Abstract

Logic gates are devices that can perform Boolean logic operations and are the basic components of integrated circuits for information processing and storage. In recent years, molecular logic gates are gradually replacing traditional silicon-based electronic computers with their significant advantages and are used in research in water quality monitoring, heavy metal ion detection, disease diagnosis and treatment, food safety detection, and biological sensors. Logic gates at the molecular level have broad development prospects and huge development potential. In this review, the development and application of logic gates in various fields are used as the entry point to discuss the research progress of logic gates and logic circuits. At the same time, the application of logic gates in quite a few emerging fields is briefly summarized and predicted.
1. Introduction
A logic gate is a kind of Boolean logic execution device that uses “1” to represent “true value” or “high signal” and “0” to represent “false value” or “low signal”.1,2 In recent years, the development of logic gates at the molecular level has received extensive attention. A slice of logic gates that perform Boolean operations at the molecular level are called molecular logic gates.2,3 As an important genetic material, DNA can be used as a molecular logic gate based on quite a few unique properties of DNA strands,4 such as low cost, easy synthesis, superior biocompatibility, high programmability,1 and base mismatch.2 DNA logic gates can solve the bottleneck problem of traditional silicon material operation speed, which cannot continue to improve, and can successfully replace traditional silicon-based logic gates5 and gradually be applied in various fields. At present, logic gates at the molecular level mainly include basic logic gates (Figure 1)1 and combinatorial logic systems. The basic logic gates include single-input logic gates (“YES” logic gate, “NOT” logic gate, “PASS 0” logic gate, and “PASS 1” logic gate), two-input logic gates (“OR” logic gate, “AND” logic gate, “XOR” logic gate, “INHIBIT” logic gate, “NOR” logic gate, “NAND” gate, “XNOR” logic gate, and“IMPLICATION” logic gate),3 and multiple-input logic gates. Combinatorial logic systems are complex logic systems composed of basic logic gates, such as half-adder, half-subtractor, and digital comparator.
Figure 1.

(A) Equivalent logic circuits of basic logic gates and (B) corresponding truth tables of two-input ones. Adapted with permission from ref (1). Copyright 1958 Dover Publications.
2. Applications of Molecular Logic Gates
2.1. Ion Detection
2.1.1. Detection of Ions in Aqueous Media
Both F– ions and AcO– ions are important anions in aqueous media.6 F– ion is related to human health problems. As a drug ingredient, it can be used for sleeping pills, anesthetics, etc., and in clinical medicine, it can also be used for tooth correction and osteoporosis treatment. An abnormal content of F– ions can lead to a variety of diseases, such as thyroid diseases, etc., and can even lead to cancer in serious cases. AcO– ion is often used in the chemical and pharmaceutical industries. It can also be used in hemodialysis. Excessive intake of AcO– ion in the human or animal body will affect the metabolism of the organism.6 Fe3+ ion plays an important role in cell metabolism and oxygen transport in the life system. An Fe3+ ion content different from the normal value will lead to anemia and other diseases.7 The Mn7+ ion in permanganate is stable and easy to treat. It is often used as an oxidant to repair polluted groundwater resources. As a necessary trace element for the human body, the existence of Mn7+ ion can maintain the normal growth and metabolism of the human body. However, if the Mn7+ ion content in the human body exceeds the normal value, it will lead to hallucinations, mental disorders, and even permanent disability.8
In view of the above introduction of several kinds of cations and anions, researchers have proposed a variety of detection methods. Taking anions as an example, researchers have proposed chromatography (e.g., ion exchange chromatography) and spectrometry (e.g., atomic absorption spectrometry, flame atomic absorption spectrometry, etc.) to quantitatively detect anions;6 the above methods often lack of economical and simple operation methods and detection processes, and the detection efficiency is relatively low. In the study by Bhat et al.,6 an “INHIBIT” gate was constructed with selective receptors for F– and AcO– ions as input and the absorbance as output. Through the colorimetric method, the change in absorbance can be used to identify the ion content. This method can judge the ion content through the change in solution color. It is easy to operate and has good development prospects.
For detection of cations, at present atomic absorption spectrometry, spectrophotometry, and polarography (e.g., direct current polarography and differential pulse polarography) can be used.8 However, like anion detection methods, these methods still have the defects of complex operation, low sensitivity, and poor selectivity. In order to detect cations in aqueous media more efficiently, a new fluorescent probe LN-1 was designed to detect Fe3+ in the study by Wen.7 The detection principle of the LN-1 probe is that the fluorescence intensity changes when Fe3+ and EDTA are incorporated into the probe. Using this principle, the researchers constructed a two-input logic gate with Fe3+ and EDTA as inputs and the fluorescence intensity as the output. If and only if Fe3+ is input alone and EDTA is not input will the fluorescence be quenched, giving a fluorescence intensity signal of “0”. This is in line with the logic relationship of the “INHIBIT” gate (Figure 2),7 proving the feasibility of selective detection of Fe3+ with the LN-1 probe. Besides, this method has high selectivity and low detection limit.
Figure 2.

(a) Logic gate behavior of the LN-1 probe with Fe3+/EDTA. (b) Truth table for the “INHIBIT” logic gate. Reproduced with permission from ref (7). Copyright 2020 Elsevier B.V.
Gon et al.8 reported a new fluorescent probe, N,S,P-CNDSac, that can detect Mn7+. The detection principle of the probe is that Mn7+ can quench the fluorescence of the probe. After the addition of l-ascorbic acid (l-AA), Mn7+ is reduced to other valence states, and the fluorescence intensity of the probe recovers. According to the change in fluorescence intensity, with Mn7+ and l-AA as the inputs and the change in fluorescence intensity of N,S,P-CNDSac as the output, an “AND” logic gate was constructed. If and only if Mn7+ and l-AA are input as “1” at the same time will the fluorescence intensity be recovered, giving a fluorescence signal of “1”, which is in accordance with the logic relationship of the “AND” gate (Figure 38). This method is simple and has a low detection limit of Mn7+ in aqueous media. It also combines the biosensor well and makes up for the long time-consuming defect of the original method.
Figure 3.

