Abstract
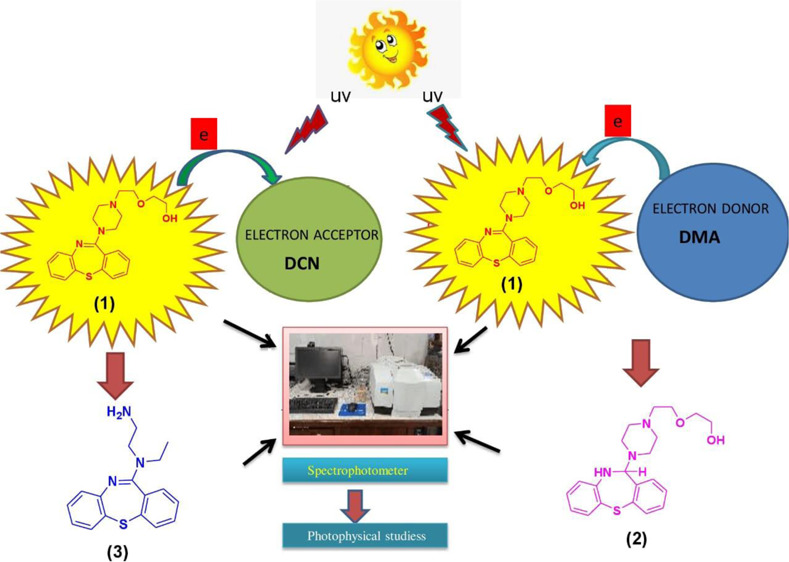
Quetiapine (QTP) (1), a psychotropic agent belonging to a chemical class, dibenzothiazepine derivatives, is photosensitive and photolabile. Its photochemistry was studied in the presence of an electron donor N,N-dimethylaniline (DMA) and an electron acceptor 1,4-dicyanobenzene (DCB) under anaerobic conditions. This resulted in photoinduced electron transfer-mediated transformation of drug QTP. Irradiation of Quetiapine (QTP, 1) in the presence of electron donor N,N-dimethylaniline (DMA) under anaerobic conditions in a photochemical reactor afforded one major photoproduct 2 when irradiation of QTP (1) was carried out in the presence of electron acceptor 1,4-dicyanobenzene (DCB) under similar conditions; it afforded 3 as a major photoproduct. These photoproducts were isolated and characterized on the basis of their spectral (IR, UV, 1H NMR, 13C NMR, and mass spectra) studies. The photophysical properties of Quetiapine were also determined in several solvents to investigate the relevance of the molecular structure in their photophysics and consequently in their photochemistry.
Introduction
Several common over-the-counter and prescription medications can trigger toxic and allergic reactions to natural or artificial ultraviolet light, a condition called photosensitivity.1 Adverse photosensitivity effects in patients are marked in responses that have been labeled as phototoxicity and photoallergy.2−8 Phototoxicity is the most common form of drug-induced photosensitivity. It may occur when the skin is exposed to the sun after certain drugs are injected, taken orally, or applied to the skin, when the drug absorbs the light; it gets activated and results in the formation of compounds that will have a direct damaging effect.
The photochemistry of pharmaceuticals is an area of growing concern, as the number of drugs found to be photosensitive and phototoxic is increasing. Photosensitivity occurs when chemicals in certain drugs absorb ultraviolet light, leading to a build-up of damaging compounds (ion radicals/photoproducts). Because all the adverse photobiological effects produced by photosensitive and phototoxic drugs are the consequences of photochemical reactions, it is important to stimulate more chemists to work on the molecular basis of photobiological problems.
The dated interest of photochemists in the properties of the electronically excited states of compounds of pharmaceutical importance has been rapidly increasing during the last decade. This is connected to the increasing number of cases of drug-photoinduced disorders, and it has also attracted considerable attention from a more fundamental photochemical standpoint. Thus, it is worthy to stress that studies performed on drugs bearing either a simple or complex chromophoric structure have provided remarkable contributions to the broad area of the molecular mechanisms of photoinitiated reactions.
Photosensitivity ensuring phototoxicity is defined as an alteration of cell function by an interaction between chemical and non-ionizing radiation, the response being linked to exaggerated sunburn. An adequate knowledge of the involved phototoxic mechanism is important to ensure safe handling, packaging, and labeling of the products to reduce the potential for adverse effects and to optimize drug therapy by developing new drug delivery systems, formulations, or therapeutic regimes.9,10 It has been reported that chemically there are two main types of photosensitization mechanisms caused by an excited sensitizer: type I (direct radical-mediated reactions) and type II (singlet oxygen-mediated reactions). Many important photosensitization reactions are type I processes, and the radical produced via the electron transfer process in photosensitization reactions has been reported for some important class of drugs including flouroquinolone,11 NSAID,12 phenothiazine,13 1-4-dihydropyridin,14 vasoregulator;15 they have been highlighted in recent publication. Hence, the study of photoinitiated electron transfer reactions in drugs is significant from a more fundamental photochemical standpoint16 to get information on molecular events of photodegradation and photosensitization.
