Abstract
Background:
The etiology of dementias and cognitive decline remain largely unknown. It is widely accepted that inflammation in the central nervous system plays a critical role in the pathogenesis of dementia. However, less is known about the role of the peripheral immune system and interactions with cortisol, though evidence suggests that these, too, may play a role.
Methods:
Using data from 1,337 participants aged 60+ years from the Sacramento Area Latino Study of Aging (observational cohort) we investigated variation in trajectories of cognitive decline by pathogen IgG and cytokine levels. Linear mixed effects models were used to examine the association between baseline Interleukin (IL)-6, C-reactive protein, tumor necrosis factor (TNF)-α, and five persistent pathogens’ IgG response and trajectories of cognition over 10 years, and to examine interactions between immune biomarkers and cortisol. Stratified cumulative incidence functions were used to assess the relation between biomarkers and incident dementia. Inverse probability weights accounted for loss-to-follow-up and confounding.
Results:
IL-6, TNF-α, and CMV IgG were statistically significantly associated with a higher log of Modified Mini-Mental State Examination errors (IL-6, β=0.0935 (95%CI: 0.055, 0.13), TNF-alpha β=0.0944 (95%CI: 0.032, 0.157), and CMV, β=0.0409 (95%CI: 0.013, 0.069)). Furthermore, cortisol interacted with HSV-1 and IL-6, and CRP for both cross-sectional cognitive function and rate of decline. No statistically significant relationship was detected between biomarkers and incidence of dementia.
Conclusions:
These findings support the theory that the peripheral immune system may play a role in cognitive decline but not incident dementia. Furthermore, they identify specific markers amenable for intervention for slowing decline.
Keywords: immune function, dementia, cognition, 3MSE, endocrine pathways
Introduction
Recent evidence has implicated senescing immune cells, inflammation, and chronic infection as potential risk factors for cognitive decline and incident dementia.(Jones et al., 2010; VanItallie, 2017) Cross-sectional and longitudinal studies have shown that higher levels of IL-6, TNF-alpha, and other cytokines are associated with lower cognitive level,(Jefferson et al., 2011; Xu et al., 2009) and higher dementia incidence.(Koyama et al., 2012; Xu et al., 2009) Furthermore, immune function has been implicated in the accumulation of amyloid-β, a key pathological feature of Alzheimer’s Disease and related dementias (ADRD), and associated with other neurodegenerative processes including microglial senescence.(Akiyama et al., 2000; Fakhoury, 2015; VanItallie, 2017) Resulting shifts in the T-cell compartment toward aged T-cell phenotypes (i.e. lower CD4+:CD8+ T-cell ratios) may also play a role.(Wikby et al., 2005) However several studies have reported no significant associations between immune markers and cognition, though comparisons between these studies are difficult given restricted populations (e.g. depressed or schizophrenic adults), limited biomarkers of immune function included, and different outcomes (e.g. incident AD, memory, etc.) examined across studies.(Elderkin-Thompson et al., 2012; Lima et al., 2013; Lurain et al., 2013; Sundelöf et al., 2009; Torniainen-Holm et al.; Yolken et al., 2011)
The stress-related elevation of cortisol and dysregulated cortisol rhythms have been implicated in numerous adverse outcomes including cardiovascular disease, Type 2 Diabetes, and ADRD.(Martocchia et al., 2016) Importantly, cortisol and immune cells are biologically connected. Cytokines can activate the HPA axis, inducing production of cortisol, but cortisol may also affect immune pathways by binding to glucocorticoid receptors on innate and adaptive cells.(Besedovsky and del Rey, 2000) Furthermore, chronic inflammation has been shown to induce glucocorticoid resistance.(Straub and Cutolo, 2016) Given the interplay documented between cortisol and immune molecules, it is critical to understand how altered cortisol levels and patterns may interact with immune markers to affect aging outcomes, particularly cognition and dementia incidence, in order to be able to intervene to prevent and delay outcomes. Yet, few studies have assessed these interactions.
This study examined the association of cytokines and pathogen IgG response with cognitive decline and dementia incidence in a Latinx population of older adults, and investigated whether salivary cortisol interacts with these biomarkers to alter the outcomes. Our study adds to the existing literature in several ways: first, we examine these associations in an understudied population for aging research; second, we include eight biomarkers of immunity, providing a more comprehensive view of immune function; third, we investigate both global cognition trajectories and incident dementia over the course of up to 10 years of follow-up; and finally, we use modern epidemiologic methods that allow us to better account for systematic biases in our observational cohort.
Methods
Study Population
This study used data from the Sacramento Area Latino Study of Aging (SALSA), a prospective, observational community-based cohort study of 1,789 self-designated Latina/Latino (designated as Latinx from here on out) adults in the Sacramento Valley, CA area who were aged 60 years or older at baseline. SALSA survey design and methods have been described previously.(Haan et al., 2003) Baseline 2-hour interviews occurred at the participants’ homes during 1998–1999. The interview included questions regarding lifestyle factors, depressive symptoms, acculturation, and medical diagnoses. Buccal swabs for DNA, blood pressure, and fasting blood samples were taken at baseline. Follow-up visits occurred every 12–15 months, with a total of 7 follow-up visits over 10 years, during which participants reported health conditions and lifestyle and sociodemographic risk factors and completed a cognitive evaluation. Our analysis used existing immune function biomarker data collected on a sub-sample of 1,574 participants at baseline. We further restricted our analysis to individuals with at least two cognitive data points and complete covariate data, for a final sample size of 1,337 (eFigure 1).
Measures
Exposures
Cytokines
Blood samples were collected from participants in their homes by trained phlebotomists and transferred on ice to be processed. Serum samples have since been stored continuously frozen at −70 ºC, and this storage protocol has been proven to have minimal effects on reliability of measurements conducted at later time points, with antibodies to viruses stable over time when continuously frozen.(Pappin et al., 1995) hs-CRP levels above 40 were excluded from analysis as high levels may indicate acute infection. For the purposes of statistical analysis, IL-6, hs-CRP, and TNF-alpha and were log transformed then modeled as continuous and dichotomized at the mean. Additional details for laboratory testing of cytokines and pathogens are provided in the Supplementary Material.
Pathogens
Baseline blood serum samples were analyzed for level of IgG antibodies to the cytomegalovirus (CMV), herpes simplex virus (HSV)-1, varicella zoster virus (VZV), toxoplasma gondii (T, gondii), and helicobacter pylori (H. pylori) using enzyme-linked immunosorbent assays (ELISA). Through a series of incubation and wash steps, the antibodies specific to our viruses of interest were linked to antibodies present in our samples and then to a secondary reactive antibody. These assays generated values on a continuous scale, reported as optical density (OD) units, that correspond to the level of IgG antibodies in the blood. In addition to the continuous measurement of IgG response, the laboratory determined whether each sample was seropositive or seronegative for each virus based on established cutoffs of IgG response. Each commercial ELISA kit contained controls and internal calibrations. For the purposes of analysis, all IgG titer variables were standardized to a mean of 0 and a SD of 1.