“AND” logic gate with a two-input logic circuit. Reproduced from ref (8). Copyright 2017 American Chemical Society.
In the work of Dwivedi et al,9 a highly selective Schiff base fluorescence “on–off” module probe was developed for the detection of Fe3+ and F– in aqueous media. The functioning of the probe was regulated by —CH=N isomerization and ligand-to-metal charge transfer (LMCT) mechanisms. The in situ RAPHN–Fe3+ complex was deployed for the selective cascade sensing of F– ions. The fluorescent probe exhibited excellent bioimaging results with no toxicity in gut tissue of Drosophila. The selectivity and cell permeability of the RAPHN probe and its Fe3+ complex in aqueous media indicated its plausible application for monitoring iron and fluoride ions in biological systems.
To sum up, we can know that the method of combining logic gate to detect anions and cations in aqueous media solves the shortcomings of the original spectroscopic and polarographic methods, such as the long time required, complex operation, and low detection efficiency. At the same time, the method of constructing logic gates is a simple process and results in a low detection limit, which decreases the cost of detecting ions in water and shortens the detection time.
2.1.2. Detection of Heavy Metal Ions
The term “heavy metal” refers to nearly 50 metals, including Pb, Hg, Cr, and Cu. The atomic weight of these metals is usually greater than 63.5 and less than 200.6, and the atomic density is usually greater than 5 g/cm3. Heavy metals are widely distributed, highly toxic, and difficult to degrade in organisms.10 Therefore, it is necessary to find a simple and feasible method to detect heavy metal ions.
Cd2+ is a kind of heavy metal ion that poses a serious threat to human health, food safety, and water environment safety. If the content of highly toxic Cd2+ exceeds the normal value, it will cause various diseases11 and can even cause cancer or death in serious cases.12 In the past, researchers usually used atomic absorption spectrometry or fluorescence spectrometry to detect Cd2+, but these methods have high cost, are time-consuming, and have low detection efficiency, which are not conducive to large-scale detection of Cd2+. The heavy metal ion Ni2+ can be detected in wastewater, which is not degradable in water. Ni2+ is often used in battery manufacturing and catalytic reactions. Excessive Ni2+ will affect human health, often leading to diarrhea, dry cough, respiratory system damage, etc., and it can even cause cancer in serious cases.12 Pb2+ not only pollutes the environment but also causes serious harm to human health, affecting the nervous system, liver, and kidney function. After excessive intake of Pb2+, people will have hallucinations, dizziness, and headache.12,13 Normal intake of Cr3+ is beneficial to human immunity and health, but excessive intake of Cr3+ will affect the normal metabolism of the human body and cause harm to the function of human cells. Less-than-normal intake of Cr3+ will damage the nervous system and cardiovascular system of the human body.14 At present, Ag+ is often used in the pharmaceutical industry and thus is often present in industrial wastewater and causes water pollution. When excessive Ag+ enters the human body, it will damage the protein structure and osmotic regulation of the body, leading to liver problems and damage to the skin and bone.4,15 Hg2+ will produce bioaccumulation with the increase of food chain level and is extremely difficult to degrade after entering the human body. It will damage the human nervous system, digestive system, cause dyspnea, damage renal function, and even lead to liver and kidney failure or cancer.4,12,13,16 In addition to the above-mentioned detection methods for Cd2+, the traditional detection methods for heavy metal ions include mass spectrometry, inductively coupled plasma mass spectrometry, and high-performance liquid chromatography.4,16 These methods have a slice of shared defects: they are time-consuming and have low efficiency, complex operation, and high cost. The methods combined with logic gates can detect heavy metal ions quickly and selectively.
In recent years, quite a few researchers have used logic gates to detect heavy metal ions. For Cd2+ as an example, Chen et al.11 prepared the fluorescent probe NMM, which has only weak fluorescence. After binding with G-quadruplex DNAzyme, the fluorescence intensity can be significantly enhanced. Therefore, the precondition for producing a high signal “1” is to produce G-quadruplex DNAzyme. According to this principle, a series of logic gates were constructed to detect Cd2+ in rice samples. The fluorescence intensity of NMM is the output, and the inputs were different. When Cd2+ and DNA 1 were used as inputs, the “AND” gate was constructed. If and only if the inputs of Cd2+ and DNA 1 are both “1” would there be the formation of G-quadruplex DNAzyme. At this time, the fluorescence intensity would be enhanced, and the output would be “1”. When Cd2+ and DNA 4 were used as inputs, the “OR” gate was constructed (Figure 411). If and only if the inputs of Cd2+ and DNA 4 were both “0” would there be no G-quadruplex DNAzyme formation, and the output would be “0”. In any other case—(0, 1), (1, 0) (1, 1)—there will be G-quadruplex DNAzyme formation, and the output of the logic gate is “1”. When constructing the “INHIBIT” gate, the researchers regarded the complex of Cd2+–DNA 1 as one input in the two-input logic gate and took DNA 5 as another input. If and only if the Cd2+–DNA 1 complex is input alone will there be the generation of G-quadruplex DNAzyme, resulting in an enhancement of the fluorescence intensity, making the output “1”. The “IMPLICATION” gate is the reverse gate of the “INHIBIT” gate. In this work, the Cd2+–DNA 6 complex and DNA 7 are used as inputs, and DNA 8 is introduced into the sensing system. At this time, if and only if the Cd2+–DNA 6 complex is input alone, that is, when the input is (1, 0), the output is “0”, and in other cases, the output is “1”, which is just opposite to the truth table of the “INHIBIT” gate. Using the Cd2+–DNA 6 complex and DNA 9 as inputs, the “NOR” gate was constructed. If and only if the input of the “NOR” gate was (0, 0) will the G-quadruplex DNAzyme be produced, giving an output of “1”. Similarly, with Cd2+–DNA 6 complex and DNA 9 as inputs, a “NAND” gate is constructed. Different from the “NOR” gate, the sensing system contains DNA 11. In this case, if and only if the input is (1, 1) will there be no significant enhancement of the fluorescence intensity, giving an output of “0”.
Figure 4.
“OR” logic gate. Reproduced from ref (11). Copyright 2020 American Chemical Society.
For Ni2+ as an example, in the work of Xiao et al.3 a single input “YES” logic gate was constructed by combining DNA template silver nanoclusters. Ni2+ was used as the input, and the fluorescence intensity of DNA Ag NCs was the output. The content of Ni2+ was detected by the change in the fluorescence intensity. Ni2+ made Ag NCs more stable and enhanced the fluorescence. In other words, when the input is “1”, the output is “1”. The logic of the “YES” gate is simple, and it can detect Ni2+ conveniently. In addition, in their study, a “INHIBIT” gate was constructed to detect Ni2+ and Hg2+. The logic gate takes Ni2+ and Hg2+ as inputs 1 and 2, respectively, and DNA Ag NCs as the output. Only when the input is (1, 0) will the fluorescence be enhanced, giving an output of “1”; that is, only when Ni2+ is input alone, will the output be a high signal, and in the other cases, the output will be “0” because Hg2+ will quench the fluorescence.
In the work of Zou et al.,13 a DNA logic gate was constructed for the simultaneous detection of Pb2+ and Hg2+. The logic gate constructed in this work is combined with the surface-enhanced Raman scattering (SERS) technology, with Hg2+ and Pb2+ as the inputs and the SERS signal as the output. In this “INHIBIT” gate, according to the research in this paper, the SERS signal intensity is directly proportional to the concentration of Hg2+ and inversely proportional to the concentration of Pb2+. For the constructed “INHIBIT” gate, when the input state is (0, 0), the output SERS signal is “1” because of the principle of base pairing; when only Pb2+ is input separately, the SERS signal shows weak intensity, which can be regarded as “0”; when Hg2+ and Pb2+ are input at the same time or only Hg2+ is input, the output SERS signal has high intensity, that is, the output is “1”.
For Fe3+ and Cr3+ as examples, a new probe L1 was prepared in the work by Das et al.14 An “INHIBIT” gate was constructed with Fe3+ as input 1 and CN– as input 2, and the change in the L1 probe emission intensity at 558 nm was the output. The principle of the logic gate is that Fe3+ can increase the emission intensity of the probe, but the emission intensity of the probe will decrease as long as CN– exists. In other words, if and only if the input is (1, 0) will the output result be a high signal “1”. In the original state (0, 0) or when the input of CN– is “1”, the output result is “0”, which is displayed as a low signal. In addition, in this work the researchers also constructed a four-input logic gate based on “OR” and “INHIBIT”, and the output of the logic gate is still based on the emission intensity change of the L1 probe at 558 nm, with Al3+, Fe3+, Cr3+, and CN– as inputs 1, 2, 3, and 4, respectively. According to the research results in this paper, we can know that in the constructed four-input logic gate, when one or two inputs of Al3+, Fe3+ are “1” and the inputs of inputs 3 and 4 are “0”, the output is “1”. When one, two, or three inputs 1, 2, and 3 corresponding to Al3+, Fe3+, and Cr3+ are “1” and input 4 is “0”, the output is also “1”. When input 4 is “1”, that is, when CN– is input, then the output is “0” when other metal ions are also input. Here, input 4 can be regarded as the logic of a “NOT” gate.
In the study by Wang et al.,4 because of the specific interaction between Hg2+ and thymine (T) and the specific interaction between Ag+ and cytosine (C), based on the principle of base mismatch, combined with a biosensor, an “OR” gate was constructed to detect Hg2+ and Ag+. The researchers prepared a DNA probe labeled with carboxyfluorescein (FAM) at one end and a quencher at the other end. When the logic gate was constructed, Hg2+ and Ag+ were used as inputs, and the fluorescence intensity of the probe was used as the output. In this logic gate, the T–Hg2+–T structure or C–Ag+–C structure generated by base mismatch will bring the fluorophore and quencher at the ends of the probe close to each other, which leads to quenching of the fluorescence and a decrease in fluorescence intensity. When one or both of Hg2+ and Ag+ are present, the fluorescence of the probe will be quenched, so the existence of these two ions can be detected by the “OR” gate.
In the work of Tripathy et al.,16 a chemical sensor L1 was prepared, and an “INHIBIT” gate was constructed to detect Hg2+. The logic gate took Hg2+ and S2– as inputs and the change in the fluorescence intensity of L1 at 566 nm as the output. If and only if the input is (1, 0) will the output of the logic gate be “1”, that is to say, only when Hg2+ is input alone will the fluorescence signal of sensor L1 be enhanced. The existence of Hg2+ can be detected by the appearance of a high signal “1” in the output, which is consistent with the logic relationship of the “INHIBIT” gate.
In the research of Cheng et al.,15 a new class of droplet digital PCR (ddPCR) logic gate that can be used in an intelligent DNA calculator was also constructed on the basis of the base mismatch principle,4 including the “YES” gate and “OR” gate that can detect Hg2+ or Ag+ separately and the “AND” gate that can detect Hg2+ and Ag+ simultaneously. In this logic gate, Hg2+ and Ag+ are used as inputs, and the display layer color is defined as “1” and the display green as “0”. The ddPCR logic gates constructed in this work, compared with the various logic gates mentioned above, can display the final results of ion detection more clearly through intuitive color change and can also be applied to the detection of Ni2+, which is a new direction of using logic gates to detect heavy metal ions and has an overwhelmingly broad development space.
Basheer et al. synthesized two novel cyclohexyl thiosemicarbazides,17 H2L1 and H3L2. The anionic and cationic sensing properties of these two receptors have been revealed as an effective ratio and colorimetric sensor for selective and rapid detection of fluoride, copper, and cobalt ions (Figure 5).17
Figure 5.

Complementary molecular logic gate of H2L1 and H3L2. Reproduced with permission from ref (17). Copyright 2019 Elsevier B.V.
The He research group18 designed and synthesized the novel Schiff base chemosensor CMTAH containing coumarin fluoride by coupling coumarin aldehyde with sulfide-2-carbohydrates. CMTAH can be used as a selective chemosensor through the chroma/ratio reaction of Cu(II)/Zn(II) ions and the fluorescence-switchable strategy in aqueous media. The molecular logic gate is established by combining CMTAH and CMTAH, which is used for intelligent recognition of Cu(II) and Zn(II) ions (Figure 6.18).
Figure 6.

Combinatorial molecular logic gates. Reproduced with permission from ref (18). Copyright 2020 Elsevier Ltd.
From the above introduction, it is not difficult to see that most of the methods combined with logic gates are used to detect Hg2+, and logic gates can be used to detect other heavy metal ions in future work.
2.1.3. Detection of Other Ions
Roy and co-workers constructed a chemical sensor using a rhodamine dye derivative (HL) as a molecular logic gate probe.19 The input signals of this probe were Fe3+, Al3+, Cr3+, and AsO43–, and the output signal was the fluorescence intensity. The above three cations were mixed with HL to form HL–Fe3+, HL–Al3+, and HL–Cr3+ complexes, respectively, and the fluorescence signal intensity could be selectively enhanced. According to the signal intensity, highly sensitive detection of the three metal cations was realized. In addition, when AsO43– is present, it will replace the Al3+ in HL–Al3+, thereby quenching the fluorescence and realizing the detection of this anion. In 2020, Du20 designed a three-input series logic gate (AND-INH) for direct visual detection of stannous ion (Sn2+) and nitrite (NO2–).
Shree et al.21 designed and synthesized a novel Schiff base probe (DISN) as a selective colorimetric sensor of Co2+ and fluorescence sensor of F– (Figure 7).21 The sensor shows excellent sensitivities for Co2+ and F–, and the sensor can be employed as a unique tool to recognize F– in toothpaste samples and living cells.
Figure 7.

