Abstract
Hair graying depends on the altered presence and functionality of hair follicle melanocytes. Melanocyte stem cells (MelSCs) reside in the bulge of hair follicles and give rise to migrating and differentiating progeny during the anagen phase. Aging, genotoxic stress, redox stress, and multiple behavior-associated acute stressors have been seen to induce hair graying by depleting the MelSC pool, a phenomenon which is accompanied by ectopic pigmentation of these cells, followed by their depletion from the stem cell niche. This aberrant differentiation produces a state from which a return to stem cell-like quiescence appears to be lost. The cellular features of stress-induced hair graying have been extensively studied in murine models. Here we describe a method to assess and quantify human hair follicle MelSC differentiation by measuring ectopically pigmented MelSCs in isolated human hair follicles exposed to specific stress signal mediators. Ionizing radiation, hydrogen peroxide, and noradrenaline have been shown to cause hair graying in mice. We demonstrate here that isolated, ex vivo cultured human hair follicles exposed to these treatments display similar ectopic pigmentation within the bulge area which is accompanied by induction of differentiated melanocytic markers. This study suggests that as in murine models, stress signaling induces closely matching phenotypic changes in human hair follicles which can be monitored and studied as a surrogate model for early steps in human hair graying.
Keywords: hair graying, ectopic pigmentation, melanocyte stem cells, hair follicles, stress
INTRODUCTION
Hair color is determined by melanin-producing cells called melanocytes. Melanocytes are derived from a stem-cell population called melanocyte stem cells (MelSCs), which reside within the bulge region of the hair follicle. The normal hair cycle is divided into regeneration (anagen), degeneration (catagen) and resting (telogen) phases.1 During the anagen phase, MelSCs from the bulge become activated and differentiate into melanocytes. These differentiated cells migrate from the bulge region in the outer root sheath downward into the bulb,2while self-renewal of MelSCs continues to maintain a stem cell pool close to the bulge.2-5 The balance between differentiation and self-renewal/maintenance of MelSCs is critical for the ongoing repigmentation component of subsequent hair follicle cycles. The follicular melanocytes are progressively lost during hair graying. This loss has been attributed to numerous impaired processes, including death of bulbar melanocytes, failure in migration of MelSCs, or depletion of MelSCs.6-12 Here, we focus on the depletion of MelSCs, which has been identified in the context of hair graying in multiple mouse models and in human aging.2,5,13-15
MelSC depletion in mice has been attributed to several factors, including, genotoxic stressors,11,16 and other acute stressors (psychological or physical nociceptive stressors).17 The prevalent mechanism of MelSC depletion was seen to involve premature differentiation of the stem cells within the niche. The stem cell state is by definition associated with maintenance of an undifferentiated state. Therefore, an aberrant signal capable of inducing premature differentiation appears to remove the stem-like state in this limited pool of MelSCs. The premature differentiation of MelSCs is evidenced by increased expression of the key regulator of melanogenesis, microphthalmia associated transcription factor (MITF), and multiple of its target genes including tyrosinase related protein-1 (TRP-1) and tyrosinase related protein-2 (TRP-2) that are involved in melanin synthesis.14,16-19 In addition, premature differentiation of MelSCs is characterized by production of ectopic pigmentation within the same cells.14,16-18 While most of the experimental results regarding MelSC maintenance are derived from animal models, only limited data on human hair follicle MelSC maintenance are available due in part to the absence of convenient experimental models for assessing the dynamics of the human follicle.
Ectopically pigmented MelSCs should be detectable using brightfield microscopy of intact hair follicles. We hypothesized that isolated human hair follicles might be capable of exhibiting similar ectopic pigmentation if exposed to known mediators of MelSC pigmentation and attrition, as derived from previous animal models. To test this hypothesis, we isolated human hair follicles and treated them ex vivo with ionizing radiation (IR), hydrogen peroxide (H2O2) or noradrenalin (NE), three treatments previously seen to induce ectopic pigmentation of MelSCs within the outer root sheath (ORS) as well as eventual hair graying in mice.16-17 Each treatment modality produced an increase in ectopically pigmented cells within the ORS, accompanied by increased expression of the melanogenic enzymes, TRP-1 and TRP-2. The results demonstrated similar findings as seen within the animal models, supporting the hypothesis that premature differentiation of the MelSCs is similarly a key fate for human MelSCs after genotoxic and acute stress signals, and providing a tractable preclinical model for testing this pathway using human hair follicles.
