Abstract
Objectives
The monoclonal anti-CD20 antibody rituximab is frequently applied in the treatment of lymphoma as well as autoimmune diseases and confers efficient depletion of recirculating B cells. Correspondingly, B cell-depleted patients barely mount de novo antibody responses during infections or vaccinations. Therefore, efficient immune responses of B cell-depleted patients largely depend on protective T cell responses.
Methods
CD8+ T cell expansion was studied in rituximab-treated rheumatoid arthritis (RA) patients and B cell-deficient mice on vaccination/infection with different vaccines/pathogens.
Results
Rituximab-treated RA patients vaccinated with Influvac showed reduced expansion of influenza-specific CD8+ T cells when compared with healthy controls. Moreover, B cell-deficient JHT mice infected with mouse-adapted Influenza or modified vaccinia virus Ankara showed less vigorous expansion of virus-specific CD8+ T cells than wild type mice. Of note, JHT mice do not have an intrinsic impairment of CD8+ T cell expansion, since infection with vaccinia virus induced similar T cell expansion in JHT and wild type mice. Direct type I interferon receptor signalling of B cells was necessary to induce several chemokines in B cells and to support T cell help by enhancing the expression of MHC-I.
Conclusions
Depending on the stimulus, B cells can modulate CD8+ T cell responses. Thus, B cell depletion causes a deficiency of de novo antibody responses and affects the efficacy of cellular response including cytotoxic T cells. The choice of the appropriate vaccine to vaccinate B cell-depleted patients has to be re-evaluated in order to efficiently induce protective CD8+ T cell responses.
Keywords: Rituximab, Arthritis, Rheumatoid, Vaccination, B-Lymphocytes, T-Lymphocyte subsets
Key messages.
What is already known about this subject?
B cell-depleted individuals cannot mount antibody responses upon vaccination; hence protection against vaccination-preventable diseases depends on CD8+ T cell responses.
What does this study add?
We found that B cell depletion strongly impairs vaccination-induced CD8+ T cell responses.
Mechanistically, B cells promote CD8+ T cell responses in a type I interferon-dependent manner.
How might this impact on clinical practice or future developments?
Patients treated with rituximab should be vaccinated when B cells have repopulated in order to mount efficient CD8+ T cell responses.
Vaccines inducing a cytokine milieu that is not dominated by type I interferon could be beneficial for B cell-depleted patients.
Introduction
Antibody responses play a key role in mediating protection against severe infections and the efficacy of the majority of currently available vaccines relies on the induction of long-lasting antibody responses. In particular during the current SARS-CoV-2 pandemic, it is discussed to which extend antibody and T cell responses contribute to protection. In some convalescent patients, rapidly decreasing antibody titres were observed. The question arose, whether such patients are still protected from SARS-CoV-2 reinfection by long-lasting T cell memory.
B cell depletion using the anti-CD20 antibody rituximab is an effective treatment of lymphoproliferative diseases such as non-Hodgkin’s lymphomas,1 various autoimmune diseases, including immune thrombocytopaenia (ITP),2 3 rheumatoid arthritis (RA),4 anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis,5 systemic lupus erythematosus,6 multiple sclerosis,7 and prevents graft failure after some solid organ transplantations.8 Since B cell depletion massively reduces the formation of SARS-CoV-2-specific antibodies, it is intensively discussed whether B cell depleting therapy with rituximab and Ocrelizumab should be postponed until SARS-CoV-2 vaccination has been performed.9 In the absence of antibody responses, CD8+ cytotoxic T cells take over important functions in protection against pathogens. For B cell-depleted patients it is therefore of utmost importance to mount functional CD8+ T cell responses upon vaccination.
Recently, it became evident that B cell depletion influences CD8+ T cell responses. In a murine model of ITP, rituximab treatment inhibited splenic CD8+ T cell proliferation and thus protected against T cell-mediated autoimmune thrombocytopaenia.10 Furthermore, it was reported that B cells promote survival of intra-islet CD8+ T cells in NOD mice and that B cell deficiency significantly delayed diabetes development.11 B cells also play a specific role in modulating the contraction of CD8+ T cell responses following immunisation with Listeria monocytogenes and in establishing efficient CD8+ T cell memory.12 Furthermore, B cells were required to prevent virus-specific CD8+ T cell memory exhaustion upon lymphocytic choriomeningitis virus infection.13
Whether B cells support T cell responses by direct cell-cell contact or via cytokine and chemokine expression is still largely unclear. A CXCR5+ subset of CD8+ T cells was shown to constitute early effector cells that migrate into B cell follicles and thus might be able to directly interact with B cells.14 Several chemokines and cytokines such as type I interferon (IFN-I) were shown to orchestrate lymphocyte responses locally or via systemic inflammatory signals. In addition to direct anti-viral function, IFN-I directly triggers the IFN-I receptor (IFNAR) of CD8+ T cells to promote their expansion.15–17
IFN-I are potent antiviral cytokines that are induced early upon various infections and thus are targeted by many viral evasion strategies. The poxvirus strains vaccinia virus (VACV) and modified vaccinia virus Ankara (MVA) are relevant vaccine models to study vaccination in vivo. In contrast to its parental strain VACV, MVA lost several IFN-I inhibitors during passaging on chicken embryo fibroblasts and therefore efficiently induces serum IFN-I responses in mice.18
Here, we studied the impact of B cell depletion on CD8+ T cell expansion during immunisation with different viruses. We found massively reduced CD8+ T cell responses in B cell-depleted RA patients upon influenza vaccination. CD8+ T cell expansion was also strongly reduced in B cell deficient mice upon influenza and MVA infection, but not upon VACV infection. Direct IFNAR signalling of B cells was necessary to trigger proper T cell activation and MHC-I upregulation, thus licensing B cells to promote CD8+ T cell expansion.
