Abstract
PURPOSE:
Evidence on the nature of the relationship between patients receiving chemotherapy as an essential part of guideline-concordant cancer care and the onset of Alzheimer's Disease (AD) and other adverse cognitive outcomes has been mixed. Biological mechanisms were proposed to support both a potentially beneficial and an adverse role. To explore the relationship between chemotherapy and onset of AD and other neurocognitive disorders (ND) in colorectal cancer survivors.
METHODS:
We conducted a retrospective cohort study of 135,834 individuals older than 65 years diagnosed with colorectal cancer between 1998 and 2007, using SEER-Medicare data. A proportional hazards model was used before and after the use of inverse probability weighting to account for populational differences between the chemotherapy and nonchemotherapy groups. Weights were normalized to the total sample size.
RESULTS:
After inverse probability weighting, chemotherapy was associated with decreased AD risk (hazard ratio [HR]: 0.791; 95% CI: 0.758 to 0.824) and lower risk for the majority of other ND including AD-related diseases (HR: 0.823; CI: 0.802 to 0.844), dementia (permanent mental disorder) (HR: 0.807; CI: 0.782 to 0.832), and dementia (senile) (HR: 0.772; CI: 0.745 to 0.801). The only adverse effect to remain significant was cerebral degeneration (excluding AD) (HR: 1.067; CI: 1.033 to 1.102). The effects for AD remained after treatment was stratified by chemotherapy agent type and remained significant for up to 6 years past diagnosis.
CONCLUSION:
Chemotherapy use in colorectal cancer survivors demonstrated an association with reduced risk for AD and other ND.
INTRODUCTION
As the population of cancer survivors grows, the question of the long-term relationship between chemotherapy and cognitive ability becomes increasingly relevent1 to the health and well-being of the nation's population of older adults. Neuropsychological studies on late cognitive functioning after cytotoxic treatment showed that survivors of breast, ovarian, and lymphoma cancers experienced a decline in cognitive function.2-6 The most frequently observed cognitive problems were within the domains of memory, processing speed, and executive functioning.7 In addition, neuroimaging studies on the effects of chemotherapy on brain structure and function found that cytotoxic treatment was further associated with long-term gray matter reductions, global and focal reduced white matter integrity, and altered brain activation during cognitive tasks.2
However, epidemiologic studies of the relationship between neurodegenerative dementia and cancer in patients with breast and prostate cancers did not provide consistent evidence.8-13 Studies reported chemotherapy to be associated with impaired cognitive function,8 decreasing Alzheimer's Disease (AD) risk,9,10 no significant effects on subsequent dementia diagnosis,11 and reduced risk of dementia limited to specific age groups.12 Most recently, a study of more than 3.5 million elderly veterans13 found that for most cancers, treatment, including chemotherapy, was associated with a lower risk of AD but an increased risk of the alternative outcomes such as non-AD dementia, stroke, osteoarthritis, and macular degeneration.
Theoretical mechanisms have been proposed to support both a beneficial and an adverse relationship between chemotherapy and subsequent dementia, but no consensus exists to date.14 Proposed mechanisms supporting an adverse relationship include direct neurotoxic effects on CNS cells caused by crossing of the blood–brain barrier of chemotherapeutic agents15 and the effect on the CNS blood vessels including reduced blood vessel density in the hippocampus.16 Alternative mechanisms supporting a potential beneficial effect were centered on the role of neoadjuvant and adjuvant chemotherapy in modulating the risk for AD17 through suppressing neuroinflammation18 and/or preventing neuronal cells from entering into the cell cycle and apoptosis.19
In general, there is conflicting evidence about the potential role of chemotherapy in the development of cognitive dysfunction of patients who receive chemotherapy.20,21 In this study, we further explore the relationship between exposure to chemotherapy and the risk of AD using a population of colorectal cancer survivors enrolled in the traditional Medicare health insurance system. Our focus on colorectal cancer was motivated by it (1) being the third most prevalent cancer after prostate and female breast cancer (for which literature on the effect of chemotherapy on AD exists8-13), (2) demonstrating comparable prevalence in males and females, (3) being free of sources of confounding related to the effects of other types of therapies (eg, the use of tamoxifen, commonly used in the treatment of female breast cancer, has been associated with cognitive decline22), and (4) demonstrating continually declining mortality rates over the last 3 decades,23,24 allowing affected individuals to live longer, thereby expanding the pool of individuals reaching ages at which AD is commonly ascertained. Finally, since cancer-related cognitive decline does not need to reach the level of an AD diagnosis to be clinically meaningful and AD often coexists with or is misdiagnosed as other neurodegenerative disorders (ND), we include a wide range of these conditions in our analysis.25
METHODS
Data drawn from the SEER program linked to administrative health insurance claims records from the Medicare health insurance system (SEER-Medicare) were used for this study.26 SEER-Medicare provides data on the date of diagnosis, histology, stage, and grade of up to 10 confirmed cancer cases as well as the therapy recommended and/or provided within 4 months of diagnosis, follow-up vital status, cause of death, and basic demographic and area-based socioeconomic characteristics. The Medicare component provides additional information on the diagnoses made (International Classification of Disease 9th edition, Clinical Modification) and procedures performed (Current Procedural Terminology, 4th edition) on all episodes of care paid for by Medicare Parts A and B on a fee-for-service basis.
