Abstract
This report describes the rationale, purpose and design of A011801 (CompassHER2 RD), an ongoing prospective, multicenter, Phase III randomized trial. Eligible patients in the United States (US) and Canada with high-risk (defined as ER-negative and/or node-positive) HER2-positive (HER2+) residual disease (RD) after a predefined course of neoadjuvant chemotherapy and HER2-directed treatment are randomized 1:1 to adjuvant T-DM1 and placebo, versus T-DM1 and tucatinib. Patients have also received adjuvant radiotherapy and/or endocrine therapy, if indicated per standard of care guidelines. The primary objective of the trial is to determine if the invasive disease-free survival (iDFS) with T-DM1 plus tucatinib is superior to iDFS with T-DM1 plus placebo; other outcomes of interest include overall survival (OS), breast cancer-free survival (BCFS), distant recurrence-free survival (DRFS), brain metastases-free survival (BMFS) and disease-free survival (DFS). Correlative biomarker, quality of life (QoL) and pharmacokinetic (PK) end points are also evaluated.
Keywords: : HER2-positive early breast cancer, postneoadjuvant, residual disease, T-DM1, tucatinib
Lay abstract
In this research study (A011801; CompassHER2 RD), patients with early stage HER2-positive breast cancer who already received treatment with chemotherapy and anti-HER2 targeted therapies followed by surgery are mainly enrolled. If cancer is still present in the breast and/or lymph nodes at the time of surgery, there is a higher risk of a recurrence in the future, and enrollment on A011801 is an option. Usually, if there is tumor remaining after chemotherapy and anti-HER2 targeted therapies, the main treatment is the use of an FDA-approved intravenous drug called T-DM1. Additional treatment may also include radiotherapy and/or medications to block the activity of estrogen. The usual treatment approach reduces the likelihood of breast cancer recurring in the future. This study has been performed to answer the following question: Is the combination of T-DM1 and a newer drug tucatinib better than usual treatment with T-DM1 alone at preventing cancer from returning? Study participants will receive treatment with T-DM1 and placebo (a pill that looks like the study drug but contains no medication) or T-DM1 and tucatinib, for up to 14 cycles, unless their breast cancer returns or the side effects become too severe. Research bloods are taken on study along with standard blood work, and we also request a stored tumor sample from the original biopsy and from the breast cancer surgery for research purposes. Optional Quality of Life Questionnaires are also included in the trial. After the study, participants finish T-DM1 and placebo, or T-DM1 and tucatinib, and their doctor will continue to follow their condition with clinic visits every 6 months for 10 years and watch for side effects and for signs of breast cancer recurring.
Clinical Trial Registration: NCT04457596 (ClinicalTrials.gov).
HER2+ breast cancer (BC) represents 10–15% of invasive BC, and historically it was associated with an aggressive clinical course and a poor prognosis [1]. The advent of the HER2-targeted monoclonal antibody trastuzumab was a major therapeutic breakthrough [2]; landmark trials evaluating the efficacy of chemotherapy plus trastuzumab versus chemotherapy alone showed striking improvements in progression-free survival (PFS) and overall survival (OS; ∼50% reduction in recurrence and ∼30% improvement in survival) in both adjuvant and metastatic HER2+ BC. These data quickly established trastuzumab-based therapy as a standard of care [1,3–5], and currently there are eight US FDA-approved HER2-targeted therapies used in clinical oncology practice [6]. Over the last two decades, polychemotherapy with trastuzumab defined the standard neoadjuvant treatment for HER2+ BC. As neoadjuvant trials evaluating dual or sequential HER2-targeted therapy demonstrated superior oncologic outcomes [7–11], the majority of neoadjuvant regimens for HER2+ BC now include 2 or 3 cytotoxic chemotherapeutic agents and 1 to 3 HER2-targeted therapies, and the duration of treatment is typically 1 or 2 years. Given the improved outcomes overall in HER2+ early breast cancer (EBC), subsequent studies have demonstrated outstanding outcomes from ‘de-escalated’ systemic therapy (e.g., less chemotherapy, shorter duration of HER2-targeted therapy) in lower risk HER2+ disease, with the goal of mitigating toxicities and maintaining excellent oncologic outcomes [12–14]. Conversely, treatment escalation is an important consideration for patients who remain at a high risk of relapse and death despite optimal systemic therapy. For example, patients with residual, invasive, HER2+ BC after neoadjuvant systemic therapy (NAST) on the NeoALTTO and cancer and leukemia Group B (CALGB) 40601 trials had recurrence rates of 35% and 16% at 6 and 7 years, respectively [7,15]. Thus, escalation in this population has been a focus of recent research efforts.
In the Phase III KATHERINE trial (ClinicalTrials.gov: NCT01772472; n = 1486), patients with residual, invasive, HER2+ BC were randomized 1:1 to receive 14 cycles of postneoadjuvant T-DM1 or trastuzumab [16]. At 3-year follow-up, patients who received T-DM1 had a 50% reduction of invasive disease recurrence or death compared with those who received trastuzumab (hazard ratio: 0.5; 95% CI: 0.39–0.64; p < 0.001). These notable results led to the FDA approval of postneoadjuvant T-DM1 in this setting and established a new standard of care. Despite these improvements, it was noted that patients with ER-negative residual disease (RD) or node-positive disease who received T-DM1 in KATHERINE still had inferior oncologic outcomes, with 3-year invasive disease-free survival (iDFS) rates of 82% and 83%, respectively; whereas patients with ER-positive, node negative disease had a 3-year iDFS of ∼91% [16]. Further, the incidence of CNS relapses was similar in the T-DM1 and control arms of KATHERINE (∼5 % each), demonstrating that the effective prevention and treatment of breast cancer brain metastases (BCBM) in this setting is an unmet clinical need. Given the relative inability of several HER2-directed therapies and chemotherapy to penetrate the blood–brain barrier (BBB), patients with residual invasive HER2+ BC after NAST remain at high risk of breast cancer brain metastases (BCBM) [17], a devastating diagnosis that is difficult to treat. Therefore, more effective treatment options to prevent both systemic and CNS recurrence is a research priority. While prevention of recurrence is of paramount importance, understanding the cost from a patient toxicity standpoint is also critical, particularly when benefits may be modest.