Salivary Cortisol
Salivary cortisol was measured in all included participants from buccal swabs collected as a waking sample at baseline. The salivary cortisol testing was collected with Salivettes (Sarstedt, Newton, NC) and assayed unextracted with DPC Coat a Count cortisol kits (DPC. Los Angeles, CA). Cortisol was log transformed for analysis.
Outcomes
Global Cognition
Global cognition was measured at baseline and each follow-up visit using the modified Mini-Mental State Examination (3MSE).34 The 3MSE tests a variety of cognitive abilities including recall, attention and calculation, registration, and orientation to time and place and is a common measure of cognitive ability in our populations. With 30 questions total, each varying in score value, the 3MSE has a final score range of 0 – 100, a sum of each individual question’s score.34,61 Due to the negatively skewed distribution, we modeled this outcome as the log of the errors on the test (i.e. log(101–3MSE)).
Clinician Adjudicated Dementia Diagnosis
Clinical diagnosis of dementia was a multistage process beginning if participants scored below the 20th percentile on the 3MSE or their score declined by >8 points since the previous exam. These participants were referred to take a neuropsychological test battery and were further referred to neuropsychologists if they received scores below the specified cutoffs. A neuropsychologist or neurologist then diagnosed type of dementia based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria.(Zeki Al Hazzouri et al., 2011)
Covariates
Sociodemographic data including years of educational attainment, sex (male/female), age (grand mean centered at baseline age, quadratic), self-reported health, and depressive symptoms (CES-D) were self-reported at baseline, and self-reported health and depressive symptoms were subsequently collected at each follow-up visit. Educational attainment was reported as the number of years attended and categorized as less than 8th grade, 8th grade diploma, or high school diploma and above for analysis, thus basing the measure on the highest degree obtained. Self-reported health was reported on a 5-point Likert scale from excellent to poor. The Center for Epidemiological Studies-Depression (CES-D) scale was used to categorize baseline depression among study participants with a cutoff at 16 points. Date of death was ascertained through mortality surveillance of the SALSA cohort.
Statistical Analyses
All statistical analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, North Carolina) and figures were created in R using the ggplot2 package. Descriptive statistics were used to characterize the study population. To estimate the relationship between biomarkers of immune function and cognitive level and decline, we used linear mixed effects models with a random intercept and slope. To assess the relationship between the exposures and incident dementia, we compared exposure-stratified cumulative incidence functions (using the Aalen-Johansen estimator) with death treated as a competing event. Inverse probability (IP) weights were used to account for informative censoring (variables included: exposure, educational attainment, age, diabetes, depression, self-rated health, and change in cognition between two prior waves) and confounding (variables included: age2, educational attainment, biological sex, depression, and self-reported health). IP weights addressed confounding by weighting the population to represent exchangeable (i.e., equivalent on confounders) exposed and unexposed populations. IP weights were also used to address loss-to-follow-up by weighting the subsequent waves’ populations to represent the initial population. This minimally sufficient adjustment set was determined a priori by directed acyclic graph (DAG) analysis. We further investigated potential biological interaction with salivary cortisol by including interaction terms between the exposure variables, salivary cortisol, and time in mixed effects models for cognitive decline. Separate models were used for each exposure measure: 1) crude models, 2) IP-weighted models, and 3) IP-weighted interaction models. Due to the sample size and limited power to detect interaction, we considered P < 0.10 statistically significant for interactions. All analyses for this study were approved by the institutional review board at the University of North Carolina at Chapel Hill.
Data Availability
Data for this study is publicly available on the Inter-University Consortium for Political and Social Research (ICPSR), hosted by the University of Michigan.
Results
Sample Characteristics
The 1,337 adults who met eligibility criteria and were included in our analysis were 58.9% female and were on average 70 years old at baseline, with an age range of 60 to 93 years old. Thirty-two and a half percent of participants received a high school diploma or higher education. Just under 46% reported their health as “very good” or “excellent”, and 25.0% reported depressive symptoms at baseline. The mean 3MSE score at baseline was 87 (SD = 10.3). 86.5% of the population was seropositive for HSV-1, while 84.5%, 29.3%, 91.1%, and 33.5% were seropositive for CMV, VZV, H. pylori, and T. gondii, respectively. The mean (SD) values for cytokines CRP, IL-6, and TNF-alpha were 5.14 (5.7), 5.17 (7.0), and 4.07 (2.5), respectively. The complete demographic and social characteristics of our study population are described in Table 1. Four hundred and thirty-one participants died over the course of follow-up.
Table 1.
Baseline Sociodemographic, Immune, and Cognitive Characteristics of Eligible SALSA Participants, N = 1,337
| Overall | ||
|---|---|---|
| N | % | |
|
|
||
| Age (mean, range) | 70 | 60, 93 |
| Sex (N, % Female) | 787 | 58.9 |
| Education | ||
| Less than 8th grade | 663 | 49.6 |
| 8th Grade diploma | 239 | 17.9 |
| High School Diploma and above | 435 | 32.5 |
| Self-rated Health | ||
| Poor | 97 | 7.3 |
| Fair | 185 | 13.8 |
| Good | 441 | 33.0 |
| Very Good | 496 | 37.1 |
| Excellent | 118 | 8.8 |
| Depressive Symptoms (N, % >=16) | 334 | 25.0 |
| 3MSE Score, Mean (SD) | 86.7 | (10.3) |
| HSV1 (% seropositive) | 946 | 86.5 |
| CMV | 924 | 84.5 |
| VZV | 320 | 29.3 |
| H. pylori | 996 | 91.1 |
| T. gondii | 366 | 33.5 |
| missing infection data | 244 | |
| CRP, Mean (SD) | 5.14 | (5.7) |
| missing | 24 | |
| IL-6, Mean (SD) | 5.17 | (7.0) |
| missing | 17 | |
| TNF-alpha, Mean (SD) | 4.07 | (2.5) |
| missing | 89 | |
SD = standard deviation
Population Mean Cognitive Function
Table 2 shows the association between continuous cytokine levels and population mean cognitive level over the course of follow-up before and after weighting. In IP-weighted models, we found that higher IL-6 and higher TNF-alpha were significantly associated with more errors on the 3MSE (i.e. lower cognitive scores). Each 1 unit increase in log(IL-6) was associated with an increase in the log of the errors of 0.0935 (95% CI: 0.055, 0.13). For each 1 unit increase in log(TNF-alpha), the log of the errors increased by 0.0944 (95% CI: 0.032, 0.157). Based on models including age as the only independent variable, these associations are roughly equivalent to cognitive differences of 3.75 years.
Table 2.