Quetiapine is a short-acting atypical or second generation’s antipsychotic (SGAs) drug belonging to the class of dibenzothiazepine approved for the treatment of schizophrenia and bipolar disorder, along with an antidepressant to treat major depressive disorder.17,18 Quetiapine is an antagonist of a broad range of neurotransmitter receptors.19 Out of various antipsychotic drugs, there are three basic classes of medications such as conventional, atypical, and dopamine partial agonist antipsychotics drugs, which act principally on dopamine systems.20−23 Specifically the D1, D2 dopamine, the α1, α2 adrenoreceptor, and 5-HT1A, 5-HT2 serotonin receptor subtypes are antagonized. Quetiapine fumarate also has an antagonistic effect on the histamine H1 receptor. It has no significant affinity for cholinergic muscarinic or benzodiazepine receptors. Drowsiness and orthostatic hypotension associated with use may be explained by its antagonism of histamine H1 and adrenergic α 1 receptors, respectively.24
Quetiapine is well tolerated and characterized by fewer extrapyramidal symptoms (EPS) than the conventional antipsychotic drugs, and it is also effective to treat patients.25 Quetiapine is a phototoxic drug. Photodegradation of drugs may lead to loss in the activity and produce adverse effects due to the formation of toxic degradation products.25−27 The phototoxic effect can appear in any patient, provided that his skin is exposed to enough light doses and the photosensitizer is present at the appropriate concentration. Usually, these reactions appear immediately after the first exposure of UV light and are confined to the exposed areas of the skin.28,29 Quetiapine and a large number of drug molecules absorb radiation in the ultraviolet and/or visible region and thus show photosensitivity toward the UV light radiation.30−33 In continuation to our research interest in the present study, we have herein investigated the photochemical behavior of QTP (1) in the presence of an electron donor, N,N-dimethylaniline (DMA), and electron acceptor, 1,4-dicynobenzene (DCB), in UV light under anaerobic conditions with the aim of isolation, identification of its photoproducts, and elucidation of the molecular mechanism. Molecular oxygen (O2) is believed to play an important role in the photochemical oxidation reactions of drugs.
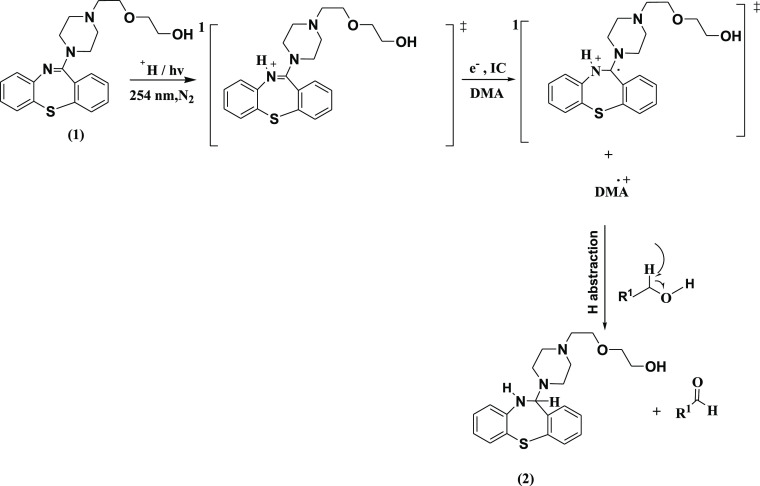
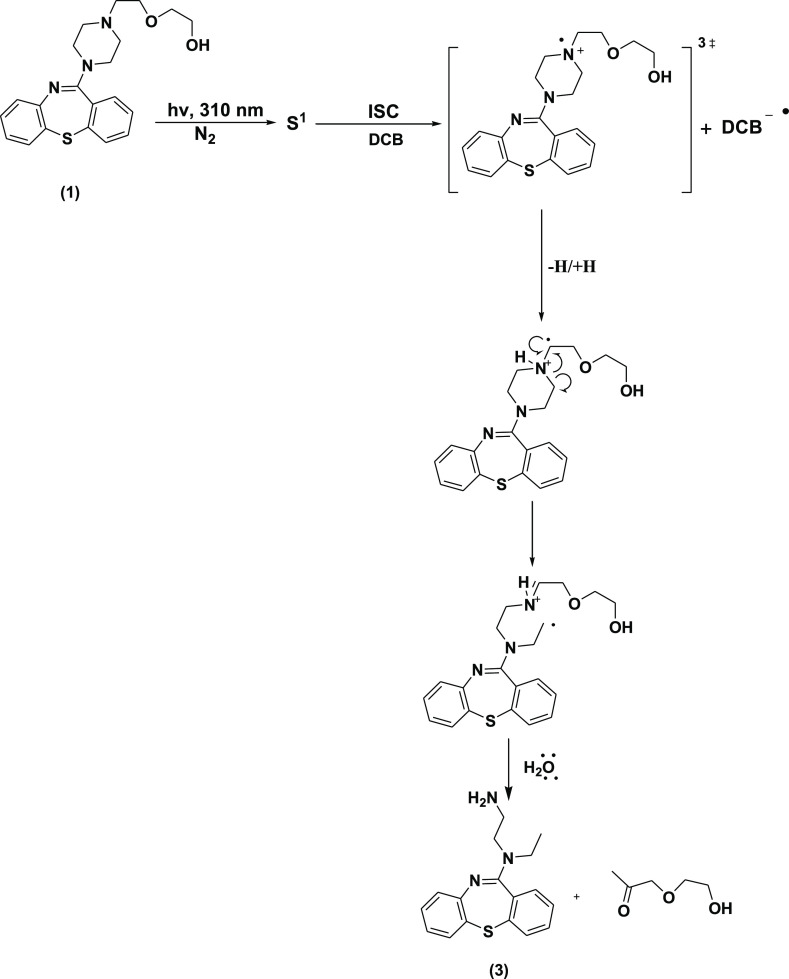