Results
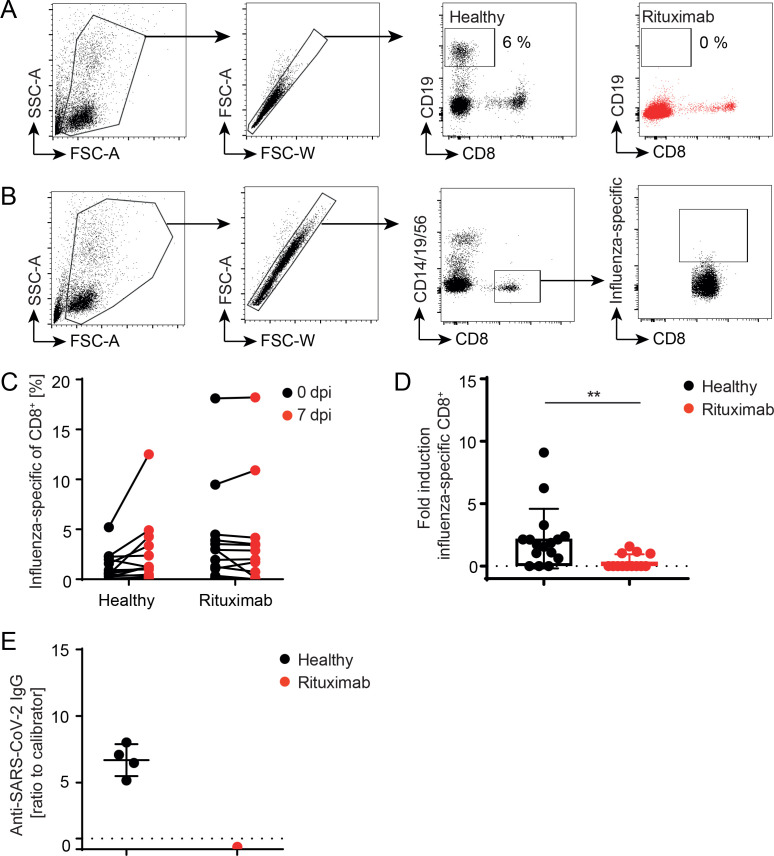
Patients suffering from rheumatic diseases are frequently treated with rituximab. Rituximab has a high depletion efficiency, which lasts for approximately 6 months (figure 1A). During a therapy cycle, vaccination against seasonal influenza is recommended, whereas the protective efficacy of influenza vaccination under conditions of B cell depletion is debated. To study the impact of B cell depletion on the induction of CD8+ T cell responses, rituximab-treated RA patients and healthy controls were human leucocyte antigen (HLA)-typed and vaccinated with Influvac. Influenza-specific T cells were determined 7 days post vaccination (figure 1B online supplemental figure 1). An increase of influenza-specific CD8+ T cells was observed in healthy individuals, but not in B cell deficient patients (figure 1C). To directly compare T cell responses of different donors, the fold induction of specific T cells post vaccination was calculated (figure 1D). Of note, the observed reduced T cell expansion in rituximab-treated patients was independent on other immunomodulatory comedication (online supplemental figure 2). Thus, B cell depleted RA patients show reduced CD8+ T cell expansion upon anti-influenza vaccination. During the current SARS-CoV-2 pandemic, such patients are particularly vulnerable and bare an enhanced mortality risk.19 20 COVID-19 vaccination of younger patients just started and is applied independently of the rituximab treatment cycle, as similarly done for influenza vaccination. One patient with granulomatosis and polyangiitis (GPA) was analysed 4 weeks after second BNT162b2 vaccination for anti-SARS-CoV-2 antibody titres (figure 1E). In contrast to healthy controls, who mount high anti-SARS-CoV-2 IgG responses, no antibody titre was detected in the serum of this patient. Of note, as SARS-CoV-2 specific HLA-multimers are not available yet, T cell expansion could not be tested.
Figure 1.
B cell depletion affects CD8+ T cell response upon influenza vaccination. Healthy subjects and rituximab-treated RA patients were vaccinated against seasonal influenza. (A) Rituximab treatment efficiently depletes circulating B cells from blood. (B) Influenza-specific CD8+ T cells were determined after excluding CD14+/CD19+/CD56+ cells by using one or more personalised MHC-I multimers (left panels). B cell depletion efficiency was monitored using flow cytometry (right panel). (C) The frequency of influenza-specific T cells of CD8+ T cells was monitored on day 0 and 7 post vaccination. (D) Fold induction was calculated for each MHC-I multimer measurement (n=10 healthy, n=5 rituximab). Healthy subjects and one rituximab-treated GpA patient were fully vaccinated against SARS-CoV-2. (E) Serum IgG against SARS-CoV-2 S1 was determined (n=4 healthy, n=1 rituximab). Titre was considered positive when >0.8 ratio to calibrator (dotted line). error bars indicate mean±SD; **p≤0.01; one-tailed Mann-Whitney U test. FSC-A, forward scatter-area; RA, rheumatoid arthritis; SSC-A side scatter-area.
annrheumdis-2021-220435supp004.pdf (70KB, pdf)
annrheumdis-2021-220435supp005.pdf (25.9KB, pdf)
Since the analysis of immune responses in RA patients is potentially confounded by generally impaired immune status due to primary diseases and concomitant immunomodulatory treatment, the molecular mechanism of how B cells affect CD8+ T cell expansion was further addressed in B cell-deficient mice. To this end, JHT mice, in which the deletion of the J elements of the immunoglobulin heavy chain locus (JHT) resulted in a premature block of B cell development, were analysed. Upon infection with the mouse adapted influenza strain PR8, JHT mice showed significantly reduced expansion of nucleoprotein- and polymerase acidic protein-specific CD8+ T cells when compared with wild type mice (figure 2A, B). Thus, B cells are needed to efficiently induce influenza-specific CD8+ T cell responses in humans and mice.
