Abstract
Background:
Ketamine is a phencyclidine derivative with dissociative anaesthetic properties. Increasing numbers of individuals in England take ketamine recreationally. Information on deaths arising from such use in England is presented.
Methods:
Cases were extracted on 31 January 2020 from the National Programme on Substance Abuse Deaths database, based on text searches of the cause of death, coroner’s verdict and positive toxicology results for the terms ‘ketamine’ or ‘norketamine’.
Findings:
During 1997–2005, there were <5 deaths p.a. in which ketamine was implicated. Numbers increased until 2009 (21), plateauing until 2016; thereafter, deaths have risen to about 30 p.a. Decedents’ characteristics (N = 283): male 84.1%, mean age 31.2 (SD 10.0) years, employed 56.5%, drug use history 79.6% and living with others 60.3%. Ketamine was detected with other substances in most cases. Main (74.6%) underlying cause of death was accidental poisoning. Ketamine may have impaired judgement in other cases.
Conclusions:
Although controlled, recreational ketamine use and related fatalities continue to increase. Consumers need to be more aware of the potentially fatal risks they face.
Keywords: Deaths, ketamine, England, drug misuse, recreational use
Introduction
The recreational use of ketamine has increased over recent decades and appears to be continuing its upward trajectory. Therefore, it is important for a range of healthcare professionals, including clinical psychiatrists, and associated experts, such as toxicologists and forensic analysts, to be as up to date as possible in terms of its uses and adverse effects.
Deaths arising from the recreational use of ketamine are on the increase, some even as a result of long-term use (Vice, 2014). However, there is little in the public domain about such fatalities.
This paper examines in detail the largest dataset of deaths involving ketamine in non-emergency healthcare and non-anaesthetic settings, for example recreational use and suicides. The focus here is on the characteristics of (a) the decedents and (b) the deaths. These fatalities are examined against the background of a ‘state-of-the-art’ review (Grant and Booth, 2009), and with a discussion of what previous studies have indicated about these types of events.
‘State-of-the-art’ review
Mode of action
Ketamine or 2-(2-chlorophenyl)-2-(methylamino)cyclohexanone, commonly known as ‘K’, ‘Special K’, and ‘Kit-kat’, is a non-competitive glutamate N-methyl-d-aspartate (NDMA) receptor antagonist, inducing dissociative anaesthetic effects by generating an electrophysiological dissociation between the thalamo-neocortical and limbic systems (Kalsi et al., 2011). This produces a cataleptic trance-like state exhibiting amnesia, deep analgesia and unconsciousness (Domino et al., 1965) probably due to agonistic activity at the α1 and β2 adrenoceptors (Bevan et al., 1997). It binds to the phencyclidine binding site preventing the ingress of neuronal Ca2+. Its S(+)-isomer has four times the affinity of the R(-)-isomer to NMDA phencyclidine (PCP) type receptors (Øye et al., 1992). Ketamine may potentiate the synaptic inhibition of gamma-aminobutyric acid (GABA) (Anis et al., 1983; Irifune et al., 2000) and trigger dopamine release (Rabiner, 2007). It has a weak agonist effect on μ (mu) opioid receptors (Øye et al., 1992; Smith et al., 1980) and has a low affinity for the sigma and mu opioid receptors. It further contributes to analgesia by inhibiting nitric oxide synthase (Curran and Morgan, 2000; Goldberg et al., 2011; Rowland, 2005). Furthermore, ketamine acts as an uptake inhibitor at the serotonergic and noradrenergic neurotransmitters, which are both concerned in descending antinociceptive pathways (Jansen, 2000; Pomarol-Clotet et al., 2006). As the molecule binds to both sigma-1 and sigma-2 receptors with mu affinities, its antidepressant properties may arise from sigma receptor-mediated neuronal remodelling (Robson et al., 2012). Recent research suggests that ketamine’s acute antidepressant effect requires opioid system activation in order to be effective in adults with treatment-resistant depression (Williams et al., 2018). At low doses (dosage undefined), ketamine produces stimulant effects (Wolff and Winstock, 2006).
Ketamine is extensively metabolised hepatically by cytochrome P450-mediated N-demethylation, principally involving not only CYP3A4 but also CYP2C9 and CYP2B6 (Hijazi and Boulieu, 2002), producing norketamine, reducing bioavailability following oral or rectal administration, a non-competitive NMDA receptor antagonist with one-third the potency of ketamine (Cohen and Trevor, 1974). Dehydronorketamine is produced by dehydrogenation of norketamine, both being subjected to hepatic conjugation. This leads to ketamine and its metabolites being renally excreted, principally as conjugates (80%), ketamine (2.3%), norketamine (1.6%) and dehydronorketamine (16.2%) (Wieber et al., 1975). These can be detected in urine as follows: ketamine 5–11 days, dehydronorketamine 10 days and norketamine 6–14 days (Adamowicz and Kala, 2005; Parkin et al., 2008). Norketamine might also exhibit enantioselective pharmacological activity, for example, (S)-norketamine has an eightfold higher affinity than (R)-norketamine (Goldberg et al., 2010). The highest bioavailability of ketamine is intravenous (IV) (100%), followed by intramuscular (IM) (93%), nasal (25%–50%), rectal (25%) and oral (17%) (Clements et al., 1982; Malinovsky et al., 1996). This high rate of first-pass metabolism may well explain why ketamine is typically not ingested. Ketamine has a distribution half-life of about 24 s, a redistribution half-life of 4–5 min and an elimination half-life of >2 h (Reich and Silvay, 1989).
Medical uses
Analgesia is produced when plasma concentrations reach 100 ng/mL, drowsiness and distortions of perception at 50–200 ng/mL, general anaesthesia at 200–3000 ng/mL, but when levels fall to 500–1000 ng/mL, awakening occurs (Sinner and Graf, 2008). Although used as a general anaesthetic, primarily in veterinary practice, it is sometimes administered to humans as well, for example in emergency medicine, paediatric anaesthesia, and in chronic pain clinics (Noppers et al., 2010; Smith et al., 2002). It has a good safety record as it only causes mild stimulation of the cardiovascular system without suppressing the respiration and gag reflexes (Sehdev et al., 2006) or affecting haemodynamic stability (Sinner and Graf, 2008).
Ketamine can be considered as an alternative to opioids for short-term pain control in Emergency Departments (EDs) as it is non-inferior to morphine in the control of acute pain (Karlow et al., 2018). A small-scale double-blind, randomised, active-placebo-controlled trial recently showed that individuals with multiple sclerosis may experience a reduction of their fatigue in the longer-term following infusions of ketamine (Fitzgerald et al., 2021).
Ketamine can be used in the management of extended epileptic seizures in the intensive care setting (Fujikawa, 1995). Levodopa is used in the treatment of Parkinson’s disease, but 40% of such patients using the drug may develop dyskinesia. A small-scale 3-year phase 1 clinical trial commenced in summer 2018 to see if ketamine can help to reduce and control such involuntary movements (Sherman et al., 2016; UAHS, 2018).
Ketamine has been used in the psychotherapy of individuals with alcohol dependence (Krupitsky and Grinenko, 1997; Krystal et al., 2003), heroin addiction (Krupitsky et al., 2007) and cocaine use (Jones et al., 2018). Recent research indicates that ketamine in conjunction with maladaptive reward memories can reduce the reinforcing effects of alcohol and long-term levels of alcohol consumption (Das et al., 2019). Sub-anaesthetic doses (0.5 mg/kg for 40 min) have been used effectively in the treatment of individuals with treatment-resistant depression (TRD) (Duman and Aghajanian, 2012; George et al., 2017; Murrough et al., 2013) and treatment-resistant bipolar depression maintained on mood stabilisers (Diazgranados et al., 2010b). The most effective IV dosages for TRD appear to be 0.5 mg/kg and 1.0 mg/kg (Fava et al., 2020). The fact that ketamine is principally administered via the IV route in hospitals and clinics means that its clinical use is restricted to such settings.
In recent years, an (S)-ketamine stereoisomer, esketamine, has shown promise in a number of potential roles and across different countries. For example, one US-based pharmaceutical company has developed a nasal application/formulation; this has shown promise in clinical trials and has now been approved by the US Food and Drug Administration for TRD (Duman, 2018; FDA, 2019; Gálvez et al., 2018; Koons and Edney, 2019), and more recently for the combination treatment of major depressive disorder (MDD) with acute suicidal ideation or behaviour (Canady, 2020), following clinical trials (Canuso et al., 2018). Esketamine may have a potential use in the treatment of unipolar and bipolar depression (Correia-Melo et al., 2017). Esketamine’s uses may also extend to counteracting opioid-related respiratory depression (Jonkman et al., 2018) and as a general anaesthetic (Himmelseher and Pfenninger, 1998).
The European Medicines Agency (EMA) gave Marketing Authorisation for esketamine for combination treatment in adults with treatment-resistant major depressive disorder on 31 December 2019 (EMA, 2019). In the United Kingdom (UK), the National Institute for Health and Care Excellence (NICE) decided, in January 2020, not to recommend this product’s use for this purpose because of uncertainties over its clinical and cost-effectiveness (NICE, 2020a). However, they opened a new public consultation on 3 September, which was due to close on 25 September 2020 (NICE, 2020b); the outcomes of this are still awaited. However, esketamine was approved for use in the Scottish NHS on 7 September 2020 (Scottish Medicines Consortium, 2020).
The antidepressant properties of ketamine appear to be useful in the treatment of post-traumatic stress disorder (PTSD) (Liriano et al., 2019), in both adults (Feder et al., 2014) and children (Donoghue et al., 2015). It appears to be a useful tool for preventing PTSD and suicide in those with burns (Escarment et al., 2017; Mion et al., 2017). Ketamine can swiftly reduce suicidal ideation (DiazGranados et al., 2010a; Price et al., 2014; Siegel et al., 2021). Low doses of ketamine can be used to recreate a number of physiological abnormalities, which are characteristic of schizophrenia (Coyle et al., 2012; Javitt et al., 2012) and has been used experimentally to develop a ‘ketamine model’ of psychosis (Fletcher and Honey, 2006; Morgan et al., 2009).
It is also important to note that illicit forms or diverted supplies of ketamine may be used by individuals to self-medicate their symptoms of depression and PTSD (Chaves et al., 2020).
Epidemiology of recreational use
Desired effects reported by recreational users include: altered senses, including auditory and visual hallucinations, enhanced colour vision, timelessness; out of body experiences; weightlessness; euphoria; empathy; escaping reality; feeling of well-being; enhancing the effect of other drugs; energy; creativity and stress release (Dillon et al., 2003). Unwanted effects include: fear of the ‘K-hole’; health risks; confusion; impaired memory; coming down; psychological problems, including anxiety and paranoia; tolerance (Dillon et al., 2003).
Reasons for ketamine’s recreational use may include: short time to effect (IV 30 s, intranasally 5–30 min, orally 20 min); duration of action (up to 3 h); low cost; unique psychotropic effects, noted as early as 1967 (Jansen, 2004); because friends use; and curiosity (Corazza et al., 2013; Dillon et al., 2003). According to the Global Drug Survey, the mean global value-for-money rating for ketamine in 2019 was 7.2 (on a scale where 1 = poor, 10 = excellent), whereas the ratings for cocaine and 3,4-methylenedioxy-methamphetamine (MDMA) powder were 4.9 and 7.2, respectively (Winstock et al., 2019).
Recreational use of ketamine started during the early 1970s in California and, during the 1990s, at first it was sold in the UK as ‘ecstasy’ (Schifano et al., 2006). Initially abused by those with access to it (veterinarians, emergency medicine practitioners, anaesthetists, etc.), ketamine’s appeal has broadened to contexts such as nightclubs, dance parties and ‘raves’. Studies of young people attending dance music clubs in a city in north-west England found that levels of ketamine use varied according to the type of music: lifetime use was highest amongst those preferring trance music (50%) compared to funky house (34%) or drum and bass (28%) (Moore and Measham, 2006). It has since evolved to become a mainstream ‘club drug’ in the ‘post-rave’ clubbing and youth dance culture (Kalsi et al., 2011). The most commonly described types of ketamine users are: regular users in the dance music scene, including electronic dance music parties (Palamar et al., 2021); psychonauts; injecting heroin users and the ‘gay’ club/party scene (Dillon et al., 2003); no recent studies have looked at this issue. However, evidence from this study (see section below) would suggest that it is also used at music festivals.
UK Prevalence
The following sub-sections outline what is known about non-healthcare use of ketamine in the UK, pointing to increased use and availability, especially in recent years.
Surveys conducted by Mixmag, a monthly magazine aimed at clubbers, during the period 1999 to 2003 showed that ketamine lifetime prevalence amongst UK clubbers rose from 25.5% to 39.8%, whereas current use of ketamine rose from 3.9% to 16.0% (McCambridge et al., 2007). In 2011, the lifetime use was 62% with last year use at 41.2% (Winstock, 2011), down from 67% and 50.7%, respectively in 2010 (Dick and Torrance, 2010). These levels then fell in 2013 to 50.6% and 31.5%, but these were still higher than for US respondents (26.3% and 15.4%, respectively) (Winstock, 2013). These higher ketamine usage rates in the UK are also borne out by the Mixmag’s successor survey – the Global Drug Survey. In 2012, the last year use of ketamine was only 5.5% for US respondents compared to 24.5% for UK respondents, rising to 40% for UK regular clubbers (Winstock, 2012); in 2014, the last year use of ketamine globally was 5.7%, compared with 19.8% in the UK as a whole and 14.7% in Scotland (Winstock, 2015b). Global last year use rates of ketamine were 6.72% in 2016, 8.6% in 2017 and 6.5% in 2018; lifetime rates for 2017 and 2018 were 11.7% and 10.4%, respectively (Winstock et al., 2016, 2017). By comparison, the last year use of ketamine in the UK was 19.8% in 2014, fell to 14.0% in 2015, before rising to 23.6% in 2016 and 26.0% in 2017 (Winstock et al., 2017).
According to the Crime Survey of England and Wales (CSEW, formerly known as the British Crime Survey (BCS)), the last year use of ketamine amongst those aged 16–59 years in households rose from 0.3% in 2006/2007 to 0.6% in 2008/2009 and remained about this level until 2013/2014 before falling to 0.3% in 2015/2016, but rose sharply to 0.8% in 2017/2018 and 2018/2019 (Flatley, 2019a). Ketamine usage rates amongst 16–24-year-olds were somewhat higher, rising from 0.8% in 2006/2007 to 2.0% in 2010/2011 before falling to 1.0% in 2015/2016, but rising sharply in 2017/2018 to 3.1% and 2.9% in 2018/2019. The estimated number of individuals aged 16–59 years ever trying ketamine in England and Wales was 1,046,000 in 2018/2019. The number using in the last year was 266,000, and the month prior to the interview was 103,000 (Flatley, 2019a).
Ketamine is not identified separately in the Northern Ireland prevalence survey. However, the Scottish Crime and Justice Survey has breakdowns for ketamine from the 2006 sweep up and including the 2017/2018 sweep. The most recent statistics available (Brown and Bolling, 2007; MacLeod et al., 2010; MacLeod and Page, 2011; Inman et al., 2012; Robertson, 2016; Robertson and Bates, 2014; Scottish Government, 2019) indicates that lifetime ketamine use for individuals aged 16–59 years rose from 1.2% in 2006 and 2008/2009 to 1.3% in 2009/2010, falling back to 1.2% in 2010/2011, before rising to 1.5% in 2012/2013 and 1.3% in 2014/2015 and 1.6% in 2017/2018. Ketamine usage rates were higher amongst males than females in every sweep, for example 2.2% versus 1.0% in 2017/2018. Similar patterns were exhibited for the last year use of ketamine: all respondents: 2008/2009 – 0.2%; 2009/2010 – 0.3%; 2010/2011 – 0.3%; 2012/2013 – 0.2%; 2014/2015 – 0.2% and 2017/2018 – 0.4% (males 0.6%, females 0.2%). In 2017/2018, the highest last year ketamine use rate was for males aged 16–24 (3.9%).
Data from the CSEW for 2012/2013 indicate that ketamine users were typically: male, single, aged 20–24, unemployed or studying and from Chinese or mixed-race ethnic backgrounds (Home Office, 2013a). Frequency of attendance at nightclubs and pubs predicts higher ketamine usage rates. For example, the last year use of ketamine amongst those who had visited a nightclub more than three times in the last month was 6.6% compared to only 0.3% for those who had not (Home Office, 2012a). The highest rates of ketamine use appear to be evidenced by clients of ‘gay-friendly’ dance clubs in South London. Here, lifetime ketamine use rose from 57% to 70% between 2010 and 2013; the last year use of ketamine rose from 46% to 51% and the last month use from 30% to 35% (Wood et al., 2012b).
The Smoking, Drinking and Drug Use among Young People in England survey series has captured information on ketamine use amongst 11- to 15-year-olds since 2005. The most recent published results from the 2018 sweep indicate that last year use of ketamine by this age-group was typically 0.4% or 0.5% over this timeframe, with higher levels being recorded in 2008 and 2009 (0.7% and 0.6%, respectively), but peaking in 2018 at 1.0% (NHS Digital, 2019). Rates for boys tended to be higher than for girls.
The Scottish Schools Adolescent Lifestyle and Substance Use Survey (SALSUS) also captures information on ketamine use by pupils aged 13 and 15 years, but only in its 2010 sweep. The data for that year indicate that 2% of boys aged 15 reported lifetime use compared to 1% of girls of that age, and 1% of boys aged 13 and 0% of girls aged 13 years. Last year ketamine use was highest at 1% for boys aged 13 and 15 years (Black et al., 2011). The 2017/2018 sweep of the SALSUS survey indicates that the proportion of 15-year-old pupils offered ketamine had doubled from 5% in 2015 to 10% in 2018; in 2018, boys (12%) were more likely than girls (7%) to have been offered the drug (Black et al., 2019).
Unfortunately, there are no national survey data available for school-age children in Wales covering ketamine. However, Holloway and Bennett (2017) reported that 6.8% of male and 2.4% of female students in seven Welsh universities used ketamine in the 12 months prior to interview in 2015/2016, with use amongst 17–20-year-olds being 4.8%.
In Northern Ireland, 2.4% of schoolchildren aged 11–16 years reported in 2019 that they had ever been offered ketamine and 0.4% that they had ever taken the drug (Foster et al., 2020). The proportion of pupils offered ketamine was about half that level in 2016 (Foster et al., 2016).
Treatment
Figures from the National Drug Treatment Monitoring System (NDTMS) indicate that in England the number of adults receiving treatment for the first time for ketamine use rose from 116 in 2005/2006 to 1043 in 2013/2014 before dropping sharply to 426 in 2014/2015, since when it has risen to 1140 in 2019/2020 (PHE, 2020). In 2019/2020, there was a total of 1660 individuals under treatment for ketamine use (PHE, 2020). Breakdowns available for young people (<18 years) indicate that the number under treatment during 2019/2020 was 549; the number receiving treatment for the first time for this drug rose from 11 in 2005/2006 to 101 in 2010/2011 before falling to 15 in 2015/2016, since when it has risen to 93 in 2019/2020 (PHE, 2021). There are no breakdowns for ketamine treatment available for the rest of the UK.