In the photodissociative study of a drug, detailed knowledge of photophysical and photochemical properties of photosensitizing drugs is essential to understand its mechanism of action in the biological system. In this connection, photodegradation of Quetiapine may have significance in rationalization of the observed phototoxicity of this drug. Irradiation of methanolic solution of QTP (1) in the presence of the electron donor (DMA) under anaerobic conditions results in the formation of one major photoproduct 2. When it was irradiated in the presence of an electron acceptor (DCB) under the same condition, photoproduct 3 was obtained. Formation of the photoproducts 2 and 3 was explained by the photoinduced electron transfer mechanism, and the results of photolyses are outlined in Schemes 1 and 2.
Scheme 1. Mechanistic Pathways for the Photodegradation of QTP in the Presence of DMA Under Anaerobic Conditions.
Scheme 2. Mechanistic Pathways for the Photodegradation of QTP in the Presence of DCB Under Anaerobic Conditions.
Results and Discussion
Irradiation of QTP (1) was carried out under anaerobic conditions at pH 4.0 in the presence of an electron donor N,N-dimethylaniline (DMA) in an immersion well-type photochemical reactor equipped with a medium pressure mercury vapor lamp. It afforded a major photoproduct 2-{2-[4-(10,11-dihydro-dibenzo [b,f] [1,4] thiazepin-11-yl)-piperazin-1-yl]-ethoxy} ethanol (2). The photoproduct was isolated and identified from its spectral (IR, 1H NMR, 13C NMR, and mass spectra) studies. The structure assigned to these products well corresponds to their observed spectral properties. The 1H NMR and 13C NMR spectra of compound 2 were similar to those of 1 except for the signals obtained due to the dibenzothiazepine ring. The IR absorption band at 3445 cm–1 (s, N–H), a broad 1H NMR signal at δ 4.024 (br. s, N–H) ppm, indicated a secondary amine functional group. A sharp 1H NMR signal for H-11 that appears at δ 5.048 ppm and its corresponding 13C NMR signal that appears at δ 70.6 ppm indicated that the imine functional group converted into a secondary amine product. This was also supported by λmax at 280 nm. The compound was thus assigned a structure similar to 2 with a molecular formula C21H27N3O2S (M+, 385). The mechanistic pathway for the formation of compound 2 is outlined in Scheme 1.
Irradiation of QTP (1) in the presence of an electron donor (DMA) under anaerobic conditions gave the photoproduct 2. The photoinduced electron transfer mechanism of 2 can be rationalized when irradiation leads to the electronic excited state of QTP (1). The excited state of QTP accepts an electron from the ground state of electron donor (DMA) molecule from corresponding radical anion (QTP•–) and radical cation (DMA•+). Subsequently, the generated quetiapine radical anion (QTP•–) undergoes photoinduced electron transfer by abstraction of the H atom from the solvent molecule followed by hydrolysis to yield photoproduct 2. The mechanism outlined in Scheme 1 well explains the observed reaction.
