Abstract
Background:
Adjuvant platinum-based chemotherapy is standard of care for patients with resected stage IIA/B or IIIA NSCLC. Overall survival is suboptimal due to the high metastatic potential of early-stage NSCLC and there is substantial clinical need for additional efficacious adjuvant treatment options.
Methods:
PubMed (all time to 4 February 2021) and related conference databases were searched using the key search terms ‘NSCLC’ AND ‘Adjuvant’ AND ‘EGFR inhibitor’ OR respective aliases.
Results:
The literature search identified five adjuvant phase III trials of EGFR inhibitors in early NSCLC. The earlier BR19 and RADIANT trials failed to demonstrate statistically significant improvements in either OS or DFS for gefitinib and erlotinib, respectively, compared with placebo in patients with EGFR mutation-unselected NSCLC. Three subsequent phase III trials, ADAURA, CTONG1104, and IMPACT, were conducted in EGFR-mutant NSCLC. IMPACT showed no statistically significant DFS benefit for adjuvant gefitinib, and although CTONG1104 did report improved DFS for gefitinib (HR = 0.56, p = 0.001), this benefit was not enduring, resulting in comparable 5-year DFS rates. Statistically significant and clinically meaningful DFS benefits were observed in ADAURA for osimertinib compared with placebo in patients with stage IB-IIIA and II-IIIA disease (7th Edition Staging), and these benefits, coupled with a meaningful improvement in 2-year CNS DFS and favorable HRQoL, make osimertinib an important new treatment option for the adjuvant treatment of EGFR exon 19 deletion or exon 21 L858R-mutated stage II-IIIA NSCLC (UICC/AJCC 8th Edition Staging), with final mature OS data eagerly awaited.
Conclusion:
Adjuvant osimertinib used alone or following platinum-based chemotherapy is now recommended in patients with stage II-IIIA EGFR-mutated NSCLC.
Keywords: adjuvant therapy, EGFR-positive, NSCLC, resected disease, targeted therapy
Introduction
Lung cancer is the leading cause of cancer-related mortality, with over 2.2 million new cases diagnosed and nearly 1.8 million deaths in 2020. 1 Non-small cell lung cancer (NSCLC) accounts for close to 85% of cases 2 and approximately 20–30% of patients present with early-stage disease with the potential for curative-intent surgical resection.3–5 Adjuvant platinum-based chemotherapy is a standard of care for appropriate patients with resected stage IIA/B or IIIA/B NSCLC using the American Joint Committee on Cancer (AJCC) 8th edition for staging of thoracic malignancies.6–9 In the previous 7th edition, 10 patients with stage IB with tumors 4 cm or greater were also identified as benefiting from adjuvant therapy while those with smaller tumors but other high-risk features such as visceral pleural invasion were not.6,11 The AJCC 8th edition categorizes all patients with tumors greater than 4 cm as having at least stage II disease, which simplifies adjuvant therapy recommendations to include only patients with resected stage IIA/B and IIIA/B disease. 8 Despite adjuvant chemotherapy, overall 5-year survival outcomes remain disappointing, ranging from 53% to 60% for stage II A/B and from 26% to 36% for stage III A/B disease. 12 There is therefore a substantial unmet clinical need for additional and more efficacious treatment options for early-stage disease.
Mutations of the epidermal growth factor receptor (EGFR) are common drivers of oncogenesis in NSCLC. Sensitizing EGFR mutations occur in approximately 15% of NSCLC cases in the United States and in up to 50% of cases diagnosed in Asian patients. 13 The most common EGFR alterations are exon 19 deletions (Ex19del, approximately 45%) and exon 21 L858R point mutations (approximately 40–45%).14–16 Tyrosine kinase inhibitors (TKIs) targeting EGFR have been used for the treatment of advanced NSCLC since 2003.17,18 Multiple generations of EGFR TKIs have shown clinical benefit in this setting, including the first-generation TKIs, erlotinib and gefitinib; the second-generation TKIs, afatinib and dacomitinib; and the third-generation TKI osimertinib.18–20 Acquired resistance to first- and second-generation TKIs occurs primarily through a secondary EGFR exon 20 T790M mutation.19,20 The third-generation EGFR TKI osimertinib was specifically designed to target both common EGFR mutations and the acquired T790M alteration. 21 Osimertinib has shown substantial activity for advanced disease in the AURA3 phase III trial,22,23 leading to its approval for metastatic EGFR T790M-mutant NSCLC following progression on another EGFR TKI in 2015 24 and in the first-line setting for common EGFR mutations in 2018. 25 Osimertinib is now a standard of care in many countries for both indications.18,26
The success of EGFR TKIs in the advanced setting has led to their assessment as adjuvant therapy for resected EGFR-positive NSCLC in five phase III trials.27–31 This review will describe outcomes from these studies and provide practical guidance on the use of EGFR inhibitors for the treatment of EGFR-positive NSCLC.
Methods
A search of published and presented literature was conducted to identify phase III trials reporting outcomes from adjuvant therapy with EGFR inhibitors for resected NSCLC. PubMed (all time to 4 February 2021), proceedings from 2019 to June 2021 of the American Society of Clinical Oncology (ASCO) the European Society for Medical Oncology (ESMO) annual meetings and World Conference on Lung Cancer (WCLC) were searched using the key search terms ‘NSCLC’ AND ‘Adjuvant’ AND ‘EGFR inhibitor’ OR respective aliases (Supplemental File S1). A supplemental bibliographic search of review articles and pooled/meta-analyses was also conducted. In addition, directed searches were performed after the database search cutoff date to ensure that the most up-to-date reports of eligible studies were considered.
English language records were vetted at abstract level and confirmed at full text as needed. Excluded studies included those that were nonoriginal research, preclinical, correlative science, not specific to NSCLC, in neoadjuvant or advanced settings, retrospective, prospective phase I, II, IV or undefined phase, studies not investigating EGFR inhibitors, and duplicate or prior reports. Studies without reported efficacy outcomes were also excluded. 32
Findings
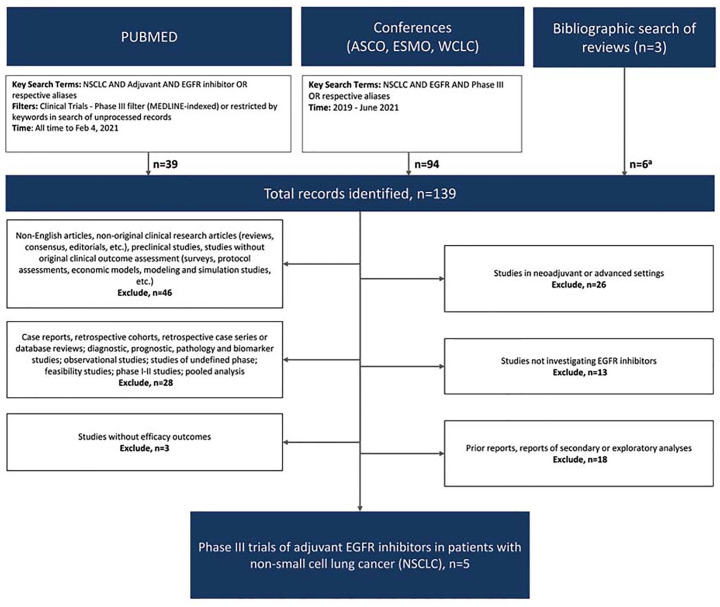
The literature search identified a total of 139 records, which resulted in a total of five phase III trials reporting efficacy outcomes on the use of adjuvant EGFR inhibitors for resected NSCLC (PRISMA, Figure 1),27,28,30,31,33 of which three were conducted exclusively in patients with EGFR-mutant disease.30,31,33
Figure 1.