The initial sample consisted of 287,967 individuals older than 65 years who were diagnosed with colorectal cancer between 1991 and 2007. Individuals without full fee-for-service Medicare Parts A and B coverage 12 months before and 6 months after diagnosis were then excluded, which reduced the sample to 197,564. Then, individuals without at least 6 months of follow-up (−36,272), with a diagnosis of AD and ND at time of diagnosis (−18,807) or with missing data for cancer stage (−6,651), were excluded. After exclusions, the final sample size was 135,834.
The presence of AD and ND, baseline comorbidities, and chemotherapy was identified from Medicare claims using the appropriate diagnosis or procedural codes (Appendix Tables A1 and A2, online only) and algorithms discussed in detail elsewhere.25,27,28 In addition to a combined measure representing any chemotherapy treatment, eight nonmutually exclusive groups representing the use of individual chemotherapy agents were defined: fluorouracil, irinotecan, oxaliplatin, cetuximab, panitumumab, capecitabine, and other or unspecified chemotherapy.
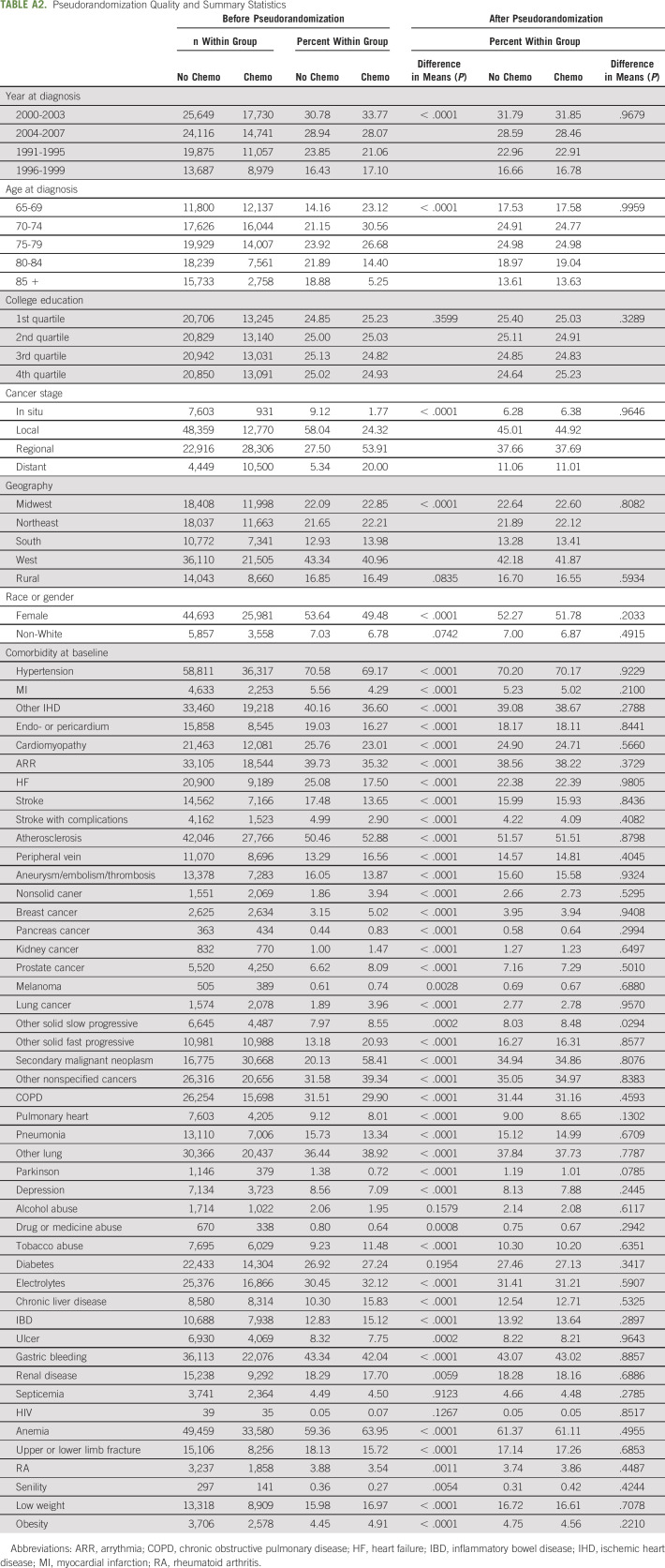
To evaluate the effect of chemotherapy, individual inverse probability weights (IPWs) were calculated as the reciprocal of the probability to have observed chemotherapy treatment. This resulted in a weighted population pseudorandomized with respect to all predictors used in the treatment model (Appendix Table A2), that is, the only difference between the two groups was the receipt of chemotherapy (and factors not included in the pseudorandomization algorithm). Pseudorandomization is one of the several propensity score–based methods29 focused on addressing selection bias in observational data. In this approach, individual weights are calculated in such a way as to provide statistical similarity between weighted groups with and without the treatment of interest. No sample loss is involved as the existence of a statistically similar case or control pair is not necessary—the effect is achieved through weighting. Significance testing of pseudorandomization quality (Appendix Table A2) showed that the process was successful. Finally, the effect of the chemotherapy was evaluated