients, clinicians, data managers, and the statistician. Performing an appropriately powered and designed prospective investigation in this population is pragmatically challenging because most operations often occur at night, which creates logistical challenges for implementation of research-related activities. Furthermore, changes in clinical culture to adopt iEPO as a potential iNO alternative was critical for the successful implementation of a parallel design with clinician blinding. In fact, protocol nonadherence occurred in only a single participant who was allocated to iEPO, switched to iloprost, a long-acting prostacyclin analogue, to accommodate a procedure, and transitioned back to iEPO.
This study was funded through our institution’s health system without external research support, and medication costs were remunerated through insurance providers in the same manner as nontrial patients because both medications constituted standard care. Although blanket approval for Centers for Medicare & Medicaid Services patients was obtained, private insurers were contacted individually, resulting in 58 eligible patients who were denied enrollment (Figure 1). Three of these patients were denied enrollment after an initial approval allowed for randomization. Furthermore, a separate documentation platform was developed to facilitate medication blinding during clinical care and unblinding for medical billing after participant hospital discharge.
After randomization, participants who were not withdrawn for clinical deterioration, insurance denial, or death were included in the intention-to-treat analysis. Modification of a classic intention-to-treat analysis has been described previously in trials of other critically ill populations37 and those undergoing other cardiothoracic operations.38 Furthermore, given the standard use of iNO or iEPO for LT at our institution, analyzing all participants as randomized without iPVD blinding and risking potential for crossover to the nonrandomized treatment could have led to significant interpretation bias. Postrandomization changes to eligibility before LT mainly included new ECMO support, which was an established exclusion criteria because pre-LT ECMO support routinely continued after LT and would have confounded PGD-3 assessment.
A post hoc analysis was performed to evaluate for PGD-3 development using 2 validated subintervals of the 72-hour assessment time frame.9,29,31 Compared with overall PGD-3 rates for the primary outcome (42.2%), lower rates were demonstrated at 48 and 72 hours (27.4%, consistent with a previous report of 30% using this time frame29) and at 72 hours (18.9%). The first 24 hours was included in the primary outcome definition because the allocated treatment could be weaned during this early postoperative period. In fact, 25% of participants were weaned by 31 hours after ICU arrival. Thus, evaluating the primary outcome at 48 or 72 hours while excluding the 24-hour mark could have potentially resulted in missed events in the early post-LT period related to interventions. Thus, we were able to capture all participants who experienced PGD-3 while receiving allocated treatment. Between-group PGD-3 risk differences at each subinterval were similar to those of the intention-to-treat analysis, suggesting similar between-group effects on PGD-3 development at these differing times along the 72-hour window.
Limitations
This study has several limitations. Although this study represents one of the largest randomized perioperative clinical trials in adult LT to date, it was of moderate size and powered for equivalence between treatment groups for the primary outcome. Second, the prespecified equivalence margin was based on a range of incidences that may have been considered too large. Although CIs for the relative risk of developing PGD-3 with iEPO vs iNO included the null hypothesis for unadjusted, adjusted, and per-protocol analyses, the risk difference point estimate favored iNO in all 3 analyses. Thus, iEPO could conceivably be clinically inferior while simultaneously meeting the statistical criteria for equivalence. Although this is a potential interpretation, the present study represents the best available randomized evidence and suggests no between-group differences were observed for PGD-3 development. Third, given the complexity of risk factors in this patient population and the anticipation that the randomization would balance most potential confounders, an adjustment model was not prespecified. Instead, a stepwise, data-driven, model-building approach was performed to adjust for significant outcome-effect modifiers. Although this approach may not account for all potential sources of confounding, it does account for the most significant of them, and ones that would have been strong enough to bias our findings. For example, delayed chest closure, which heralds a more complex clinical course, occurred more often in the iNO group and was identified as a PGD-3 effect modifier and used to adjust the intention-to-treat analysis. Fourth, given the unique funding mechanism, a multicenter investigation could not be supported. Fifth, this study was not placebo controlled. However, iPVD use constituted standard care at our institution, and placebo would have led to considerable crossover to the treatment arm, thus complicating the interpretation between intention-to-treat and per-protocol analyses. Sixth, although prophylactic iPVD administration is considered standard care, this practice is not universal, and some high-volume LT centers may selectively initiate iPVD in high-risk patients or after lung-allograft reperfusion injury. Nevertheless, high-risk patients were represented in this study and balanced between groups. Last, this report was limited to 90-day outcomes, and 1-year follow-up with a cost-effectiveness analysis is under way.