PRISMA diagram of eligible studies.
ASCO, American Society of Clinical Oncology; ESMO, European Society for Medical Oncology; WCLC, World Conference on Lung Cancer.
aPrimary or associated reports of eligible studies that were not identified through database search.
The phase III BR 19 study planned to enroll 1242 patients with resected stage IB-IIIA NSCLC (AJCC 6th edition), unselected for EGFR mutation status, and patients were randomized 1:1 to receive either gefitinib or placebo with overall survival (OS) the primary endpoint. The trial was halted by the data safety and monitoring board following negative results from the ISEL and S0023 studies.34,35 Among the 503 patients randomized prior to study closure, 52%, 35%, and 13% of patients had stage IB, II, and IIIA disease, respectively, and 17% of patients in each arm had received prior adjuvant chemotherapy. After a median follow-up of 56.4 months, no OS benefit was seen for gefitinib versus placebo [OS, median 61.2 months versus not yet reached (NYR), hazard ratio (HR) = 1.24, 95% confidence interval (CI) = 0.94–1.64; p = 0.14] or for the secondary endpoint of disease-free survival (DFS, median 50.4 months versus NYR, HR = 1.22, 95% CI = 0.93–1.61, p = 0.15) (Table 1). 27 EGFR mutation status was evaluated in 359 patients and was not associated with OS (HR = 0.57, 95% CI = 0.14–2.33, p = 0.43) or DFS (HR = 0.95, 95% CI = 0.300–3.01, p = 0.93), although only 4% of patients had EGFR mutation-positive tumors. Treatment discontinuation due to toxicity occurred in 15.3% of patients receiving gefitinib versus 3.3% of those receiving placebo, with the most common grade 3/4 adverse events (AEs) in the gefitinib versus placebo arms being dyspnea (12.4% versus 7.8%), rash/acne (8.4% versus 0.4%), diarrhea (7.2% versus 2.0%), and fatigue (6.8% versus 2.9%) (Table 2). Deaths due to AEs occurred in 1.2% and 0% of patients in the gefitinib and placebo arms, respectively.
Table 1.
Efficacy outcomes from phase III trials of adjuvant EGFR inhibitors in early EGFR-positive NSCLC.
| Trial Phase |
Key eligibility criteria | Regimen(s) | n | Median Follow-up (months) [range] |
Median disease-free
survival Months HR (95% CI) |
Median overall survival Months HR (95% CI) |
|---|---|---|---|---|---|---|
| BR 19 Phase III 27 |
Stage IB, II or IIIA NSCLC 4% EGFR mutated |
Gefitinib 250 mg once daily × 2 years | 251 | 56.4 |
50.4
HR 1.22 (0.93–1.61) p = 0.15 |
61.2
HR 1.24 (0.94–1.64) p = 0.14 |
| Placebo × 2 years | 252 | NYR | NYR | |||
| RADIANT Phase III 28 |
Stage IB-IIIA NSCLC 16.5% EGFR mutated |
Erlotinib 150 mg once daily × 2 years | 623 | 47 |
50.5
HR 0.90 (0.74–1.10) p = 0.32 |
NS
HR 1.13 (0.9–1.5) p = 0.34 |
| Placebo × 2 years | 350 | 48.2 | NS | |||
| CTONG1104 Phase III 29 |
Stage II–IIIA (N1-N2) NSCLC 100% at least an EGFR exon 19 deletion or L858R |
Gefitinib 250 mg once daily × 2 years | 111 | 80.0 |
30.8
HR 0.56 (0.40–0.79) p = 0.001 |
75.5
HR 0.92 (0.62–1.36) p = 0.67 |
| Vinorelbine 25 mg/m2 D1,8 + cisplatin 75 mg/m2 D1 q3w × 4 cycles | 111 | 19.8 | 62.8 | |||
| ADAURA Phase III 30 |
Stage IB, II, or IIIA NSCLC 100% at least an EGFR exon 19 deletion or L858R |
Osimertinib 80 mg once daily × 3 years | 339 | 22.1 |
NYR
HR 0.20 (0.14–0.30) p < 0.001 |
NYR |
| Placebo once daily × 3 years | 343 | 14.9 | 27.5 | NYR | ||
| IMPACT Phase III 31 |
Stage IIA-IIIB completely resected NSCLC 100% at least an EGFR exon 19 deletion or L858R without T790M |
Gefitinib 250 mg once daily × 2 years | 116 | 70.1 |
35.9
HR 0.92 (NR) p = 0.63 |
NYR HR 1.03 (NR) p = 0.89 |
| Cisplatin 80 mg/m2 D1 + vinorelbine 25 mg/m2 D1,8 q3w × 4 cycles | 116 | 25.0 | NYR |
CI, confidence interval; D1, day 1; EGFR, epidermal growth factor receptor; HR, hazard ratio; n, number of patients; NSCLC, non-small cell lung cancer; NR, not reported; NS, not significant; NYR, not yet reached.
Efficacy outcomes of phase III targeted therapy trials of adjuvant EGFR inhibitors in early NSCLC. Ordered chronologically with primary endpoints in bold.
Table 2.
Phase III trials assessing safety of adjuvant EGFR inhibitors in early NSCLC.
| Trial Phase |
BR 19 Phase III 27 |
RADIANT Phase III 28 |
CTONG1104 Phase III 33 |
ADAURA Phase III 30 |
IMPACT Phase III 31 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment algorithm | Gefitinib | Placebo | Erlotinib | Placebo | Gefitinib | Vinorelbine + Cisplatin | Osimertinib | Placebo | Gefitinib | Cisplatin + Vinorelbine |
| Safety population (n) | 249 | 243 | 611 | 343 | 106 | 87 | 337 | 343 | 115 | 115 |
| Overall | ||||||||||
| Any grade AE | NR | NR | 98.0 | 89.5 | 61 (57.5) | 70 (80.5) | 97.6 | 89.2 | NR | NR |
| Grade ⩾ 3 AEs | NR | NR | NR | NR | 13 (12.3) | 42 (48.3) | 68 (20.2) | 46 (13.4) | NR | NR |
| AEs leading to discontinuation of any
treatment n (%) |
15.3 | 3.3 | 33.6 | 8.5 | 3 (2.8) | 5 (5.7) | 37 (11.0) | 10 (2.9) | NR | NR |
| AE- or treatment-associated deaths n (%) |
3 (1.2) TRAE |
0 (0) TRAE |
0 | 0 | 0 (0) | 0 (0) | 0 (0) | 1 (0.3) | 0 (0) | 3 (2.6) |
| Select grade ⩾ 3 AEs | ||||||||||
| Most common grade ⩾ 3 AEs | Dyspnea (12.4%) Rash/acne (8.4%) Diarrhea (7.2%) Fatigue (6.8%) |
Dyspnea (7.8%) Fatigue (2.9%) Diarrhea (2.0%) Infection (1.2%) |
Rash (22.3%) Diarrhea (6.2%) Pneumonia (1.3%) Pruritis (1.3%) Dyspnea (1.1%) |
Weight gain (4.1%) Dyspnea (1.5%) Headache (1.5%) Abdominal pain (1.5%) Fatigue (0.9%) |
Elevated ALT (1.9%) Elevated AST (1.9%) Rash (0.9%) Diarrhea (0.9%) |
Neutropenia (34.5%) Leucopenia (16.1%) Vomiting (10.0%) Nausea (6.9%) |
Diarrhea (2.4%) Stomatitis (1.8%) Paronychia (0.9%) Upper respiratory tract infection (0.6%) Decreased appetite (0.6%) |
Diarrhea (0.3%) | Elevated ALT (27.0%) Elevated AST (15.7%) Dermatitis acneiform (4.3%) Rash (4.3%) Paronychia (3.5%) |
Neutropenia (87.0%) Leucopenia (57.4%) Anorexia (13.9%) Anemia (9.6%) Hyponatremia (9.6%) |
AEs, adverse events; ALT, alanine transaminase; AST, aspartate transaminase; EGFR, epidermal growth factor receptor; n, number of patients; NR, not reported; NSCLC, non-small cell lung cancer; TRAE, treatment-related adverse events.
