Abstract
Background:
Healthcare workers (HCW) are at increased risk of being infected with SARS-CoV-2; while PCR test remains gold standard for diagnosis of COVID19 infection, antigen based rapid detection tests have been recently approved by OMS.
Methods:
We pooled data on occupational surveillance of 6,397 asymptomatic HCW and other employees who were tested for SARS-CoV-2 infection at the University Hospital in Bologna using rapid antigen test between November 16, 2020 and January 29, 2021.
Findings:
A total of 17,993 rapid tests were performed, of which 704 for contact with an infected person and 17,289 for voluntary screening. Among 17,732 tests with valid results, 87 tested positive (0.49%) and 17 weakly positive (0.10%). The sensitivity of the antigenic test was 88.6% (81.1-96.1), the specificity was 93.4% (89-97.8), the positive predictive value, given a prevalence of infection of 42.1%, was 90.7% (84.8-96.6).
Keywords: Antigenic Swab, healthcare workers, sensitivity, specificity
Introduction
In January 2020 a new coronavirus, SARS-CoV-2, was isolated for the first time in the city of Wuhan, the capital of Hubei province, after many cases of atypical pneumonia diagnosed during the last months of 2019. The virus spread quickly all over the world causing an increasing number of COVID-19 cases and resulting in the most important problem in terms of public health and social-economic impact due to an infectious disease in the last 100 years.
From the beginning of pandemic spread to 22 March 2021, more than 120 million cases of COVID-19 were counted globally, including more than 2.7 million deaths. During the same period, more than 3.3 million cases and more than 120,000 deaths were recorded in Italy (1). During the last year, many public health interventions were implemented to stop viral spread, including lockdown, contact tracing, quarantine and home isolation, but the single most important action to stop viral transmission is the early detection of cases, in order to isolate them and break the chain of transmission. This highlights the importance of highly sensitive and specific tests, that are crucial to identify and manage COVID-19.
Real time reverse transcription polymerase chain reaction (RT-qPCR) tests in respiratory mucosa samples are the operational gold standard for detecting SARS-CoV-2 infection disease in clinical practice (2, 3). In performing the RT-qPCR, the indicator of detectable amplification of the viral RNA is graphically known as quantitation cycle, commonly reported as cycle threshold value (CT) (4).
Different CT values have been used for the diagnosis of COVID-19, ranging between 16.9 and 38.8 for various clinical samples. Although CT values < 40 are generally recommended as indicator of SARS-CoV-2 RNA positivity, some Authors reported that samples with CT values > 33.33 or 35, or ≥ 39.2 or 40 could be considered as negative [5].
Some aspects related to CT remain unclear: indeed, many authors highlighted a relationship between CT and clinical and infectious pattern (6).
While PCR test remains the gold standard for diagnosis of SARS-CoV-2 infection, antigen based rapid detection tests or point of care tests have been approved by the World Health Organization (7) and are of widespread use. These methods were conceived to detect SARS-CoV-2 virus particles using immunoassays (8), in particular focused on coronavirus NP, predominant virus-derived structural protein, released in large amounts into serum, nasopharyngeal aspirate, throat wash samples, fecal material, and urine during the early period of infection (9). Antigen tests are performed on nasopharyngeal or nasal swab specimens placed directly into the assay’s extraction buffer or reagent. Recently, antigen tests based on saliva samples have been introduced.
The clinical performance of antigen diagnostic tests largely depends on the circumstances in which they are used. Both antigen tests and nucleic acid amplification tests (NAATs) perform best on patients with high viral load. They also may be particularly informative in circumstances of known exposure to an infected person, in which a result is rapidly needed.
Accuracy of immunoassay tests is not fully understood because information regarding sensitivity and specificity are limited to those reported by manufacturing companies. Indeed, only few Authors reported some information about test performance comparing antigenic and RT-qPCR test results in the same patient (10).
The objectives of the present study are to analyze the prevalence of antigenic test positivity by period of time, and to calculate the accuracy of the test using RT-qPCR test as reference.
