Abstract
A practical route to 2-(2-(2-methyl-1,3-dioxolan-2-yl)ethyl)cyclohexan-1-one was developed, featuring the use of inexpensive starting materials/reagents and readily attainable reaction conditions. The overall transformation was achieved in 53% yield with one chromatographic purification via NaOH-mediated aldol condensation, ethylene glycol protection of the ketone group in the presence of HC(OEt)3/concd HCl, saturation of the C=C bond and the benzene ring with Al–Ni alloy in aqueous KOH, and oxidation of the intermediate cyclohexanol with aqueous NaClO/TEMPO/KBr.
Introduction
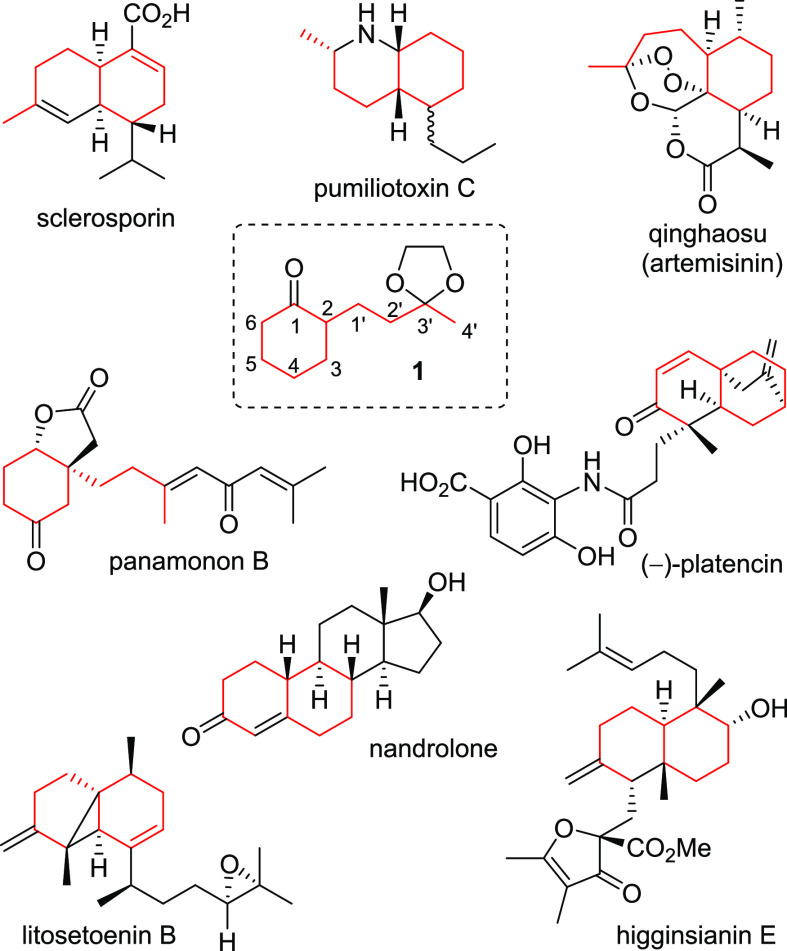
During our studies on synthesis1,2 of organic peroxides/natural products and development of synthetic methodologies, at times, we found ourselves in need of a monosubstituted cyclohexanone, 2-(2-(2-methyl-1,3-dioxolan-2-yl)ethyl)cyclohexan-1-one (1, Figure 1), either as a convenient starting material for constructing various target molecules or as a suitable substrate for testing synthetic methodologies. Through the repeated accidental demand for 1 in different projects over the years, we gradually come to realize the so far largely unrecognized potential of this small compound as a versatile building block in synthesis in general. Indeed, a great many natural products and bioactive compounds contain a cyclohexane motif and in quite a few cases, it is even possible to identify an intact carbon framework of 1 incorporated in the given target structures as exemplified by sclerosporin,3a pumiliotoxin C (alkaloid cis-195 J),3b qinghaosu,4 panamonon B,1c platencin,5 nandrolone,6 litosetoenin B,7a and higginsianin E7b (Figure 1). Also, ketone 1 indeed allows for many different ways to incorporate itself into various target structures. For example, the methylene groups α to the carbonyl group in 1 can be regioselectively deprotonated under either kinetic8 or thermodynamic9 control to offer the possibility of reacting at either C-2 or C-6. Alternatively, the enolates may be further converted to the corresponding silyl enol ether and treated with Pd (OAc)210 or IBX11 to install a C=C bond conjugate to the carbonyl group at either C2/C-3 or C-5/C-6 to activate either C-3 or C-5 as desired. Also, in either case, C-4 would be activated as it becomes an allylic position. The silyl enol ethers may be coupled with ring-expansion12 (mediated by a 3/6 ring fused bicyclic species) to generate the corresponding cycloheptenones. By using Stoltz5,13 allylation, even enantioselective installation of a quaternary center at C-2 is possible. In addition, the cyclohexanone ring of 1 may be regioselectively oxidized to furnish the corresponding lactone and/or linear multifunctionalized product(s). Finally, the functionalized sidechain of 1 may also open up many additional options, including annulation to construct a bicyclic system and attachment of other subunits to the desired positions of the chain.