Legal status
Ketamine is an acrylcyclohexylamine and listed as an essential medicine by the WHO. It is not under international control. In the UK, it became controlled as a Class C drug under the Misuse of Drugs Act 1971 from 1 January 2006 (Home Office, 2005) and was reclassified as a Class B drug from 10 June 2014 (Home Office, 2014).
Confiscations/seizures
Although diversion of legitimate supplies from medical and veterinary establishments has long been a source of illicit ketamine, there is also evidence of illicit supplies being produced in China and Southeast Asia, including the Golden Triangle in Malaysia, destined for export to China and Thailand; it is easily purchased online (UNODC, 2018a, 2018b). Global seizures of ketamine increased greatly between 2012 and 2015, mainly due to confiscations in East and Southeast Asia. Seizures totalled 22 tonnes in 2015 but fell in 2016 due to a large decline in quantities seized in China, including Hong Kong (UNODC, 2018a), and totalled 12 tonnes in 2017 (UNODC, 2019). In the period 2001–2017, 47 countries and territories reported seizures of ketamine (UNODC, 2019).
The first seizures of ketamine in England and Wales were reported in 1990, steadily increasing to over 250 in 1998, and 195 in 2003 (ACMD, 2004). Although ketamine became a controlled drug in the UK in 2006, it was grouped in ‘other class C’ and not separately identified until 2008/2009 for police forces in England and Wales and 2009/2010 for the Border Force. The number of seizures of ketamine in England and Wales rose from 1269 in 2008/2009 to 1793 in 2010/2011 before falling slightly to around 1500–1600 per year until 2013/2014 and then falling to 558 in 2014/2015, but rising to 726 in 2017/2018 and 959 in 2018/2019 (Flatley, 2019b). In Scotland, the number of seizures ranged from 19 to 34 per year between 2010/2011 and 2013/2014 but fell to only 2 in both 2014/2015 and 2015/2016, only to rise to 7 in both 2016/2017 and 2017/2018; the amount seized is typically low at about 100 g, but in 2016/2017 10 kg was confiscated compared to 199 g in 2017/2018 (personal communication from Justice Analytical Services, Scottish Government, to first author, 3 July 2017 and 27 March 2019). The number of ketamine seizures in Northern Ireland has varied between 1 and 11 in the period since it became a controlled drug in 2006 up to 2016/2017 but rose to 15 in 2017/2018 with 131 g being seized, followed in 2018/2019 by 17 seizures amounting to 44 g (personal communication from Police Service of Northern Ireland, to first author, 8 April and 5 June 2019).
Her Majesty’s Customs and Excise first noticed a trend emerging in 2001 of ketamine being imported covertly into the UK, initially in bulk, in a variety of products, including massage oils and rosewater, from India (ACMD, 2004). This method and route of importation appears to have decreased in 2003/2004 (ACMD, 2004). The quantity of ketamine seized in England and Wales rose from 3 kg in 2006/2007 to 221 kg in 2010/2011 but fell abruptly to 12 kg in 2011/2012 and continued at a low level until 2015/2016, before rising to 132 kg in 2017/2018 and 155 kg in 2018/2019 (Flatley, 2019b). The fall in the number of seizures in 2014/2015 follows the reclassification of ketamine to a Class B drug in 2013, and stronger controls on production and exports from India (Narcotics Control Bureau, 2013).
Price, purity and availability
The UK street price of ketamine (1 g) fell from £28 in 2006 (Eaton et al., 2008) to £25 in 2010–2011, and £20 in the period 2012 to 2018 (Crawford et al., 2018; UK Focal Point on Drugs, 2020). The most recent report on the EU drugs market indicates that the median price for ketamine offered on darknet sites between July 2017 and June 2018 was 23€ (EMCDDA & Europol, 2019).
According to the Global Drug Survey 2016, the most common sources of supply of ketamine in the last 12 months were: friends 29.2%; dealers that you know 26.2%; friends of friends 15.7%; darknet markets (purchased directly) 12.9%; on the street/festivals 6.4%; another source 3.2%; darknet markets (indirect) 2.9%; open websites 1.5%; WhatsApp 1.1%; other social media apps 0.6% and shop fronts (adult store) 0.3%.
Globally speaking, in 2015, only 11.4% of respondents obtained their ketamine via the darknet, but by 2018 this had risen to 15.5% and increased to 24.1% in 2019 (Winstock et al., 2019). There are no UK national records for diversion of ketamine from legitimate human and veterinary sources. However, occasional incidents do come to light (ACMD, 2013).
There is little recent published information on the purity of ‘street’ ketamine. The ACMD (2013) reported that in the first half of 2012, the purity of ketamine samples seized in the UK was in the range of 76%–100% (n = 12). Typical other active ingredients found at this point in time were: amphetamine, benzocaine, cocaine, MDMA, methoxetamine, paracetamol, piperazines and synthetic cathinones (mephedrone, 4-methylethcathinone (4-MEC), 4-methylmethcathinone (4-MMC)) (ACMD, 2013; SOCA, 2013).
An analysis was conducted by the first author, using Microsoft® Excel 365® on 26 August 2020 of Wedinos (http://www.wedinos.org) analysis and results posted online between 15 October 2013 and 31 July 2020 where the purchase intent was ketamine on its own. This revealed that 321/362 submissions (88.7%) actually contained ketamine as a major or minor component; ketamine on its own was present in 273 cases.
Additional components were identified in 48 further cases, as follows: 2C-B (1), alprazolam (1), amphetamine (1), benzocaine (2), caffeine (2), cannabis (1), chlorpheniramine (3), cocaine (18), creatine (1), dextromethorphan (1), levisamole (10), MDMA (16), mephedrone (1), opiate metabolites (1), paracetamol (2) and quetiapine (1). Twelve cases had more than one additional component. About 8.8% (32/362) of submissions did not contain any ketamine but consisted of other components, as follows: 4-CEC (1), 6-APB (1), amphetamine (1), benzocaine (1), benzoylecgonine (1), beta-hydroxyfentanyl (1), caffeine (1), cocaine (10), flephedrone (2), lidocaine (1), MDAI (1), MDMA (4), methoxetamine (9) in the period 2014–2016, norcaine (1), tamoxifen (1), tramadol (1) and xylazine (1). The remaining nine submissions were accounted for by: no active component (3), nothing detected (2), unable to identify (2) and insufficient amount to identify (2). It appears that either ketamine derivatives or other stimulants are typically substituted for ketamine in such cases. In passing, it was noted that ketamine was often substituted for other stimulants such as cocaine and MDMA.
A study reporting anonymous testing by the Spanish-based International Drug Testing Service of drug purchases made using cryptomarkets between March 2014 and March 2015 found that ketamine purity was 71.3% ± 38.4%, range 27%–95% and that 3/6 samples only contained ketamine, 2/6 contained additional substances and one purchase contained no ketamine at all (Caudevilla et al., 2016).
The 2017 report from the Drugs Information and Monitoring System (DIMS) in the Netherlands indicated that ketamine samples analysed were 64% pure; 86% contained only ketamine, with the commonest additions being: chlorpheniramine (5%); lidocaine (3%); 2-fluorodeschloroketamine (1%); other (2%); no active ingredient (3%) (Trimbos Institute, 2017). These results echo those presented above from Wedinos.
Forms and route of use
Ketamine’s lipid- and water-solubility means that it can be misused in many ways. When misused, ketamine is usually used as a powder through nasal insufflation or snorting (Dillon et al., 2003; Wolff, 2016); the 2013 Mixmag/Global Drug Survey reported that 96% of ketamine users snorted it (Winstock, 2013). Ketamine can be smoked, vapourised in solution and inhaled (EMCDDA, 2002), dissolved in water and taken orally. Occasionally, it may be injected either intravenously or intramuscularly (Dillon et al., 2003; Wolff, 2016); such users are likely to be a different ‘at-risk’ group composed of psychonauts (ACMD, 2004). It may also be administered rectally (Sinner and Graf, 2008). It can also be found in capsule or tablet form, sometimes with other psychoactive ingredients (EMCDDA, 2002). The possibility of ketamine being ‘vaped’ has been aired in online user fora (https://treato.com/Ketamine,Vaporizer/); there is some research evidence beginning to emerge supporting this as a route of administration (Blundell et al., 2018; Thurtle et al., 2017).
Ketamine is often consumed as part of a polysubstance scene, often with other ‘club drugs’, alcohol or stimulants (Kalsi et al., 2011). Simultaneous co-administration of ketamine with other substances appears to be common, with 48% of 2011/2012 CSEW respondents reporting that they used at least one other drug the last time they took ketamine: 8% cannabis only, 77% with alcohol and other substances (Home Office, 2012a). BCS data in 2009/2010 suggest that past use of other substances is higher among ketamine users than for consumers of other drugs (Hoare and Moon, 2010).
Dosages
Low doses of ketamine (0.1–0.5 mg/kg/h) can be used for local anaesthesia and co-analgesia (Lynch et al., 2005). For general anaesthesia induction, the typical dosages are: IV 0.5–2 mg/kg, IM 2–4 mg/kg, nasal 5 mg/kg; rectal 8–10 mg/kg (Sinner and Graf, 2008). A typical recreational dose is equivalent to 10%–25% of the effective general anaesthetic one (Jansen, 2000). This translates into a single IV dose of 50–100 mg, IM 75–125 mg and oral 200–300 mg (EMCDDA, 2002). Sub-anaesthetic doses (0.1–0.5 mg/kg) can induce dissociative and schizotypal symptoms in healthy volunteers (Krystal et al., 1994). The route of administration affects the duration of effects: IM and IV – 30–45 min, nasal 45–60 min and oral 60–120 min (Dalgarno and Shewan, 1996; Kalsi et al., 2011). The onset of effects following IV administration is practically instantaneous (seconds), whereas for IM it is 1–5 min, nasal 5–10 min and oral 15–20 min (Sinner and Graf, 2008).
A typical intranasal dose reported by Scottish users was 125 mg (Dalgarno and Shewan, 1996). The 2010 Mixmag survey reported that 43.7% of its respondents took 250 mg or less during an average session, with 7.6% taking 2 g or more (Dick and Torrance, 2010). This is in line with a follow-up study to the 2009 sweep: 31% reporting using <125 mg, 35% using 250–500 mg, 34% using >1 g and 5% >3 g in a typical session (Winstock and Mitcheson, 2012).
A snorted low dose of 10–75 mg may lead to “the mild, trippy euphoria that has led to its sale as an alternative to Ecstasy. Smells and tastes seem muted. Visual perception and sense of touch amplified”; a medium dose of 60–125 makes it seem as if “everything is in slow motion, there’s a buzzing or ringing in the ears, disconnection from your surroundings, loss of coordination and in less than ten minutes you’ll yourself hardly able to move” (Thegooddrugsguide, 2019). A large dose of 100–250 mg leads to a range of effects including: feeling light, losing track of time, body dysmorphia, being at one with the universe (Corazza et al., 2013) and perhaps culminating in the ‘K-hole’, in which users undergo out-of-body experiences (Pomarol-Clotet et al., 2006). A recent animal study has proposed that a high IV dose caused the complete cessation of cortical EEG activity for several minutes, akin to the ‘K-hole’ in humans (Nicol and Morton, 2020).
Non-physical, or psychological, dependence (Dillon et al., 2003), tolerance and withdrawal symptoms including flashbacks may all develop with long-term users (Peyton et al., 1988). The need to increase doses to achieve the same effect as previously (Dillon et al., 2003; Morgan et al., 2010) may lead to ‘binge’ sessions with associated risks. Even if ketamine use is stopped, perceptual distortions, schizotypal symptoms and distortions of perception may continue (Curran and Morgan, 2000). Only 4.9% of respondents to the 2018 Global Drugs Survey gave ketamine a Severity of Dependence Scale score of 4, compared to 24.7% for crystal methamphetamine, 24.5% for synthetic cannabinoids at one extreme and 7.4% for MDMA (Winstock et al., 2018).
Effects
Due to its sympathomimetic activity, ketamine causes only mild stimulation of the cardiovascular system without suppressing respiration and gag reflexes (Sehdev et al., 2006). Ketamine does not affect haemodynamic stability (Sinner and Graf, 2008) and has only a minimal effect on pupil dilation and bronchodilation. The drug, therefore, has a good safety record when used in medical and veterinary contexts. However, when high doses are used, for example for recreational purposes, cardiovascular (raised heart rate and blood pressure) and respiratory toxicity (dose-dependent respiratory depression) effects can be caused (Moore et al., 1997). In acute toxicity cases, the cardiovascular effect most often recorded is a sinus tachycardia, which resolves of its own accord though palpitations and chest pains can also occur (Weiner et al., 2000; Wood et al., 2008).
Recreational ketamine users may experience numbness of the limbs, analgesia, hyperthermia, pyrexia, impaired conscious level, nausea and vomiting (which can cause asphyxiation). Difficulty with balance, dizziness, lack of coordination, combined with numbness, muscle weakness and impaired perception can result in falls, trauma or burns (Dillon et al., 2003; Jansen, 2000; Ng et al., 2010).
Risks from impaired awareness or perception, flawed risk assessment, dissociative effects, perceptual distortions and blurred vision include: drowning, death by hypothermia from lying outside in winter, extended exposure in water, falls from height, road traffic accidents and becoming a crime victim (e.g. ‘date rape’) (Dillon et al., 2003; Jansen, 2000; Rowland, 2005; Scott-Ham and Burton, 2005). The risk of physical harm can also arise from ketamine-induced aggression or agitation (Kalsi et al., 2011). Such impairments can obviously be enhanced by co-administration of other psychoactive substances.
Other acute effects include impaired handling of information with episodic and working memory, and deficits in semantic processing (Morgan and Curran, 2006; Morgan et al., 2006, 2009, 2010).
Acute administration of ketamine can lead to “schizophrenia-like or psychotomimetic symptoms with large effect sizes” (Beck et al., 2020). Long-term users can experience constant and strong schizophrenic-type symptoms (Kalsi et al., 2011). Chronic users can also suffer cognitive impairment in visual and verbal memory as well as in executive function (Zhang et al., 2020).
Adverse and serious effects
If administered quickly IV, ketamine can cause apnoea (Schifano et al., 2008). When co-administered with stimulants, such as amphetamines and MDMA, it can lead to high blood pressure (Curran and Morgan, 2000). Other complications, such as respiratory depression, could arise from the concurrent use of central nervous system (CNS) depressants, for example alcohol, benzodiazepines and opioids (EMCDDA, 2002; Wolff and Winstock, 2006).
Reported bladder problems include: urinary cystitis caused by repeated dosing or long-term use; incontinence; painful bladder; bladder shrinkage; damage to the kidney and urethral obstruction, which can lead to the removal of the bladder; dilated common bile ducts and epigastric pain (Bhattacharya, 2011; Colebunders and Van Erps, 2008; Ng et al., 2010; Wood et al., 2012a). Some ketamine-related urinary dysfunction can occur after relatively short periods of use (Schifano et al., 2020). Longer exposure to ketamine and depression appear to increase the risk of lower urinary tract symptoms (Chen et al., 2018). The possible cause(s) of bladder pathology in ketamine users appear to be very complex and varied (Jhang et al., 2015).
Other adverse effects reported include: anxiety, panic attacks, palpitations, tachycardia, chest pains, depression, aggravated symptoms of existing mental health issues, slurred speech and inability to speak (Chu et al., 2008; Corazza, 2008; Corazza and Schifano, 2010; Dillon et al., 2003; Mason et al., 2010; Ng et al., 2010; Tsai et al., 2009; Wood et al., 2011). A presumptive case of ketamine-induced serotonin syndrome has also been reported (Gueits and Witkin, 2020).
Heavy ketamine use can lead to loss of appetite and stimulant-like weight loss (Corazza et al., 2013). Gastric and hepatic problems have been reported in long-term ketamine users (Kalsi et al., 2011).
It has been postulated that the antidepressant effects of drugs may be realised, in part, through synaptic plasticity and signalling of brain-derived neurotrophic factors (BDNFs; Colucci-D’Amato et al., 2020). Chronic use of ketamine leads to higher BDNF levels in serum (Ricci et al., 2011). Higher expression of BDNFs through the use of ketamine may lead to an increase in synaptic plasticity in the prefrontal cortex (Woelfer et al., 2020). Ketamine use also appears to reduce the resting-state functional connectivity of the dorsomedial prefrontal cortex. As a result, memory processes may be impacted adversely, and the induction of long-term potentiation in the hippocampus may be hindered (Ricci et al., 2011).
The literature on hospital presentations because of ketamine use is very scant. In Hong Kong, ketamine users accounted for 16% of all drug abuse attendees at Accident and Emergency Departments in the last 6 months of 2005, but this proportion rose to 40% in the first 6 months of 2008 (Ng et al., 2010). One-tenth of clients had co-ingested alcohol; other common substances co-used were: MDMA (6%), methamphetamine (6%), benzodiazepines (4%), cocaine (4%) and zopiclone (4%). The most common abnormal physical findings were: high blood pressure (40%), tachycardia (39%), abdominal tenderness (18%), hyperthermia (14%) and decreased level of consciousness (13%). A central London Emergency Department (ED) reported that 89% of 116 clients in 2006–2007 with acute recreational ketamine-induced toxicity had used at least one or other recreational drug or alcohol (Wood et al., 2008): alcohol (38.7%), GHB/GBL (47.3%), cocaine (19%) and MDMA (52.6%). Hypertension (38.8%), tachycardia (29.3%) and agitation/aggression (25%) were key outcomes.
More recently, a study in the metropolitan area of Bologna, Emilia-Romagna region, northern Italy during the period 1 January 2009 to 30 June 2019 describes 74 ED visits (Pavarin et al., 2019). The key demographics were: male 70.3%, mean age 25.6 years (males 26.4 vs. females 23.7 years); snorting ketamine 94.6%. Ketamine use on its own was reported by 42%. Use of other substances was reported by 46%, as follows: cocaine (18.9%), heroin (17.6%), cannabis (12.2%), amphetamines and MDMA (both 9.5%) and LSD (4.1%). Alcohol abuse was reported by one-quarter (25.7%) of patients. The researchers noted the following neurological issues: soporific state (17.6%; females 31.8%, males 11.5%), “agitation (13.5%), confusional state (6.8%), panic attacks (6.8%) mydriasis (6.8%) and tremors (6.8%)” (Pavarin et al., 2019). Other symptoms of a neurological nature reported were: blurred speech; coma; dizziness; epilepsy; hallucinations; neck pain. Abdominal pains were reported by 15%, vomiting by 10.8% (females 22.7%, males 5.8%) and urological complications (6.8%). Cardiac symptoms noted included palpitations and chest pain (both 5.4%) and hyperpyrexia (4.1%).