Methods
Starting on November 16, 2020, at the Sant’Orsola Malpighi University Hospital in Bologna, Emilia-Romagna region, we started testing asymptomatic health care workers (HCW) and other asymptomatic employees using the SARS-CoV-2 Rapid Antigenic Swab (SARS CoV-2FLUO – 63181- Liaison Diasorin).
The program consisted of two different parts: (i) test following high-risk contact with patient or colleague, with the test performed 3-4 days after the contact, and a RT-qPCR test 10 days after the contact; (ii) screening on a voluntary basis of HCW and other employees every 15 to 30 days depending on the specific risk of the unit of employment. Tests were carried out between November 16, 2020 and January 29, 2021.
Results of the tests were classified as positive (cut-off index [COI]>10 units), weakly positive (3<COI≤10 units), uninterpretable (1<COI≤3 units), and negative (COI≤1 unit). The measures resulting from the results provided by the microbiology laboratory are shown in Figure 1. For subjects that performed one or more RT-qPCR tests after the antigenic test, the result of the first RT-qPCR test after the antigenic test were also collected.
Figure 1.
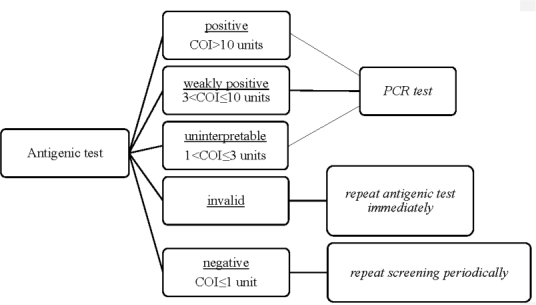
Operating Protocol
Prevalence of positivity of antigenic test, including its 95% confidence interval (CI), was calculated by time period and reason for the test; specificity of the antigenic test was calculated using the result of RT-qPCR test as gold standard.
Results
From November 16, 2020 to January 29, 2021 a total of 17,993 antigenic tests were performed among 6,397 workers, of which 704 for high-risk contacts and 17,289 for voluntary screening.
A total of 43 samples were found to be invalid and excluded from the analysis. Among the remaining 17,950 samples, 87 tested positive and 17 weakly positive; and 218 results were not interpretable.
While the overall positivity was 0.58% (95% CI 0.49-0.67), this proportion was higher among subjects tested in December 2020 (0.84%), especially when the test was conducted following a high-risk contact (2.95%). In January 2021, the positivity dropped to 0.38% (Table 2).
Table 2.
Rate of positivity by month
| Contact Antigenic Swab | Screening Antigenic Swab | Total Antigenic Swab | |
| November | 0/2 (0%) | 1/1324 (<0.1%) | 1/1326 (<0.1%) |
| December | 14/474 (2.95%) | 58/8074 (0.72%) | 72/8548 (0.84%) |
| January | 1/228 (0.44%) | 30/7891 (0.38%) | 31/8119 (0.38%) |
| Total | 15/704 (2.13%) | 89/17289 (0.51%) | 104/17993 (0.58%) |
Table 1.
Results of antigenic tests
| Contact Antigenic Swab | Screening Antigenic Swab | Total Antigenic Swab | |
| Positive | 11 | 76 | 87 |
| Low Positive | 4 | 13 | 17 |
| Uninterpretable Result | 7 | 211 | 218 |
| Invalid | 2 | 41 | 43 |
| Negative | 680 | 16948 | 17628 |
| Total Results | 704 | 17289 | 17993 |
Results on both RT and qPCR and antigenic test were available for 209 subjects with positive or uninterpretable RT result (Table 3). Considering uninterpretable results as negative, the sensitivity of the antigenic test was 88.6% (95% CI 81.1-96.1), the specificity was 93.4% (95% CI 89-97.8), the positive predictive value, given a prevalence of infection of 42.1%, was 90.7% (95% CI 84.8-96.6).
Table 3.