MATERIALS AND METHODS
Isolation of human scalp hair follicles
Human scalp specimens were anonymized, discarded tissue samples obtained from elective face-lift surgeries. The Mass General Brigham Institutional Review Board approved the anonymized tissue study. Individual hair follicles were isolated as previously described.20 Briefly, the tissues were rinsed with wash solution (0.1 M phosphate buffered saline pH 7.4, 1000 U/mL Penicillin, 1mg/mL streptomycin, 25 μg/mL Fungizone) until all traces of blood and debris were removed. The hair follicles were dissected from surrounding dermal, subcutaneous tissue using a fine tweezers (#5, Roboz Surgical Instrument, Switzerland) and a scalpel (#15, Covidien, MA, USA) under the dissection microscope. The hair follicles were individually cultured in a semi-solid agarose medium containing William’s E medium (Invitrogen) supplemented with 2 mM glutamine, 10 ng/mL hydrocortisone, 10 μg/mL insulin, and 2% agarose. Only the hairs from the anagen VI phase with matured and pigmented hair shafts were used for the research. Follicle cycle stages were determined using professional criteria that include considerations of hair bulb status as well as pigmentation, melanin distribution and features of the hair matrix.21 Damaged hair follicles (e.g., unpigmented and artificial colored hairs and hairs in catagen or telogen stages) were discarded, as described previously.20,22-25
The induction of ectopic pigmentation
Ectopic pigmentation was induced by γ-ray exposure at doses of 4, 8, or 12 Gy (Cesium Irradiator). Hair follicles were treated with 1%, 2%, or 3% H2O2, then incubated in a semi-solid agarose William’s E medium for the indicated periods of time. Ectopic pigmentation of MelSCs was identified by observing the hair follicles under brightfield microscopy (Nikon, SMZ1500, Japan). The percentage of hair follicles with ectopic pigmentation was calculated by dividing the number of hair follicles with ectopic pigmentation by the total number of follicles analyzed.
Tissue immunohistochemistry analysis
For whole mount preparation, individual hair follicles were fixed with 4% paraformaldehyde (PFA) (Thermo Fisher Scientific, 50980487) for 30 minutes at room temperature and incubated for 1 hour in a blocking buffer containing 10% goat serum (Sigma Aldrich, G9D23) and 5% BSA (Sigma Aldrich, A3294). The hair follicles were subsequently incubated with diluted primary antibodies overnight at 4°C. TRP-2 (abcam, ab74073; 1:100), Ki67 (abcam, ab15580; 1:100), TRP-1 (abcam, ab186929; 1:100), and γH2AX (abcam, ab81299; 1:100) were used as the diluted primary antibodies. The hair follicles were then incubated with 1:500 diluted secondary antibodies for 1 hour at room temperature. Alexa Fluor 594 (goat anti-rabbit IgG; Thermo Fisher Scientific, A-11012) and Alexa Fluor 488 (goat anti-rabbit IgG; Thermos Fisher Scientific, A-11008) were used as the diluted secondary antibodies. Nuclei was labeled with VECTASHIELD Mounting Medium containing DAPI (Vector Laboratories, H-1200).
In the TUNEL assay, cell death was detected by TUNEL staining (TdT-mediated dUTP-digoxigenin nick end labeling technique) using the “in situ cell death detection kit” (Roche Diagnostics, 11684795910). Images were captured using confocal microscopy (Zeiss Axio Observer Z1 Inverted Phase Contrast Fluorescence microscope). Standard microscopy techniques were used to adjust brightness, contrast, focus, and image capture.
Immunolabeling for γ-H2AX and CD200
Tissue samples were embedded in OCT compound (Thermo Fisher Scientific, 23730625). 10 μm-thick tissue sections were cut longitudinally along the hair follicles. Cryosections were fixed in 4% PFA at room temperature for 10 minutes and incubated in blocking buffer (5% normal goat serum in PBS containing 0.5% Triton X-100 (Sigma-Aldrich, T9284) for 1 hour at room temperature. Cryosections were incubated with diluted primary antibodies overnight at 4°C. Sections were washed in PBS containing 0.01% Tween 20 (Sigma-Aldrich, P7949) and incubated with diluted secondary antibody for 2 hours at room temperature. Sections were washed in PBS containing 0.01% Tween 20 and mounted with VECTASHIELD Mounting Medium containing DAPI (Vector Laboratories, H-1200). Images were captured with rhodamine (CD200; Thermo Fisher Scientific, LS-C149902), Cy5 (γ-H2AX), DAPI and bright field (pigment) using confocal microscopy (Zeiss Axio Observer Z1 Inverted Phase Contrast Fluorescence microscope). Standard microscopy techniques were used to adjust brightness, contrast, focus, and image capture.
Detection of cellular reactive oxygen species (ROS)
The redox-sensitive fluorescent dye chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA; Life Technologies, C6827) was used to measure intracellular ROS accumulation. Hair follicles were isolated from the scalp. The follicles were then subject to hydrogen peroxide treatment (3% H2O2) for 2 hours, and CM-H2DCFDA was added to the samples to assess overall ROS production. After diffusion into the cells, CM-H2DCFDA was deacetylated and subsequently oxidized by ROS to form CM-DCF, a highly fluorescent product positively proportional to the degree of ROS, which can be assessed by confocal microscopy. Both brightfield and fluorescence images were obtained from the same follicle regions.
Noradrenaline treatments of hair follicles
Noradrenaline (Sigma-Aldrich, 489350) stock solution was prepared freshly by dissolving in a sterile aqueous solution containing 0.1% ascorbic acid and 0.9% NaCl to a final concentration of 10 mM. Butoxamine (Sigma-Aldrich, B1385) was dissolved in ultrapure water to a final concentration of 10 mM as a stock solution. Isolated human hair follicles were incubated with William’s E medium containing 0.1 mM noradrenaline or 0.1 mM Butoxamine.