Data from the Global Drug Survey indicate that globally 0.5% of ketamine users who responded to the 2015 sweep sought emergency medical treatment after using the drug in the previous year, the rate for males (0.3%) being lower than for females (1.0%) (Winstock, 2015a). The ketamine usage rates for 2016 were: 0.4%, 0.3% and 0.6%, respectively (Winstock et al., 2016); those for 2018 were 0.6%, 0.5% and 0.5% (Winstock et al., 2018). Ketamine ranked 10th in the list of drugs for which respondents to the 2019 sweep of the Global Drug Survey reported seeking emergency treatment during the previous year; the rate was 1.1% for females, 0.6% for males and 0.8% overall (Winstock et al., 2019). The most common ketamine bladder symptoms reported in the 2018 Global Drug Survey were: urine frequency 38%; pain in abdomen 25%; burning when urinating 18%; incontinence 7%; and blood 3% (Winstock et al., 2018). These appear to be in line with the 2010 Mixmag Survey results where 30% of ketamine users reported getting stomach pains or ‘K cramps’, and 20% experienced cystitis or other urinary tract problems, with such issues being more common among women (Dick and Torrance, 2010).
Information from the UK National Poisons Information Service (NPIS) indicates that it has been a steady increase over the past decade in the number of both telephone enquiries and TOXBASE® accesses, but at an increasing rate for the latter over the past 5 years. The number of TOXBASE® accesses increased from 1918 in 2015/2016 to 4126 in 2019/2020 (NPIS, 2020). (TOXBASE® https://www.toxbase.org/ is a poisons information database created and maintained by the NPIS for healthcare professionals seeking poisons information in the UK.)
Rationale for studying deaths associated with recreational use of ketamine
Although the recreational use of ketamine has increased, relatively few reports of fatalities attributed to ketamine poisoning, either alone or in combination, have been documented to date. Post-mortem toxicological reports citing ketamine are uncommon, and deaths attributed to the drug are considered quite rare (Watterson, 2015).
A search of the literature using Google Scholar and PubMed using the search terms ‘ketamine’ + ‘death’ or ‘fatal’ or ‘toxicity’ revealed that few substantive or other large-scale studies have been published on ketamine fatalities, either for the UK or elsewhere.
The largest non-UK studies up until June 2020 were: (a) reports on 15 deaths that occurred in 1997–1999 in New York City (Gill and Stajíc, 2000), and (b) very basic summary information on 11 deaths in Wuhan, China, in 2005–2017 (Li et al., 2020). However, Darke et al. (2020) have recently examined 68 cases of self-administered ketamine-related death in Australia, 2000–2019; of which, 22 were considered suicides.
Due to what we considered to be the general lack of ketamine misuse mortality data, we previously collated available data for the UK for the period 1993–2006 on 23 deaths (Schifano et al., 2008) and 1997–2013 in respect to cases reported to the National Programme on Substance Abuse Deaths (NPSAD) (ACMD, 2013).
This paper helps to fill the gaps in knowledge still persisting in respect to deaths associated with recreational drug use. Given the continuing growth of recreational ketamine use, it is important that information on such events is available to both (potential) recreational users of ketamine and health professionals. In turn, it is hoped that it will contribute to a reduction in the ever-rising number of such deaths in different parts of the world, especially in the UK.
Methods
On account of the paucity of ketamine misuse mortality data, the aim of this report was to focus on those cases that were available for England. The NPSAD regularly receives information from coroners on a voluntary basis on deaths related to drugs in both those dependent and non-dependent on them; currently 80.5% of coroners in England are regularly reporting to NPSAD. Data for deaths occurring in England are much more complete than for other parts of the UK (i.e. Wales, Scotland and Northern Ireland). Since July 1997, details of more than 35,100 deaths had been received from English jurisdictions and processed by 1 February 2020.
To be recorded in the NPSAD database, at least one of the following criteria must be met: (a) the presence of one or more psychoactive substances directly implicated in death; (b) the history of dependence or abuse of drugs and (c) the presence of controlled drugs at post-mortem (Corkery et al., 2014). Ethical approval is not required in the UK for studies whose subjects are deceased but solely involves retrospective reviews of death records.
A range of documents are contained in coronial inquest files, although the variety differs from case to case. Typically, the coroner has access to: statements from witnesses, family and friends; General Practitioner records (if the deceased is registered with one); reports from ambulance, police or other emergency services; hospital EDs and clinical ward reports; psychiatric and substance abuse team reports and post-mortem and toxicology reports.
Relevant information from the NPSAD data collection and the sources outlined above are held in a relational database management system held on an encrypted secure server. Ketamine-related deaths were defined here as: text searches of the cause of death, coroner’s verdict and toxicology results for the terms ‘ketamine’ or ‘norketamine’. Cases where ketamine was present post-mortem as a result of being administered for medical reasons, for example as an anaesthetic or in emergency situations, were excluded from this study, as were cases where the source of ketamine could not be determined. Data were extracted retrospectively from the NPSAD database at St George’s, University of London for the period 1 July 1997 to 31 January 2020. Data analyses were performed using IBM® SPSS™ Statistics for Windows (version 26; IBM SPSS, Armonk, NY, USA) employing descriptive statistics, and Microsoft® Excel 365® (Microsoft Corp., Redmond, WA, USA).
Some 18 of the cases included in this dataset have been previously reported on by NPSAD (Schifano et al., 2008) and 83 (including the 18 afore-mentioned cases) by the ACMD in its earlier assessment of ketamine (ACMD, 2004), as well as in its most recent review (ACMD, 2013). NPSAD data were drawn on in compiling the ACMD’s report, but only key summary results were presented in their report (ACMD, 2013: 30).
Results
Deaths reported to NPSAD from England in the period ending January 2020 where ketamine was detected at post-mortem and/or implicated in causing the death mostly occurred in the period from 2005 onwards, with numbers increasing over time and appearing to peak first in 2009 and plateauing until 2016. However, since then, numbers have increased to a higher level (Figure 1). During the period July 1997 to January 2020, 283 relevant deaths were identified in the NPSAD database where illicitly sourced ketamine was implicated in death. Furthermore, in addition to this increase in raw number of deaths, an increasing proportion of deaths reported to NPSAD where ketamine was detected is evident. As the average time between death and conclusion of coronial inquest for deaths where ketamine was present was approximately 7 months, it is anticipated that further deaths will be reported to NPSAD that occurred in 2019. Based on jurisdiction reporting trends, a projected number of ketamine-related deaths expected to be received by NPSAD has been extrapolated. Both the raw number (Figure 1) and the proportion (Figure 2) of deaths involving ketamine are expected to rise.
Figure 1.
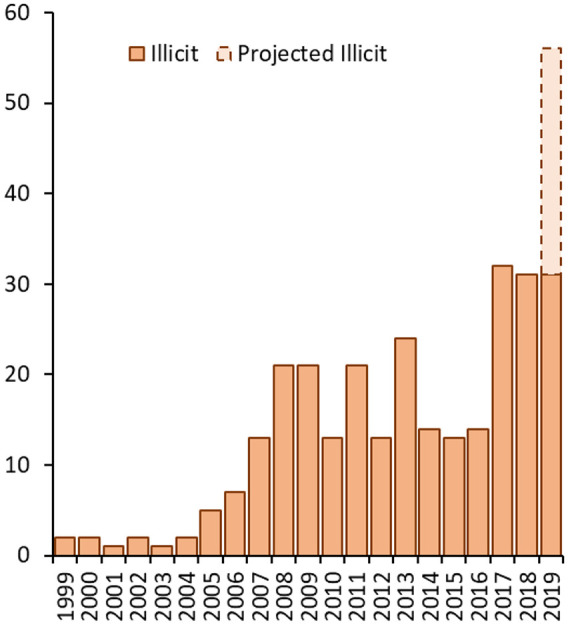
Deaths reported to NPSAD from England in the period ending 31 January 2020 where ketamine was detected at post-mortem and/or implicated in causing the death.
Note: There were no relevant deaths in 1997 or 1998. As inquests take on average 7−10 months to be concluded, more deaths are expected to be received (as the cut-off date for this analysis was January 2020).
Figure 2.
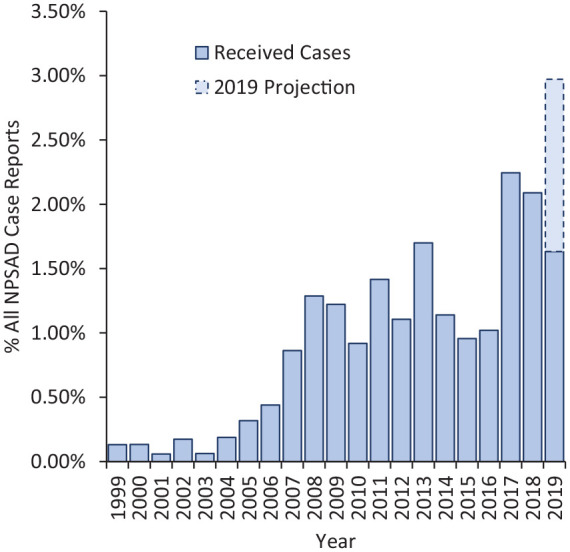
Percentage of all deaths reported to NPSAD from England that were ketamine-related.
Socio-demographics
Table 1 shows that most victims were males (84.1%). The mean age of decedents was 31.2 years; males 31.1, females 32.2. Where known, about half (56.5%) were employed and three-fifths (60.3%) lived with others. Where ethnicity was known, the majority (94.4%) of decedents were White. Where known, four-fifths (79.6%) had a history of drug use, of which 16.5% were known to have injected drugs. About three-tenths (28.6%) of decedents were known to have been prescribed psychoactive drugs, the majority of these being benzodiazepines, hypnotics/sedatives, opioids and antipsychotics (Table 2).
Table 1.
Comparison of socio-demographics of ketamine-related versus all NPSAD deaths, England, 1999−2019.
| Variable | Category | Illicit ketamine-related deaths |
All NPSAD deaths |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Total | 283 (100.0) | 33,186 (100.0) | |
| Gender | Male | 238 (84.1) | 24,299 (73.2) |
| Female | 45 (15.9) | 8887 (26.8) | |
| Employment status | Unemployed | 74 (26.1) | 16,256 (49.0) |
| Employed | 140 (49.5) | 9261 (27.9) | |
| House-person | 2 (0.7) | 568 (1.7) | |
| Student | 28 (9.9) | 640 (1.9) | |
| Retired/invalidity | 4 (1.4) | 3201 (9.7) | |
| Not known | 35 (12.4) | 3260 (9.8) | |
| Living arrangements | Alone | 87 (30.7) | 13,765 (41.5) |
| With others | 144 (50.9) | 12,899 (38.9) | |
| No fixed abode | 5 (1.8) | 1355 (4.1) | |
| Hostel | 3 (1.1) | 747 (2.3) | |
| Not known | 44 (15.5) | 3957 (11.9) | |
| Other (prison, hotel, B&B, rehab, hospital, nursing home, boat, shed and workplace) | – | 463 (1.4) | |
| History of drug use | Yes | 164 (57.9) | 18,749 (56.5) |
| Of which, injectors | 27 (16.5) | 2892 (15.4) | |
| No | 42 (14.9) | 8422 (25.4) | |
| Not known | 77 (27.2) | 6015 (18.1) | |
| Age at death (years) | Male | Mean 31.1; Min 15, Max 67, SD 9.7 | Mean 38.9 (SD 12.4) |
| Female | Mean 32.2; Min 15, Max 56, SD 11.6 | Mean 44.2 (SD 15.9) | |
| All | Mean 31.2; Min 15, Max 67, SD 10.0 | Mean 40.2 (SD 13.7) |
Table 2.
Prescribed psychoactive drugs.
| Variable | Category | Number (%) |
|---|---|---|
| Total | 283 (100.0) | |
| Number of drugs | 0 | 118 (41.7) |
| 1 | 52 (18.4) | |
| 2 | 16 (5.7) | |
| 3 | 9 (3.2) | |
| 4 | 2 (0.7) | |
| 5 | 2 (0.7) | |
| Unknown | 84 (29.7) | |
| Medication type | ||
| Anti-depressants | 50 | |
| Amitriptyline | 2 | |
| Citalopram | 16 | |
| Dothiepin | 1 | |
| Fluoxetine | 9 | |
| Mirtazapine | 7 | |
| Sertraline | 11 | |
| Trazodone | 1 | |
| Venlafaxine | 2 | |
| Unspecified antidepressant | 1 | |
| Anxiolytics and sedatives | 27 | |
| Diazepam | 16 | |
| Lorazepam | 1 | |
| Temazepam | 2 | |
| Zopiclone | 7 | |
| Unspecified benzodiazepine | 1 | |
| Opioids | 23 | |
| Codeine | 4 | |
| Methadone | 10 | |
| Morphine | 4 | |
| Tramadol | 4 | |
| Oxycodone | 1 | |
| Anti-psychotics | 14 | |
| Aripiprazole | 2 | |
| Flupenthixol | 1 | |
| Haloperidol | 1 | |
| Olanzapine | 5 | |
| Pericyazine | 1 | |
| Promazine | 1 | |
| Quetiapine | 2 | |
| Risperidone | 1 | |
| Gabapentinoids | 6 | |
| Pregabalin | 6 | |
| Anti-epileptics | 4 | |
| Carbamazepine | 1 | |
| Clonazepam | 1 | |
| Levetiracetam | 1 | |
| Lamotrigine | 1 | |
| Anti-histamines | 2 | |
| Cyclizine | 1 | |
| Fexofenadine | 1 | |
| Stimulants | 1 | |
| Methylphenidate | 1 | |
| Treatment of bipolar disorder | 1 | |
| Lithium | 1 | |
| Treatment of substance dependence | 1 | |
| Varenicline | 1 | |
Characteristics of deaths
Two-thirds of deaths (66.8%) occurred in an individual’s home or another residential address (Table 3); in many instances, the death followed recreational use at a pub or at home or in the home of another. However, a substantial proportion (15.9%) of cases died in hospital, suggesting that friends/family members tried to intervene. Other deaths occurred in a variety of locations including in the street, in open spaces and open water.
Table 3.
Characteristics of deaths.
| Variable | Category | Illicit ketamine-related deaths |
All NPSAD deaths |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Total | 283 (100.0) | 33,186 (100.0) | |
| Place of death | At home | 130 (45.9) | 18,383 (55.4) |
| Other private residence | 49 (17.3) | 3451 (10.4) | |
| Hospital | 45 (15.9) | 6571 (19.8) | |
| Street/road | 13 (4.6) | 730 (2.2) | |
| Open space/woodland/campsite | 12 (4.2) | 498 (1.5) | |
| River/stream/lake/sea | 8 (2.8) | 232 (0.7) | |
| Music festival site | 3 (1.1) | 3 (<0.1) | |
| Pub/club | 2 (0.7) | 66 (0.2) | |
| Hotel | 3 (1.1) | 398 (1.2) | |
| Hostel | 2 (0.7) | 232 (0.7) | |
| Grounds of a church | 1 (0.4) | 33 (0.1) | |
| Public toilets | 1 (0.4) | 166 (0.5) | |
| Derelict property | 1 (0.4) | 66 (0.2) | |
| Bus/railway station | 1 (0.4) | 100 (0.3) | |
| Car park | 1 (0.4)) | 133 (0.4) | |
| Motor vehicle | 1 (0.4) | 100 (0.3) | |
| Not specified | 10 (3.5) | 1560 (4.7) | |
| Other (public building/place, care home, workplace, boat, prison, garage/shed) | 0 (0.0) | 464 (1.4) | |
| Manner of death | Accidental | 234 (82.7) | 25,620 (77.2) |
| Suicidal | 16 (5.6) | 4148 (12.5) | |
| Undetermined | 31 (11.0) | 2721 (8.2) | |
| Natural (combined drug-related) | 2 (0.7) | 631 (1.9) | |
| Homicidal | 0 (0.0) | 66 (0.2) | |
| Number of post-mortem drugs | Mean 4.6; Min 0, Max 13, SD 1.30 | Mean 3.3; Min 0; Max 14, SD 2.22 |
The majority (82.7%) of deaths were deemed accidental in nature. However, there were 16 suicides and 31 cases where the intent was unclear – but where ketamine use could be assumed to have been deliberate. The violent means used in several of these cases suggest the possibility of suicidal or depressive thoughts, as seen with mephedrone (and other stimulants such as cocaine, amphetamine, MDMA and cannabis). There was one case where a history of bladder problems was mentioned, and another where ketamine use had necessitated a nephrectomy.
In terms of the underlying cause of death (Table 4), the principal ones were: accidental poisoning (74.6%), poisoning of undetermined intent (4.6%), intoxication 1.4%) and hanging (4.2%). However, examining the other cause of death fields reveals further details of the way(s) in which some of these decedents died. There were 16 instances of drowning or immersion where ketamine ingestion had caused intoxication/poisoning; intoxication may have impaired judgement, as was the case in six falls and three road traffic accidents. There were 15 deaths that could be considered violent following use of ketamine: six falls, four hangings, three road traffic accidents, one person being hit by a train and one instance of intentional self-harm by use of a knife. In addition, autoerotic asphyxiation was involved in one case.
Table 4.