Comparison of antigenic and RT-qPCR test results
| Result of antigenic test | Result of RT-qPCR test | ||
| Positive* | Negative | Total | |
| Positive* | 78 | 8 | 86 |
| Uninterpretable | 10 | 113 | 123 |
| Total | 88 | 121 | 209 |
* Including weakly positive
Discussion
HCWs have been exposed to numerous risks during this pandemic; hazards include pathogen exposure, long working hours, psychological distress, fatigue, burnout, stigma, and physical and psychological violence. All health personnel should be alert to the risk of COVID-19 in a wide variety of occupations, and not only HCWs. These occupational groups can be protected by good infection control practices (11).
During the first epidemiological wave of the COVID-19 epidemic in Italy in spring 2020, PCR tests were limited to symptomatic HCWs, and eventually also to HCWs reporting a contact, while screening for asymptomatic HCW with no risk contacts was not available due to shortage of these tests. Early reports on prevalence and determinants of infection were therefore based on results of PCR tests (12).
In another Italian seroprevalence study, no differences in seropositivity were observed by sex, while older HCWs had higher positivity than other groups, and nurses had higher positivity compared to physicians, but not other HCWs (13).
In the current emergency context, the early identification of subjects affected by COVID-19 is essential for the control of the infection, as well as for the assistance and treatment of confirmed cases (14).
Several strategies have been proposed, including point-of care tests such as antigenic rapid assays. It is important to point out that the success of each strategy is profoundly impacted by the pre-test probability of the infection. It is essential that the evaluation of the infection also takes into account the symptoms, to complement the result of diagnostic tests: negative results cannot exclude infection if the patient is experiencing Covid-like symptoms (15).
Since the end of 2020, the antigenic test has become increasingly important, because of speed of execution and result, excellent sensitivity in the initial viral phase of the infection and excellent specificity in the final phase of infection (a phase in which the PCR test can detect numerous false positives (16-18). Starting in January, 2021, results of antigenic test has been considered sufficient for a molecular diagnosis of COVID-19 (19).
One of the main strengths of this study lies in the fact that it is based on a database of about 18,000 antigenic swab results carried out in less than 3 months, in the midst of the second epidemiological wave of COVID-19, on asymptomatic HCWs and other employees.
The positivity rate found was rather low, reaching its peak in December 2020 (0.84%); during the same period of the year, according to data from the Italian government, in the Emilia-Romagna region the positivity rate for all swabs performed (including PCR tests) ranged from 5% to 13% [20]; while these latter figures reflect the prevalence in a high-risk population, the prevalence estimated from our data is closer to that of the unselected population of the region.
Another reason why there is an underestimation of the prevalence is that as of November 16, 2020, 210 employees had already been infected with SARS-CoV2 and therefore were less likely to reinfect.
Estimating an infection rate among the asymptomatic population of the Emilia Romagna region equal to that found in December among HCWs (0.84%), in the months of November and December we would have an estimate of just over 30,000 additional cases to those already registered.
During these 2 months, from the data of the Civil Protection we recorded about 120,000 positive swabs in the region, which correspond to about 60,000 new cases of infection (considering an average of 2 positive swabs for each case confirmed before healing).
Finally, taking into account that a large percentage of positive molecular swabs refers to close asymptomatic contacts, we can conclude that during those 2 months, at the height of the epidemiological wave, 66.7% of about 90,000 cases were intercepted (the remaining 33.3% would have been recognized only by testing the screened population) and the ratio between symptomatic and asymptomatic tends to 1.
The PCR test, recognized as the gold standard, has a sensitivity around 95% in the first 5 days after symptom onset and has an estimated specificity of >99% (21).
We estimated a sensitivity of the antigenic test of 88.6%; this value is likely to be an underestimate of the real sensitivity of the test, as it was not calculated on the basis of negative results but on “uninterpretable results” value in the antigen test.
