Abstract
PURPOSE:
The proposed legislation Verifying Accurate and Leading-edge In vitro clinical test Development (VALID) clarifies the US Food and Drug Administration's authority to regulate laboratory-developed tests. Many stakeholders have pointed out that the lack of direct US Food and Drug Administration oversight has led to erroneous results that have serious patient consequences—in particular for patients with cancer. Technology Certification is a key provision proposed in VALID to navigate the balance between safety, patient access, and innovation; however, the maintenance cost of the proposed framework after implementation is unclear.
METHODS:
On the basis of 2019 retrospective data from a laboratory-developed test–based cancer diagnostics laboratory, we expressed laboratory complexity by the number and complexity of assays and in vitro diagnostic technologies. We estimated the national health care cost increase by modeling three stringencies of complying with the Act. We performed sensitivity analysis of our regulatory stringency model taking into account number of patients tested, materials, submission cost, and labor using extra cost per patient as the output.
RESULTS:
We estimate the national health care cost increase to range from $33M US dollars (USD) to $1,110M USD or $0.21 USD to $0.70 USD per employed person in the United States. Sensitivity analysis demonstrates that regulatory stringency is the primary driver of extra cost per patient. Cancer testing does not reflect all areas of in vitro diagnostics affected by VALID; nonetheless, concrete cost models are paramount in informing the ongoing legislative negotiations.
CONCLUSION:
Our findings show the critical importance of clarity in the legislative language to ensure balance between VALID's goals of assuring high-quality test performance and the burden to laboratories and overall health care cost.
INTRODUCTION
The COVID-19 pandemic has pushed the longstanding debate regarding diagnostic test regulation into the public conscience.1,2 Currently, regulatory authority of the US Food and Drug Administration (FDA) extends to manufactured in vitro clinical tests (IVCTs), but the agency has historically exercised nonenforcement discretion for tests that are developed and used in the same clinical laboratory (so-called laboratory-developed tests [LDTs]).3-8 The Verifying Accurate and Leading-edge IVCT Development (VALID Act, H.R. 6102/S.3404),9,10 introduced in March 2020, is bipartisan, bicameral legislation that aims to ensure the quality and safety of all diagnostic tests by establishing a framework for FDA oversight in the least burdensome manner to facilitate continued innovation (an alternative proposal [the VITAL Act] has recently been realized via appropriate guidance).11,12 Regulatory guidance documents or specific regulations on how VALID will be implemented remain to be determined (Fig 1A); however, VALID would eliminate the status quo of nonenforcement discretion and would codify FDA's authority to regulate LDTs (Fig 1B).
FIG 1.

VALID Act: context, regulatory pathways, technology certification, and stringency definition. (A) VALID is a proposed bill that, if passed into law, the Executive Branch would be responsible for implementing through the various regulatory mechanisms illustrated on the left. aUnlike guidelines, policies are enforceable and mandatory, formalized statements that apply to a specific task; employees who violate a policy may be disciplined. Note, regulations for VALID will have to be issued. (B) Comparison of the regulatory pathway for FDA-reviewed tests versus the LDTs. Under VALID, FDA will enforce regulatory oversight over LDTs through so-called technology certification. (C) VALID and the proposed technology certification applies to high-complexity testing. For certification, IVCTs have to be representative. Depending on the stringency of regulatory implementation, the oversight may differ in terms of how many submissions the regulator will require as an adequate representation of the technologies administered in a laboratory. CGH, comparative genomic hybridization; CLIA, Clinical Laboratory Improvement Amendments; FDA, US Food and Drug Administration; FISH, fluorescence in situ hybridization (a molecular test technology); IVCT, in vitro clinical test; LDTs, laboratory-developed tests; NGS, next-generation sequencing; PCR, polymerase chain reaction; VALID Act, Verifying Accurate Leading-edge In vitro clinical test Development Act.