The major prognostic biomarker in KATHERINE, and in the recently activated Alliance for Clinical Trials in Oncology A011801 trial, (CompassHER2 RD: postneoadjuvant T-DM1 + tucatinib/placebo in patients with residual HER2-positive invasive breast cancer; ClinicalTrials.gov: NCT04457596) is RD after NAST, which has been associated with a less favorable prognosis based on results from several neoadjuvant trials in HER2+ EBC [7,18–20]. However, because most patients with RD do not relapse, accurate identification of high-risk patients is important to personalize treatment decisions [21]. Predictive and prognostic biomarkers could assist oncologists to identify patients at the highest risk of recurrence and to tailor postneoadjuvant therapy accordingly. Although no biomarker has demonstrated clinical utility in HER2+ EBC beyond HER2 and HR status [22], there is increasing evidence supporting the clinical validity of intrinsic subtyping by PAM50 analysis and stromal tumor infiltrating-lymphocytes (TILs) [23,24]. Specifically, either the HER2-E subtype or high TILs appears to be associated with a high response to HER2-targeted treatments in the neoadjuvant setting. Further, the presence of TILs in tumor tissue has been associated with prognostic outcomes in breast cancer [25]. Krop et al. performed a comprehensive biomarker analysis on 1023 patient samples from the Phase III APHINITY trial (ClinicalTrials.gov: NCT01358877), which randomized 4805 patients with HER2+ EBC to adjuvant chemotherapy and trastuzumab, plus pertuzumab or placebo. It was noted that higher levels of immune markers and HER2 appeared to be associated with better prognosis and greater benefit from trastuzumab and pertuzumab [26]. Other potential biomarkers of response that might be useful to tailor HER2-targeted therapy in the curative intent setting include HER2 levels and heterogeneity, HER3 and DNA mutations [27].
The oral potent HER2-specific tyrosine kinase inhibitor (TKI) tucatinib is approved for later-line therapy of HER2+ metastatic breast cancer (MBC). Tucatinib selectively inhibits HER2, compared with other HER2-specific TKIs, which are dual inhibitors of both EGFR and HER2. Based on encouraging preclinical data and results from early-phase trials [28,29], the randomized Phase II HER2CLIMB study (ClinicalTrials.gov: NCT02614794) was launched; results were reported in late 2019 and led the way for the April 2020 FDA approval of tucatinib (TUKYSA™), in combination with trastuzumab and capecitabine in patients with HER2+ MBC. In HER2CLIMB, 612 persons with HER2+ advanced breast cancer who had progressed on trastuzumab, pertuzumab and T-DM1 were randomized 2:1 to receive trastuzumab, capecitabine and tucatinib versus trastuzumab and capecitabine alone [30]. Forty-eight percent of participants had brain metastases at enrollment, including a significant proportion with active metastases. Progression-free survival (PFS) at 1 year was 33.1% in the tucatinib arm and 12.3% in the placebo arm (HR: 0.54; 95% CI: 0.42 to 0.71; p < 0.001), and the median PFS duration was 7.8 and 5.6 months, respectively. OS at 2 years was 44.9% in the tucatinib arm and 26.6% in the placebo arm (HR: 0.66; 95% CI: 0.50 to 0.88; p = 0.005), and the median OS was 21.9 and 17.4 months, respectively. In the BCBM subgroup, PFS at 1 year was 24.9% in the tucatinib arm and 0% in the placebo arm (HR: 0.48; 95% CI: 0.34 to 0.69; p < 0.001), and the median PFS was 7.6 and 5.4 months, respectively. Common side events in the tucatinib arm included diarrhea, palmar–plantar erythrodysesthesia, nausea, fatigue and vomiting. Diarrhea and elevated aminotransferase levels ≥grade 3 were more common in the tucatinib arm than in the placebo arm. Given the remarkable activity of tucatinib, trastuzumab and capecitabine in persons with pretreated HER2+ MBC, including those with active or stable BCBM, evaluation of tucatinib in other settings, including the HER2+ postneoadjuvant space, was a logical progression.
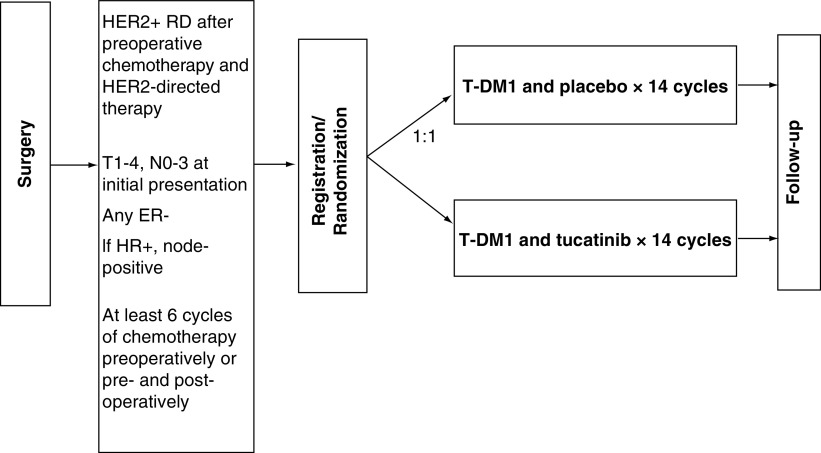
In the CompassHER2 trials (comprehensive use of pathologic response assessment to escalate and de-escalate therapy in HER2-positive breast cancer), it is anticipated that use of preoperative single-agent taxane and dual HER2-targeted therapy with trastuzumab and pertuzumab (THP) will enable use of pathologic complete response (pCR) as a functional biomarker to determine the subsequent risk of recurrence and to tailor additional therapy in clinical stage II–III HER2+ BC. Although scientifically linked and developed in tandem, CompassHER2 pCR and CompassHER2 RD are separate trials. In CompassHER2 pCR (EA1181, led by ECOG-ACRIN; ClinicalTrials.gov: NCT04266249), the goal is systemic therapy de-escalation if there is a pCR after NAST with THP. This article focuses on CompassHER2 RD (A011801, led by the Alliance). In CompassHER2 RD, eligible patients with high-risk residual HER2+ disease defined as any residual ER-negative disease or node-positive ER-positive disease) are randomized to adjuvant T-DM1 and placebo, versus adjuvant T-DM1 and tucatinib (Figure 1). The primary aim of CompassHER2 RD is to further improve iDFS outcomes in this high-risk patient population. Further, along with CompassHER2 pCR, A011801 will evaluate rational approaches to de-escalating and escalating therapy in HER2+ BC, and will address several important questions:
-
1.
