This cohort study examines differing types of total pancreatectomy and presents 4 proposed categories based on extent, complexity, and technical aspects of the procedure.
Key Points
Question
Are differences in the extent, complexity, and technical aspects of total pancreatectomy (TP) relevant for postoperative outcomes after the procedure?
Findings
In a cohort study of 1451 patients who underwent TP, postoperative morbidity and mortality rates gradually and statistically significantly increased from (1) standard TP to (2) TP with venous resection, (3) TP with multivisceral resection, and (4) TP with arterial resection.
Meaning
The findings of this study suggest that TP comprises heterogeneous procedures; categorization of 4 different types of TP may be useful for better risk stratification and more accurate comparisons of future studies.
Abstract
Importance
Comparability of morbidity and mortality rates after total pancreatectomy (TP) reported by different surgical centers is limited. Procedure-specific differences, such as the extent of resection, including additional vascular or multivisceral resections, are rarely acknowledged when postoperative outcomes are reported.
Objectives
To evaluate postoperative outcomes after TP and categorize different types of TP based on the extent, complexity, and technical aspects of each procedure.
Design, Setting, and Participants
This single-center study included a retrospective cohort of 1451 patients who had undergone TP between October 1, 2001, and December 31, 2020. Each patient was assigned to 1 of the following 4 categories that reflect increasing levels of procedure-related difficulty: standard TP (type 1), TP with venous resection (type 2), TP with multivisceral resection (type 3), and TP with arterial resection (type 4). Postoperative outcomes among the groups were compared.
Main Outcomes and Measures
Categorization of different types of TP based on the procedure-related difficulty and differing postoperative outcomes.
Results
Of the 1451 patients who had undergone TP and were included in the analysis, 840 were men (57.9%); median age was 64.9 (IQR, 56.7-71.7) years. A total of 676 patients (46.6%) were assigned to type 1, 296 patients (20.4%) to type 2, 314 patients (21.6%) to type 3, and 165 patients (11.4%) to type 4 TP. A gradual increase in surgical morbidity was noted by TP type (type 1: 255 [37.7%], type 2: 137 [46.3%], type 3: 178 [56.7%], and type 4: 98 [59.4%]; P < .001), as was noted for median length of hospital stay (type 1: 14 [IQR, 10-19] days, type 2: 16 [IQR, 12-23] days, type 3: 17 [IQR, 13-29] days, and type 4: 18 [IQR, 13-30] days; P < .001), and 90-day mortality (type 1: 23 [3.4%], type 2: 17 [5.7%], type 3: 29 [9.2%], and type 4: 20 [12.1%]; P < .001). In the multivariable analysis, type 3 (TP with multivisceral resection) and type 4 (TP with arterial resection) were independently associated with an increased 90-day mortality rate.
Conclusions and Relevance
The findings of this study suggest there are significant differences in postoperative outcomes when the extent, complexity, and technical aspects of the procedure are considered. Classifying TP into 4 different categories may allow for better postoperative risk stratification as well as more accurate comparisons in future studies.
Introduction
Total pancreatectomy (TP) is considered a standard procedure in pancreatic surgery and is regularly performed to achieve negative resection margins in extensive pancreatic tumors.1,2,3,4 Other indications for TP include intraductal papillary mucinous neoplasms involving the main pancreatic duct of the whole pancreas, multicentric mixed-type or side-branch intraductal papillary mucinous neoplasms, chronic pancreatitis, neuroendocrine tumors with multicentric tumor growth, and, less frequently, multifocal pancreatic metastases (eg, of clear cell renal cell carcinoma).5,6,7,8,9,10,11 In rare cases, surgeons may consider TP for technical reasons, such as to avoid the need for a high-risk pancreatoenteric anastomosis in soft pancreata with extremely friable tissue texture and small-caliber pancreatic ducts, or in patients with pancreatic cancer with complex vascular resections, to prevent postoperative pancreatic fistula and its potentially deleterious sequelae, especially erosional postpancreatectomy hemorrhage (PPH).12
Individual surgical expertise, quality of interdisciplinary perioperative patient care, and the successful management of perioperative complications are the primary factors associated with short-term TP outcomes. Morbidity rates range from 30% to 87%.2,4,13,14 Likewise, postoperative mortality rates after TP differ considerably, ranging from 0% to as high as 23%.2,3,4,15,16,17,18,19,20,21 Hospital volume has an inverse association with adverse surgical outcome.22 However, even among specialized high-volume institutions, morbidity and mortality rates vary greatly, with reported perioperative mortality rates between 0% and 10%.2,3,4,15,16,17 On the other hand, similar short-term postoperative outcomes do not necessarily reflect comparable quality of pancreatic surgery because the procedure’s demands may vary substantially, a fact seldom accounted for in the literature. The aim of this study was to assess postoperative outcomes after TP and break the procedure down into different category types of TP based on the extent, complexity, and technical aspects of each procedure.