Phase III safety data are ordered chronologically. Grade ⩾ 3 AE treatment-related adverse events were summarized when available.
The phase III RADIANT trial randomized 973 patients with stage IB-IIIA NSCLC (AJCC 6th edition) with EGFR expression or EGFR gene amplification 2:1 to receive adjuvant erlotinib or placebo for 2 years following resection, with 51%, 33%, and 16% of patients having stage IB, II, and IIIA disease, and 53% of patients receiving prior adjuvant chemotherapy. After a median follow-up of 47 months, there was no statistically significant difference in the primary endpoint of DFS for erlotinib versus placebo (median 50.5 versus 48.2 months, HR = 0.90, 95% CI = 0.74–1.10, p = 0.32), with immature OS data showing no difference between treatment arms (HR = 1.13, 95% CI = 0.88–1.45, p = 0.34) (Table 1). 28 Among the 16.5% of patients with confirmed EGFR mutations, median DFS was 46.4 versus 28.5 months favoring erlotinib (HR = 0.61, 95% CI = 0.38–0.98), although it was not statistically significant based on hierarchal testing and with no difference in OS between treatment arms (HR = 1.09, 95% CI = 0.55–2.16, p = 0.82). AEs leading to treatment discontinuation occurred more frequently in the erlotinib arm (33.6% versus 8.5%), with the most common grade ⩾ 3 AEs in the erlotinib arm being rash (22.3% versus 0.3%), diarrhea (6.2% versus 0.3%), pneumonia (1.3% versus 0.6%), pruritus (1.3% versus 0), and dyspnea (1.1% versus 1.5%) (Table 2). No treatment-related deaths were reported.
The phase III CTONG1104 trial randomized 222 patients with resected stage II-IIIA NSCLC (AJCC 7th edition) containing an EGFR-activating mutation (exon 19 deletion or exon 21 L858R) in a 1:1 ratio to gefitinib for 2 years versus cisplatin plus vinorelbine for four cycles with 33%, and 64% of patients having stage II and IIIA disease, and study treatment starting 3–6 weeks following resection. With a median follow-up of 36.5 months, a statistically significant improvement in the primary end point of DFS was observed for gefitinib versus chemotherapy in the intent-to-treat population (HR = 0.60, p = 0.005) which was consistent across all subgroups analyzed 33 and persisted with a longer follow-up of 80.0 months (median DFS 30.8 versus 19.8 months, HR = 0.56, 95% CI = 0.40–0.79; p = 0.001) (Table 1). 29 Despite this, there was no statistically significant improvement in OS (median 75.5 versus 62.8 months, HR = 0.92, 95% CI = 0.62–1.36; p = 0.67). In the safety population (n = 193), discontinuation due to toxicity occurred in 2.8% of those receiving gefitinib versus 5.7% of patients receiving cisplatin plus vinorelbine. 33 The most common grade 3/4 AEs in the gefitinib versus combination arm were elevated alanine transaminase (ALT) and aspartate transaminase (AST, 1.9% versus 0% each), rash, and diarrhea (0.9% versus 0% each) (Table 2). No treatment-related deaths were reported in either group.
The placebo-controlled phase III ADAURA study randomized 682 patients with resected stage IB-IIIA NSCLC (AJCC 7th edition) and confirmed EGFR-activating mutation (exon 19 deletion or exon 21 L858R), 60% of whom received adjuvant chemotherapy to receive osimertinib or placebo in a 1:1 ratio for 3 years. Disease stages were IB, II, and IIIA in 32%, 34%, and 34–35% patients, respectively. Randomization occurred within a maximum of 26 or 10 weeks after surgery with or without adjuvant chemotherapy, respectively. At a median follow-up of 22.1 months in the osimertinib arm and 14.9 months in the placebo arm, the study was unblinded by an independent data monitoring committee due to a statistically significant improvement in the primary endpoint of DFS among patients with stage II-IIIA disease for osimertinib versus placebo (n = 470, median NYR versus 19.6 months, HR = 0.17, 99.06% CI = 0.11–0.26; p < 0.001). 30 OS data are immature, although a statistically significant DFS improvement for the overall population was also seen (stage IB-IIIA, median NYR versus 27.5 months, HR = 0.20, 99.12% CI = 0.14–0.30; p < 0.001; Table 1). DFS benefit was apparent in all subgroups analyzed. Health-related quality of life (HRQoL) physical and mental component summaries (SF-36 PCS and MCS, respectively) were maintained in the osimertinib arm through 96 weeks (mean change from baseline, PCS 1.13, 95% CI = 0.54–1.72 and MCS 1.34, 95% CI = 0.60–2.08), with no clinically meaningful changes compared with placebo.30,36 Discontinuation due to AEs occurred in 11.0% of those receiving osimertinib versus 2.9% in those receiving placebo, with the most common osimertinib-related grade 3 AEs being diarrhea (2.4% versus 0.3%), stomatitis (1.8% versus 0%), paronychia (0.9% versus 0%), upper respiratory tract infection, and decreased appetite (0.6% versus 0% for both) (Table 2). 30 No grade 4 or 5 AEs were reported in the osimertinib arm, with one patient dying in the placebo group due to a pulmonary embolism (0.3%).
The phase III IMPACT study randomized 232 patients with stage II-III NSCLC and a confirmed EGFR-activating mutation (exon 19 deletion or exon 21 L858R and not T790M) in a 1:1 ratio to gefitinib for 2 years or platinum-based chemotherapy following surgical resection. At a median follow-up of 70.0 months, there was no statistically significant difference in the primary endpoint of DFS (median 35.9 versus 25.0 months gefitinib versus chemotherapy respectively, HR = 0.92, p = 0.63) or OS (HR = 1.03, p = 0.89) (Table 1). 31 The most common grade ⩾ 3 AEs in the gefitinib versus chemotherapy arms were ALT increase (27.0% versus 3.5%), AST increase (15.7% versus 0.9%), dermatitis acneiform (4.3% versus 0%), rash (4.3% versus 0%), and paronychia (3.5% versus 0%) (Table 2). No treatment-related deaths were observed in the gefitinib arm versus three (2.6%) in the chemotherapy arm.
Discussion
What is the clinical benefit of adjuvant EGFR inhibition in resected EGFR-mutant NSCLC?