Linear Mixed Effects Model Estimates of Cytokines and Cognitive Decline in the SALSA, n=1,337
| Crude Models | IP Weighted Models | Cortisol Interaction Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | 95% | CI | Estimate | 95% | CI | Estimate | 95% | CI | interaction p-value | |
| Interleukin - 6 | Intercept | 1.88 | 1.83 | 1.94 | 1.94 | 1.88 | 2.00 | 2.05 | 1.73 | 2.37 | |
| IL-6 | 0.13 | 0.09 | 0.16 | 0.09 | 0.06 | 0.13 | −0.16 | −0.36 | 0.04 | ||
| IL-6*Age | 0.00 | −0.01 | 0.01 | 0.01 | 0.00 | 0.02 | 0.04 | −0.03 | 0.10 | ||
| Age | 0.01 | −0.01 | 0.02 | 0.01 | −0.01 | 0.02 | −0.09 | −0.19 | 0.01 | ||
| Age2 | 0.00 | 0.00 | 0.00 | ||||||||
| Cortisol | −0.04 | −0.17 | 0.08 | ||||||||
| Age*Cortisol | 0.04 | 0.00 | 0.08 | ||||||||
| IL-6*Cortisol | 0.10 | 0.03 | 0.18 | 0.0091 | |||||||
| IL-6*Age*Cortisol | −0.01 | −0.04 | 0.01 | 0.3503 | |||||||
| Tumor Necrosis Factor - alpha | Intercept | 1.84 | 1.76 | 1.92 | 1.95 | 1.86 | 2.03 | 1.74 | 1.33 | 2.16 | |
| TNF-alpha | 0.17 | 0.11 | 0.23 | 0.09 | 0.03 | 0.16 | 0.10 | −0.23 | 0.43 | ||
| TNF-alpha*Age | 0.00 | −0.02 | 0.01 | 0.01 | −0.01 | 0.03 | −0.07 | −0.17 | 0.04 | ||
| Age | 0.02 | −0.01 | 0.04 | 0.01 | −0.01 | 0.03 | 0.04 | −0.09 | 0.17 | ||
| Age2 | 0.00 | 0.00 | 0.00 | ||||||||
| Cortisol | 0.08 | −0.08 | 0.25 | ||||||||
| Cortisol*Age | −0.01 | −0.06 | 0.04 | ||||||||
| TNF-alpha*Cortisol | −0.01 | −0.14 | 0.12 | 0.9297 | |||||||
| TNF-alpha*Age*Cortisol | 0.03 | −0.01 | 0.07 | 0.1698 | |||||||
| C-reactive Protein | Intercept | 2.02 | 1.99 | 2.05 | 2.04 | 2.00 | 2.08 | 1.98 | 1.76 | 2.19 | |
| CRP | 0.02 | 0.00 | 0.05 | 0.02 | 0.00 | 0.04 | −0.16 | −0.28 | 0.04 | ||
| CRP*Age | 0.00 | −0.01 | 0.00 | 0.00 | −0.01 | 0.00 | −0.01 | −0.04 | 0.03 | ||
| Age | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 | 0.03 | −0.04 | −0.10 | 0.02 | ||
| Age2 | 0.00 | 0.00 | 0.00 | ||||||||
| Cortisol | 0.02 | −0.06 | 0.11 | ||||||||
| Age*Cortisol | 0.03 | 0.00 | 0.05 | ||||||||
| CRP*Cortisol | 0.07 | 0.02 | 0.12 | 0.0042 | |||||||
| CRP*Age*Cortisol | 0.00 | −0.01 | 0.01 | 0.9804 | |||||||
Table 3 shows the association between standardized IgG response to pathogens and population mean cognitive level over the course of follow-up before and after weighting. We found that higher CMV IgG was associated with more errors. Each 1 SD increase in CMV IgG was associated with a 0.0409 (95% CI: 0.013, 0.069) increase in the log errors. This pattern is borne out also when looking at the associations between CMV seropositivity and cognitive level (data in eTable 2).
Table 3.
Fixed Effects Estimates from Linear Mixed Effects Models of Pathogens and Cognitive Decline in the SALSA, n=1,093
| Crude Models | IP Weighted Models | Cortisol Interaction Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | 95% | CI | Estimate | 95% | CI | Estimate | 95% | CI | interaction p-value | |
| HSV-1 IgG | Intercept | 2.01 | 1.99 | 2.04 | 2.04 | 2.02 | 2.07 | 1.79 | 1.61 | 1.96 | |
| HSV-1 | −0.03 | −0.06 | −0.01 | −0.03 | −0.06 | 0.00 | 0.24 | 0.06 | 0.42 | ||
| HSV-1*Age | 0.00 | −0.01 | 0.01 | 0.00 | −0.01 | 0.01 | 0.05 | 0.00 | 0.11 | ||
| Age | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | −0.04 | −0.09 | 0.01 | ||
| Age2 | 0.00 | 0.00 | 0.00 | ||||||||
| Cortisol | 0.10 | 0.03 | 0.17 | ||||||||
| Age*Cortisol | 0.02 | 0.00 | 0.04 | ||||||||
| HSV-1*Cortisol | −0.11 | −0.18 | −0.04 | 0.0028 | |||||||
| HSV-1*Age*Cortisol | −0.02 | −0.04 | 0.00 | 0.046 | |||||||
| BIC | 13983.7 | 13135.5 | 13032.2 | ||||||||
| CMV IgG | Intercept | 2.02 | 1.99 | 2.05 | 2.04 | 2.01 | 2.06 | 1.74 | 1.57 | 1.92 | |
| CMV | 0.07 | 0.05 | 0.10 | 0.04 | 0.01 | 0.07 | 0.12 | −0.07 | 0.31 | ||
| CMV*Age | 0.00 | −0.01 | 0.01 | 0.00 | 0.00 | 0.01 | −0.04 | −0.10 | 0.01 | ||
| Age | 0.01 | 0.00 | 0.02 | 0.02 | 0.01 | 0.03 | −0.04 | −0.09 | 0.01 | ||
| Age2 | 0.00 | 0.00 | 0.00 | ||||||||
| Cortisol | 0.11 | 0.05 | 0.18 | ||||||||
| Age*Cortisol | 0.02 | 0.00 | 0.04 | ||||||||
| CMV*Cortisol | −0.03 | −0.10 | 0.04 | 0.42 | |||||||
| CMV*Age*Cortisol | 0.02 | 0.00 | 0.04 | 0.10 | |||||||
| BIC | 13959.3 | 13267.3 | 13172.2 | ||||||||
| VZV IgG | Intercept | 2.01 | 1.98 | 2.04 | 2.03 | 2.01 | 2.06 | 1.81 | 1.63 | 1.98 | |
| VZV | −0.05 | −0.08 | −0.03 | −0.02 | −0.05 | 0.01 | 0.02 | −0.16 | 0.20 | ||
| VZV*Age | 0.00 | −0.01 | 0.01 | 0.00 | −0.01 | 0.01 | 0.00 | −0.06 | 0.05 | ||
| Age | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | −0.04 | −0.09 | 0.01 | ||
| Age2 | 0.00 | 0.00 | 0.00 | ||||||||
| Cortisol | 0.09 | 0.02 | 0.16 | ||||||||
| Age*Cortisol | 0.02 | 0.00 | 0.04 | ||||||||
| VZV*Cortisol | −0.01 | −0.08 | 0.06 | 0.70 | |||||||
| VZV*Age*Cortisol | 0.00 | −0.02 | 0.02 | 0.82 | |||||||
| BIC | 13973.4 | 13237.6 | 13145.4 | ||||||||
