Abstract
PURPOSE:
Systemic therapy use in the last 30 days of life (DOL) for patients with advanced cancer is a low-value medical practice. We hypothesized that systemic therapy use in the last 30 DOL increased after approval of antiprogrammed cell death protein 1 immune checkpoint inhibitors (ICIs) and has contributed to increased health care utilization and spending.
METHODS:
We investigated the change in prevalence of any systemic therapy use in the last 30 DOL among patients with advanced solid tumors in the 4 years before and after antiprogrammed cell death protein 1 ICI approval in 2014. We used cases from the Western Washington Cancer Surveillance System linked to commercial and Medicare insurance. We calculated the difference in prevalence between the pre- and post-ICI periods. We also calculated the annual prevalence of any systemic therapy and ICI use in the last 30 DOL and measured health care utilization (emergency department visits and hospitalizations) and costs during the last 30 DOL.
RESULTS:
Eight thousand eight hundred seventy-one patients (median age 73 years) were included; 34% and 66% in the pre-and post-ICI period, respectively. Systemic therapy use in the last 30 DOL was lower in the post-ICI versus pre-ICI period (12.4% v 14.4%; difference −2.0% [95% CI, −3.5 to −0.5]). The annual prevalence of systemic therapy use in the last 30 DOL also declined, although ICI use rose. Patients treated with ICIs in last 30 DOL had more emergency department visits, hospitalizations, and higher costs.
CONCLUSION:
Systemic therapy use in the last 30 DOL was lower in the period after ICI approval. However, ICI use rose over time and had higher utilization and costs in the last 30 DOL. Systemic therapy use in the last 30 DOL warrants monitoring, especially as more ICI indications are approved.
INTRODUCTION
The use of systemic chemotherapy in patients with advanced cancer and poor prognosis approaching the end of life (EOL) has been associated with significant toxicity and worse quality of life compared with supportive care.1,2 Therefore, this practice has been discouraged by ASCO and is a metric of low-value care by Choosing Wisely.3 The EOL treatment landscape, however, may be changing with the emergence of immune checkpoint inhibitor (ICI) therapy in oncology. Medications from this class have been approved by the US Food and Drug Administration (FDA) for a wide variety of solid tumors and hematologic malignancies. Although the first agent (ipilimumab, targeting cytotoxic T-lymphocyte–associated protein 4) was approved in 2011, more widespread use came after the FDA approval of agents targeting programmed cell death protein 1 (PD-1) or its ligand (PD-L1), with the first FDA approval for melanoma on September 4, 2014.4
The promising aspects of these drugs, however, may also contribute to their potential use near EOL. For example, the favorable toxicity profile of ICIs relative to conventional cytotoxic chemotherapy may enable use in patients with advanced disease, older age, severe comorbidities, and poor performance status, who might not have been considered optimal chemotherapy candidates.5,6 Furthermore, the potential for durable response may contribute to patients’ and providers’ overestimation of the benefit of ICIs relative to actual benefit observed in clinical trials.7,8 However, ICI use near the EOL may be associated with high cost and more aggressive interventions, like emergency department (ED) visits and inpatient hospitalizations.6 More frequent use of subacute rehabilitation, delays in hospice referrals, and an increase in in-hospital deaths have also been reported.6,9,10 Immune-related toxicities associated with ICIs are also likely more common outside of clinical trials11 and can lead to unnecessary medical and financial burdens for patients and the health care system.
We hypothesized that because of availability of ICIs, systemic therapy use in the last 30 days of life (DOL) would have increased since anti-PD-1 approval in 2014. To test this hypothesis, we conducted a study to understand the change in prevalence of any systemic therapy use in the last 30 DOL before and after anti-PD-1 ICI approval in 2014. We also investigated the populations treated with ICIs and estimated costs associated with ICI use in the last 30 DOL.
METHODS
Study Design and Population
The primary objective was to compare the prevalence of any systemic therapy use in the last 30 DOL before and after ICI drug approval. For our primary analysis, we included all patients (rather than only those with a prior ICI indication) as we suspected there would be high off-label use. Previous studies have reported off-label use of ICIs to range from 18% to 30%.12,13 In addition, in a preliminary analysis from our data, more than 30% of patients who died before 2017 and received an ICI in the last 30 DOL did not have an approved ICI indication. Given this high rate of off-label use, we not only included all patients in our primary analysis but also performed a sensitivity analyses to focus on the populations with early approvals.
We conducted a cross-sectional study using the Hutchinson Institute for Cancer Outcomes Research (HICOR) database14 that links a population-based cancer registry to health insurance claims for multiple regional and national insurers (Medicare, Premera Blue Cross, Regence BlueShield, and Uniform Medical Plan). This database draws cancer diagnosis information from the Fred Hutchinson Cancer Research Center's Cancer Surveillance System (CSS), which collects information on cancer staging, initial treatment, and survival for all persons diagnosed with cancer, excluding nonmelanoma skin cancer, who are residents of 13 counties of western Washington state when diagnosed.15 Among the 234,143 cases in CSS between 2011 and 2018, 85% have a linked file in the HICOR database.
We included adult cancer cases in the HICOR database with American Joint Committee on Cancer (AJCC) stage III or IV or unknown staged solid tumor who died between 2011 and 2018 and had 6 months of continuous insurance enrollment before death. We excluded patients with more than one tumor and those with incomplete treatment information (eg, diagnosis made by autopsy or death certificate or lack of outpatient pharmacy plan enrollment). The study had institutional review board (IRB) approval by the Fred Hutchinson Cancer Research Center IRB (#8135).