Conclusions
Among patients undergoing LT, use of iEPO was associated with similar risks for PGD-3 development and other outcomes compared with those who received iNO. Although the results of this investigation have changed practice at our institution, future directions may include implementation of a larger multicenter trial to substantiate these findings.
Trial Protocol
eMethods. Protocols for ECMO Management
eTable 1. Multivariable Model
eTable 2. Adverse Events Separated by Allocated Treatment
eTable 3. Primary Outcome by Randomization Strata
eFigure 1. Time-to-Event Analysis for Duration of Mechanical Ventilation
eFigure 2. Daily Mean Pulmonary Arterial Pressure Values According to Treatment Groups
Statistical Analysis Plan
Nonauthor Collaborators. The INSPIRE-FLO nonauthor collaborators
Data Sharing Statement
References
- 1.Pinsky DJ, Naka Y, Chowdhury NC, et al. The nitric oxide/cyclic GMP pathway in organ transplantation: critical role in successful lung preservation. Proc Natl Acad Sci U S A. 1994;91(25):12086-12090. doi: 10.1073/pnas.91.25.12086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricciardolo FL. Multiple roles of nitric oxide in the airways. Thorax. 2003;58(2):175-182. doi: 10.1136/thorax.58.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shargall Y, Guenther G, Ahya VN, Ardehali A, Singhal A, Keshavjee S; ISHLT Working Group on Primary Lung Graft Dysfunction . Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant. 2005;24(10):1489-1500. doi: 10.1016/j.healun.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 4.Bacha EA, Sellak H, Murakami S, et al. ; Paris-Sud University Lung Transplantation Group . Inhaled nitric oxide attenuates reperfusion injury in non–heartbeating-donor lung transplantation. Transplantation. 1997;63(10):1380-1386. doi: 10.1097/00007890-199705270-00002 [DOI] [PubMed] [Google Scholar]
- 5.Gielis JF, Quirynen L, Briedé JJ, Roelant E, Cos P, Van Schil PEY. Pathogenetic role of endothelial nitric oxide synthase uncoupling during lung ischaemia-reperfusion injury. Eur J Cardiothorac Surg. 2017;52(2):256-263. doi: 10.1093/ejcts/ezx125 [DOI] [PubMed] [Google Scholar]
- 6.Minamoto K, Pinsky DJ, Fujita T, Naka Y. Timing of nitric oxide donor supplementation determines endothelin-1 regulation and quality of lung preservation for transplantation. Am J Respir Cell Mol Biol. 2002;26(1):14-21. doi: 10.1165/ajrcmb.26.1.4649 [DOI] [PubMed] [Google Scholar]
- 7.Moreno I, Vicente R, Mir A, et al. Effects of inhaled nitric oxide on primary graft dysfunction in lung transplantation. Transplant Proc. 2009;41(6):2210-2212. doi: 10.1016/j.transproceed.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 8.Snell GI, Yusen RD, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: definition and grading-A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1097-1103. doi: 10.1016/j.healun.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 9.Christie JD, Bellamy S, Ware LB, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29(11):1231-1239. doi: 10.1016/j.healun.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507-513. doi: 10.1164/rccm.200608-1079OC [DOI] [PubMed] [Google Scholar]