Underlying cause of death.
| ICD 10 code | Number (%) |
|---|---|
| F13.0 Mental disorders due to sedatives or hypnotics – intoxication | 2 (0.7) |
| F15.0 Mental disorders due to stimulants – intoxication | 5 (1.8) |
| F15.5 Mental disorders due to stimulants – psychotic disorder | 1 (0.4) |
| F19.0 Mental disorders due to drugs unspecified – intoxication | 1 (0.4) |
| F19.1 Mental and behavioural disorders due to multiple drug use and use of other psychoactive substances – harmful use | 2 (0.7) |
| I25.1 Atherosclerotic heart disease | 1 (0.4) |
| I49.9 Cardiac arrhythmia, unspecified | 1 (0.4) |
| R09.0 Asphyxia general | 1 (0.4) |
| R99 Unascertained | 2 (0.7) |
| S09.9 Head injuries, unspecified | 2 (0.7) |
| T07 Unspecified multiple injuries | 1 (0.4) |
| T71 Asphyxiation | 1 (0.4) |
| V23 Motorcycle rider injured in collision with car, pick-up truck or van | 1 (0.4) |
| V43.5 Car driver injured in collision with car, pick-up truck or van | 2 (0.7) |
| W17 Other fall from one level to another | 1 (0.4) |
| W19 Unspecified fall | 2 (0.7) |
| W26 Contact with knife, sword or dagger (stab wound) | 1 (0.4) |
| W69 Drowning and submersion while in natural water | 3 1.1) |
| W73 Drowning in reservoir | 1 (0.4) |
| W74 Drowning and submersion, unspecified | 1 (0.4) |
| W76 Hanging | 1 (0.4) |
| X41 Accidental poisoning by and exposure to antiepileptic, sedative-hypnotic, antiparkinsonism and psychotropic drugs, not elsewhere classified | 21 (7.4) |
| X42 Accidental poisoning by narcotics and psychodysleptics | 143 (50.5) |
| X44 Accidental poisoning by other unspecified drugs | 44 (15.5) |
| X45 Accidental poisoning by alcohol | 3 (1.1) |
| X62 Intentional self-poisoning by narcotics and psychodysleptics | 5 (1.8) |
| X64 Intentional self-poisoning by and exposure to other and unspecified drugs, medicaments and biological substances | 1 (0.4) |
| X70 Hanging, intentional | 8 (2.8) |
| Y10 Poisoning by and exposure to nonopioid analgesics, antipyretics and antirheumatics, undetermined intent | 1 (0.4) |
| Y11 Poisoning by and exposure to antiepileptic, sedative-hypnotic, antiparkinsonism and psychotropic drugs, not elsewhere classified, undetermined intent | 4 (1.4) |
| Y12 Poisoning by and exposure to narcotics and psychodysleptics [hallucinogens], not elsewhere classified, undetermined intent | 2 (0.7) |
| Y14.0 Poisoning by other and unspecified drugs – undetermined intent | 6 (2.1) |
| Y20 Hanging, strangulation and suffocation, undetermined intent | 3 (1.1) |
| Y30 Falling, jumping or pushed from a high place, undetermined intent | 1 (0.4) |
| Z90.5 Acquired absence of kidney | 1 (0.4) |
Polysubstance use is evident in the majority of cases (Table 5), but deaths involving ketamine on its own are well represented (32/283 cases). It appears that the use of other stimulants, such as cocaine, MDMA, amphetamines and synthetic cathinones (as well as other recreational drugs) often occurs, as does the use of opiates/opioids such as heroin/morphine and methadone, anxiolytics and hypnotics and antidepressants (Table 6).
Table 5.
Implicated drugs and ketamine post-mortem levels (mg/L).
| Combination | N | N with values | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|---|
| K implicated alone and no drug in PM | 1 | 0 | – | – | – | – |
| K implicated alone and sole drug in PM | 14 | 10 | 0.107 | 10.60 | 3.59 | 3.03 |
| K implicated alone and other drugs in PM | 17 | 12 | 0.330 | 32.00 | 5.73 | 8.74 |
| K and Alc implicated alone and only substances in PM | 7 | 3 | 0.780 | 2.70 | 1.54 | 1.02 |
| K and Alc implicated alone and other substances in PM | 11 | 9 | 0.150 | 2.30 | 0.95 | 0.77 |
| K and other drugs implicated and no Alc in PM | 76 | 41 | 0.011 | 6.90 | 1.05 | 1.68 |
| K and other drugs implicated and Alc in PM | 74 | 44 | 0.020 | 24.73 | 1.89 | 4.05 |
| K implicated overall | 182 | 107 | 0.011 | 32.00 | 2.17 | 4.35 |
Table 6.
Substances found at post-mortem.
| Substance | Number | % |
|---|---|---|
| Total | 283 | 100.0 |
| Alcohol | 117 | 41.3 |
| Illicit substances | ||
| Ketamine | 279 | 98.6 |
| Illicit stimulants | 251 | 88.7 |
| Cannabis | 51 | 18.0 |
| Hallucinogens | 6 | 2.1 |
| Synthetic cannabinoid receptor agonists | 3 | 1.1 |
| Volatile substances | 2 | 0.7 |
| Other illicit | 23 | 8.1 |
| Psychoactive substances | ||
| Opioids (prescribed and illicit) | 190 | 67.1 |
| Anxiolytics and hypnotics (prescribed and illicit) | 150 | 53.0 |
| Anti-depressants | 56 | 19.8 |
| Anti-psychotics | 24 | 8.5 |
| Gabapentinoids | 17 | 6.0 |
| Anti-histamine | 16 | 5.7 |
| Anti-epileptic | 13 | 4.6 |
| ADHD | 4 | 1.4 |
| Anti-emetic | 4 | 1.4 |
| Anti-Parkinson’s | 2 | 0.7 |
| Anaesthetics and muscle relaxants | 16 | 5.6 |
| Non-psychoactive substances | ||
| Non-opioid analgesics | 29 | 10.2 |
| Anti-bacterials/protozolas/fungals/virals | 12 | 4.2 |
| Cardiac drugs | 10 | 3.5 |
| Anti-inflammatories | 8 | 2.8 |
| Ulcer healing drugs | 7 | 2.5 |
| Drugs used in impotence | 6 | 2.1 |
| Bronchodilators | 3 | 1.1 |
| Anti-diarrhoeal | 2 | 0.7 |
| Anti-coagulants | 1 | 0.4 |
| Diabetic drugs | 1 | 0.4 |
Deaths involving ketamine alone
Looking at the 32 cases where ketamine was the sole drug implicated (Table 7), the majority of decedents were male (90.6%). The mean age at death was 30.8 years. Where known, most were White (93.1%), employed (65.6%), lived with others (61.3%) and had a history of drug use (71.4%). Most deaths occurred as a result of an incident in residential premises (59.4%). Most deaths (81.3%) were accidental in nature with the main (56.3%) underlying cause being accidental poisoning. In addition, some accidents occurred in situations where ketamine may have impaired judgement. There were also a number of suicides where ketamine was the only drug implicated. These characteristics are in line with those of the sample as a whole.
Table 7.
Characteristics of ketamine-related deaths involving ketamine alone.
| Characteristic | Number |
|---|---|
| Total | 32 |
| Gender | Male = 29; female = 3 |
| Ethnicity | White = 27; Indian = 1; Pakistani = 1; not known = 3 |
| Age at death (years) | Mean = 30.8; Min = 19; Max = 67, SD = 11.4 |
| Employment status | Employed = 21; unemployed = 10; student = 1 |
| Living arrangements | Alone = 13; with others = 19; not known = 1 |
| History of drug use | Yes = 15; No = 6; Not known = 11 |
| Place of incident leading to death | Home = 17; Other private residence = 2; Hospital = 2; River/stream/lake = 2; Street/road = 2; Derelict property = 1; Railway station = 2; Open space/woodland/camping site = 1 |
| Underlying cause of death (ICD-10 code) | F13.0 Mental disorders due to sedatives or hypnotics – intoxication = 2; F19.0 Mental disorders due to drugs unspecified – intoxication = 4; R09.0 Asphyxia general = 1; V23 Motorcycle rider injured in collision with car, pick-up truck or van = 1; V43.5 Car driver injured in collision with car, pick-up truck or van = 1; W19 Unspecified fall = 1; X41 Accidental poisoning by and exposure to antiepileptic, sedative-hypnotic, antiparkinsonism and psychotropic drugs, not elsewhere classified = 3; X42 Accidental poisoning by narcotics and psychodysleptics = 1; X44 Accidental poisoning by other unspecified drugs = 13; X70 Hanging, intentional = 4; Z90.5 Acquired absence of kidney = 1 |
| Manner of death | Accidental = 26; suicidal = 4; undetermined = 1; natural = 1 |
Discussion
To the best of our knowledge, at the time of writing, the present report constitutes the largest available collection of ketamine misuse mortality data from both the UK and elsewhere (see Table 8).
Table 8.
Post-mortem ketamine drug levels in previous studies.
| Study | Nature of death (if known) | Ketamine levels | Other substances (levels) |
|---|---|---|---|
| Centini et al. (1987) | M 34 | Liver 12.2 µg/g; brain 3.7 µg/g; lung 16.4 µg/g; kidney 15.5 µg/g; spleen 14.1 µg/g | None |
| Norketamine: liver 0.15 µg/g; lung 0.17 µg/g; kidney 0.70 µg/g; spleen 0.12 µg/L | |||
| Peyton et al. (1988) | F 31 Accidental intravenous overdose (probably 900 mg) | Bl 7.0 mg/L; liver 6.3 mg/kg; kidney 3.2 mg/kg; lung 1.6 mg/kg; heart 2.4 mg/kg | None |
| Licata et al. (1994) | Homicide for homosexual ends. Multiple i.m. doses, with probable total of 1g | Bl 27.4 µg/mL, ur 8.51 µg/mL; bile 15.2 µg/g; brain 3.24 µg/g; liver 6.6 µg/g; kidney 3.38 µg/g | Norketamine detected but not quantified in all specimens |
| Moore et al. (1997) | Mixed-drug fatality | Bl 1.8 mg/L; ur 2.0 mg/L; brain 4.3 mg/kg; spleen 6.1 mg/kg; liver 4.9 mg/kg; kidney 3.6 mg/kg | Ethanol bl 170 mg/dl |
| Gaillard and Pepin (1998) | F Polydrug addict | Bl 1.4 µg/mL; hair 11.3 µg/g | Chloroform bl 30.7 µg/mL; cocaine: bl 0.576 µg/mL; hair 5.5 µg/g; Benzoylecgonine hair 1.5 µg/g; Methylecgonine ester bl 0.78 µg/mL, hair 1.0 µg/g; Anhydroecgonine ester bl 0.12 µg/mL; codeine: bl 0.013 µg/mL, 6-acetylmorphine hair 4.4 µg/g; morphine0.42 µg/mL, hair 3.4 µg/g; thiopental: bl 1.97 µg/mL, hair 5.3 µg/g; pentobarbital bl 2.64 µg/mL, hair 10.0 µg/g; diazepam: bl 0.07 µg/mL, hair 1.2 µg/g; nordiazepam bl 0.2 µg/mL, hair 0.1 µg/g; oxazepam – present |
| Norketamine hair 1.0 µg/g | |||
| Cording et al. (1999) | M 45 Mixed drug intoxication (ketamine, tiletamine, zolazepam) | Bl 0.037 µg/mL; ur 0.381 µg/mL | Tiletamine: bl 0.295 µg/mL; ur 0.682 µg/mL; liver 0.196 µg/g |
| Zolazepam: bl 1.71 µg/mL; ur 1.33 µg/mL; liver 15.5 µg/g | |||
| Gill and Stajíc (2000) | M 41 White, seen to collapse morning after a party; pronounced dead on arrival at hospital without therapeutic intervention. Right and left ventricles including the septum of the heart were replaced by a firm white infiltrating mass. Microscopic examination showed fibrosis, non-caseating granulomas, and multinucleated giant cells. Slight granulomatous involvement of the lung. Proximate cause of death was sarcoidosis, contributed to by acute intoxication | Bl <0.1 mg/L | MDA 0.1 mg/L, MDMA 0.5 mg/L |
| F 18 White, found on fire in her bedroom by a female roommate. History of ketamine abuse and cigarette smoking. Pronounced dead at the scene. PM showed thermal injuries with charring of 95% body surface area with inhalational injury and soot in the trachea; carboxyhaemoglobin 12%. Ketamine was detected in blood and urine. Fire was deemed accidental due to heat from smoking material. Decedent was an art student and had paint supplies in the room | Bl 2.1 mg/L | ||
| M 28 White, body was found in the back of a nightclub, to which he had earlier gained admission, in the early morning by an employee outside on a break. Access restricted to employees, but open access to the seven-storey roof above the deceased. Two vials of white powder were found on his person and white powder was visible in nostrils. PM showed rib fractures with lung contusions and haemothorax, pelvic fractures and lacerations to the spleen and kidneys. There was peritoneal, retroperitoneal and subdural haemorrhage. Pronounced dead on arrival at hospital without therapeutic intervention. Cause of death – blunt injuries (fall from height) | Bl 1.0 mg/L | MDA < 0.1 mg/L, MDMA 1.3 mg/L | |
| M 18 White, collapsed and had seizures outside a dance club. Unsuccessful resuscitation attempts. PM rectal temperature, measured 21⁄2 after the collapse, was 103°F (39.4°C). No gross or microscopic disease at PM. Vitreous chemistries included a creatinine of 0.7 mg/dl and urea nitrogen of 10 mg/dl. Proximate cause of death was acute multidrug intoxication, leading to hyperthermia | Bl 0.2 mg/L | MDA < 0.1 mg/L, MDMA 1.7 mg/L, cannabinoids | |
| M 20 White, acute intoxication | Bl 1.1 mg/L | Opiates 0.5 mg/L [free morphine 0.2 mg/L], cocaine < 0.1 mg/L, methamphetamine < 0.1 mg/L, benzylecgonine < 0.1 mg/L | |
| M 25 White, acute intoxication | Bl < 0.1 mg/L | Opiates 0.3 mg/L (free morphine < 0.1 mg/L) | |
| M 42 White, acute intoxication | Bl 0.2 mg/L | Opiates 0.2 mg/L (free morphine < 0.1 mg/L), cocaine 0.2 mg/L, benzoylecgonine 0.2 mg/L, methamphetamine 0.1 mg/L, ethanol 0.2 g% | |
| M 21 White, acute intoxication | Bl < 0.1 mg/L | Opiates 0.3 mg/L, diazepam 0.2 mg/L | |
| Breitmeier et al. (2002) | M 28 Autoerotic accident involving a fatal combination of asphyxia by suffocation and intoxication with self-administered IV ketamine | Femoral blood 2.5 µg/mL | None |
| Lalonde and Wallage (2004) | M 26 Accidental ketamine intoxication | Femoral bl 1.8 mg/L; heart 6.9 mg/L | Femoral blood ethanol 14.0 mg/dl |
| M 20 Asthma and presence of ketamine | Femoral bl 0.6 mg/L; heart 1.6 mg/L | Femoral blood ethanol 13.0 mg/dl | |
| Cheng et al. (2005) | Fatal traffic crash (Hong Kong) | Bl 0.21 µg/mL | MDMA bl 0.68 µg/mL; alcohol bl 25 mg/dl; VH 24 mg/dL |
| Tao et al. (2005) | F 34 Chronic ketamine poisoning (homicide). 200 mg administered immediately prior to death; but had been administered over a long period at lower doses | Bl 3.8 µg/mL; ur 1.2 µg/mL; gastric 21 µg/mL | None |
| Dinis-Oliveira et al. (2010) | M 29 Suicide by hanging under influence of ketamine | Femoral blood 1.3 mg/L | Femoral blood ethanol 66 g/L |
| Gerace et al. (2014) | M 25 Accidental multidrug overdose. Pulmonary oedema and multivisceral congestion | Hair 1.90 ng/mg | Cardiac blood: mephedrone 1.33 mg/L, ethanol 0.13 g/L, cocaethylene 18 ng/mL, lidocaine, phenacetin, paracetamol, levamisole positive; urine: mephedrone 144 mg/L, ethanol 0.43 g/L, benzoylecgonine 34.5 mg/L, cocaine 6.97 mg/L, cocaethylene 3.10 mg/L, lidocaine, paracetamol, levamisole positive; gastric contents: mephedrone 4.52 mg/L, ethanol 0.23 g/L; hair: mephedrone 0.25 ng/mg, cocaine: 0.78 ng/mg, benzoylecgonine 0.49 ng/mg, MDMA 0.23 ng/mg; bile: mephedrone 1.29 mg/L; lung: mephedrone 0.79 mg/kg; brain: mephedrone 0.89 mg/kg |
| San Nicolas and Lemos (2015) | N = 25, M = 22. Ethnicity: White 19, Black 4, White Hispanic 1, Asian 1 | Peripheral bl (n = 14): mean 0.91, median 0.29, range 0.01−3.71, SD 1.24 mg/L | Amphetamines 8; cocaine 7; morphine/codeine 6; ethanol 4; methadone, oxycodone, GHB, diazepam, diphenhydramine – all 3 |
| Age: mean 40.1, range 19–64, years | Detected in peripheral bl = 21, of which 12 detected in ur, 2 detected solely in ur, 1 solely in muscle, 1 solely in liver | ||
| Manner of death: accident 17, natural 4, undetermined 2, suicide 1, homicide 1 | Norketamine: bl (n = 5) range 0.05−0.16 mg/L; ur (n = 4) | ||
| Nadesan et al. (2017) | M 21. Malay ethnicity. | Urine 0.06 µg/mL | Bl MDMA 0.01 µg/mL |
| Heat-stroke aggravated by MDMA and Ketamine | |||
| Darke et al. (2020) | N = 68, M = 52. Age (mean years) 35.2 (SD = 11.5, range = 16–63); employed/student = 40; married/de-facto relationship = 18; decedent prescribed ketamine = 6; documented history of injecting drug use = 28; documented history of chronic pain = 6; circumstance of death: accidental drug toxicity = 40; suicide = 22; deliberate toxicity = 16; violent means = 6; traumatic accident = 6; homicide = 0 | Detected in blood = 60 | Other drugs present (n = 63) |
| Opioids = 39: morphine = 21; codeine = 14; oxycodone = 9; tramadol = 8; fentanyl or analogues = 7; other opioids (hydromorphone, methadone, buprenorphine, pethidine) = 10. | |||
| Location of fatal incident: private = 49; public = 19; injected ketamine at fatal incident = 22; resuscitation attempted = 16; hospitalised prior to death = 12 | Present in blood and quantitated (median mg/L) 0.2 (range = 0.02–6.9) | Hypnosedatives = 38; diazepam = 23; temazepam = 17; alprazolam = 9; oxazepam = 9; other hypnosedatives (clonazepam, nitrazepam, midazolam, pentobarbital, zolpidem) = 9 | |
| Psychostimulants = 33: methamphetamine = 19; MDMA = 16; cocaine = 15 | |||
| Pathology (n = 45): pulmonary oedema = 37; pneumonia = 12; emphysema/asthma = 6; cardiovascular – coronary atherosclerosis (moderate–severe) = 6; cardiomegaly < 5; myocardial fibrosisa (indicative of previous ischaemia) < 5; hepatic inflammation = 11; renal nephrosclerosis = 7 | Not detected = 9 | Alcohol = 18. | |
| Antidepressants = 19 | |||
| Detected but not quantified = 11 (0.01–0.50 mg/L = 30), (0.51–1.00 mg/L = 8), >1.00 mg/L = 9) | Δ9 THC = 12 | ||
| Antipsychotics = 6 | |||
| Present only in other biomarker (urine/hair) = 8 | New psychoactive drugs (synthetic cannabinoids, stimulants/hallucinogens, new depressants) = 0 | ||
| Other substances (GHB, LSD, meprobamate, pregabalin) = 5 |
The present study demonstrates that there has been an increase in the annual number of deaths involving recreational use of ketamine in England from less than five between 1997 and 2005, with a gradual increase to the end of the 2000s, since when there are about 30 fatalities a year. The principal characteristics of those dying were: mostly male (84%), mean age 31.2 (SD 10.0) years, more than half (57%) were employed, four-fifths (80%) had a drug use history and three in five (60%) were living with someone else at the time of their death. Most deaths involved ketamine with other substance, with three-quarters (75%) being attributed to an underlying cause of death of accidental poisoning. Impaired judgement may have been caused by ketamine use in other cases, such as accidents, and contributed to a state of mind leading to suicide.