Determination of Systemic Therapy, ICI Use, and Costs
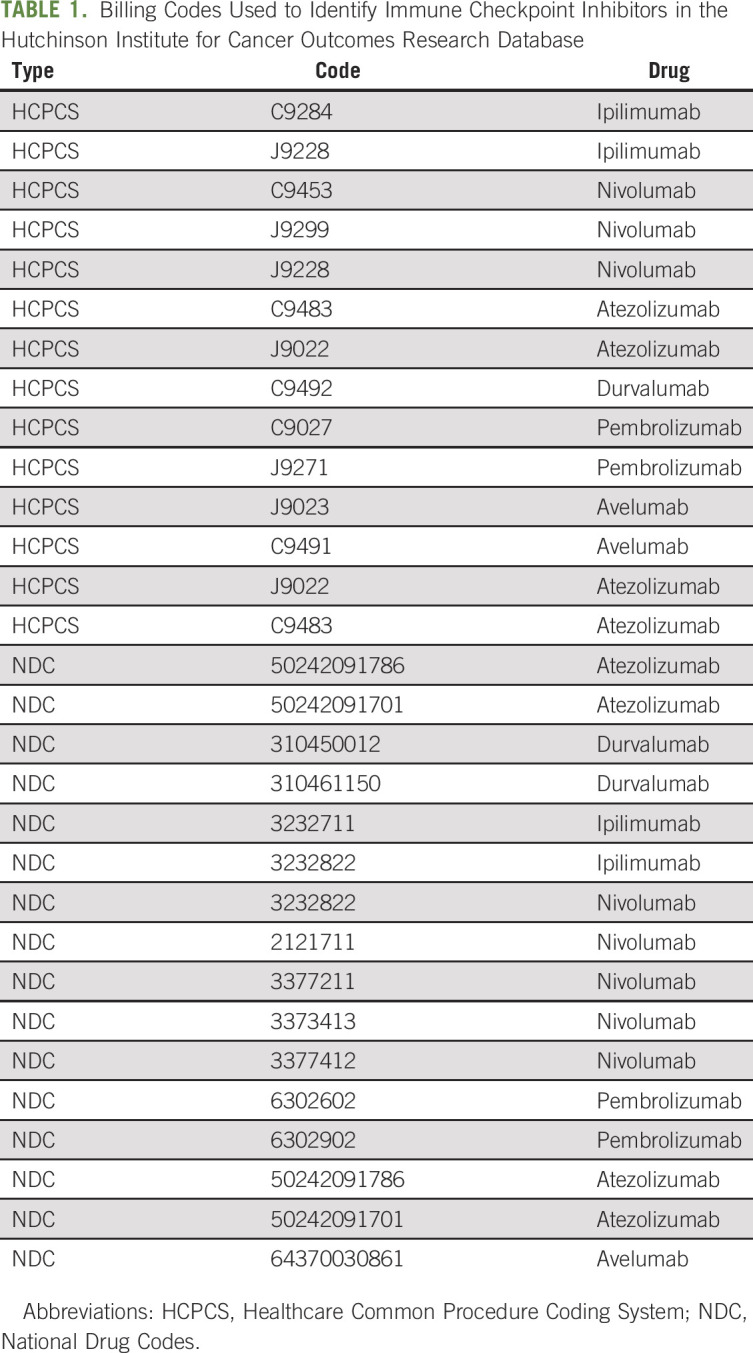
Systemic therapy, ICI use, and costs were assessed using insurance claims. Systemic therapy was identified as any claim of any anticancer therapy (including oral medications, intramuscular, subcutaneous, and IV infusions). ICI use, including ipilimumab and anti-PD(L)-1 agents, was identified on the basis of Healthcare Common Procedure Coding System and National Drug Codes listed in Table 1. We also identified infusion billing codes without an associated medication and calculated prevalence in the pre- and post-ICI periods as a potential surrogate for off-label or compassionate care use not captured by insurance claims.
TABLE 1.
Billing Codes Used to Identify Immune Checkpoint Inhibitors in the Hutchinson Institute for Cancer Outcomes Research Database
Statistical Analysis
Baseline information included demographic characteristics (age at death, sex, and race), cancer type, AJCC staging, Charlson Comorbidity Index, and insurance type. The Charlson Comorbidity Index16 was first developed as a weighted index to predict risk of death within 1 year of hospitalization for patients with specific comorbid conditions; we identified the individual Charlson comorbidities using claims in the 6-month period before death and then categorized patients into three groups on the basis of the number of noncancer comorbidities (0, 1 and ≥ 2). Descriptive statistics were used to summarize the baseline information in the two exposure groups (pre- and post-ICI).
We hypothesized that systemic therapy in the last 30 DOL would be higher after the approval of anti-PD(L)1 ICIs. The first anti-PD(L)1 ICI was approved on September 4, 2014 (pembrolizumab for melanoma), so we categorized cases as pre-ICI approval if the date of death occurred between January 1, 2011, and September 4, 2014, and post-ICI if the date of death occurred between September 5, 2014, and December 31, 2018. We estimated the prevalence of systemic therapy use in the last 30 DOL in the pre- and post-ICI groups, regardless of whether or not patients had a diagnosis for which there was an FDA-approved ICI indication. We then calculated the difference and corresponding CI between the two time periods and also fit a multivariable model using Poisson regression to estimate the prevalence ratio (PR) of systemic therapy use in the last 30 DOL adjusted for covariates.17 Covariates considered for the multivariable model included demographic characteristics (age, sex, and race), Charlson Comorbidity Index, and insurance type. Each covariate was included individually in the regression model and retained in the final model if the covariate resulted in 10% or greater change in the adjusted PR.