| H. pylori IgG | Intercept | 2.01 | 1.99 | 2.04 | 2.04 | 2.02 | 2.07 | 1.74 | 1.57 | 1.92 | |
| H. pylori | 0.05 | 0.02 | 0.07 | 0.03 | 0.00 | 0.05 | 0.05 | −0.13 | 0.23 | ||
| H. pylori*Age | −0.01 | −0.02 | 0.00 | −0.01 | −0.02 | 0.00 | −0.04 | −0.10 | 0.02 | ||
| Age | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | −0.03 | −0.09 | 0.02 | ||
| Age2 | 0.00 | 0.00 | 0.00 | ||||||||
| Cortisol | 0.12 | 0.05 | 0.18 | ||||||||
| Age*Cortisol | 0.02 | 0.00 | 0.04 | ||||||||
| H. pylori*Cortisol | −0.01 | −0.08 | 0.06 | 0.82 | |||||||
| H. pylori*Age*Cortisol | 0.01 | −0.01 | 0.03 | 0.28 | |||||||
| BIC | 13972.8 | 13160.2 | 13059.6 | ||||||||
| T. gondii IgG | Intercept | 2.01 | 1.99 | 2.04 | 2.04 | 2.01 | 2.07 | 1.78 | 1.61 | 1.95 | |
| T. gondii | 0.05 | 0.02 | 0.08 | −0.01 | −0.04 | 0.01 | −0.01 | −0.18 | 0.15 | ||
| T. gondii*Age | 0.00 | −0.01 | 0.00 | 0.00 | −0.01 | 0.03 | 0.00 | −0.05 | 0.05 | ||
| Age | 0.01 | 0.01 | 0.02 | 0.02 | 0.01 | 0.03 | −0.04 | −0.09 | 0.02 | ||
| Age2 | 0.00 | 0.00 | 0.00 | ||||||||
| Cortisol | 0.10 | 0.03 | 0.17 | ||||||||
| Age*Cortisol | 0.02 | 0.00 | 0.04 | ||||||||
| T. gondii*Cortisol | 0.00 | −0.06 | 0.07 | 0.98 | |||||||
| T. gondii*Age*Cortisol | 0.00 | −0.02 | 0.02 | 0.94 | |||||||
| BIC | 13974.3 | 13198.3 | 13108.0 | ||||||||
Interestingly, we also found that higher HSV-1 IgG was associated with fewer errors on the 3MSE in IP-weighted models. Each 1 SD increase in HSV-1 IgG was associated with 0.0308 (95% CI: 0.004, 0.058) decrease in the log errors. This relationship, in the opposite direction of that hypothesized, is corroborated in results analyzing the association between HSV-1 seropositivity and cognitive function, seen in eTable 2). CRP and VZV, T. gondii, and H. pylori IgG levels were not significantly associated, in either direction, with number of errors on the 3MSE.
Cognitive Change over Time
Tables 2 and 3 also show the association between continuous cytokine and pathogen IgG levels and cognitive decline over the course of follow-up before and after weighting. H. pylori, however, was significantly associated with rate of cognitive change over follow-up; for each year of age, a 1 SD higher H. pylori IgG level was associated with 0.0086 (95% CI: 0.001, 0.016) fewer log errors. Full results can be seen in Table 3. However, neither IL-6 nor TNF-alpha were significantly associated with the rate of cognitive change over follow-up, nor was CRP. eTable 1 shows results for the association of categories of high or low cytokine levels with cognitive level and change over follow-up. Full results can be seen in Table 2. Neither HSV-1 nor CMV IgG levels were associated with rate of decline, nor were VZV or T. gondii.
Cortisol Interactions
Overall, higher cortisol levels were associated with lower cognitive function in this population. We found statistically significant interactions (p < 0.10) between cortisol and HSV-1, IL-6, and CRP for cognitive level as well as between cortisol and HSV-1 for rate of decline. These results are demonstrated visually in Figure 1, and results for all biomarkers are shown as ‘Cortisol Interaction Models’ in Tables 2 and 3. Those with higher cortisol (e.g. log cortisol values equal to 3), exhibited, on average, more errors on the 3MSE than those with lower cortisol. For younger age groups, this relationship did not appear to change substantially with increasing levels of biomarkers. However, at ages 80 and above, the predicted log(3MSE errors) were different by level of biomarker. In Figure 1a we see that as levels of IL-6 increase, 3MSE errors increase as well, though the change is steeper for those with lower cortisol. In Figure 1c we see that as age increases, the HSV-1 IgG and 3MSE error relationship becomes stronger, with those with high cortisol actually decreasing in errors while those with lower cortisol increasing. For IL-6, CRP, and HSV-1, the relationship between biomarker and cognition was similar by levels of cortisol for younger ages.
Figure 1.
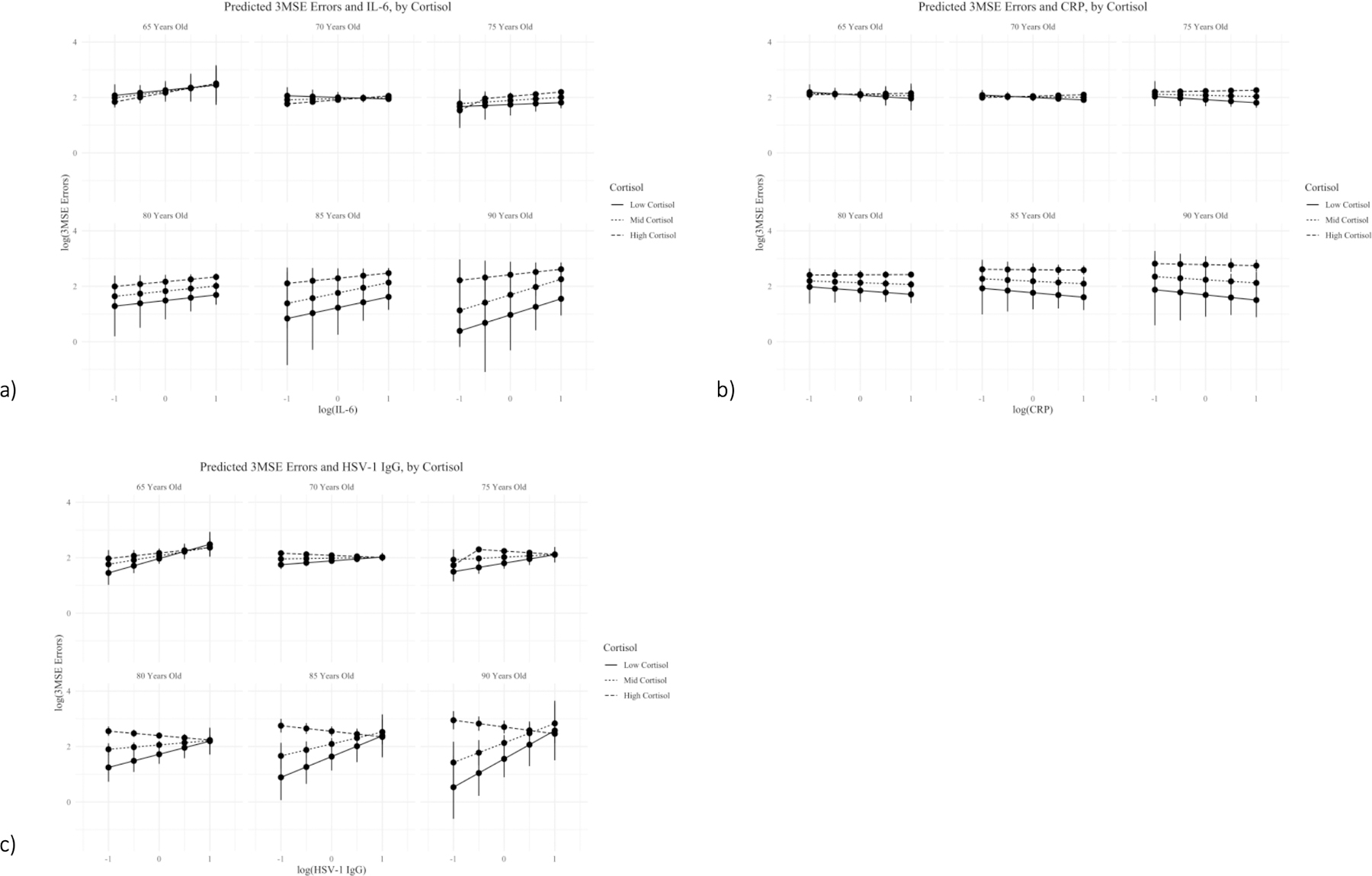
This figure demonstrates relationship between continuous level of immune biomarker and log of the errors on the Modified Mini-Mental State Examination (3MSE). Output are the predicted 3MSE errors for incremental increases in biomarkers, categorized by estimated cortisol levels and paneled by age. Panel a) shows the relationships for IL-6, Panel b) shows the relationships for CRP, and Panel c) shows the relationships for HSV-1 IgG. All predicted values come from final, IP-weighted cortisol interaction linear mixed effects regression models. Only those biomarkers for which there was a statistically significant interaction with cortisol are shown here.