For patients with hormone receptor (HR)-negative HER2+, or high-risk HR-positive HER2+ BC who do not achieve a pCR after NAST, can treatment with T-DM1 and tucatinib (+/- endocrine therapy, if applicable) improve outcomes compared to adjuvant T-DM1 therapy and placebo (+/- endocrine therapy, if applicable)?
-
2.
Can we identify biomarkers in tumor, blood, or the microenvironment to enhance our ability to estimate patient prognoses after HER2-directed therapy, or identify persons who benefit from adjuvant T-DM1 and tucatinib versus T-DM1 and placebo?
-
3.
From the patient's perspective, does the addition of tucatinib to T-DM1 result in increased symptomatic toxicity and a reduced quality of life compared with T-DM1 and placebo?
Figure 1. . A011801 (CompassHER2 RD) study scheme.
1 cycle = 21 days.
HR: Hormone receptor; RD: Residual disease.
Construction & content
Study design
A011801 is a prospective, double-blinded, multicenter, Phase III superiority trial being conducted in the US and Canada (ClinicalTrials.gov: NCT04457596). It is anticipated that ∼15–30% of enrollees have participated in EA1181, and therefore will have received a de-escalated neoadjuvant regimen (4 cycles of THP). EA1181 patients or patients treated on a similar preoperative de-escalation trial must complete ≥2 additional cycles of adjuvant chemotherapy (doxorubicin and cyclophosphamide [AC] or THP), prior to enrolling on CompassHER2 RD. Most participants in A011801 will have received a standard neoadjuvant chemotherapy and HER2-directed therapy regimen; therefore, it is likely that they will have completed ≥6 cycles of NAST preoperatively and may enroll directly on CompassHER2 RD postoperatively. Based on the adjuvant APHINITY trial, which noted only a minimal (1–2%) absolute benefit of pertuzumab added to trastuzumab and chemotherapy [10], A011801 permits, but does not require, pertuzumab use preoperatively. Radiotherapy and endocrine therapy, if recommended, can be given in conjunction with adjuvant T-DM1 and tucatinib/placebo. Further, selected patients who have commenced/completed radiotherapy and/or who have already commenced adjuvant T-DM1 may also be candidates for this study. The target accrual is 1031 patients, who are enrolled across >500 sites in the US; A011801 is anticipated to open in Canada under the auspices of the Canadian Cancer Trials Group (CCTG) in the fall of 2021.
Patients are randomized 1:1 to receive 14 cycles of T-DM1 and placebo or 14 cycles of T-DM1 and tucatinib. Comprehensive guidelines for dose modifications for both T-DM1 and tucatinib, if required, are accessible in the A011801 study protocol. If indicated, patients will also receive radiation therapy and/or endocrine therapy per standard of care guidelines. The study assessments include routine tests and observations, ECHO/MUGA every 3 months, adverse event (AE) assessments, review of patient medication diaries and standard of care laboratory testing. If available, tumor tissue obtained pre- and post-NAST will be evaluated for tumor infiltrating lymphocytes (TIL) levels (mandatory). Blood will be submitted for mandatory circulating biomarker and PK analysis. Tissue and blood samples for biobanking are optional and may include intrinsic subtyping of tumor tissue and ctDNA analyses (pending a future protocol amendment).
Primary & secondary objectives
The primary objective of A011801 is to determine if the iDFS with T-DM1 and tucatinib are superior to the iDFS in the control arm (T-DM1 + placebo) when administered to high-risk patients with HER2+ RD after NAST. The secondary objectives are to evaluate whether treatment with T-DM1 plus tucatinib compared with T-DM1 plus placebo improves OS, breast cancer free survival (BCFS), distant recurrence-free survival (DRFS), disease-free survival (DFS) and brain metastases-free survival (BMFS) in A011801 participants. The study will also evaluate whether treatment with T-DM1 plus tucatinib compared with T-DM1 plus placebo reduces the incidence of BCBM.
Correlative biomarker & local regional exploratory objectives
The objectives of correlative biomarkers include the following: (1) the association of TIL levels in both the primary tumor and the RD specimen with iDFS; (2) whether there is differential treatment benefit of T-DM1 and tucatinib versus T-DM1 and placebo in high versus low TIL cancers (assessed in both preneoadjuvant and residual cancer tissue); (3) the association between iDFS and the presence of detectable circulating tumor cells (CTC at baseline, at completion of study therapy and/or 1 year after completion of study therapy and (4) the difference in absolute magnitude of benefit of tucatinib (in terms of iDFS) in patients with detectable CTC at baseline versus in patients without detectable CTC at baseline. The local regional exploratory objectives are to determine local regional recurrence following breast conservation (i) based on margin width (no ink on tumor, close, >2 mm), and (ii) based on treatment, with or without radiation boost.
Eligibility criteria
Patients who satisfy the following key inclusion criteria are eligible for enrollment on A011801:
-
1.