Statistical analysis
GraphPad Prism software (Version 8.4.3 (471)) was used to perform two-tailed Student’s t-test, two-way ANOVA test and Fisher’s exact test (as indicated in the figure legends). P < 0.05 is considered statistically significant.
RESULTS
Genotoxic stress induces ectopic pigmentation in isolated human hair follicles
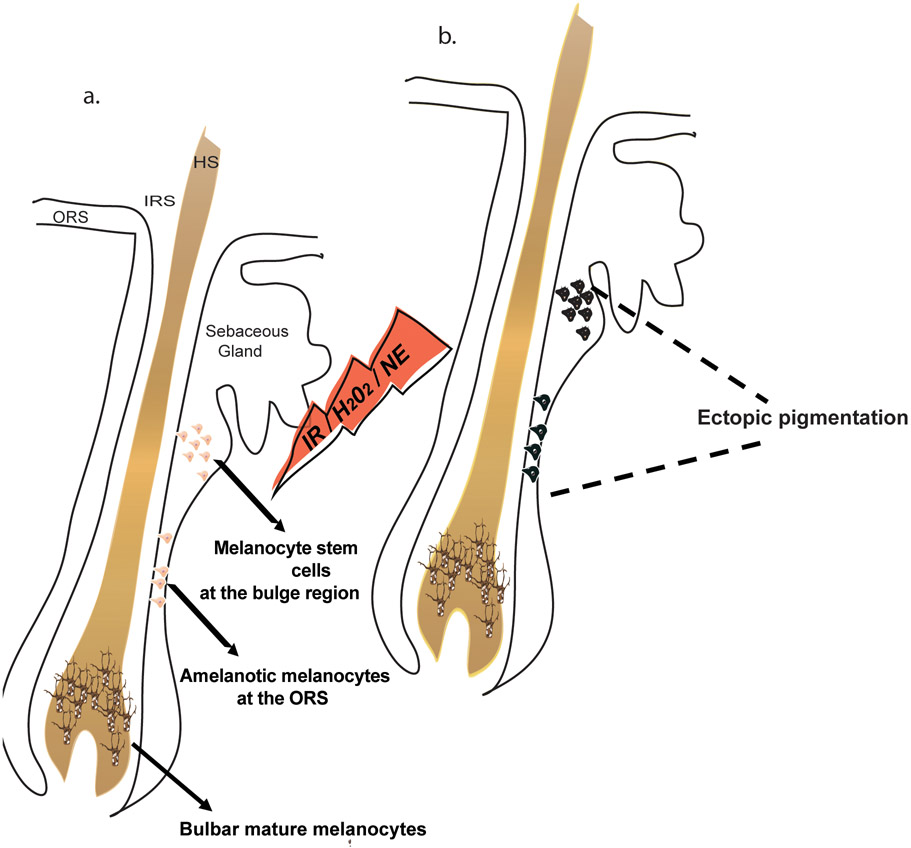
Premature differentiation of MelSCs resulted in the depletion of the MelSC pool followed by hair graying in mice and included the appearance of ectopic pigmentation.14,26 Based on this mechanism, we developed an assay to evaluate MelSC pigmentation and expression of differentiation genes within human hair follicles. We reasoned that signals inducing premature differentiation may be observable by brightfield microscopy as hyperpigmented cells within the ORS or bulge area due to the increase of melanin production (Figure 1) as observed in vivo within aging or stress-associated hair follicles in mice and in human aging hair follicles.14,16-17
Figure 1. A schematic for evaluation of melanocyte stem cell and amelanotic melanocyte differentiation.
Exposure of hair follicles to irradiation (IR), hydrogen peroxide (H2O2) or norepinephrine (NE) induces differentiation of melanocyte stem cells to pigmented cells within the niche, which appear as darkly pigmented cells under light microscopy. a. Hair follicle before the exposure, where the melanocyte stem cells at the bulge and the amelanotic melanocytes at the ORS are unpigmented. b. Hair follicle after exposure to a stressor, where the melanocyte stem cells and the amelanotic melanocytes can be observed as pigmented, dark cells. ORS, outer root sheath; IRS, inner root sheath; HS, hair shaft.