In the scientific literature, a good sensitivity of the test is also described (72.6%) (22), especially in the initial phase of infection (viral phase), where in consideration of the higher viral load it is more likely to detect the viral antigen; the nasopharyngeal COVID-19 antigen test performed at point-of-care is highly sensitive in symptomatic patients, particularly with CT<30 and older age. The test is useful to identify asymptomatic patients with lower CT values and therefore at risk of being contagious (23).
We found a specificity of the antigenic test of 93.4%, a result in line with the literature.
In other studies, COVID-19 Ag Rapid Test had 100% specificity, and a sensitivity above 95% for nasopharyngeal samples when using CT-values <32 cycles as cut-off for RT-qPCR test positivity (24).
In another study, specificity was 100%, overall sensitivity was 72.6% and 95.2% when using a CT-value of 32 as cut-off (22).
Our findings suggest that large-scale SARS-CoV-2 Ag based testing can be considered for detecting potentially infective individuals and reducing the virus spread (10).
A limitation of this analysis lies in not having been able to have the CT values of the molecular swabs; the number of PCR amplifications is in fact an indirect and inversely proportional value of the viral load and having this data available could have given us the possibility of having a correlation between the positivity of the test and the value of CT, as a semi-quantitative measure of the viral load present in the sample (6).
Another limitation of the study concerns the fact that it has no data on the variants of the virus that raged all over the world at the beginning of 2021; however as regards the English variant (VOC 202012/01, lineage B.1.1.7), an assessment by Public Health England found that five SARS-CoV-2 rapid antigen tests evaluated were all able to successfully detect the variant; for South African and Brazilian variants no evaluation studies have been carried out to confirm that test performance is not affected, but no major performance deficits are anticipated (25).
Lastly, the limitation on the study design, having this research performed PCR confirmatory test only on positive ones, leads to subsequent potential risk of spreading of the infection by asymptomatic/false negative subjects.
Although the number of asymptomatic HCW positive for SARS-CoV-2 detected with the RT was modest (N=88), they represented a potential source of outbreak both in and outside the workplace.
Conflict of interest:
No potential conflict of interest relevant to this article was reported by the authors
Authors' contribution:
PB, GV: design of the study. PB, FV: supervision of data collection. GV, CZ: data collection. PB, GV, CZ: data harmonization, statistical analysis. PB, GV, CZ: drafting of the manuscript
References
- 1.Center for Systems Science and Engineering (CSSE) COVID-19 Dashboard at Johns Hopkins University (JHU) Accessed June 12 2020 [Google Scholar]
- 2.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. Erratum in: Euro Surveill. 2020;25(14): Erratum in: Euro Surveill. 2020;25(30): Erratum in: Euro Surveill. 2021;26(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OMS Laboratory Testing Strategy Recommendations for COVID-19. Interim Guidance 21 March [Google Scholar]
- 4.Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–22. doi: 10.1373/clinchem.2008.112797. doi: 10.1373/clinchem.2008.112797. Epub 2009 Feb 26. [DOI] [PubMed] [Google Scholar]
- 5.Vogels CBF, Brito AF, Wyllie AL, et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT-qPCR primer-probe sets. Nat Microbiol. 2020;5(10):1299–1305. doi: 10.1038/s41564-020-0761-6. doi: 10.1038/s41564-020-0761-6. Epub 2020 Jul 10. PMID: 32651556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tom MR. Mina MJ. To Interpret the SARS-CoV-2 Test, Consider the Cycle Threshold Value. Clin Infect Dis. 2020;71(16):2252–2254. doi: 10.1093/cid/ciaa619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO/2019-nCoV/Antigen_Detection/2020.1 [Google Scholar]