The VALID Act has tremendous implications for the practice of oncology. As of 2019, 61% of oncology clinical trials included biomarker measurement and 33% of personalized medicine drugs are for cancer.13,14 Forty (93%) of the 43 companion diagnostics authorized by FDA guide the use of oncology drugs and identify patients with an increased likelihood to respond to 41 different targeted therapies.15 After FDA authorization, many companion diagnostics are implemented or replicated as LDTs. All clinical laboratories that offer diagnostic tests—both locally developed (LDTs) and FDA-authorized—fall under federal regulations that apply to their operating procedures for performing medical tests in the United States (CLIA88). However, although Clinical Laboratory Improvement Amendments (CLIA) provides for proficiency in laboratory performance, it does not formally mandate assessment of clinical validity—albeit most laboratories include clinical validity assessment in their validations—in particular with high-complexity tests. Most cancer diagnostics and in particular companion diagnostics are considered class III devices by the FDA because these tests identify those patients at increased risk for serious adverse effects.16,17 Laboratories are trusted to select and provide the best testing approaches (whether LDT or FDA-authorized).18 VALID underscores the vital role of clinical laboratories in driving innovation and supporting public health needs by changing the governance framework of LDTs.19,20 VALID prioritizes FDA resources for review of the highest risk tests that have the greatest potential to expose patients to serious or irreversible harms. Stakeholders have pointed out that the lack of FDA oversight has led to erroneous results leading to serious patient consequences,21 and an FDA report highlighted the increased risk of error and patient harm as tests become more complex.22 Nonetheless, LDTs play an important role in test availability. VALID is intended to reform the regulation of diagnostics and ameliorate LDT safety concerns without limiting access to novel diagnostics.
The financial implication of VALID has not been systematically assessed. Although many groups and professional societies have shared their views,23-26 these statements are usually opinions rather than factual evidence.27,28 Whether the VALID framework is financially sustainable is a direct function of the maintenance cost; the cost of a VALID-regulated laboratory system has been unclear. Cost modeling at the national level is an accepted tool to foresee the economic impacts of proposed legislation.29 For example, the Congressional Budget Office (CBO) and the Center for Medicare and Medicaid Innovation (CMMI) produce dynamic financial analyses (which balance costs with savings) when relevant. These top-down analyses are complex and require a significant amount of time and resources.30 Therefore, these reports are reserved for a small number of major proposals. Given that the legislation is in flux and affects a technically difficult domain, it is understandable that national cost modeling is currently pending. But methods applied by the CBO and CMMI are publicly available and stakeholders can use retrospective data to project fiscal impacts.31 We aim to provide, to our knowledge, the first such model of VALID in this report.
We used a simplified version of the CBO and CMMI cost-estimation approach, built on retrospective data from a high-complexity molecular diagnostics laboratory and modeled the cost for three stringency levels of the VALID framework. Modeling the national maintenance cost is critical during the early stages of the legislative process to inform whether the proposed legislation is financially sustainable.
METHODS
Setting, Data, and Design
To assess the practical and financial impact of the VALID Act, we used retrospective volume and patient data (January 1, 2019-December 12, 2019) from a high-complexity clinical molecular diagnostic laboratory. The institutional review board–approved project was conducted in a CLIA-certified laboratory at the Massachusetts General Hospital as part of an ongoing prospective quality-improvement initiative to assess implications of regulatory updates.
Application of VALID Concepts and Terminology
VALID would amend the Federal Food, Drug, and Cosmetic Act to provide balanced regulation for IVCTs through technology certification, where technology is defined as a group of IVCTs that do not significantly differ in control mechanisms, energy sources, or operating principles (Fig 1C) and for which design, deployment, and validation would be addressed in a similar manner. To reflect the test menu of our laboratory in VALID terms, we categorized tests with the definitions and terminology proposed in VALID by determining the specific control mechanisms, energy sources, and operating principles. We did not allow a method of analysis to fall under multiple operating principles or grouped technologies (Table 1). For example, polymerase chain reactions (PCRs) are part of next-generation sequencing (NGS); however, we classified such tests only in the higher-complexity group (ie, NGS).
TABLE 1.
In Vitro Clinical Tests of One High-Complexity Laboratory
Stringency Models
From a laboratory perspective, the effort of undergoing technology certification varies with the interpretation of single technology. Lacking explicit regulatory guidance, we created a measure for how rigidly the technology certification framework may be enforced by the FDA. We chose a three-tiered stringency system (low, moderate, and high; Table 1) reflecting how many assays are needed to represent all the IVCT technologies in the laboratory. In the low-stringency Model I, we selected one assay per grouped technology (n = 3 IVCTs, excluding PCR because of inclusion of this technology in NGS). In the moderate-stringency Model II, we additionally accounted for three operating principles of PCRs (n = 6 IVCTs). In the high-stringency Model III, we selected one IVCT per method of analysis (n = 9 IVCTs); premarket review for all assays was considered Model IV.