using the Cox proportional hazards model with the time-independent indicator of chemotherapy as the only explanatory variable (since all other observable covariates were controlled for in the pseudorandomization process). Individual follow-up was started from the date of colorectal cancer diagnosis. The effect of age was accounted for nonparametrically with age serving as a timescale variable. In this model design, age dependence of AD and ND risks is included in the baseline hazard only. Such models are preferable when age is a strong predictor of the outcome,30,31 as was the case with AD, for which age is the strongest nongenetic risk factor. Visualizations of group-specific survival functions and log-negative-log survival functions were used to ensure that the proportionality assumption required by the Cox model was satisfied.
RESULTS
Before pseudorandomization, the chemotherapy and nonchemotherapy groups differed significantly across 50 of the 57 variables included in the IPW model (Appendix Table A2). The exceptions were college education, rural residence, non-White race, and the presence of alcohol abuse, diabetes mellitus, septicemia, and HIV at baseline. After pseudorandomization, only one statistically significant difference remained: the presence of other slow-progressing tumor at baseline. The sample pool was 52% female and 93% White; about 50% of the sample was between 70 and 80 years old at baseline, with only 14% older than 85 years. The overwhelming majority of patients were diagnosed with local (45%) or regional (38%) stage cancer, with in situ (6%) and distant (11%) being relatively rare. Only three chemotherapy treatment patterns occurred with sufficient frequency to power further analysis: fluorouracil alone (54%), fluorouracil and irinotecan (9%), and fluorouracil and oxaliplatin (8%). The study-wide incidence rates of AD and ND were AD (7.22%), Alzheimer's disease–related dementias (ADRDs) (18.40%), ADRD with AD excluded (17.40%), dementia/permanent mental disorder (13.02%), dementia/senile (9.35%), vascular dementia (3.28%), cerebral degeneration with AD excluded (11.27%), cognitive deficits or late effects (5.10%), and encephalopathy or not elsewhere classified (4.87%).