Figure 1.
Structures of 1 and a few representatives of a large number of natural products/bioactive compounds that apparently incorporate the carbon framework (red) of ketone 1.
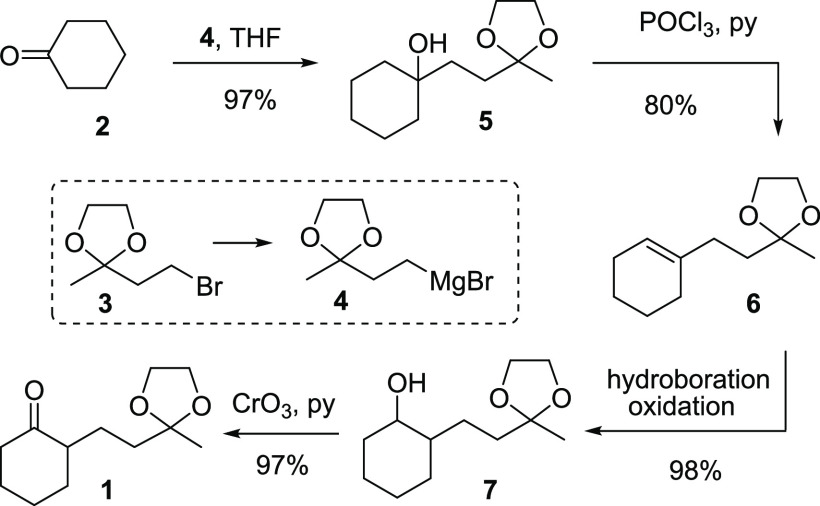
However, despite its unassuming structure, ketone 1 is far not as readily attainable as one would presume, especially when needed in large quantities (e.g., >10 g).14 The first15 documented synthesis of 1 appeared in 1976, which was a four-step sequence: (1) the addition of the Grignard reagent 4 to cyclohexanone, (2) dehydration, (3) hydroboration/oxidation,16 and (4) Collins17 oxidation (Scheme 1).
Scheme 1. The First Route to Ketone 1 Documented in the Literature.
Later, in 1992, Moeller and Tiano18 reported another route that relied on the alkylation of enamine 9 with iodide 10(19) (Scheme 2). This approach is simpler than the first one, but still suffered from serious drawbacks, with the yield for the alkylation step being only 31%.20 In a previous1a work, we attempted direct alkylation of cyclohexanone 2 with iodide 10 and obtained 1 in 58% yield from 2 (or <28%21 overall from 11). However, because of the involvement of LDA (lithium diisopropylamide), HMPA (N-hexamethyl phosphoric triamide, a known carcinogen), and strict moisture-free/oxygen-free conditions, that approach also has apparent confines and is thus only suitable for small-scale synthesis. The limitations of the existing methods, together with the hidden problem common to all iodide 10-based routes, i.e., the capricious22 (often lower than originally reported) yield of iodide 10, made it rather difficult to acquire 1 in ample quantities for large-scale and/or multistep syntheses. All these prompted us to seek a more practical route to ketone 1, which may be readily adapted for multi-ten gram or even larger scales without involving either expensive reagents or moisture-free/oxygen-free conditions.
Scheme 2. Two Previous Routes to 1, with the Most Convenient/Practical Entry to the Alkylating Agent 10 Shown at the Bottom (Boxed).
Results and Discussion
The eventually developed sequence is shown in Scheme 3. The desired carbon framework was derived from the inexpensive and readily available salicylaldehyde 13 and acetone.
Scheme 3. The Present Route to Ketone 1.