(a) “YES” gate and “INH” gate and (b) corresponding truth table. Adapted with permission from ref (21). Copyright 2019 Elsevier B.V.
2.2. Biomedical Applications
2.2.1. Disease Diagnosis and Treatment
The commonly used chemotherapy methods in the treatment of cancer can show positive therapeutic effect in the initial treatment, but after a multitude of times of chemotherapy, the therapeutic effect is greatly reduced. The reason can be summarized as the increased drug resistance of cancer cells to anticancer drugs.22 Early detection of cancer can improve the success rate of treatment and reduce the cost of treatment.23 As an exceedingly important cancer biomarker, microRNA is extremely important for the application of logic gates in disease treatment and diagnosis. Logic-gate-based sensors can detect different types of cancer cells by detecting different expression patterns of microRNA. Yue et al.22 developed a drug delivery system triggered by dual microRNA, which can be used in chemotherapy and gene therapy. This system has contributed to prolonging the drug time and enhancing the therapeutic effect.
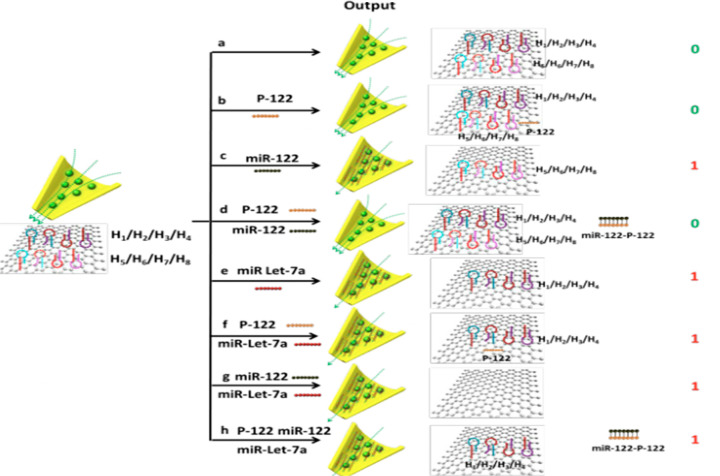
MicroRNA is directly related to the differentiation and apoptosis of hepatoma cells.24 In the work of Zhang et al.,24 we constructed a cascade of “INHIBIT-OR” gate, which can be used for intelligent detection or differentiation of miR-122 and miR Let-7a related to liver cancer. In the construction of this cascade logic gate, the researchers designed a sensing platform including H1, H2, H3, H4, H5, H6, H7, and H8 (Figure 8) and graphene oxide (GO).24 Without affecting the detection of miR Let-7a, P-122 complementary to miR-122 was introduced to suppress the signal changes caused by miR-122 to construct the “INHIBIT” gate. In the “INHIBIT” gate, P-122 and miR-122 are taken as inputs 1 and 2, respectively, and the high signal “1” and low signal “0” are taken as output by the rectifier ratio of the ion current. Only when miR-122 is input alone is the output a high signal. In addition, in the cascaded “INHIBIT-OR” gate, miR Let-7a is used as input 3. This cascade logic gate has a low cost and high detection speed.
Figure 8.
Schematic illustration of INH-OR cascaded logic gates for the multiplexed analysis of miR-122 and miR Let-7a in various input combinations. Reproduced from ref (24). Copyright 2020 American Chemical Society.
In the study by Sanjabi and Jahanian,23 a multi-input, multithreshold logic gate was designed to detect mir-2 and miR-122 related to cancer in mammalian cells. In this work, a new logic gate was designed that is fast and automatic and can be used for the simultaneous detection of microRNAs with different thresholds with high efficiency.
In the work of Yue et al.,25 a logic platform denoted as GQN was designed that can be used to distinguish the microRNA patterns of specific cancer cell types. The logic platform takes two microRNAs (miRNA-21 and miRNA-122) as inputs and quantum dot (QD) photoluminescence (PL) as the output to form an “AND” gate (Figure 9).25 Only when both microRNAs are input will the photoluminescence of GQN not be quenched giving the high signal “1” as the output signal.
Figure 9.
Schematic diagram of an “AND” logic gate. Reproduced from ref (25). Copyright 2018 American Chemical Society.
Wu et al.26 used the fluorescence intensity change of 2-AP as the output signal and adenosine triphosphate (ATP), K+, and the genes P53 and K-ras as the input signals to create a four-input “AND” logic platform (Figure 10).26 This was constructed to specifically detect P53 and K-ras genes related to pancreatic cancer. All four inputs are indispensable, as only when the input is (1, 1, 1, 1) will 2-AP show the high fluorescence signal “1”.
Figure 10.
(a) Four-input logic gate and (b) truth table. Reproduced with permission from ref (26). Copyright 2018 Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, published by Elsevier B.V.
In the study by Peng et al.,27 an “AND” gate was constructed using two miRNAs, miRNA-21 and miRNA-155, as inputs and the change in the fluorescence intensity of the signal probe (SP) labeled with the quenching agent Dabcyl and the fluorophore FAM as the output. In addition, T3 and T4 (complementary to SP function recognition regions I and II) were selected as inputs and the fluorescence signal was selected as the output to construct an “INHIBIT” gate. These two kinds of double-input logic gates (Figure 11)27 can simultaneously detect multiple microRNAs from tumor cells and can be used for disease diagnosis.
Figure 11.

(a) Schematic diagram of the “AND” logic gate operation with miRNA-21 and miRNA-155 as the inputs and (b) the corresponding truth table. (c) Schematic diagram of the “INHIBIT” logic gate operation with T3 and T4 as the inputs and (d) the corresponding truth table. Adapted with permission from ref (27). Copyright 2018 Elsevier B.V.
In addition to microRNA detection, mammalian cell synthesis is also associated with disease diagnosis. In the research work of Matsuura et al.,28 a series of double-input logic gates were constructed with two kinds of miRNA, miR-21 and miR-302a, as inputs and apoptotic genes as outputs. At the same time, miR-21, miR-302a, and miR-206 were used as inputs and apoptotic genes were used as outputs. Compared with the previous logic gates, the constructed logic gates can detect miRNAs with multiple inputs, which is conducive to applications in the medical field. Similarly, in the work of Li et al.,29 a dual-input “AND” logic gate based on mammalian cells was constructed. This work also has great application potential in the field of bioengineering.
Seelig et al.30 constructed an mRNA-based computing system that combined the recognition ability of nucleic acid bases with machine learning technology and successfully realized the identification of different gene expression cases of viral and bacterial respiratory system infection in host cells. In 2018, an intelligent nucleic acid system, called the adaptive immune response simulator (AIRS),31 was constructed by using nucleic acids and enzymes to simulate the basic functions of an acquired immune system. The system can intelligently recognize pathogen DNA and produce a specific immune response and memory effect on the pathogen sequence. The artificial immune system can play a role in prototype cells (Figure 12).31
Figure 12.

Schematic of an artificially acquired immune system built into a prototype cell. Reproduced from ref (31). Copyright 2018 American Chemical Society.
The Chang research group32 established a programmable molecular logic gate whose principle is to detect multiple single nucleotide polymorphisms (SNPs) by using the amplification effect of the fluorescence signal mediated by the CRISPR/Cas9 system. First, the CRISPR/Cas9 system identifies and slices the target strand to form a nucleic acid fragment containing a sticky terminal. Second, under the action of DNA polymerase and endocleticase, a signal amplification reaction was triggered to produce a large number of guanine-rich single-stranded DNAs, forming G-quadruplexes. Finally, thioflavin T (ThT) and G-quadruplexes combine to produce a fluorescence signal and complete the detection of SNPs. In addition, this principle was used to construct separate “AND”, “NOT”, and “OR” logic gates. The three logic gates realize the accurate diagnosis of gene loci and have the potential to detect SNPs (Figure 13).32
Figure 13.
Schematic diagram of multi-SNP detection based on the CRISPR/Cas9 system and related molecular logic gates. Reproduced from ref (32).
In 2019, the Wang research group33 designed a double-amplification cascade logic gate AND circuit controlled by double miRNA for the detection of cancer cells. In 2020, Cheng34 designed a logic gate detection system based on the programmable and easily assembled characteristics of DNA nanomachines. The in vitro detection of miRNA-21 and miRNA-155 and its application in the detection of tumor cells can selectively analyze multiple endogenous miRNAs in living cells for the identification of tumor cells, so as to improve the accuracy and reliability of clinical diagnosis. In the same year, Chen et al. designed a DNA logic gate35 that combines the recognition of multiple biomarkers with signal amplification to provide accurate, sensitive analysis of circulating tumor cells. It also has the potential to analyze rare cells that exist in small amounts but are of great significance (e.g., stem cells) in the fields of clinical diagnosis and biomedicine.
2.2.2. Pharmaceutical Analysis
Zhang et al.36 established a novel fluorescent typing method based on SNPs with logic gates. The mutation of one base in a single nucleotide fragment may involve two to four genotypes. Genotypes with a certain concentration are taken as the input signal, and changes in the relative fluorescence intensity of the fluorescent small molecule 2-amino-7-methyl-1,8-naphthyridine are taken as the output signal. Three kinds of molecular logic gates for SNP typing were constructed. The relative value of the output signal obtained from the experimental results is used to set the value to be judged. If the output signal is greater than the threshold value, it is defined to be “1”; otherwise, it is defined to be “0”. The results show that the constructed fluorescence detection system and the three designed logic gates can be used to distinguish the second-class or fourth-position SNPs genotypes logically and determine whether there are mutants and the number of mutants in the samples.
In the study by Ma et al.,37 a NAND molecular logic gate based on molecularly imprinted functionalized quantum dot composites (QDs@MIPs) was constructed for the simultaneous detection of streptomycin sulfate (SS) and kanamycin sulfate (KS). The molecularly imprinted dual-emission quantum dot composites DEQDs@MIPs were used as fluorescent probes for the rapid and simultaneous detection of SS and KS. In this system, the input of SS and KS and the output of the fluorescence value coincided with the NAND logic gate.
In 2019, Tavallali et al.38 designed a reversible colorimetric fluorescence chemical sensor for the determination of ascorbate (AscH–) in water/DMSO (9:1 v/v, 1.0 mM HEPES, pH 7.0). The “on–off” fluorescence and colorimetric response of the ion association complex to AscH– are based on the displacement mechanism. The linear ranges for the determination of AscH– by UV–vis and fluorescence spectroscopy are 3.9–62.6 and 9–85.4 μmol·L–1, respectively. The detection limits of both were also calculated as 0.4 and 0.2 μmol L–1. The method has also been successfully applied to the rapid identification of ascorbic acid in juice samples, human serum, and supplementary product formulations (Figure 14).38
Figure 14.