Incidence of Dementia
IP-weighted cumulative incidence functions for the age range of the study population over follow-up show no statistically significant relationship between pathogen seropositivity and incidence of dementia. Figure 2b shows the full CIFs for each pathogen. There is some indication that H.pylori seropositivity may be associated with decreased incidence of dementia. However, due to the small number of incident dementia cases in this sample (n = 112 total but only 78 among those with infection data) and the high prevalence of the pathogens, particularly CMV and HSV-1, our analyses were underpowered to detect associations here. Similarly, there was no statistically significant associations between IL-6, TNF-alpha, or CRP and the incidence of dementia. The confidence intervals for each CIF substantially overlap indicating no significant association. Figure 2b shows the full CIFs for each cytokine.
Figure 2. Exposure-Stratified Cumulative Incidence Functions.
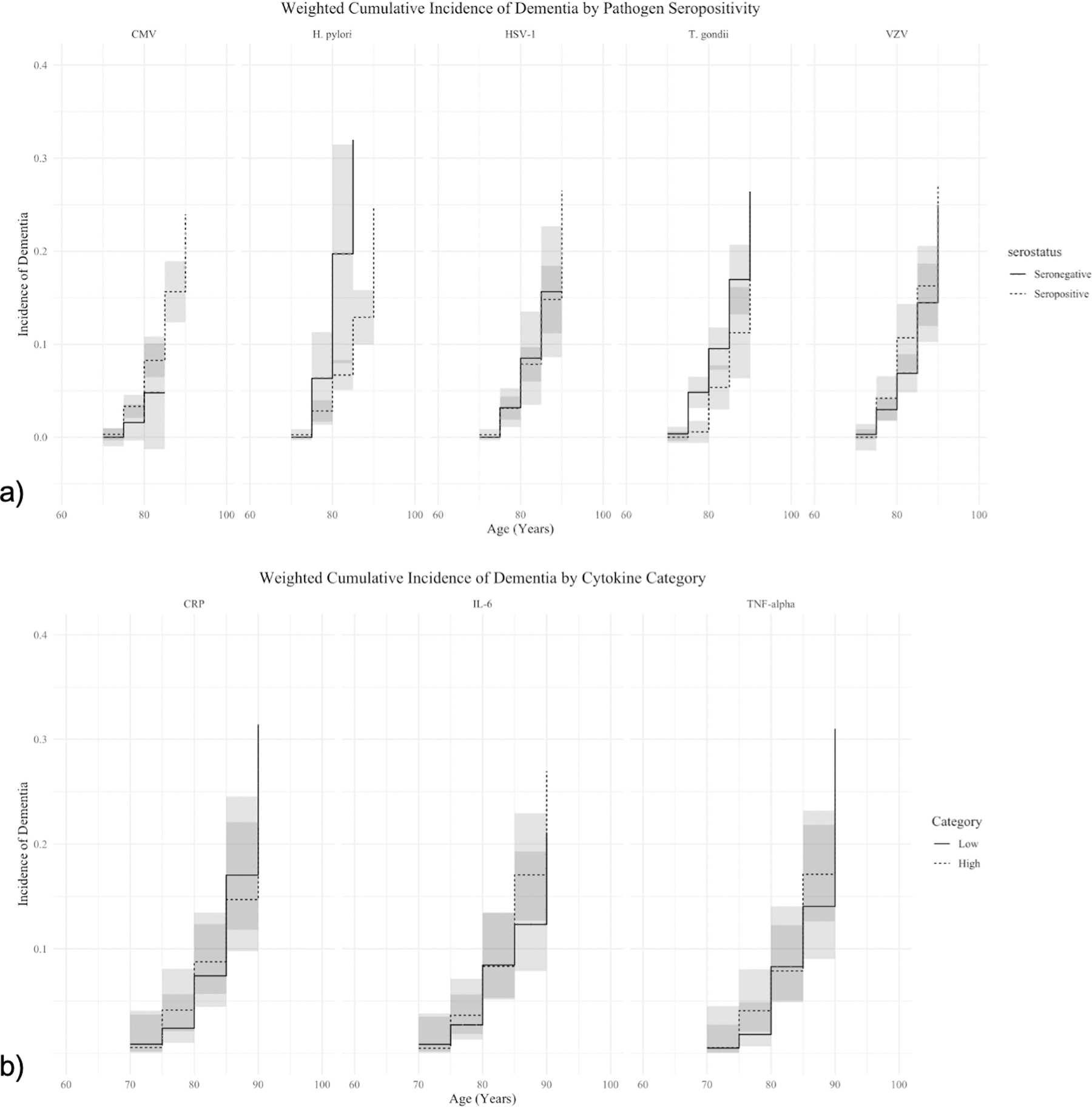
This figure demonstrates the relationship between category of immune biomarker and the incidence of dementias. Outputs are the IP-weighted, stratified cumulative incidence functions (95% CIs in grey) for each biomarker. Panel a) shows the relationships for pathogen seropositivity and Panel b) shows the relationships for high and low levels of cytokines. All values come from final, IP-weighted cumulative incidence models were estimated using the Aalen-Johansen estimator.