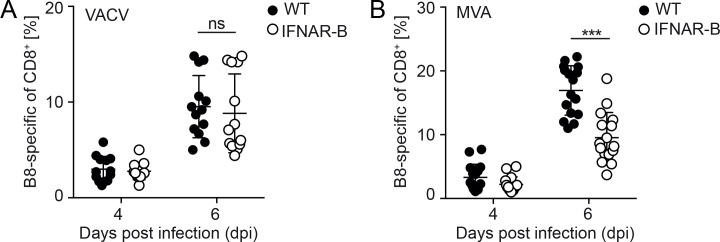
Figure 2.
B cell deficient mice show reduced virus-specific CD8+ T cell response upon influenza and MVA, but not VACV infection. (A) Wild type (WT) and JHT mice were infected with 5×103 ffu mouse adapted influenza virus for 7 days. (B) Influenza-specific CD8+ T cells were determined by using nucleoprotein (NP) or polymerase acidic protein (PAP) specific MHC-I multimers. WT and JHT mice were infected with 105 pfu of (C) VACV or (D) MVA and B8-specific CD8+ T cells were determined by using a MHC-I multimer. Data shown are pooled from 2 to 3 experiments with n=3–4. JHT mice were reconstituted with (E) 107 B cells or (F) 300 µL serum of WT mice 1 day prior to MVA infection and B8-specific T cell expansion was monitored. One out of two independent experiments is shown. Error bars indicate mean±SD; *p≤0.05, ***p≤0.001; one-tailed Mann-Whitney U test. MVA, modified vaccinia virus Ankara; ns, not significant; VACV, vaccinia virus.
To analyse whether the impact of B cells on T cell expansion is a unique feature on influenza infection, wild-type mice and JHT mice were infected with VACV, which is known to induce particularly strong T cell responses. The expansion of VACV-specific T cells was measured using an major histocompatibility complex (MHC)-I multimer loaded with the immune-dominant peptide B8. Upon VACV infection, wild type and JHT mice showed similar T cell expansion (figure 2C). Following MVA infection the expansion of B8-specific CD8+ T cells was significantly increased in wild type mice compared with JHT mice (figure 2D). To analyse whether B cell reconstitution of B cell-deficient mice restored T cell responses, splenic B cells of wild type mice were adoptively transferred into JHT mice 1 day prior to MVA infection. In B cell-reconstituted JHT mice the expansion of B8-specific CD8+ T cells was comparable with that in wild type mice (figure 2E), whereas adoptive transfer of serum from wild type mice, which contains natural antibodies but no B cells, had no impact (figure 2F). These data indicate that B cells support the induction of B8-specific CD8+ T cell responses on MVA infection, whereas during VACV infection B cells are not needed. Thus, the capacity of B cells to modulate CD8+ T cell responses is dependent on the properties of the pathogen/vaccine.
MVA and VACV induce distinct cytokine milieus upon infection: While MVA induces systemic IFN-I responses, VACV efficiently inhibits systemic IFN-I responses and rather induces an IL-12 dominated cytokine milieu.15 18 To test whether IFN-I responses affect B and T cell responses, we made use of conditional CD19-Cre+/-IFNARflox/flox mice (IFNAR-B) in which the IFNAR is selectively deleted on B cells. Upon VACV infection, the expansion of B8-specific CD8+ T cells was similar in IFNAR-B and wild type mice (figure 3A), whereas upon MVA infection the expansion of T cells was significantly reduced in IFNAR-B mice (figure 3B). To test whether B cells are directly triggered by IFN-I, B cells from Mx2-luc reporter mice expressing a luciferase reporter upon IFNAR triggering were adoptively transferred into albino C57BL/6 mice. Upon MVA infection, a strong luciferase signal was detected by in vivo imaging particularly in the spleen and lymph nodes, which declined within the following day (figure 4). These results indicated that B cells were directly triggered by IFN-I early after MVA infection, which is in accordance with the fast onset of MVA induced IFN-I responses.21
Figure 3.
IFNAR depletion on B cells affects B8-specific CD8+ T cell responses upon MVA, but not VACV infection. Wild type (WT) and CD19-Cre+/-IFNARflox/flox (IFNAR-B) mice were infected with 105 pfu (A) VACV or (B) MVA. B8-specific CD8+ T cells were determined by using an MHC-I multimer. Data shown are pooled from 3 to 4 experiments with n=3–4. Error bars indicate mean±SD; ***p≤0.001; one-tailed Mann-Whitney U test. IFNAR, type I interferon receptor; MVA, modified vaccinia virus Ankara; ns, not significant; VACV, vaccinia virus.
Figure 4.
MVA-induced IFN-I responses directly trigger B cells in vivo. 107 B cells isolated from Mx2-luc reporter mice were adoptively transferred into albino C57BL/6 wild type mice 1 day prior to infection. Upon treatment with phosphate buffered saline (PBS) (first mouse per row) or infection with 105 pfu MVA (mouse 2–4 per row), luciferase reporter expression in adoptively transferred B cells was monitored after luciferin administration by in vivo imaging at different days (d) postinfection (scale=p/sec/cm2/sr). one out of two independent experiments is shown. IFN-I, type I interferon; MVA, modified vaccinia virus Ankara.