The aldol condensation reaction was performed in aqueous NaOH at ambient temperature, affording the known23 enone-phenol 14. The side products caused by unavoidable concurrent self-condensation of acetone were effectively removed by extraction of the basic reaction mixture with Et2O before final acidification with HCl to generate phenol 14 from the corresponding enolate. The crude 14 thus obtained was proven to be pure enough and used as such in the next step of reaction.
Subsequent protection of the ketone group was achieved via reaction with HO(CH2)2OH in THF with concd HCl as the catalyst and HC(OEt)3 as a water scavenger. To remove the undesired non-phenolic side products, the acidic reaction mixture was first basified to pH 13–14 by addition of NaOH and extracted with Et2O. The strongly basic aqueous phase was then neutralized very carefully with diluted HCl to pH 6 to give crude phenol-ketal 15,24 which was pure enough for the next step.25
Saturation of the C=C bond and the phenyl ring in 15 was first attempted by hydrogenation over conventionally employed catalysts including Ra–Ni or 10% Pd–C with or without an acetyl protecting group at the phenol OH. However, the yield of the desired cyclohexanol 7 varied drastically from run to run without any obvious causes. Involvement of H2 in the experiment also imposed additional inconvenience.26 For these reasons, later, we turned our attention to the inexpensive Al–Ni alloy (the starting material for preparation of Raney Nickel), which had been reported to be effective in the saturation of various phenols in aqueous KOH or NaOH at 90 °C.27 Use of Al–Ni alloy to saturate the C=C bond conjugate to either a phenyl ring or a carbonyl group is also known, though the reactions were performed under slightly different conditions (without added KOH or NaOH).28,29
Gratifyingly, stirring of crude ketal 15 with Ni–Al alloy in aqueous KOH at 90–91 °C for 14 h indeed led to full reduction30 of both the phenyl ring and the C=C bond, giving the expected alcohol 7 as a 1:1 mixture of two diastereomers along with small amounts of side product(s)31,32 that were substantially more polar than 7.
Direct use of the crude 7 in the subsequent oxidation was then examined under the NaClO/TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl)/KBr33 conditions.34 The reaction proceeded smoothly as expected. However, the product 1 thus obtained was very difficult to purify because of the similar polarity of ketone 1 and the side product (which later was proven to be the known35 dione 18). In comparison, the polarity difference observed between the components of crude 7 was much larger. Therefore, the chromatographic purification initially planned to be done at the end of the whole synthetic sequence was then performed before the final oxidation.
Removal of the impurity (i.e., diol 16) from crude 7 indeed solved the problem. Thus, oxidation of the 1:1 mixture of cis- and trans-7 afforded clean 1 in 91% yield after removal of the TEMPO-related colored species by filtration through silica gel.36
Conclusions
We have developed an alternative approach to the synthesis of ketone 1, featuring the use of inexpensive starting materials/reagents, readily attainable reaction conditions, and rather simple operations. As a consequence, ketone 1 now becomes much easier37 to access than ever before; the main barrier to the broad application of this versatile bifunctional building block in synthesis thus has been removed.
Experimental Section
General Remarks
All solvents and reagents were used as received from commercial sources. Column chromatography was performed on silica gel (300–400 mesh) under slightly positive pressure. PE stands for petroleum ether (b.p. 60–90 °C). The Al–Ni alloy had an Al/Ni ratio of 50/50. Melting points were uncorrected (measured on a hot-stage melting point apparatus equipped with a microscope). IR spectra were measured with a Nicolet 380 infrared spectrophotometer. NMR spectra were recorded with an Agilent DD2 500 NMR (operating at 500 MHz for 1H). HRMS (EI) data for 15 were obtained with a Waters Premier GC-TOF-MS spectrometer. HRMS (FI) data for 7 were obtained with a JEOL-AccuTOF-GCv4G-GCT MS spectrometer.