Comparison of decedents’ characteristics with other NPSAD cases
It is considered appropriate to make a comparison here in the Discussion section of the sample examined by the present study with other NPSAD cases, rather than to include it in the Results section thereby avoiding possible confusion on the part of the reader. Comparisons with other study populations are made below.
When the characteristics of these decedents who experienced ketamine-related deaths in England are compared with all other NPSAD cases in England (Tables 1 and 3) over the period 1999–2019, several differences become apparent. The proportions for ketamine-related deaths are higher in respect to male gender, being employed, living with someone else, having a history of drug use and dying an accidental death. These differences are extremely statistically significant, based on one-tailed Z-tests (https://www.socscistatistics.com/tests/ztest/default2.aspx; Table 9). However, there is no significant difference in the proportion dying at home. The mean age of those undergoing a ketamine-related death is 9 years less than that for other NPSAD deaths over this period. Based on unpaired t-tests (https://www.graphpad.com/quickcalcs/ttest2/), this difference is extremely statistically significant both overall and for males and females separately (Table 10). Ketamine users tend to be male and younger than opiate/opioid or stimulant users (the majority group of NPSAD cases). This is also true in other countries (Li et al., 2020; Uosukainen et al., 2015).
Table 9.
Comparison of characteristics between ketamine-related deaths and other NPSAD deaths, England, 1999−2019.
| Characteristic | Ketamine-related deaths |
Other NPSAD deaths |
One-tailed Z test score | p-Value | Significance level | ||||
|---|---|---|---|---|---|---|---|---|---|
| Enumerator | Denominator | Proportion (%) | Enumerator | Denominator | Proportion (%) | ||||
| Male gender | 238 | 283 | 84.1 | 24,299 | 33,186 | 73.2 | 4.1197 | < 0.00001 | < 0.01 |
| Employed | 140 | 248 | 56.5 | 9261 | 29,926 | 30.9 | 8.6369 | < 0.00001 | < 0.01 |
| Living with others | 144 | 239 | 60.3 | 12,899 | 29,229 | 44.1 | 4.997 | < 0.00001 | < 0.01 |
| Drug use history | 164 | 206 | 79.6 | 18,749 | 27,171 | 69.0 | 5.9831 | < 0.00001 | < 0.01 |
| Accidental manner of death | 242 | 283 | 85.5 | 25,620 | 33,186 | 77.2 | 3.3221 | 0.00045 | < 0.01 |
| Dying at home | 179 | 273 | 65.6 | 21,836 | 31,626 | 69.0 | -1.2369 | 0.10749 | Nil |
Table 10.
Comparison of age characteristics between ketamine-related deaths and other NPSAD deaths, England, 1999−2019.
| Characteristic |
Ketamine-related deaths |
Other NPSAD deaths |
Mean of ketamine related deaths minus other NPSAD deaths | 95% CI | Unpaired t-test score | Degrees of freedom | Standard error of difference | p-Value | Significance level | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Mean | SD | Denominator | Mean | SD | Denominator | |||||||
| Male | 31.1 | 9.7 | 238 | 38.9 | 12.4 | 24,299 | –7.800 | –9.384 to –6.216 | 9.6752 | 24,535 | 0.806 | < 0.00001 | < 0.01 |
| Female | 32.2 | 11.6 | 45 | 44.2 | 15.9 | 8887 | –12.000 | –16.663 to –7.337 | 5.0559 | 8930 | 2.373 | < 0.00001 | < 0.01 |
| All persons | 31.2 | 10.0 | 283 | 40.2 | 13.7 | 33,186 | –9.000 | –10.604 to –7.396 | 11.0262 | 33,467 | 0.816 | < 0.00001 | < 0.01 |
When deprivation, as measured by The English Indices of Deprivation 2019 (Penney, 2019), is taken into consideration, ketamine-related decedents resided on average in less deprived areas than the average NPSAD case dying in England (Figure 3). The indices used here are based on 39 separate indicators, organised across seven distinct domains of deprivation (i.e. income, employment, health deprivation and disability, education and skills training, crime, barriers to housing and services and living environment). These are combined and then weighted to calculate an Index of Multiple Deprivation (Penney, 2019). Data for individual ‘small areas or neighbourhoods’ in which decedents lived were extracted from the Indices of Deprivation 2019 local authority dashboard (https://www.gov.uk/guidance/english-indices-of-deprivation-2019-mapping-resources#indices-of-deprivation-2019-explorer-postcode-mapper). Although there is no substantive research into the relationships between employment, income, housing or deprivation in general in terms of resulting ketamine use, there is some evidence that ketamine use can result in detrimental or negative experiences in respect to economic status, employment and education (Vidal Giné et al., 2016).
Figure 3.
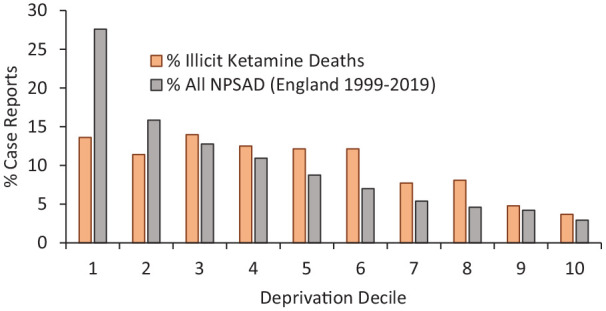
Decile of deprivation where decedents were residing at time of death.
Comparison with other studies
Gill and Stajíc (2000) reviewed 87 ketamine-positive deaths occurring in New York City during 1997–1999. Only 12 were non-hospital deaths due to acute polydrug misuse intoxications; in no case was the fatal intoxication caused exclusively by ketamine. San Nicolas and Lemos (2015) reported on 25 post-mortem cases involving ketamine between 1997 and 2013 in San Francisco. Ketamine on its own was involved in only 8% of cases. The main characteristics were: male (88%), White (75%), mean age 40.1 years and accidental (68%). The commonest drugs found were: amphetamines (32%), cocaine (28%), morphine/codeine (24%), ethanol (16%) and methadone, oxycodone, GHB, diphenhydramine and diazepam (all 12%). In terms of the characteristics of the decedents, the NPSAD findings with regard to gender and ethnicity are similar to those obtaining in San Francisco; however, the average age of the NPSAD sample is about 9 years younger than that of the Californian study. The latter is more in line with the remainder of NPSAD deaths during the period 1999–2019.
Darke et al. (2020) examined 68 cases of self-administered ketamine-related death in Australia during 2000–2019, following principles introduced and advocated by NPSAD over two decades ago, and using similar coronial sources of information. Compared to the Australian cases, NPSAD ketamine-related decedents tended to be younger (31.2 vs. 35.2 years), more likely to be male (84% vs. 77%) and to die of an accidental overdose/toxicity (83% vs. 59%). By contrast, NPSAD fatalities were less likely to be employed (50% vs. 59%), have a history of injecting drug use (13% vs. 41%) and die at home or in a private residential property (63% vs. 72%). The profile of substances identified in toxicology was: opioids (59%), hypnotics/sedatives (58%, diazepam 38%), psychostimulants (50%, methamphetamine 29%, MDMA 24%, cocaine 23%), antidepressants (29%), alcohol (27%) and cannabis (18%).
Just under one-third (29%) of decedents had been prescribed psychoactive drugs, typically suggesting they had mental health issues such as psychoses, anxiety and depression. Furthermore, there were 16 suicides, including hanging or other violent means, echoing the suicide by hanging reported by Dinis-Oliveira et al. (2010), as well the 22 cases reported by Darke et al. (2020), the majority of which were due to deliberate drug toxicity. Indeed six of the 68 cases reported by Darke et al. (2020) had been prescribed ketamine for chronic pain. It is also of note that the proportion of suicides involving ketamine is half that of all NPSAD where other drugs were involved. These findings are of particular interest currently, given that ketamine is now being used to help treat individuals with depression, PTSD and to prevent suicide. Clinicians who prescribe ketamine for self-administration should be alerted to the potential risks of deliberate overdose (Darke et al., 2020), especially where other drugs may be prescribed as drug–drug interactions may occur (see next section).
The mean number of post-mortem drugs for the ketamine cases in our study was 4.6 compared to 3.3 for NPSAD cases as a whole; this reflects the use of other substances with ketamine noted by the BCS (Hoare and Moon, 2010). In other studies, the average/mean number of post-mortem drugs recorded in ketamine-related deaths is not provided. The profile of drugs found at post-mortem overlaps with both those found in San Francisco (San Nicolas and Lemos, 2015) and Australia (Darke et al., 2020). The main substances recorded in the NPSAD post-mortem toxicology were: alcohol (41%), cocaine (41%), heroin/morphine (31%), diazepam (28%), MDMA (20%), cannabis (18%) and methadone (10%). Although there are commonalties in the wide range of substances taken in conjunction with ketamine, there are geographical variations between the three regions compared here.
Safety of ketamine
In this report, we describe 32 cases (Table 7) where ketamine was detected on its own (11.3% of the total), somewhat questioning the high safety profile for ketamine suggested elsewhere (Degenhardt et al., 2005) and contradicts the findings in the other above-mentioned large-scale studies. Although Darke et al. (2020) do not provide any information on whether any fatalities were due solely to ketamine, they do state that fewer than 5% of their cases had only taken ketamine and concede that, outside a controlled clinical setting, ketamine ‘may be toxic when self-administered in uncontrolled dosage and rapid administration’. One could argue, however, that these 32 cases were possibly related to enhanced likelihood of accidents caused by some level of impaired judgement of risk, which was in turn associated with the dissociative effects caused by ketamine. It is possible that the real cause of death, in polysubstance cases, was due to a particular pharmacokinetic interaction (including the possibility of cytochrome P450 activity) and/or to a synergistic effect of the different self-administered drugs (Dinis-Oliveira et al., 2010). In addition, several of these cases were suicides, reflecting the possible impact of ketamine on suicidal ideation noted above.
This study indicates that the underlying cause of death (initial condition or cause in the train of events leading to death, as determined by a pathologist or medical examiner) was typically accidental poisoning/intoxication (Table 4). Unfortunately, underlying (distal) cause of death is not given in other comparable studies (Darke et al., 2020; Gill and Stajíc, 2000; San Nicolas and Lemos, 2015). However, the immediate (proximate) causes of death are in alignment with these studies, for example accidental intoxication.
This study found that the most common manner of death (i.e. the determination by a coroner or medical examiner as to how a disease or injury led to death) recorded in England during the period 1999–2019 for recreational ketamine-related deaths was accidental (83%) and accounted for a much higher proportion of deaths than in comparable studies: San Nicolas and Lemos (2015) = 17/25; Darke et al. (2020) = 40/68, whereas other studies have recorded higher levels of suicides, for example 32% by Darke et al. (2020) compared to our study (< 6%), the case definitions are not identical.
Consumption of ketamine may expose individuals to external factors that contribute to death, such as hypothermia, drowning and smoke inhalation from fires (e.g. Hitchenor and Mills, 2019; Murphy-Bates, 2019). In fact, three deaths noted here took place in nightclubs and another two at ‘raves’, where high temperatures may have been experienced; five deaths also occurred at or shortly after music festivals. One death in this study featured hypothermia. Six of the cases reported by Darke et al. (2020) occurred at festivals dance clubs. Impaired judgement could also play a part in deaths associated with such scenarios, a factor that has also been identified in road traffic accidents, both in the present study and other research (Giorgetti et al., 2015; Li et al., 2011), and accidents whilst under the influence of ketamine (Hansen et al., 1988; Morgan and Curran, 2012).
Breitmeier et al. (2002) suggest that a recreational dose of ketamine is equivalent to a low anaesthetic dose of 50–120 mg IM, and that the therapeutic range is 1.0–6.0 µg/mL. Toxic levels or doses of ketamine do not appear to have been documented to date. The range of fatal levels in blood of ketamine previously reported in the literature range from 2.1 to 27.4 mg/L for cases where ketamine alone was implicated, and from 0.01 to 3.71 mg/L where it was implicated with other substances (Table 8). In the present study, in cases where ketamine was the only substance implicated the mean level was 4.757 (range 0.107–32.000) mg/L, whereas for cases where ketamine was implicated with other substances, the mean was 1.418 (range 0.011–24.730) mg/L. Thus, higher levels of ketamine were implicated in deaths where it was the sole substance, compared to deaths involving ketamine with other substances. Individuals may develop tolerance due to long-term use of ketamine; therefore, it would not be surprising to there being some overlap between higher recreational doses and those associated with death.
Lethality of ketamine
Polysubstance use may mean that a lower level of ketamine may cause or contribute to death than in cases where it is the sole substance implicated, especially if respiratory depression was a mechanism of death. This is more likely to occur if taken with central nervous system depressants such as opiates/opioids and hypnotics (such as benzodiazepines).Table 6 indicates how common such classes were in the NPSAD cases reported here as separate drug classes, but the high proportions of these two specific classes leads to their appearance in combination in many deaths. Ketamine appears to have a lethality index, based on several different approaches, similar to that of stimulants, such as MDMA, amphetamine and mephedrone (King and Corkery, 2018). The ratio in the King and Corkery study was 1:2.659 (44/117) for England and Wales reported by the Office for National Statistics (ONS) for deaths registered between 1993 and 2016; ONS are unable to provide any further updates (personal communication to lead author, 24 September 2020). The ratio of sole to any mention of ketamine in the cause of death fields of the NPSAD cases examined here is 1:9.154 (13/119), suggesting that ketamine was less lethal in the sample examined by the present study. This difference underlines the differences that exist in the coverage, quality and depth of information available to these two types of registries (Corkery et al., 2017).
Data from the UK General Mortality Registers, such as the ONS, show that deaths involving ketamine have continued, despite it being made a Class C drug in 2006 and being reclassified to Class B in 2014, following the recommendation on this point from the ACMD (2013). That said, the number of deaths registered in England and Wales involving ketamine fell in 2010 and remained below their 2009 levels before spiking again in 2014. This was followed by a fall in 2015, but from 2016 to 2018, there was a fourfold increase to a new peak (Figure 4). This overall pattern for deaths registered in a calendar year is broadly similar to the pattern evidenced by NPSAD cases occurring in a calendar, even allowing for other differences such as cases definition, geographical coverage and completeness of reporting.
Figure 4.
UK deaths registered by UK general register offices where ketamine was mentioned in the cause of death.
Deaths resulting from medical treatment employing ketamine may be included in these totals.
Sources: Office for National Statistics (2015, 2016, 2017, 2019a, 2019b); personal communications from Northern Ireland Statistics and Research Agency and National Records of Scotland to lead/corresponding author.
The decline in deaths between 2010 and 2013 may be due, in part, to the appearance of methoxetamine in 2010 and other ketamine derivatives, for example N-ethylnorketamine, 3-MeO-PCE, 3-MeO-PCP in 2012 and/or fears about bladder/urinary problems (Dargan et al., 2014; Wang et al., 2017). However, these analogues quickly lost favour following several deaths in 2012 (Chiappini et al., 2015). (NPSAD recorded 14 deaths involving methoxetamine between 2011 and 2016, eight of them in 2011 and 2012.) These deaths, in part, led to methoxetamine being placed under a Temporary Class Drug Order (TCDO) on 5 April 2012 (Home Office, 2012b), followed by control of methoxetamine and other related ketamine derivatives as Class B substances under the Misuse of Drugs Act 1971 from 26 February 2013 (Home Office, 2013b).
Against a background of increasing and historically high levels of ketamine use across all ages in England and Wales (Flatley, 2019a; NHS Digital, 2019), alertness to possible dangers should be a watchword for potential users of all substances, they may not know what they are consuming. In recent years, ecstasy tablets containing both MDMA and ketamine have been in circulation (Baynes, 2017; Devon & Cornwall Police, 2018), although this is not a new concern, dating back at least a couple of decades (e.g. Cole et al., 2002; Wolff et al., 1995). This may also turn out to be the case in a cluster of recent deaths in the northeast of England (BBC, 2020).
During the period 2010–2018, a total of 116 deaths involving ketamine (unspecified type of use) were registered in England and Wales, an average of 12.88 deaths p.a. During the period 2010/2011 to 2018/2019, in England and Wales, there was an average estimated population, aged 16–59 years, of 176,444 using ketamine in the last year (using CSEW figures). This yields an annual death rate of 7.3 per 100,000 users in this period.
The trends over time in terms of increasing numbers abusing ketamine, seeking treatment for dependence and dying from its recreational use although of concern do not warrant any change in its status under the Misuse of Drugs Act 1971. This is because numbers are still relatively low in the UK context, especially when compared with other Novel Psychoactive Substances such as ‘designer benzos’, synthetic cannabinoids and synthetic cathinones.
The COVID-19 pandemic that emerged towards the start of 2020 may be having an impact on the consumption of ketamine in the UK. There are anecdotal reports of a ketamine drought in northern England (Lawn and Skumlien, 2020). There are indications that drug users in Australia have stopped or reduced their consumption of the drug as a reaction to the pandemic (Peacock et al., 2020; Sutherland et al., 2020). By contrast, it is reported from the USA that some individuals are trying ketamine for the first time in an attempt to block out some of the anxiety caused by the pandemic, whereas others have increased their consumption during longer periods spent at home during lockdowns (Lhooq, 2020). It remains to be seen if any of these changes have been or will be mirrored in the UK.
Strengths and limitations of this study
This study only looked at deaths where the recreational use of ketamine was implicated in or contributed to death, that is, excluded cases where ketamine was present because of medical procedures. That said, the range of deaths was as comprehensive as possible, not being limited to ketamine poisonings or overdoses. To our knowledge, this is the largest case-series of ketamine-related fatalities reported to date.
In England and Wales, the proportion of inquests held into deaths reported to coroners fell from 61% in 1995 to 39% in 2019. During the same period, the proportion of inquests opened in which post-mortem examinations were conducted fell from 98% to 59%. The proportion of post-mortem examinations involving toxicology rose from 13% in 2011 to 24% in 2019 (Ministry of Justice, 2020). It should be noted that at a local level, there are differences in all of the above rates. Taking the national rates outlined above, approximately 9.15% of all deaths reported to coroners in 2019 had toxicology testing done.
It would appear that 94% of forensic laboratories responding to a recent EMCDDA survey have the analytical capacity to routinely screen for ketamine, and another 4% on request; however, typically ketamine is routinely screened for in toxicology tests only in a number of highly specialised laboratories and/or upon specific request (EMCDDA, 2019). Coroners (and procurators fiscal in Scotland) should be encouraged to consider more routine screening for recreational drugs, including ketamine, in unexpected deaths.