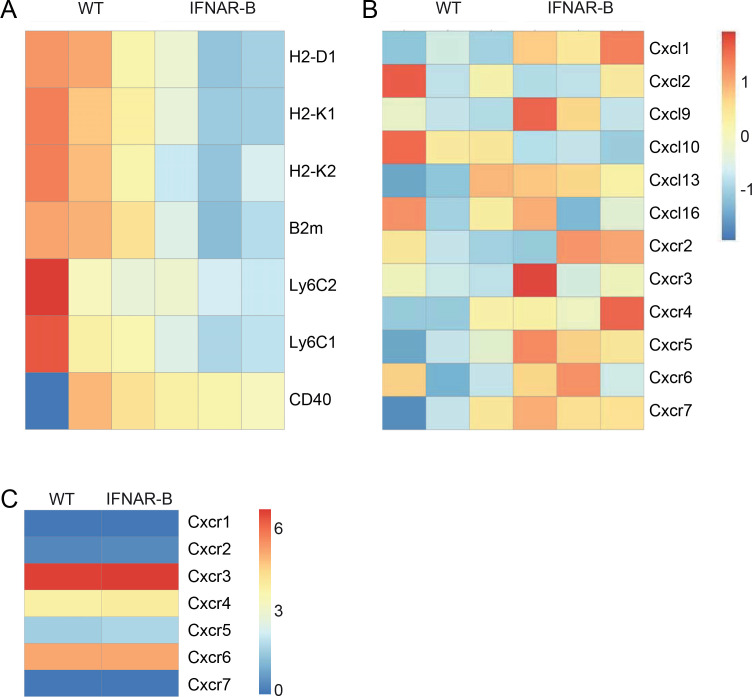
To study effects of direct IFNAR signalling, B cells were isolated from spleens of MVA-infected wild type and IFNAR-B mice and analysed for differential gene expression by RNA sequencing. B cells of wild type mice expressed higher messenger RNA (mRNA) levels of MHC-I, β−2-microglobulin, and Ly6C than B cells of IFNAR-B mice (figure 5A). Furthermore, IFNAR-deficient B cells highly upregulated many chemokine receptors as well as CXCL1, CXCL9, and CXCL13, while CXCL10 was down-modulated when compared with wild type B cells (figure 5B). Thus, direct IFNAR-triggering of B cells modulates pathways involved in antigen presentation and tissue homoeostasis.
Figure 5.
MVA-induced IFN-I responses activate B cells, but do not affect CXCR5+CD8+ T cell responses. Wild type (WT) and IFNAR-B mice were infected with 105 pfu MVA and B cells were isolated 1 day post infection via untouched magnetic cell separation and prepared for mRNA sequencing. Differentially regulated (A) surface molecules and (B) chemokine as well as chemokine receptor expression profiles are shown. n=3 (C) WT and IFNAR-B mice were infected with 105 pfu MVA and B8-specific CD8+ T cells were FACS-sorted six days post infection from spleens using a B8-specific MHC-I multimer. RNA sequencing samples were pooled from three different mice and chemokine expression profiles were analysed. IFN-I, type I interferon; IFNAR, IFN-I receptor; MVA, modified vaccinia virus Ankara.
To test whether virus-specific CD8+ T cells showed distinct chemokine receptor expression, MVA-specific T cells were sorted by fluorescence activated cell sorting (FACS) using an MHC-I multimer and mRNA was sequenced. Of note, no differences in chemokine receptor expression were found comparing B8-specific CD8+ T cells of wild type and IFNAR-B mice (figure 5C). Even being less frequent, B8-specific CD8+ T cells showed very similar gene expression profiles when compared with T cells from wild type mice.
In accordance with sequencing data, B cells’ surface expression of MHC-I and the B8 presenting haplotype H2-Kb was significantly increased upon direct IFNAR triggering, while MHC-II expression was upregulated upon infection IFNAR-independently (figure 6A–C). In addition, MVA infection induced CD86 and CD69 expression on wild type B cells, which was significantly reduced on IFNAR-deficient B cells (figure 6D–E). Thus, direct IFNAR signalling activates B cells and induces the expression of MHC-I as well as costimulatory molecules, and thus has a major impact on the capacity for antigen presentation of B cells.
Figure 6.
MVA-induced IFN-I responses modulate antigen presentation in B cells. Wild type (WT) and IFNAR-B mice were infected with 105 pfu MVA and splenocytes were isolated 48 hours post infection. Expression of (A) MHC-II, (B) MHC-I, (C) H2-kb, (D) CD86, and (E) CD69 was analysed by flow-cytometry. Data shown are pooled from 2 to 3 experiments with n=2–4. Error bars indicate mean±SD; *p≤0.01; ***p≤0.001; one-tailed Mann-Whitney U test. DPI, days post infection; IFN-I, type I interferon; IFNAR, IFN-I receptor; MVA, modified vaccinia virus Ankara; MFI, mean fluorecscence intensity; NS, not significant.
Discussion
Here, we report that B cell depletion can affect the expansion of virus-specific CD8+ T cells, depending on the T cell stimulating pathogen/vaccine. The underlying mechanism is mediated via direct IFNAR signalling of B cells, which showed enhanced MHC-I, CD69, and CD86 expression, increased activation, and a distinct chemokine expression profile.
Most RA patients treated with rituximab received an immunomodulatory comedication and thus are therapeutically immunosuppressed. Since rituximab is not licensed as first-line RA treatment, the patients received other immunomodulatory treatments earlier. Additionally, RA patients were recently shown to harbour exhausted CD4+ T cells,22 which might influence the outcome of CD8+ T cell responses as well. Furthermore, patients are not immunologically naïve, since they were previously vaccinated against seasonal influenza virus or were in contact with the pathogen itself. The analysis of T cell expansion upon vaccination reflects a reactivation of memory CD8+ T cells rather than a primary response. The question remains, whether upon other diseases than RA B cell depletion influence CD8+ T cells responses as well. To prove that reduced expansion of CD8+ T cells in patients treated with rituximab was not caused by such secondary effects, we studied the result of B cell depletion on T cell responses in mice.