(E)-4-(2-Hydroxyphenyl)but-3-en-2-one (14)
To a solution of salicylic aldehyde (20.00 g, 163.77 mmol) in acetone (118 mL) stirred at ambient temperature was added a freshly prepared aq. NaOH (2.0 M, 98 mL, ca. 196 mmol). The colorless solution of salicylic aldehyde first turned yellow, then burgundy, and finally dark-red. The turbid mixture was stirred at ambient temperature for 13 h (TLC on silica gel plate, developed with 3:1 PE/EtOAc, showed full consumption of 13). Acetone was removed by rotary evaporation under an aspirator vacuum. Water (100 mL) was added to the dark-red residue (containing some precipitates), followed by Et2O (100 mL). The mixture was stirred at ambient temperature for ca. 20 min before being transferred to a separatory funnel. The dark-red aqueous layer was drained, and the yellow upper (ethereal) layer was washed with small portions of 2.0 M NaOH and H2O (until TLC showed no more 14 in the ethereal phase). The combined aqueous layers (in a beaker) were carefully acidified with 2% HCl (ca. 160 mL). Some yellow solids formed during the acidification. To the mixture was added EtOAc (400 mL). The two-phase mixture was transferred to a separatory funnel. The lower (aqueous) layer (ca. pH 5) was drained. The yellow organic layer in the funnel was washed with brine (30 mL) and dried over anhydrous Na2SO4. Removal of the drying agent by filtration and the solvent by rotary evaporation left crude 14 as a yellow solid (26.66 g, 164 mmol, 100% from 13), which was pure enough and used as such in the next step. For comparison, the following data were also collected on crude 14: m.p. 139–142 °C. 1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 16.4 Hz, 1H), 7.52–7.47 (a lump, 0.4H, OH), 7.47 (br dd, J = 7.6, 1.2 Hz, 1H), 7.263 (br s, 0.6H, OH), 7.260 (br dt, J = 1.5, 7.5, 1H), 7.05 (br d, J = 16.4 Hz, 1H), 6.93 (br d, J = 7.5 Hz, 1H), 6.92 (br t, J = 7.5 Hz, 1H), 2.43 (s, 3H); 13C{1H} NMR (125 MHz, CDCl3) δ 201.4, 156.2, 141.0, 132.1, 129.9, 127.9, 121.6, 120.8, 116.7, 27.0. FT-IR (KBr) 3355, 3065, 3023, 1673, 1641, 1618, 1603, 1499, 1462, 1420, 1356, 1326, 1305, 1253, 1223, 1191, 1159, 1092, 1042, 1008, 972, 862, 795, 761 cm–1.
(E)-2-(2-(2-Methyl-1,3-dioxolan-2-yl)vinyl)phenol (15)
To a yellow solution of the above-obtained crude enone 14 (22.11 g, 136 mmol) in THF (220 mL) stirred at ambient temperature were added HO(CH2)2OH (76 mL, 1.36 mol), HC(OEt)3 (45 mL, 272 mmol), and concd HCl (36%, 0.14 mL). The dark-red mixture was stirred at ambient temperature for 7 h (TLC showed completion of the reaction after stirring for 5 h). Freshly prepared aq. NaOH (2.0 M, 40 mL) was carefully added. Most of the organic volatiles in the mixture were removed by rotary evaporation under aspirator vacuum. To the residue was added H2O (20 mL) followed by Et2O (100 mL). The two-phase mixture was stirred at ambient temperature for 15 min before being transferred to a separatory funnel. The dark-red lower (aqueous) layer was drained. The upper yellow ethereal layer was washed with small portions of 2.0 M NaOH and H2O until TLC showed the absence of 15 in the ethereal phase. The combined aqueous layers were acidified very carefully with 2% HCl to pH 7 (further acidification would lead to significant hydrolysis of the ketal). The red-brown mixture was transferred to a separatory funnel. EtOAc (400 mL) was added. Small portions of 2% HCl were then introduced slowly with the funnel shaken after each addition until two yellow clear phases clearly formed on standing. The lower (aqueous) layer was drained (ca. pH 6) and extracted with EtOAc (300 mL). The combined organic layers were washed with water (40 mL × 3) and brine (30 mL × 3) and dried over anhydrous Na2SO4. Removal of the drying agent by filtration and the solvent by rotary evaporation left crude 15 as a yellow solid (23.97 g, 116 mmol, 85% from 14, m.p. 116–121 °C with the 1H and 13C{1H} NMR spectra for this crude sample shown in the Supporting Information for comparison), which was used as such in the next step.