Standard therapy for appropriate patients with resected stage II-IIIA NSCLC is platinum-based chemotherapy.7,37 Over the last 8 years, five phase III clinical trials have assessed adjuvant EGFR inhibition in this setting.27–31 All trials assessed an anti-EGFR TKI, three with a placebo-control arm (BR19, RADIANT, and ADAURA)27,28,30 and two compared with chemotherapy (CTONG1104 and IMPACT).29,31 OS was the primary endpoint in BR19 27 and all other trials used DFS as the primary endpoint.28–31 BR19 and RADIANT failed to demonstrate a statistically significant improvement in either OS or DFS for gefitinib and erlotinib, respectively, versus placebo in patients with EGFR mutation-unselected NSCLC (Table 1).27,28 For BR19, early halting of the study may have led to an underpowered statistical analysis and the number of EGFR-positive patients was extremely low (4.2%), potentially limiting conclusions. 27 An adjusted post hoc exploratory analysis of EGFR-positive patients in RADIANT revealed a 40% reduction in the risk of progression for erlotinib compared with placebo which was consistent with a planned unadjusted analysis (39% risk reduction), although the difference did not reach statistical significance due to a hierarchical statistical design. 28
Stage II–IIIA
Three subsequent phase III trials, CTONG1104, ADAURA, and IMPACT, assessed EGFR inhibitors in EGFR-mutant resected stage II-IIIA NSCLC.29–31 Gefitinib failed to significantly reduce the risk of recurrence or death compared with platinum chemotherapy in the IMPACT trial (n = 232). 31 While CTONG1104 showed a statistically significant 40% reduction in the risk of recurrent disease or death favoring 2 years of gefitinib versus chemotherapy (n = 222, p = 0.0054),33 with a median follow-up of 80.0 months the two arms of the DFS log-rank curve returned together at 4 years (5-year DFS was 22.8% for vinorelbine and 22.6% for gefitinib),29 suggesting a lack of durable effect and, not surprisingly, no OS benefit was observed.
The larger ADAURA trial (n = 683) reported a statistically significant DFS benefit for osimertinib compared with placebo in patients with resected stage IB-IIIA NSCLC (AJCC 7th edition) and a confirmed EGFR activating mutation (exon 19 deletion or exon 21 L858R). 30 The remainder of this discussion will thus focus on the clinical benefit observed in the positive ADAURA study. At a median follow-up of 22.1 versus 14.9 months for the osimertinib and placebo arms, the ADAURA trial observed a statistically significant 83% reduction in the risk of disease recurrence or death favoring osimertinib (p < 0.001) in patients with resected stage II-IIIA disease.
It is unclear why the DFS benefit of osimertinib in ADAURA was so pronounced compared with that seen with gefitinib in CTONG1104.29,30 Comparisons between these studies should be made cautiously due to variable design, with CTONG1104 comparing gefitinib with platinum-based chemotherapy and ADAURA comparing osimertinib with placebo, in addition to different patient populations and length of therapy. Part of the explanation could, however, potentially be attributed to the greater activity and central nervous system (CNS) penetrance of osimertinib compared with gefitinib.21,30,38–40 It is also possible that the additive benefit of adjuvant chemotherapy in CTONG1104 blunted the observed differences between the treatment arms, given the overall benefit of adjuvant chemotherapy observed in other trials. In ADAURA, prior adjuvant chemotherapy did not seem to influence the benefit of adjuvant osimertinib (HR = 0.16 and 0.23, prior chemotherapy versus not, respectively), although neither prior chemotherapy nor type of surgery was stratified. 30 Treatment initiation with osimertinib was also started later than gefitinib due to adjuvant chemotherapy receipt (up to 26 versus 6 weeks following surgery) and administered for longer (3 versus 2 years).29,30 Recent data from the ICOMPARE randomized phase II trial as well as a subgroup analysis from CTONG1104 suggest that longer EGFR TKI exposure is associated with improved adjuvant outcomes among stage II-IIIA patients with EGFR-mutant NSCLC (DFS for icotinib 1 versus 2 years and OS for gefitinib < 18 versus ⩾18 months),41,42 which may also in part explain differences in effect. The optimal length of adjuvant treatment and the importance of agent-specific toxicities weighed against clinical efficacy remain unknown and needs to be explored further.
The magnitude of benefit observed with adjuvant EGFR TKIs in selected EGFR Stage II-IIIA NSCLC is robust and at least comparable with that observed in IMpower010 for adjuvant atezolizumab in programmed cell death ligand 1 (PD-L1) overexpressing (TC ⩾ 1%) stage II-IIIA NSCLC (AJCC 7th edition), with a 34% reduction in the risk of recurrence or death in patients with EGFR-unselected tumors (95% CI: 0.50–0.88, p = 0.004) and a suggestion of a 43% reduction in patients with EGFR-mutant tumors (9% of patients, 95% CI: 0.26–1.24) favoring the addition of atezolizumab. 43
Adjuvant EGFR TKIs have yet to demonstrate an OS improvement although are associated with some HRQoL gain in EGFR-mutant NSCLC. CTONG1104 was not powered to detect OS differences and patients on the placebo arm received more subsequent therapy than those receiving gefitinib (73.6% versus 68.4%, p = 0.50), 29 although a significant delay in time to deterioration in HRQoL favoring gefitinib was observed (TOI: median 164 versus 9 weeks, p = 0.001). 44 OS data for ADAURA are currently immature, although the early and wide separation of the osimertinib and placebo arms of the DFS log-rank curves are suggestive of a possible survival benefit, which may end up being partly obscured by unblinding upon progression resulting in subsequent osimertinib therapy in the placebo arm. 30 Osimertinib was not associated with a decrease in HRQoL versus placebo despite 3 years of therapy. 36
Stage III
The DFS benefit observed for osimertinib in ADAURA was more pronounced among patients with stage IIIA disease (88% reduction in risk of recurrence or death, 95% CI: 0.7–0.20). Although CTONG1104 did not separate outcomes by disease stage, 29 the randomized phase II EVAN trial reported a 68% reduced risk of death for erlotinib compared with chemotherapy among 102 patients with stage IIIA disease (95% CI: 0.15–0.67, p = 0.0015). 45
Stage IB
Unlike CTONG1104, ADAURA included patients with resected AJCC 7th edition stage IB NSCLC, therefore including patients with tumors of at least 4 cm,33,46 with a 61% reduction in the risk of recurrence or death favoring osimertinib (95% CI:0.18–0.76). 30 Adjuvant erlotinib did not result in a DFS advantage among stage IB patients in RADIANT (AJCC 6th edition, HR = 0.98, 95% CI = 0.71–1.35) 28 and subgroup results for stage IB disease were not reported for gefitinib in BR19. 27 The phase II SELECT trial, however, reported similar rates of recurrence (35%) for patients with stage IB and IIIA (AJCC 7th edition) disease receiving adjuvant erlotinib, suggesting activity in patients with lower disease stage. 47 Although editions of the staging system have changed since CTONG1104 and ADAURA (AJCC 7th edition), available data support consideration of adjuvant osimertinib in patients with stage IB NSCLC (AJCC 7th edition). To further explore the efficacy of adjuvant EGFR TKIs in lower stage disease, a phase II study examining adjuvant osimertinib in resected stage IA/B-IIIA EGFR-mutant NSCLC has been initiated (NCT03433469).