Methods
Data on all 1451 consecutive patients undergoing elective TP or elective completion pancreatectomy at the Heidelberg University Hospital, Heidelberg, Germany, between October 1, 2001, and December 31, 2020, were extracted from a prospectively maintained database. All procedures were performed by open surgery. The study was approved by the institutional review board of the Ethics Committee of the Medical Faculty, University of Heidelberg; written informed consent for data collection and analysis had been obtained from each patient at the time of admission. Patient characteristics and operative details, including age, sex, body mass index, American Society of Anesthesiologists performance status, operation time, estimated blood loss, and histologic findings were analyzed. Technical details, such as pylorus preservation, pylorus resection or (partial) gastrectomy, additional venous resection, multivisceral resection, and arterial resection were also identified. Postoperative outcome analysis comprised medical and surgical morbidity, including PPH, bile leakage, leakage of the gastroenterostomy, chyle leakage, intra-abdominal fluid collection, delayed gastric emptying, surgical site infection, and reoperation, as well as length of hospital stay and 90-day mortality. Chyle leak and delayed gastric emptying were classified according to International Study Group of Pancreatic Surgery definitions.23,24 Reoperations were performed to control PPH, bile leakage, gastroenterostomy leakage, surgical site infection, burst abdomen, ischemia, postoperative bowel obstruction, small-bowel and/or colon perforation, portal vein thrombosis, arterial thrombosis, and abdominal sepsis. Planned reoperations for 2-step reconstruction with hepaticojejunostomy and duodenojejunostomy or gastrojejunostomy were also included. Based on the extent, complexity, and technical aspects of TP, the following 4-stage classification system, which has been used and recently published for pancreatoduodenectomy, was established25: type 1, standard TP (without vascular or adjacent organ resection); type 2, TP with portal vein and/or superior mesenteric vein resection; type 3, TP with multivisceral resection; and type 4, TP with arterial resection.
Procedures requiring multiple vessel or organ resections were placed in the higher category into which they fell (eg, a TP requiring both venous and multivisceral resections was classified as type 3 instead of type 2). Multivisceral resections were defined as TPs that extended beyond pylorus preserving, pylous resecting, and TPs with antrectomy and included the partial or total resection of additional organs with the exception of partial or total splenectomy as defined by the International Study Group of Pancreatic Surgery. In their definition, extended pancreatectomy is defined as involving (1) more than the antrum or distal half of the stomach; (2) the colon and/or mesocolon with relevant vascular structures of the transverse mesocolon (ileocolic, right, or middle colic vessels); (3) the small bowel beyond the first segment of jejunum; (4) the portal, superior mesenteric, and/or inferior mesenteric vein; (5) the hepatic artery, celiac trunk, and/or superior mesenteric artery; (6) the inferior vena cava; (7) the right and/or left adrenal gland; (8) the right and/or left kidney and/or its vasculature; (9) the liver; and/or (10) the diaphragmatic crura.26 For TP with liver resection, procedures were categorized as type 1 or 3 depending on the extent of liver resection. If only an excisional biopsy or wedge resection was performed, TP was categorized as type 1 (standard). If the liver resection required a larger atypical, segmental, bisegmental or major (hemihepatectomy) resection, the operation was categorized as type 3 (multivisceral). Details on the procedures are provided in eTable 1 in the Supplement.
Statistical Analysis
Data management and statistical analysis were performed using SAS, version 9.4 (SAS Institute Inc). This classification system was validated for postoperative morbidity and mortality. Patient characteristics, perioperative parameters, and postoperative outcomes were summarized using absolute and relative frequencies for categorical parameters and median (IQR) for continuous parameters. For comparisons, either the Fisher exact test or χ2 test was performed, as appropriate, along with the Kruskal-Wallis test. All tests were used to compare the 4 groups as overall testing and the resulting P values were interpreted together with the observed differences of the respective parameter. Univariable risk factor analysis for 90-day mortality was performed using a logistic regression analysis. The odds ratios (ORs) and corresponding 95% CIs are presented. Subsequently, we examined a multivariable logistic regression model comprising risk factors with a univariable P value <.20. Variable selection and model building were performed using the Akaike information criterion and no missing values were imputed. A 2-sided value of P ≤ .05 was considered statistically significant. Owing to the exploratory nature of the analyses performed, results were interpreted carefully and P values have been used descriptively.
Results
Population and Short-term Outcomes
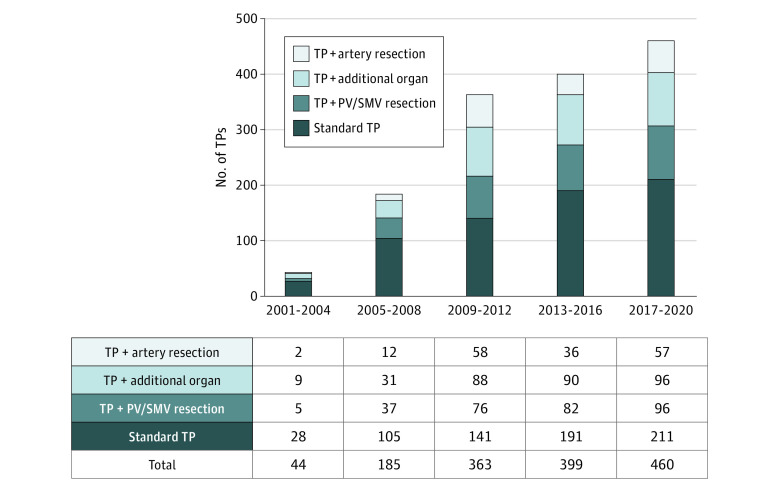
During the study period, 1451 patients underwent elective TP, including 171 patients who underwent elective completion pancreatectomy. The median age was 64.9 (IQR, 56.7-71.7) years, 840 patients (57.9%) were men, and 611 patients (42.1%) were women. The most frequent indication for TP was pancreatic adenocarcinoma (874 [60.2%]), followed by intraductal papillary mucinous neoplasms (223 [15.4%]), chronic pancreatitis (89 [6.1%]), and neuroendocrine tumors (75 [5.2%]). The median operation time was 355 (IQR, 285-427) minutes and the median estimated blood loss was 1000 (IQR, 600-1800) mL. Most patients underwent standard TP (type 1: 676 [46.6%]). Total pancreatectomy with resection of the portal vein/superior mesenteric vein (type 2) was performed in 296 patients (20.4%). Reconstruction after venous resection was performed by end-to-end anastomosis in 226 patients (76.4%). A venous interposition graft or conduit was used in 29 patients (9.8%). Partial venous excision with direct closure (venorrhaphy) was possible in 34 patients (11.5%) and required a patch in 7 patients (2.4%). A total of 314 patients (21.6%) underwent multivisceral resection (type 3). Specific details regarding the additionally resected organs are presented in eTable 1 in the Supplement. Total pancreatectomy with arterial resection (type 4) was done in 165 patients (11.4%). Arterial resections included 37 superior mesenteric artery resections, 93 hepatic artery resections, 2 aortic resections, and 38 resections of the celiac axis. Five patients had resections of multiple arteries. Arterial reconstruction was performed by end-to-end anastomosis (n = 84), interposition graft/conduit (n = 32), or transposition of the splenic artery to the superior mesenteric artery or hepatic artery (n = 45). In 9 patients, no arterial reconstruction was performed. One hundred fifty-two patients who underwent TP with venous resections and 83 with multivisceral resections were assigned to higher groups because they also required multivisceral (type 3) or arterial (type 4) resections. The number of TPs increased continuously during the study period (2001-2004: 44, 2005-2008: 185, 2009-2012: 363, 2013-2016: 399, and 2017-2020: 460) (Figure). Further detailed patient characteristics are reported in Table 1.