- 11.Tomasi R, Betz D, Schlager S, et al. Intraoperative anesthetic management of lung transplantation: center-specific practices and geographic and centers size differences. J Cardiothorac Vasc Anesth. 2018;32(1):62-69. doi: 10.1053/j.jvca.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 12.Rao V, Ghadimi K, Keeyapaj W, Parsons CA, Cheung AT. Inhaled nitric oxide (iNO) and inhaled epoprostenol (iPGI2) use in cardiothoracic surgical patients: is there sufficient evidence for evidence-based recommendations? J Cardiothorac Vasc Anesth . 2018;32(3):1452-1457. doi: 10.1053/j.jvca.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 13.Khan TA, Schnickel G, Ross D, et al. A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. J Thorac Cardiovasc Surg. 2009;138(6):1417-1424. doi: 10.1016/j.jtcvs.2009.04.063 [DOI] [PubMed] [Google Scholar]
- 14.Fiser SM, Cope JT, Kron IL, et al. Aerosolized prostacyclin (epoprostenol) as an alternative to inhaled nitric oxide for patients with reperfusion injury after lung transplantation. J Thorac Cardiovasc Surg. 2001;121(5):981-982. doi: 10.1067/mtc.2001.115668 [DOI] [PubMed] [Google Scholar]
- 15.Torbic H, Szumita PM, Anger KE, Nuccio P, Lagambina S, Weinhouse G. Clinical and economic impact of formulary conversion from inhaled Flolan to inhaled Veletri for refractory hypoxemia in critically ill patients. Ann Pharmacother. 2016;50(2):106-112. doi: 10.1177/1060028015621308 [DOI] [PubMed] [Google Scholar]
- 16.Dhanani J, Fraser JF, Chan HK, Rello J, Cohen J, Roberts JA. Fundamentals of aerosol therapy in critical care. Crit Care. 2016;20(1):269. doi: 10.1186/s13054-016-1448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Preston IR, Sagliani KD, Roberts KE, et al. Comparison of acute hemodynamic effects of inhaled nitric oxide and inhaled epoprostenol in patients with pulmonary hypertension. Pulm Circ. 2013;3(1):68-73. doi: 10.4103/2045-8932.109916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Heerden PV, Barden A, Michalopoulos N, Bulsara MK, Roberts BL. Dose-response to inhaled aerosolized prostacyclin for hypoxemia due to ARDS. Chest. 2000;117(3):819-827. doi: 10.1378/chest.117.3.819 [DOI] [PubMed] [Google Scholar]
- 19.Torbic H, Szumita PM, Anger KE, Nuccio P, LaGambina S, Weinhouse G. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care. 2013;28(5):844-848. doi: 10.1016/j.jcrc.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 20.Ammar MA, Bauer SR, Bass SN, Sasidhar M, Mullin R, Lam SW. Noninferiority of inhaled epoprostenol to inhaled nitric oxide for the treatment of ARDS. Ann Pharmacother. 2015;49(10):1105-1112. doi: 10.1177/1060028015595642 [DOI] [PubMed] [Google Scholar]
- 21.DeGrado JR, Szumita PM, Schuler BR, et al. Evaluation of the efficacy and safety of inhaled epoprostenol and inhaled nitric oxide for refractory hypoxemia in patients with coronavirus disease 2019. Crit Care Explor. 2020;2(10):e0259. doi: 10.1097/CCE.0000000000000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonti R, Pike CW, Cobb N. Responsiveness of inhaled epoprostenol in respiratory failure due to COVID-19. J Intensive Care Med. 2021;36(3):327-333. doi: 10.1177/0885066620976525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray AL, Mulvihill MS, Hartwig MG. Lung transplantation at Duke. J Thorac Dis. 2016;8(3):E185-E196. doi: 10.21037/jtd.2016.02.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johannes T, Ince C, Klingel K, Unertl KE, Mik EG. Iloprost preserves renal oxygenation and restores kidney function in endotoxemia-related acute renal failure in the rat. Crit Care Med. 2009;37(4):1423-1432. doi: 10.1097/CCM.0b013e31819b5f4e [DOI] [PubMed] [Google Scholar]
- 25.Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294(1):F1-F9. doi: 10.1152/ajprenal.00424.2007 [DOI] [PubMed] [Google Scholar]