Reporting to NPSAD is on a voluntary basis, varying over time in terms of compliance by different coroners’ areas and in terms of geographical coverage. Further cases may be notified as coroners’ staff submit late cases and as inquests on deaths for earlier periods are completed. Further cases may remain to be submitted for inquests already completed. In addition, there may have been further deaths, which have occurred during the period covered but for which the formal investigations have not yet been concluded. The numbers presented should therefore be regarded as a minimum for ketamine.
Further deaths have been registered by the General Register Offices since these data were compiled, but these databases do not capture the wealth of information available to specialist mortality registers, such as NPSAD. Indeed, some of the deaths registered by the General Mortality Registers will include cases where ketamine was administered for medical reasons.
Conclusions
The data presented here considerably extend our previous paper (Schifano et al., 2008) and our contribution to the ACMD’s advice on ketamine (ACMD, 2013), by bringing the information up to date and providing more in-depth analysis. At the time of writing, this paper is the largest published study conducted on deaths related to recreational ketamine use.
Polysubstance consumption is inherently risky and can lead to serious adverse consequences, especially mixing ketamine with other CNS depressants. However, due to geographical variations in use patterns, warnings need to be tailored to the local population(s). Ketamine bought on the street is also likely to contain a range of undeclared psychoactive substances. Ketamine on its own can kill, so caution should be exercised when it is used recreationally.
It is likely that recreational use of ketamine will continue, but hopefully the dangers flagged up by this study may lead to a reduction in avoidable and premature deaths. It should dispel the myth that ketamine-related deaths are rare events.
Acknowledgments
The authors are grateful to Her Majesty’s Coroners in England, Wales, Northern Ireland, Guernsey, Jersey and the Isle of Man for notifying cases to NPSAD. The Department of Health (England) and the National Treatment Agency for Substance Misuse (England) and Public Health England provided part-funding for the work of NPSAD from 2004 to 2019. We are also grateful to staff at the following agencies for providing unpublished data: National Records of Scotland, Northern Ireland Statistics and Research Agency, Office for National Statistics, Police Service of Northern Ireland and Justice Analytical Services, Scottish Government.
Footnotes
Author contributions: The original UK data were compiled and analysed in 2012 by JC on behalf of the then NPSAD team (in alphabetical order: Salvatore Casula, Hugh Claridge (HC), John Corkery (JC), Carla Gimeno Clemente, Christine Goodair (CG); Barbara Loi and Fabrizio Schifano (FS)) for the ACMD’s Ketamine Working Group. Updated data for England were prepared by CG, HC and CC. Data extraction and analyses were undertaken by CC and W-CH. JC undertook additional analysis of NPSAD data, Wedinos data, and compiling the epidemiological information. JC had the original idea and prepared the paper. JC was a co-founder of the NPSAD in 1997; CC is the current Director of the NPSAD programme. The present paper was contributed to and agreed by all named authors.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JC, CC, HC and CG are members of the Novel Psychoactive Substances Committee of the UK’s Advisory Council on the Misuse of Drugs (ACMD). FS was a full ACMD member from 2013 to 2019; JC is also a member of its Technical Committee. JC was also a member of the ACMD Ketamine sub-committee in 2003–2004. All views expressed here are solely those of the authors, and do not necessarily represent those of their employers or of the ACMD.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Department of Health, National Treatment Agency for Substance Misuse (England) and Public Health England provided part-funding of NPSAD 2004–2019.
ORCID iD: John Martin Corkery  https://orcid.org/0000-0002-3849-817X
https://orcid.org/0000-0002-3849-817X
References
- ACMD (2004) ACMD Technical Committee: Report on Ketamine. London: Advisory Council on the Misuse of Drugs. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/119098/ketamine-report.pdf (accessed 2 November 2020). [Google Scholar]
- ACMD (2013) Ketamine: A Review of Use and Harm. London: Advisory Council on the Misuse of Drugs. 10 December. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/264677/ACMD_ketamine_report_dec13.pdf (accessed 2 November 2020). [Google Scholar]
- Adamowicz P, Kala M. (2005) Urinary excretion rates of ketamine and norketamine following therapeutic ketamine administration: Method and detection window considerations. J Anal Toxicol 29(5): 376–382. [DOI] [PubMed] [Google Scholar]
- Anis NA, Berry SC, Burton NR, et al. (1983) The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol 79(2): 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes C. (2017) Woman who died after taking MDMA on night out was young mother-of-two, neighbours say. Evening Standard, 4 September. Available at: https://www.standard.co.uk/news/london/woman-who-died-after-taking-mdma-on-night-out-was-young-mother-neighbours-say-a3626211.html (accessed 2 November 2020).
- BBC (2020) Student drug deaths: Four young people die in North East. BBC, 6 October. Available at: https://www.bbc.co.uk/news/uk-england-tyne-54413820 (accessed 2 November 2020).
- Beck K, Hindley G, Borgan F, et al. (2020) Association of ketamine with psychiatric symptoms and implications for its therapeutic use and for understanding schizophrenia: A systematic literature review and meta-analysis. JAMA Netw Open 3(5): e204693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan RK, Rose MA, Duggan KA. (1997) Evidence for direct interaction of ketamine with alpha 1- and beta 2-adrenoceptors. Clin Exp Pharmacol Physiol 24(12): 923–926. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S. (2011) Chronic ketamine use kills bladder cells. New Scientist, 18 June, 2817. Available at: https://www.newscientist.com/article/mg21028174.100-chronic-ketamine-use-kills-bladder-cells/ (accessed 2 November 2020).
- Black C, Eunson J, Sewel K, et al. (2011) Scottish schools adolescent lifestyle and substance use survey (SALSUS) national report: Smoking, drinking and drug use among 13 and 15 year olds in Scotland in 2010, 20 December. Edinburgh: Ipsos MORI & Information Services Division, Scottish Government. Available at: https://www.isdscotland.org/Health-Topics/Public-Health/SALSUS/Previous-Reports/_docs/2010/SALSUS_2010_National_Report.pdf (accessed 2 November 2020). [Google Scholar]
- Black C, Hutcheson L, Hockaday C. (2019) Scottish Schools Adolescent Lifestyle and Substance Use Survey (SALSUS) Drug Use Report (2018). Edinburgh: The Scottish Government. Available at: https://www.gov.scot/binaries/content/documents/govscot/publications/statistics/2019/11/scottish-schools-adolescent-lifestyle-substance-use-survey-salsus-drug-use-report-2018/documents/scottish-schools-adolescent-lifestyle-substance-use-survey-salsus-drug-use-report-2018/scottish-schools-adolescent-lifestyle-substance-use-survey-salsus-drug-use-report-2018/govscot%3Adocument/scottish-schools-adolescent-lifestyle-substance-use-survey-salsus-drug-use-report-2018.pdf?forceDownload=true (accessed 2 November 2020). [Google Scholar]
- Blundell M, Dargan P, Wood D. (2018) A cloud on the horizon-a survey into the use of electronic vaping devices for recreational drug and new psychoactive substance (NPS) administration. QJM 111(1): 9–14. [DOI] [PubMed] [Google Scholar]
- Breitmeier D, Passie T, Mansouri F, et al. (2002) Autoerotic accident associated with self-applied ketamine. Int J Legal Med 116(2): 113–116. [DOI] [PubMed] [Google Scholar]
- Brown M, Bolling K. (2007) Drugs Misuse in Scotland: Findings from the 2006 Scottish Crime and Victimisation Survey. Edinburgh: The Scottish Government. 26 September. Available at: https://www2.gov.scot/Resource/Doc/198856/0053157.pdf (accessed 2 November 2020). [Google Scholar]
- Canady VA. (2020) FDA approves esketamine treatment for MDD, suicidal ideation. Mental Health Weekly 30(31): 6–7. [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, et al. (2018) Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: Results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry 175(7): 620–630. [DOI] [PubMed] [Google Scholar]
- Caudevilla F, Ventura M, Fornís I, et al. (2016) Results of an international drug testing service for cryptomarket users. Int J Drug Policy 35: 38–41. [DOI] [PubMed] [Google Scholar]
- Centini F, Gabbrielli M, Fineshi V, et al. (1987) Fatal ketamine abuse: Report of a case and analytical determination by gas liquid chromatography, mass spectrometry. In: Piemonte G, Tagliaro F, Marigo M, Frigerio A. (eds) Developments in Analytical Methods in Pharmaceutical, Biomedical and Forensic Sciences. New York, Plenum Press Co, pp.147–153. [Google Scholar]
- Chaves TV, Wilffert B, Sanchez ZM. (2020) The use of ketamine to cope with depression and post-traumatic stress disorder: A qualitative analysis of the discourses posted on a popular online forum. Am J Drug Alcohol Abuse 46(5): 613–624. [DOI] [PubMed] [Google Scholar]
- Chen IC, Lee MH, Chen WC, et al. (2018) Risk factors of lower urinary tract syndrome among ketamine users. Low Urin Tract Symptoms 10(3): 281–286. [DOI] [PubMed] [Google Scholar]
- Cheng JY, Chan DT, Mok VK. (2005) An epidemiological study on alcohol/drugs related fatal traffic crash cases of deceased drivers in Hong Kong between 1996 and 2000. Forensic Sci Int 153(2−3): 196–201. [DOI] [PubMed] [Google Scholar]
- Chiappini S, Claridge H, Corkery JM, et al. (2015) Methoxetamine-related deaths in the UK: An overview. Hum Psychopharmacol 30(4): 244–248. [DOI] [PubMed] [Google Scholar]
- Chu PS, Ma WK, Wong SC, et al. (2008) The destruction of the lower urinary tract by ketamine abuse: A new syndrome? BJU Int 102(11): 1616–1622. [DOI] [PubMed] [Google Scholar]
- Clements JA, Nimmo WS, Grant S. (1982) Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci 71: 539–542. [DOI] [PubMed] [Google Scholar]
- Cohen ML, Trevor AJ. (1974) On the cerebral accumulation of ketamine and the relationship between metabolism of the drug and its pharmacological effects. J Pharmacol Exp Ther 189(2): 351–358. [PubMed] [Google Scholar]
- Cole JC, Bailey M, Sumnall HR, et al. (2002) The content of ecstasy tablets: Implications for the study of their long-term effects. Addiction 97(12): 1531–1536. [DOI] [PubMed] [Google Scholar]
- Colebunders B, Van Erps P. (2008) Cystitis due to the use of ketamine as a recreational drug: A case report. J Med Case Rep 2: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci-D’Amato L, Speranza L, Volpicelli F. (2020) Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci 21(20): 7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazza O. (2008) Near-Death Experiences: Exploring the Mind-Body Connection. London/New York: Routledge. 30 June. [Google Scholar]
- Corazza O, Assi S, Schifano F. (2013) From “special K” to “special M”: The evolution of the recreational use of ketamine and methoxetamine. CNS Neurosci Ther 19(6): 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazza O, Schifano F. (2010) Near-death states reported in a sample of 50 misusers. Subst Use Misuse 45(6): 916–924. [DOI] [PubMed] [Google Scholar]
- Cording CJ, DeLuca R, Camporese T, et al. (1999) A fatality related to the veterinary anesthetic telazol. J Anal Toxicol 23(6): 552–555. [DOI] [PubMed] [Google Scholar]
- Corkery JM, Claridge H, Goodair C, et al. (2017) An exploratory study of information sources and key findings on UK cocaine-related deaths. J Psychopharmacol 31(8): 996–1014. [DOI] [PubMed] [Google Scholar]
- Corkery J, Claridge H, Loi B, et al. (2014) Drug-related deaths in the UK: Annual report 2013. Drug-related deaths reported by Coroners in England, Wales, Northern Ireland, Guernsey, Jersey and the Isle of Man; Police forces in Scotland; & the Northern Ireland Statistics and Research Agency – Annual report January-December 2012, 12 February. London: International Centre for Drug Policy, St George’s University of London. Available at: https://www.re-solv.org/wp-content/uploads/2016/02/Drug-related-deaths-in-the-UK-2013.pdf (accessed 2 November 2020). [Google Scholar]
- Correia-Melo FS, Argolo FC, Araújo-de-Freitas L, et al. (2017) Rapid infusion of esketamine for unipolar and bipolar depression: A retrospective chart review. Neuropsychiatr Dis Treat 13: 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Basu A, Benneyworth M, et al. (2012) Glutamatergic synaptic dysregulation in schizophrenia: Therapeutic implications. Handb Exp Pharmacol 213: 267–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C, Clare T, Sharpe C, et al. (eds) (2018) United Kingdom drug situation: Focal point annual report 2017, March. London: Public Health England. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/713101/Focal_Point_Annual_Report.pdf (accessed 2 November 2020). [Google Scholar]
- Curran HV, Morgan C. (2000) Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 95(4): 575–590. [DOI] [PubMed] [Google Scholar]
- Dalgarno PJ, Shewan D. (1996) Illicit use of ketamine in Scotland. J Psychoactive Drugs 28(2): 191–199. [DOI] [PubMed] [Google Scholar]
- Dargan PI, Tang HC, Liang W, et al. (2014) Three months of methoxetamine administration is associated with significant bladder and renal toxicity in mice. Clin Toxicol (Phila) 2(3): 176–180. [DOI] [PubMed] [Google Scholar]
- Darke S, Duflou J, Farrell M, et al. (2020) Characteristics and circumstances of death related to the self-administration of ketamine. Addiction 116(2): 339–345. [DOI] [PubMed] [Google Scholar]
- Das RK, Gale G, Walsh K, et al. (2019) Ketamine can reduce harmful drinking by pharmacologically rewriting drinking memories. Nat Commun 10(1): 5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Copeland J, Dillon P. (2005) Recent trends in the use of “club drugs”: An Australian review. Subst Use Misuse 40(9−10): 1241–1256. [DOI] [PubMed] [Google Scholar]
- Devon & Cornwall Police (2018) Squeezing drug dealers and new youth concerns. 12 October. Available at: https://www.devon-cornwall.police.uk/your-area/teams/Exeter/Priorities/70ce07c5-b873-4ac1-b5f0-58cd0e121512 (accessed 2 November 2020).
- DiazGranados N, Ibrahim LA, Brutsche NE, et al. (2010. a) Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71(12): 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, et al. (2010. b) A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67(8): 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D, Torrance C. (2010) Mixmag drugs survey. Mixmag 225: 44–53. [Google Scholar]
- Dillon P, Copeland J, Jansen K. (2003) Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend 69(1): 23–28. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Carvalho F, Duarte JA, et al. (2010) Suicide by hanging under the influence of ketamine and ethanol. Forensic Sci Int 202(1−3): e23−e27. [DOI] [PubMed] [Google Scholar]
- Domino EF, Chodoff P, Corssen G. (1965) Pharmacologic effects of CI-581, a new dissociative anesthetic in man. Clin Pharmacol Ther 6: 279–291. [DOI] [PubMed] [Google Scholar]
- Donoghue AC, Roback MG, Cullen KR. (2015) Remission from behavioral dysregulation in a child with PTSD after receiving procedural ketamine. Pediatrics 136(3): e694−e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. (2018) Ketamine and rapid-acting antidepressants: A new era in the battle against depression and suicide. F1000Res 7: F1000 Faculty Rev-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. (2012) Synaptic dysfunction in depression: Potential therapeutic targets. Science 338(6103): 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton G, Davies C, English L, et al. (eds) (2008) United Kingdom drug situation: Annual report to the European monitoring centre for drugs and drug addiction (EMCDDA) 2008. Available at: http://www.emcdda.europa.eu/system/files/publications/524/NR_2008_UK_168554.pdf (accessed 2 November 2020).