Figure. Number and Type of Total Pancreatectomies (TPs) Performed per 4-Year Interval and Type of TP.
PV indicates portal vein; SMV, superior mesenteric vein.
Table 1. Patient Characteristics According to Type of Total Pancreatectomy.
| Characteristic | Total | Type 1 (standard TP) | Type 2 (TP + PV/SMV resection) | Type 3 (TP + additional organ resection) | Type 4 (TP + artery resection) | P value |
|---|---|---|---|---|---|---|
| No. (%) | 1451 | 676 (46.6) | 296 (20.4) | 314 (21.6) | 165 (11.4) | |
| Age, median (IQR), y | 64.9 (56.7-71.7) | 65.8 (57.4-72.5) | 64.9 (58.5-72.7) | 64.4 (57.1-71.2) | 62.1 (53.5-68.7) | <.001 |
| Sex | ||||||
| Female | 611 (42.1) | 271 (40.1) | 141 (47.6) | 127 (40.4) | 72 (43.6) | .15 |
| Male | 840 (57.9) | 405 (59.9) | 155 (52.4) | 187 (59.6) | 93 (56.4) | |
| BMI, median (IQR) | 24.5 (22.3-27.5) | 24.7 (22.5-27.8) | 24.9 (22.6-27.6) | 24.3 (22.1-27.1) | 23.9 (21.9-26.3) | .007 |
| Missing | 60 | 29 | 3 | 24 | 4 | |
| ASA classification | ||||||
| I | 48 (3.5) | 24 (3.8) | 14 (5.0) | 5 (1.7) | 5 (3.1) | .07 |
| II | 728 (53.4) | 338 (53.8) | 146 (51.8) | 158 (53.6) | 86 (54.1) | |
| III | 581 (42.6) | 265 (42.2) | 122 (43.3) | 127 (43.1) | 67 (42.1) | |
| IV | 7 (0.5) | 1 (0.2) | 0 (0.3) | 5 (1.7) | 1 (0.6) | |
| Missing | 87 | 48 | 14 | 19 | 6 | |
| Total pancreatectomy | ||||||
| With gastrectomya | 674 (46.5) | 204 (30.2) | 186 (62.8) | 174 (55.4) | 110 (66.7) | <.001 |
| Pylorus | ||||||
| Preserving | 491 (33.8) | 318 (47.0) | 68 (23.0) | 68 (21.7) | 37 (22.4) | |
| Resecting | 115 (7.9) | 58 (8.6) | 30 (10.1) | 22 (7.0) | 5 (3.0) | |
| Elective completion pancreatectomy | 171 (11.8) | 96 (14.2) | 12 (4.1) | 50 (15.9) | 13 (7.9) | |
| Operation time, median (IQR), min | 355 (285-427) | 314 (259-380) | 377 (321-442) | 383 (305-465) | 437 (373-509) | <.001 |
| Estimated blood loss, median (IQR), mL | 1000 (600-1800) | 800 (500-1300) | 1200 (800-2000) | 1200 (700-2200) | 1700 (1000-3000) | <.001 |
| Histologic findings | ||||||
| Pancreatic carcinoma | 874 (60.2) | 286 (42.3) | 255 (86.1) | 188 (59.9) | 145 (87.9) | <.001 |
| Periampullary cancer | 58 (4.0) | 40 (5.9) | 7 (2.3) | 9 (2.9) | 2 (1.2) | |
| IPMN | 223 (15.4) | 185 (27.4) | 8 (2.7) | 27 (8.6) | 3 (1.8) | |
| NET | 75 (5.2) | 44 (6.5) | 9 (3.0) | 16 (5.1) | 6 (3.6) | |
| SCN/MCN | 14 (1.0) | 10 (1.5) | 2 (0.7) | 0 | 2 (1.2) | |
| Chronic pancreatitis | 89 (6.1) | 60 (8.9) | 8 (2.7) | 20 (6.4) | 1 (0.6) | |
| Other | 118 (8.2) | 51 (7.5) | 7 (2.4) | 54 (17.2) | 6 (3.6) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MCN, mucinous cystic neoplasm; IPMN, intraductal papillary mucinous neoplasm; NET, neuroendocrine tumor; SCN, serous cystic neoplasm; TP, total pancreatectomy.