A small portion of the above crude 15 was purified by column chromatography (6:1 PE/EtOAc) on silica gel to give the following data for pure 15: m.p. 118–120 °C. 1H NMR (500 MHz, CDCl3) δ 7.37 (br dd, J = 7.8, 1.4 Hz, 1H), 7.13 (br dt, J = 7.8, 1.6 Hz, 1H), 6.96 (br d, J = 16.1 Hz, 1H), 6.90 (br dt, J = 7.3 Hz, 1H), 6.78 (br d, J = 8.0 Hz, 1H), 6.23 (d, J = 16.1 Hz, 1H), 5.54–5.50 (a lump, 1H, OH), 4.06–3.96 (m, 4H), 1.59 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 153.5, 131.1, 129.1, 128.0, 124.6, 123.4, 121.0, 116.1, 108.1, 64.7, 25.4. FT-IR (KBr) 3356, 2985, 2894, 1644, 1619, 1601, 1499, 1454, 1371, 1357, 1043, 1266, 1181, 1159, 1089, 1040, 989, 955, 940, 851, 816, 761 cm–1. MS (EI) m/z (%): 206 (M+, 16), 191 (M+–CH3, 100), 145 (45), 131 (10), 115 (13), 103 (29); HRMS (EI positive) m/z: M+ calcd for C12H14O3 206.0937, found 206.0941.
(1R*,2R*)-2-(2-(2-Methyl-1,3-dioxolan-2-yl)ethyl)cyclohexan-1-ol (cis-7) and (1S*,2R*)-2-(2-(2-Methyl-1,3-dioxolan-2-yl)ethyl)cyclohexan-1-ol (trans-7)
A solution of KOH (6.38 g, 113.7 mmol) in H2O (425 mL) was added to a 1 L three-necked flask equipped with a mechanical stirrer38 and a condenser. The above-obtained crude 15 (19.54 g, 94.74 mmol) was then introduced with stirring at ambient temperature through the side neck. The mixture turned dark-red soon (still with some undissolved solids). Stirring was continued at ambient temperature for 20 min. Al–Ni alloy (58.62 g) was added in small portions over ca. 10 min. Stirring was continued at ambient temperature for another 30 min and then in an oil bath (103 °C). The temperature of the reaction mixture gradually reached 90–91 °C and remained stable throughout the whole reaction time. The dark-red mixture gradually turned first yellow, then gray, and finally milky (with some dark solids precipitated at the bottom). After heating/stirring for 4 h, a yellow oily layer gradually formed and floated on the aqueous mixture. Another portion of Al–Ni (19.0 g) was then added in small portions. Stirring was continued at the same temperature for 5.5 h before an additional portion of Al–Ni alloy (5.0 g) was added carefully. Stirring/heating was then continued for another 4 h, when TLC (2:1 PE/EtOAc) showed completion of the reaction. The oil bath was removed. After the mixture was cooled to ambient temperature, EtOAc (100 mL) was added. The mixture was stirred for 10 min and then allowed to stand still overnight. Most of the yellow oily upper layer (crude 7) was transferred to a beaker using a pipette. The mixture was then filtered through Celite. The filter cake was washed in turn with H2O (200 mL × 3) and EtOAc (200 mL × 3). The combined biphasic filtrate/washings were transferred to a separatory funnel. The phases were separated. The aqueous layer was extracted with EtOAc (200 mL × 2). The combined organic layers (including the initial yellow oil taken out before filtration) were washed with brine (60 mL) and dried over anhydrous Na2SO4. Removal of the drying agent and the solvent left a yellowish oil (19.02 g), containing mostly 7 and small amounts of diol 16 as well as traces of other unidentified side-products.
A major portion of the above-obtained yellowish oil (16.07 g) was purified by column chromatography on silica gel (4:1 PE/EtOAc) to give a ca. 1:1 mixture of cis-7 and trans-7 as a yellowish oil (11.89 g, 55.48 mmol, 69.3% from 15, with the 1H and 13C NMR shown in the Supporting Information), along with (eluting with 1:1 PE/EtOAc) diol 16 (3.073 g, 17.8 mmol, 22.2% from 15) as a yellowish oil.