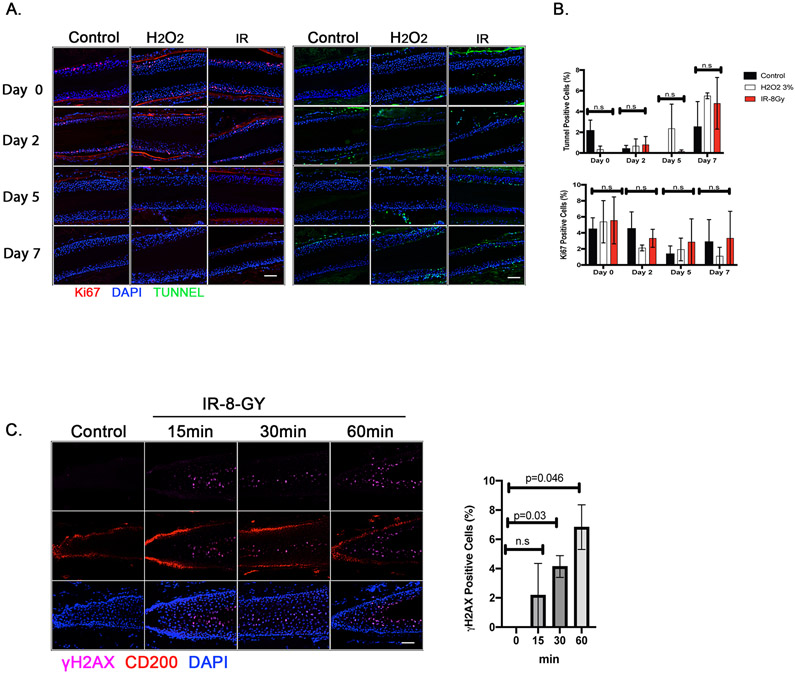
In order to establish a human ex vivo experimental protocol for monitoring abnormal MelSC differentiation, isolated human hair follicles were first exposed to ionizing radiation (IR) or hydrogen peroxide, two treatment modalities previously shown to potently trigger premature differentiation (Figure 1).16 Upon either treatment, Ki-67- and TUNEL-positive cells exhibited no significant changes (Figures 2A and 2B). Although increases in proliferation or apoptosis after the treatments were not seen, IR-induced DNA damage signaling was increased as evidenced by the increase in γH2AX-positive cells within the bulge area (CD200-positive area; Figure 2C).
Figure 2. Effect of genotoxic stress (hydrogen peroxide and ionizing radiation) on viability and DNA damage in isolated human hair follicles.
Human hair follicles (HFs) were isolated and irradiated (8 Gy) or treated with 3% hydrogen peroxide. A. Viability was tested at days 0, 2, 5 and 7 post treatment using immunofluorescent staining for Ki-67 (a proliferation marker; red) and TUNNEL assay (apoptosis; green) in the ORS (Scale bar; 50μm). B. Ki-67-and TUNNEL-positive cells were quantified and normalized to DAPI-positive ORS cell counts. Data are mean ± standard error of mean (SEM). n = 2 human donors; 48-50 hair follicles from each donor were analyzed; n.s., not significant; p values were determined by two-way ANOVA. C. Immunofluorescent staining of hair follicles for the DNA damage marker (γ-H2AX; purple), for hair follicle at the bulge (CD200; red), and for nuclei (DAPI). Scale bar, 50μm. The percentage of γ-H2AX-positive cells were calculated out of the total DAPI-positive cells at the bulge area (CD200-positive area). Data are mean ± SEM. 48-50 hair follicles were analyzed from each of 2 independent donors. p values were calculated by the two-tailed Student’s t-test; p < 0.05 is considered statistically significant.
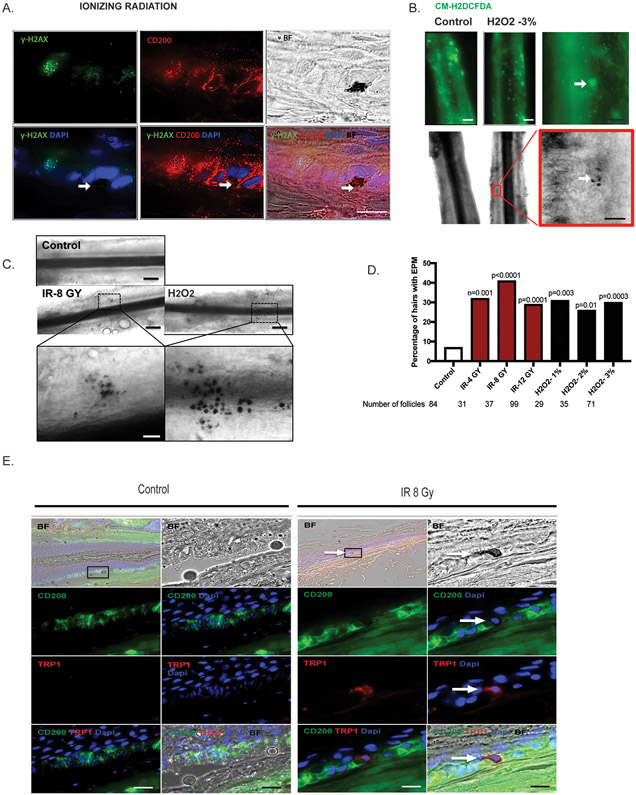
Thirty minutes after IR, pigmented cells were observed by brightfield microscopy in the ORS region where γH2AX-positive cells (green) were also visible (Figure 3A). Correspondingly, pigmented cells also appeared around ROS-positive cells in the ORS after H2O2 treatment (Figure 3B). The proportions of follicles with ectopic pigmentation in the ORS area after ionizing radiation or H2O2 treatments were greatly increased, when compared with control follicles (Figures 3C and 3D). To confirm whether the pigmented cells expressed differentiation-associated melanocyte factors, we examined the expression of the melanogenic enzyme, TRP-1, which is expressed in differentiated melanocytes, but not in melanocyte stem cells.18-19,27 Co-localization of TRP-1-positive cells with the pigmented cells was seen (Figure 3E). These data suggest that the pigment-containing cells in the ORS area are indeed ectopically pigmented melanocytes and that genotoxic stress after IR or hydrogen peroxide treatments triggered their differentiation in the stress-induced ex vivo human hair follicle model.
Figure 3. Induction of ectopic pigmentation by IR and H2O2 in the niche of human HFs.