When QTP (1) was irradiated in the presence of DCB under anaerobic conditions, it afforded the corresponding product 3. The structure of the photoproduct was confirmed on the basis of their spectral (IR, 1H NMR, 13C NMR, UV, and mass spectra) properties. The 1H NMR and 13C NMR spectra of compound 3 were similar to those of 1 except for the signals obtained due to the aliphatic side chain. Two sharp single IR absorption bands at 3445 and 3226 cm–1 (s, 2N–H) and a broad 1H NMR signal at δ 2.122 (s, 2H, NH2) ppm indicated the presence of primary amine functional groups. The signals appeared due to the piperazine ring effect at δ 2.592 and 2.772 for H-13 and H-15, respectively. While 13C NMR for C-13 and C-15 appears at δ 40.5, 51.8 ppm indicated the cleavage of the piperazine ring. A more characteristic NMR signal for H-14 at δ 1.058 ppm and that for C-14 at δ 13.8 ppm indicated the formation of the CH3 group in the piperazine ring, and other signals due to the aliphatic side chain was missing from 3. This indicates that the piperazine ring was cleaved through the photoinduced electron transfer mechanism. This was also confirmed by λmax at 246 nm. The compound was thus assigned a structure similar to 3 with a molecular formula C17H19N3S (M+, 297).
A plausible mechanism for the observed reaction involves a photoinduced intermolecular electron transfer between the photoexcited state of QTP (1) and the ground state of the electron acceptor (DCB) molecule. It afforded the formation of the radical cation (QTP•+) and radical anion (DCB•–). Subsequently, the generated quetiapine radical cation (QTP•+) undergoes the hydrolysis of imine (>C=N) in the alcoholic solvent and a back electron transfer to afford the corresponding photoproduct 3. The abovementioned results are in good agreement with the mechanism, as depicted in Scheme 2.
Photophysical Properties
Effect of the Concentration of Photoproducts on the Absorption Intensity
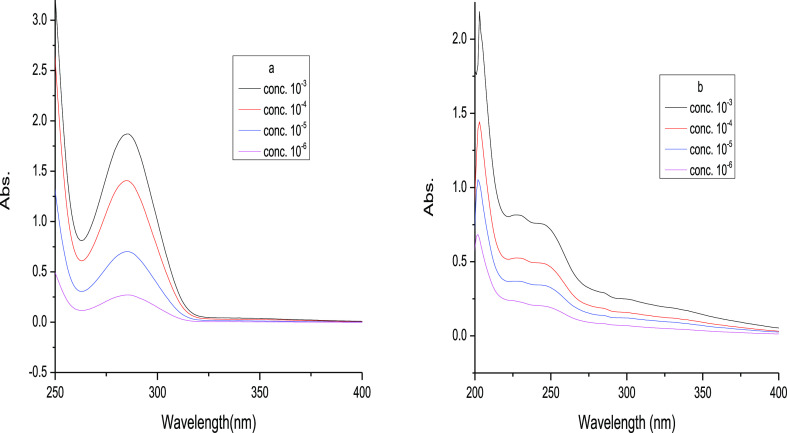
Figure 1 represents the absorption spectra of the photoproducts 2 and 3 with the continuous increase of the molar concentration in the range of 200–300 nm. The spectra of the photoproducts are different from the parent drug molecule, which suggested the formation of new photoproducts. From the figure, it is clear that the peak intensity of the photoproducts continuously increases with the increasing concentration of photoproducts; there is no shift in the absorption maxima, and the nature of the absorption profile remains almost constant, as shown in Figure 1.
Figure 1.
Absorption intensity vs wavelength plot of photoproducts 2 and 3 with continuously increasing molar conc.; 10–3 to 10–6 M, temp.; 15–20 °C.
Effect of Concentration of Photoproducts on Fluorescence Intensity
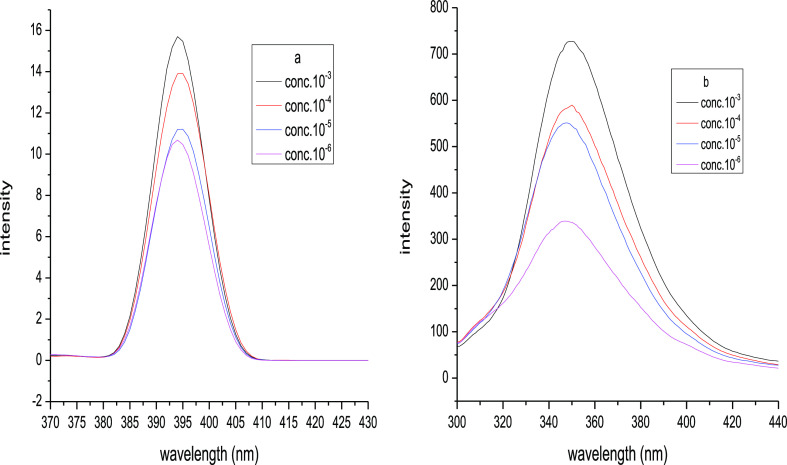
The fluorescence spectra of the molecules deal with the excited state of the molecule. The spectra of the photoproducts were carried out with continuously increasing the molar concentrations of the compound at a fixed excited wavelength of the molecule in the range of 300–450 nm. From the figure, it is clear that the peak intensity of photoproducts increases by about 10 times on increasing the concentration of the photoproduct. The photoproducts 2 and 3 were excited at different wavelengths 280 and 246 nm, respectively. The fluorescence intensity vs wavelength plot of the photoproducts is shown in Figure 2.