Included patients with antrectomy, partial, subtotal, and total gastrectomy.
Postoperative surgical complications occurred in 668 patients (46.0%) and included PPH necessitating radiologic/endoscopic intervention or relaparotomy (n = 86), bile leakage (n = 74), gastroenterostomy leakage (n = 57), chyle leakage (n = 121), delayed gastric emptying (n = 286), and intra-abdominal fluid collection (n = 193). Reoperation was necessary in 216 patients. The median postoperative length of hospital stay was 15 (IQR, 11-22) days. The overall mortality rates for the entire patient cohort were 3.4% for 30-day mortality and 6.1% for 90-day mortality. From 2017 to 2020, the 30-day mortality rate in 460 patients decreased to 2.2% and the 90-day mortality rate decreased to 3.7%. Postoperative outcomes are summarized in Table 2.
Table 2. Postoperative Outcomes According to Extent of TP.
| Outcome | No. (%)a | P value | ||||
|---|---|---|---|---|---|---|
| Total | Type 1 | Type 2 | Type 3 | Type 4 | ||
| No. | 1451 | 676 | 296 | 314 | 165 | |
| Mortality | ||||||
| 30-d | 49 (3.4) | 18 (2.7) | 7 (2.4) | 14 (4.5) | 10 (6.1) | .02 |
| 90-d | 89 (6.1) | 23 (3.4) | 17 (5.7) | 29 (9.2) | 20 (12.1) | <.001 |
| Surgical morbidity | 668 (46.0) | 255 (37.7) | 137 (46.3) | 178 (56.7) | 98 (59.4) | <.001 |
| PPH | 86 (5.9) | 29 (4.3) | 13 (4.4) | 25 (8.0) | 19 (11.5) | .002 |
| Interventional/endoscopic | 16 (1.1) | 5 (0.7) | 2 (0.7) | 8 (2.6) | 1 (0.6) | .09 |
| Operative | 70 (4.8) | 24 (3.6) | 11 (3.7) | 17 (5.4) | 18 (10.9) | .003 |
| Bile leakage | 74 (5.1) | 25 (3.7) | 19 (6.4) | 16 (5.1) | 14 (8.5) | .05 |
| Gastroenterostomy leakage | 57 (3.9) | 9 (1.3) | 15 (5.1) | 21 (6.7) | 12 (7.3) | <.001 |
| Chyle leak | 121 (8.3) | 59 (8.7) | 19 (6.4) | 27 (8.6) | 16 (9.7) | .56 |
| Delayed gastric emptying | 286 (19.7) | 98 (14.5) | 63 (21.3) | 90 (28.7) | 35 (21.2) | <.001 |
| Intra-abdominal fluid collection | 193 (13.3) | 55 (8.1) | 40 (13.5) | 60 (19.1) | 38 (23.0) | <.001 |
| Relaparotomy | 216 (14.9) | 57 (8.4) | 41 (13.9) | 72 (22.9) | 46 (27.9) | <.001 |
| ICU stay ≥2 d | 453 (31.2) | 173 (25.6) | 100 (33.8) | 111 (35.4) | 69 (41.8) | <.001 |
| Length of hospital stay, median (IQR), d | 15 (11-22) | 14 (10–19) | 16 (12–23) | 17 (13–29) | 18 (13–30) | <.001 |
Abbreviations: ICU, intensive care unit; PPH, postpancreatectomy hemorrhage; PV, portal vein; SMV, superior mesenteric vein; TP, total pancreatectomy.
Type 1, standard TP; type 2, TP plus PV and/or SMV resection; type 3, TP plus additional organ resection; and type 4, TP plus artery resection.
Type 2 TP was associated with postoperative portal vein/superior mesenteric vein thrombosis in 18 patients (6.1%), including 11 patients with end-to-end anastomosis, 6 patients with interposition graft/conduit, and 1 patient with venorrhaphy. Management of postoperative portal vein/superior mesenteric vein thrombosis included relaparotomy with thrombectomy in 11 patients. Seven patients were treated nonsurgically with only anticoagulation therapy. Type 3 TP included 120 colon resections (38.2%). A primary anastomosis was performed in 71 patients and anastomotic leakage was found in 9 patients (12.7%). After type 4 TP, 21 patients (12.7%) developed severe liver perfusion failure that was associated with major systemic complications. Eleven patients underwent relaparotomy with thrombectomy of the reconstructed artery. In 10 patients, the therapeutic approach was nonsurgical and included only radiologic intervention or anticoagulation. In total, 7 of these 21 patients died in the sequalae of liver ischemia. Furthermore, 3 patients had gastric ischemia. Outcomes differed regarding the artery that was resected during TP. The 30-day mortality rates associated with resection were 6.7% for the superior mesenteric artery, 9.1% for the hepatic artery, 2.7% for the celiac axis, and 20.0% for multiple arteries; no mortality was noted with aortic resection. The 90-day mortality rates associated with resection were 8.1% for the superior mesenteric artery, 15.9% for the hepatic artery, 5.9% for the celiac axis, and 20.0% for multiple arteries; no mortality was noted with aortic resection.