Here, we report a reduced in vivo expansion of antigen-specific CD8+ T cells in B cell-deficient mice upon infection with different viruses, suggesting the presence of a species-independent mechanism of immune cell cross-talk. This phenomenon is remarkable, as dendritic cells (DCs) are broadly accepted to be the main APC responsible for T cell priming.
Guo et al showed that on anti-CD20 treatment, splenic CD8+ T cell proliferation was inhibited in a murine model of ITP.10 In that study, B cell depletion led to increased numbers of FOXP3+, CD4+, and CD8+ T cells within the spleen and lymph nodes, while splenic CD8+ T cells showed a reduced proliferation upon in vitro stimulation.10 In our experiments, the impaired T cell expansion was restored by adoptive transfer of B cells. B1 cell-derived natural antibodies, which are present in the serum of naïve mice, were shown to decorate antigen rather unspecifically and to enhance antigen presentation by antigen trapping.23 However, we found that serum transfer was not effective in restoring the deficit in CD8+ T cell expansion in B cell deficient mice.
Upon MVA infection, the lack of IFNAR expression exclusively on B cells resulted in reduced T cell expansion as similarly detected in B cell deficient mice. Thus, besides serving as a direct third signal for T cell responses15 IFN-I can also increase CD8+ T cell responses indirectly via B cells. IFN-I responses were shown to critically modulate the overall cytokine milieu and in particular, to inhibit IL-12 responses.15 24 25 Furthermore, IL-12 was shown to serve as third signal in T cell activation as well,26 27 which might explain why in the absence of IFN-I responses B cells are dispensable for CD8+ T cell expansion. Direct IFNAR triggering on B cells induced the activation of the STAT1 pathway and enhanced the expression of Ly6C and CD69. Moreover, MHC-I and CD86 were induced, thus facilitating adequate antigen presentation. Interestingly, B cells were described before to cross-present MHC-I restricted antigen, although less efficiently than DC.28 Thus, IFN-I is a key mediator to promote efficient interaction between B cells and CD8+ T cells. Of note, virus-induced IFN-I was also reported to confer disintegration of B cell follicles29 and to drive B cell reduction by differentiating B cells into short-lived antibody-secreting cells.30 This mechanism called ‘B cell decimation’ was independent of B cell-intrinsic IFN-I sensing.30
Whether B cells and CD8+ T cells are in direct contact within secondary lymphoid organs has been discussed controversially. B cell follicles and T cell zones are organised in separate compartments in secondary lymphoid organs. In human (HIV) and simian immunodeficiency virus (SIV) infection, B cell follicle sanctuaries were shown to permit a persistent infection reservoir due to the absence of protective CD8+ T cell responses.31–33 Quigley et al showed that a CXCR5+ subset of CD8+ T cells infiltrates B cell areas of tonsils.14 During chronic viral infection with lymphocytic choriomeningitis virus or SIV, CXCR5+CD8+ T cells migrate into B cell follicles and critically contribute to the control of viral replication.34–36 Upon MVA infection, IFNAR deficient B cells showed enhanced expression of CXCL13, which was previously shown to attract CXCR5+CD8+ T cells.14 Of note, CXCR5 expression of sorted MVA-specific CD8+ T cells was very similar in wild type and IFNAR-B mice. These data suggest that in IFNAR-B mice, CXCR5+CD8+ T cells initially infiltrate B cell follicles, but cross-talk with B cells may be reduced. CD4+ T cells can directly interact with B cells, critically increase CD8+ T cell responses by providing help,37 38 and are activated in a spatially distinct compartment of lymph nodes before encountering CD8+ T cells.39 Thus, CD4+ T cells might function as a link between B cell and CD8+ T cell responses.
Of note, rituximab treatment of RA patients not only depletes recirculating B cells, but also a CD20+ terminally differentiated T cell subset with immune-regulatory and proinflammatory function.40 Nevertheless, the frequency of CD20+CD8+ T cells is very low in humans and might not be the primary cause for reduced T cell expansion in rituximab-treated patients.
Here, we studied the immune response against an influenza vaccine in B cell depleted RA patients. It is possible that antigen-specific T cell responses are also reduced in rituximab-treated patients after vaccination against other diseases. Of note, SARS-CoV-2 infection induces only mild IFN-I responses due to active IFN-I blockade41 42 and patients with severe COVID-19 displayed a highly impaired IFN-I response when compared with patients with moderate COVID-19 courses.43 44 Among the available COVID-19 vaccines, the mRNA-based vaccines induce IFN-I dominated cytokine milieus.45 In contrast, for adenovirus-based vaccines it was shown that excessive IFN-I responses rather inhibit transgene expression, and as a consequence, vectors inducing only minor IFN-I responses were chosen for the development of an immunogenic vaccine.46 47 Among SARS-CoV-2 adenoviral vectors, HAd5-based vaccines most likely induce less IFN-I compared with ChAdOx1-based vaccines. Considering a reduced CD8+ T cell responses in the presence of IFN-I with simultaneous absence of B cells, the non-IFN-I inducing adenovirus based vaccines could be even better suited to induce decent CD8+ T cell responses in B cell-depleted patients compared with mRNA-based vaccines.46 48
Patients treated with rituximab were reported to bare an enhanced mortality risk if infected with SARS-CoV-2.19 20 With regard to COVID-19 disease, it appears therefore not advisable to delay vaccination of such patients a few months after rituximab suspension, when naïve B cells have repopulated. In contrast to other vaccines, COVID-19 vaccine should rather be administered as soon as available. In order to induce at least protective CD8+ T cell responses, the usage of vaccines inducing a cytokine milieu that is not dominated by IFN-I could be beneficial for such patients.