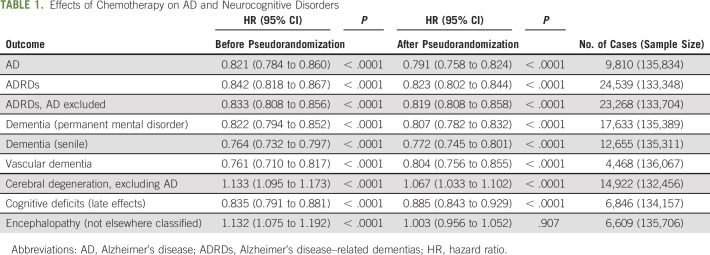
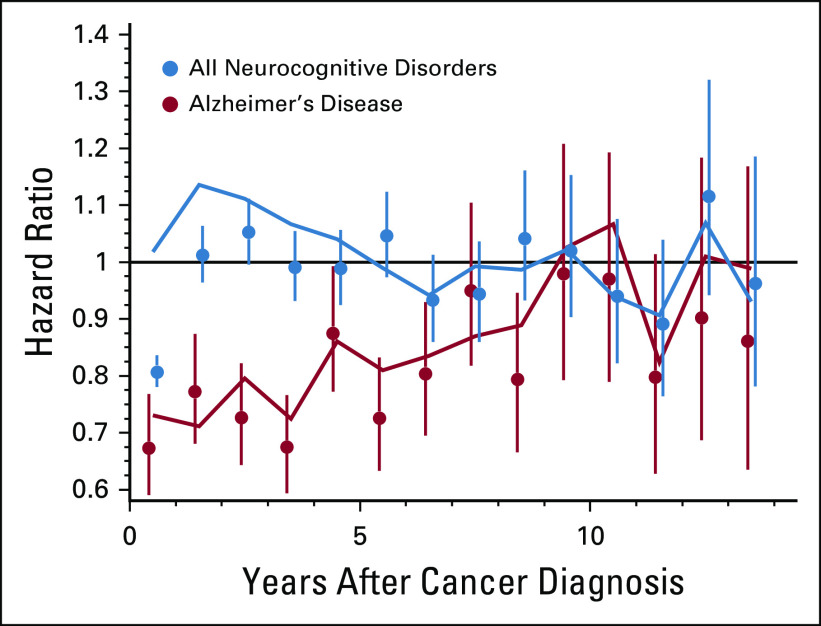
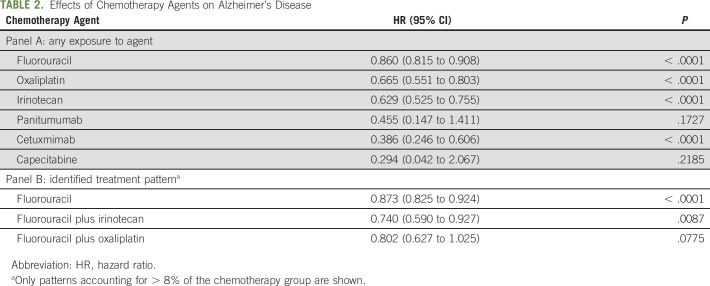
Effects of chemotherapy on AD and ND before and after pseudorandomization are presented in Table 1. After pseudorandomization, chemotherapy was associated with decreased AD risk (hazard ratio [HR]: 0.791; 95% CI: 0.758 to 0.824) and lower risk for the majority of other ND including ADRD (HR: 0.823; CI: 0.802 to 0.844), dementia (permanent mental disorder) (HR: 0.807; CI: 0.782 to 0.832), and dementia (senile) (HR: 0.772; CI: 0.745 to 0.801). The only adverse association to remain significant was cerebral degeneration (excluding AD) (HR: 1.067; CI: 1.033 to 1.102). The protective effect for the onset of AD was time dependent (Fig 1): the effect decreased over time, and 7-9 years after colorectal cancer diagnosis, it was no longer significant. When the effect of chemotherapy on AD onset was stratified by the presence of an individual agent (Table 2; Panel A) in the treatment plan or by mutually exclusive agent combinations (Table 2; Panel B), the protective association was consistent across all strata where significant.
TABLE 1.
Effects of Chemotherapy on AD and Neurocognitive Disorders
FIG 1.
Time-dependent hazard ratios (HRs) associated with exposure to chemotherapy. HRs with 95% CIs after pseudorandomization for Alzheimer's disease (AD) (red dots) and a composite measure of all neurocognitive disorders (ND) (blue dots). HRs with 95% confidence intervals before pseudorandomization for AD (red lines) and a composite measure of all ND (blue lines).
TABLE 2.
Effects of Chemotherapy Agents on Alzheimer's Disease
DISCUSSION
Our study showed that exposure to chemotherapy was associated with a lower long-term risk for AD. An important finding was that the impacts of chemotherapy varied between specific chemotherapy medications. The association of chemotherapy with reduced risk was also observed, although to a lesser extent, in some other ND. Although before pseudorandomization, receipt of chemotherapy was associated with higher risk for the development of cerebral degeneration and encephalopathy, only the effect associated with cerebral degeneration remained after pseudorandomization.
Our findings show an association between lower risk of AD and ND and chemotherapy receipt in patients with cancer and provide additional independent support to previous findings in this area of study.9,12,13 Although a recent work already showed that patients with a history of mood disorder who received chemotherapy had significantly lower risk of AD, vascular dementia, and other nonspecified dementia than those without such a therapy,9 these findings were potentially subject to confounding by unmeasured factors that might have influenced the choice of chemotherapy. In contrast, our analysis included pseudorandomization of patients with cancer, thus mitigating this source of confounding. Furthermore, this study makes a number of novel contributions not found in the literature: we found that lower risk of senile dementia, cognitive deficit as a late effect of cerebral hemorrhage or infarction, and higher risk of cerebral degeneration (excluding AD) was associated with receipt of chemotherapy.