Perioperative Details and Postoperative Outcomes Stratified by Type of TP
Patients with more advanced surgery (types 2-4 TP) were younger (type 1: 65.8 [IQR, 57.4-72.5] years, type 2: 64.9 [IQR, 58.5-72.7] years, type 3: 64.4 [IQR, 57.1-71.2] years, and type 4: 62.1 [IQR 53.5-68.7] years; P < .001). Median estimated blood loss was higher (type 1: 800 [IQR, 500-1300] mL, type 2: 1200 [IQR, 800-2000] mL, type 3: 1200 [IQR, 700-2200] mL, and type 4: 1700 [1000-3000] mL; P < .001) and operation time longer (type 1: 314 [IQR, 259-380] minutes, type 2: 377 [IQR, 321-442] minutes, type 3: 383 [IQR, 305-465] minutes, and type 4: 437 [IQR, 373-509] minutes; P < .001) in patients with more complex TP. The overall surgical morbidity gradually increased with the complexity of TP (type 1: 255 [37.7%], type 2: 137 [46.3%], type 3: 178 [56.7%], and type 4: 98 [59.4%]; P < .001) as did the rates of complications, such as PPH (from type 1: 29 [4.3%] to type 4: 19 [11.5%]), gastroenterostomy leakage (from type 1: 9 [1.3%] to type 4: 12 [7.3%]), intra-abdominal fluid collection (from type 1: 55 [8.1%] to type 4: 38 [23.0%]), and relaparotomy (from type 1: 57 [8.4%] to type 4: 46 [27.9%]) (Table 2). Accordingly, the median length of hospital stay increased throughout the 4 TP categories (type 1: 14 [IQR, 10-19] days, type 2: 16 [IQR, 12-23] days, type 3: 17 [IQR, 13-29] days, and type 4: 18 [IQR, 13-30] days; P < .001). The mortality rates likewise increased for 30-day mortality (type 1: 2.7%, type 2: 2.4%, type 3: 4.5%, and type 4: 6.1%; P = .02) and 90-day mortality (type 1: 3.4%, type 2: 5.7%, type 3: 9.2%, and type 4: 12.1%; P < .001). A detailed comparison of postoperative outcomes is reported in Table 2.
In the univariable analysis, patients with American Society of Anesthesiologists III/IV stages, patients categorized as type 3 (314 [9.2%]; P < .001) and type 4 (165 [12.1%]; P < .001) TP, patients undergoing TP between 2009 and 2012 (363 [9.6%]; P = .02), and those who had received neoadjuvant therapy (256 [10.2%]; P = .004) had significantly higher 90-day mortality. In the multivariable analysis, age older than 70 years (OR, 1.922; 95% CI, 1.146-3.195; P = .01), American Society of Anesthesiologists stages III and IV (OR, 1.844; 95% CI, 1.132-3.038; P = .01), neoadjuvant therapy (OR, 2.067; 95% CI, 1.172-3.577; P = .01), and type 4 vs types 1 and 2 of TP (OR, 3.572; 95% CI, 1.840-6.792; P < .001) were independently associated with 90-day mortality. Patients requiring multivisceral (type 3) and arterial resection (type 4) had a 2.2- and 3.6-fold risk of dying within 90 days. Data from univariable and multivariable analyses are presented in Table 3.
Table 3. Logistic Regression Analyses Associated With 90-Day Mortality After TP.
| Variable | No. (% events) | OR (95% CI) | P value |
|---|---|---|---|
| Univariable analysis | |||
| Sex | |||
| Male | 840 (6.4) | 1 [Reference] | .58 |
| Female | 611 (5.7) | 0.884 (0.566-1.365) | |
| Age, y | |||
| <50 | 167 (4.2) | 1 [Reference] | |
| 50-69 | 838 (5.6) | 1.358 (0.642-3.340) | .46 |
| ≥70 | 446 (7.8) | 1.946 (0.898-4.861) | .12 |
| BMI | |||
| <18.5 | 52 (7.7) | 1 [Reference] | |
| 18.5-<25.0 | 713 (5.3) | 0.676 (0.258-2.322) | .47 |
| 25.0-<30.0 | 454 (5.7) | 0.729 (0.270-2.547) | .57 |
| ≥30.0 | 172 (4.7) | 0.585 (0.176-2.269) | .40 |
| ASA classification | |||
| I/II | 776 (3.9) | 1 [Reference] | |
| III/IV | 588 (7.3) | 1.962 (1.220-3.194) | .007 |
| Type of TPa | |||
| 1 | 676 (3.4) | 1 [Reference] | |
| 2 | 296 (5.7) | 1.730 (0.897-3.274) | .09 |
| 3 | 314 (9.2) | 2.889 (1.646-5.125) | <.001 |
| 4 | 165 (12.1) | 3.917 (2.079-7.324) | <.001 |
| TP procedure | |||
| Pylorus | |||
| Preserving | 491 (5.7) | 1 [Reference] | |
| Resecting | 115 (2.6) | 0.443 (0.105-1.279) | .19 |
| Gastrectomyb | 674 (7.6) | 1.354 (0.847-2.205) | .21 |
| Elective completion pancreatectomy | 171 (4.1) | 0.706 (0.279-1.561) | .42 |
| Year of operation | |||
| 2001-2008 | 229 (4.4) | 1 [Reference] | |
| 2009-2012 | 363 (9.6) | 2.337 (1.175-5.071) | .02 |
| 2013-2016 | 399 (6.8) | 1.590 (0.778-3.507) | .22 |
| 2017-2020 | 460 (3.7) | 0.840 (0.385-1.933) | .67 |
| Carcinoma | |||
| No | 378 (5.6) | 1 [Reference] | |
| Yes | 1073 (6.3) | 1.150 (0.707-1.947) | .59 |
| Neoadjuvant therapy | |||
| No | 1195 (5.3) | 1 [Reference] | |
| Yes | 256 (10.2) | 2.031 (1.240-3.241) | .004 |
| Multivariable analysis | |||
| Age, y | |||
| ≥70 vs <70 | NA | 1.922 (1.146-3.195) | .01 |
| ASA classification | |||
| III and IV vs I and II | NA | 1.844 (1.132-3.038) | .01 |
| Neoadjuvant therapy | |||
| Yes vs no | NA | 2.067 (1.172-3.577) | .01 |
| Type of TPa | |||
| Type 3 vs 1 and 2 | NA | 2.177 (1.200-3.886) | .009 |
| Type 4 vs 1 and 2 | NA | 3.572 (1.840-6.792) | <.001 |
| Year of operation | |||
| 2017-2020 vs 2001-2016 | NA | 0.461 (0.249-0.810) | .01 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; OR, odds ratio; TP, total pancreatectomy.