A. Human HFs were isolated and irradiated (8 Gy). The pigmented cells are observable under brightfield microscopy (black spots). Human HFs were immunolabeled with CD200 (bulge; red) and γH2AX (a DNA damage maker; green). Scale bar, 100μm. The pigmentated cells are indicated by white Arrow. B. Isolated human HFs were treated with 3% H2O2 for 2 hours. ROS-positive cells were immunolabeled with CM-H2DCFDA (green) at the bulge area of the hair follicles. The black-pigmented cells appear around the ROS-positive cells (white arrow). White line-scale bar, 20μm; black line-scale bar, 100μm C. Representative follicle showing ectopic pigmentation after IR and H2O2 treatment. EPM, ectopically pigmented melanocytes. Black line-scale bar, 20μm; white line-scale bar, 100μm. D. Quantification of pigmentation efficiency (percentage of HFs with EPM divided by the total number of HFs) 3 days post treatment. n = 8 human donors, 29 to 99 hair follicles. p values were calculated by the Fisher’s exact test; p < 0.05 is considered statistically significant. E. Immunofluorescent staining of TRP-1 (red) for human HFs after IR (8 Gy). Arrow indicates ectopic pigmentation co-localized with TRP-1 staining.
Noradrenalin-induced ectopic pigmentation can be blocked by β-2 antagonist, butoxamine
Recently, we have reported a link between acute stress and hair graying.17 We showed in mouse models that acute stress triggered the sudden release of the neurotransmitter noradrenalin (NE) from sympathetic neurons. This caused a shift of MelSCs from quiescent to proliferative states, which were followed by their rapid differentiation and depletion from the niche.17 We wished to test whether this adrenergic signal might similarly induce ectopic pigmentation of MelSCs in the human ex vivo hair follicle assay.
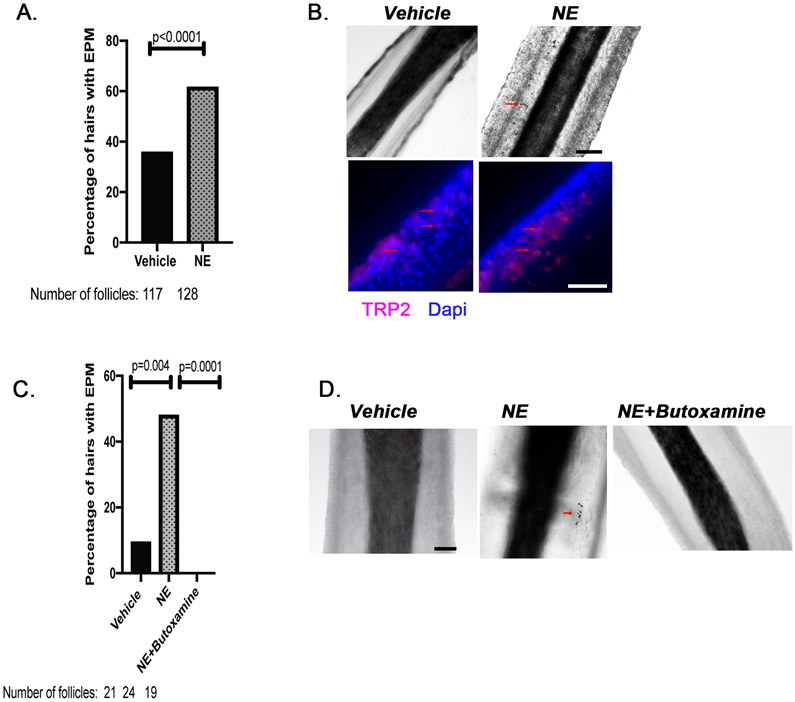
Isolated human HFs were treated with either NE or vehicle for 24 hours. The NE treatment significantly increased the number of hair follicles with pigmented cells at the ORS (Figures 4A and 4B). We further measured the melanogenic enzyme TRP-2. The TRP-2-positive cells increased in the ORS of NE-treated HFs (Figure 4B). These results confirmed that the pigmented cells observed under the microscope were indeed melanocytes and that NE could trigger the premature differentiation as indicated by the presence of dark pigment.
Figure 4. Noradrenaline induces ectopic pigmentation in the ORS, which can be prevented by blocking the β-2 adrenergic receptor.
A. Human HFs were isolated and treated with 0.1mM noradrenaline, or vehicle (deionized distilled water) for 24 hours. n = 2 different human donors, 117 to 128 hair follicles. p value was calculated by the Fisher’s exact test; p < 0.05 is considered statistically significant. B. Immunofluorescence staining of hair follicles for the melanocytic marker, TRP-2 (Red). Brightfield images of the isolated hair follicles with ectopic pigmentation observed as black cells as indicated by red arrows. Scale bar, 20μm. C. Isolated human HFs were treated with vehicle (deionized distilled water), noradrenalin (0.1 mM), or noradrenalin (0.1 mM) + butoxamine (0.1 mM) for 24 hours. EPM, ectopically pigmented melanocytes. n = 1 human donor, 19 to 24 hair follicles. p values were calculated by the Fisher’s exact test; p < 0.05 is considered statistically significant. D. Representative brightfield images of the isolated HFs. Scale bar, 20μm.