(a) Equivalent logic circuit and (b) corresponding truth table for the fluorescent sensor. Reproduced with permission from ref (38). Copyright 2019 Elsevier B.V.
2.2.3. Application to Cell Imaging
In this work,39 an economical and effective fluorescein-based fluorescent chemical sensor was developed for the selective determination of Zn2+. INHIBIT molecular logic gates were designed with Zn2+ and EDTA as chemical inputs by monitoring the emission intensity at 520 nm. The sensor successfully enabled real-time detection of Zn2+ in thin-layer chromatography (TLC)-based paper tape. Cell imaging of African green monkey kidney cells (Vero cells) was used to determine exogenous Zn2+ by immunofluorescence assay (IFA) (Figure 15).39
Figure 15.

Truth table and circuit diagram of an INHIBIT logic gate. Reproduced with permission from ref (39). Copyright 2019 Elsevier B.V.
In 2020, Wang40 designed an ATP adapter (ABA27) and I-motif in TSP’s opposite DNA using four-prism (TSP) as the framework and built an DNA framework nucleic acid (FNA) nanostructure with a double strand containing antisense oligonucleotide (ASO) as the sensor element of thymidine kinase 1 (TK1) mRNA. Two cascading logic gates (OR-AND and AND-AND) were constructed to produce different responses to ATP and H+. After FNA endocytosis into the target cell, the intracellular ATP molecule and H+ effectively controlled the release of the load ASO double chain from the four-prism (TSP) framework, which reacted with TK1 mRNA to generate fluorescence signals to realize the control of TK1 intracellular imaging of mRNA.
2.3. Food Safety Testing
In their work on detection of Cd2+ heavy metal ions, Chen et al.11 constructed logic gates to detect Cd2+ in rice samples, indicating the feasibility of combining logic gates in food safety detection. Biofilms formed by the self-protection mechanism of fungi produce various toxins that can lead to food pollution, which in turn leads to liver and kidney damage, cancer, and harm to human health. In addition, a large number of mycotoxins cause damage to the economy and reduce crop yields. In the study by Wang et al.,5 zearalenone (ZEN) and ochratoxin A (OTA) were detected. There are HPLC, TLC, and other methods to detect these two toxins, but most of these methods are time-consuming and costly. Therefore, it is necessary to develop a rapid and low-cost biosensor to detect toxins. In this work, the researchers constructed an aptasensor graphene oxide fluorescence resonance energy transfer (APT-GO FRET) platform to detect ZEN and OTA by combining the construction of logic gates. APT1 and APT2 were specifically recognized by ZEN and OTA, respectively. With GO and APT as inputs and compounds and fluorescence signals as outputs, respectively, “AND” gates and “INHIBIT” gates were constructed. This method can be used in the field of controlling biofilm toxins.
2.4. Application of Logic Gates Combined with Biosensors
In addition to the combination of logic gates and biosensors in the detection of various ions mentioned above,4,11,16 there are also the following studies.
Qin et al.41 constructed a new type of nanosensor, PEIN, with Cu2+ as input 1 and cysteine (Cys) as input 2. On the basis of the IMPLICATION operation, the fluorescence signal of PEIN at 444 nm is changed into an output for a two-input logic gate (Figure 16).41 This two-input logic gate consists of a “NOT”gate and an “OR”gate. The principle of the logic gate is that the fluorescence of PEIN at 444 nm can be quenched by Cu2+ but can be recovered after addition of the biological thiol Cys. In other words, only when the input is (1, 0) will the output be “0”. The logic gate constructed in this study can detect Cu2+ and Cys in sequence quickly.
Figure 16.

(a) Schematic diagram of the two-input logic gate and (b) the corresponding truth table. Reproduced with permission from ref (41). Copyright 2020 Elsevier B.V.
In the study by Chen et al.,42 polythymine-templated copper nanoparticles (polyT40-CuNPs) were prepared and combined with the method of constructing logic gates to detect Cr3+, P2O7– (PPi), and alkaline phosphatase (ALP). In the presence of Cr3+ alone, the fluorescence of the prepared polyT40-CuNPs is quenched; after addition of PPi to the system of polyT40-CuNPs and Cr3+, the fluorescence is recovered; after addition of ALP, PPi is hydrolyzed to Pi(PO43–), and therefore, Cr3+ is released, resulting in quenching of the polyT40-CuNPs fluorescence. On the basis of the above principles, the researchers constructed a series of combinatorial logic gates to detect Cr3+, PPi, and ALP. First, a “NOT-OR” gate was constructhed with Cr3+ as input 1, PPi as input 2, and the fluorescence intensity as the output (Figure 17a,b).42 Only when the input is (1, 0) is the output “0”; in other cases, the output of the logic gate is “1”. Then a “NOT-AND” gate was constructed with Cr3+ and PPi as input 1, ALP as input 2, and the fluorescence intensity as the output (Figure 18c,d).42 Only when the input is (1, 0) is the output is “1”; in other cases, the output of the logic gate is “0”. The constructed logic gate can be used to detect Cr3+, PPi, and ALP in human serum.
Figure 17.
(a) Schematic diagram and (b) truth table for the “NOT-OR” gate. (c) Schematic diagram and (d) truth table for the “NOT-AND” gate. Adapted with permission from ref (42). Copyright 2019 Elsevier B.V.
Figure 18.