Maintenance Cost Model
We identified the specific dollar amount (Centers for Medicare and Medicaid Services [CMS] fee schedules) by linking each IVCT to an appropriate current procedural terminology code (AMA, Chicago, IL; Data Supplement, online only). For each test in each model, we multiplied the CMS fee schedule price by the number of tests required for regulatory submission (n = 50 for non-NGS or n = 100 for NGS; Data Supplement) and by an ancillary cost factor. The ancillary cost factor (Data Supplement) accounts for regulatory submission costs that are not accounted for in CMS pricing.32 The ancillary cost factor was derived from a microcost analysis of a representative assay (Data Supplement) and the regulatory submission costs using a fixed direct and four-tiered indirect cost model (Data Supplement).
Sensitivity Analysis
Some factors other than regulatory stringency (Models I-IV) contribute to the net cost increase under VALID. To calculate the relative contribution of factors other than regulatory stringency, we started at Model II (realistic midpoint) and varied one factor at a time while keeping all other inputs constant. The sensitivity analysis incorporates a 10% bidirectional error in number of patients tested, materials (eg, reagents, kits, etc), ancillary cost factor, and labor, plotting the absolute ranges between min and max as a tornado diagram.
National Impact Projection
We estimated the 3-year national health care cost by multiplying the total maintenance cost by the number of cancer hospitals in the United States (n = 886). A different approach would allow each state to have one VALID-compliant laboratory. We accounted for this (hypothetical) scenario by estimating the maintenance cost for one laboratory per state/DC/PR (n = 52). To make estimates relatable to oncology practice, we expressed the cost increase as a distributed cost per patient with newly diagnosed late-stage cancer in 2019 (NCI SEER; disease-specific fiscal estimate) using American Joint Committee on Cancer stage III or IV cases and alternatively per employed person in the United States (Bureau of Labor Statistics from 2019; fiscal estimate).
RESULTS
Cost Modeling VALID
The 2019 annual volume of 12,241 tests from 10,295 patients in the prototype laboratory was mapped across the laboratory's full menu of 40 assays. Mapping of the 40 IVCTs in our test menu according to the proposed VALID concepts resulted in four grouped technologies: NGS, fluorescent in situ hybridization (FISH), PCR, and comparative genome hybridization (CGH). In addition, we assigned six operating principles and nine methods of analysis (Table 1). As an example, we provide the total submission cost of a representative qPCR test (median ∼$20k in US dollars [USD]; range: $12k-32k USD; Data Supplement). The submission-related ancillary cost factor is at least 1.85 (Data Supplement) and thereby exceeds the published33 median maintenance cost of non–FDA-authorized tests (1.19; range, 1.09-1.41; Data Supplement). We therefore consider the ancillary cost factor of 1.85, albeit conservative, a realistic estimate to account for costs related to submission efforts in our models.
Laboratory and National Cost Estimates
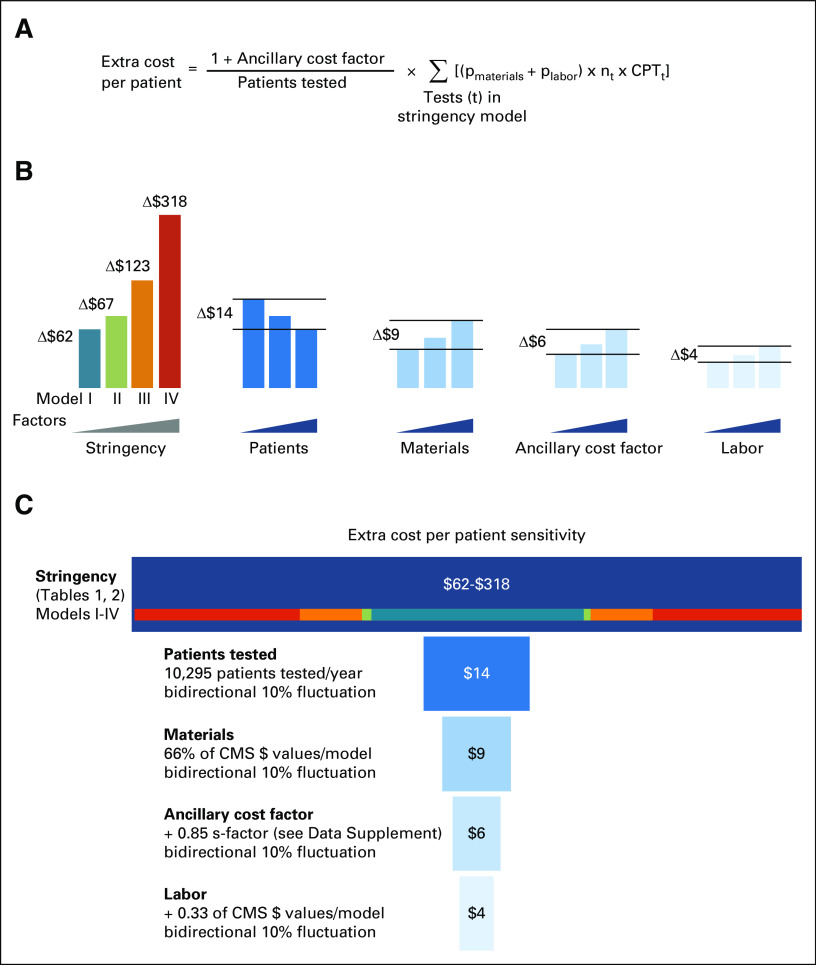
The total cost per model is derived from the sum of the maintenance costs of all IVCTs per model (Table 2; Fig 1; Data Supplement). The lower-stringency models require fewer financial resources and effort for maintenance (Table 2). FDA technology certification for three assays was estimated at $638k USD or an increased cost of $62 USD/patient (Data Supplement). Technology certification for six or nine assays costs $684k USD (+$67 USD/patient) or $1.2M USD (+$122 USD/patient), respectively (Data Supplement). Specifically, when comparing Model I ($638k USD) with Model III ($1.2M USD), the cost nearly doubled and the overall time commitment was 2.75 times as much (Table 2; Data Supplement). In a separate sensitivity analysis (Fig 2; Data