NE binds to the ADRB2 receptor (β-2 adrenergic receptor) on MelSCs to mediate stress-induced hair graying.17 We therefore asked whether treatment with a β-2 adrenergic antagonist could prevent the induction of premature differentiation/pigmentation by NE. Although the initial treatment with NE increased the number of pigmented cells in the ORS (Figures 4C and 4D), concurrent treatment with NE and butoxamine, a β-2-selective antagonist, abolished formation of the NE-induced pigmented cells. These data indicate that NE, similar to genotoxic stress, induced premature differentiation of MelSCs and/or the amelanotic melanocytes within the human hair follicle niche; moreover, blocking the β-2 receptor prevents this stress-induced endpoint of the early stage of the hair graying pathway within human follicles.
DISCUSSION
The studies reported here established an ex vivo hair follicle model for monitoring abnormal differentiation of the MelSCs in which dissected human follicles were incubated with or without known hair-graying associated triggers, and ectopic pigmentation/differentiation of melanocytes was assessed. Hyperpigmentation associated with the premature differentiation of MelSCs was originally identified as a key event accompanying the progressive loss of MelSCs in the aging hair follicles of mice and man.14,16 This phenotype of ectopically pigmentated melanocytes at the bulge was observed in genetic mouse models associated with multiple hair-graying conditions.11,14,16,18,28-30 Prior analyses demonstrated appearance of pigmented cells within the bulge region that accompanied hair graying, suggesting that the ectopic pigmentation is a potentially useful marker for incomplete MelSC maintenance.1,14,16,18,28-30
It should be noted that although human and murine hair follicles share most of the essential features, structure and signaling pathways, there are several fundamental differences between the species.6,22 For instance, the human scalp hair can remain in anagen for several years whereas in mice the dorsal hair is at anagen for only 2-3 weeks. Furthermore, the reaction to several stimulators of hair growth is sometimes different. One striking example is the opposite reaction between mouse and human hair follicles to prolactin and estrogen.31 While pregnant and lactating mice have prolonged telogen phase32 in the scalp, the proportion of anagen HFs increased.33 Our approach reveals that, similar to the in vivo context in mice, human hair follicles can respond to genotoxic, reactive oxygen species, or sympathetic neurotransmitter stress signals with measurable induction of premature differentiation of MelSCs and/or the amelanotic cells, a mechanism which has been shown in mice to result in depletion of the MelSC pool. However, other mechanisms could in principle also contribute to melanocyte depletion, and they have not been specifically examined in the current study.6,13,34-35 For example, it will be interesting to examine the question of whether gender may contribute differentially to the behavior of hair follicles using this assay.
The identification of MelSCs has been mainly based upon their unique geographic localization in the bulge–sub-bulge area and on the absence of melanin pigments.2 However, other populations of unpigmented immature melanocytes exist in the mid-lower ORS and even in the periphery of the bulb (amelanotic melanocytes). These populations also lack visible melanin pigments, do not express TRP-1, and are considered to be partially differentiated melanocytes. Therefore, they have been suggested as progenitor melanocytes derived from MelSCs.19,36-37 However, the role of these populations is incompletely understood and will be valuable to further elucidate.19,36
Here, we have identified the bulge using CD200;38-40 however, we cannot exclude the ORS amelanotic cells from among the cells that were analyzed in this model. Nevertheless, the combination of the increase in pigmentation and expression of TRP-1 and TRP-2, indicate premature melanocytic differentiation of the cells.
The ex vivo hair follicle assay described here might facilitate future studies involving tests of various conditions that could affect premature/ectopic melanocytic differentiation. For example, deficiencies in certain nutrients (e.g., vitamins B-6, B-12, D and E, and biotin) and the use of certain drugs (e.g., chloroquine) can contribute to premature graying through unknown mechanisms.41-42 It is also important to consider that certain forms of hair graying do not involve melanocyte stem cell loss13,34,43 (or perhaps involve a replenishment of these cells) such as hair repigmentation that has been observed after c-Kit-targeted therapy or various other exposures.44-45
In conclusion, we present here a preclinical model of ex- vivo cultured human hair follicles that is amenable to experimental manipulation and appears to recapitulate early steps in the stress- and aging-associated gray hair pathway in which melanocyte stem cells become prematurely differentiated. While the role of incomplete MelSC maintenance in hair graying has been demonstrated in multiple experimental or aging-related contexts, effective and predictable therapeutic strategies to prevent or reverse hair graying in humans have yet to be developed. The system described herein may provide a useful tool for such research.
ACKNOWLEDGEMENTS
We thank Dr. W. Robert Liu for reviewing and editing the manuscript and Sharon K. Germana, Vivien Igras and Yiqun Su for helping with human tissue administration and technical assistance. Y.-C.H. is a Pew Scholar and a NYSCF – Robertson Investigator. B.Z. is awarded the Charles A. King Trust Postdoctoral Research Fellowship. D.E.F. acknowledges grant support from NIH (R01 AR043369-23).