We also estimated the annual (year-by-year) prevalence of systemic therapy and ICI use in the last 30 DOL and calculated the proportion of last 30 DOL systemic therapy that was an ICI.
We conducted a sensitivity analysis estimating prevalence of systemic therapy use in the last 30 DOL before and after 2014 for all cancer types with an early ICI indication and in each specific cancer subgroup with an early ICI indication. We defined early ICI indication as a cancer type with an FDA-approved ICI indication before December 31, 2017. Five cancer types met this definition: melanoma, non–small-cell lung cancer (NSCLC), renal cell carcinoma, urothelial carcinoma, and head and neck squamous cell carcinoma. We identified each of the five cancer subgroups using International Classification of Diseases-O-3 site and histology codes from CSS. For the analyses by specific cancer type, the baseline and exposure periods were adjusted on the basis of the time of FDA approval for the specific cancer.
In addition to above, we compared baseline characteristics and health care utilization and direct medical costs in the last 30 DOL between patients who were treated with ICI and non-ICI systemic therapy in the last 30 DOL. For health care utilization, we calculated proportion of patients with 0, 1, and ≥ 2 ED visits and inpatient hospital admissions in the last 30 DOL. For medical costs, we used diagnosis codes and procedure codes to identify all medical services used in the last 30 DOL and calculated each individual patient's direct medical costs by summing all paid claims. We also calculated drug costs, which were the subset of direct medical costs that were directly drug related.
RESULTS
Patient Population
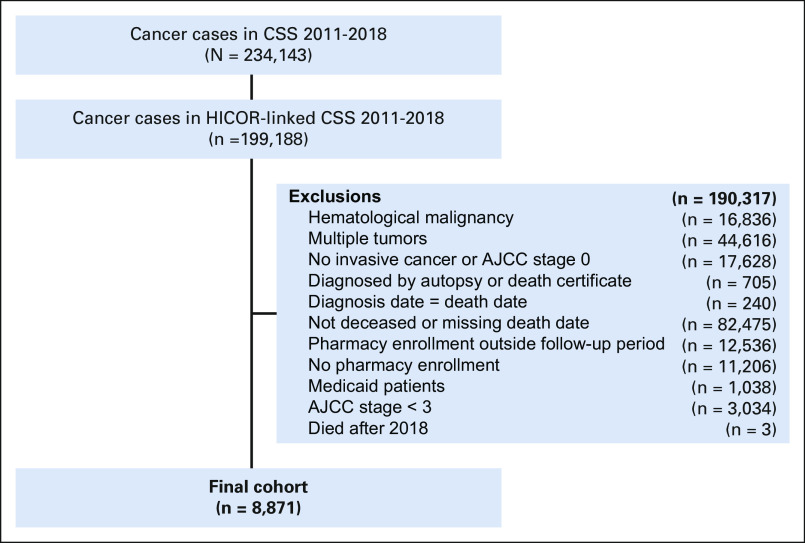
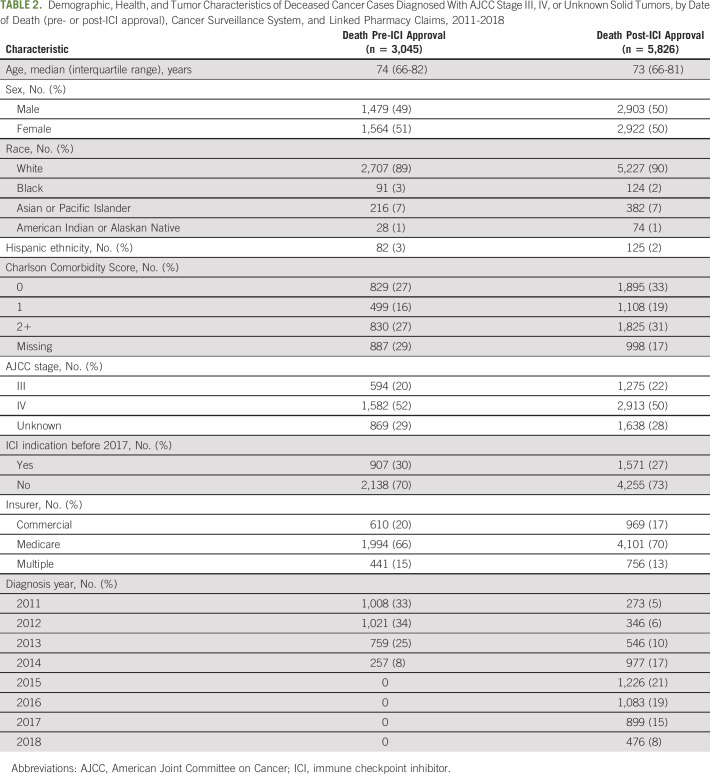
A total of 8,871 patients with cancer met eligibility criteria and were included in the study; 3,045 (34%) died between January 1, 2011, and September 4, 2014 (pre-ICI approval), and 5,826 (66%) died between September 5, 2014, and December 31, 2018 (post-ICI approval). Figure 1 shows a CONSORT18 diagram for the population included. Patients in the pre- and post-ICI groups had similar demographic characteristics, comorbidity scores, stage distribution, proportion with early ICI indication, and insurance type (Table 2).
FIG 1.