In our study, chemotherapy use was associated with a higher risk for cerebral degeneration, which is a disorder characterized by gradual and progressive loss of neural tissue and neurologic function. There are several potential etiological factors identified for cerebral degeneration including because of alcoholism, cerebrovascular disease, neoplastic disease, Parkinson's disease, and vitamin B12 deficiency. The multifactorial origin and underlying mechanisms of cerebral degeneration in combination with chemotherapy, therefore, highlight the need for future studies with focus on a better characterization of the complex association between exposure to chemotherapy, preexisting conditions, and the risk of cerebral degeneration.
We found an association between chemotherapy and increased risk of encephalopathy (without pseudorandomization) that became nonsignificant after pseudorandomization. Other studies of encephalopathy in patients with cancer were focused on ifosfamide-induced encephalopathy: these studies showed the risk being significantly increased.32,33 Ifosfamide is an isomer of a cyclophosphamide that is used to treat gynecological, testicular, and head and neck cancers, sarcomas, and lymphomas.34 Ifosfamide is not used to treat patients with colorectal cancer, and therefore, in our study, no effect was expected. However, another type of encephalopathy discussed in the literature is a posterior reversible encephalopathy syndrome associated with cytotoxic therapies: several studies showed that treatment with irinotecan, leucovorin, and 5-fluorouracil,35 oxaliplatin and fluoropyrimidine,36 and capecitabine37 had neurotoxic effect and increased the risk of encephalopathy. The suggested mechanism for capecitabine was that medication crosses the blood-brain barrier in the form of 5′-DFUR (doxifluridine) and is transformed to 5-fluorouracil in the brain.37 Our study suggests that the risk of encephalopathy, at least as related to 5-fluorouracil,35 irinotecan, leucovorin, and capecitabine,37 is not present after population heterogeneity is accounted for through pseudorandomization.
The results of our study showed that although capecitabine (antineoplastic antimetabolite agent) and cetuximab (anti-epidermal growth factor [EGF] receptor monoclonal antibody agent) were associated with the lowest risk of AD (0.294), the impacts of irinotecan (cytotoxic quinolone–based alkaloid prodrug, HR: 0.629), oxaliplatin (platinum compound, cytotoxic compound, and inhibitor of DNA replication and transcription, HR: 0.665), and fluorouracil (antineoplastic antimetabolite agent, HR: 0.860) were less pronounced. Some previous studies, predominantly on animal models,38-40 suggested potential links between AD risk and chemotherapy agent–specific mechanisms. For chemotherapy agents that showed the highest HRs in our study (capecitabine, cetuximab, and panitumumab), no studies focused on associations with AD have been published; however, it has been shown that treatment of older women with stage I-III breast cancer with capecitabine was not associated with a cognitive decline over a 24-month period of observation.41 In addition, several studies described participation of these agents in pathways that could lead to a lower risk of neurodegeneration. For example, it has been shown that panitumumab and cetuximab as well as several other anticancer EGF receptor inhibitors target a heparin binding EGF-like growth factor gene that has been strongly associated with late-onset AD.42 This chemotherapy agent has been proposed for retargeting for use in the treatment of injuries of nervous system.