- EMA (2019) Spravato. European Medicines Agency. 31 December 2019. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/spravato#authorisation-details-section (accessed 2 November 2020). [Google Scholar]
- EMCDDA (2002) Report on the risk assessment of ketamine in the framework of the joint action on new synthetic drugs, June. Luxembourg: Office for Official publications of the European Communities. Available at: http://www.emcdda.europa.eu/system/files/publications/173/Risk3_62941.pdf_en (accessed 23 November 2020). [Google Scholar]
- EMCDDA (2019) An analysis of post-mortem toxicology practices in drug-related death cases in Europe. Technical report, European Monitoring Centre for Drugs and Drug Addiction, April. Luxembourg: Publications Office of the European Union. Available at: http://www.emcdda.europa.eu/system/files/publications/10544/Analysis%20of%20practices%20of%20PM%20toxicology%20of%20DRD%20in%20Europe_EMCDDA%20Technical%20report.pdf (accessed 2 November 2020). [Google Scholar]
- EMCDDA & Europol (2019) EU drug markets report 2019, November. Luxembourg: Publications Office of the European Union. Available at: http://www.emcdda.europa.eu/system/files/publications/12078/20192630_TD0319332ENN_PDF.pdf (accessed 2 November 2020). [Google Scholar]
- Escarment J, Wey PF, Martinez JY, et al. (2017) An effective tool for preventing suicide in burned combat veterans. J Burn Care Res 38(3): e689. [DOI] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, et al. (2020) Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 25(7): 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2019) FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic. Press Release, 5 March. Silver Spring, MD: U.S. Food & Drug Administration. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm632761.htm (accessed 2 November 2020). [Google Scholar]
- Feder A, Parides MK, Murrough JW, et al. (2014) Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: A randomized clinical trial. JAMA Psychiatry 71(6): 681–688. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KC, Morris B, Soroosh A, et al. (2021) Pilot randomized active-placebo-controlled trial of low-dose ketamine for the treatment of multiple sclerosis–related fatigue. Mult Scler J 27(6): 942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatley J. (ed.) (2019. a) Drug Misuse: Findings from the 2018/19 Crime Survey for England and Wales. Statistical Bulletin 21/19. London: Home Office. 19 September. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832533/drug-misuse-2019-hosb2119.pdf, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832332/drug-misuse-1819-tables.xlsx (accessed 2 November 2020). [Google Scholar]
- Flatley J. (ed.) (2019. b) Seizures of Drugs, England and Wales, Financial Year Ending 2019, 2nd edn. London: Home Office. 5 December. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/870323/seizures-drugs-mar2019-hosb3119.pdf, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/870289/seizures-drugs-mar2019-hosb3119-tables.ods (accessed 2 November 2020). [Google Scholar]
- Fletcher PC, Honey GD. (2006) Schizophrenia, ketamine and cannabis: Evidence of overlapping memory deficits. Trends Cogn Sci 10(4): 167–174. [DOI] [PubMed] [Google Scholar]
- Foster C, Scarlett M, Stewart B. (2016) Young Persons’ Behaviour and Attitude Survey 2016 – Health Modules. Belfast: Department of Health. June. Available at: https://www.health-ni.gov.uk/sites/default/files/publications/health/bulletin-16-ypbas.pdf (accessed 2 November 2020). [Google Scholar]
- Foster C, Scarlett M, Stewart B. (2020) Young Persons’ Behaviour and Attitudes Survey 2019 - Substance Use - (Smoking, Alcohol & Drugs). Belfast: Department of Health. 22 September. Available at: https://www.health-ni.gov.uk/sites/default/files/publications/health/summary-19-ypbas.pdf https://www.health-ni.gov.uk/sites/default/files/publications/health/tables-19-ypbas_1.xlsx (accessed 2 November 2020). [Google Scholar]
- Fujikawa DG. (1995) Neuroprotective effect of ketamine administered after status epilepticus onset. Epilepsia 36(2): 186–195. [DOI] [PubMed] [Google Scholar]
- Gaillard Y, Pépin G. (1998) Evidence of polydrug use using hair analysis: A fatal case involving heroin, cocaine, cannabis, chloroform, thiopental and ketamine. J Forensic Sci 43(2): 435–438. [PubMed] [Google Scholar]
- Gálvez V, Li A, Huggins C, et al. (2018) Repeated intranasal ketamine for treatment-resistant depression - the way to go? Results from a pilot randomised controlled trial. J Psychopharmacol 32(4): 397–407. [DOI] [PubMed] [Google Scholar]
- George D, Gálvez V, Martin D, et al. (2017) Pilot randomized controlled trial of titrated subcutaneous ketamine in older patients with treatment-resistant depression. Am J Geriatr Psychiatry 25(11): 1199–1209. [DOI] [PubMed] [Google Scholar]
- Gerace E, Petrarulo M, Bison F, et al. (2014) Toxicological findings in a fatal multidrug intoxication involving mephedrone. Forensic Sci Int 243: 68–73. [DOI] [PubMed] [Google Scholar]
- Gill JR, Stajíc M. (2000) Ketamine in non-hospital and hospital deaths in New York City. J Forensic Sci 45(3): 655–658. [PubMed] [Google Scholar]
- Giorgetti R, Marcotulli D, Tagliabracci A, et al. (2015) Effects of ketamine on psychomotor, sensory and cognitive functions relevant for driving ability. Forensic Sci Int 252: 127–142. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Torjman MC, Schwartzman RJ, et al. (2010) Pharmacodynamic profiles of ketamine (R)- and (S)- with 5-day inpatient infusion for the treatment of complex regional pain syndrome. Pain Physician 13: 379–387. [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Torjman MC, Schwartzman RJ, et al. (2011) Enantioselective pharmacokinetics of (R)- and (S)-ketamine after a 5-day infusion in patients with complex regional pain syndrome. Chirality 23(2): 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MJ, Booth A. (2009) A typology of reviews: An analysis of 14 review types and associated methodologies. Health Info Libr J 26: 91–108. [DOI] [PubMed] [Google Scholar]
- Gueits C, Witkin M. (2020) Ketamine and serotonin syndrome: A case report. Current Psychiatry 19(3): e1−e2. [Google Scholar]
- Hansen G, Jensen SB, Chandresh L, et al. (1988) The psychotropic effect of ketamine. J Psychoactive Drugs 20(4): 419–425. [DOI] [PubMed] [Google Scholar]
- Hijazi Y, Boulieu R. (2002) Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos 30(7): 853–858. [DOI] [PubMed] [Google Scholar]
- Himmelseher D, Pfenninger E. (1998) Die klinische Anwendung von S-E.,(+)-Ketamin - eine standortbestimmung [The clinical use of S-(+)-ketamine – a determination of its place]. Anaesthesiol Intensivmed Notfallmed Schmerztherapie 33(12): 764–770. [DOI] [PubMed] [Google Scholar]
- Hitchenor T, Mills KA. (2019) Woman, 18, and boyfriend, 21, die in barn conversion after electrical cable overheats. The Mirror, 24 September. Available at: https://www.mirror.co.uk/news/uk-news/woman-18-boyfriend-21-die-20166379 (accessed 3 November 2020).
- Hoare J, Moon D. (ed.) (2010) Drug Misuse Declared: Findings from the 2009/10 British Crime Survey: England and Wales. Home Office Statistical Bulletin 13/10. London: Home Office Research, Development and Statistics. July. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/116321/hosb1310.pdf (accessed 3 November 2020). [Google Scholar]
- Holloway K, Bennett T. (2017) Characteristics and correlates of drug use and misuse among university students in Wales: A survey of seven universities. Addict Res Theory 26(1): 11–19. [Google Scholar]
- Home Office (2005) The misuse of drugs (amendment) (No. 3) regulations 2005. SI 2005 No. 3372. Available at: http://www.legislation.gov.uk/uksi/2005/3372/pdfs/uksi_20053372_en.pdf (accessed 3 November 2020).
- Home Office (2012. a) Drug Misuse Declared: Findings from the 2011/12 Crime Survey for England and Wales, 2nd edn. London: Home Office. 27 September. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/147938/drugs-misuse-dec-1112-pdf.pdf (accessed 3 November 2020). [Google Scholar]
- Home Office (2012. b) The misuse of drugs act 1971 (temporary class drug) order 2012. SI 2012 No. 980. Available at: http://www.legislation.gov.uk/uksi/2012/980/pdfs/uksi_20120980_en.pdf (accessed 3 November 2020).
- Home Office (2013. a) Drug Misuse: Findings from the 2012/13 Crime Survey for England and Wales. London: Home Office. 25 July. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/225122/Drugs_Misuse201213.pdf (accessed on 3 November 2020). [Google Scholar]
- Home Office (2013. b) The misuse of drugs act 1971 (amendment) order 2013. SI 2013 No. 239. Available at: http://www.legislation.gov.uk/uksi/2013/239/pdfs/uksi_20130239_en.pdf (accessed 3 November 2020).
- Home Office (2014) The misuse of drugs act 1971 (ketamine etc.) (amendment) order 2014. SI 2014 No. 1106. Available at: http://www.legislation.gov.uk/uksi/2014/1106/pdfs/uksi_20141106_en.pdf (accessed 3 November 2020).
- Inman L, Carr J, Hupert W, et al. (2012) 2010/11 Scottish Crime and Justice Survey: Drug Use. Edinburgh: The Scottish Government. 26 March. Available at: https://www2.gov.scot/Resource/0039/00390472.pdf, https://www2.gov.scot/Resource/0039/00391934.xls (accessed 3 November 2020). [Google Scholar]
- Irifune M, Sato T, Kamata Y, et al. (2000) Evidence for GABA(A) receptor agonistic properties of ketamine: Convulsive and anesthetic behavioral models in mice. Anesth Analg 91(1): 230–236. [DOI] [PubMed] [Google Scholar]
- Jansen KL. (2000) A review of the nonmedical use of ketamine: Use, users and consequences. J Psychoactive Drugs 32(4): 419–433. [DOI] [PubMed] [Google Scholar]
- Jansen KLR. (2004) Ketamine: Dreams and Realities, 2nd edn. Sarasota, FL: Multidisciplinary Assoc. For Psychedelic Studies. Available at: https://www.maps.org/images/pdf/books/K-DreamsKJansenMAPS.pdf (accessed 3 November 2020). [Google Scholar]
- Javitt DC, Zukin SR, Heresco-Levy U, et al. (2012) Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 38: 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhang JF, Hsu YH, Kuo HC. (2015) Possible pathophysiology of ketamine-related cystitis and associated treatment strategies. Int J Urol 22(9): 816–825. [DOI] [PubMed] [Google Scholar]
- Jones JL, Mateus CF, Malcolm RJ, et al. (2018) Efficacy of ketamine in the treatment of substance use disorders: A systematic review. Front Psychiatry 9: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman K, van Rijnsoever E, Olofsen E, et al. (2018) Esketamine counters opioid-induced respiratory depression. Br J Anaesth 120(5): 1117–1127. [DOI] [PubMed] [Google Scholar]
- Kalsi SS, Wood DM, Dargan PI. (2011) The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use. Emerg Health Threats J 4: 7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlow N, Schlaepfer CH, Stoll CRT, et al. (2018) A systematic review and meta-analysis of ketamine as an alternative to opioids for acute pain in the emergency department. Acad Emerg Med 25(10): 1086–1097. [DOI] [PubMed] [Google Scholar]
- King LA, Corkery JM. (2018) An index of fatal toxicity for new psychoactive substances. J Psychopharmacol 32(7): 793–801. [DOI] [PubMed] [Google Scholar]
- Koons C, Edney A. (2019) First big depression advance since prozac nears FDA approval. Bloomberg.com, 12 February. Available at: https://www.bloomberg.com/news/articles/2019-02-12/first-big-depression-advance-since-prozac-nears-fda-approval (accessed 3 November 2020).
- Krupitsky EM, Burakov AM, Dunaevsky IV, et al. (2007) Single versus repeated sessions of ketamine-assisted psychotherapy for people with heroin dependence. J Psychoactive Drugs 39(1): 13–19. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Grinenko AY. (1997) Ketamine psychedelic therapy (KPT): A review of the results of ten years of research. J Psychoactive Drugs 29(2): 165–183. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, et al. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51(3): 199–214. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Krupitsky E, et al. (2003) NMDA receptor antagonism and the ethanol intoxication signal: From alcoholism risk to pharmacotherapy. Ann N Y Acad Sci 1003: 176–184. [DOI] [PubMed] [Google Scholar]
- Lalonde BR, Wallage HR. (2004) Postmortem blood ketamine distribution in two fatalities. J Anal Toxicol 28(1): 71–74. [DOI] [PubMed] [Google Scholar]
- Lawn W, Skumlien M. (2020) How is the COVID-19 pandemic changing our use of illegal drugs? An overview of ongoing research. Society for the Study of Addiction Blog, 28 May. Available at: https://www.addiction-ssa.org/how-is-the-covid-19-pandemic-changing-our-use-of-illegal-drugs-an-overview-of-ongoing-research/ (accessed 27 March 2021).
- Lhooq M. (2020) People Are Using Ketamine at Home to Escape Their Pandemic Reality. Vice. 4 November. Available at: Available at: https://www.vice.com/en/article/akddya/people-are-using-ketamine-to-escape-pandemic-reality-covid-19 (accessed 27 March 2021). [Google Scholar]
- Li F, Liu J, Yip PSF, et al. (2020) Mortalities of methamphetamine, opioid, and ketamine abusers in Shanghai and Wuhan, China. Forensic Sci Int 306: 110093. [DOI] [PubMed] [Google Scholar]
- Li JH, Vicknasingam B, Cheung YW, et al. (2011) To use or not to use: An update on licit and illicit ketamine use. Subst Abuse Rehabil 2: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata M, Pierini G, Popoli G. (1994) A fatal ketamine poisoning. J Forensic Sci 39(5): 1314–1320. [PubMed] [Google Scholar]
- Liriano F, Hatten C, Schwartz TL. (2019) Ketamine as treatment for post-traumatic stress disorder: A review. Drugs Context 8: 212305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Clark AJ, Sawynok J, et al. (2005) Topical amitriptyline and ketamine in neuropathic pain syndromes: An open-label study. J Pain 6(10): 644–649. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Winstock A, Hunt N. (2007) 5-Year trends in use of hallucinogens and other adjunct drugs among UK dance drug users. Eur Addict Res 13(1): 57–64. [DOI] [PubMed] [Google Scholar]
- MacLeod P, Page L. (2011) 2009/10 Scottish Crime and Justice Survey: Drug Use. Edinburgh: The Scottish Government. 14 February. Available at: https://www2.gov.scot/Resource/Doc/933/0113642.pdf, https://www2.gov.scot/Resource/Doc/933/0112298.xls (accessed 3 November 2020). [Google Scholar]
- MacLeod P, Page L, Kinver A, et al. (2010) 2008-09 Scottish Crime and Justice Survey: Drug Use. Edinburgh: The Scottish Government. 19 February. Available at: https://www2.gov.scot/Resource/Doc/303253/0095109.pdf, https://www2.gov.scot/Resource/Doc/933/0094658.xls (accessed 3 November 2020). [Google Scholar]
- Malinovsky JM, Servin F, Cozian A, et al. (1996) Ketamine and norketamine plasma concentrations after i.v., nasal and rectal administration in children. Br J Anaesth 77(2):203–207. [DOI] [PubMed] [Google Scholar]
- Mason K, Cottrell AM, Corrigan AG, et al. (2010) Ketamine-associated lower urinary tract destruction: A new radiological challenge. Clin Radiol 65(10): 795–800. [DOI] [PubMed] [Google Scholar]
- Ministry of Justice (2020) Coroners Statistics 2019: England and Wales. London: Ministry of Justice. 29 May. Available at: https://www.gov.uk/government/publications/coroners-statistics-2019/coroners-statistics-2019-england-and-wales (accessed 3 November 2020). [Google Scholar]
- Mion G, Le Masson J, Granier C, et al. (2017) Ketamine for the prevention of posttraumatic stress disorder in burned patients. J Burn Care Res 38(3): e690. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kilbane EM, Jones R, et al. (1997) Tissue distribution of ketamine in a mixed drug fatality. J Forensic Sci 42(6): 1183–1185. [PubMed] [Google Scholar]
- Moore K, Measham F. (2006) Ketamine use: Minimising problems and maximising pleasure. Drugs Alcohol Today 6(3): 29–32. [Google Scholar]
- Morgan CJ, Curran HV. (2006) Acute and chronic effects of ketamine upon human memory: A review. Psychopharmacology (Berl) 188(4): 408–424. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV; Independent Scientific Committee on Drugs (2012) Ketamine use: A review. Addiction 107(1): 27–38. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. (2009) Ketamine use, cognition and psychological wellbeing: A comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction 104(1): 77–87. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. (2010) Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: A 1-year longitudinal study. Addiction 105(1): 121−133. Erratum in: Addiction 105(4): 766. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Rossell SL, Pepper F, et al. (2006) Semantic priming after ketamine acutely in healthy volunteers and following chronic self-administration in substance users. Biol Psychiatry 59(3): 265–272. [DOI] [PubMed] [Google Scholar]
- Murphy-Bates S. (2019) Champion swimmer, 18, and her boyfriend, 21, died when blaze tore through their barn conversion as they slept after taking cocktail of drugs. Daily Mail, 24 September. Available at: https://www.dailymail.co.uk/news/article-7498107/Champion-swimmer-18-boyfriend-21-roasted-death-barn-went-flames.html (accessed 3 November 2020).
- Murrough JW, Iosifescu DV, Chang LC, et al. (2013) Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiatry 170(10): 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadesan K, Kumari C, Afiq M. (2017) Dancing to death: A case of heat stroke. J Forensic Leg Med 50: 1–5. [DOI] [PubMed] [Google Scholar]
- Narcotics Control Bureau (2013) Annual report 2013. New Delhi: Narcotics Control Bureau, Ministry of Home Affairs, Government of India. Available at: http://narcoticsindia.nic.in/upload/download/document_id8d09e4b85c783cbc30c9b8ae175f2d33.pdf (accessed 27 October 2019). [Google Scholar]
- Ng SH, Tse ML, Ng HW, et al. (2010) Emergency department presentation of ketamine abusers in Hong Kong: A review of 233 cases. Hong Kong Med J 16(1): 6–11. [PubMed] [Google Scholar]
- NHS Digital (2019) Smoking, drinking and drug use among young people in England 2018. NHS Digital, 20 August. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/smoking-drinking-and-drug-use-among-young-people-in-england/2018 https://files.digital.nhs.uk/EE/E28E35/sdd-2018-tab8.xlsx (accessed 3 November 2020).
- NICE (2020. a) Nasal Spray Medicine for Treatment-Resistant Depression Not Recommended by NICE. London: National Institute for Health and Care Excellence. 18 January. Available at: https://www.nice.org.uk/news/article/nasal-spray-medicine-for-treatment-resistant-depression-not-recommended-by-nice (accessed 3 November 2020). [Google Scholar]
- NICE (2020. b) Esketamine for Treatment-Resistant Depression (ID1414). London: National Institute for Health and Care Excellence. 3 September. Available at: https://www.nice.org.uk/guidance/indevelopment/gid-ta10371/consultation/html-content-3 (accessed 3 November 2020). [Google Scholar]
- Nicol AU, Morton AJ. (2020) Characteristic patterns of EEG oscillations in sheep (Ovis aries) induced by ketamine may explain the psychotropic effects seen in humans. Sci Rep 10: 9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppers I, Niesters M, Aarts L, et al. (2010) Ketamine for the treatment of chronic non-cancer pain. Expert Opin Pharmacother 11(14): 2417–2429. [DOI] [PubMed] [Google Scholar]
- NPIS (2020) National Poisons information service report 2019/20, October. London: Public Health England. Available at: https://www.npis.org/Download/NPIS%20Report%202019-20.pdf (accessed 26 October 2020). [Google Scholar]
- ONS (2015) Drug-related deaths registered in England and Wales in 2014. Ad hoc data request No 005096, released 10 December 2015, Office for National Statistics, Newport Gwent. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/005096drugrelateddeathsregisteredinenglandandwalesin2014 Drug-related deaths registered in England and Wales in 2014 (accessed 3 November 2020).
- ONS (2016) Drug related deaths involving new psychoactive substances, England and Wales, 2015 registrations. Ad hoc data request No 006158, released 29 September 2016, Office for National Statistics, Newport Gwent. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/adhocs/006158drugrelateddeathsinvolvingnewpsychoactivesubstancesenglandandwales2015registrations, https://www.ons.gov.Uk/file?uri=/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/adhocs/006158drugrelateddeathsinvolvingnewpsychoactivesubstancesenglandandwales2015registrations/drugrelateddeaths2015final.xls (accessed 3 November 2020).
- ONS (2017) Deaths related to drug poisoning involving specific substances, England and Wales, deaths registered in 2016. Ad hoc data request No 007307, released 4 August 2017, Office for National Statistics, Newport Gwent. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/007307deathsrelatedtodrugpoisoninginvolvingspecificsubstancesenglandandwalesdeathsregisteredin2016, https://www.ons.gov.Uk/file?uri=/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/007307deathsrelatedtodrugpoisoninginvolvingspecificsubstancesenglandandwalesdeathsregisteredin2016/2016drugrelateddeathsbyspecificsubstances.xls (accessed 3 November 2020).
- ONS (2019. a) Deaths related to drug poisoning involving specific substances, England and Wales, deaths registered in 2017. Ad hoc data request No 009738, released 6 March 2019, Office for National Statistics, Newport Gwent. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/009738deathsrelatedtodrugpoisoninginvolvingspecificsubstancesenglandandwalesdeathsregisteredin2017, https://www.ons.gov.Uk/file?uri=/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/009738deathsrelatedtodrugpoisoninginvolvingspecificsubstancesenglandandwalesdeathsregisteredin2017/finalfile.xls (accessed 3 November 2020).