Type 1, standard TP; type 2, TP plus PV and/or superior mesenteric vein resection; type 3, TP plus additional organ resection; and type 4, TP plus artery resection.
Included patients with antrectomy, partial, subtotal, and total gastrectomy.
Discussion
To our knowledge, this was the largest single-center study reporting on short-term postoperative outcomes after TP. In a cohort of 1451 patients, TP was performed with acceptable surgical morbidity and mortality. The overall surgical morbidity was 46.0%, the 30-day mortality rate was 3.4%, and the 90-day mortality rate was 6.1%. Between 2017 and 2020, as TP use increased, the mortality rates consistently decreased to 2.2% for 30-day and 3.7% for 90-day mortality. We also noted significant differences in postoperative outcomes when the surgical complexity of each procedure was taken into consideration. Surgical morbidity and 90-day mortality increased with TP category. Patients who had undergone TP and concomitant arterial resection (type 4) had the highest rates of both surgical morbidity and 90-day mortality. We believe that these data present evidence that TP must not be regarded as a homogeneous procedure because the results depend on surgical complexity and can be specified by differentiating types of resections. Therefore, the risk for an adverse outcome is not comparable among all TPs and the extent of resection should be acknowledged separately. The proposed categorization into 4 different types of TP may be important for operative risk stratification and standardized outcome reporting and also may enhance the comparability of future studies.
Pancreatic surgery is technically demanding and requires a high level of surgical expertise. Pancreatic surgeons must be able to handle the superior mesenteric artery and the celiac axis with its major branches to the liver, spleen, and stomach. In addition to this surgical challenge, more advanced resections entail a higher risk of perioperative complications that are not commonly experienced after standard resections, for example, anastomotic leakage after colon resection with primary anastomosis, arterial occlusion with intestinal or liver ischemia, or venous congestion. These differences in extent, complexity, and technical difficulty are rarely acknowledged in the literature. Therefore, the comparability of short-term postoperative outcomes after TP is limited.
To address this issue, the International Study Group of Pancreatic Surgery has published the concept of standard pancreatectomy and extended pancreatectomy.26 Although its definition is clear, the term extended pancreatectomy summarizes a great variety of additional vascular and organ resections requiring different technical skills and expertise. Therefore, data on comparisons of morbidity and mortality following extended resections vary widely among different centers.27 We believe the term extended pancreatectomy remains vague. Our 4-category classification of TP enables a subtler discrimination among different types of TP while remaining simple enough for everyday use. The present study noted a clear gradual difference in both postoperative surgical morbidity and mortality rates across all 4 categories.
Hospital volume has been accepted as a major determinant of postoperative outcome after major pancreatectomy. Hata et al22 analyzed 13 studies based on nationwide databases from 11 countries including a total of 58 023 patients undergoing pancreatoduodenectomy. Their meta-analysis provided evidence for an inverse association between hospital volume and mortality. In line with these findings, Nimptsch et al16 reported a 23% in-hospital mortality rate after TP in a nationwide inpatient database that included data from low-volume hospitals. Krautz et al28 found a significantly lower risk-adjusted in-hospital mortality rate of 6.5% after major pancreatic resection in very high-volume hospitals compared with 11.5% in very low-volume hospitals. Their analysis was based on national hospital discharge data. In a recent multicenter pan-European snapshot study (June 2018-June 2019), Latenstein et al8 found a 25% major complication rate and a 90-day mortality of 8% in 277 patients undergoing TP in 43 centers. The rate of venous resection was 21% and the rate of arterial resection was 4%. Annual volume of less than 60 TPs, increasing patient age, and an estimated blood loss of 2 L or more were associated with higher in-hospital mortality. Hunger and Mantke,29 who analyzed a nationwide patient discharge database of 43 213 patients from 800 hospitals over a 5-year observation period, found that hospitals with the highest volume quintile for classic pancreatoduodenectomy had the lowest adjusted in-hospital mortality rate of 8.3%. However, the analysis also revealed that 44% of the low-volume hospitals performed equal to or better than the hospitals of the highest volume quintile.