Further detailed analysis of such associations, including the studies of medical records and chemotherapy protocols in patients with different cancers, is needed to investigate the stability of the results obtained in our study. If certain chemotherapy agents have a persistent association with a lower risk of AD, then this information could be useful for further studies on AD treatment. Searching for AD therapies among medications used for cancer treatment is a growing study direction.43 Structural similarities have been described for AD tau and prostate cancer cell tau, with a correlation between tau levels and cancer response to microtubule-targeting chemotherapy drugs.44 Neuroprotective effects have been reported for some cancer chemotherapy agents,45 eg, taxanes have been proposed as potential therapeutic agents for AD,38 bexarotene was effective in clearing amyloid from the brains of mouse models of AD39,46 (however, bexarotene was not effective in AD treatment or prevention in recent in vivo studies47,48), carmustine reduced beta-amyloid generation and plaque burden in mice,40 and imatinib reduced amyloid burden and promoted neuroprotection.49,50 Future investigations are expected to shed light on the spectrum of benefit-to-harm ratio of the effects of chemotherapeutic compounds on CNS along with the alteration of blood-brain barrier and the response of adjacent or other tissues.14
Despite the inverse association between chemotherapy and AD observed in our study and others9,12 and the proposed biological mechanisms, several potential methodological shortcomings should be taken into consideration when interpreting our results. Because of its retrospective nature and reliance on administrative data (which can, for example, misclassify chemotherapy use and contain other data errors), our study outcomes only included late-stage cognitive impairments. However, we believe that the change between the lack of symptoms consistent with a diagnosis and the presence of sufficient symptoms to warrant a diagnosis is a clinically meaningful cognitive change. We were unable to ascertain the severity of AD and the impact of chemotherapy on the development of milder forms of cognitive impairment. Indeed, a potentially important effect of chemotherapy and related surgical exposures on development of cognitive impairment cannot be completely ruled out. Nevertheless, if such an effect exists, based on our findings, it is unlikely that any cognitive impairment related to exposure to chemotherapy results in progression to AD. In addition, Medicare claims have limited information on the dose of chemotherapy used, which could influence the occurrence and severity of cognitive impairment. We can envision at least two important scenarios that could lead to the effects observed in our study artificially.
First, our findings can be caused by the competing risk of death. Indeed, in our study, the observed short-term beneficial effect of exposure to chemotherapy can also be explained by premature death, which is assumed to be a censoring event independent of the risk of AD. This dependence can be generated and explained by a simple mechanism: administration of chemotherapy could imply that individuals in poor baseline physical health status are highly likely to die prematurely, and, therefore, these individuals will not have the time to develop AD. In addition, individuals with advanced cancer stages (especially those with metastatic cancer disease) could be less likely to undergo diagnostic testing for AD. We explored these possibilities through a series of sensitivity analyses. Specifically, we estimated the Fine-Gray model, a more realistic model in which deceased individuals continue to contribute to the set of individuals at risk (in the denominator of the partial likelihood) with individual weights dependent on the prevalence of individuals with AD diagnosis in the cohort. The estimate in this case was (HR: 0.725; CI: 0.696 to 0.756) also consistent with our primary findings. Next, we stratified our sample by cancer stage and repeated the analyses (where power allowed). The results were consistent with our primary findings. For example, the associations between receipt of chemotherapy and the risk of AD onset were in situ (HR: 0.642; CI: 0.479 to 0.860), localized (HR: 0.868; CI: 0.803 to 0.939), regional (HR: 0.785; CI: 0.730 to 0.844), and distant (HR: 0.659; CI: 0.522 to 0.834).
Second, individuals with higher cognitive ability could choose chemotherapy more often. However, the frequency of chemotherapy is independent of the quartiles of area-based education measures at the zip code level (39.1%, 38.7%, 38.3%, and 38.5%) and this distribution is further improved after pseudorandomization of chemotherapy groups. We acknowledge the limitation of using area-based measures in lieu of individual-level measures, but the latter were not available in our data.
Finally, in a retrospective study such as ours, there is always a concern that selection of patients for chemotherapy treatment might have been influenced by patient- and disease-specific factors. To control for the potential of such selection bias, we opted for using IPW—a methodology designed to adjust for such inherent differences.
In conclusion, the results of our study support the hypothesis that receipt of chemotherapy in colorectal cancer survivors is associated with reduced risk for AD after adjusting for patient-, cancer-, and treatment-related characteristics. Furthermore, our findings demonstrated that the association between chemotherapy exposure and AD was not affected by competing risk of long-term mortality. Although additional validation is required, such findings may be used to reduce the potential treatment-related anxiety among patients with cancer worried about the potential adverse effects of guidance-concordant care.
ACKNOWLEDGMENT
The authors are grateful to Anatoliy Yashin for interesting discussions of the methodological and substantive aspects of our analysis and comments.
Appendix
TABLE A1.
Administrative Codes Used
TABLE A2.