Study CONSORT diagram. AJCC, American Joint Committee on Cancer; CSS, Cancer Surveillance System; HICOR, Hutchinson Institute for Cancer Outcomes Research.
TABLE 2.
Demographic, Health, and Tumor Characteristics of Deceased Cancer Cases Diagnosed With AJCC Stage III, IV, or Unknown Solid Tumors, by Date of Death (pre- or post-ICI approval), Cancer Surveillance System, and Linked Pharmacy Claims, 2011-2018
Prevalence of Systemic Therapy Near EOL
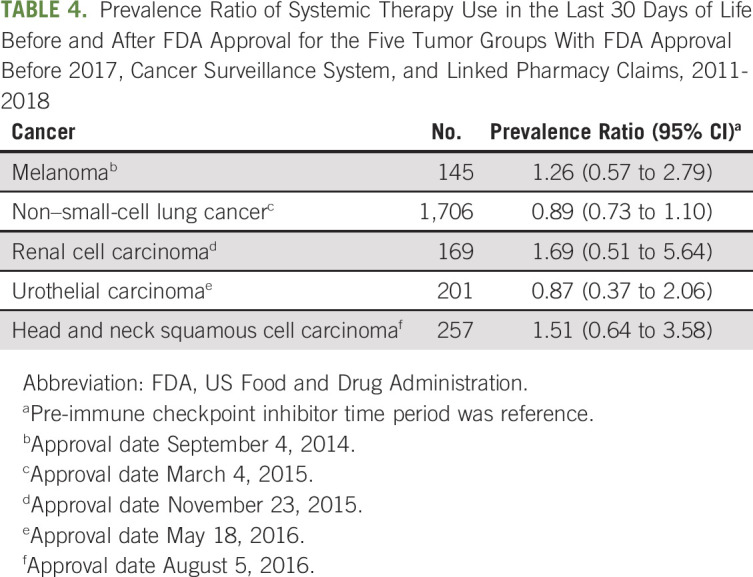
The prevalence of systemic therapy use in the last 30 DOL in the overall study population was 14.4% before ICI approval and 12.4% after ICI approval for a difference of −2.0% (95% CI, −3.5 to −0.5) and a PR of 0.86 (95% CI, 0.77 to 0.96; Table 3). Among the cases with an early FDA-approved ICI indication, the PR (0.92 [95% CI, 0.77 to 1.11]) and the difference between the two time periods were not significantly different (−1.4% [95% CI, −4.4 to 1.7]). Among the five diagnoses with an early indication, there was more prevalent use of systemic therapy in the last 30 DOL in the post-ICI period versus pre-ICI period for patients with melanoma, renal cell carcinoma, and head and neck squamous cell carcinoma, but less prevalent use with NSCLC and urothelial carcinoma (Table 4). However, none of the tumor-specific differences among the early ICI indication tumors were statistically significant.
TABLE 3.
Prevalence of Systemic Therapy Use in the Last 30 Days of Life by Date of Death (pre- or post-ICI approval) for Deceased Cancer Cases Diagnosed With AJCC Stage 3, 4, or Unknown Solid Tumors, Cancer Surveillance System, and Linked Pharmacy Claims, 2011-2018
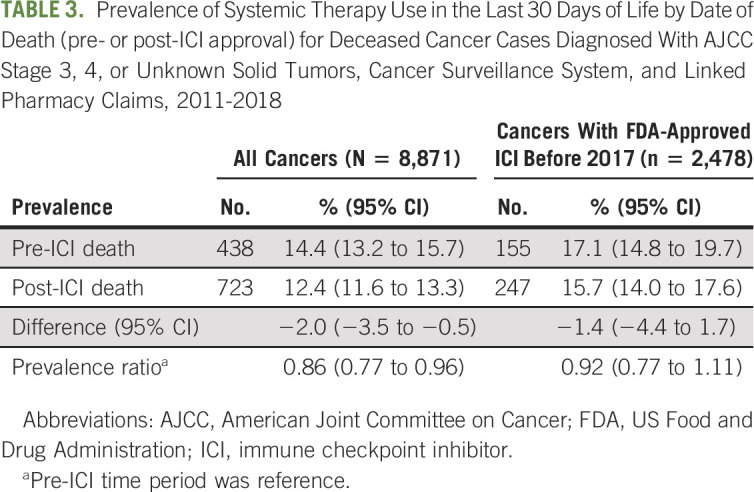
TABLE 4.
Prevalence Ratio of Systemic Therapy Use in the Last 30 Days of Life Before and After FDA Approval for the Five Tumor Groups With FDA Approval Before 2017, Cancer Surveillance System, and Linked Pharmacy Claims, 2011-2018
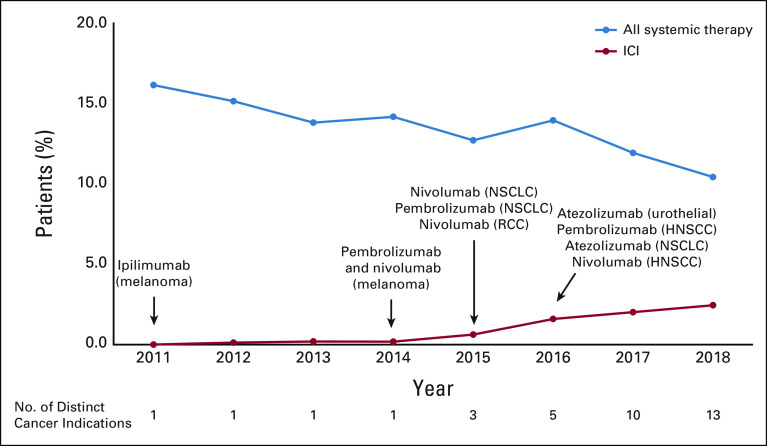
We also calculated the annual (year-by-year) prevalence of systemic therapy and ICI use in the last 30 DOL. Although the annual prevalence of systemic therapy in the last 30 DOL decreased from 16.1% in 2011 to 10.4% in 2018, ICI use in the last 30 DOL rose from 0% to 2.4% over the same time period (Fig 2). Notably, ICI use made up 23% of all systemic therapy use in the last 30 DOL in 2018 compared with 1% in 2014.