HER2+ EBC based on pretreatment biopsy material and defined as an immunohistochemistry (IHC) score of 3+ and/or positive by in situ hybridization (ISH) according to current ASCO/CAP guidelines.
-
2.
Patients with residual HR-negative, HER2+ disease in the breast and/or lymph nodes per the surgical pathology report are eligible; however, patients with HR-positive HER2+ cancers must have node-positive residual disease to qualify for the study.
-
3.
Prior neoadjuvant chemotherapy with one of the following regimens: THP, TMP, AC-TH(P); TCH(P); FAC-TH(P), or FEC-TH(P).
-
4.
Prior treatment with ≥6 cycles of chemotherapy and HER2-directed therapy, total duration ≥12 weeks, including ≥9 weeks of preoperative taxane and trastuzumab with or without pertuzumab (or FDA-approved biosimilars). Patients who received ≥9 weeks of preoperative taxane, pertuzumab and margetuximab are eligible if they received ≥6 cycles of chemotherapy prior to enrollment.
-
5.
All systemic chemotherapy should have been completed preoperatively unless participating in EA1181 (CompassHER2 pCR) or the BIG DECRESCENDO trial (which is very similar to EA1181 in terms of the study design, drugs and eligibility criteria).
-
6.
Patients who participated in EA1181 or received similar preoperative treatment and proceeded to surgery immediately after the de-escalated trial regimen must receive postoperative chemotherapy to complete a total of ≥6 cycles of systemic treatment prior to enrollment on A011801 (e.g., 4 cycles preoperatively and 2 cycles post-operatively).
-
7.For EA1181 participants, both of the following points must be true.
-
–An interval of no more than 12 weeks may have elapsed between the completion date of the last definitive treatment (which includes postoperative chemotherapy) and the date of registration, AND
-
–Patients must be registered on study within ≤180 days of the date of the most recent definitive breast cancer surgery (not including reconstructive surgery).
-
–
-
8.
Patients who received NAST which included experimental HER2-directed therapy are potentially eligible, however HER2-targeted antibody–drug conjugates (e.g., T-DM1 or trastuzumab deruxtecan) or HER2-targeted TKIs (e.g., tucatinib, lapatinib, neratinib) are not permitted.
-
9.
Patients may have received ≤1 cycle of T-DM1 in the postneoadjuvant setting prior to enrolling on A011801.
Statistical analyses
This trial is a two-arm, multicenter, randomized, double-blinded, placebo-controlled, Phase III superiority trial to compare the efficacy and safety of standard postneoadjuvant therapy with T-DM1 and placebo (14 cycles) compared with T-DM1 and tucatinib (14 cycles) in patients with HER2+ BC who have RD present in the breast and/or axillary lymph nodes following NAST. Eligible patients will be randomized 1:1 to receive either T-DM1 and placebo or T-DM1 and tucatinib. Patients on both arms will receive endocrine therapy and/or radiation as well if indicated per standard of care guidelines. The primary end point is iDFS.
Sample size, accrual time and study duration
The total sample size is 981 evaluable patients based on a two-tailed test of hypothesis at a two-sided alpha level of 0.05 and with 80% power. We assumed that the average monthly accrual rate will be 25 patients and that the 3-year iDFS rate for the control arm (T-DM1 and placebo) is 82%. The minimum detectable hazard ratio is 0.70. There will be one interim analysis for futility, which will occur when 50% of the iDFS events have been observed.
The final analysis will be performed when 248 iDFS events are observed. Up to a 5% over-accrual will be allowed to account for patients who cancel/withdraw prior to treatment or are lost to follow-up. The maximum accrual to the trial will be 1031 patients with a goal of 981 evaluable patients. Stratification factors are: (i) receipt of postoperative CT (Y/N), (ii) hormone receptor status (+/-), (iii) pathologic lymph node status (+/-). The study targets an absolute difference of 5% in the 3-year iDFS rate (control vs experimental arm iDFS 82% and 87%, respectively). It is expected that patients will be recruited over 40 months; projected study duration 83 months.
BMFS is defined as the time from randomization to documentation of involvement of the CNS by metastatic cancer including parenchymal brain and spinal cord metastases as well as leptomeningeal carcinomatosis. Patients who are alive at the time of analysis without documentation of a brain metastasis as defined above will be censored at the time of last follow-up for disease status. Patients who died prior to any brain-metastases recurrence will be censored at date of death. Patients will be followed for 10 years after registration or until death, whichever comes first.
Recruitment plan
Currently, 735+ sites in the National Cancer Trials Network (NCTN) are participating in A011801; CCTG sites will open later in 2021. The projected accrual rate is 25 patients per month; this is likely feasible given the current rate of accrual to EA1181 and planned participation of all North American cooperative groups. Further, in the post-KATHERINE era, the concept of postneoadjuvant therapy improving outcomes in patients with HER2+ RD after NAST is widely accepted. Given the knowledge that selected high-risk patients who receive adjuvant T-DM1 still have suboptimal iDFS outcomes, oncologists and eligible patients will likely be highly motivated to participate in A011801 when clinically appropriate.
Study variables
The following variables are collected and reviewed for all patients: patient demographics; study site characteristics; general medical history and baseline concomitant medications; BC diagnosis history; disease status and characteristics; ECOG performance status; NAST regimen administered; specimen submission; treatment intervention; adverse events (AE); as well as clinical and survival follow-up data. Data will be collected from the treating providers' routine clinical assessments per local standard of care at registration. Optional substudy A011801-HO1 participants will complete questionnaires (booklet or ePRO) addressing (i) Quality of Life (QoL) by use of the Functional Assessment of Cancer Therapy-Breast [FACT-B], (ii) symptoms by use of the Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE™) and (iii) adherence to oral therapy (DOSE-Nonadherence) at registration, cycle (C) 2D1, C5D1, C9D1, C14D1 and at 18 and 24 months post-registration. Consenting patients will provide blood samples pretreatment, after the completion of treatment, one year after the completion of treatment and at recurrence (if applicable) for additional correlative analyses (including ctDNA; Table 1).