Supplement), we examined the added cost per patient in relation to typical drivers of laboratory cost, that is, number of patients tested, materials cost, the ancillary (submission) cost, and labor (Fig 2B). We noted that these factors—even when taken together—change the extra cost per patient by only ±$33 USD (Fig 2B). In other words, our sensitivity analysis shows that the cost variance caused by these typical drivers has relatively minor effects when compared with the variance in extra cost per patient under the four stringency models for maintaining technology certification under VALID (Fig 2C).
TABLE 2.
Cost Models Under VALID
FIG 2.
Sensitivity analysis. (A) Formula used to estimate extra cost per patient takes into account: an ancillary cost factor (accounting for regulatory submission costs that are not accounted for in CMS pricing), number of patients tested, the fractional costs for material and labor, the number of tests, and the current procedural terminology (CPT) billing codes for the tests in each of the four stringency models (details, see Data Supplement). (B) The sensitivity analysis accounts for four factors in addition to stringency. The cost variance (delta) is derived from a ±10% change to model II (midpoint assumption). Note that increasing numbers of patients tested results in a decreased unit cost and an inverse cost variance relationship. (C) Tornado diagram. The model is influenced by five inputs, and the source of the largest differences in model output is sorted from top to bottom. Assumption stringency at midpoint; 10% error in both directions. CMS, Centers for Medicare and Medicaid Services; CPT, current procedural terminology; nt, number of test equivalents.
To make the maintenance cost under VALID more relatable to oncology practice, we expressed the laboratory cost estimates as added cost per patient with stage III-IV cancer in 2019 (Data Supplement) and expressed the cost at a state or cancer center level (Data Supplement). FDA technology certification for the four regulatory stringency models is ranging from $43 USD to $3,700 USD per patient with stage III-IV cancer (Data Supplement). This broad range illustrates, in practically relatable terms, the cost impact of the regulatory stringency under VALID. We also expressed the cost to the labor pool of the United States (Data Supplement). Specifically, for the hypothetical state-based level, with one center per state/DC/PR undergoing technology certification (n = 52; Data Supplement), multiplied by the low-stringency cost of certifying three assays, the 3-year national health care cost would increase by $33M USD or +$43 USD/patient with new late-stage cancer (n = 775k) or +$0.21 USD/employed person (n = 158M). More stringent technology certification for 52 centers for six or nine assays would cost $36M USD (+$45 USD/patient, +$0.22 USD/employed person) or $65M USD (+$83 USD/patient, +$0.41 USD/employed person), respectively. Pivoting to the cancer center level, there are approximately 886 cancer centers and the increased cost was estimated at <$7 USD/employed person.
DISCUSSION
We conducted a cost modeling study of maintaining the technology certification framework outlined in VALID. Using retrospective data, and making assumptions about the technologic implementation of VALID, we estimated the overall health care costs of maintaining technology certification for cancer diagnostics. The findings highlight the importance of maintenance cost considerations as Congress considers this legislation. Our data show the critical importance of balanced legislative language and corresponding regulatory interpretation that is needed to ensure patient access to validated cancer diagnostics, while supporting continued innovation through clinical laboratories. The findings are critical for value-based health care investments in general, and the forced allocation of health care funds to different kinds of work, one of them being regulatory operations under VALID.