FIG 2.
Annual (year-by-year) prevalence of systemic therapy and ICI use among patients with cancer in Western Washington in the last 30 days of life between 2007 and 2018, Cancer Surveillance System, and Linked Pharmacy Claims. HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; NSCLC, non–small-cell lung cancer; RCC, renal cell carcinoma.
Characteristics of Patients Receiving ICI
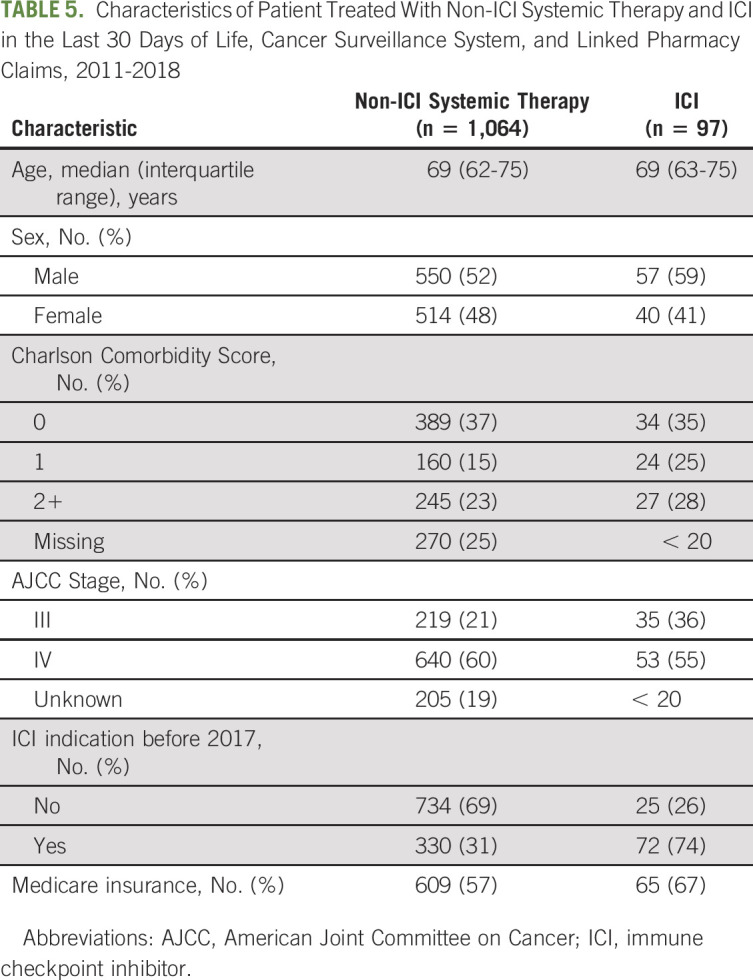
Characteristics of patients receiving ICI and non-ICI systemic therapy in the last 30 DOL are shown in Table 5. A substantially higher proportion of patients receiving ICI in the last 30 DOL had a diagnosis with an early (pre-2017) ICI approval (74% v 31%). Otherwise, the population receiving ICI in the last 30 DOL had similar characteristics to the population receiving non-ICI therapy in the last 30 DOL.
TABLE 5.
Characteristics of Patient Treated With Non-ICI Systemic Therapy and ICI in the Last 30 Days of Life, Cancer Surveillance System, and Linked Pharmacy Claims, 2011-2018
Cost of ICI
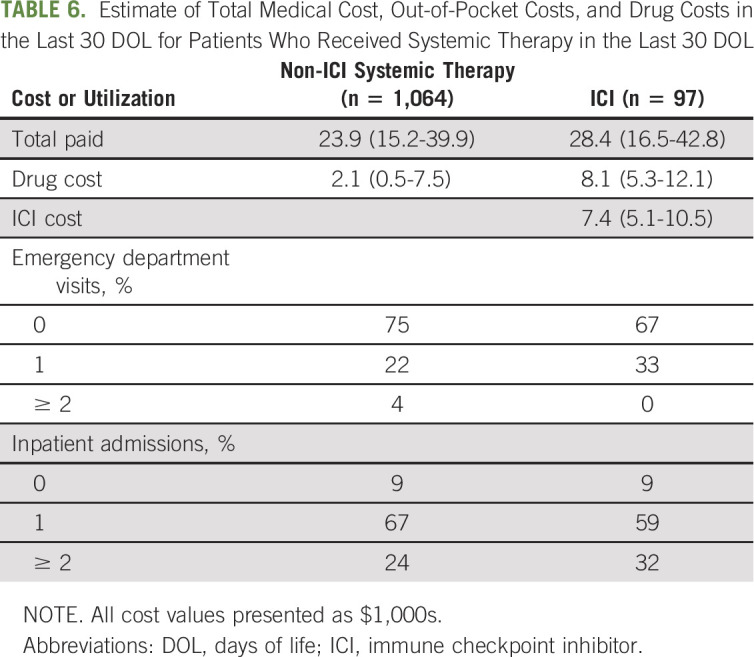
Estimates of direct medical costs in the last 30 DOL and use of other intensive interventions (ED visits and hospital admissions) for patients receiving ICI and non-ICI systemic therapy in the last 30 DOL are shown in Table 6. Patients receiving ICI had higher overall and out-of-pocket costs. This was most notable with higher drug costs ($8,100 v $2,100 US dollars), with 88% of drug costs attributable to ICI. Patients receiving ICI also had higher prevalence of ≥ 1 ED visits and ≥ 2 inpatient admissions.