Table 1. . A011801 study calendar.
| Prior to registration† | Day 1 of each 21-day cycle (-2 or +3 days) | Every 4 cycles (+/- 7 days) | Post-treatment follow-up‡ | At recurrence, withdrawal or removal‡ | |
|---|---|---|---|---|---|
| Tests & observations | |||||
| – History and physical, weight, PS§ | X | X | X | X | |
| – Height | X | ||||
| – Pulse, blood pressure | X | X | |||
| – Echo/MUGA | X¶ | X¶ | X¶ | ||
| – Adverse event assessment | X# | X# | |||
| – Patient medication diary | X†† | ||||
| Laboratory studies | |||||
| – Complete blood count, differential, platelets | X | X | |||
| – Serum creatinine | X | X | |||
| – Albumin, glucose | X | X | |||
| – AST, ALT, ALP, Bilirubin | X | ¶¶ | |||
| – Serum HCG | X‡‡ | ||||
| – Tissue submission for TIL analysis (if available) | X§§ | ||||
| – Blood submission for CTC and PK analysis | X§§ | ||||
| Correlative studies: for patients who consent to participate | |||||
| – QOL assessment | See Section 6.3 for a schedule of QOL assessments. To be completed using ePRO. See Section 4.3.2 and Appendix I for further instructions. |
||||
| – Tissue and blood samples for biobanking | See Section 6.2 for a schedule of tissue and blood sample submission. | ||||
To be completed ≤28 days before registration: All laboratory studies, history and physical.
Labs completed prior to registration may be used for day 1 of cycle 1 tests if obtained ≤21 days prior to treatment. For subsequent cycles, labs, scans, tests and observations may be obtained no more than 3 days prior to day 1 of treatment.
ECHO or MUGA is required 30 days after the last dose of study treatment, unless already done within 12 weeks prior. Physical examinations are required every 6 months (+/- 30 days) until 10 years after registration or a CNS disease event. Thereafter, survival information is required every 12 months until 10 years following registration. See also Section 12.0.
Drug dosages need not be changed unless the calculated dose changes by ≥10% or weight changes by >10%.
The same test (ECHO or MUGA) should be used to monitor cardiac function for an individual patient during the trial.
Please refer to Appendix XI and Section 9.1 for PRO-CTCAE items (for patients who consent to A011801-HO1).
The diary must begin the day the patient starts taking the medication and must be completed per protocol and returned to the treating institution OR compliance must be documented in the medical record by any member of the care team.
For women of childbearing potential (see Section 3.2). Must be done ≤7 days prior to registration.
See Sections 6.2 and 14.2.
To be collected on day 1 of each cycle and on day 12 of cycles 1 and 2 (+/- 2 days).
Correlative biomarker analyses
A011801 has two secondary (and mandatory) correlative biomarker objectives, involving the studies of TILs and CTCs outlined below. Biobanking is optional. Additionally, the trial aims to include several to-be-determined correlative objectives, with the goal of evaluating the association of ctDNA tumor-specific mutations, tumor intrinsic subtypes and other transcriptional signatures with iDFS.
CTC analyses
The presence of RD after NAST is a negative prognostic factor in EBC (including HER2+ disease) [31], but not all patients relapse. Additional biomarkers to identify patients at the highest risk of recurrence would be clinically useful and may assist with adjuvant therapy selection. Based on the available evidence [32], we hypothesize that the detection of CTCs in blood will be associated with iDFS in A011801. Although the CTC detection rate has been traditionally low in EBC, the Epic Sciences platform enables detection of CTCs with high sensitivity. In A011801, peripheral blood is collected for CTC analysis at (1) baseline (pretreatment); (2) after completion of study therapy, (3) 1 year after completion of study therapy and (4) at recurrence (if applicable). These tests are mandatory for A011801 participants. We hypothesize that (i) patients with detectable CTC at baseline will have inferior iDFS compared to patients without detectable CTC at baseline, (ii) the magnitude of benefit of the addition of tucatinib will be greater in patients with detectable CTC at baseline than in patients without detectable CTC at baseline and (iii) patients with detectable CTC after completing study therapy and/or 1 year after completing study therapy will have inferior iDFS compared to patients without detectable CTC at both time points.
TILs analyses
An association between the detection of TILs in tumor tissue and improved prognostic outcomes in BC has been described [33]. There are limited data regarding the significance of TIL levels in the residual disease specimen after preoperative systemic therapy [34–38], therefore there is interest in studying the role of TILs in patients treated with NAST, in both the pretreatment tumor tissue and in RD after therapy [36]. In A011801, we will evaluate the association of TIL levels in both the primary tumor and the RD specimen with iDFS and determine whether TIL levels are associated with treatment benefit of T-DM1 and tucatinib versus T-DM1 and placebo. We hypothesize that: (1) patients with higher TILs in preneoadjuvant tumor tissue will have superior iDFS compared to patients with lower TILs; (2) the benefit of tucatinib compared to placebo will be greater in patients with increased TIL levels in preneoadjuvant tumor tissue; (c) Patients with higher TILs in residual cancer tissue (postneoadjuvant therapy) will have superior iDFS compared to patients with lower TILs and d) the benefit of tucatinib compared to placebo will be greater in patients with increased TIL levels in residual cancer tissue. The objectives are to determine (i) the association of TIL levels in both the primary tumor and the RD specimen with iDFS and (ii) whether there is differential treatment benefit of T-DM1 and tucatinib compared to T-DM1 and placebo in high TIL cancers compared to low TIL cancers (assessed in both the preneoadjuvant tumor and the residual cancer tissue).