- ONS (2019. b) Deaths related to drug poisoning involving specific substances, England and Wales, deaths registered in 2018. Ad hoc data request No 10582, released 23 September 2019, Office for National Statistics, Newport Gwent. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/10582deathsrelatedtodrugpoisoninginvolvingspecificsubstancesenglandandwalesdeathsregisteredin2018, https://www.ons.gov.Uk/file?uri=/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/adhocs/10582deathsrelatedtodrugpoisoninginvolvingspecificsubstancesenglandandwalesdeathsregisteredin2018/selectedsubstances.xls (accessed 3 November 2020). [Google Scholar]
- Øye I, Paulsen O, Maurset A. (1992) Effects of ketamine on sensory perception: Evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 260(3): 1209–1213. [PubMed] [Google Scholar]
- Palamar JJ, Salomone A, Rutherford C, et al. (2021) Extensive underreported exposure to ketamine among electronic dance music party attendees. J Gen Intern Med 36(1): 235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin MC, Turfus SC, Smith NW, et al. (2008) Detection of ketamine and its metabolites in urine by ultra high pressure liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 876(1): 137–142. [DOI] [PubMed] [Google Scholar]
- Pavarin RM, Marani M, Turino E. (2019) Ketamine abusers referring to emergency departments in northern Italy: A cross-sectional study. Ann Ist Super Sanità 55(4): 338–344. [DOI] [PubMed] [Google Scholar]
- Peacock A, Price O, Dietze P, et al. (2020) Impacts of COVID-19 and Associated Restrictions on People Who Use Illicit Stimulants in Australia: Findings from the Ecstasy and Related Drugs Reporting System 2020. Drug Trends Bulletin Series. Sydney: National Drug and Alcohol Research Centre, UNSW. Available at: https://ndarc.med.unsw.edu.au/sites/default/files/ndarc/resources/COVID%20EDRS%20bulletin_National_20200917.pdf (accessed 19 October 2020). [Google Scholar]
- Penney B. (2019) The English Indices of Deprivation 2019 (IoD2019). London: Ministry of Housing, Communities & Local Government. 26 September. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/835115/IoD2019_Statistical_Release.pdf (accessed 1 September 2020). [Google Scholar]
- Peyton SH, Couch AT, Bost RO. (1988) Tissue distribution of ketamine: Two case reports. J Anal Toxicol 12(5): 268–269. [DOI] [PubMed] [Google Scholar]
- PHE (2020) Adult substance misuse treatment statistics 2019 to 2020: Report, 26 November. London: Public Health England. Available at: https://www.gov.uk/government/statistics/substance-misuse-treatment-for-adults-statistics-2019-to-2020/adult-substance-misuse-treatment-statistics-2019-to-2020-report, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/955313/NDTMS_annual_report_supporting_tables_2019-20.ods (accessed 27 March 2021). [Google Scholar]
- PHE (2021) Young people’s substance misuse treatment statistics 2019 to 2020: Report, 28 January. London: Public Health England. Available at: https://www.gov.uk/government/statistics/substance-misuse-treatment-for-young-people-statistics-2019-to-2020/young-peoples-substance-misuse-treatment-statistics-2019-to-2020-report#trends-over-time, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/955863/NDTMS_YP_Annual_Report_Data_Tables_2019-20.xlsx (accessed 27 March 2021). [Google Scholar]
- Pomarol-Clotet E, Honey GD, Murray GK, et al. (2006) Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry 189: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Iosifescu DV, Murrough JW, et al. (2014) Effects of ketamine on explicit and implicit suicidal cognition: A randomized controlled trial in treatment-resistant depression. Depress Anxiety 31(4): 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiner EA. (2007) Imaging of striatal dopamine release elicited with NMDA antagonists: Is there anything there to be seen? J Psychopharmacol 21(3): 253–258. [DOI] [PubMed] [Google Scholar]
- Reich DL, Silvay G. (1989) Ketamine: An update on the first twenty-five years of clinical experience. Can J Anaesth 36(2): 186–197. [DOI] [PubMed] [Google Scholar]
- Ricci V, Martinotti G, Gelfo F, et al. (2011) Chronic ketamine use increases serum levels of brain-derived neurotrophic factor. Psychopharmacology 215: 143–148. [DOI] [PubMed] [Google Scholar]
- Robertson L. (2016) Scottish Crime and Justice Survey 2014/15: Drug Use. Edinburgh: The Scottish Government. 28 June. Available at: https://www.gov.scot/publications/scottish-crime-justice-survey-2014-15-drug-use/, https://www2.gov.scot/Resource/0050/00502480.xls (accessed 3 November 2020). [Google Scholar]
- Robertson L, Bates E. (2014) Scottish Crime and Justice Survey 2012/13: Drug Use. Edinburgh: The Scottish Government. 24 June. Available at: https://www.gov.scot/binaries/content/documents/govscot/publications/statistics/2014/06/scottish-crime-justice-survey-2012-13-drug-use/documents/scottish-crime-justice-survey-2012-13-drug-use-report/scottish-crime-justice-survey-2012-13-drug-use-report/govscot%3Adocument/00455131.pdf?forceDownload=true, https://www2.gov.scot/Resource/0045/00454707.xls (accessed 3 November 2020). [Google Scholar]
- Robson MJ, Elliott M, Seminerio MJ, et al. (2012) Evaluation of sigma (r) receptors in the antidepressantlike effects of ketamine in vitro and in vivo. Eur Neuropsychopharmacol 22(4): 308–317. [DOI] [PubMed] [Google Scholar]
- Rowland LM. (2005) Subanesthetic ketamine: How it alters physiology and behavior in humans. Aviat Space Environ Med 76(7 Suppl): C52−C58. [PubMed] [Google Scholar]
- San Nicolas AC, Lemos NP. (2015) A survey of human performance and post-mortem cases involving ketamine in San Francisco between 1997 and 2013. Toxicology Section, Abstract K68. American academy of forensic sciences 2015 annual meeting, Orlando, Florida, 16−21 February 2015. Proc Am Acad Forensic Sci 21: 1141−1142. Available at: https://www.aafs.org/common/Uploaded%20files/Resources/Proceedings/2015_Proceedings.pdf (accessed 8 September 2020). [Google Scholar]
- Schifano N, Chiappini S, Castiglione F, et al. (2020) Is medicinal ketamine associated with urinary dysfunction issues? Assessment of both the European Medicines Agency (EMA) and the UK Yellow Card Scheme pharmacovigilance database-related reports. Low Urin Tract Symptoms. Epub ahead of print 9 October 2020. DOI: 10.1111/luts.12355. [DOI] [PubMed] [Google Scholar]
- Schifano F, Corkery J, Deluca P, et al. (2006) Ecstasy (MDMA, MDA, MDEA, MBDB) consumption, seizures, related offences, prices, dosage levels and deaths in the UK (1994-2003). J Psychopharmacol 20(3): 456–463. [DOI] [PubMed] [Google Scholar]
- Schifano F, Corkery J, Oyefeso A, et al. (2008) Trapped in the “K-hole”: Overview of deaths associated with ketamine misuse in the UK (1993-2006). J Clin Psychopharmacol 28(1): 114–116. [DOI] [PubMed] [Google Scholar]
- Scott-Ham M, Burton FC. (2005) Toxicological findings in cases of alleged drug-facilitated sexual assault in the United Kingdom over a 3-year period. J Clin Forensic Med 12(4): 175–186. [DOI] [PubMed] [Google Scholar]
- Scottish Government (2019) Scottish Crime and Justice Survey 2017-2018: Main Findings. Edinburgh: Justice Directorate, Scottish Government. 26 March. Available at: https://www.gov.scot/publications/scottish-crime-justice-survey-2017-18-main-findings/, https://www.gov.scot/binaries/content/documents/govscot/publications/statistics/2019/03/scottish-crime-justice-survey-2017-18-main-findings/documents/scottish-crime-justice-survey-2017-18-main-findings/scottish-crime-justice-survey-2017-18-main-findings/govscot%3Adocument/scottish-crime-justice-survey-2017-18-main-findings.pdf?forceDownload=true (accessed 3 November 2020). [Google Scholar]
- Scottish Medicines Consortium (2020) Esketamine (Spravato). Glasgow: Scottish Medicines Consortium. 7 September. Available at: https://www.scottishmedicines.org.uk/medicines-advice/esketamine-spravato-full-smc2258/ (accessed 26 March 2021). [Google Scholar]
- Sehdev RS, Symmons DA, Kindl K. (2006) Ketamine for rapid sequence induction in patients with head injury in the emergency department. Emerg Med Australas 18(1): 37–44. [DOI] [PubMed] [Google Scholar]
- Sherman SJ, Estevez M, Magill AB, et al. (2016) Case reports showing a long-term effect of subanesthetic ketamine infusion in reducing l-DOPA-induced dyskinesias. Case Rep Neurol 8(1): 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AN, Di Vincenzo JD, Brietzke E, et al. (2021) Antisuicidal and antidepressant effects of ketamine and esketamine in patients with baseline suicidality: A systematic review. J Psychiatr Res 137: 426–436. [DOI] [PubMed] [Google Scholar]
- Sinner B, Graf BM. (2008) Ketamine. Handb Exp Pharmacol 182: 313–333. [DOI] [PubMed] [Google Scholar]
- Smith KM, Larive LL, Romanelli F. (2002) Club drugs: Methylenedioxymethamphetamine, flunitrazepam, ketamine hydrochloride, and gamma-hydroxybutyrate. Am J Health Syst Pharm 59(11): 1067–1076. [DOI] [PubMed] [Google Scholar]
- Smith DJ, Pekoe GM, Martin LL, et al. (1980) The interaction of ketamine with the opiate receptor. Life Sci 26(10): 789–795. [DOI] [PubMed] [Google Scholar]
- SOCA (2013) An Intelligence Update on Ketamine. London: Serious and Organised Crime Agency, cited in ACMD (2013). [Google Scholar]
- Sutherland R, Baillie G, Memedovic S, et al. (2020) Key Findings from the ‘Australians’ Drug Use: Adapting to Pandemic Threats (ADAPT)’ Study. ADAPT Bulletin No. 1. Sydney: National Drug and Alcohol Research Centre, UNSW. Available at: https://6d4c02d1-3362-4c6f-a837-b46833d5b1a5.filesusr.com/ugd/8a9f74_c264d95a82f14b0fbd68031668d6d77b.pdf (accessed 19 October 2020). [Google Scholar]
- Tao Y, Chen XP, Qin ZH. (2005) A fatal chronic ketamine poisoning. J Forensic Sci 50(1): 173–176. [PubMed] [Google Scholar]
- Thegooddrugsguide (2019) Ketamine – Dosage. Available at: https://www.thegooddrugsguide.com/ketamine/dosage.htm (accessed 3 November 2020).
- Thurtle N, Abouchedid R, Archer JRH, et al. (2017) Prevalence of use of electronic nicotine delivery systems (ENDS) to vape recreational drugs by club patrons in South London. J Med Toxicol 13(1): 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimbos Institute (2017) Annual Report 2017. Drugs Information and Monitoring System (DIMS). Utrecht, Netherlands: Trimbos Institute. Available at: https://assets-sites.trimbos.nl/docs/b182f333-9363-4fef-9273-ffa050e1a1fe.pdf (accessed 3 November 2020). [Google Scholar]
- Tsai TH, Cha TL, Lin CM, et al. (2009) Ketamine-associated bladder dysfunction. Int J Urol 16(10): 826–829. [DOI] [PubMed] [Google Scholar]
- UAHS (2018) UA Clinical Trial to Repurpose Ketamine for Parkinson’s Patients. The University of Arizona Health Sciences. 18 July. Available at: http://uahs.arizona.edu/news/ua-clinical-trial-repurpose-ketamine-parkinsons-patients (accessed 3 November 2020). [Google Scholar]
- UK Focal Point on Drugs (2020) United Kingdom drug situation: Focal point annual report, 10 August. London: Public Health England. Available at: https://www.gov.uk/government/publications/united-kingdom-drug-situation-focal-point-annual-report/uk-drug-situation-2019-summary, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/908403/UK_Focal_Point_annual_report_2019_data_tables.ods (accessed 3 November 2020). [Google Scholar]
- UNODC (2018. a) Myanmar hosts talks on Asia Pacific strategy to control drug making chemicals. Press Release, 7 November. Vienna: United Nations Office for Drugs and Crime. Available at: http://www.unodc.org/southeastasiaandpacific/en/myanmar/2018/11/high-level-regional-precursor-control-conference/story.html (accessed 3 November 2020). [Google Scholar]
- UNODC (2018. b) World Drug Report 2018, volume 3 analysis of drug markets, 26 June. Vienna: United Nations Office on Drugs and Crime. Available at: http://www.unodc.org/pdf/opioids-crisis/WDR18_Booklet_3_DRUG_MARKETS.PDF (accessed 3 November 2020). [Google Scholar]
- UNODC (2019) World Drug Report 2019, volume 5 Cannabis and hallucinogens, 26 June. Vienna: United Nations Office on Drugs and Crime. Available at: https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_5_CANNABIS_HALLUCINOGENS.pdf (accessed 3 November 2020). [Google Scholar]
- Uosukainen H, Tacke U, Winstock AR. (2015) Self-reported prevalence of dependence of MDMA compared to cocaine, mephedrone and ketamine among a sample of recreational poly-drug users. Int J Drug Policy 26(1): 78–83. [DOI] [PubMed] [Google Scholar]
- Vice (2014) The Sad Demise of Nancy Lee, One of Britain’s Ketamine Casualties. Vice. 23 July. Available at: https://www.vice.com/en_uk/article/av4njj/ketamine-slowly-ruins-your-bladder-and-kills-you-863 (accessed 3 November 2020). [Google Scholar]
- Vidal Giné C, Fernández Calderón F, López Guerrero J. (2016) Patterns of use, harm reduction strategies, and their relation to risk behaviour and harm in recreational ketamine users. Am J Drug Alcohol Abuse 42(3): 358–369. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wu Q, Wang J, et al. (2017) Ketamine analog methoxetamine induced inflammation and dysfunction of bladder in rats. Int J Mol Sci 18(1): E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson J. (2015) Postmortem toxicology of ketamine. In: Yew DT. (ed.) Ketamine: Use and Abuse. Abingdon, Oxon: CRC Press, pp.243–248. [Google Scholar]
- Weiner AL, Vieira L, McKay CA, et al. (2000) Ketamine abusers presenting to the emergency department: A case series. J Emerg Med 18(4): 447–451. [DOI] [PubMed] [Google Scholar]
- Wieber J, Gugler R, Hengstmann JH, et al. (1975) Pharmacokinetics of ketamine in man. Anaesthesist 24(6): 260–263. [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, et al. (2018) Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 175(12): 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock A. (2011) The 2011 Mixmag drugs survey. Mixmag Magazine, March. Available at: https://issuu.com/mixmagfashion/docs/drugsurvey (accessed 3 November 2020).
- Winstock A. (2012) Mixmag global drug survey. Mixmag Magazine. Available at: https://issuu.com/mixmagfashion/docs/drugs_survey_2012_2 (accessed 3 November 2020).
- Winstock A. (2013) Mixmag global drug survey 2013. Mixmag Magazine, May. Available at: https://issuu.com/mixmagfashion/docs/mm_may13_drug_survey (accessed 3 November 2020).
- Winstock A. (2015. a) The Global Drug Survey 2014 Findings - Reflections on the Results of the World’s Biggest Ever Drug Survey. Global Drug Survey. 7 June. Available at: https://www.globaldrugsurvey.com/the-global-drug-survey-2014-findings/ (accessed 3 November 2020).
- Winstock AR. (2015. b) The Global Drug Survey 2015 Findings. Global Drug Survey. Available at: https://www.globaldrugsurvey.com/the-global-drug-survey-2015-findings/ (accessed 3 November 2020). [Google Scholar]
- Winstock A, Barratt M, Ferris J, et al. (2016) Global drug survey 2016: What we learned from GDS2016 – An overview of our key findings. Available at: https://www.globaldrugsurvey.com/wp-content/uploads/2016/06/TASTER-KEY-FINDINGS-FROM-GDS2016.pdf (accessed 3 November 2020).
- Winstock A, Barratt M, Ferris J, et al. (2017) Global drug survey 2017: Global overview and highlights. Available at: https://www.globaldrugsurvey.com/wp-content/themes/globaldrugsurvey/results/GDS2017_key-findings-report_final.pdf (accessed 3 November 2020).
- Winstock AR, Barrett MJ, Maier LJ, et al. (2019) Global drug survey (GDS) 2019 key findings report, 16 May. London: Global Drug Survey. Available at: https://issuu.com/globaldrugsurvey/docs/gds2019_key_findings_report_may_16_ (accessed 3 November 2020). [Google Scholar]
- Winstock AR, Barratt MJ, Maier LJ, et al. (2018) GDS2018 key findings report, 9 May. London: Global Drug Survey. Available at: https://www.globaldrugsurvey.com/gds-2018/ (accessed 3 November 2020). [Google Scholar]
- Winstock AR, Mitcheson L. (2012) New recreational drugs and the primary care approach to patients who use them. BMJ 344: e288. [DOI] [PubMed] [Google Scholar]
- Woelfer M, Li M, Colic L, et al. (2020) Ketamine-induced changes in plasma brain-derived neurotrophic factor (BDNF) levels are associated with the resting-state functional connectivity of the prefrontal cortex. World J Biol Psychiatry 21(9): 696–710. [DOI] [PubMed] [Google Scholar]
- Wolff K. (2016) Ketamine: The pharmacokinetics and pharmacodynamics in misusing populations. In: Wolff K, White J, Karch S. (eds) The SAGE Handbook of Drug & Alcohol Studies: Biological Approaches. Thousand Oaks, CA: Sage, pp.177–193. [Google Scholar]
- Wolff K, Hay AW, Sherlock K, et al. (1995) Contents of “ecstasy”. Lancet 346(8982): 1100–1101. [DOI] [PubMed] [Google Scholar]
- Wolff K, Winstock AR. (2006) Ketamine - From medicine to misuse. CNS Drugs 20(3): 199–218. [DOI] [PubMed] [Google Scholar]
- Wood DM, Bishop CR, Greene SL, et al. (2008) Ketamine-related toxicology presentations to the ED. Abstract 122. Abstracts of the 2008 North American Congress of Clinical Toxicology annual meeting, 11−16 September 2008, Toronto, Canada. Clin Toxicol 46(7): 630. [Google Scholar]
- Wood D, Cottrell A, Baker SC, et al. (2011) Recreational ketamine: From pleasure to pain. BJU Int 107(12): 1881–1884. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Puchnarewicz M, et al. (2012. a) Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine. Eur J Clin Pharmacol 68(5): 853–856. [DOI] [PubMed] [Google Scholar]
- Wood DM, Measham F, Dargan PI. (2012. b) ‘Our favourite drug’: Prevalence of use and preference for mephedrone in the London night-time economy 1 year after control. J Subst Use 17(2): 91–97. [Google Scholar]
- Zhang C, Xu Y, Zhang B, et al. (2020) Cognitive impairment in chronic ketamine abusers. Psychiatry Res 291: 113206. [DOI] [PubMed] [Google Scholar]