Figure 2.
Fluorescence intensity vs. wavelength plot of photoproducts 2 and 3 with continuously increasing molar conc.; 10–3 to 10–6 M, temp.; 15–20 °C.
Conclusions
In conclusion, the drug Quetiapine (1) undergoes photochemical transformations in the presence of an electron donor (DMA) and an electron acceptor molecule (DCB) under anaerobic conditions through a photoinduced electron transfer mechanism. The importance of the photophysical studies is that at higher concentrations, photoproducts absorb a large fraction of light radiation and the excited state of molecule may interact with the biological system and the photoproducts may undergo further dissociation in the presence of UV light and exhibit phototoxicities comparable to or even higher than that of the parent drug at the same concentration. On the basis of obtained results, the electron transfer plays a significant role in the photodegradation of Quetiapine. The involvement of radical ions may account for the phototoxic effects, sometimes observed, in therapeutic uses of the drug.
Therefore, the obtained data confirmed that the adequate light protection should be adopted for the handling and storage of Quetiapine, which suggested that excessive sunlight should be avoided after the drug consumption.
Experimental Section
Materials and Chemicals
All chemicals used were of analytical grade and pharmaceutical grade and were purchased from the commercial store Qutipin (Symbiosis Pharmaceuticals, India). The purity of the drug extracted was checked by TLC, and its melting point was compared with the literature value. N,N-dimethylaniline (DMA) and 1,4-dicyanobenzene (DCB) were purchased from Sigma-Aldrich (India).
Apparatus
Photochemical reactions were carried out in a quartz fitted immersion well photochemical reactor equipped with a 400 W medium pressure mercury vapor lamp with continuous supply of water. The incident photon flux of the irradiation setup was 8.72107 E/min, as determined by using ferrioxalate actinometry.34 IR spectra were recorded in KBr discs on a PerkinElmer model spectrum RX1. 1H NMR and 13C NMR spectra were recorded on a Bruker DRX-300 spectrometer using SiMe4 as internal standard. Electron ionization mass spectrometry (EIMS) was obtained on a VG-ZAB-HS mass spectrometer. High-resolution mass spectra were obtained with a VG-ZAB-BEQ9 spectrometer at 70 eV ionization voltage. Column chromatography was performed on silica gel 60 (70–230 mesh). TLC was carried on Merck silica gel 60 F254 (0.2 mm-thick plates). UV absorbance spectra were performed on a double beam Shimadzu UV1800 spectrophotometer (Shimadzu, Kyoto, Japan) equipped with a 150 W deuterium lamp, and fluorescence spectra were recorded on a Hitachi F-2500 (Hitachi, Tokyo, Japan) spectrophotometer equipped with a 150 W Xenon lamp using a quartz cell of 1.0 cm path length. The widths of both the excitation slit and emission slit were set at 5.0 and 10.0 nm.
General Photoirradiation Procedure
An alcoholic solution of Quetiapine (QTP, 1) dissolved in methanol was stirred and flushed with nitrogen for 1 h before irradiation and was kept bubbling during the irradiations in an immersion well-type photoreactor (quartz). The progress of reaction was monitored by thin layer chromatography on precoated silica gel TLC plates using a chloroform–methanol (94:06) mixture. After the completion of reaction (when desired conversions have reached), the solvent was removed in a rotary evaporator and products were purified by silica gel column chromatography.
Irradiation of QTP in the Presence of DMA under Anaerobic Conditions
A methanolic solution of QTP (1) (1.0 gm, 0.5 mM) was irradiated at 254 nm in the presence of 2.5 mole equivalents of electron donor35,36 (DMA) with continuous stirring at 30–40 °C for 5–6 h under a N2 atmosphere. After following the steps as described in the general photoirradiation procedure, the photoproduct 2 was obtained, which exhibit the following spectral properties.