What is the clinical benefit of adjuvant EGFR inhibitors preventing CNS metastases?
CNS metastases are common in NSCLC and confer poor prognosis, with EGFR mutations potentially increasing the frequency of CNS disease in patients receiving adjuvant TKIs.48–50 RADIANT assessed CNS recurrence and observed an increased risk of CNS metastases for those with an EGFR mutation in the erlotinib arm, although the numbers were small (n = 13, 37.1% versus n = 4, 12.9%; erlotinib versus placebo, respectively) with no difference observed in the EGFR-unselected overall trial population (n = 48, 20.9% versus n = 26, 17.1%). 28 Although a post hoc analysis of CTONG 1104 observed an initial delay in CNS events with adjuvant gefitinib compared with chemotherapy, by the end of the third year CNS events were comparable in the two arms (27.4% versus 24.1%, p = 0.61). 51 In contrast, ADAURA observed a reduction in both distant (2.9% versus 22.7%) and CNS recurrence (1.2% versus 9.6%) for patients receiving adjuvant osimertinib versus placebo, with a clinically meaningful 98% CNS DFS at 24 months compared with 85% for placebo.30,52 These findings support preclinical data which suggest greater brain penetrance for osimertinib compared with other TKIs38–40,53 as well as clinical data from the phase III FLAURA trial which observed a 52% reduced risk of CNS progression with osimertinib compared with gefitinib or erlotinib in the first-line treatment of advanced NSCLC (95% CI: 0.26–0.86, p = 0.014).54,55 Although promising, these results might be partly explained by a lack of systematic brain imaging in ADAURA as it was only performed if patients became symptomatic. 30
Taken together, these results suggest that adjuvant osimertinib may reduce CNS recurrence for patients with resected stage IB-IIIA EGFR-positive NSCLC (7th Edition Staging).22,23,30 Given the overall DFS benefit observed and the potential for reducing CNS metastases, we recommend use of osimertinib in patients with stage II-IIIA EGFR-mutant NSCLC (exon 19 deletion or exon 21 L858R, Union for International Cancer Control (UICC)/AJCC 8th Edition Staging). 8 Mature OS data are awaited, and consistent with this recommendation, osimertinib was approved by the United States Food and Drug Administration on 18 December 2020, 56 by the European Medicines Agency on 22 April 2021 57 for the adjuvant treatment of EGFR exon 19 deletion or exon 21 L858R mutated following resection, and is not yet approved in Canada. 58
What is the safety of adjuvant EGFR inhibition in resectable EGFR-positive NSCLC?
No unexpected safety signals were seen for gefitinib and osimertinib in CTONG1104 and ADAURA, respectively, and reported toxicities were primarily grade 1/2.29,30 Grade 3/4 toxicities from osimertinib were rare (1–2%), with no treatment-emergent toxicities despite the extended 3-year treatment. 30 Rates of dose reductions were low for both agents (9–11%), although discontinuation due to AEs was higher for osimertinib versus gefitinib (11% versus 3%).29,30 Interstitial lung disease was not a substantial clinical issue in either trial, with no cases reported for gefitinib in CTONG1104 29 and 10 mild or moderate cases reported for osimertinib in ADAURA (3.0%). 30
What is the place of adjuvant EGFR inhibition in resectable EGFR-positive NSCLC therapy?
Treatment selection should take into account patient and treatment history, disease characteristics, personal preference, and other available clinical trials, with reflex mutational testing employed to ensure early detection of EGFR mutations. When selecting adjuvant EGFR TKIs, caution should be exercised when considering use in patients with Eastern Cooperative Oncology Group PS 2 or the very elderly ( > 75 years), as these patients were not well represented in the adjuvant EGFR TKI trials.27,28,30,31,33 Based on available evidence, adjuvant osimertinib either used alone or after chemotherapy is recommended for patients with resected stage II-IIIA EGFR-mutant NSCLC (UICC/AJCC 8th edition). 8
Re-challenging with osimertinib is an appropriate option for recurrent disease if the disease-free interval is greater than 12 months, based on results from the INSIGHT and SELECT trials where few patients expressed the T790M mutation in the advanced setting following early-stage TKI exposure.47,59 For patients who recur within 6 to 12 months, a liquid or tissue biopsy should be considered to detect the T790M resistance mutation.60,61 Either osimertinib re-challenge or platinum-based chemotherapy should be considered based on results.
What are future directions for targeted-inhibition in early NSCLC?
Multiple target-directed therapies are being evaluated as adjuvant therapy in patients with NSCLC molecular alterations. The randomized Adjuvant Lung Cancer Enrichment Marker Identification and Sequencing Trials (ALCHEMIST) trial is a platform designed by the National Cancer Institute that uses biomarker analysis to test novel adjuvant therapies for high-risk resected NSCLC within a randomized trial context. 62 Patients with stage IB to IIIA disease harboring EGFR mutations may be assigned 2 years of erlotinib versus observation with the primary end point of OS (NCT02193282, A081105) while patients with anaplastic lymphoma kinase (ALK) fusions may receive adjuvant crizotinib versus observation, and those with biomarker negative disease may be randomized to the nivolumab versus observation regardless of PD-L1 status (Table 3). Although this study will inform the use of adjuvant targeted therapy in high-risk NSCLC, results will be limited by the use of earlier generation EGFR and ALK TKIs. Other ongoing trials include one evaluating the third-generation T790M resistance mutation-specific TKI almonertinib compared with placebo for stage II-IIIA disease (HS-10296-302, NCT04687241) or almonertinib added to chemotherapy compared with almonertinib monotherapy and chemotherapy alone (APEX, NCT04762459), with preliminary findings from both trials expected in 2026. First- and second-generation adjuvant TKIs are also being evaluated in patients with stage II-III disease. Gefitinib added to chemotherapy is being compared with chemotherapy alone (4-2016-0763, NCT03381066) and the highly selective first-generation EGFR TKI icotinib is being assessed either following adjuvant chemotherapy (ICTAN, NCT01996098) or compared with chemotherapy as initial adjuvant therapy (EVIDENCE, NCT02448797). Results from these studies will provide additional insight into the benefit of EGFR-directed approaches for earlier stage EGFR-positive NSCLC and will help determine whether the sequential or concurrent administration of EGFR targeted therapies with chemotherapy is the optimal approach in this setting.
Table 3.