Patient reported outcomes & quality-of-life outcomes
A011801 has one optional substudy (A011801-HO1), which assesses QoL, self-reported patient adherence to the assigned treatment arm, reasons for nonadherence and patient-reported symptoms. The primary and secondary objectives are to compare QoL between the A011801 study arms after approximately 8 and 13 cycles of treatment, respectively, as assessed by the FACT-B Trial Outcome Index. It is hypothesized that QoL will be non-inferior in the T-DM1 + tucatinib arm compared to the T-DM1 + placebo arm at cycle 8, day 1 and cycle 13 day 1, respectively. Exploratory objectives will aim to compare the study arms as it relates to (i) various QoL domains after ∼8 and ∼13 cycles of treatment (5 subscales of FACT-B questionnaire), (ii) patient adherence and reasons for nonadherence after 1, 4, 8 and 13 cycles (DOSE-Nonadherence instrument) and (iii) symptomatic adverse events after 1, 4, 8 and 13 cycles (PRO-CTCAE).
A011801 is powered to detect an absolute difference in the 3-year iDFS rate of 5% between the investigational and placebo arms. However, a survey of BC advocates conducted when the study was initially designed showed that a 3% difference in iDFS would be considered clinically meaningful. Finding this difference is not feasible within the study timeframe and with the anticipated recruited numbers. Therefore, there is a preplanned joint analysis with the Breast International Group (BIG), to determine whether a smaller benefit of the investigational therapy is observed, which would allow for the identification of smaller differences deemed meaningful by patient advocates. If a smaller benefit is noted, the results of the QoL analysis could inform clinical decision making.
Pharmacokinetic objectives
The PK objectives of A011801 are to characterize the PK of (i) T-DM1 in all patients; (ii) tucatinib in tucatinib-treated patients, and (iii) to investigate exposure–effect (efficacy and safety) relationships in tucatinib-treated patients. PK labs for T-DM1 and tucatinib will be drawn immediately before T-DM1 infusions on C2 and C3 D1, PK labs for T-DM1 will be drawn again after the infusion at both time points. Individual (subject) tucatinib and T-DM1 concentrations at each sampling time will be listed; corresponding summary statistics will also be calculated. Additional exploratory population PK and exposure–response analyses will be conducted by Seagen Inc. and specified separately in a pharmacometrics analysis plan.
Ethical considerations
A011801 is conducted in accordance with local legal and regulatory requirements to ensure protection of patients' personal data, including collection of informed consent. Separate consent is required for biospecimen acquisition for correlative analyses, as well as for the A011801-HO1 substudy. Additionally, A011801 has been reviewed and approved by the Alliance for Clinical Trials in Oncology Scientific Review Committee, the National Cancer Institute (NCI) central Institutional Review Board and applicable local institutional review boards prior to local study activation. Of note, emergency unblinding is permitted if an A011801 participant relapses with intra and/or extracranial metastases to determine if they received tucatinib or placebo, as this would influence subsequent systemic therapy choices.
Governance structure
A011801 is supported by the NCI Cancer Therapy Evaluation Program (CTEP) and Seagen Inc. The study is sponsored and led by the NCI funded Alliance for Clinical Trials in Oncology (ALLIANCE) with participation of other NCI funded NCTN groups. A collaboration with ECOG-ACRIN also exists, as they are leading the scientifically related CompassHER2 pCR trial (EA1181), the objectives of which are complementary to those of A011801. The trial's registrational intent is sponsored by the Alliance Breast Committee and study leadership. The Breast Committee, study leadership, Alliance central operations office and Alliance Statistics and Data Center work together to provide expert guidance regarding all A011801 proceedings, including the monitoring, analysis and interpretation of clinical trial data. Scientific oversight is provided by the Alliance Breast Committee and study leadership and their specific responsibilities include the provision of expert guidance as regards the protocol design and subsequent amendments to achieve the study objectives; ensuring the scientific merit of the study; providing input regarding the plans for the statistical analysis; as well as reviewing and appraising abstracts and manuscripts describing the study findings. The Breast Committee members have well defined roles and meet at least twice annually or more frequently as required.
Methodological limitations
Several of the methodological limitations inherent to any prospective randomized clinical trial (RCT) will apply to A0111801 (e.g., missing, inaccurate, or incomplete data, selection or enrollment bias). However, the RCT remains the most effective way to determine the effectiveness of the study intervention (i.e., the additive benefit of tucatinib and T-DM1 compared with T-DM1 and placebo) as the processes used during the conduct of a RCT minimize the risk of confounding factors impacting the results. In addition, this is a double-blind trial, so patients and physicians will not know the arm to which a patient was assigned. However, it is possible that the blinding is compromised due to observed adverse events. However, this would only occur rarely, if at all. Finally, the patients may not want to be randomized with the chance of receiving placebo. The proportion of patients unwilling to be randomized will be relatively small and have had successful experience randomizing patients to placebo in other Alliance trials.
Utility & discussion
Major advances have been made in the management of patients with HER2+ BC over the past two decades, with unprecedented improvements in DFS and OS in the era of HER2-targeted therapy. However, although treatment with postneoadjuvant T-DM1 has improved overall iDFS for patients with residual invasive disease after NAST, those with ER-negative or lymph node-positive disease have inferior iDFS outcomes despite optimal systemic therapy and may benefit from trials evaluating therapy escalation. Further, as the incidence of BCBM was not improved with postneoadjuvant T-DM1 compared with trastuzumab alone in KATHERINE [16], there is a great need to incorporate novel systemic therapy options with better CNS penetrance into our treatment regimens (e.g., novel HER2-targeted TKIs, antibody–drug conjugates, others). A011801 aims to further improve iDFS for patients with RD after NAST by randomizing participants to T-DM1 and tucatinib or T-DM1 and placebo; the incidence of BCBM in both treatment arms will be recorded. Given the results from HER2CLIMB, which showed notable PFS and OS gains in a pretreated population of patients with HER2+ MBC (∼50% of whom had BCBM, mostly active) [30], it is hoped that the addition of tucatinib to postneoadjuvant T-DM1 will improve iDFS and reduce the incidence of BCBM. Our correlative analyses may identify the patients most likely to benefit from tucatinib and may also determine the magnitude of benefit they receive; these valuable insights could allow oncologists to further individualize therapy in this setting. Other postneoadjuvant trials in HER2+ RD include ClinicalTrials.gov: NCT04197687, a Phase II study that aims to evaluate immune-related biomarkers for pCR in HER2+ BC. Participants with RD after NAST are randomized 2:1 to receive either T-DM1 and a multiepitope vaccine, or T-DM1 and placebo; the primary study end points are iDFS and safety. In the Phase III DESTINY-Breast05 trial (ClinicalTrials.gov: NCT04622319), patients with HER2+ BC with RD after NAST are randomized to postneoadjuvant T-DM1 or to trastuzumab deruxtecan (which was FDA approved for the treatment of HER2+ MBC in 2020); the primary end point is iDFS. Results from these important trials will be beneficial for future research in high-risk HER2+ BC and potentially lead to FDA approvals in the postneoadjuvant space.