Pseudorandomization Quality and Summary Statistics
SUPPORT
Supported by National Institute of Health/National Institute on Aging grants R01-AG-066133 (PI: I.A.), RF1-AG-046860 (PI: A.I. Yashin; Co-PI: I. Akushevich), and R01-AG-057801 (PIs: J.K. and I.A.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Igor Akushevich
Administrative support: Arseniy P. Yashkin
Collection and assembly of data: Igor Akushevich, Arseniy P. Yashkin
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Chemotherapy and the Risk of Alzheimer's Disease in Colorectal Cancer Survivors: Evidence From the Medicare System
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Snyder HM, Ahles T, Calderwood S, et al. : Exploring the nexus of Alzheimer's disease and related dementias with cancer and cancer therapies: A convening of the Alzheimer's Association & Alzheimer's Drug Discovery Foundation Alzheimers Demen 13:267–273, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koppelmans V, Breteler MM, Boogerd W, et al. : Late effects of adjuvant chemotherapy for adult onset non-CNS cancer; cognitive impairment, brain structure and risk of dementia Crit Rev Oncol Hematol 88:87–101, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Correa DD, Ahles TA: Neurocognitive changes in cancer survivors Cancer J 14:396–400, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Vodermaier A: Breast cancer treatment and cognitive function: The current state of evidence, underlying mechanisms and potential treatments Womens Health 5:503–516, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Wefel JS, Saleeba AK, Buzdar AU, et al. : Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer Cancer 1163348–3356, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Wefel JS, Schagen SB: Chemotherapy-related cognitive dysfunction Curr Neurol Neurosci Rep 12:267–275, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Wefel JS, Vardy J, Ahles T, et al. : International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer Lancet Oncol 12:703–708, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Heck JE, Albert SM, Franco R, et al. : Patterns of dementia diagnosis in surveillance, epidemiology, and end results breast cancer survivors who use chemotherapy J Am Geriatr Soc 56:1687–1692, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Du XL, Xia R, Hardy D: Relationship between chemotherapy use and cognitive impairments in older women with breast cancer: Findings from a large population-based cohort Am J Clin Oncol 33:533–543, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Baik SH, Kury FSP, McDonald CJ: Risk of Alzheimer's disease among senior medicare beneficiaries treated with androgen deprivation therapy for prostate cancer J Clin Oncol 35:3401, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raji MA, Tamborello LP, Kuo Y-F, et al. : Risk of subsequent dementia diagnoses does not vary by types of adjuvant chemotherapy in older women with breast cancer Med Oncol 26:452–459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baxter NN, Durham SB, Phillips KA, et al. : Risk of dementia in older breast cancer survivors: A population‐based cohort study of the association with adjuvant chemotherapy J Am Geriatr Soc 57:403–411, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Frain L, Swanson D, Cho K, et al. : Association of cancer and Alzheimer's disease risk in a national cohort of veterans Alzheimers Demen 13:1364–1370, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monacelli F, Cea M, Borghi R, et al. : Do cancer drugs counteract neurodegeneration? Repurposing for Alzheimer's disease J Alzheimers Dis 55:1295–1306, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Dietrich J, Han R, Yang Y, et al. : CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo J Biol. 5:22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seigers R, Timmermans J, van der Horn HJ, et al. : Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release Behav Brain Res 207:265–272, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Driver JA, Beiser A, Au R, et al. : Inverse association between cancer and Alzheimer's disease: Results from the Framingham Heart Study BMJ 344:e1442, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zotova E, Nicoll JA, Kalaria R, et al. : Inflammation in Alzheimer's disease: Relevance to pathogenesis and therapy Alzheimers Res Ther 2:1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webber KM, Raina AK, Marlatt MW, et al. : The cell cycle in Alzheimer disease: A unique target for neuropharmacology Mech Ageing Dev 126:1019–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Wang XM, Walitt B, Saligan L, et al. : Chemobrain: A critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy Cytokine 72:86–96, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hess LM, Huang HQ, Hanlon AL, et al. : Cognitive function during and six months following chemotherapy for front-line treatment of ovarian, primary peritoneal or fallopian tube cancer: An NRG oncology/gynecologic oncology group study Gynecol Oncol 139:541–545, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schilder C, Schagen S: Effects of hormonal therapy on cognitive functioning in breast cancer patients: A review of the literature Minerva Ginecol 59:387, 2007 [PubMed] [Google Scholar]