Footnotes
CONFLICT OF INTERESTS
D.E.F. has a financial interest in Soltego, a company developing salt inducible kinase inhibitors for topical skin-darkening treatments that might be used for a broad set of human applications. The interests of D.E.F. were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. A patent application has been filed related to this work (applicants: President and Fellows of Harvard College and The General Hospital Corporation; inventors: Y.-C.H., B.Z., D.E.F. and I.R.; PCT Serial Number: PCT/US2020/024772; status: filed; aspect covered: methods and compositions for controlling hair graying). J.H.L., J.S., I.J., and Y.I.L. have no disclosures or conflicts of interest to report.
REFERENCES
- 1.Paus R, Foitzik K. In search of the "hair cycle clock": a guided tour. Differentiation. December 2004;72(9-10):489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura EK. Melanocyte stem cells: a melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. June 2011;24(3):401–10. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang CY, Pasolli HA, Giannopoulou EG, et al. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature. March 7 2013;495(7439):98–102. doi: 10.1038/nature11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabbani P, Takeo M, Chou W, et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. June 10 2011;145(6):941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimura EK, Jordan SA, Oshima H, et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. April 25 2002;416(6883):854–60. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan JDB, Nicu C, Picard M, et al. The biology of human hair greying. Biol Rev Camb Philos Soc. February 2021;96(1):107–128. doi: 10.1111/brv.12648. [DOI] [PubMed] [Google Scholar]
- 7.Tobin DJ, Paus R. Graying: gerontobiology of the hair follicle pigmentary unit. Exp Gerontol. January 2001;36(1):29–54. doi: 10.1016/s0531-5565(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 8.Tobin DJ. Aging of the hair follicle pigmentation system. Int J Trichology. July 2009;1(2):83–93. doi: 10.4103/0974-7753.58550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiberg M. Age-induced hair greying - the multiple effects of oxidative stress. Int J Cosmet Sci. December 2013;35(6):532–8. doi: 10.1111/ics.12090. [DOI] [PubMed] [Google Scholar]
- 10.Campiche R, Daniltchenko M, Imfeld D, Peters EMJ. Effects of the selective TrkA agonist gambogic amide on pigmentation and growth of human hair follicles in vitro. PLoS One. 2019;14(8):e0221757. doi: 10.1371/journal.pone.0221757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arck PC, Overall R, Spatz K, et al. Towards a "free radical theory of graying": melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. July 2006;20(9):1567–9. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 12.Sikkink SK, Mine S, Freis O, Danoux L, Tobin DJ. Stress-sensing in the human greying hair follicle: Ataxia Telangiectasia Mutated (ATM) depletion in hair bulb melanocytes in canities-prone scalp. Sci Rep. October 30 2020;10(1):18711. doi: 10.1038/s41598-020-75334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Commo S, Gaillard O, Bernard BA. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br J Dermatol. March 2004;150(3):435–43. doi: 10.1046/j.1365-2133.2004.05787.x. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. February 4 2005;307(5710):720–4. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 15.Qiu W, Chuong CM, Lei M. Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: Biological patterning and therapeutic potentials. Exp Dermatol. April 2019;28(4):395–405. doi: 10.1111/exd.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inomata K, Aoto T, Binh NT, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. June 12 2009;137(6):1088–99. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Ma S, Rachmin I, et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. January 2020;577(7792):676–681. doi: 10.1038/s41586-020-1935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris ML, Levy DJ, Watkins-Chow DE, Pavan WJ. Ectopic differentiation of melanocyte stem cells is influenced by genetic background. Pigment Cell Melanoma Res. March 2015;28(2):223–8. doi: 10.1111/pcmr.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horikawa T, Norris DA, Johnson TW, et al. DOPA-negative melanocytes in the outer root sheath of human hair follicles express premelanosomal antigens but not a melanosomal antigen or the melanosome-associated glycoproteins tyrosinase, TRP-1, and TRP-2. J Invest Dermatol. January 1996;106(1):28–35. doi: 10.1111/1523-1747.ep12326989. [DOI] [PubMed] [Google Scholar]
- 20.Tobin DJ, Colen SR, Bystryn JC. Isolation and long-term culture of human hair-follicle melanocytes. J Invest Dermatol. January 1995;104(1):86–9. doi: 10.1111/1523-1747.ep12613573. [DOI] [PubMed] [Google Scholar]