Conclusion
Overall, the outlook for patients with HER2+ EBC has dramatically improved since the advent of HER2-targeted therapy, leading to steady incremental progress in the field since the initial FDA approval of adjuvant trastuzumab in 2005. However, the risk of intra- and extra-cranial disease recurrence is still considerable in those with suboptimal response to NAST. The authors assume that the results from novel clinical trials, including A011801, which seek to address the challenges noted above, will continue to improve clinical oncologic outcomes in this patient population.
Executive summary.
Background & rationale
Patients with HER2+ early breast cancer (EBC) and invasive residual disease (RD) after neoadjuvant systemic therapy (NAST) have a higher risk of relapse than those with a pCR.
Postneoadjuvant T-DM1 has improved invasive disease-free survival (iDFS), but patients with estrogen receptor (ER)-negative and/or node positive RD have suboptimal outcomes.
CNS relapses are a challenge, signifying the need for systemic therapies with better CNS penetration.
Tucatinib, trastuzumab and capecitabine improved PFS and OS compared with trastuzumab and capecitabine in patients with pretreated HER2+ MBC participating in the HER2CLIMB trial (∼50% patients had BCBM).
A011801 is an escalation trial examining the addition of tucatinib to postneoadjuvant T-DM1 for patients with high risk HER2+ RD after NAST.
Construction & content
A011801 is a prospective, double-blinded, multicenter, Phase III superiority trial to enroll 1031 (981 evaluable) patients (USA and Canada).
Randomization is 1:1 to T-DM1+ placebo, versus T-DM1 + tucatinib (with adjuvant RT +/- ET, if indicated per standard of care).
Primary objective: to determine if iDFS is ≥5 % higher with the addition of T-DM1 to tucatinib in pts with HER2+ EBC with RD after NAST.
-
Key eligibility criteria:
HER2+ invasive RD (ER-negative, node-positive, or both) after NAST.
completion of ≥6 cycles of NAST, including ≥9 weeks of T and H +/- P.
All neoadjuvant chemotherapy to be completed preoperatively unless participating in EA1181; these patients must receive postoperative chemotherapy to complete ≥6 cycles prior to A011801 enrollment.
Patients who received HER2-targeted TKIs or antibody–drug conjugates as NAST are ineligible.
Correlative analyses will determine the association of (i) TILs in the primary tumor and RD with iDFS, (ii) TILs with tucatinib benefit, (iii) the association between iDFS and CTCs at serial timepoints and (iv) the magnitude of benefit of tucatinib (iDFS) in pts with/without detectable pretreatment CTCs.
Utility & discussion
Patients with invasive HER2+ RD after NAST (ER-, node-positive, both) have inferior outcomes and are at higher risk of BCBM, a challenging clinical scenario which reflects an unmet clinical need.
As such, A011801 aims to improve iDFS and reduce the incidence of BCBM in high-risk pts with HER2+ EBC.
Conclusion
Hopefully, T-DM1 and tucatinib will improve iDFS outcomes and reduce the incidence of BCBM in pts with high risk HER2+ EBC compared with T-DM1 and placebo.
Correlative analysis, PK and QoL studies may also assist in clinical decision making and future research endeavors in the postneoadjuvant HER2+ space.
Footnotes
Financial & competing interests disclosure
Research reported in this publication was supported by the National Cancer Institute of the NIH under award numbers: U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233180, UG1CA233290, UG1CA233329, UG1CA233373 and UG1CA232760. https://acknowledgments.alliancefound.org. Also supported in part by Seagen. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr O'Sullivan has received research funding to institution from Eli Lilly, Sermonix, Bavarian Nordic and Seagen Inc. Dr Goetz reports other from Eagle pharmaceuticals, other from Lilly, other from Biovica, other from Novartis, other from Sermonix, grants from Pfizer, grants from Lilly, other from Pfizer, other from Biotheranostics, grants from Sermonix, other from AstraZeneca, other from Blueprint Medicines, other from Research to Practice, other from Clinical Education Alliance, outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353(16), 1673–1684 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Slamon D, Eiermann W, Robert N et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365(14), 1273–1283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez EA, Romond EH, Suman VJ et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 32(33), 3744–3752 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel C, Cobleigh MA, Tripathy D et al. First-line, single-agent Herceptin (trastuzumab) in metastatic breast cancer: a preliminary report. Eur. J.Cancer 37(Suppl. 1), S25–S29 (2001). [PubMed] [Google Scholar]
- 5.Vogel CL, Cobleigh MA, Tripathy D et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 20(3), 719–726 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Choong GM, Cullen GD, O'Sullivan CC. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J. Clin. 70(5), 355–374 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Baselga J, Bradbury I, Eidtmann H et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, Phase 3 trial. Lancet 379(9816), 633–640 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneeweiss A, Chia S, Hickish T et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized Phase II cardiac safety study (TRYPHAENA). Ann. oncol. 24(9), 2278–2284 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Gianni L, Pienkowski T, Im YH et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (neosphere): a randomised multicentre, open-label, Phase 2 trial. Lancet Oncol. 13(1), 25–32 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Von Minckwitz G, Procter M, De Azambuja E et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377(2), 122–131 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin M, Holmes FA, Ejlertsen B et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 18(12), 1688–1700 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Tolaney SM, Barry WT, Dang CT et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N. Engl. J. Med. 372(2), 134–141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto AC, Ades F, De Azambuja E, Piccart-Gebhart M. Trastuzumab for patients with HER2 positive breast cancer: delivery, duration and combination therapies. Breast (Edinburgh, Scotland) 22(Suppl. 2), S152–155 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Conte P, Frassoldati A, Bisagni G et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the Phase III randomized Short-HER study. Ann. Oncol. 29(12), 2328–2333 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Martinez A, Krop IE, Hillman DW, Polley MY, Parker JS, Huebner L et al. Survival, pathologic response and genomics in CALGB 40601 (Alliance), a neoadjuvant Phase III trial of paclitaxel-trastuzumab with or without lapatinib in HER2-positive breast cancer. J.Clin. Oncol. 38(35), 4184–4193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Minckwitz G, Huang CS, Mano MS et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380(7), 617–628 (2018). [DOI] [PubMed] [Google Scholar]; •• Trial which led to FDA approval of postneoadjuvant T-DM1 in persons with residual invasive breast cancer.