Discussion
We found that overall, IL-6, TNF-alpha, and CMV IgG levels were associated with poorer cognitive function in this study population. The magnitude of these associations was roughly equivalent to the change in cognitive function seen over 3.75 years of age in our study population for the cytokines and 1.5 years for CMV. Only H. pylori IgG was significantly associated with the rate of cognitive change over 10 years of follow-up, with a 1 SD increase H. pylori IgG associated with a small but statistically significant change in log errors (slope=0.0086 (95% CI: 0.001, 0.016). Moreover, increases in HSV-1 IgG, were associated with better cognitive function. Additionally, IL-6, TNF-alpha, and HSV-1 interacted with cortisol to alter the relationships with cognitive level and age. Overall, we found that higher cortisol levels were associated with lower cognitive function in this population and that cortisol interacted with HSV-1, IL-6, and CRP for cognitive level as well as with HSV-1 for rate of decline. For younger age groups, this relationship did not change much with increasing levels of biomarkers. However, at ages 80 and above, cognitive function is different by level of immune biomarker. As levels of IL-6 increase, cognitive function decreases, though more steeply for those with lower cortisol, and as age increases, the slope of the HSV-1 IgG and 3MSE error relationship becomes stronger, with those with high cortisol actually decreasing in errors while those with lower cortisol increasing. We did not find any statistically significant associations between peripheral immune system biomarkers and dementia incidence.
The findings of negative associations between biomarkers of immune function and cognition are consistent with the previous literature indicating associations between biomarkers of immune function and cognition. Several studies have shown that dementia patients have higher CRP levels in the blood compared to non-dementia patients, as well as similar associations between CRP and cognitive level.(Ge et al., 2013; Jefferson et al., 2011; Koyama et al., 2012; Mancinella et al., 2009; Wersching et al., 2010; Xu et al., 2009) However, both cross-sectional and longitudinal studies have shown mixed results, as do our analyses, with several reporting no such associations.(Chen et al., 2016; Elderkin-Thompson et al., 2012; Lima et al., 2013; Luciano et al., 2009; Sundelöf et al., 2009) These associations and mixed results have been seen with other inflammatory markers, including cytokines (i.e. IL-6 and TNF-α) (Duarte et al., 2017; Palta et al., 2014; Rizzi and Roriz‐Cruz, 2017; Singh-Manoux et al., 2014; Uslu et al., 2012) and CMV IgG response.(Kawasaki et al., 2016) Our results here are also mixed, with some cytokines and infection IgG responses being associated with worse cognition, but with some not being associated with cognition at all, and even one (HSV-1) associated with better cognition. However, as much of the literature in this area focuses on CRP only, it is important that we have investigated a more comprehensive set of indicators of peripheral immune function than previous research.
The research on the relationship between immune system aging and cognition is based on biological mechanisms that have been studied extensively in humans and animal models.(Fakhoury, 2015; Perry and Holmes, 2014; Rawji et al., 2016; Tuppo and Arias, 2005; VanItallie, 2017) As the human brain ages, the presence of misfolded proteins, free radicals, and epigenetic changes increase(Perry and Holmes, 2014; Rawji et al., 2016) and microglia become senescent, leading to an elevation in the production of pro-inflammatory cytokines, impaired phagocytosis, reduced motility, and reversing the demyelination of axons, which is a common change in neurodegenerative diseases.(Fakhoury, 2015; Rawji et al., 2016) The presence of misfolded proteins (such as amyloid-β) elevates production of pro-inflammatory cytokines in microglia,(Perry and Holmes, 2014; Rawji et al., 2016; VanItallie, 2017) but may additionally inhibit the ability of microglia to secrete anti-inflammatory cytokines.(Rawji et al., 2016) However, there is also evidence that an inflammatory response activates microglia and astrocytes, leading to increased production of amyloid-β, and as such, it is unclear which is the triggering event for neurodegeneration leading to AD.(Fakhoury, 2015; Tuppo and Arias, 2005)
Studies comparing the immune profiles of individuals with AD to those of healthy controls have found evidence of advanced peripheral immunosenescence in individuals with AD, though these studies are small and results have been conflicting.(Androsova et al., 1995; Schindowski et al., 2006; Zhang et al., 2003) While advanced peripheral immunosenescence could itself be integral in the pathogenesis of dementia, it would require accompanying perturbations in the blood brain barrier (BBB) to allow movement of immune molecules into the central nervous system (CNS). Disruptions in the BBB can lead to increased permeability allowing the transport of aged immune cells, inflammatory cytokines and chemokines, and infectious agents, all of which are critical to the disease process.
As cytokines have neuromodulatory properties including roles in neurogenesis and neuronal survival,(Vezzani and Viviani, 2015) the imbalance of pro- and anti-inflammatory cytokines resulting from senescent microglia can lead to physiological changes in the brain. The overproduction and extended presence of pro-inflammatory cytokines in the brain can further damage the BBB,(Vezzani and Viviani, 2015) which deteriorates naturally with increasing age.(VanItallie, 2017) Leakage in the BBB has been observed early on in cases of AD(VanItallie, 2017; Zlokovic, 2008) and allows the passage of peripheral immune cells and inflammatory mediators into the central nervous system.(Fakhoury, 2015) While the BBB naturally deteriorates with age,(VanItallie, 2017) environmental stimuli can also accelerate this process. However, it could be that the natural deterioration of the BBB that is in seen with age is in part driven by both peripheral and central immunosenescence. For example, as cytokines have neuromodulatory properties including roles in neurogenesis and neuronal survival,(Marin and Kipnis, 2013; Vezzani and Viviani, 2015) the imbalance of pro- and anti-inflammatory cytokines resulting from senescent microglia can lead to physiological changes in the brain. The overproduction and extended presence of pro-inflammatory cytokines in the brain can further damage the BBB.(Vezzani and Viviani, 2015) Specifically, IL-β activates IL-1 receptor 1 (IL-1R1), which can induce the deterioration of membrane phospholipids with as few as 2–3% of the receptors occupied.(Dinarello, 1996; Vezzani and Viviani, 2015) The imbalance of cytokines can alter neurotransmitter release, astrocyte function, and the activity of voltage-gated ion channels and receptor-coupled ion channels, which has been shown to have long-term effects on cognition.(Vezzani and Viviani, 2015) Cortisol binding to immune cells may alter this relationship, as demonstrated in our analyses. Our results provide further evidence that measurements of peripheral immune function, including cytokines and persistent pathogen IgG response, may lead to and therefore be indicative of decreased cognitive function and/or accelerated cognitive decline in populations over 65 years old.
Our study had several strengths, including the participants, representative of the Sacramento, CA area LatinX population, and longitudinal data covering up to 10 years of follow-up for participants. The availability of baseline IgG response to five common persistent pathogens as well as three cytokines allowed us to examine a better picture of participants’ immune function, as opposed to a single marker. Additionally, the use of markers of both the adaptive and innate immune system captures a wider spectrum of immune function in the participants.