TABLE 6.
Estimate of Total Medical Cost, Out-of-Pocket Costs, and Drug Costs in the Last 30 DOL for Patients Who Received Systemic Therapy in the Last 30 DOL
DISCUSSION
ICIs are a well-established and valuable treatment option for patients with a broad range of cancers and have revolutionized cancer care. However, there is still potential for overuse, especially near the EOL, as previously suggested.19,20 In our study, we investigated changes in the prevalence of systemic therapy in the last 30 DOL before and after the first anti-PD-(L)1 ICI was FDA-approved among patients with solid tumors diagnosed between 2011 and 2018. Contrary to our hypothesis of higher systemic therapy use in the last 30 DOL, we noted a significantly lower prevalence of systemic therapy use in the last 30 DOL after ICI approval in 2014.
Some studies investigating ICI use near the EOL have estimated similar use as noted in our study,10 whereas most have measured higher use.21-23 For example, a recent study by Riaz et al22 using the Flatiron Health Database noted higher ICI use near the EOL. In that study, patients with melanoma or NSCLC had an increase in systemic therapy use near the EOL after ICI approval, whereas those with microsatellite stable colon cancer (a cancer type without ICI indication) did not note a similar rise. Most of the change in systemic therapy noted was due to ICIs. In addition, an earlier study with the Flatiron Health Database also noted high use of ICIs in patients with urothelial carcinoma near the EOL.23 Notably, in our study, when we limited our population to those with an early ICI indication (including melanoma, NSCLC, and urothelial carcinoma), we still did not find an increase in prevalence of systemic therapy use in the last 30 DOL. A possible explanation is the different patient populations included. In the study by Riaz et al, 28.8% of patients with NSCLC and 32.3% of patients with melanoma were > 75 years age and 53.4% and 44.2% had no comorbidities, whereas the median age in our population was 74 and 73 years for the pre- and post-ICI periods and only 27% and 33% had no comorbidities, suggesting that our population was older with more comorbidities. Looking at specific characteristics of those treated with ICI near the EOL in our study, we noted a slightly higher proportion with a higher comorbidity score than noted in those receiving alternate systemic therapy near the EOL. This is consistent with ICI being less toxic and more tolerable than cytotoxic chemotherapy.
Our findings are consistent with previous trends reported from SEER-Medicare. A previous study by Fang et al reported a steady decline in systemic therapy use in the last 14 days and 30 DOL from 2007 to 2013.24 The prevalence of systemic therapy in the last 30 DOL in that study was approximately 15%. In our study, in the pre-ICI period from 2011 to 2014, we note a similar 14.4% prevalence of systemic therapy and a steady trend to lower use continued even after ICI approvals. Fang et al concluded from their study that the decline in systemic therapy use near the EOL likely suggested recognition by oncologists that this was a low-value practice, suggesting success of efforts by CMS, the National Quality Forum, and ASCO Choosing Wisely to draw attention to this issue.3,25,26 Our findings showing a continuous decline despite widespread ICI approvals continue to support this assertion. Given these other interventions to lower systemic therapy use near the EOL, we cannot attribute the decline noted in our study to be directly because of ICI approval.
Although overall, the decline in systemic therapy use in the last 30 DOL remains promising, it is notable that ICI therapy made up an increasing proportion of systemic therapy use in the last 30 DOL in our study. By 2018, ICIs made up 25% of systemic therapy in the last 30 DOL. This is consistent with the rise in ICI indications during that time. Between 2015 and 2018, the proportion of patients with cancer diagnoses eligible for ICIs increased from 26.9% to 44.6%.12 Recent tumor-agnostic FDA approvals for pembrolizumab for metastatic, microsatellite instability-high, or mismatch repair–deficient tumors and for tumors with tumor mutational burden high (≥ 10 mutations/megabase) likely further increase the population eligible for ICIs.27,28 However, although more cancer indications have received FDA approval, there has also been a trend to move ICI therapy to earlier lines of treatment, including perioperatively for earlier-stage tumors (mainly in clinical trials) and in earlier lines for metastatic cancers. This shift to earlier treatments may also reduce the use of ICI near EOL as patients might have already been treated with these agents earlier in their disease course. Ultimately, the true impact of ICIs on treatment near the EOL will require further follow-up.
One additional finding in our study was the higher utilization and cost of care with ICI therapy. More than two thirds of the annualized cost for medical services for patients with cancer have been estimated to be spent in the last year of life, making the EOL phase of cancer care the most costly.29 The cost of cancer care also puts patients with cancer at greater risk for bankruptcy and has been associated with patients turning to crowd funding to cover financial obligations.30,31 In our study, patients receiving ICI therapy in the last 30 DOL had higher total medical costs and drug costs than those receiving other systemic therapy in the last 30 DOL. In addition, patients receiving ICI in the last 30 DOL also had more ED presentations and inpatient hospitalizations in the last 30 DOL. These findings suggest that the use of ICI therapy may also be more costly and lead to a more intense EOL experience for patients.