- 17.Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS metastases in breast cancer: old challenge, new frontiers. Clin. Cancer Res. 19(23), 6404–6418 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey LA, Berry DA, Cirrincione CT et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized Phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J. Clin. Oncol. 34(6), 542–549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this trial, tissue analysis showed a high degree of intertumoral heterogeneity with respect to both tumor genomics and tumor microenvironment that significantly influenced pathologic complete response rates, highlighting the need for an individualized approach in the treatment of HER2+ early breast cancer.
- 19.File D, Curigliano G, Carey LA. Escalating and de-escalating therapy for early-stage HER2-positive breast cancer. Am. Soc. Clin. Oncol. Educ. Book 40, 1–11 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Von Minckwitz G, Untch M, Blohmer JU et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30(15), 1796–1804 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Loibl S, Gianni L. HER2-positive breast cancer. Lancet 389(10087), 2415–2429 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Curigliano G, Burstein HJ, Winer EP et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 28(8), 1700–1712 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prat A, Pascual T, De Angelis C et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J. Natl Cancer Inst. 112(1), 46–54 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salgado R, Denkert C, Campbell C et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol. 1(4), 448–454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savas P, Salgado R, Denkert C et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev. Clin. Oncol. 13(4), 228–241 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Krop IE, Paulson J, Campbell C et al. Genomic correlates of response to adjuvant trastuzumab (H) and pertuzumab (P) in HER2+ breast cancer (BC): biomarker analysis of the APHINITY trial. J. Clin. Oncol. 37(Suppl. 15), 1012–1012 (2019).30811295 [Google Scholar]
- 27.Dieci MV, Miglietta F, Griguolo G, Guarneri V. Biomarkers for HER2-positive metastatic breast cancer: beyond hormone receptors. Cancer Treat. Rev. 88, 102064 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Borges VF, Ferrario C, Aucoin N et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a Phase 1b clinical trial. JAMA Oncol. 4(9), 1214–1220 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Trial which provided Phase I evidence for the safety, efficacy and tolerability of T-DM1 + tucatinib in HER2+ advanced breast cancer.
- 29.Murthy R, Borges VF, Conlin A et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, Phase 1b study. Lancet Oncol. 19(7), 880–888 (2018). [DOI] [PubMed] [Google Scholar]; •• Trial describing the effectiveness of treatment with tucatinib, capecitabine and trastuzumab in persons with metastatic breast cancer with or without brain metastases.
- 30.Murthy RK, Loi S, Okines A et al. Tucatinib, trastuzumab and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382(7), 597–609 (2019). [DOI] [PubMed] [Google Scholar]; •• Trial which led to FDA approval of tucatinib, trastuzumab and capecitabine in persons with advanced or metastatic HER2+ breast cancer.
- 31.Symmans WF, Wei C, Gould R et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J. Clin. Oncol. 35(10), 1049–1060 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rack B, Andergassen U, Janni W, Neugebauer J. CTCs in primary breast cancer (I). Recent Results Cancer Res. 195, 179–185 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Salgado R, Denkert C, Demaria S et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann. Oncol. 26(2), 259–271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieci MV, Radosevic-Robin N, Fineberg S et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the international immuno-oncol biomarker working group on breast cancer. Semin Cancer Biol 52(Pt 2), 16–25 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Huober JB, Holmes EC, Baselga J, De Azambuja E, Untch M, Fumagalli D et al. Survival outcomes of the NeoALTTO study: updated results of a randomized multicenter Phase III neoadjuvant trial. J. Clin. Oncol. 35(Suppl. 15), 512–512 (2017). [Google Scholar]
- 36.Hamy AS, Pierga JY, Sabaila A et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann. Oncol. 28(9), 2233–2240 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Kurozumi S, Inoue K, Matsumoto H et al. Prognostic utility of tumor-infiltrating lymphocytes in residual tumor after neoadjuvant chemotherapy with trastuzumab for HER2-positive breast cancer. Sci. Rep. 9(1), 1583 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Data supporting the potential role of tumor infiltrating lymphocytes in residual invasive HER2+ early breast cancer to determine prognostic outcomes.
- 38.Meisel JL, Zhao J, Suo A et al. Clinicopathologic factors associated with response to neoadjuvant anti-HER2-directed chemotherapy in HER2-positive breast cancer. Clin. Breast Cancer 20(1), 19–24 (2020). [DOI] [PubMed] [Google Scholar]