2-{2-[4-(10,11-Dihydro-dibenzo [b,f] [1,4] Thiazepin-11-yl)-piperazin-1-yl]-ethoxy} Ethanol (2)
Yield: 50 mg (5.0%); UV λmax (MeOH) 280 nm (w); HRMS calcd. for (M+) C21H27N3O2S, 385.1825; found, 385.1824, IR (KBr/cm–1) 3445 (s, N–H), 3310 (br. O–H), 3045, 3012, 2980, 2950, 1478, 1365, 1212, 978, 818, 764; 1H NMR (DMSO-d6, 400 MHz): δ 6.823 (d, 1H, J = 8.0, Ar–H), 7.112 (m, 1H, Ar–H), 7.208 (d, 1H, J = 7.6, Ar–H), 7.228–7.434 (m, 4H, Ar–H), 7.508 (m, 1H, Ar–H), 5.048 (s, 1H, 11), 4.024 (br. s, N–H, 10), 3.702 (t, 2H, 20), 3.568 (t, 2H, 19), 3.454 (t, 2H, 17), 2.534 (t, 2H, 16), 2.468–2.548 (m, 8H, 13,13′,14,14′), 2.122 (s, 1H, 21) ppm; 13C NMR (DMSO-d6, 400 MHz): δ 146.4, 138.2, 132.3, 132.0, 131.2, 130.8, 129.8, 127.9, 127.7, 126.5, 115.8, 112.6, 72.8, 67.3, 60.3, 56.8, 52.4 ppm; MS: m/z: 386 (M+ + 1), 385 (M+), 322 (M+ – 63), 211 (M+ – 174).
Irradiation of QTP in the Presence of DCB under Anaerobic Conditions
A methanolic solution of QTP (1) (1.0 g, 0.5 mM) was irradiated at 310 nm in the presence of 2.0 mole equivalents of electron acceptor37,38 (DCB) with continuous stirring at 30–40 °C for 5–6 h under a N2 atmosphere. After following the steps as described in the general photoirradiation procedure, the photoproduct 3 was obtained, which exhibit the following spectral properties.
N1-Dibenzo [b,f][1,4] Thiazepin-11-yl-N1-ethyl-ethane-1,2-diamine (3)
Yield: 38 mg (3.8%); UV λmax (MeOH) 246 nm (s); HRMS calcd. for (M+) C17H19N3S, 297.4179; found, 297.4178, IR (KBr/cm–1) 3445, 3226 (s, 2 N–H), 3030, 3010, 1648 (>C=N), 1415, 1337, 1308, 1256, 970, 838, 794, 765; 1H-NMR (DMSO-d6, 400 MHz, ppm): δ 7.554 (m, 1H, Ar–H), 7.254–7.402 (m, 4H, Ar–H), 7.204 (d, 1H, J = 7.6, Ar–H), 7.102 (m, 1H, Ar–H), 6.854 (d, 1H, J = 8.0, Ar–H), 2.814 (t, 2H, J = 6.4, CH2), 2.772 (t, 2H, J = 6.4, CH2), 2.598 (q, 2H, J = 6.8, CH2), 2.122 (s, 2H, NH2), 1.058 (t, 3H, J = 6.8, CH3) ppm; 13C-NMR (DMSO-d6, 400 MHz): δ 164.4, 156.7, 135.4, 132.3, 131.1, 130.5, 129.2, 128.3, 127.6, 127.1, 126.5, 125.3, 122.6, 51.8, 42.1, 40.5, 13.8 ppm; MS: m/z: 298 (M+ + 1), 297 (M+), 296 (M+ – 1), 281 (M+ – 16), 211 (M+ – 86).
Acknowledgments
The author acknowledges the facilities provided by the Chairman, Department of Chemistry, Aligarh Muslim University, Aligarh, India, and are thankful to Prof. Jawed Iqbal for utmost support throughout the preparation of this manuscript.
The authors declare no competing financial interest.