Material and methods
Patients and healthy controls
After immunisation withInfluvac season 2012/2013 or 2013/2014 (Mylan Healthcare) PBMC were isolated on day 0 and 7, and frozen at −80°C. The frequency of influenza virus specific CD8+ T cells was determined using HLA matched pentamers (Proimmune) (online supplemental table 1). Five RA patients (one male, four female, average age 63 years) and 10 healthy controls (five male, five female, average age 31 years) were identified with one or more matching HLA subtypes. After BNT162b2 vaccination, 1 GPA patient (female, age 20 years) and four healthy controls (two female, 1 male, average age 33 years) were recruited. Characteristics of patients are indicated (online supplemental table 2).
annrheumdis-2021-220435supp002.pdf (302.1KB, pdf)
annrheumdis-2021-220435supp003.pdf (334.9KB, pdf)
Mice
C57BL/6 (wild type) and albino C57BL/6BrdCrHsd-Tyrc (C57BL/6 albino) mice were purchased from Harlan Winkelmann or Envigo. IFNAR-/-, 49 JHT,50 CD19-Cre+/-IFNARflox/flox (IFNAR-B),51 and Mx2-luc reporter mice52 were described before. All mice were bred under specific pathogen free conditions at the central animal facility of TWINCORE and the Helmholtz Centre for Infection Research, Brunswick, Germany, or the Paul-Ehrlich-Institut, Langen, Germany. Mouse experimental work was carried out using 8 to 16 week old mice in compliance with regulations of the German animal welfare law (F107/64, 09/1655, 10/0265, 10/0266, 11/0367, 12/0939, 13/1073).
Viruses and infection
MVA and VACV strain Western Reserve (originally provided by Bernard Moss, NIH, Bethesda, Maryland, USA)53 were propagated and titrated on chicken embryonic fibroblasts and purified by sucrose density gradient centrifugation. Mouse-adapted influenza A/PR/8/34 (H1N1 PR8)54 was propagated in the chorio-allantoic fluid of 10 days old pathogen free embryonated chicken eggs at 37°C55 and was kindly provided by Dr. P. Blazejewska, Dr. K. Schughart, and Carlos A. Guzmάn (Helmholtz Centre for Infection Research Brunswick, Germany). In all infection experiments, mice were treated with 105 pfu MVA/VACV, or 5×103 ffu influenza virus dissolved in PBS intravenously.
Adoptive cell and serum transfer experiments
B cells were isolated from spleens, via untouched magnetic B cell separation kit (Miltenyi). 107 B cells with a purity of 90%–98% were adoptively transferred into recipient mice. For serum transfer, 300 µL serum pooled from different wild-type animals was injected 1 day prior to infection.
In vivo imaging
Reporter mice were intravenously injected with 3 mg of D-luciferin (PerkinElmer) diluted in PBS and anaesthetised using 2.5% isoflurane (Abbott). The emitted light signals were measured in the in vivo imaging system IVIS SpectrumCT (Calliper) and analysed with Living Image 4.5 software (Calliper).
Flow cytometric analysis and cell sorting
All antibodies were purchased from eBioscience or BD-Pharmingen. Cells were measured using flow cytometry (LSR II, BD) and data were analysed by FlowJo software. FACS sorting was conducted using a MoFlo XDP cell sorter (Becton Dickinson).
ELISA
Anti-SARS-CoV-2 IgG antibody titres were determined from serum using an ELISA (Euroimmun AG, EI 2606–9601 G) according to the manufacturer’s instructions. The ratio of the optical density to the calibrator was used to classify the samples as negative (ratio <0.8) or positive (ratio ≥1.1).
Deep sequencing and pathway analysis
After 24 hours of MVA infection, B cells were isolated from spleens of C57BL/6 and IFNAR-B mice using the untouched magnetic B cell separation kit (Miltenyi). FACS sorting of B8-specific CD8+ T cells from spleens was conducted using a MoFlo XDP cell sorter (Becton Dickinson). After RNA isolation using tNucleoSpin RNA kit (Macherey-Nagel) mRNA sequencing was performed at TRON (Translational Oncology Mainz, Germany). Pathway analysis was performed as described in online supplemental methods section.
annrheumdis-2021-220435supp001.pdf (909.5KB, pdf)
Statistical analysis
Statistical analyses were performed using GraphPad Prism V.6 software as indicated.
Acknowledgments
We kindly thank all blood donors for participating in this study, Dr.Elena Grabski for critical discussion, Dr.Elisabeth Janecek-Erfurth for critical reading of the manuscript, and Jennifer Skerra for organising the breeding of the involved transgenic mouse lines.
Footnotes
Handling editor: Josef S Smolen
Contributors: TG, UK, TW and RES planned the study and TG and UK designed experiments. TG, KB, MK, and MB performed and analyzed experiments. LG and TW were involved in treatment and recruitment of the patients. HM and LV analyzed sequencing data and performed statistics. MV and MH performed HLA-typing of patients. CAG and GS provided virus stocks. TG, UK, and TW wrote the manuscript. All authors critically reviewed the manuscript.
Funding: This project was funded by DZIF TTU 07.806_00 (German Centre for Infection Research), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy – EXC 2155 “RESIST” – Project ID 39087428 to RS, TW, and UK, the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project ID 158989968 - SFB 900 to UK, and the German Federal Ministry of Education and Research (BMBF, 01GM1910E) to RS.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary Information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Human subjects were asked to participate in the study approved by the local ethical committee (No. 6259) and provided written informed consent. Mouse experimental work was carried out in compliance with regulations of the German animal welfare law (F107/64, 09/1655, 10/0265, 10/0266, 11/0367, 12/0939, 13/1073).