Calculation of “AND” on a cell membrane surface. Reproduced from ref (50). Copyright 2018 American Chemical Society.
In addition, in the work of Cao et al.,43 the application of copper nanomaterials (CuNMs) based on DNA scaffolds in the field of logic gates is reviewed. CuNMs can be used as the input of logic gates and the output of logic gates, which has a broad application prospect in the field of biosensors.
In the work of Wang et al.,44 a logic circuit was constructed using a G-quadruplex/hemin complex as a quencher and a fluorescence sensing platform of the electron transfer mechanism. On the basis of the split G-quadruplex molecular beacon (SGMB) platform, ssDNA was used as the input and the change in the fluorescence signal caused by photoinduced electron transfer (PET) as the output, and a series of dual-input logic gates were constructed, including “OR”, “INHIBIT”, “AND”, and “XOR” logic gates, along with a combinational logic “INHIBIT-OR” gate. It has great potential in the field of biological computing. Fan et al.45 successfully constructed an upconversion luminescent DNA logic library for the first time, and they also constructed a universal biosensor.
Using functional and structural DNA nanotechnology, Peng46 designed and constructed several DNA devices of different types and functions, which are applied in ionic or molecular sensing, subcellular fluorescence imaging, and cross-membrane logical transport of anticancer target molecules. Lan constructed a marker free biosensor system47 and applied it to the analysis and detection of tetrotropic toxin, detection of the small molecule ATP, kanamycin ultrasensitive detection, Ag+- and Cys-sensitive analysis, and detection of a variety of substances. Du48 developed an advanced DNA fluorescent switch that catalyzes the conversion of “DNA fuel strands” into fluorescent output signals, which can be amplified through the fuel chain for accurate identification of low-concentration target DNA strands in complex solutions. In addition, a series logic gate bioluminescence sensor based on a DNA logic circuit “AND” gate was designed. The two sets of DNA fluorescence switches showed good recognition and differentiation of target oligonucleotides with slightly different base sequences, and the linear detection range was 1–100 nm.
There are a sea of examples of logic gates combined with biosensors to detect the target. Using this method to detect the target is simple and fast and has high selectivity and sensitivity. The application prospect of logic gates in this aspect is intensely broad.
2.5. DNA Logical Computing Models
2.5.1. DNA Logic Calculation Model Based on Chain Replacement Reaction
Morihiro et al.49 designed a logic device with miRNA as the input and small-molecule release as the output. At the same time, basic logic gates were cascaded to release the corresponding small-molecule outputs in response to the combination of three miRNA inputs. In order to complete the complex computation function, the structural complexity of the DNA logic gate itself is also increasing gradually. Peng et al.50 built an aptamer-based 3D DNA logic machine to perform “AND” logic calculations on the surface of cancer cell membranes to specifically identify overexpressed cancer markers (Figure 18).50 The special 3D structure integrates the recognition module and the calculation module together, which greatly improves the efficiency and accuracy of the whole system.
In 2017, Massey et al.51 used luminescent lanthanide complexes (LLCs) as markers to replace traditional fluorescent markers. When a chain replacement reaction occurred, a specific FRET-based fluorescence detection device marked on the end of the DNA was automatically triggered. A series of logical calculation models (“AND” (Figure 19),51 “NAND”, “OR”, and “NOR”) were designed, and their arbitrary cascade to form more complex DNA molecular logic circuits was realized. Qu et al.52 first constructed a DNA-based chemical reaction network in vitro to program the adhesion of mammalian cells on a chip. Then a series of DNA molecular logic gates (“AND”, “OR”, “XOR”, “AND-OR”) were constructed to regulate the whole reaction network through a DNA chain replacement reaction. This research provided a highly universal tool for the self-formalization of biological systems.
Figure 19.
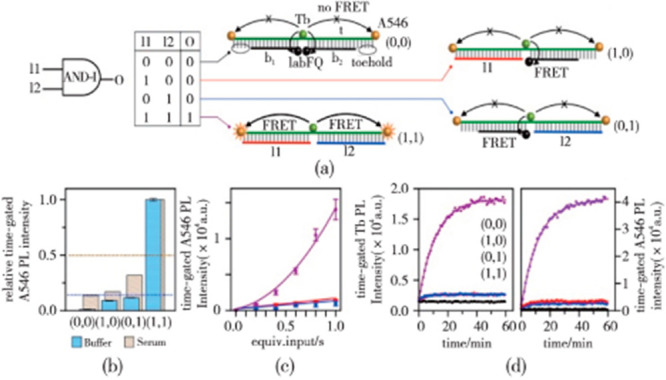
Novel “AND” computing model based on DNA strand substitution: the AND-I gate. (a) Truth table and DNA structures. The template (t), blocking (b1, b2), and input (I1, I2) oligonucleotides, Tb, A546, and IabFQ are labeled. Toeholds for strand displacement by the inputs are circled. Efficient FRET is shown as arrows. Suppressed FRET is shown with x-arrows. (b) Relative time-gated A546 PL intensity when the gate was challenged with 1 equiv of one or both inputs in buffer and 90% v/v serum (normalized to mimic truth-table-like output). The “false/true” threshold is the dashed line. (c) Calibration curve for challenges with different amounts of one or both inputs. (d) Kinetic traces of time-gated Tb and A546 PL intensities when the gate was challenged with one or both inputs. The data in panels (c) and (d) are with buffer. Reproduced from ref (51). Copyright 2017 American Chemical Society.
Song et al.53 reported another strategy to realize the square root operation of the DNA loop, in which the DNA logic gate is composed of single-stranded DNA, and the realization of the logic function is based on the chain substitution reaction mediated by DNA polymerase. The authors successfully constructed “AND” and “OR” gates, two- and three-layer cascading loops, and fan-in and fan-out DNA loops. One of the most complex DNA loops can also produce the largest square root integer of any four-digit binary number. Focusing on increasing the computation speed and reducing the sequence complexity enabled the logical operation of the square root to be realized with only 37 DNA strands, and the response time of the operation was about 25 min.
2.5.2. DNA Logic Calculation Model Based on G-Quadruplex
In 2017, Zhong et al.54 made two “INHIBIT” doors by adjusting the combination of G4 and corresponding hemin using the pH and the K+ and Pb2+ concentrations. The principle is to combine hemin with G4 between guanine and KB(OH)4 to construct an artificial enzyme hydrogel (AEH) system. The overall sensing strategy is based on the decrease of AEH system activity induced by Pb2+. As Pb2+ has a higher stabilization efficiency of G-quadruplex structure than K+, Pb2+ replaces K+ and triggers the a transformation of the G-quadruplex structure to release hemin, resulting in conversion of the enzyme structure and loss of enzyme activity (Figure 20).54 Another important element of hemin binding is that the pH is strictly controlled at 8. Both acidity and alkalinity affect the binding efficiency. “INHIBIT” doors based on the above principles can be used to check Pb2+.
Figure 20.
Artificial enzyme hydrogel (AEH) system. Reprinted from ref (54). Copyright 2018 American Chemical Society.
In 2018, Bader and Cockroft55 modulated the conformation of a G-quadruplex by modulating its structure. A series of DNA logic gates (“YES”, “AND”, “OR”) were prepared using the G4 structure which could bind specifically with thioflavin T and release obvious fluorescence as a detection method, and they could be run simultaneously in the system without causing mutual influence. In the same year, Wang et al.56 made a series of logic gates (“OR”, “INHIBIT”, “AND”, “XOR”) by regulating the structure of a G-quadruplex, which can be expanded from two-input logic gates to four-input logic gates. This work shows the great potential of G-quadruplexes in DNA logic gate design. In the same year, Lin et al.57 developed a special fluorescence reaction with N-methyl mesoporphyrin IX based on the G-quadruplex structure combined with the assisted detection of silver nanoclusters. A set of logic doors (“YES”, “AND”, “OR”, “XOR”, “INHIBIT”) were made, and because of the double output mechanism of the logic door, they combined and made functional molecular logic circuits, such as a half-adder, half-subtractor, multiplier, and divider. This molecular logic circuit was designed without the involvement of any enzymes and is very simple and efficient to regulate. G-quadruplex structures have also been found to form in the presence of exonucleases without affecting the properties and functions of the G-quadruplex structures.
2.5.3. Logical Computing Model Based on DNA Self-Assembly
In 2017, Campbell et al.58 constructed a set of logical computing models (“AND”, “NOT”, “X-NOR”, “OR”) based on controllable switching of the DNA tile structure. When the input signal number is high, the system recognizes the DNA input and accepts it. When the input signal is low, the cross-forked structure disintegrates to form discrete DNA. Moreover, logic computing models can be cascaded to each other to form more complex logic computing circuits, which can realize more complex logic computing functions
2.5.4. DNA Logic Computing Models Based on Other Techniques
Morihiro et al.59 designed a special logic calculation model of DNA. Different from the previous model that used single-stranded oligonucleotides as output signals, it could generate an organic small molecule as the output signal (Figure 21),59 and the Staudinger reduction reaction was used as a trigger to release and activate the small-molecule fluorophore. “AND” and “OR” gates were successfully constructed and used to make specific responses to the input miRNA to achieve the detection purpose. These logic gates can also be cascaded into more complex DNA logic circuits, which have been able to provide small-molecule outputs for three specific combinations of miRNA (miR-21, miR-122, miR-125b). In addition, such a logic computing circuit design can easily be multiplexed, so that two independent DNA logic computing circuits can run simultaneously without interfering with each other. This construction method not only makes up for the vacancy of biological or chemical molecule output triggered by DNA but also puts forward a new method for the research of DNA logic computation in artificial biology and nanotechnology fields
Figure 21.

miRNA-based logic computing circuits. Reproduced from ref (59). Copyright 2017 American Chemical Society.
In 2018, Yu et al.60 selected miRNA-141 abnormally expressed in prostate cancer and miRNA-21 found in many cancer cells as detection targets and designed a logical calculation model. The self-assembled hairpin -modified gold nanoparticles (HP-AuNPs), which could bind AuNPs, were used as the base material for a polymerization reaction. Duplex-specific nuclease (DSN) was used to retrieve and amplify the trigger chain of the polymerization reaction, and finally a large gold nanopolymer was formed on the surface of Au. The “AND” door and “INHIBIT” door built based on the above principles can be used for the detection of miRNA-141 and miRNA-21. The unlabeled electrochemical biosensor with high sensitivity and selectivity for detection of miRNAs has been successfully applied in the detection of miRNAs in different cancer cells. Ran et al.61 designed a logical computing model based on a hybrid chain reaction (HCR) that could be used to detect miRNAs (miRNA-200c and miRNA-605) associated with prostate cancer. The miRNA can be accurately detected by a simple DNA chain substitution reaction with a specific transition chain as the intermediate product, and then a molecular polymer can be generated by the HCR, combined with the auxiliary detection of AuNPs. An “AND” gate was designed to effectively detect miRNA-200c and miRNA-605.
2.6. DNA Cascaded Logic Circuits
Chatterjee et al.62 developed a “DNA domino” structure in which DNA hairpins on the surface of origami are activated along a specified signal transmission route. In the domino architecture, DNA hairpins interact preferentially with neighboring components, whose pattern of arrangement determines signal propagation, and the same DNA hairpins can be reused as local components. Experiments showed that basic logic gates and multi-input logic circuits can be implemented in a few minutes and are more robust and faster than systems running in solution.
3. The Latest Research Progress on Logic Gates
3.1. Nanorobots
Nanorobots can overcome some major challenges of modern medicine and have broad application prospects in cancer treatment and drug delivery. Because of the high stability of antibodies in whole blood, it is possible to develop logic gates in vivo using biological computing.63 At present, nanorobots have been used in disease diagnosis (e.g., tumor diagnosis). We plan to apply nanorobots in the field of human health monitoring (Figure 22).
Figure 22.

Application of nanorobots combined with logic gates in the medical field. Reproduced from ref (63). Copyright 2018 American Chemical Society.
Llopis-Lorente et al.64 designed a nanorobot that can read the information on glucose, NAD+, and urea from the environment can perform some basic Boolean logic operations.
3.2. Application of Logic Gates in Smart Phones
Chang et al.65 used the method of smart phone imaging (Figure 23)65 combined with logic gate construction to detect microRNA (miR-21, miR-31, and miR-141). The logic gate can be used in the field of disease diagnosis.
Figure 23.
Nucleic acid detection by smartphone imaging. Reproduced from ref (65). Copyright 2020 American Chemical Society.
3.3. Biological Detection
Liu et al.66 presented a novel method for monitoring oxidative stress and subsequent mitophagy with a single fluorescent molecular logic gate probe by caging two fluorophores, 1,8-naphthalimide and rhodamine, with AP and spirolactam, respectively. Changing the recognition unit allows the molecular logic system to be extended to a general method for monitoring mitochondrial autophagy induced by oxidative stress (Figure 24).66
Figure 24.
How Mito-PN works in the mitophagy process. Reproduced from ref (66). Copyright 2021 American Chemical Society.
3.4. Organic Molecule Detection
Terbium-based metal–organic frameworks (TB MOFs)67 have been successfully prepared in a simple and effective way. These well-structured TB MOFs exhibit the characteristics of Tb3+, and the green-emitting ions are excited by UV light. It is worth noting that TB MOFs can be used as convenient and efficient luminescence sensors for volatile organic compounds (VOCs). In particular, TB MOFs show high selectivity and excellent sensitivity to styrene solution and vapor through a fluorescence quenching mechanism. TB MOFs can realize the rapid detection of styrene vapor, and the response time is 30 s (Figure 25).67
Figure 25.