However, our study does have limitations. We expect measurement error in the cognitive measures, both measured cognitive level and incidence of dementia. Also, the low incidence of dementia limits our power to detect differences by biomarker. The 3MSE collected over the course of follow-up has limited sensitivity to detect differing levels of cognitive function among study participants or decline of function in all cognitive domains within an individual, though it is widely used in large cohort studies. The 3MSE is also subject to retest effects whereby the participant may become familiar with the test and actually appear to improve over subsequent administrations, or remain constant over subsequent administrations, rather than decline in performance, as would be expected over time with cognition. A final measurement error limitation is in our use of biomarkers in this study. Biomarkers are subject to imperfect sensitivity and specificity as well as further error induced by the natural fluctuation of these biological markers in humans, which can depend on time of day of measurement,(Bauer et al., 1994) recent exercise,(Pedersen, 2000) and diet.(Aziz et al., 1999) All biomarkers gathered in SALSA were taken at a fasting time period and cortisol measurements were all taken as a waking sample, offsetting concerns about consistency in the timing of these measures and fasting influences. However, the magnitude of cortisol change over the course of the day may be a better indication of the physiologic embodiment of stress. SALSA does not information on particpants’ cortisol levels throughout the day, so we were unable to use that measure. The small magnitude of the associations may be a result of survival bias in our dataset. It is possible that those who were the least healthy and had higher cytokine levels and higher pathogen IgG levels did not survive to join the study. Finally, there is potentially bias in our results due to unmeasured confounding.
Functional and cognitive aging present a unique health care challenge for the country, given that an estimated 5.2 million people over 65 years in the U.S. had an ADRD as of 2016.(Hebert et al., 2013) In 2016 alone, the U.S. spent $162.7 billion on long-term healthcare, which is approximately 5% of all U.S. healthcare costs.(Mongan, 2017) This burden will only continue to grow through the next several decades, including in the Hispanic population, with an estimated 14 million cases of AD projected in the U.S. by 2050,(Hebert et al., 2013) underscoring the importance of long term, preventive measures. Besides introducing a new burden on the healthcare system simply by requiring extensive care for routine activities, those suffering from dementia are at an elevated risk of several other negative health outcomes due to their increased risk of exposure to infections and diminished ability to care for themselves, contributing significantly to healthcare costs.(Frahm‐Falkenberg et al., 2016) Those suffering from dementia and other memory disorders are more likely to have comorbidities such as diabetes, infection, injury, and depression.(Bunn et al., 2014) All of these secondary health conditions that occur partially as a result of the cognitive and functional impairment of older adults exert an additional burden on the healthcare system. As such, it is imperative that researchers investigate novel determinants of dementias. It will continue to be important to examine factors that either accelerate cognitive decline, increase risk of dementia, or both, in order to identify potential targets for intervention and prevention. Of note, Mexican Americans experience a disproportionate prevalence of these persistent infections than the white non-Latinx US population,(Stebbins et al., 2019) which may enhance associations we observed, compared to a nation-wide samples.
Thus, our results have implications for the prevention and delay of cognitive decline, but not the incidence of dementia, in older populations. Currently, there are documented disparities in the distribution of AD and other dementias across the US, including by sex, race and ethnicity, income, and educational attainment.(Plassman et al., 2007) All of these factors are deeply intertwined and difficult to disentangle. However, mechanisms associated with immune function could present a target for intervention, to delay the onset of cognitive impairment and slow decline. As dementia is a progressive disease, prevention prior to onset is essential to reducing the subsequent health care burdens. Understanding the mechanisms through which these social and environmental factors may interact to affect cognitive decline and onset of dementia will move the field forward and allow us to design targeted interventions to reduce disparities in and the overall incidence of dementia.
Supplementary Material
Highlights.
Investigated immune biomarkers & cognition in a 10-year longitudinal study of older Latinos in Sacramento County, CA.
Higher IL-6, TNF-α, and CMV IgG associated with lower cognition, using linear-mixed effects models with inverse probability weights to account for confounding and informative censoring..
Cortisol interacted with HSV-1 and IL-6, and CRP for cross-sectional cognitive function or rate of decline.
No statistically significant relationship was detected between biomarkers and incidence of dementia.
Funding:
This work was supported by the Infectious Disease Society of America Grant: The Role of Dementia-Associated Pathogen Burden in the Development of Alzheimer’s Disease (AD) and Other Dementias, and NIH grants K99AG062749 to GN and R01AG012975.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT Author Statement:
Rebecca Stebbins: conceptualization, methodology, software, formal analysis, writing – original draft, visualization. Jessie Edwards: methodology, software, writing – review & editing. Brenda Plassman: methodology, writing – review & editing. Yang Claire Yang: methodology, formal analysis, writing – review & editing. Grace Noppert: conceptualization, writing – review & editing. Mary Haan: investigation, writing – review & editing, funding acquisition. Allison Aiello: conceptualization, supervision, writing – review & editing, funding acquisition, visualization.
Declarations of Interest: none.
Conflict of Interest: none declared.
Contributor Information
Rebecca C. Stebbins, Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Jessie K. Edwards, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC
Brenda L. Plassman, Departments of Psychiatry and Neurology, Duke University School of Medicine, Durham, NC
Y. Claire Yang, Department of Sociology, Lineberger Cancer Center University of North Carolina at Chapel Hill; Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, NC
Grace A. Noppert, Social Environment and Health, Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, MI
Mary Haan, Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, CA
Allison E. Aiello, Department of Epidemiology, University of North Carolina at Chapel Hill; Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, NC
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, 2000. Inflammation and Alzheimer’s disease. Neurobiology of aging 21, 383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androsova LV, Sekirina TP, Selezneva ND, Koliaskina GI, Gavrilova SI, 1995. [Changes in the immunological parameters in Alzheimer’s disease: their relation to disease severity]. Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova 95, 24–27. [PubMed] [Google Scholar]
- Aziz N, Nishanian P, Mitsuyasu R, Detels R, Fahey JL, 1999. Variables that affect assays for plasma cytokines and soluble activation markers. Clinical and diagnostic laboratory immunology 6, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Hohagen F, Ebert T, Timmer J, Ganter U, Krieger S, Lis S, Postler E, Voderholzer U, Berger M, 1994. Interleukin-6 serum levels in healthy persons correspond to the sleep-wake cycle. Journal of Molecular Medicine 72, 315–315. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A, 2000. The cytokine-HPA axis feed-back circuit. Zeitschrift fur Rheumatologie 59 Suppl 2, Ii/26–30. [DOI] [PubMed] [Google Scholar]
- Bunn F, Burn AM, Goodman C, Rait G, Norton S, Robinson L, Schoeman J, Brayne C, 2014. Comorbidity and dementia: a scoping review of the literature. BMC medicine 12, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Oakley AE, Monteiro M, Tuomela K, Allan LM, Mukaetova-Ladinska EB, O’Brien JT, Kalaria RN, 2016. Multiplex analyte assays to characterize different dementias: brain inflammatory cytokines in poststroke and other dementias. Neurobiology of aging 38, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, 1996. Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147. [PubMed] [Google Scholar]