A very small portion of the above-obtained crude yellowish oil (after removal of the solvent, before column chromatography) was subjected to preparative TLC (developing with 2:1 PE/EtOAc) to give samples of cis-7 and trans-7, from which the following spectroscopic data were collected. Data for cis-7 (the less polar isomer, a colorless oil): 1H NMR (500 MHz, CDCl3) δ 3.96–3.89 (m, 4H), 3.88–3.85 (unresolved m, 1H), 2.00–1.81 (a lump, 1H, OH), 1.81–1.74 (m, 1H), 1.70–1.60 (m, 3H), 1.59–1.50 (m, 1H), 1.48–1.37 (m, 4H), 1.37–1.30 (m, 3H), 1.30 (s, 3H), 1.27–1.16 (m, 1H); 13C{1H} NMR (125 MHz, CDCl3) δ 110.4, 69.0, 64.7, 41.6, 36.5, 33.1, 26.9, 26.1, 25.3, 23.9, 20.6. FT-IR (film of a concd solution in CH2Cl2) 3462, 2981, 2930, 2862, 1447, 1377, 1317, 1253, 1222, 1157, 1135, 1109, 1077, 1046, 975, 948, 859 cm–1. HRMS (FI positive) m/z: M+ calcd for C12H22O3 214.1563, found 2014.1567. Data for trans-17 (the more polar isomer, a colorless oil): 1H NMR (500 MHz, CDCl3) δ 3.97–3.90 (m, 4H), 3.22 (dt, J = 4.5, 9.5 Hz, 1H), 1.97–1.91 (m, 1H), 1.86–1.69 (m, 5H), 1.66–1.55 (m, 2H), 1.31 (s, 3H), 1.29–1.10 (m, 5H), 0.92 (br dq, J = 3.8, 12.9 Hz, 1H); 13C{1H} NMR (125 MHz, CDCl3) δ 110.5, 74.6, 64.8, 64.7, 45.3, 36.0, 35.7, 30.4, 26.4, 25.7, 25.1, 23.9. FT-IR (film of a concd solution in CH2Cl2) 3440, 2981, 2927, 2857, 1449, 1377, 1343, 1296, 1255, 1221, 1158, 1103, 1050, 947, 912, 861 cm–1. HRMS (FI positive) m/z: M+ calcd for C12H22O3 214.1563, found 2014.1562.
2-(2-(2-Methyl-1,3-dioxolan-2-yl)ethyl)cyclohexan-1-one (1)
A commercially available aqueous NaClO39 solution (containing >5.2% of active chlorine, with free base 7–8%, 100 mL) was diluted with distilled H2O (100 mL). To the resulting solution was added powdered NaHCO3 (36.00 g, 428 mmol). The mixture was stirred at ambient temperature until most NaHCO3 was dissolved. The mixture was then allowed to stand, and the yellowish supernatant (ca. 200 mL, pH 9–10)40 was ready to use in the following oxidation.
TEMPO (401 mg, 2.57 mmol) was added to a solution of alcohol 7 (the above-obtained 1:1 mixture of cis and trans isomers, 11.00 g, 51.33 mmol) in CH2Cl2 (103 mL) stirred in an ice-water bath (giving a light orange solution) followed by a solution of KBr (611 mg, 5.13 mmol) in distilled H2O (10 mL). The above-obtained supernatant of NaClO (pH 9–10, estimated to be 0.4 M in NaClO, ca. 200 mL) was then introduced slowly. The addition first resulted in a dark-brown color, which faded gradually to orange. After completion of the addition of NaClO, stirring was continued in the ice-water bath for another 2 h, when TLC (2:1 PE/EtOAc) showed full consumption of the starting alcohol 7. To the yellow mixture was added Et2O (30 mL) followed by H2O (15 mL). The phases were separated. The aqueous layer was back-extracted with Et2O (200 mL × 2). The combined organic layers were washed with saturated aqueous Na2SO3 (50 mL × 2) and brine (50 mL) and dried over anhydrous Na2SO4. Removal of the drying agent and the solvent left a light-brown/gray crude oil (11.91 g, 52.71 mmol, 103% yield from 7), which was filtered through a short column of silica gel (ϕ 8 × 10 cm, rinsing with 2–3 L of 7:1 PE/EtOAc) to remove the colored TEMPO-related species (remained on top of the silica gel column). The combined filtrate/washings were rotary evaporated to give the known 1 as a yellowish oil (9.907 g, 46.67 mmol, 91% from 7).