Our study has several limitations inherent to the study nature, along with potential selection and confounding biases. The relatively small sample size of our study introduces the potential that we did not have sufficient power to detect a statistical difference between time periods. It is also possible that we are not capturing all ICI use near the EOL if patients received ICIs on clinical trials or under compassionate use protocols not billed to insurance. We think that it is unlikely that many patients near the EOL would be on clinical trials, and when we used alternate insurance billing codes to identify compassionate use cases, there were no meaningful changes in our results. For these reasons, we think that misclassification of ICI use is unlikely. We also do not have certain clinical data available in the electronic medical record (eg, Eastern Cooperative Oncology Group performance status, laboratory results, or molecular or pathologic biomarkers [like PD-L1 staining]), which limits the characterization of factors associated with ICI use. Similarly, although we measure systemic therapy use near the EOL, we are unable to assess the appropriateness of that therapy or the predictability of death, so it is possible that in a number of cases, therapy was appropriate.32 Finally, our data were limited to patients diagnosed after 2011, which might have introduced some bias between the two populations as there may be more patients with indolent cancers present in the post-ICI population. However, since our analysis focused on the use of systemic therapy in the last 30 DOL, we do not suspect that this imbalance will have a substantial effect on our conclusions.
Despite these limitations, the strengths of our study include the use of a robust and credible data set focusing on contemporary analysis of changes in systemic therapy near the EOL and the inclusion of cost data to estimate the financial burden of ICI use near the EOL. Our results can contribute to the ongoing discussions about value-based care and further support cost-effectiveness and pragmatic studies.
In summary, we showed that overall systemic therapy use in the last 30 DOL for patients with solid tumors declined after the first anti-PD-(L)1 ICI approval in September 2014. However, ICI use near the EOL is slowly increasing and was more costly than other systemic therapy near the EOL. Future studies with a larger population are needed to validate our findings. In addition, further work to characterize patient populations with high use of ICI near the EOL can help identify interventions to curb low-value utilization practices.
Ali Raza Khaki
Stock and Other Ownership Interests: Merck, Sanofi
Petros Grivas
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Clovis Oncology, EMD Serono, Seattle Genetics, Foundation Medicine, Pfizer, Janssen, Genzyme, Mirati Therapeutics, Exelixis, Roche, GlaxoSmithKline, Genentech, Immunomedics, Dyania Health, Infinity Pharmaceuticals, QED Therapeutics, 4D Pharma
Research Funding: Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Bristol Myers Squibb, Debiopharm Group, Merck Sharp & Dohme, QED Therapeutics, Kure It Cancer Research, GlaxoSmithKline, Mirati Therapeutics
Scott D. Ramsey
Employment: Flatiron Health
Consulting or Advisory Role: Bayer, Genentech, Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Grail, Pfizer, Seattle Genetics, Biovica
Research Funding: Bayer, Bristol Myers Squibb, Microsoft
Travel, Accommodations, Expenses: Bayer Schering Pharma, Bristol Myers Squibb, Flatiron Health, Bayer, Grail
Veena Shankaran
Honoraria: Proteus Digital Health, Taiho Pharmaceutical
Research Funding: Amgen, Merck Sharp & Dohme, Bayer, Bristol Myers Squibb, AstraZeneca, Genentech/Roche, Apexigen
Travel, Accommodations, Expenses: Proteus Digital Health, Taiho Pharmaceutical
No other potential conflicts of interest were reported.
SUPPORT
A.R.K. was supported by the National Cancer Institute under Training Grant Award No. T32CA009515. P.G. acknowledges Kure It Cancer Research.
AUTHOR CONTRIBUTIONS
Conception and design: Ali Raza Khaki, Stephen M. Schwartz, Veena Shankaran
Provision of study materials or patients: Stephen M. Schwartz
Collection and assembly of data: Ali Raza Khaki, Shasank Chennupati, Catherine Fedorenko, Li Li, Stephen M. Schwartz, Veena Shankaran
Data analysis and interpretation: Ali Raza Khaki, Catherine Fedorenko, Li Li, Qin Sun, Petros Grivas, Scott D. Ramsey, Stephen M. Schwartz, Veena Shankaran
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Utilization of Systemic Therapy in Patients With Cancer Near the End of Life in the Pre- Versus Postimmune Checkpoint Inhibitor Eras
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ali Raza Khaki
Stock and Other Ownership Interests: Merck, Sanofi
Petros Grivas
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Clovis Oncology, EMD Serono, Seattle Genetics, Foundation Medicine, Pfizer, Janssen, Genzyme, Mirati Therapeutics, Exelixis, Roche, GlaxoSmithKline, Genentech, Immunomedics, Dyania Health, Infinity Pharmaceuticals, QED Therapeutics, 4D Pharma
Research Funding: Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Bristol Myers Squibb, Debiopharm Group, Merck Sharp & Dohme, QED Therapeutics, Kure It Cancer Research, GlaxoSmithKline, Mirati Therapeutics
Scott D. Ramsey
Employment: Flatiron Health
Consulting or Advisory Role: Bayer, Genentech, Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Grail, Pfizer, Seattle Genetics, Biovica
Research Funding: Bayer, Bristol Myers Squibb, Microsoft
Travel, Accommodations, Expenses: Bayer Schering Pharma, Bristol Myers Squibb, Flatiron Health, Bayer, Grail
Veena Shankaran
Honoraria: Proteus Digital Health, Taiho Pharmaceutical
Research Funding: Amgen, Merck Sharp & Dohme, Bayer, Bristol Myers Squibb, AstraZeneca, Genentech/Roche, Apexigen
Travel, Accommodations, Expenses: Proteus Digital Health, Taiho Pharmaceutical
No other potential conflicts of interest were reported.