- 23.Siegel RL, Miller KD, Fedewa SA, et al. : Colorectal cancer statistics, 2017 CA Cancer J Clin 67:177–193, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Arnold M, Sierra MS, Laversanne M, et al. : Global patterns and trends in colorectal cancer incidence and mortality Gut 66:683–691, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Akushevich I, Yashkin AP, Kravchenko J, et al. : Time trends in the prevalence of neurocognitive disorders and cognitive impairment in the United States: The effects of disease severity and improved ascertainment J Alzheimers Dis 64:137–148, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren JL, Klabunde CN, Schrag D, et al. : Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population Med Care 40:IV3–IV18, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Akushevich I, Kravchenko J, Arbeev KG, et al. : Health effects and Medicare trajectories: Population-based analysis of morbidity and mortality patterns Biodemography Aging:47–93, 2016 [Google Scholar]
- 28.Akushevich I, Kravchenko J, Ukraintseva S, et al. : Age patterns of incidence of geriatric disease in the US elderly population: Medicare‐based analysis J Am Geriatr Soc 60:323–327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson SR, Tomlinson GA, Hawker GA, et al. : Propensity score methods for bias reduction in observational studies of treatment effect Rheum Dis Clin 44:203–213, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Korn EL, Graubard BI, Midthune D: Time-to-event analysis of longitudinal follow-up of a survey: Choice of the time-scale Am J Epidemiol 145:72–80, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Canchola AJ, Stewart SL, Bernstein L, et al. : Cox Regression Using Different Time-Scales San Francisco, CA, Western Users of SAS Software, 2003 [Google Scholar]
- 32.Rieger C, Fiegl M, Tischer J, et al. : Incidence and severity of ifosfamide-induced encephalopathy Anticancer Drugs 15:347–350, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Ajithkumar T, Parkinson C, Shamshad F, et al. : Ifosfamide encephalopathy Clin Oncol 19:108–114, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Kerbusch T, de Kraker J, Keizer HJ, et al. : Clinical pharmacokinetics and pharmacodynamics of ifosfamide and its metabolites Clin Pharmacokinet 40:41–62, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Plavetić ND, Rakušić Z, Ozretić D, et al. : Fatal outcome of posterior “reversible” encephalopathy syndrome in metastatic colorectal carcinoma after irinotecan and fluoropyrimidine chemotherapy regimen World J Surg Oncol 12:264, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Femia G, Hardy TA, Spies JM, et al. : Posterior reversible encephalopathy syndrome following chemotherapy with oxaliplatin and a fluoropyrimidine: A case report and literature review Asia Pac J Clin Oncol 8:115–122, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Formica V, Leary A, Cunningham D, et al. : 5-Fluorouracil can cross brain–blood barrier and cause encephalopathy: Should we expect the same from capecitabine? A case report on capecitabine-induced central neurotoxicity progressing to coma Cancer Chemother Pharmacol 58:276, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Brunden KR, Yao Y, Potuzak JS, et al. : The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer's disease and related tauopathies Pharmacol Res 63:341–351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cramer PE, Cirrito JR, Wesson DW, et al. : ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models Science 335:1503–1506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayes CD, Dey D, Palavicini JP, et al. : Striking reduction of amyloid plaque burden in an Alzheimer's mouse model after chronic administration of carmustine BMC Med 11:81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman RA, Pitcher B, Keating NL, et al. : Cognitive function in older women with breast cancer treated with standard chemotherapy and capecitabine on Cancer and Leukemia Group B 49907 Breast Cancer Res Treat 139:607–616, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwok MK, Lin SL, Schooling CM: Re-thinking Alzheimer's disease therapeutic targets using gene-based tests EBioMedicine 37:461–470, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araki W: Potential repurposing of oncology drugs for the treatment of Alzheimer's disease BMC Med 11:82, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Souter S, Lee G: Tubulin-independent tau in Alzheimer's disease and cancer: Implications for disease pathogenesis and treatment Curr Alzheimer Res 7:697–707, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Ganguli M: Cancer and dementia: It's complicated Alzheimer Dis Assoc Disord 29:177, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fantini J, Di Scala C, Yahi N, et al. : Bexarotene blocks calcium-permeable ion channels formed by neurotoxic Alzheimer's β-amyloid peptides ACS Chem Neurosci 5:216–224, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balducci C, Paladini A, Micotti E, et al. : The continuing failure of bexarotene in Alzheimer's disease mice J Alzheimers Dis 46:471–482, 2015 [DOI] [PubMed] [Google Scholar]
- 48.O'Hare E, Jeggo R, Kim E-M, et al. : Lack of support for bexarotene as a treatment for Alzheimer's disease Neuropharmacology 100:124–130, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Hussain I, Fabrègue J, Anderes L, et al. : The role of γ-secretase activating protein (GSAP) and imatinib in the regulation of γ-secretase activity and amyloid-β generation J Biol Chem 288:2521–2531, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu J, Lauretti E, Craige CP, et al. : Pharmacological modulation of GSAP reduces amyloid-β levels and tau phosphorylation in a mouse model of Alzheimer's disease with plaques and tangles J Alzheimers Dis 41:729–737, 2014 [DOI] [PubMed] [Google Scholar]