- 21.Kloepper JE, Sugawara K, Al-Nuaimi Y, Gaspar E, van Beek N, Paus R. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol. March 2010;19(3):305–12. doi: 10.1111/j.1600-0625.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 22.Oh JW, Kloepper J, Langan EA, et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J Invest Dermatol. January 2016;136(1):34–44. doi: 10.1038/JID.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magerl M, Kauser S, Paus R, Tobin DJ. Simple and rapid method to isolate and culture follicular papillae from human scalp hair follicles. Exp Dermatol. August 2002;11(4):381–5. doi: 10.1034/j.1600-0625.2002.110414.x. [DOI] [PubMed] [Google Scholar]
- 24.Langan EA, Philpott MP, Kloepper JE, Paus R. Human hair follicle organ culture: theory, application and perspectives. Exp Dermatol. December 2015;24(12):903–11. doi: 10.1111/exd.12836. [DOI] [PubMed] [Google Scholar]
- 25.Philpott MP. Culture of the human pilosebaceous unit, hair follicle and sebaceous gland. Exp Dermatol. May 2018;27(5):571–577. doi: 10.1111/exd.13669. [DOI] [PubMed] [Google Scholar]
- 26.Harris ML, Fufa TD, Palmer JW, et al. A direct link between MITF, innate immunity, and hair graying. PLoS Biol. May 2018;16(5):e2003648. doi: 10.1371/journal.pbio.2003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Commo S, Bernard BA. Melanocyte subpopulation turnover during the human hair cycle: an immunohistochemical study. Pigment Cell Res. August 2000;13(4):253–9. doi: 10.1034/j.1600-0749.2000.130407.x. [DOI] [PubMed] [Google Scholar]
- 28.Aubin-Houzelstein G, Djian-Zaouche J, Bernex F, et al. Melanoblasts' proper location and timed differentiation depend on Notch/RBP-J signaling in postnatal hair follicles. J Invest Dermatol. November 2008;128(11):2686–2695. doi: 10.1038/jid.2008.120. [DOI] [PubMed] [Google Scholar]
- 29.Harris ML, Buac K, Shakhova O, et al. A dual role for SOX10 in the maintenance of the postnatal melanocyte lineage and the differentiation of melanocyte stem cell progenitors. PLoS Genet. 2013;9(7):e1003644. doi: 10.1371/journal.pgen.1003644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanimura S, Tadokoro Y, Inomata K, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. February 4 2011;8(2):177–87. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 31.Langan EA, Foitzik-Lau K, Goffin V, Ramot Y, Paus R. Prolactin: an emerging force along the cutaneous-endocrine axis. Trends Endocrinol Metab. September 2010;21(9):569–77. doi: 10.1016/j.tem.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Hodgson SS, Neufeld Z, Villani RM, Roy E, Khosrotehrani K. Transgenic flash mice for in vivo quantitative monitoring of canonical Wnt signaling to track hair follicle cycle dynamics. J Invest Dermatol. June 2014;134(6):1519–1526. doi: 10.1038/jid.2014.92. [DOI] [PubMed] [Google Scholar]
- 33.Gizlenti S, Ekmekci TR. The changes in the hair cycle during gestation and the post-partum period. J Eur Acad Dermatol Venereol. July 2014;28(7):878–81. doi: 10.1111/jdv.12188. [DOI] [PubMed] [Google Scholar]
- 34.O'Sullivan JDB, Nicu C, Picard M, et al. The biology of human hair greying. Biol Rev Camb Philos Soc. September 23 2020;doi: 10.1111/brv.12648. [DOI] [PubMed] [Google Scholar]
- 35.Sarin KY, Artandi SE. Aging, graying and loss of melanocyte stem cells. Stem Cell Rev. Fall 2007;3(3):212–7. doi: 10.1007/s12015-007-0028-0. [DOI] [PubMed] [Google Scholar]
- 36.Tobin DJ. Human hair pigmentation--biological aspects. Int J Cosmet Sci. August 2008;30(4):233–57. doi: 10.1111/j.1468-2494.2008.00456.x. [DOI] [PubMed] [Google Scholar]
- 37.Peters EM, Liezmann C, Spatz K, et al. Profiling mRNA of the graying human hair follicle constitutes a promising state-of-the-art tool to assess its aging: an exemplary report. J Invest Dermatol. May 2013;133(5):1150–60. doi: 10.1038/jid.2012.462. [DOI] [PubMed] [Google Scholar]
- 38.Inoue K, Aoi N, Sato T, et al. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab Invest. August 2009;89(8):844–56. doi: 10.1038/labinvest.2009.48. [DOI] [PubMed] [Google Scholar]
- 39.Ohyama M, Terunuma A, Tock CL, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. January 2006;116(1):249–60. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer KC, Klatte JE, Dinh HV, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. November 2008;159(5):1077–85. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- 41.Shaw NA, Dickey HC, Brugman HH, Blamberg DL, Witter JF. Zinc deficiency in female rabbits. Lab Anim. January 1974;8(1):1–7. doi: 10.1258/002367774780943913. [DOI] [PubMed] [Google Scholar]
- 42.Donovan JC, Price VH. Images in clinical medicine. Chloroquine-induced hair hypopigmentation. N Engl J Med. July 22 2010;363(4):372. doi: 10.1056/NEJMicm0912609. [DOI] [PubMed] [Google Scholar]
- 43.Takada K, Sugiyama K, Yamamoto I, Oba K, Takeuchi T. Presence of amelanotic melanocytes within the outer root sheath in senile white hair. J Invest Dermatol. November 1992;99(5):629–33. doi: 10.1111/1523-1747.ep12668031. [DOI] [PubMed] [Google Scholar]
- 44.Robert C, Spatz A, Faivre S, Armand JP, Raymond E. Tyrosine kinase inhibition and grey hair. Lancet. March 22 2003;361(9362):1056. doi: 10.1016/S0140-6736(03)12805-X. [DOI] [PubMed] [Google Scholar]
- 45.Yale K, Juhasz M, Atanaskova Mesinkovska N. Medication-Induced Repigmentation of Gray Hair: A Systematic Review. Skin Appendage Disord. January 2020;6(1):1–10. doi: 10.1159/000504414. [DOI] [PMC free article] [PubMed] [Google Scholar]