Ongoing phase III clinical trials of neoadjuvant and adjuvant EGFR and ALK inhibitors in early NSCLC.
| Experimental agent(s) | Trial ID (NCT#) |
Key eligibility criteria | Experimental regimen | Comparator | Primary endpoint(s) | Estimated PCD |
|---|---|---|---|---|---|---|
| Neoadjuvant NSCLC | ||||||
| Osimertinib | NeoADAURA (NCT04351555) |
Resectable EGFR-mutant nsNSCLC | Osimertinib ± platinum-based chemotherapy | Placebo + platinum-based chemotherapy | MPR | March 2024 |
| Adjuvant NSCLC, EGFR inhibitors | ||||||
| Icotinib |
ICTAN
(NCT01996098) |
Stage IIA-IIIA EGFR-mutant NSCLC | 6- or 12-month Icotinib following chemotherapy | Chemotherapy | DFS | January 2020 |
| Icotinib | EVIDENCE (NCT02448797) |
Stage II-IIIA EGFR-mutant NSCLC | Icotinib | Vinorelbine + Cisplatin a | DFS | December 2020 |
| Erlotinib | ALCHEMIST Treatment Trial A081105 (NCT02193282) |
Completely resected stage IB-IIIA EGFR-mutant nsNSCLC | Erlotinib | Placebo | OS | November 2021 |
| Gefitinib | 4-2016-0763 (NCT03381066) |
Completely resected stage IIA to IIIB EGFR-mutant nsNSCLC |
Gefitinib + Pemetrexed + Cisplatin | Vinorelbine + Cisplatin | DFS | December 2022 |
| Furmonertinib | FORWARD (NCT04853342) |
Completely resected stage II-IIIA EGFR-mutant NSCLC | Furmonertinib | Placebo | DFS | December 2023 |
| Almonertinib | HS-10296-302 (NCT04687241) |
Stage II-IIIB EGFR-mutant NSCLC | Almonertinib | Placebo | DFS | January 2026 |
| Almonertinib | APEX (NCT04762459) |
Completely resected stage II-IIIA EGFR-mutant nsNSCLC | Almonertinib + Pemetrexed + Cisplatin | Pemetrexed + Cisplatin | DFS | May 2026 |
| Adjuvant NSCLC, ALK inhibitors | ||||||
| Crizotinib | ALCHEMIST Treatment Trial A081105 (NCT02201992) |
Completely resected stage IB-IIIA ALK-mutant NSCLC | Crizotinib | Placebo | OS | May 2022 |
| Alectinib | BO40336 (NCT03456076) |
Completely resected stage IB-IIIA ALK-mutant NSCLC | Alectinib | Platinum-based chemotherapy | DFS | June 2023 |
ALK, anaplastic lymphoma kinase; DFS, disease-free survival; EGFR, epidermal growth factor receptor; PCD, primary completion date; MPR, major pathological response; ns, non-squamous; NSCLC, non-small cell lung cancer; OS; overall survival.
Ongoing (trials that are actively recruiting for which efficacy outcomes are not yet available and records have been updated in the last 2 years) phase III trials on neoadjuvant and adjuvant treatment with EGFR and ALK inhibitors for NSCLC listed at CT.gov on 17 March 2021 ordered by treatment setting and estimated primary completion date.
Pemetrexed plus cisplatin for adenocarcinoma.
Neoadjuvant
Neoadjuvant EGFR inhibition is also showing promise as demonstrated by outcomes of the randomized phase II CTONG 1103 trial (EMERGING, NCT01407822), which reported a near doubling in median progression-free survival for neoadjuvant erlotinib versus chemotherapy (21.5 versus 11.4 months, HR: 0.36, 95% CI: 0.21–0.61, p < 0.001) among patients with stage IIIA N2 disease. 63 Osimertinib is also being assessed as neoadjuvant therapy with or without chemotherapy for patients with stage II-IIIB disease in the placebo-controlled NeoADAURA trial (NCT04351555). The observational LCMC4 study will also examine the proportion of patients with stage IA2-III lung cancers who possess a variety of actionable oncogenic driver mutations (NCT04712877).
Summary
Up to 3 years of the third-generation EGFR TKI osimertinib has demonstrated an unprecedented, statistically significant DFS benefit compared with placebo in patients with stage II-IIIA EGFR-mutant NSCLC (exon 19 deletion or exon 21 L858R, UICC/AJCC 8th edition). A clinically meaningful reduction in CNS recurrence was also reported and HRQoL was maintained despite the prolonged treatment duration of 3 years. Although OS data are immature, adjuvant osimertinib used alone or following platinum-based chemotherapy is recommended in these patients. This is a rapidly evolving field and ongoing trials will define the expanding role of EGFR inhibition for early-stage NSCLC.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211056306 for The dawn of a new era, adjuvant EGFR inhibition in resected non-small cell lung cancer by Barbara Melosky, Parneet Cheema, Rosalyn A. Juergens, Natasha B. Leighl, Geoffrey Liu, Paul Wheatley-Price, Adrian Sacher, Stephanie Snow, Ming-Sound Tsao, Deanna McLeod and Quincy Chu in Therapeutic Advances in Medical Oncology
Acknowledgments
We would like to thank Ilidio Martins and Paul Card from Kaleidoscope Strategic Inc., Bristol-Myers Squibb Canada, Roche Canada, and AstraZeneca Canada Inc. for their support.
Footnotes
Author contributions: All authors contributed to concept development and/or data collection, and substantive drafting/editing; reviewed for accuracy and final approval; and agreed to be accountable for work.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BM has received honoraria from and served in a compensated consultancy or advisory role for Merck, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Novartis, and AstraZeneca, and has received research funding from Roche. PC has served in a compensated consultancy or advisory role for Roche, AstraZeneca, Amgen, Merck, Bayer, Novartis, Pfizer, EMD Serono, Takeda, and Bristol-Myers SquibbBMS; has received honoraria from AstraZeneca, Pfizer, Merck, and Novartis; and has received research funding from AstraZeneca, Roche, and Turning Point. RAJ has served in a compensated consultancy or advisory role for AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, BMSBristol-Myers Squibb, EMD Serono, Fusion Pharmaceuticals, Merck, Novartis, Pfizer, Roche, and Takeda; has received honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, BMSBristol-Myers Squibb, EMD Serono, Merck, Novartis, Pfizer, Roche, and Takeda; and has received research funding from AstraZeneca, BMSBristol-Myers Squibb, and Merck. NBL has received research funding from Pfizer, Roche, Bristol-Myers Squibb Canada, Array, Guardant Health, Amgen, Eli Lilly, Takeda, MSD, and Bayer. GL has served in a compensated consultancy or advisory role for Takeda, Merck, BMSBristol-Myers Squibb, Novartis, Pfizer, Roche, and AstraZeneca; has received honoraria from Takeda, AstraZeneca, and Pfizer; and has received research funding from Takeda, Boehringer Ingelheim, AstraZeneca, and Roche. PW-P has served in a compensated consultancy or advisory role for Sanofi, EMD Serono, Merck, Astra Zeneca, Pfizer, Bayer, BMSBristol-Myers Squibb, Roche, and Novartis; has received honoraria from AstraZeneca, Pfizer, Bayer, Merck, and Takeda; and has received research funding from Roche, AbbVie, GSK, Turning Point, Pfizer, and Merck. ASAdrian Sacher has served in a compensated consultancy or advisory role for Amgen, AstraZeneca, Bayer, Genentech-Roche, Bristol-Myers SquibbBMS, and KisoJi; has received honoraria from Amgen, AstraZeneca, Merck, Genentech-Roche, Bayer, Bristol-Myers SquibbBMS, Pfizer, Tesaro, KisoJi, Iovance, and Galvanize Therapeutics; and has received research funding from AstraZeneca, Amgen, Genentech, Merck, Lilly, Pfizer, Bayer, Bristol-Myers SquibbBMS, Spectrum, GlaxoSmithKline?GSK, Iovance, CRISPR Therapeutics, and RAIN Therapeutics. SS has served in a compensated consultancy or advisory role for AstraZeneca, Amgen, Bayer, Bristol-Myers SquibbBMS, EMD Serono, Eisai, Merck, Novartis, Pfizer, Roche, Sanofi, Takeda, and Taiho; has received honoraria from AstraZeneca, Amgen, Bayer, BMSBristol-Myers Squibb, and Merck; and has received research funding from AstraZeneca, BMSBristol-Myers Squibb, Merck, Novartis, and Takeda. M-ST has served in a compensated consultancy or advisory role for AstraZeneca, Pfizer, Bayer, Takeda, Amgen, Roche, and Eli Lilly; has received honoraria from Bayer and Roche; and has received research funding from Bayer. DM has nothing to disclose. QC has received honoraria from and served in a compensated consultancy or advisory role for AbbVie, AstraZeneca, BIBoehringer Ingelheim, BMSBristol-Myers Squibb, Eli Lilly, Eisai, Merck, Novartis, Pfizer, Roche, and Takeda, and has received research funding from Astra Zeneca and BMSBristol-Myers Squibb.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this review was provided through unrestricted educational grants from AstraZeneca Canada Inc. and Pfizer Canada. No discussion or viewing of review content was permitted with sponsors at any stage of review development.