References
- 1. Hainsworth JD, Burris HA, Morrissey LH, et al. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood 2000;95:3052–6. 10.1182/blood.V95.10.3052 [DOI] [PubMed] [Google Scholar]
- 2. Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med 2007;146:25–33. 10.7326/0003-4819-146-1-200701020-00006 [DOI] [PubMed] [Google Scholar]
- 3. Khellaf M, Charles-Nelson A, Fain O, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood 2014;124:3228–36. 10.1182/blood-2014-06-582346 [DOI] [PubMed] [Google Scholar]
- 4. Edwards JCW, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81. 10.1056/NEJMoa032534 [DOI] [PubMed] [Google Scholar]
- 5. Thiel J, Troilo A, Salzer U, et al. Rituximab as induction therapy in eosinophilic granulomatosis with polyangiitis refractory to conventional immunosuppressive treatment: a 36-month follow-up analysis. J Allergy Clin Immunol Pract 2017;5:1556–63. 10.1016/j.jaip.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 6. Iwata S, Saito K, Hirata S, et al. Efficacy and safety of anti-CD20 antibody rituximab for patients with refractory systemic lupus erythematosus. Lupus 2018;27:802–11. 10.1177/0961203317749047 [DOI] [PubMed] [Google Scholar]
- 7. Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol 2018;75:320–7. 10.1001/jamaneurol.2017.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 2008;359:242–51. 10.1056/NEJMoa0707894 [DOI] [PubMed] [Google Scholar]
- 9. Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology 2020;95:e1999–2008. 10.1212/WNL.0000000000010380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo L, Kapur R, Aslam R, et al. CD20+ B-cell depletion therapy suppresses murine CD8+ T-cell-mediated immune thrombocytopenia. Blood 2016;127:735–8. 10.1182/blood-2015-06-655126 [DOI] [PubMed] [Google Scholar]
- 11. Brodie GM, Wallberg M, Santamaria P, et al. B-Cells promote intra-islet CD8+ cytotoxic T-cell survival to enhance type 1 diabetes. Diabetes 2008;57:909–17. 10.2337/db07-1256 [DOI] [PubMed] [Google Scholar]
- 12. Shen H, Whitmire JK, Fan X, et al. A specific role for B cells in the generation of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol 2003;170:1443–51. 10.4049/jimmunol.170.3.1443 [DOI] [PubMed] [Google Scholar]
- 13. Thomsen AR, Johansen J, Marker O, et al. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J Immunol 1996;157:3074–80. [PubMed] [Google Scholar]
- 14. Quigley MF, Gonzalez VD, Granath A, et al. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur J Immunol 2007;37:3352–62. 10.1002/eji.200636746 [DOI] [PubMed] [Google Scholar]
- 15. Frenz T, Waibler Z, Hofmann J, et al. Concomitant type I IFN receptor-triggering of T cells and of DC is required to promote maximal modified vaccinia virus Ankara-induced T-cell expansion. Eur J Immunol 2010;40:2769–77. 10.1002/eji.201040453 [DOI] [PubMed] [Google Scholar]
- 16. Aichele P, Unsoeld H, Koschella M, et al. Cd8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol 2006;176:4525–9. 10.4049/jimmunol.176.8.4525 [DOI] [PubMed] [Google Scholar]
- 17. Le Bon A, Thompson C, Kamphuis E, et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol 2006;176:2074–8. 10.4049/jimmunol.176.4.2074 [DOI] [PubMed] [Google Scholar]
- 18. Waibler Z, Anzaghe M, Frenz T, et al. Vaccinia virus-mediated inhibition of type I interferon responses is a multifactorial process involving the soluble type I interferon receptor B18 and intracellular components. J Virol 2009;83:1563–71. 10.1128/JVI.01617-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guilpain P, Le Bihan C, Foulongne V, et al. Rituximab for granulomatosis with polyangiitis in the pandemic of covid-19: lessons from a case with severe pneumonia. Ann Rheum Dis 2021;80:e10. 10.1136/annrheumdis-2020-217549 [DOI] [PubMed] [Google Scholar]
- 20. Schulze-Koops H, Krueger K, Vallbracht I, et al. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis 2021;80:e67. 10.1136/annrheumdis-2020-218075 [DOI] [PubMed] [Google Scholar]
- 21. Waibler Z, Anzaghe M, Konur A, et al. Excessive CpG 1668 stimulation triggers IL-10 production by cDC that inhibits IFN-alpha responses by pDC. Eur J Immunol 2008;38:3127–37. 10.1002/eji.200838184 [DOI] [PubMed] [Google Scholar]
- 22. Frenz T, Grabski E, Buschjäger D, et al. CD4(+) T cells in patients with chronic inflammatory rheumatic disorders show distinct levels of exhaustion. J Allergy Clin Immunol 2016;138:e510:586–9. 10.1016/j.jaci.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 23. Ochsenbein AF, Fehr T, Lutz C, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science 1999;286:2156–9. 10.1126/science.286.5447.2156 [DOI] [PubMed] [Google Scholar]
- 24. Cousens LP, Peterson R, Hsu S, et al. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med 1999;189:1315–28. 10.1084/jem.189.8.1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dalod M, Salazar-Mather TP, Malmgaard L, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med 2002;195:517–28. 10.1084/jem.20011672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chouaib S, Chehimi J, Bani L, et al. Interleukin 12 induces the differentiation of major histocompatibility complex class I-primed cytotoxic T-lymphocyte precursors into allospecific cytotoxic effectors. Proc Natl Acad Sci U S A 1994;91:12659–63. 10.1073/pnas.91.26.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol 2002;169:6842–9. 10.4049/jimmunol.169.12.6842 [DOI] [PubMed] [Google Scholar]
- 28. Heit A, Huster KM, Schmitz F, et al. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol 2004;172:1501–7. 10.4049/jimmunol.172.3.1501 [DOI] [PubMed] [Google Scholar]