The purpose of our study is to make government departments, stakeholders, and domain experts aware of the scale of costs that the proposed regulation could impose. As US health care evolves into a value-based system, the field is tasked to emphasize quality over cost and use evidence-based data to optimize care delivery in a financially viable fashion. Although the explicit patient value (in terms of reduced adverse events, reduction of erroneous results, or improved therapy selection—all leading to improved health outcomes and value) of VALID-regulated tests is uncertain, the increasing complexity of diagnostics, including data-driven technologies alongside new regulations,34-36 mandates reform of the decades-old system for regulatory oversight of LDTs. Many patients will be affected by VALID. Specifically, the assays with highest regulation under VALID are particularly relevant in immuno-oncology or precision oncology (precision oncology encompasses prevention and treatment strategies that take individual variability into account)37,38 where identification of biomarker alterations affects treatment options and outcomes. Clinical trials of novel compounds serve to validate a drug and the biomarker used to select the patient but not the validity or reproducibility of different assays.39 Notably, initial trial and subsequent clinical implementation of these assays rely almost entirely on LDTs. Under current practice, as LDTs are typically not subjected to uniform FDA oversight, this may result in unintended variations in quality for different tests developed and used by different institutions. Central oversight by FDA would ensure that all tests with the same intended use meet similar quality standards—thereby providing confidence in the quality of tests for patients. The technology certification approach is added onto unchanged CLIA requirements. Under CLIA, and under the proposed paradigm of CLIA plus VALID, the accuracy and reliability of test results of a given diagnostic test will remain the responsibility of the medical director.1,3,4,8,22,40-43 Thus, who will cover the cost of maintaining VALID? One common misperception in health care technology is that FDA authorization equals reimbursement. In fact, reimbursement coverage decisions and price levels are continuously in flux at local, federal, and private payer levels. Thus, our data provide a concrete starting point for the scale of costs the system will need to endure to maintain VALID—if implemented as proposed. As currently drafted VALID does not propose a link between FDA authorization and reimbursement; linking VALID to reimbursement would create a powerful incentive for adoption.
Limitations of our study are largely based on specific assumptions. First, we did not account for costs during the transition to a new regulatory framework. Given the complexity of transition at the federal, local, and laboratory level, we find this appropriate. Second, the total number of LDTs to be certified under VALID will likely demand a third-party review process extramural to the FDA.9,10 The current version of the VALID Act has built-in language for allowing Accredited Persons to review IVCT applications.9,10 Professional societies could provide the required domain expertise (after appropriate accreditation). We did not account for the cost of these extramural accreditation services; however, we point out that the CLIA governance (accreditation, inspection, and certification) including proficiency and competency assessment would still apply (Fig 1).9,10,40 Similarly, we did not include the initial assay development cost or whether tests have been clinically implemented as LDTs or as FDA-authorized tests or devices. Third, we made cost assumptions on the basis of current reimbursement levels for the tests and the associated administrative burden; however, depending on the final wording of the law, these estimates might change. Fourth, we limited our analysis to cancer tests and did a breakout analysis and focused on one specific setting (ie, late-stage cancer testing) because this is where a large volume of high-risk tests will fall. However, VALID if implemented as proposed will affect many different settings (eg, earlier-stage cancer, infectious diseases, neurology, and inherited conditions) and the total cost of maintaining VALID will be higher than what is accounted for in our study. Nonetheless, our findings are relevant because high-complexity laboratories will have the highest level of regulatory review and have to bear the most burden from VALID. In our sensitivity analysis, we accounted for variations in volume, materials, overhead, and labor by incorporating a 10% bidirectional error that exceeds the scale of 3 standard deviations in historical data.44 In addition to not modeling the transition from status quo, we did not model the opportunity cost of maintaining technical certification versus other investments. The structures and flexibilities for change in different complex laboratories is no doubt highly variable, so inability to dollarize transition period burdens in terms of time, effort, and cost is a serious limitation. Finally, current procedural terminology code–derived prices are an imperfect surrogate for the net technical and professional costs in a laboratory.