Disclaimer: This review was prepared according to ICMJE standards with editorial assistance from Kaleidoscope Strategic Inc.
ORCID iDs: Barbara Melosky  https://orcid.org/0000-0003-2865-659X
https://orcid.org/0000-0003-2865-659X
Quincy Chu  https://orcid.org/0000-0003-4814-3126
https://orcid.org/0000-0003-4814-3126
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Barbara Melosky, Medical Oncology, BC Cancer Agency–Vancouver Centre, 600 West 10th Avenue, Vancouver, BC V5Z 4E6, Canada.
Parneet Cheema, William Osler Health System, Brampton, ON, Canada; University of Toronto, Toronto, ON, Canada.
Rosalyn A. Juergens, Juravinski Cancer Centre, McMaster University, Hamilton, ON, Canada
Natasha B. Leighl, Princess Margaret Cancer Centre, University of Toronto, Toronto, ON, Canada
Geoffrey Liu, Princess Margaret Cancer Centre, University of Toronto, Toronto, ON, Canada.
Paul Wheatley-Price, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, ON, Canada.
Adrian Sacher, Princess Margaret Cancer Centre, University of Toronto, Toronto, ON, Canada.
Stephanie Snow, QEII Health Sciences Centre, Dalhousie University, Halifax, NS, Canada.
Ming-Sound Tsao, Princess Margaret Cancer Centre, University Health Network, University of Toronto, Toronto, ON, Canada.
Deanna McLeod, Kaleidoscope Strategic Inc., Toronto, ON, Canada.
Quincy Chu, Cross Cancer Institute, University of Alberta, Edmonton, AB, Canada.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008; 359: 1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Datta D, Lahiri B. Preoperative evaluation of patients undergoing lung resection surgery. Chest 2003; 123: 2096–2103. [DOI] [PubMed] [Google Scholar]
- 4. Cagle PT, Allen TC, Olsen RJ. Lung cancer biomarkers: present status and future developments. Arch Pathol Lab Med 2013; 137: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 5. Le Chevalier T. Adjuvant chemotherapy for resectable non-small-cell lung cancer: where is it going? Ann Oncol 2010; 21: vii196–vii198. [DOI] [PubMed] [Google Scholar]
- 6. Pignon J-P, Tribodet H, Scagliotti GV, et al. Lung adjuvant Cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol 2008; 26: 3552–3559. [DOI] [PubMed] [Google Scholar]
- 7. Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol 2017; 35: 2960–2974. [DOI] [PubMed] [Google Scholar]
- 8. Nicholson AG, Tsao MS, Travis WD, et al. Staging of thoracic malignancies: implications for the reporting pathologist. Arch Pathol Lab Med 2018; 142: 645–661. [DOI] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer (version 3.2020). National Comprehensive Cancer Network, 2020, https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.Pdf (accessed 21 October 2021). [Google Scholar]
- 10. Amin MB, Edge SB. AJCC cancer staging manual. Cham: Springer, 2017. [Google Scholar]
- 11. Butts CA, Ding K, Seymour L, et al. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non–small-cell lung cancer: updated survival analysis of JBR-10. J Clin Oncol 2010; 28: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 13. Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015; 4: 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gazdar A. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene 2009; 28: S24–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nature Rev Cancer 2007; 7: 169–181. [DOI] [PubMed] [Google Scholar]
- 16. Chintala L, Kurzrock R. Epidermal growth factor receptor mutation and diverse tumors: case report and concise literature review. Mol Oncol 2010; 4: 306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen MH, Williams GA, Sridhara R, et al. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 2003; 8: 303–306. [DOI] [PubMed] [Google Scholar]
- 18. Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2021; 39: 1040–1091. [DOI] [PubMed] [Google Scholar]
- 19. Gelatti AC, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer 2019; 137: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H. Three generations of epidermal growth factor receptor tyrosine kinase inhibitors developed to revolutionize the therapy of lung cancer. Drug Des Devel Ther 2016; 10: 3867–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malapelle U, Ricciuti B, Baglivo S, et al. Osimertinib. Recent Results Cancer Res 2018; 211: 257–276. [DOI] [PubMed] [Google Scholar]
- 22. Mok TS, Wu Y-L, Ahn M-J, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med 2017; 376: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Papadimitrakopoulou V, Mok T, Han J-Y, et al. Osimertinib versus platinum–pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol 2020; 31: 1536–1544. [DOI] [PubMed] [Google Scholar]
- 24. OncLive.com. FDA approves osimertinib for EGFR T790M-positive NSCLC, https://www.onclive.com/view/fda-approves-osimertinib-for-egfr-t790m-positive-nsclc (accessed 16 March 2021).
- 25. AstraZeneca.com. US FDA approves Tagrisso as 1st-line treatment for EGFR-mutated non-small cell lung cancer, https://www.astrazeneca.com/media-centre/press-releases/2018/us-fda-approves-tagrisso-as-1st-line-treatment-for-EGFR-mutated-non-small-cell-lung-cancer.html#! (accessed 16 March 2021).
- 26. FDA.gov. TAGRISSO® (osimertinib), https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208065s008lbl.pdf (accessed 11 March 2021).