- 8.Ji T, Liu Z, Wang G, et al. Detection of COVID-19: A review of the current literature and future perspectives. Biosens Bioelectron. 2020;166:112455. doi: 10.1016/j.bios.2020.112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das D, Kammila S, Suresh MR. Development, characterization, and application of monoclonal antibodies against severe acute respiratory syndrome coronavirus nucleocapsid protein. Clin Vaccine Immunol. 2010;17(12):2033–6. doi: 10.1128/CVI.00293-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohmer N, Toptan T, Pallas C, et al. The Comparative Clinical Performance of Four SARS-CoV-2 Rapid Antigen Tests and Their Correlation to Infectivity In Vitro. J Clin Med. 2021;10(2):328. doi: 10.3390/jcm10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutti A. Occupational Medicine in the time of COVID-19. Med Lav. 2020;111(2):83–86. doi: 10.23749/mdl.v111i2.9546. doi: 10.23749/mdl.v111i2.9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boffetta P, Violante F, Durando P, et al. Working Group on SARS-CoV-2 Infection in Italian Healthcare Workers. Determinants of SARS-CoV-2 infection in Italian healthcare workers: a multicenter study. Sci Rep. 2021;11(1):5788. doi: 10.1038/s41598-021-85215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visci G, Lodi V, Bonfiglioli R, et al. Serologic SARS-CoV-2 Testing in Healthcare Workers with Positive RT-PCR Test or Covid-19 Related Symptoms. Archives of Clinical and Biomedical Research 5. 2021:427–436. [Google Scholar]
- 14.Società Italiana di Medicina del Lavoro, 2020. Esami di laboratorio per SARS-CoV-2 nella gestione in ambito occupazionale della pandemia COVID 19. Posizione della Società Italiana di Medicina del Lavoro. MedLav. 2020;111(2):151–154. DOI:https://doi.org/10.23749/mdl.v111i2.9667. [Google Scholar]
- 15.Ferrari L, Nigro S, Bordini L, Carugno M, Bollati V. SARS-CoV-2 tests in occupational settings: what you look for is what you get. Med Lav. 2021;112(3):183–189. doi: 10.23749/mdl.v112i3.11472. Available from: https://mattioli1885journals.com/index.php/lamedicinadellavoro/article/view/11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaafar R, Aherfi S, Wurtz N, et al. Correlation Between 3790 Quantitative Polymerase Chain Reaction-Positives Samples and Positive Cell Cultures, Including 1941 Severe Acute Respiratory Syndrome Coronavirus 2 Isolates. Clin Infect Dis. 2021;72(11):e921. doi: 10.1093/cid/ciaa1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferson T, Spencer EA, Brassey J, Heneghan C. Viral cultures for COVID-19 infectious potential assessment-a systematic review. Clin Infect Dis. 2020:ciaa1764. doi: 10.1093/cid/ciaa1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee C, Kanjilal S, Baker M, Klompas M. Duration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infectivity: When Is It Safe to Discontinue Isolation? Clin Infect Dis. 2021;72(8):1467–1474. doi: 10.1093/cid/ciaa1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=78155&parte=1%20&serie=null] last access on 22nd March 2021. [Google Scholar]
- 20.https://statistichecoronavirus.it/coronavirus-italia] last access on 22nd March 2021 [Google Scholar]
- 21.Miller TE, Garcia Beltran WF, Bard AZ, et al. Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital. FASEB J. 2020;34(10):13877–13884. doi: 10.1096/fj.202001700RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gremmels H, Winkel BMF, Schuurman R, et al. Real-life validation of the Panbio™ COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine. 2021;31:100677. doi: 10.1016/j.eclinm.2020.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masiá M, Fernández-González M, Sánchez M, et al. Nasopharyngeal Panbio COVID-19 Antigen Performed at Point-of-Care Has a High Sensitivity in Symptomatic and Asymptomatic Patients With Higher Risk for Transmission and Older Age. Open Forum Infect Dis. 2021;8(3):ofab059. doi: 10.1093/ofid/ofab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gremmels H, Winkel BMF, Schuurman R, et al. Real-life validation of the Panbio™ COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine. 2021;31:100677. doi: 10.1016/j.eclinm.2020.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. https://www.finddx.org/covid-19/novel-variants/ last access on 22nd March 2021. [Google Scholar]