In addition to the discrepancies in postoperative morbidity and mortality rates deriving from varying quality in surgical expertise and medical care, the question of the association between surgical complexity and outcomes has not been extensively investigated. Crippa et al3 reported no postoperative mortality in a series of 65 patients undergoing TP. In comparison with other studies on outcomes after TP, the authors did not specify whether more extended resections, such as venous, multivisceral, or arterial, were performed in this cohort. High perioperative morbidity and mortality rates after major pancreatic resections with arterial resections have also been published.30,31 In a 100-patient series on perioperative outcomes after TP, Reddy et al2 reported a 30-day mortality rate of 8%. In this study, extended resections were performed, namely, 14 patients (14%) with concomitant venous resections and 7 patients (7%) with multivisceral resections. No arterial resections were reported. Hartwig et al4 reported an in-hospital mortality of 7.8%. In this study of 434 patients, the rates of venous (32%) and multivisceral (19%) resections were substantially higher, and arterial resections were performed in 15% of the cases. In 2019, Pulvirenti et al17 published the short-term outcomes of a bi-institutional series of 329 patients. The authors reported a morbidity rate of 59.3%, a 30-day mortality rate of 2.1%, and a 90-day mortality rate of 3.3%. In this study, 13.1% of patients undergoing TP had venous resections and 3.3% had multivisceral resections. In a 145-patient series from Sweden, the authors reported a major morbidity rate of 34.5% and a 90-day mortality rate of 5.5%.15 In this series, 64 patients (44.1%) underwent simultaneous venous resection and 23 patients (15.9%) had arterial resections. Collectively, these studies show that the reasons for different outcomes, especially surgical complexity, are not elucidated adequately in the literature, making results difficult to compare. Applying our proposed classification of TP may help address this drawback.
Our study cohort included 171 elective completion pancreatectomies. Although elective completion pancreatectomy after pancreatoduodenectomy or distal pancreatectomy is technically a different procedure than TP, the functional result equals that of TP, and the technical challenges, pitfalls, and limitations (including tissue dissection along the visceral arteries and portal vein/superior mesenteric vein axis, adequate oncologic lymphadenectomy, vascular resection in case of tumor encasement of visceral arteries or of the portal vein/superior mesenteric vein axis) are comparable. Details on earlier surgical procedures and postoperative outcomes according to the extent of elective completion pancreatectomy are presented in eTable 2 in the Supplement.
Limitations
This study has limitations. The limitations of our study originate from the design being a single-center study from a highly specialized pancreatic surgery institution. This narrow focus limits the generalizability of our results. Further studies (eg, prospective multicenter trials) are warranted to validate our data. Given the retrospective study design, there may be some heterogeneity in baseline patient characteristics with respect to the different types of TP. However, the study cohort may reliably represent the patient population in daily practice in other national and international pancreas centers.
Conclusions
Our proposal to categorize TP according to its extent, difficulty, and technical demands suggests an association of postoperative outcomes with each of the 4 types of TP. In this study, postoperative morbidity and mortality rates gradually increased from standard TP to TP with venous resection, TP with multivisceral resection, and TP with arterial resection. Our classification of TP into these 4 categories is easy to apply in daily routine and may improve accuracy not only in postoperative risk stratification but also among comparisons of future studies.
eTable 1. Total Pancreatectomy With Multivisceral Resections
eTable 2. Prior Surgical Procedures and Postoperative Outcomes According to the Extent of Elective Completion Pancreatectomy
References
- 1.Rockey EW. Total pancreatectomy for carcinoma: case report. Ann Surg. 1943;118(4):603-611. doi: 10.1097/00000658-194310000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy S, Wolfgang CL, Cameron JL, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250(2):282-287. doi: 10.1097/SLA.0b013e3181ae9f93 [DOI] [PubMed] [Google Scholar]
- 3.Crippa S, Tamburrino D, Partelli S, et al. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149(1):79-86. doi: 10.1016/j.surg.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 4.Hartwig W, Gluth A, Hinz U, et al. Total pancreatectomy for primary pancreatic neoplasms: renaissance of an unpopular operation. Ann Surg. 2015;261(3):537-546. doi: 10.1097/SLA.0000000000000791 [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Fernández-del Castillo C, Adsay V, et al. ; International Association of Pancreatology . International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183-197. doi: 10.1016/j.pan.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 6.Del Chiaro M, Verbeke C, Salvia R, et al. ; European Study Group on Cystic Tumours of the Pancreas . European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45(9):703-711. doi: 10.1016/j.dld.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 7.Griffin JF, Poruk KE, Wolfgang CL. Is it time to expand the role of total pancreatectomy for IPMN? Dig Surg. 2016;33(4):335-342. doi: 10.1159/000445019 [DOI] [PubMed] [Google Scholar]