References
- Condorelli G.; Constanzo L. L.; De Guidi G.; Giuffrida S.; Sortino S. Molecular Mechanism of Drug Photosensitization. 7. Photocleavage of DNA sensitized by suprofen. Photochem. Photobiol. 1995, 62, 155–161. 10.1111/j.1751-1097.1995.tb05252.x. [DOI] [PubMed] [Google Scholar]
- Großkopf J.; Kartz T.; Rigotti T.; Bach T. Enantioselective photochemical reactions enabled by triplet energy transfer. Chem. Rev. 2021, 10.1021/acs.chemrev.1c00272. [DOI] [PubMed] [Google Scholar]
- Tong Y.; Liu Y.; Chen Q.; Mo Y.; Ma Y. Long lived triplet excited state bischrmophopric iridium photocatalyst for controlled photo-mediated atom transfer radical polymerization. Macromol 2021, 54, 6117–6126. 10.1021/acs.macromol.1c00482. [DOI] [Google Scholar]
- Delgado J. A. C.; Correia J. T. M.; Pissinati E. F.; Paixão M. W. Biocompatible photoinduced alkylation of dehydroalanine for the synthesis of unsaturated a- amino acid. Org. Lett. 2021, 23, 5251–5255. 10.1021/acs.orglett.1c01781. [DOI] [PubMed] [Google Scholar]
- Rehan Zaheer M.; Gupta A.; Iqbal J.; Zia Q.; Ahmad A..; Roohi; Owais M.; Hashlamon A.; Setapar S. H. M.; Ashraf G. M.; Alieve G. Molecular mechanism of drug photodegradation and photosensitization. Curr. Pharm. Des. 2016, 22, 768–782. 10.2174/1381612822666151209151408. [DOI] [PubMed] [Google Scholar]
- Sortino S.; Petralia R.; Darcy R.; Donohue R.; Mazzaglia A. Photochemical outcome modification of diflunisal by a novel cationic amphiphilic cyclodextrin. New J. Chem. 2003, 27, 602–608. 10.1039/b209157g. [DOI] [Google Scholar]
- Foote C. S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. 10.1111/j.1751-1097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- Onoue S.; Yamauchi Y.; Kojima T.; Igarashi N.; Tsuda Y. Analytical studies on photochemical behavior of phototoxic substances; effect of detergent additives on singlet oxygen generation. Pharm. Res. 2008, 25, 861–868. 10.1007/s11095-007-9383-4. [DOI] [PubMed] [Google Scholar]
- Sortino S.; Scaiano J. C.; Giuffrida S. Transient photochemistry of naphazoline in a protein environment. New J. Chem. 1999, 23, 1159–1162. 10.1039/a906513j. [DOI] [Google Scholar]
- Sortino S.; Cosa G.; Scaiano J. C. pH Effect on the efficiency of the photodeactivation pathways of naphazoline: a combined steady state and time resolved study. New J. Chem. 2000, 24, 159–163. 10.1039/b000712i. [DOI] [Google Scholar]
- Barraja P.; Caracausi L.; Diana P.; Diana A.; Montalbano A.; Cirrincione G.; Brun P.; Palù G.; Castagliuolo I.; Dall’Acqua F.; Vedaldi D.; Salvador A. Synthesis of pyrrolo [3, 2-h] quinolinones with good photochemotherapeutic activity and no DNA damage. Bioorg. Med. Chem. 2010, 18, 4830–4843. 10.1016/j.bmc.2010.04.080. [DOI] [PubMed] [Google Scholar]
- Boscá F.; Miranda M. A.; Carganico G.; Mauleón D. Photochemical and photobiological properties of ketoprofen associated with the benzophenone chromophore. Photochem. Photobiol. 1994, 60, 96–101. 10.1111/j.1751-1097.1994.tb05073.x. [DOI] [PubMed] [Google Scholar]
- García C.; Piñero L.; Oyola R.; Arce R. Photodegradation of 2-chloro substituted phenothiazines in alcohols. Photochem. Photobiol. 2009, 85, 160–170. 10.1111/j.1751-1097.2008.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y. J.; Fang J. G.; Ma L. P.; Yang L.; Liu Z. L. Inhibition of free radical-induced peroxidation of rat liver microsomes by resveratrol and its analogues. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2003, 1637, 31–38. 10.1016/s0925-4439(02)00174-6. [DOI] [PubMed] [Google Scholar]
- Miranda M. A.; Garcia H. 2, 4, 6-Triphenylpyrylium tetrafluoroborate as an electron-transfer photosensitizer. Chem. Rev. 1994, 94, 1063–1089. 10.1021/cr00028a009. [DOI] [Google Scholar]
- Alexander G. C.; Gallagher S. A.; Mascola A.; Moloney R. M.; Stafford R. S. Increasing off-label use of antipsychotic medications in the United States, 1995–2008. Pharmacoepidemiol Drug Saf 2011, 20, 177–184. 10.1002/pds.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinon B. J.; Noordsy D. L.; Liu-Seifert H.; Gulliver A. H.; Ascher-Svanum H.; Kollack-Walker S. Randomized, double-blind 6-month comparison of olanzapine and quetiapine in patients with schizophrenia or schizoaffective disorder with prominent negative symptoms and poor functioning. J. Clin. Psychopharmacol. 2006, 26, 453–461. 10.1097/01.jcp.0000236658.16286.25. [DOI] [PubMed] [Google Scholar]
- Andrezina R.; Josiassen R.-C.; Marcus R.-N.; Oren D.-A.; Manos G.; Stock E.; Carson W.-H.; Iwamoto T. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double-blind, placebo-controlled comparison with intramuscular haloperidol. Psychopharma 2006, 188, 281–292. 10.1007/s00213-006-0541-x. [DOI] [PubMed] [Google Scholar]
- Miyamoto S.; Duncan G.E.; Marx C.E.; Lieberman J.A. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 2005, 10, 79–104. 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Meltzer H. Y.; Li Z.; Kaneda Y.; Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1159–1172. 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Guzman F.Citalopram Vs. Escitalopram; Psychopharmacology Institute, 5Sept, 2014.