The key question is how stringently VALID will be implemented. Generally speaking, the assessment of test complexity has been established at the individual test level using a point system42,45; however, VALID explicitly focusses on high-complexity tests (ie, those with the highest risk of harm). Therefore, test complexity of an individual test alone is an imperfect measure for the complexity of the entire laboratory operation and the risk carried by it. Furthermore, laboratories across the nation differ substantially in their test menu composition and there are currently no established approaches to formally compare laboratory complexity. VALID accounts for these variations by proposing a technology certification pathway where the review of selected, representative technologies reflects the overall laboratory complexity. In the absence of specific regulatory guidance, we expressed laboratory complexity by the number and complexity of assays and technologies. Specifically, we projected three different interpretations of VALID in the form of three levels of regulatory stringency with increasing numbers of technologies used to stratify the complexity of a given laboratory. This approach and our cost model can be considered a framework to assess laboratory complexity and regulatory stringency that should enable other groups to corroborate our findings in their setting. Under the lowest possible stringency, just one assessment would be submitted for the most complex test within a laboratory. Although cost-efficient, we do not believe that this can accurately reflect the complexity and risk carried by a laboratory. A stringent implementation of VALID may result in a disproportionate burden in cost and time, especially if the associated quality gains are similar between varied interpretation stringency. Notably, the explicit patient value (in terms of reduced adverse events or improved therapy selection) of VALID-regulated tests is uncertain, so this remains difficult to quantify. However, we can all acknowledge that allowing substandard tests to be regularly used in patient care would be inappropriate, be undesirable, and reduce care quality. A system that does not mandate steps to ensure clinical validity of tests prior to the clinical application is at increased risk for substandard quality of care to inadvertently occur. In absence of these quantifiable metrics, we interpreted the cost and effort of full premarket review for all assays as unrealistic and not recommended unless an appropriate technology certification program is established. Furthermore, many clinical disorders do not affect significant number of patients—and IVCT manufacturers may not produce enough sales to recoup the upfront investment in an expensive premarket review.46 To account for this, an orphan disease rule has been integrated in VALID.9 These considerations clearly argue that VALID should be implemented following a moderate stringency approach, and our data clearly show that the cost burden of the proposed technology certification pathway in laboratories nationwide will directly depend on the definition of the term technology. We made best judgments for three stringencies, working from existing regulatory paradigms, and provide the data to enable evidence-based legislative decision making.47 We believe that clear enumeration of stringency categories should be made explicit in VALID to ensure optimal implementation. Although it may be challenging to provide further guidance and definitions in VALID language, it is crucial to the cost impact imposed on the health care system and finite laboratory budgets.
In conclusion, data from our cost model emphasize the critical importance of concretely defining the stringency of regulation via appropriate legislative language in the VALID Act to optimize alignment of the intent (ie, to ensure accurate diagnostic results for patients) with the burden to laboratories and the associated overall health care cost.
Laura Lasiter
Employment: Regulatory and Quality Solutions, AgNovos, Qiagen, Smith & Nephew
Bruce Quinn
Consulting or Advisory Role: Illumina, Thermo Fisher Scientific, InVitae, Guardant Health, Natera, Adaptive Biotechnologies, Genelex, Eurofins
Roberto Salgado
Consulting or Advisory Role: Roche, AstraZeneca, Bristol-Myers Squibb
Research Funding: Merck, Puma Biotechnology, Roche
Travel, Accommodations, Expenses: Roche, Merck
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or any other organization.
SUPPORT
Supported by NIH (R37 CA225655) to J.K.L.
AUTHOR CONTRIBUTIONS
Conception and design: Richard Huang, Laura Lasiter, Christina Young, Jochen K. Lennerz
Administrative support: Jochen K. Lennerz
Financial support: Jochen K. Lennerz
Provision of study materials or patients: Jochen K. Lennerz
Collection and assembly of data: Richard Huang, Adam Bard, Jochen K. Lennerz
Data analysis and interpretation: Richard Huang, Laura Lasiter, Adam Bard, Bruce Quinn, Roberto Salgado, Jeff Allen, Jochen K. Lennerz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