- 8.Latenstein AEJ, Scholten L, Al-Saffar HA, et al. ; Scientific, Research Committee of the European-African Hepato-Pancreato-Biliary Association (E-AHPBA) . Clinical outcomes after total pancreatectomy: a prospective multicenter pan-European snapshot study. Ann Surg. 2020. doi: 10.1097/SLA.0000000000004551 [DOI] [PubMed] [Google Scholar]
- 9.Müller MW, Friess H, Kleeff J, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246(6):966-974. doi: 10.1097/SLA.0b013e31815c2ca3 [DOI] [PubMed] [Google Scholar]
- 10.Linehan IP, Lambert MA, Brown DC, Kurtz AB, Cotton PB, Russell RC. Total pancreatectomy for chronic pancreatitis. Gut. 1988;29(3):358-365. doi: 10.1136/gut.29.3.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrman SW, Mulloy M. Total pancreatectomy for the treatment of chronic pancreatitis: indications, outcomes, and recommendations. Am Surg. 2006;72(4):297-302. doi: 10.1177/000313480607200403 [DOI] [PubMed] [Google Scholar]
- 12.Marchegiani G, Perri G, Burelli A, et al. High-risk pancreatic anastomosis vs. total pancreatectomy after pancreatoduodenectomy: postoperative outcomes and quality of life analysis. Ann Surg. 2021. Published online March 24, 2021. doi: 10.1097/SLA.0000000000004840 [DOI] [PubMed] [Google Scholar]
- 13.Satoi S, Murakami Y, Motoi F, et al. Reappraisal of total pancreatectomy in 45 patients with pancreatic ductal adenocarcinoma in the modern era using matched-pairs analysis: multicenter study group of pancreatobiliary surgery in Japan. Pancreas. 2016;45(7):1003-1009. doi: 10.1097/MPA.0000000000000579 [DOI] [PubMed] [Google Scholar]
- 14.Nikfarjam M, Low N, Weinberg L, Chia PH, He H, Christophi C. Total pancreatectomy for the treatment of pancreatic neoplasms. ANZ J Surg. 2014;84(11):823-826. doi: 10.1111/ans.12640 [DOI] [PubMed] [Google Scholar]
- 15.Stoop TF, Ateeb Z, Ghorbani P, et al. Surgical outcomes after total pancreatectomy: a high-volume center experience. Ann Surg Oncol. 2021;28(3):1543-1551. doi: 10.1245/s10434-020-08957-x [DOI] [PubMed] [Google Scholar]
- 16.Nimptsch U, Krautz C, Weber GF, Mansky T, Grützmann R. Nationwide in-hospital mortality following pancreatic surgery in Germany is higher than anticipated. Ann Surg. 2016;264(6):1082-1090. doi: 10.1097/SLA.0000000000001693 [DOI] [PubMed] [Google Scholar]
- 17.Pulvirenti A, Pea A, Rezaee N, et al. Perioperative outcomes and long-term quality of life after total pancreatectomy. Br J Surg. 2019;106(13):1819-1828. doi: 10.1002/bjs.11185 [DOI] [PubMed] [Google Scholar]
- 18.Vollmer CM Jr, Sanchez N, Gondek S, et al. ; Pancreatic Surgery Mortality Study Group . A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg. 2012;16(1):89-102. doi: 10.1007/s11605-011-1753-x [DOI] [PubMed] [Google Scholar]
- 19.Nathan H, Wolfgang CL, Edil BH, et al. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99(2):87-92. doi: 10.1002/jso.21189 [DOI] [PubMed] [Google Scholar]
- 20.Balcom JH IV, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136(4):391-398. doi: 10.1001/archsurg.136.4.391 [DOI] [PubMed] [Google Scholar]
- 21.Schmidt CM, Glant J, Winter JM, et al. Total pancreatectomy (R0 resection) improves survival over subtotal pancreatectomy in isolated neck margin positive pancreatic adenocarcinoma. Surgery. 2007;142(4):572-578. doi: 10.1016/j.surg.2007.07.016 [DOI] [PubMed] [Google Scholar]
- 22.Hata T, Motoi F, Ishida M, et al. Effect of hospital volume on surgical outcomes after pancreaticoduodenectomy: a systematic review and meta-analysis. Ann Surg. 2016;263(4):664-672. doi: 10.1097/SLA.0000000000001437 [DOI] [PubMed] [Google Scholar]
- 23.Besselink MG, van Rijssen LB, Bassi C, et al. ; International Study Group on Pancreatic Surgery . Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161(2):365-372. doi: 10.1016/j.surg.2016.06.058 [DOI] [PubMed] [Google Scholar]
- 24.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761-768. doi: 10.1016/j.surg.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 25.Mihaljevic AL, Hackert T, Loos M, et al. Not all Whipple procedures are equal: proposal for a classification of pancreatoduodenectomies. Surgery. 2021;169(6):1456-1462. doi: 10.1016/j.surg.2020.11.030 [DOI] [PubMed] [Google Scholar]
- 26.Hartwig W, Vollmer CM, Fingerhut A, et al. ; International Study Group on Pancreatic Surgery . Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery. 2014;156(1):1-14. doi: 10.1016/j.surg.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 27.Hartwig W, Gluth A, Hinz U, et al. Outcomes after extended pancreatectomy in patients with borderline resectable and locally advanced pancreatic cancer. Br J Surg. 2016;103(12):1683-1694. doi: 10.1002/bjs.10221 [DOI] [PubMed] [Google Scholar]
- 28.Krautz C, Nimptsch U, Weber GF, Mansky T, Grützmann R. Effect of hospital volume on in-hospital morbidity and mortality following pancreatic surgery in Germany. Ann Surg. 2018;267(3):411-417. doi: 10.1097/SLA.0000000000002248 [DOI] [PubMed] [Google Scholar]
- 29.Hunger R, Mantke R. Outcome quality beyond the mean—an analysis of 43,231 pancreatic surgical procedures related to hospital volume. Published online November 23, 2020. Ann Surg. doi: 10.1097/SLA.0000000000004315 [DOI] [PubMed] [Google Scholar]
- 30.Loos M, Kester T, Klaiber U, et al. Arterial resection in pancreatic cancer surgery: effective after a learning curve. Ann Surg. 2020. doi: 10.1097/SLA.0000000000004054 [DOI] [PubMed] [Google Scholar]
- 31.Tee MC, Krajewski AC, Groeschl RT, et al. Indications and perioperative outcomes for pancreatectomy with arterial resection. J Am Coll Surg. 2018;227(2):255-269. doi: 10.1016/j.jamcollsurg.2018.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Total Pancreatectomy With Multivisceral Resections
eTable 2. Prior Surgical Procedures and Postoperative Outcomes According to the Extent of Elective Completion Pancreatectomy