Scheme and logic circuit for a VOC luminous sensor. Reproduced with permission from ref (67). Copyright 2019 Elsevier B.V.
3.5. Intelligent DNA Molecular Machine
Thubagere et al.68 designed and reported a DNA molecular robot that could perform the task of cargo sorting at the nanoscale. The DNA robot consisted of several simple building blocks (one arm and one hand for picking up goods and one leg and two feet for walking) and integrated a simple algorithm. Under the condition of no energy supply, it can walk autonomically on the two-dimensional DNA origami experimental field driven by a chain replacement reaction, pick up various types of goods that are disorderly at the beginning, and carry out cargo sorting to deliver different types of goods to different destinations. Chao et al.69 further explored the development of intelligent molecular machines based on a DNA origami platform. They defined a single-path maze on DNA origami and designed a DNA single-molecule navigation system that could perform a deep parallel-first search operation on a two-dimensional origami platform to find the correct solution to the maze. The maze has a unique entrance (A), a unique exit (J), and four nonexit ends (C, F, G, H), which is equivalent to a three-node root tree with 10 vertices, with the entrance of the maze as the “root” and one of the leaves as its exit. During the search of the maze, the DNA molecular cruisers on each of the DNA origami structures would start from a starting point and go to an end point, possibly an exit or a dead end.
Wang et al.70 proposed a novel and powerful DNA nanomachine that can detect DNA by combining strand displacement reactions with DNA walkers. The electrochemical signals generated from different input combinations can be used to distinguish multiple target DNAs (Figure 26).70
Figure 26.