REFERENCES
- 1.Prigerson HG, Bao Y, Shah MA, et al. : Chemotherapy use, performance status, and quality of life at the end of life JAMA Oncol 1:778–784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Earle CC, Landrum MB, Souza JM, et al. : Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 26:3860–3866, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Care at the End of Life for Advanced Cancer Patients. Choosing Wisely; https://www.choosingwisely.org/patient-resources/care-at-the-end-of-life-for-advanced-cancer-patients/ [Google Scholar]
- 4.First Approval of PD-1 Inhibitor: Pembrolizumab in Unresectable or Metastatic Melanoma. The ASCO Post; https://www.ascopost.com/issues/october-15-2014/first-approval-of-pd-1-inhibitor-pembrolizumab-in-unresectable-or-metastatic-melanoma/ [Google Scholar]
- 5.Grivas P, Plimack ER, Balar AV, et al. : Pembrolizumab as first-line therapy in cisplatin-ineligible advanced urothelial cancer (KEYNOTE-052): Outcomes in older patients by age and performance status Eur Urol Oncol 3:351–359, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glisch C, Saeidzadeh S, Snyders T, et al. : Immune checkpoint inhibitor use near the end of life: A single-center retrospective study J Palliat Med 23:977–979, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Cancer Treatment Hype Gives False Hope to Many Patients. USA TODAY; https://www.usatoday.com/story/news/2017/04/27/cancer-treatment-hype-gives-false-hope-many-patients/100972794/ [Google Scholar]
- 8.Weeks JC, Catalano PJ, Cronin A, et al. : Patients’ expectations about effects of chemotherapy for advanced cancer N Engl J Med 367:1616–1625, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh JC, Knight LS, Kane J, et al. : Has there been a shift in use of subacute rehabilitation instead of hospice referral since immunotherapy has become available? J Oncol Pract 15:e849–e855, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Khaki AR, Li A, Diamantopoulos LN, et al. : Impact of performance status on treatment outcomes: A real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors Cancer 126:1208–1216, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suresh K, Voong KR, Shankar B, et al. : Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: Incidence and risk factors J Thorac Oncol 13:1930–1939, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Haslam A, Prasad V: Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs JAMA Netw Open 2:e192535, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Souza JA, Duong YY: Off-label immunotherapy prescription: Financial implications for payers and patients J Clin Oncol 35, 2017suppl; abstr 6 [Google Scholar]
- 14.Hutchinson Institute for Cancer Outcomes Research : Community Cancer Care in Washington State: Quality and Cost Report 2018, 2018https://www.fredhutch.org/content/dam/public/labs-projects/hicor/CCCReport/HICOR%20Community%20Cancer%20Care%20Report%20May%202018.pdf [Google Scholar]
- 15.Seattle—Puget Sound—SEER Registries. SEER; 2020. https://seer.cancer.gov/registries/sps.html . [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation J Chronic Dis 40:373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Barros AJ, Hirakata VN: Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio BMC Med Res Methodol 3:21, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D: CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials J Clin Epidemiol 63:834–840, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Kolata G: “Desperation Oncology”: When Patients Are Dying, Some Cancer Doctors Turn to Immunotherapy The New York Times, 2018https://www.nytimes.com/2018/04/26/health/doctors-cancer-immunotherapy.html [Google Scholar]
- 20.Fojo T: Desperation oncology Semin Oncol 45:105–106, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Petrillo LA, El‐Jawahri A, Nipp RD, et al. : Performance status and end-of-life care among adults with non–small cell lung cancer receiving immune checkpoint inhibitors Cancer 126:2288–2295, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Riaz F, Gan G, Li F, et al. : Adoption of immune checkpoint inhibitors and patterns of care at the end of life JCO Oncol Pract 16:e1355–e1370, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parikh RB, Galsky MD, Gyawali B, et al. : Trends in checkpoint inhibitor therapy for advanced urothelial cell carcinoma at the end of life: Insights from real‐world practice Oncologist 24:e397–e399, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang P, Jagsi R, He W, et al. : Rising and falling trends in the use of chemotherapy and targeted therapy near the end of life in older patients with cancer J Clin Oncol 37:1721–1731, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quality Measures. CMS; https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/QualityMeasures/index . [Google Scholar]
- 26.NQF : Palliative Care and End-of-Life Care, 2020https://www.qualityforum.org/Projects/Palliative_Care_and_End-of-Life_Care.aspx [Google Scholar]
- 27.Center for Drug Evaluation and Research : FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication FDA, 2019http://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication [Google Scholar]
- 28.Center for Drug Evaluation and Research : FDA approves pembrolizumab for adults and children with TMB-H solid tumors FDA, 2020https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors [Google Scholar]
- 29.Mariotto AB, Enewold L, Zhao J, et al. : Medical care costs associated with cancer survivorship in the United States Cancer Epidemiol Biomarkers Prev 29:1304–1312, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsey S, Blough D, Kirchhoff A, et al. : Washington state cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis Health Aff 32:1143–1152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen AJ, Brody H, Patino G, et al. : Use of an online crowdfunding platform for unmet financial obligations in cancer care JAMA Intern Med 179:1717–1720, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Einav L, Finkelstein A, Mullainathan S, et al. : Predictive modeling of U.S. health care spending in late life Science 360:1462–1465, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]