- 26.Gamble C, Krishan A, Stocken D, et al. Guidelines for the content of statistical analysis plans in clinical trials. JAMA. 2017;318(23):2337-2343. doi: 10.1001/jama.2017.18556 [DOI] [PubMed] [Google Scholar]
- 27.Cornfield DN, Milla CE, Haddad IY, Barbato JE, Park SJ. Safety of inhaled nitric oxide after lung transplantation. J Heart Lung Transplant. 2003;22(8):903-907. doi: 10.1016/S1053-2498(02)00809-4 [DOI] [PubMed] [Google Scholar]
- 28.Botha P, Jeyakanthan M, Rao JN, et al. Inhaled nitric oxide for modulation of ischemia-reperfusion injury in lung transplantation. J Heart Lung Transplant. 2007;26(11):1199-1205. doi: 10.1016/j.healun.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Diamond JM, Lee JC, Kawut SM, et al. ; Lung Transplant Outcomes Group . Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187(5):527-534. doi: 10.1164/rccm.201210-1865OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Liu Y, Su L, Jiang SJ. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One. 2014;9(3):e92773. doi: 10.1371/journal.pone.0092773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171(11):1312-1316. doi: 10.1164/rccm.200409-1243OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yusen RD, Edwards LB, Dipchand AI, et al. ; International Society for Heart and Lung Transplantation . The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant. 2016;35(10):1170-1184. doi: 10.1016/j.healun.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 33.McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26. doi: 10.1177/1060028015608865 [DOI] [PubMed] [Google Scholar]
- 34.Van Raemdonck D, Hartwig MG, Hertz MI, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part IV: prevention and treatment: a 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2017;36(10):1121-1136. doi: 10.1016/j.healun.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 35.US Dept of Health and Human Services , Food & Drug Administration. FDA noninferiority clinical trials to establish effectiveness: guidance for industry. November 2016. Accessed February 12, 2017. https://www.fda.gov/media/78504/download
- 36.Department of Health and Human Services . Equivalence testing for SE evaluations. Accessed March 23, 2017. https://www.fda.gov/media/124669/download
- 37.Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the Oxygen-ICU Randomized Clinical Trial. JAMA. 2016;316(15):1583-1589. doi: 10.1001/jama.2016.11993 [DOI] [PubMed] [Google Scholar]
- 38.Mathew JP, Mackensen GB, Phillips-Bute B, et al. ; Neurologic Outcome Research Group (NORG) of the Duke Heart Center . Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40(3):880-887. doi: 10.1161/STROKEAHA.108.531236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med. 2018;6(5):357-367. doi: 10.1016/S2213-2600(18)30136-X [DOI] [PubMed] [Google Scholar]
- 40.Shashaty MGS, Forker CM, Miano TA, et al. The association of post-lung transplant acute kidney injury with mortality is independent of primary graft dysfunction: a cohort study. Clin Transplant. 2019;33(10):e13678. doi: 10.1111/ctr.13678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods. Protocols for ECMO Management
eTable 1. Multivariable Model
eTable 2. Adverse Events Separated by Allocated Treatment
eTable 3. Primary Outcome by Randomization Strata
eFigure 1. Time-to-Event Analysis for Duration of Mechanical Ventilation
eFigure 2. Daily Mean Pulmonary Arterial Pressure Values According to Treatment Groups
Statistical Analysis Plan
Nonauthor Collaborators. The INSPIRE-FLO nonauthor collaborators
Data Sharing Statement