DNA nanomeds for multiple DNA test schematics. Reproduced with permission from ref (70). Copyright 2018 Elsevier B.V.
Woods et al.71 reported a reprogrammable general-purpose self-assembly computing system that ran a total of 21 algorithms, including copying, sorting, recognition of palindromes and multiples of 3, random walking, simulation of cellular automata, generation of deterministic and random patterns, etc., with an overall error rate of less than 1/3000 per SST.
Chen et al. carried out deep research on DNA origami rings. DNA origami technology can be used in urban buildings.72 Using a hierarchical approach to reduce the join edges, the researchers used only one pair of logical controls to form a two-by-two array and three pairs of logical controls to form a four-by-four array based on a two-by-two array. In their report, the feasibility of using minimal pairs of DNA linkage chains to realize algorithm-based self-assembly of finite DNA is discussed. The same researchers73 controlled the cross-linking angle by using an oblique linking strategy for cross-shaped DNA origami, which allowed the creation of DNA origami rings of different sizes. This method can be used in the field of super-resolution microscopy and is simple and convenient.
4. Conclusions and Future Prospects
In this review, we have summarized the application of logic gates in an ocean of fields in the last 5 years and briefly introduced research on applications of logic gates in the emerging field of nanomaterials. Through the summary of the application of logic gates in the past few years, we can know that logic gates are more used in ion detection, disease diagnosis, and biosensors and less in the field of food safety detection. In the future, we can increase the strength of research on food safety. Also, in various fields there are many studies on binary logic gates, among which the construction of double-input logic gates is the most freqent, especially the “INHIBIT” gate and “AND” gate. There are extremely few applications of single-input logic gates, with only a few studies on “YES” gates. Research on multi-input and multidomain-valued logic gates is also extremely scarce but can be expanded by combination with nanodevices. Logic gates now have a pleasurable development prospect in the field of medicine. In the future, we can study logic gates in combination with medicine.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants 21864020 and 51503106), the Natural Science Foundation of Inner Mongolia (Grants 2018MS02012 and 2019MS02014), the Collaborative Innovation Center for Water Environmental Security of the Inner Mongolia Autonomous Region, China, (Grant XTCX003), and the Young Science and Technology Talents Program (Leading Person) in Inner Mongolia Autonomous Region Colleges and Universities (Grant NJYT-19-A04).
Biographies
Lijun Liu is currently a Master’s student at Inner Mongolia Normal University (with Professor Jun Ai). She is currently focused on research into bionanomaterials and DNA logic gate construction.
Pingping Liu is currently a Master’s student at Inner Mongolia Normal University (with Professor Jun Ai). She is currently focused on research into aptamer functionalization materials and biosensor construction.
Lu Ga obtained her B.Sc. degree in Chemistry in 2000 from Inner Mongolia Normal University and her M.S. degree in Medical Chemistry in 2005 from Inner Mongolia Medical University. At present, she is an associate professor and works at Inner Mongolia Medical University. Her current research is focused on targeted drugs and Mongolian medical nanomaterials.
Jun Ai obtained his B.Sc. and M.S. degrees in Chemistry in 2000 and 2004 from Inner Mongolia Normal University and Inner Mongolia University, respectively. He then worked at Inner Mongolia Normal University and at present he is a professor. In 2009, he joined Prof. Erkang Wang’s group and received his Ph.D. degree in Analytical Chemistry in January 2013 from Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. His studies are centered on DNA sensor construction and fabrication of nanobiomaterials.
The authors declare no competing financial interest.
References
- Boole G.An Investigation of the Laws of Thought; Dover: New York, 1958. [Google Scholar]
- Feng C.; Chen T.; Mao D.; Zhang F.; Tian B.; Zhu X. L. Construction of a Ternary Complex Based DNA Logic Nanomachine for a Highly Accurate Imaging Analysis of Cancer Cells. ACS Sensors 2020, 5 (10), 3116–3123. 10.1021/acssensors.0c01166. [DOI] [PubMed] [Google Scholar]
- Xiao Z.; Huang D.; Bian M.; Yuan Y.; Nie J. Fluorescence Detection Based on DNA-templated Silver Nanoclusters and the Construction of Multi-level Logic Gate. Chem. J. Chin. Univ. 2020, 41 (1), 102–110. [Google Scholar]
- Wang L.; Zhang Y. Y.; Dong Y. F. A Multifunctional Molecular Probe for Detecting Hg2+ and Ag2+ Based on Ion-Mediated Base Mismatch. Sensors 2018, 18 (10), 3280. 10.3390/s18103280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Yang Q.; Wu W. Graphene-Based Steganographic Aptasensor for Information Computing and Monitoring Toxins of Biofilm in Food. Front. Microbiol. 2020, 10, 3139. 10.3389/fmicb.2019.03139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M. P.; Vinayak S.; Yu J.; Jung H. Y.; Kurkuri M. Colorimetric Receptors for the Detection of Biologically Important Anions and Their Application in Designing Molecular Logic Gate. ChemistrySelect 2020, 5 (42), 13135–13143. 10.1002/slct.202003147. [DOI] [Google Scholar]
- Wen X.; Yan L.; Fan Z. Multi-responsive fluorescent probe based on AIE for the determination of Fe3+, total inorganic iron, and CN– in aqueous medium and its application in logic gates. J. Photochem. Photobiol., A 2021, 405, 112969. 10.1016/j.jphotochem.2020.112969. [DOI] [Google Scholar]
- Gong X.; Li Z.; Hu Q.; Zhou R. X.; Shuang S. M.; Dong C. N,S,P Co-Doped Carbon Nanodot Fabricated from Waste Microorganism and Its Application for Label-Free Recognition of Manganese (VII) and l-Ascorbic Acid and AND Logic Gate Operation. ACS Appl. Mater. Interfaces 2017, 9 (44), 38761–38772. 10.1021/acsami.7b11170. [DOI] [PubMed] [Google Scholar]
- Dwivedi R.; Singh D. P.; Singh S.; Singh A. K.; Chauhan B. S.; Srikrishna S.; Singh V. P. Logic gate behavior and intracellular application of a fluorescent molecular switch for the detection of Fe3+ and cascade sensing of F– in pure aqueous media. Org. Biomol. Chem. 2019, 17 (32), 7497–7506. 10.1039/C9OB01398A. [DOI] [PubMed] [Google Scholar]
- Shao Y.; Dong Y.; Fan L.; Wang L.; Yuan X.; Zhang M.; Liu B.; Li D.; Zhao S. Review on Nanomaterial-based Electrochemical Aptasensors for Heavy Metal Detection in Food. Sci. Technol. Food Ind. 2021, 42 (19), 418–528. 10.13386/j.issn1002-0306.2020080068. [DOI] [Google Scholar]
- Chen J.; Pan J.; Liu C. Versatile Sensing Platform for Cd2+ Detection in Rice Samples and Its Applications in Logic Gate Computation. Anal. Chem. 2020, 92 (8), 6173–6180. 10.1021/acs.analchem.0c01022. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Experimental study on the treatment of nickel tartrate wastewater by alkaline earth metal enhanced precipitation. Guangdong University of Technology, 2019. [Google Scholar]
- Zou Q.; Li X.; Xue T.; Zheng J.; Su Q. SERS detection of mercury (II)/lead (II): A new class of DNA logic gates. Talanta 2019, 195, 497–505. 10.1016/j.talanta.2018.11.089. [DOI] [PubMed] [Google Scholar]
- Das D.; Alam R.; Katarkar A.; Ali M. A differentially selective probe for trivalent chemosensor upon single excitation with cell imaging application: potential applications in combinatorial logic circuit and memory devices. Photochem. Photobiol. Sci. 2019, 18 (1), 242–252. 10.1039/C8PP00381E. [DOI] [PubMed] [Google Scholar]
- Cheng N.; Zhu P.; Xu Y.; Huang K. L.; Luo Y. B.; Yang Z. S.; Xu W. T. High-sensitivity assay for Hg (II) and Ag (I) ion detection: A new class of droplet digital PCR logic gates for an intelligent DNA calculator. Biosens. Bioelectron. 2016, 84, 1–6. 10.1016/j.bios.2016.04.084. [DOI] [PubMed] [Google Scholar]
- Tripathy M.; Subuddhi U.; Patel S. A styrylpyridinium dye as chromogenic and fluorogenic dual mode chemosensor for selective detection of mercuric ion: Application in bacterial cell imaging and molecular logic gate. Dyes Pigm. 2020, 174, 108054. 10.1016/j.dyepig.2019.108054. [DOI] [Google Scholar]
- Basheer S. M.; Bhuvanesh N. S. P.; Sreekanth A. Analytical and computational investigation on host-guest interaction of cyclohexyl based thiosemicarbazones: Construction of molecular logic gates using multi-ion detection. Mater. Sci. Eng., C 2019, 105, 110127. 10.1016/j.msec.2019.110127. [DOI] [PubMed] [Google Scholar]
- He X. J.; Xie Q.; Fan J. Y.; Xu C. C.; Xu W.; Li Y. H.; Ding F.; Deng H.; Chen H.; Shen J. L. Dual-functional chemosensor with colorimetric/ratiometric response to Cu(II)/Zn(II) ions and its applications in bioimaging and molecular logic gates. Dyes Pigm. 2020, 177, 108255. 10.1016/j.dyepig.2020.108255. [DOI] [Google Scholar]
- Roy P.; et al. Rhodamine based chemosensor for trivalent cations: Synthesis, spectral properties, secondary complex as sensor for arsenate and molecular logic gates. Sens. Actuators, B 2017, 246, 518–534. 10.1016/j.snb.2017.02.094. [DOI] [Google Scholar]
- Du J.Research on Construction and Application of Colorimetric Sensing System Based on Molybdenum Trioxide. Southwest University of Science and Technology, 2020. [Google Scholar]
- Shree G. J.; Murugesan S.; Siva A. A highly sensitive and selective Schiff-base probe as a colorimetric sensor for Co2+ and a fluorimetric sensor for F– and its utility in bio-imaging, molecular logic gate and real sample analysis. Spectrochim. Acta, Part A 2020, 226, 117613. 10.1016/j.saa.2019.117613. [DOI] [PubMed] [Google Scholar]
- Yue R.; Chen M.; Ma N. Dual MicroRNA-Triggered Drug Release System for Combined Chemotherapy and Gene Therapy with Logic Operation. ACS Appl. Mater. Interfaces 2020, 12 (29), 32493–32502. 10.1021/acsami.0c09494. [DOI] [PubMed] [Google Scholar]
- Sanjabi M.; Jahanian A. Multi-threshold and Multi-input DNA Logic Design Style for Profiling the MicroRNA Biomarkers of Real Cancers. IET Nanobiotechnol. 2019, 13 (7), 665–673. 10.1049/iet-nbt.2018.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Cheng J.; Shi W.; Li K. B.; Han D. M.; Xu J. J. Fabrication of a biomimetic nanochannel logic platform and its applications in the intelligent detection of miRNA related to liver cancer. Anal. Chem. 2020, 92 (8), 5952–5959. 10.1021/acs.analchem.0c00147. [DOI] [PubMed] [Google Scholar]
- Yue R.; Li Z.; Wang G.; Li J.; Ma N. Logic Sensing of MicroRNA in Living Cells Using DNA-Programmed Nanoparticle Network with High Signal Gain. ACS Sens. 2019, 4 (1), 250–256. 10.1021/acssensors.8b01422. [DOI] [PubMed] [Google Scholar]
- Wu S.; Peng P.; Wang H. H.; Li T. Stimuli-Triggered Strand Displacement-Based Multifunctional Gene Detection Platform Controlled by A Multi-Input DNA Logic Gate. Chin. J. Anal. Chem. 2018, 46 (5), e1832–e1837. 10.1016/S1872-2040(18)61084-9. [DOI] [Google Scholar]
- Peng Y.; Zhou W.; Yuan R.; Xiang Y. Dual-input molecular logic circuits for sensitive and simultaneous sensing of multiple microRNAs from tumor cells. Sens. Actuators, B 2018, 264, 202–207. 10.1016/j.snb.2018.02.043. [DOI] [Google Scholar]
- Matsuura S.; Ono H.; Kawasaki S.; Kuang Y.; Fujita Y.; Saito H. Synthetic RNA-based logic computation in mammalian cells. Nat. Commun. 2018, 9, 4847. 10.1038/s41467-018-07181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.; Su W.; Zhang C. Linear Double-Stranded DNAs as Innovative Biological Parts to Implement Genetic Circuits in Mammalian Cells. FEBS J. 2019, 286 (12), 2341–2354. 10.1111/febs.14816. [DOI] [PubMed] [Google Scholar]