Data for 1: 1H NMR (500 MHz, CDCl3, assigned with the aid of COSY and HSQC) δ 3.96–3.89 (m, 4H, H-ketal), 2.38 (br dtd, J = 13.4, 4.1, 1.1 Hz 1H, H-6 equatorial), 2.281 (br ddd, J = 13.4, 11.3, 4.1 Hz, 1H, H-6 axial), 2.280 (br ddt, J = 12.5, 6.8, 6.4 Hz, 1H, H-2), 2.10 (br double multiplet with the J for the doublet = 14.1 Hz, 1H, H-3 equatorial), 2.02 (br double multiplet with the J for the doublet = 14.1 Hz, 1H, H-5 equatorial), 1.89 (ddd, J = 13.4, 6.5, 5.2 Hz, 1H, H-2′), 1.88–1.82 (m, 1H, H-1′), 1.68–1.63 (m, 1H, H-5 axial), 1.69–1.61 (m, 1H, H-4 equatorial), 1.64–1.59 (m, 1H, H-1′), 1.61 (partially resolved dtd, J = 13.5, 11.5, 5.0 Hz, 1H, H-4 axial), 1.39 (br dq, J = 11.0, 4.1 Hz, 1H, H-3 axial), 1.32 (s, 3H, H-4′), 1.29 (partially resolved ddd, J = 13.5, 11.5, 6.2 Hz, 1H, H-2′); 13C{1H} NMR (125 MHz, CDCl3, assigned with the aid of DEPT, COSY, and HSQC) δ 213.2, 110.2, 64.8, 50.8, 42.2, 36.6, 34.2, 28.2, 25.0, 24.1, 23.9.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21372248, 21532002, 21672244).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c05159.
1H and 13C NMR spectra of all compounds and DEPT, COSY, HSQC, HMBC of 1, IR and HRMS of cis-7, trans-7, and 15 (PDF)
Author Contributions
† Q.Z. and X.A. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- a Zhang Q.; Wu Y. Simplified analogues of qinghaosu (artemisinin). Tetrahedron 2007, 63, 10407–10414. 10.1016/j.tet.2007.08.018. [DOI] [Google Scholar]; b Hao H.-D.; Wittlin S.; Wu Y. Potent Antimalarial 1,2,4-Trioxanes through Perhydrolysis of Epoxides. Chem. – Eur. J. 2013, 19, 7605–7619. 10.1002/chem.201300076. [DOI] [PubMed] [Google Scholar]; c An X.; Wu Y. Synthesis of (+)-Panamonon B, 7-epi-Panamonon B, and Their (Z)-Isomers. J. Org. Chem. 2021, 86, 11948–11959. 10.1021/acs.joc.1c01344. [DOI] [PubMed] [Google Scholar]
- a Han W.-B.; Wu Y. Facile Perhydrolysis of Oxetanes Catalyzed by Molybdenum Species. Org. Lett. 2014, 16, 5706–5709. 10.1021/ol502785u. [DOI] [PubMed] [Google Scholar]; b Li Y.; Hao H.-D.; Wittlin S.; Wu Y. Simple Analogues of Qinghaosu (Artemisinin). Chem. – Asian J. 2017, 7, 1881–1886. 10.1002/asia.201200166. [DOI] [PubMed] [Google Scholar]; c An X.; Zha Q.; Wu Y. Perhydrolysis in Ethereal H2O2 Mediated by MoO2(acac)2: Distinct Chemoselectivity between Ketones, Ketals, and Epoxides. Org. Lett. 2019, 21, 1542–1546. 10.1021/acs.orglett.9b00425. [DOI] [PubMed] [Google Scholar]
- a Kitahara T.; Kurata H.; Matsuoka T.; Mori K. Synthesis of both the enantiomers of sclerosporin and sclerosporal, sporogenic substance of Sclerotinia fructicola. Tetrahedron 1985, 41, 5475–5485. 10.1016/S0040-4020(01)91347-X. [DOI] [Google Scholar]; b Veliu R.; Schneider C. Stereoselective Synthesis of the Decahydroquinoline Alkaloid cis-195J. J. Org. Chem. 2021, 86, 11960–11967. (pumiliotoxin C) 10.1021/acs.joc.1c01346. [DOI] [PubMed] [Google Scholar]
- a Hao H.-D.; Li Y.; Han W.-B.; Wu Y. A Hydrogen Peroxide Based Access to Qinghaosu (Artemisinin). Org. Lett. 2011, 13, 4212–4215. and refs therein 10.1021/ol2015434. [DOI] [PubMed] [Google Scholar]; b Zanetti A.; Schwertz G.; de Oliveira M. N.; Fernandez M. A. G.; Amara Z.; Cossy J. Palladium-Catalyzed Regioselective Allylic Oxidation of Amorphadiene, a Precursor of Artemisinin. J. Org. Chem. 2021, 86, 7603–7608. 10.1021/acs.joc.1c00653. [DOI] [PubMed] [Google Scholar]
- Defieber C.; Mohr J. T.; Grabovyi G. A.; Stoltz B. M. Short Enantioselective Formal Synthesis of (−)-Platencin. Synthesis 2018, 50, 4359–4368. (platencin) 10.1055/s-0037-1610437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shimizu I.; Naito Y.; Tsuji J. Synthesis of optically active (+)-19-nortestoerone by asymmetric bis-annulation reaction. Tetrahedron Lett. 1980, 21, 487–490. (synthesis of nandrolone) 10.1016/S0040-4039(00)71440-7. [DOI] [Google Scholar]; (b) Levy D. E.; WO2016/44559, 2016, A1 (to Prevacus, Inc.; synthesis of nandrolone).