- 29. Daugan M, Murira A, Mindt BC, et al. Type I interferon impairs specific antibody responses early during establishment of LCMV infection. Front Immunol 2016;7:564. 10.3389/fimmu.2016.00564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fallet B, Narr K, Ertuna YI, et al. Interferon-Driven deletion of antiviral B cells at the onset of chronic infection. Sci Immunol. 2016;1. 10.1126/sciimmunol.aah6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015;21:132–9. 10.1038/nm.3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Connick E, Folkvord JM, Lind KT, et al. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol 2014;193:5613–25. 10.4049/jimmunol.1401161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Connick E, Mattila T, Folkvord JM, et al. Ctl fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol 2007;178:6975–83. 10.4049/jimmunol.178.11.6975 [DOI] [PubMed] [Google Scholar]
- 34. He R, Hou S, Liu C, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 2016;537:412–28. 10.1038/nature19317 [DOI] [PubMed] [Google Scholar]
- 35. Mylvaganam GH, Rios D, Abdelaal HM, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci U S A 2017;114:1976–81. 10.1073/pnas.1621418114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leong YA, Chen Y, Ong HS, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol 2016;17:1187–96. 10.1038/ni.3543 [DOI] [PubMed] [Google Scholar]
- 37. Kremer M, Suezer Y, Volz A, et al. Critical role of perforin-dependent CD8+ T cell immunity for rapid protective vaccination in a murine model for human smallpox. PLoS Pathog 2012;8:e1002557. 10.1371/journal.ppat.1002557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wiesel M, Oxenius A. From crucial to negligible: Functional CD8 + T-cell responses and their dependence on CD4 + T-cell help. Eur J Immunol 2012;42:1080–8. 10.1002/eji.201142205 [DOI] [PubMed] [Google Scholar]
- 39. Eickhoff S, Brewitz A, Gerner MY, et al. Robust anti-viral immunity requires multiple distinct T Cell-Dendritic cell interactions. Cell 2015;162:1322–37. 10.1016/j.cell.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilk E, Witte T, Marquardt N, et al. Depletion of functionally active CD20+ T cells by rituximab treatment. Arthritis Rheum 2009;60:3563–71. 10.1002/art.24998 [DOI] [PubMed] [Google Scholar]
- 41. Triggle CR, Bansal D, Ding H, et al. A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic. Front Immunol 2021;12:631139. 10.3389/fimmu.2021.631139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Onodi F, Bonnet-Madin L, Meertens L, et al. SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4. J Exp Med 2021;218. 10.1084/jem.20201387. [Epub ahead of print: 05 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020;181:e1039:1036–45. 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020;369:718–24. 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krienke C, Kolb L, Diken E, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science 2021;371:145–53. 10.1126/science.aay3638 [DOI] [PubMed] [Google Scholar]
- 46. Coughlan L. Factors which contribute to the immunogenicity of non-replicating adenoviral vectored vaccines. Front Immunol 2020;11:909. 10.3389/fimmu.2020.00909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hensley SE, Cun AS, Giles-Davis W, et al. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol Ther 2007;15:393–403. 10.1038/sj.mt.6300024 [DOI] [PubMed] [Google Scholar]
- 48. Dicks MDJ, Spencer AJ, Coughlan L, et al. Differential immunogenicity between HAdV-5 and chimpanzee adenovirus vector ChAdOx1 is independent of fiber and penton RGD loop sequences in mice. Sci Rep 2015;5:16756. 10.1038/srep16756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Müller U, Steinhoff U, Reis LF, et al. Functional role of type I and type II interferons in antiviral defense. Science 1994;264:1918–21. 10.1126/science.8009221 [DOI] [PubMed] [Google Scholar]
- 50. Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 1993;73:1155–64. 10.1016/0092-8674(93)90644-6 [DOI] [PubMed] [Google Scholar]
- 51. Kamphuis E, Junt T, Waibler Z, et al. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 2006;108:3253–61. 10.1182/blood-2006-06-027599 [DOI] [PubMed] [Google Scholar]
- 52. Pulverer JE, Rand U, Lienenklaus S, et al. Temporal and spatial resolution of type I and III interferon responses in vivo. J Virol 2010;84:8626–38. 10.1128/JVI.00303-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Meyer H, Sutter G, Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol 1991;72 (Pt 5):1031–8. 10.1099/0022-1317-72-5-1031 [DOI] [PubMed] [Google Scholar]
- 54. Blazejewska P, Koscinski L, Viegas N, et al. Pathogenicity of different PR8 influenza A virus variants in mice is determined by both viral and host factors. Virology 2011;412:36–45. 10.1016/j.virol.2010.12.047 [DOI] [PubMed] [Google Scholar]
- 55. Vashist N, Trittel S, Ebensen T, et al. Influenza-Activated ILC1s contribute to antiviral immunity partially influenced by differential GITR expression. Front Immunol 2018;9:505. 10.3389/fimmu.2018.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2021-220435supp004.pdf (70KB, pdf)
annrheumdis-2021-220435supp005.pdf (25.9KB, pdf)
annrheumdis-2021-220435supp002.pdf (302.1KB, pdf)
annrheumdis-2021-220435supp003.pdf (334.9KB, pdf)
annrheumdis-2021-220435supp001.pdf (909.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. All data relevant to the study are included in the article or uploaded as supplementary Information.