- Lopez R.; Wang R.; Seelig G. A molecular multi-gene classifier for disease diagnostics. Nat. Chem. 2018, 10 (07), 746–754. 10.1038/s41557-018-0056-1. [DOI] [PubMed] [Google Scholar]
- Lyu Y. F.; Wu C. C.; Heinke C.; Han D.; Cai R.; Teng I. T.; Liu Y.; Liu H.; Zhang X. B.; Liu Q. L.; Tan W. H. Constructing Smart Protocells with Built-in DNA Computational Core to Eliminate Exogenous Challenge. J. Am. Chem. Soc. 2018, 140 (22), 6912–6920. 10.1021/jacs.8b01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.; Chen H.; Chen S.; Liu Y.; Liu W.. Based on the CRISPR/Cas9 system-mediated signal amplification strategy to construct programmable molecular logic gates to achieve multiple SNP detection. In Abstracts of the 11th Chinese National Conference on Chemical Biology; Professional Committee of Chemical Biology of the Chinese Chemical Society, 2019; Vol. 3, p 1. [Google Scholar]
- Quan K.; Li J.; Wang J.; Xie N.; Wei Q.; Tang J.; Yang X.; Wang K.; Huang J. Dual-microRNA-controlled double-amplified cascaded logic DNA circuits for accurate discrimination of cell subtypes. Chem. Sci. 2019, 10 (5), 1442–1449. 10.1039/C8SC04887H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B.Construction of a new DNA nanomachine and its application in the Raman detection of tumor cells. Qingdao University of Science and Technology, 2020. [Google Scholar]
- Chen T. S.; Fu X.; Zhang Q. Q.; Mao D. S.; Song Y. C.; Feng C.; Zhu X. L. A DNA logic gate with dual-anchored proximity aptamers for the accurate identification of circulating tumor cells. Chem. Commun. 2020, 56 (51), 6961–6964. 10.1039/D0CC00564A. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Hou S.; Lu W. Study on the Fluorescence Typing Method of Single Nucleotide Polymorphisms Based on Logic Gates. Anal. Lab. 2018, 37 (12), 1451–1456. [Google Scholar]
- Ma Y.; Jin X.; Xing Y.; Ni G.; Peng J. Construction of an NAND logic gate based on molecularly imprinted dual-emission quantum dot composites for the detection of antibiotics. Anal. Methods 2019, 11 (15), 2033–2040. 10.1039/C9AY00101H. [DOI] [Google Scholar]
- Tavallali H.; Deilamy-Rad G.; Mosallanejad N. A reversible and dual responsive sensing approach for determination of ascorbate ion in fruit juice, biological, and pharmaceutical samples by use of available triaryl methane dye and its application to constructing a molecular logic gate and a set/reset memorized device. Spectrochim. Acta, Part A 2019, 215, 276–289. 10.1016/j.saa.2019.02.094. [DOI] [PubMed] [Google Scholar]
- Das B.; Jana A.; Mahapatra A. D.; Chattopadhyay D.; Dhara A.; Mabhai S.; Dey S. Fluorescein derived Schiff base as fluorimetric zinc (II) sensor via ‘turn on’ response and its application in live cell imaging. Spectrochim. Acta, Part A 2019, 212, 222–231. 10.1016/j.saa.2018.12.053. [DOI] [PubMed] [Google Scholar]
- Wang H.Design and application of intelligent DNA logic platform based on aptamer molecular recognition. University of Science and Technology of China, 2020. [Google Scholar]
- Qin X.; Tong Q.; Chang M.; Liang S. A hydrophilic polymer-based bifunctional nanosensor for sequential fluorescence sensing of Cu2+ and biothiols and constructing molecular logic gate. J. Photochem. Photobiol., A 2020, 402, 112792. 10.1016/j.jphotochem.2020.112792. [DOI] [Google Scholar]
- Chen C.; Geng F. H.; Wang Y. X.; Yu H. D.; Li L.; Yang S.; Liu J. H.; Huang W. Design of a nanoswitch for sequentially multi-species assay based on competitive interaction between DNA-templated fluorescent copper nanoparticles, Cr3+ and pyrophosphate and ALP. Talanta 2019, 205, 120132. 10.1016/j.talanta.2019.120132. [DOI] [PubMed] [Google Scholar]
- Cao Q.; Li J.; Wang E. Recent advances in the synthesis and application of copper nanomaterials based on various DNA scaffolds. Biosens. Bioelectron. 2019, 132, 333–342. 10.1016/j.bios.2019.01.046. [DOI] [PubMed] [Google Scholar]
- Wang S.; Sun J.; Zhao J.; Lu S. S.; Yang X. R. Photo-Induced Electron Transfer-Based Versatile Platform with G-Quadruplex/Hemin Complex as Quencher for Construction of DNA Logic Circuits. Anal. Chem. 2018, 90 (5), 3437–3442. 10.1021/acs.analchem.7b05145. [DOI] [PubMed] [Google Scholar]
- Fan D.; Wang E.; Dong S. Upconversion-chameleon-driven DNA computing: the DNA-unlocked inner-filter-effect (DU-IFE) for operating a multicolor upconversion luminescent DNA logic library and its biosensing application. Mater. Horiz. 2019, 6 (2), 375–384. 10.1039/C8MH01151F. [DOI] [Google Scholar]
- Peng P.Construction and application of functional DNA molecular devices. University of Science and Technology of China, 2020. [Google Scholar]
- Lan Y.Construction and analysis application of label-free multifunctional nucleic acid aptamer sensing system based on signal amplification strategy. Shanxi University, 2020. [Google Scholar]
- Du J.Construction and Application of DNA Fluorescent Probes Based on Cross-Pivot Mediated Strand Replacement. Xiangtan University, 2020. [Google Scholar]
- Morihiro K.; Ankenbruck N.; Lukasak B.; Deiters A. Small molecule release and activation through DNA computing. J. Am. Chem. Soc. 2017, 139 (39), 13909–13915. 10.1021/jacs.7b07831. [DOI] [PubMed] [Google Scholar]
- Peng R. Z.; Zheng X. F.; Lyu Y. F.; Xu L. J.; Zhang X. B.; Ke G. L.; Liu Q. L.; You C. J.; Huan S. Y.; Tan W. H. Engineering a 3D DNA-logic gate nanomachine for bispecific recognition and computing on target cell surfaces. J. Am. Chem. Soc. 2018, 140 (31), 9793–9796. 10.1021/jacs.8b04319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey M.; Medintz I. L.; Ancona M. G.; Algar W. R. Time-gated FRET and DNA-based photonic molecular logic gates: AND, OR, NAND, and NOR. ACS Sens. 2017, 2 (8), 1205–1214. 10.1021/acssensors.7b00355. [DOI] [PubMed] [Google Scholar]
- Qu X. M.; Wang S. P.; Ge Z. L.; Wang J. B.; Yao G. B.; Li J.; Zuo X. L.; Shi J. Y.; Song S. P.; Wang L. H.; Li L.; Pei H.; Fan C. H. Programming cell adhesion for on-chip sequential Boolean logic functions. J. Am. Chem. Soc. 2017, 139 (30), 10176–10179. 10.1021/jacs.7b04040. [DOI] [PubMed] [Google Scholar]
- Song T. Q.; Eshra A.; Shah S. L.; Bui H.; Fu D.; Yang M.; Mokhtar R.; Reif J. Fast and compact DNA logic circuits based on single-stranded gates using strand-displacing polymerase. Nat. Nanotechnol. 2019, 14 (11), 1075–1081. 10.1038/s41565-019-0544-5. [DOI] [PubMed] [Google Scholar]
- Zhong R. B.; Xiao M. S.; Zhu C. F.; Shen X. Z.; Tang Q.; Zhang W. J.; Wang L. H.; Song S. P.; Qu X. M.; Pei H.; Wang C.; Li L. Logic catalytic interconversion of G-molecular hydrogel. ACS Appl. Mater. Interfaces 2018, 10 (5), 4512–4518. 10.1021/acsami.7b17926. [DOI] [PubMed] [Google Scholar]
- Bader A.; Cockroft S. L. Simultaneous G-quadruplex DNA logic. Chem. - Eur. J. 2018, 24 (19), 4820–4824. 10.1002/chem.201800756. [DOI] [PubMed] [Google Scholar]
- Wang S.; Sun J.; Zhao J. H.; Lu S. S.; Yang X. R. Photo-induced electron transfer-based versatile platform with G-quadruplex /Hemin complex as quencher for construction of DNA logic circuits. Anal. Chem. 2018, 90 (05), 3437–3442. 10.1021/acs.analchem.7b05145. [DOI] [PubMed] [Google Scholar]
- Lin X.; Liu Y.; Deng J.; Lyu Y.; Qian P.; Li Y.; Wang S. Multiple advanced logic gates made of DNA-Ag nanocluster and the application for intelligent detection of pathogenic bacterial genes. Chem. Sci. 2018, 9 (7), 1774–1781. 10.1039/C7SC05246D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. A.; Peterson E.; Kolpashchikov D. M. Self-assembling molecular logic gates based on DNA crossover tiles. ChemPhysChem 2017, 18 (13), 1730–1734. 10.1002/cphc.201700109. [DOI] [PubMed] [Google Scholar]
- Morihiro K.; Ankenbruck N.; Lukasak B.; Deiters A. Small molecule release and activation through DNA computing. J. Am. Chem. Soc. 2017, 139 (39), 13909–13915. 10.1021/jacs.7b07831. [DOI] [PubMed] [Google Scholar]
- Yu S.; Wang Y. Y.; Jiang L. P.; Bi S.; Zhu J. J. Cascade amplification mediated in situ hot-spot assembly for MicroRNA detection and molecular logic gate operations. Anal. Chem. 2018, 90 (7), 4544–4551. 10.1021/acs.analchem.7b04930. [DOI] [PubMed] [Google Scholar]
- Ran X.; Wang Z. Z.; Ju E. G.; Pu F.; Song Y. Q.; Ren J. S.; Qu X. G. An intelligent 1:2 demultiplexer as an intracellular theranostic device based on DNA/Ag clusters gated nanovehicles. Nanotechnology 2018, 29 (06), 065501. 10.1088/1361-6528/aaa09a. [DOI] [PubMed] [Google Scholar]
- Chatterjee G.; Dalchau N.; Muscat R. A.; Phillips A.; Seelig G. A spatially localized architecture for fast and modular DNA computing. Nat. Nanotechnol. 2017, 12 (9), 920–927. 10.1038/nnano.2017.127. [DOI] [PubMed] [Google Scholar]
- Tregubov A. A.; Nikitin P. I.; Nikitin M. P. Advanced Smart Nanomaterials with Integrated Logic-Gating and Biocomputing: Dawn of Theranostic Nanorobots. Chem. Rev. 2018, 118 (20), 10294–10348. 10.1021/acs.chemrev.8b00198. [DOI] [PubMed] [Google Scholar]
- Llopis-Lorente A.; de Luis B.; Garcia-Fernandez A.; Jimenez-Falcao S.; Orzáez M.; Sancenon F.; Villalonga R.; Martinez-Manez R. Hybrid Mesoporous Nanocarriers Act by Processing Logic Tasks: Toward the Design of Nanobots Capable of Reading Information from the Environment. ACS Appl. Mater. Interfaces 2018, 10 (31), 26494–26500. 10.1021/acsami.8b05920. [DOI] [PubMed] [Google Scholar]
- Chang D.; Kim K. T.; Lindberg E.; Winssinger N. Smartphone DNA or RNA Sensing Using Semisynthetic Luciferase-Based Logic Device. ACS Sens. 2020, 5 (3), 807–813. 10.1021/acssensors.9b02454. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Sun Q.; Yan M.; Zhang C.; Yuan H.; He W. Activity-Based Fluorescent Molecular Logic Gate Probe for Dynamic Tracking of Mitophagy Induced by Oxidative Stress. Anal. Chem. 2021, 93 (7), 3502–3509. 10.1021/acs.analchem.0c04854. [DOI] [PubMed] [Google Scholar]
- Feng L.; Dong C. L.; Li M. F.; Li L. X.; Jiang X.; Gao R.; Wang R. J.; Zhang L. J.; Ning Z. L.; Gao D. J.; Bi J. Terbium-based metal-organic frameworks: highly selective and fast respond sensor for styrene detection and construction of molecular logic gate. J. Hazard. Mater. 2020, 388, 121816. 10.1016/j.jhazmat.2019.121816. [DOI] [PubMed] [Google Scholar]
- Thubagere A. J.; Li W.; Johnson R. F.; Chen Z.; Doroudi S.; Lee Y. L.; Izatt G.; Wittman S.; Srinivas N.; Woods D.; Winfree E.; Qian L. A cargo-sorting DNA robot. Science 2017, 357 (6356), eaan6558. 10.1126/science.aan6558. [DOI] [PubMed] [Google Scholar]
- Chao J.; Wang J. B.; Wang F.; Ouyang X. Y.; Kopperger E.; Liu H. J.; Li Q.; Shi J. Y.; Wang L. H.; Hu J.; Wang L. H.; Huang W.; Simmel F. C.; Fan C. H. Solving mazes with single-molecule DNA navigators. Nat. Mater. 2019, 18 (03), 273–279. 10.1038/s41563-018-0205-3. [DOI] [PubMed] [Google Scholar]
- Wang K.; He M.-Q.; Zhai F.-H.; Wang J.; He R.-H.; Yu Y.-L. Autonomous DNA nanomachine based on cascade amplification of strand displacement and DNA walker for detection of multiple DNAs. Biosens. Bioelectron. 2018, 105, 159. 10.1016/j.bios.2018.01.044. [DOI] [PubMed] [Google Scholar]
- Woods D.; Doty D.; Myhrvold C.; Hui J.; Zhou F.; Yin P.; Winfree E. Diverse and robust molecular algorithms using reprogrammable DNA self-assembly. Nature 2019, 572 (7771), E21–E21. 10.1038/s41586-019-1378-x. [DOI] [PubMed] [Google Scholar]
- Chen C.; Xu J.; Shi X. Multiform DNA origami arrays using minimal logic control. Nanoscale 2020, 12 (28), 15066–15071. 10.1039/D0NR00783H. [DOI] [PubMed] [Google Scholar]
- Chen C.; Xu J.; Shi X. Adjusting Linking Strands to Form Size-Controllable DNA Origami Rings[J]. IEEE Trans. NanoBiosci. 2020, 19 (2), 167–172. 10.1109/TNB.2020.2964061. [DOI] [PubMed] [Google Scholar]