- 27. Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non–small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013; 31: 3320–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol 2015; 33: 4007–4014. [DOI] [PubMed] [Google Scholar]
- 29. Zhong W-Z, Wang Q, Mao W-M, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol 2021; 39: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu Y-L, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non–small-cell lung cancer. N Engl J Med 2020; 383: 1711–1723. [DOI] [PubMed] [Google Scholar]
- 31. Tada H, Mitsudomi T, Yamanaka T, et al. Adjuvant gefitinib versus cisplatin/vinorelbine in Japanese patients with completely resected, EGFR-mutated, stage II-III non-small cell lung cancer (IMPACT, WJOG6410L): a randomized phase 3 trial. Philadelphia, PA: Wolters Kluwer Health, 2021. [Google Scholar]
- 32. Tsuboi M, Kato H, Nagai K, et al. Gefitinib in the adjuvant setting: safety results from a phase III study in patients with completely resected non-small cell lung cancer. Anticancer Drugs 2005; 16: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 33. Zhong W-Z, Wang Q, Mao W-M, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018; 19: 139–148. [DOI] [PubMed] [Google Scholar]
- 34. Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005; 366: 1527–1537. [DOI] [PubMed] [Google Scholar]
- 35. Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non–small-cell lung cancer: SWOG S0023. J Clin Oncol 2008; 26: 2450–2456. [DOI] [PubMed] [Google Scholar]
- 36. Majem M, Goldman J, John T, et al. OA06.03 patient-reported outcomes from ADAURA: osimertinib as adjuvant therapy in patients with resected EGFR mutated (EGFRm) NSCLC. J Thorac Oncol 2021; 16: S112–S113. [DOI] [PubMed] [Google Scholar]
- 37. Postmus P, Kerr K, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28: iv1–iv21. [DOI] [PubMed] [Google Scholar]
- 38. Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016; 22: 5130–5140. [DOI] [PubMed] [Google Scholar]
- 39. Colclough N, Ballard P, Barton P, et al. Preclinical comparison of the blood brain barrier (BBB) permeability of osimertinib (AZD9291) with other irreversible next generation EGFR TKIs. Eur J Cancer 2016; 69: S28. [Google Scholar]
- 40. Vishwanathan K, Varrone A, Varnas K, et al. Abstract CT013: osimertinib displays high brain exposure in healthy subjects with intact blood-brain barrier: a microdose positron emission tomography (PET) study with 11C-labelled osimertinib. Philadelphia, PA: AACR, 2018. [Google Scholar]
- 41. Lyu C, Wang R, Li S, et al. Different exposure duration of adjuvant icotinib in stage II-IIIA non-small cell lung cancer patients with positive EGFR mutation (ICOMPARE study): a randomized, open-label phase 2 study. Philadelphia, PA: Wolters Kluwer Health, 2021. [Google Scholar]
- 42. Wu Y-L, Zhong W, Wang Q, et al. CTONG1104: adjuvant gefitinib versus chemotherapy for resected N1-N2 NSCLC with EGFR mutation—final overall survival analysis of the randomized phase III trial 1 analysis of the randomized phase III trial. Alexandria, VA: American Society of Clinical Oncology, 2020. [Google Scholar]
- 43. Wakelee HA, Altorki NK, Zhou C, et al. IMpower010: primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). Philadelphia, PA: Wolters Kluwer Health, 2021. [Google Scholar]
- 44. Zeng J, Mao W-M, Chen Q-X, et al. Quality of life with adjuvant gefitinib versus vinorelbine plus cisplatin in patients with completely resected stage II–IIIA (N1–N2) EGFR-mutant non-small-cell lung cancer: results from the ADJUVANT (CTONG1104) study. Lung Cancer 2020; 150: 164–171. [DOI] [PubMed] [Google Scholar]
- 45. Yue D, Xu S-D, Wang Q, et al. Updated overall survival (OS) and exploratory analysis from the randomized, phase II EVAN study of erlotinib (E) versus vinorelbine plus cisplatin (NP) as adjuvant therapy in Chinese patients with stage IIIA EGFR+ NSCLC. Philadelphia, PA: Wolters Kluwer Health, 2021. [Google Scholar]
- 46. Wu Y-L, Herbst RS, Mann H, et al. ADAURA: phase III, double-blind, randomized study of osimertinib versus placebo in EGFR mutation-positive early-stage NSCLC after complete surgical resection. Clin Lung Cancer 2018; 19: e533–e536. [DOI] [PubMed] [Google Scholar]
- 47. Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor–mutant non–small-cell lung cancer. J Clin Oncol 2019; 37: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang W-Y, Wu Y-L, Su P-L, et al. The impact of EGFR mutations on the incidence and survival of stages I to III NSCLC patients with subsequent brain metastasis. PLoS ONE 2018; 13: e0192161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahluwalia MS, Becker K, Levy BP. Epidermal growth factor receptor tyrosine kinase inhibitors for central nervous system metastases from non-small cell lung cancer. Oncologist 2018; 23: 1199–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hsu F, De Caluwe A, Anderson D, et al. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer 2016; 96: 101–107. [DOI] [PubMed] [Google Scholar]
- 51. Xu S-T, Xi J-J, Zhong W-Z, et al. The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: a post hoc analysis of the ADJUVANT trial (CTONG 1104). J Thorac Oncol 2019; 14: 503–512. [DOI] [PubMed] [Google Scholar]
- 52. Tsuboi M, Wu Y, He J, et al. LBA1 Osimertinib adjuvant therapy in patients (pts) with resected EGFR mutated (EGFRm) NSCLC (ADAURA): central nervous system (CNS) disease recurrence. Ann Oncol 2020; 31: S1177. [Google Scholar]
- 53. Yang JC, Kim S-W, Kim D-W, et al. Osimertinib in patients with epidermal growth factor receptor mutation–positive non–small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol 2020; 38: 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020; 382: 41–50. [DOI] [PubMed] [Google Scholar]
- 55. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol 2018; 36: 3290–3297. [DOI] [PubMed] [Google Scholar]
- 56. FDA.gov. FDA approves osimertinib as adjuvant therapy for non-small cell lung cancer with EGFR mutations, https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-osimertinib-adjuvant-therapy-non-small-cell-lung-cancer-egfr-mutations (accessed 18 May 2021).
- 57.ESMO.org. EMA RECOMMENDS EXTENSION OF THERAPEUTIC INDICATIONS FOR OSIMERTINIB, https://www.esmo.org/oncology-news/ema-recommends-extension-of-therapeutic-indications-for-osimertinib (accessed 28 June 2021).
- 58.AstraZeneca.com. Health Canada approves Tagrisso® (osimertinib) as first-line treatment for EGFR-mutated non-small cell lung cancer, https://www.astrazeneca.ca/en/media/press-releases/2018/health-canada-approves-tagrisso-osimertinib-as-first-line-tre.html# (accessed 29 June 2021).
- 59. Wu Y-L, Cheng Y, Zhou J, et al. Tepotinib plus gefitinib in patients with EGFR-mutant non-small-cell lung cancer with MET overexpression or MET amplification and acquired resistance to previous EGFR inhibitor (INSIGHT study): an open-label, phase 1b/2, multicentre, randomised trial. Lancet Respir Med 2020; 8: 1132–1143. [DOI] [PubMed] [Google Scholar]
- 60. Nagasaka M, Uddin MH, Al-Hallak MN, et al. Liquid biopsy for therapy monitoring in early-stage non-small cell lung cancer. Mol Cancer 2021; 20: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guibert N, Hu Y, Feeney N, et al. Amplicon-based next-generation sequencing of plasma cell-free DNA for detection of driver and resistance mutations in advanced non-small cell lung cancer. Ann Oncol 2018; 29: 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sands J, Mandrekar SJ, Oxnard GR, et al. ALCHEMIST: adjuvant targeted therapy or immunotherapy for high-risk resected NSCLC. Alexandria, VA: American Society of Clinical Oncology, 2020. [Google Scholar]
- 63. Wu Y-L, Zhong W, Chen K-N, et al. CTONG1103: final overall survival analysis of the randomized phase 2 trial of erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non–small cell lung cancer. Philadelphia, PA: Wolters Kluwer Health, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211056306 for The dawn of a new era, adjuvant EGFR inhibition in resected non-small cell lung cancer by Barbara Melosky, Parneet Cheema, Rosalyn A. Juergens, Natasha B. Leighl, Geoffrey Liu, Paul Wheatley-Price, Adrian Sacher, Stephanie Snow, Ming-Sound Tsao, Deanna McLeod and Quincy Chu in Therapeutic Advances in Medical Oncology