- Duarte P.d.O., Duarte MG, Pelichek A, Pfrimer K, Ferriolli E, Moriguti JC, Lima NK, 2017. Cardiovascular risk factors and inflammatory activity among centenarians with and without dementia. Aging clinical and experimental research 29, 411–417. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Irwin MR, Hellemann G, Kumar A, 2012. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. The American Journal of Geriatric Psychiatry 20, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhoury M, 2015. Role of immunity and inflammation in the pathophysiology of neurodegenerative diseases. Neurodegenerative Diseases 15, 63–69. [DOI] [PubMed] [Google Scholar]
- Frahm‐Falkenberg S, Ibsen R, Kjellberg J, Jennum P, 2016. Health, social and economic consequences of dementias: a comparative national cohort study. European journal of neurology 23, 1400–1407. [DOI] [PubMed] [Google Scholar]
- Ge X, Xu X. y., Feng C. h., Wang Y, Li Y. l., Feng B, 2013. Relationships among serum C-reactive protein, receptor for advanced glycation products, metabolic dysfunction, and cognitive impairments. BMC neurology 13, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ, 2003. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc 51, 169–177. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA, 2013. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Massaro JM, Beiser AS, Seshadri S, Larson MG, Wolf PA, Au R, Benjamin EJ, 2011. Inflammatory markers and neuropsychological functioning: the Framingham Heart Study. Neuroepidemiology 37, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Holmans PA, Hamshere ML, Harold D, Moskvina V, Ivanov D, Pocklington A, Abraham R, Hollingworth P, Sims R, 2010. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PloS one 5, e13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M, Arai Y, Takayama M, Hirata T, Takayama M, Abe Y, Niimura H, Mimura M, Takebayashi T, Hirose N, 2016. Carotid atherosclerosis, cytomegalovirus infection, and cognitive decline in the very old: a community-based prospective cohort study. Age 38, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A, O’Brien J, Weuve J, Blacker D, Metti AL, Yaffe K, 2012. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: a meta-analysis. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 68, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima TA, Adler AL, Minett T, Matthews FE, Brayne C, Marioni RE, Function MRCC, Study A, 2013. C-reactive protein, APOE genotype and longitudinal cognitive change in an older population. Age and ageing 43, 289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Marioni RE, Gow AJ, Starr JM, Deary IJ, 2009. Reverse causation in the association between C-reactive protein and fibrinogen levels and cognitive abilities in an aging sample. Psychosomatic medicine 71, 404–409. [DOI] [PubMed] [Google Scholar]
- Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, Bennett DA, Schneider JA, 2013. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis 208, 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinella A, Mancinella M, Carpinteri G, Bellomo A, Fossati C, Gianturco V, Iori A, Ettorre E, Troisi G, Marigliano V, 2009. Is there a relationship between high C-reactive protein (CRP) levels and dementia? Archives of gerontology and geriatrics 49, 185–194. [DOI] [PubMed] [Google Scholar]
- Marin I, Kipnis J, 2013. Learning and memory… and the immune system. Learning & memory 20, 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martocchia A, Stefanelli M, Falaschi GM, Toussan L, Ferri C, Falaschi P, 2016. Recent advances in the role of cortisol and metabolic syndrome in age-related degenerative diseases. Aging clinical and experimental research 28, 17–23. [DOI] [PubMed] [Google Scholar]
- Mongan E, 2017. National long-term care spending hits all-time high at $163 billion, McKnight’s Long-Term Care News [Google Scholar]
- Palta P, Xue Q-L, Deal JA, Fried LP, Walston JD, Carlson MC, 2014. Interleukin-6 and C-reactive protein levels and 9-year cognitive decline in community-dwelling older women: the women’s health and aging study II. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 70, 873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappin A, Grissom M, Mackay W, Huang Y, Yomtovian R, 1995. Stability of cytomegalovirus antibodies in plasma during prolonged storage of blood components. Clinical and diagnostic laboratory immunology 2, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, 2000. Exercise and cytokines. Immunology and cell biology 78, 532–535. [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C, 2014. Microglial priming in neurodegenerative disease. Nature Reviews Neurology 10, 217. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, 2007. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW, 2016. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain 139, 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi L, Roriz‐Cruz M, 2017. Cerebrospinal fluid inflammatory markers in amnestic mild cognitive impairment. Geriatrics & gerontology international 17, 239–245. [DOI] [PubMed] [Google Scholar]
- Schindowski K, Peters J, Gorriz C, Schramm U, Weinandi T, Leutner S, Maurer K, Frolich L, Muller WE, Eckert A, 2006. Apoptosis of CD4+ T and natural killer cells in Alzheimer’s disease. Pharmacopsychiatry 39, 220–228. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, Kivimaki M, 2014. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 83, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins RC, Noppert GA, Aiello AE, Cordoba E, Ward JB, Feinstein L, 2019. Persistent socioeconomic and racial and ethnic disparities in pathogen burden in the United States, 1999–2014. Epidemiology and Infection 147, e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Cutolo M, 2016. Glucocorticoids and chronic inflammation. Rheumatology 55, ii6–ii14. [DOI] [PubMed] [Google Scholar]
- Sundelöf J, Kilander L, Helmersson J, Larsson A, Rönnemaa E, Degerman-Gunnarsson M, Basun H, Lannfelt L, Basu S, 2009. Systemic inflammation and the risk of Alzheimer’s disease and dementia: a prospective population-based study. Journal of Alzheimer’s Disease 18, 79–87. [DOI] [PubMed] [Google Scholar]
- Torniainen-Holm M, Suvisaari J, Lindgren M, Harkanen T, Dickerson F, Yolken RH, 2018. Association of cytomegalovirus and Epstein-Barr virus with cognitive functioning and risk of dementia in the general population: 11-year follow-up study. Brain Behav Immun 69, 480–485. [DOI] [PubMed] [Google Scholar]
- Tuppo EE, Arias HR, 2005. The role of inflammation in Alzheimer’s disease. The international journal of biochemistry & cell biology 37, 289–305. [DOI] [PubMed] [Google Scholar]
- Uslu S, Akarkarasu ZE, Ozbabalik D, Ozkan S, Çolak O, Demirkan ES, Ozkiris A, Demirustu C, Alatas O, 2012. Levels of amyloid beta-42, interleukin-6 and tumor necrosis factor-alpha in Alzheimer’s disease and vascular dementia. Neurochemical research 37, 1554–1559. [DOI] [PubMed] [Google Scholar]
- VanItallie TB, 2017. Alzheimer’s disease: innate immunity gone awry? Metabolism 69, S41–S49. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Viviani B, 2015. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, 70–82. [DOI] [PubMed] [Google Scholar]
- Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, Conty M, Minnerup J, Ringelstein E, Berger K, 2010. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 74, 1022–1029. [DOI] [PubMed] [Google Scholar]
- Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Lofgren S, Nilsson BO, Ernerudh J, Pawelec G, Johansson B, 2005. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. The journals of gerontology. Series A, Biological sciences and medical sciences 60, 556–565. [DOI] [PubMed] [Google Scholar]
- Xu G, Zhou Z, Zhu W, Fan X, Liu X, 2009. Plasma C-reactive protein is related to cognitive deterioration and dementia in patients with mild cognitive impairment. Journal of the neurological sciences 284, 77–80. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB, 2011. Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophrenia research 128, 61–65. [DOI] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A, Haan MN, Kalbfleisch JD, Galea S, Lisabeth LD, Aiello AE, 2011. Life-course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: results from the Sacramento area Latino study on aging. Am J Epidemiol 173, 1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kong Q, Zhang Z, Ge P, Ba D, He W, 2003. Telomere dysfunction of lymphocytes in patients with Alzheimer disease. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology 16, 170–176. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, 2008. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study is publicly available on the Inter-University Consortium for Political and Social Research (ICPSR), hosted by the University of Michigan.