National Maintenance Cost for Precision Diagnostics Under the Verifying Accurate Leading-Edge In Vitro Clinical Test Development (VALID) Act of 2020
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Laura Lasiter
Employment: Regulatory and Quality Solutions, AgNovos, Qiagen, Smith & Nephew
Bruce Quinn
Consulting or Advisory Role: Illumina, Thermo Fisher Scientific, InVitae, Guardant Health, Natera, Adaptive Biotechnologies, Genelex, Eurofins
Roberto Salgado
Consulting or Advisory Role: Roche, AstraZeneca, Bristol-Myers Squibb
Research Funding: Merck, Puma Biotechnology, Roche
Travel, Accommodations, Expenses: Roche, Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Shah A, Shuren J, Hahn S: FDA Support for Expedited Access to COVID-19 Diagnostics Health Affairs 2020 https://www.healthaffairs.org/do/10.1377/hblog20200814.376610/full/ [Google Scholar]
- 2.Evans BJ, Clayton EW: Deadly Delay: The FDA's Role in America's COVID-19 Testing Debacle, Volume 130Yale LJF, 2020 [Google Scholar]
- 3.Genzen JR: Regulation of laboratory-developed tests Am J Clin Pathol 152:122–131, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller VA: FDA regulation of laboratory-developed tests Clin Adv Hematol Oncol 14:305–306, 2016 [PubMed] [Google Scholar]
- 5.Caliendo AM, Couturier MR, Ginocchio CC, et al. : Maintaining life-saving testing for patients with infectious diseases: Infectious diseases Society of America, American Society for Microbiology, and Pan American Society for Clinical Virology recommendations on the regulation of laboratory-developed tests Clin Infect Dis 63:151–154, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Caldwell L, Terry SF: 21st-century healthcare policy and the regulation of laboratory-developed tests Genet Test Mol Biomarkers 19:467–468, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Ferreira-Gonzalez A, Emmadi R, Day SP, et al. : Revisiting oversight and regulation of molecular-based laboratory-developed tests: A position statement of the Association for Molecular Pathology J Mol Diagn 16:3–6, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Hockett RD, Close SL: Regulation of laboratory-developed tests: The case for utilizing professional associations Clin Pharmacol Ther 88:743–745, 2010 [DOI] [PubMed] [Google Scholar]
- 9.DeGette D: H.R.6102—VALID Act of 2020 2020https://www.congress.gov/bill/116th-congress/house-bill/6102 [Google Scholar]
- 10.Burr R: S.3404—VALID Act of 2020 2020https://www.congress.gov/bill/116th-congress/senate-bill/3404 [Google Scholar]
- 11.Paul R: S.3512—VITAL Act of 2020 2020https://www.govtrack.us/congress/bills/116/s3512 [Google Scholar]
- 12.Health and Human Services : Rescission of Guidances and Other Informal Issuances Concerning Premarket Review of Laboratory Developed Tests 2020 [Google Scholar]
- 13.Tufts Center for the Study of Drug Development Impact Reports Personalized Medicine Gains Traction But Still Faces Multiple Challenges, Volume 17, 2015https://csdd.tufts.edu/impact-reports [Google Scholar]
- 14.Personalized Medicine Coalition : The Personalized Medicine Report: Opportunity, Challenges, and the Future 2020http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/PMC_The_Personalized_Medicine_Report_Opportunity_Challenges_and_the_Future.pdf [Google Scholar]
- 15.US Food and Drug Administration : List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools) 2020https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools [Google Scholar]
- 16.US Food and Drug Administration : In Vitro Companion Diagnostic Devices Guidance for Industry and Food and Drug Administration Staff 2018https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-companion-diagnostic-devices [Google Scholar]
- 17.US Food and Drug Administration : Overview of IVD Regulation 2019https://www.fda.gov/medical-devices/ivd-regulatory-assistance/overview-ivd-regulation#8 [Google Scholar]
- 18.Graden KC, Bennett SA, Delaney SR, et al. : A high-level overview of the regulations surrounding a clinical laboratory and upcoming regulatory challenges for laboratory developed tests Lab Med lmaa086, 2020 [DOI] [PubMed] [Google Scholar]
- 19.American Association for Clinical Chemistry : New Legislation Would Jeopardize Patient Access to Medical Tests Across the Board by Restricting Policy that Removed Barriers to Coronavirus Testing CISION, PR Newswire, 2020https://www.prnewswire.com/news-releases/new-legislation-would-jeopardize-patient-access-to-medical-tests-across-the-board-by-restricting-policy-that-removed-barriers-to-coronavirus-testing-301019179.html [Google Scholar]
- 20.Friends of Cancer Research : New COVID-19 Diagnostics Evidence Accelerator Applies Real-World Data to Answer Urgent Questions on SARS-CoV-2 Testing 2020 https://www.focr.org/news/new-covid-19-diagnostics-evidence-accelerator-applies-real-world-data-answer-urgent-questions [Google Scholar]