- Kapur S.; Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? A new hypothesis. Am. J. Psychiatry 2001, 158, 360–369. 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- Dedania Z. R.; Sheth N. R.; Dedania R. R. Stability indicating high-performance thin-layer chromatographic determination of Quetiapine Fumarate. Int. J. Res. Pharm. Sci. 2013, 4, 2406–2414. [Google Scholar]
- Cheer S. M.; Wagstaff A. J. Quetiapine. A review of its use in the management of schizophrenia. CNS Drugs 2004, 18, 173–199. 10.2165/00023210-200418030-00004. [DOI] [PubMed] [Google Scholar]
- Aman W.; Thoma K. The influence of formulation and manufacturing process on the photostability of tablets. Int. J. Pharm. 2002, 243, 33–41. 10.1016/s0378-5173(02)00110-2. [DOI] [PubMed] [Google Scholar]
- Tønnesen H. H. Formulation and stability testing of photolabile drugs. Int. J. Pharm. 2001, 225, 1–14. 10.1016/s0378-5173(01)00746-3. [DOI] [PubMed] [Google Scholar]
- Dubakiene R.; Kupriene M.; Kaunas M. Scientific problems of photosensitivity. Medicina 2006, 42, 619–624. [PubMed] [Google Scholar]
- White Ian R.Phototoxic and Photoallergic Reactions. Textbook of contact dermatitis; Springer: Berlin, Heidelberg, 1995; pp 75–88. [Google Scholar]
- Ahmad I.; Fasihullah Q.; Vaid F. H. M. Effect of light intensity and wavelengths on photodegradation reactions of riboflavin in aqueous solution. J. Photochem. Photobiol., B 2006, 82, 21–27. 10.1016/j.jphotobiol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Greenhill John V.; McLelland M. A. In Progress in Medicinal Chemistry; Ellis G. P., West G. B., Eds.; Elsevier: Amsterdam, 1990; pp 51–121. [DOI] [PubMed] [Google Scholar]
- Boreen A. L.; Arnold W. A.; McNeill K. Photodegradation of pharmaceuticals in the aquatic environment: a review. Aquat. Sci. 2003, 65, 320–341. 10.1007/s00027-003-0672-7. [DOI] [Google Scholar]
- Singh S.; Bakshi M. Guidance on the conduct of stress tests to determine inherent stability of drugs. Pharm. Tech. On-Line India 2000, 24, 1–14. [Google Scholar]
- Trivedi R. K.; Patel M. C. Development and validation of a stability indicating RP-UPLC method for determination of quetiapine in pharmaceutical dosage form. Sci. Pharm. 2011, 79, 97–111. 10.3797/scipharm.1009-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchard C. G.; Parker C. A. A new sensitive chemical actinometer. II Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. London, Ser. A 1956, 153, 518–536. 10.1098/rspa.1956.0102. [DOI] [Google Scholar]
- Scherer P. O. J. Intramolecular reorganization of the electron donor N, N-dimethylaniline. J. Phys. Chem. A 2003, 107, 8327–8329. 10.1021/jp027855d. [DOI] [Google Scholar]
- Boilet L.; Buntinx G.; Lefumeux C.; Poizat O. Ultrafast photoinduced electron transfer from N, N, N′, N′-tetramethyl-p-phenylenediamine and N, N, N′, N′-tetramethylbenzidine to dichloromethane. J. Photochem. Photobiol., A 2004, 163, 529–536. 10.1016/j.jphotochem.2004.02.011. [DOI] [Google Scholar]
- Yoshimi Y.; Hayashi S.; Nishikawa K.; Haga Y.; Maeda K.; Morita T.; Itou T.; Okada Y.; Ichinose N.; Hatanaka M. Influence of solvent, electron acceptors and arenes on photochemical decarboxylation of free carboxylic acids via single electron transfer (SET). Molecules 2010, 15, 2623–2630. 10.3390/molecules15042623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould I. R.; Godleski S. A.; Zielinski P. A.; Farid S. Aminosilanes as two-electron donors: A technological application of radical cation chemistry. Can. J. Chem. 2003, 81, 777–788. 10.1139/v03-073. [DOI] [Google Scholar]