- 21.Epner PL, Gans JE, Graber ML: When diagnostic testing leads to harm: A new outcomes-based approach for laboratory medicine BMJ Qual Saf 22:ii6–ii10, 2013suppl 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration : The Public Health Evidence for FDA Oversight of Laboratory Developed Tests: 20 Case Studies—the Real and Potential Harms to Patients and to Public Health from Certain Laboratory Developed Tests (LDTs) 2015http://wayback.archive-it.org/7993/20171115144712/https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Reports/UCM472777.pdf [Google Scholar]
- 23.College of American Pathologists : CAP Statement on the Introduction of the VALID Act CAP, 2020https://www.cap.org/news/2020/cap-statement-on-the-introduction-of-the-valid-act [Google Scholar]
- 24.Association for Molecular Pathology : Laboratory Developed Testing Procedures (LDPs) 2020https://www.amp.org/advocacy/advocacy-resources/laboratory-developed-testing-procedures-ldps/ [Google Scholar]
- 25.American Comparative Literature Association : ACLA Statement on the VALID Act of 2020 2020https://www.acla.com/acla-statement-on-the-valid-act-of-2020/ [Google Scholar]
- 26.Friends of Cancer Research : Statement from Friends of Cancer Research on the VALID Act 2020https://www.focr.org/news/statement-friends-cancer-research-valid-act [Google Scholar]
- 27.Infectious Diseases Society of America : Open Letter from the Infectious Disease Society of America 2020www.idsociety.org/globalassets/idsa-valid-act-2020-comments.pdf [Google Scholar]
- 28.The Journal of Precision Medicine : Potential Impact of the VALID Act on IVD Manufacturers 2020https://www.thejournalofprecisionmedicine.com/potential-impact-of-the-valid-act-on-ivd-manufacturers/ [Google Scholar]
- 29.Congressional Budget Office : Frequently Asked Questions about CBO Cost Estimates 2020https://www.cbo.gov/about/products/ce-faq [Google Scholar]
- 30.Congressional Budget Office : Processes 2020https://www.cbo.gov/about/processes [Google Scholar]
- 31.Congressional Budget Office : CBO Cost Estimates 2017-2018 2020https://www.cbo.gov/system/files/115th-congress-2017-2018/reports/53519-costestimates.pdf [Google Scholar]
- 32.Clinical and Laboratory Standards Institute : Basic Cost Accounting for Clinical Services; Approved Guideline. NCCLS Document GP11-A1998 1998 [Google Scholar]
- 33.Sabatini LM, Mathews C, Ptak D, et al. : Genomic Sequencing procedure Microcosting analysis and health economic Cost-impact analysis: A report of the association for Molecular pathology J Mol Diagn 18:319–328, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration : 21st Centry Cures Act 2020https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act [Google Scholar]
- 35.Office of the National Coordinator for Health Information Technology : 21st Century Cures Act 2020https://www.healthit.gov/curesrule/ [Google Scholar]
- 36.European Union : General Data Protection Regulation, 2016 [Google Scholar]
- 37.Wong KM, Capasso A, Eckhardt SG: The changing landscape of phase I trials in oncology Nat Rev Clin Oncol 13:106–117, 2016 [DOI] [PubMed] [Google Scholar]
- 38.La Thangue NB, Kerr DJ: Predictive biomarkers: A paradigm shift towards personalized cancer medicine Nat Rev Clin Oncol 8:587–596, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Salgado R, Bellizzi AM, Rimm D, et al. : How current assay approval policies are leading to unintended imprecision medicine Lancet Oncol 21:1399–1401, 2020 [DOI] [PubMed] [Google Scholar]
- 40.US Food and Drug Administration : Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs) Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories 2014https://www.fda.gov/regulatory-information/search-fda-guidance-documents/framework-regulatory-oversight-laboratory-developed-tests-ldts [Google Scholar]
- 41.Halling KC, Schrijver I, Persons DL: Test verification and validation for molecular diagnostic assays Arch Pathol Lab Med 136:11–13, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Schreier J, Feeney R, Keeling P: Diagnostics reform and harmonization of clinical laboratory testing J Mol Diagn 21:737–745, 2019 [DOI] [PubMed] [Google Scholar]
- 43.Centers for Medicare and Medicaid Services : Clinical Laboratory Improvement Amendments (CLIA) Laboratory Director Responsibilities, 2003www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Downloads/brochure7.pdf [Google Scholar]
- 44.Grafarend EW: Linear and Nonlinear Models: Fixed Effects, Random Effects, and Mixed Models Walter de Gruyter, 2006 [Google Scholar]
- 45.US Food and Drug Administration : CLIA Categorizations 2020https://www.fda.gov/medical-devices/ivd-regulatory-assistance/clia-categorizations [Google Scholar]
- 46.Zehnbauer B: Integration standardization and diagnostics oversight of laboratory testing J Mol Diagn 21:735–736, 2019 [DOI] [PubMed] [Google Scholar]
- 47.Matthew DB: Two threats to precision medicine equity Ethn Dis 29:629–640, 2019suppl 3 [DOI] [PMC free article] [PubMed] [Google Scholar]