- a Li S.-W.; Mudianta I. W.; Cuadrado C.; Li G.; Yudasmara G. A.; Setiabudi G. I.; Daranas A. H.; Guo Y.-W. Litosetoenins A–E, Diterpenoids from the Soft Coral Litophyton setoensis, Backbone-Rearranged through Divergent Cyclization Achieved by Epoxide Reactivity Inversion. J. Org. Chem. 2021, 86, 11771–11781. 10.1021/acs.joc.1c01218. [DOI] [PubMed] [Google Scholar]; b Masi M.; Cimmino A.; Salzano F.; Di Lecce R.; Górecki M.; Calabrò V.; Pescitelli G.; Evidente A. Higginsianins D and E, Cytotoxic Diterpenoids Produced by Colletotrichum higginsianum. J. Nat. Prod. 2020, 83, 1131–1138. 10.1021/acs.jnatprod.9b01161. [DOI] [PubMed] [Google Scholar]
- a Kita Y.; Yasuda H.; Haruta J.-i.; Segawa J.; Tamura Y. The Chemistry of O-Silylated Ketene Acetals 1; A Mild and Facile Preparation of Trimethylsilyl Enol Ethers and of Cyclic O,O-, O,S-, and S,S-Acetals from Enolizable Carbonyl Compounds. Synthesis 1982, 14, 1089–1091. [Google Scholar]; b Destabel C.; Kilburn J. D.; Knight J. Alkyl radical cyclisations of methylenecyclopropane derivatives. Tetrahedron 1994, 50, 11267–11288. 10.1016/S0040-4020(01)89429-1. [DOI] [Google Scholar]; c Hollmann C.; Eilbracht P. Tandem Hydroformylation/Aldol Addition of Silyl Enol Ethers Bearing Remote Olefinic Functionalities. Tetrahedron 2000, 56, 1685–1692. 10.1016/S0040-4020(00)00071-5. [DOI] [Google Scholar]
- a Lee T. V.; Okonkwo J. O. A regiospecific synthesis of γ-keto esters: The alkylation of O-silylated enolates with methyl α-chloro-α-phenylthioacetate. Tetrahedron Lett. 1983, 24, 323–326. 10.1016/S0040-4039(00)81396-9. [DOI] [Google Scholar]; b Cheol H. C.; Yamamoto H. A Brønsted Acid Catalyst for the Enantioselective Protonation Reaction. J. Am. Chem. Soc. 2008, 130, 9246–9247. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Takasu K.; Ueno M.; Inanaga K.; Ihara M. Catalytic (2 + 2)-Cycloaddition Reactions of Silyl Enol Ethers. A Convenient and Stereoselective Method for Cyclobutane Ring Formation. J. Org. Chem. 2004, 69, 517–521. 10.1021/jo034989u. [DOI] [PubMed] [Google Scholar]
- a Ito Y.; Hirao T.; Saegusa T. Synthesis of .alpha.,.beta.-unsaturated carbonyl compounds by palladium(II)-catalyzed dehydrosilylation of silyl enol ethers. J. Org. Chem. 1978, 43, 1011–1013. 10.1021/jo00399a052. [DOI] [Google Scholar]; b Larock R. C.; Hightower T. R.; Kraus G. A.; Hahn P.; Zheng D. A Simple, Effective, New, Palladium-Catalyzed Conversion of Enol Silanes to Enones and Enals. Tetrahedron Lett. 1995, 36, 2423–2426. 10.1016/0040-4039(95)00306-W. [DOI] [Google Scholar]
- a Nicolaou K. C.; Gray D. L. F.; Montagnon T.; Harrison S. T. Oxidation of Silyl Enol Ethers by Using IBX and IBX·N-Oxide Complexes: A Mild and Selective Reaction for the Synthesis of Enones. Angew. Chem., Int. Ed. 2002, 41, 996–1000. . [DOI] [PubMed] [Google Scholar]; b Nicolaou K. C.; Montagnon T.; Baran P. S.; Zhong Y.-L. Iodine(V) Reagents in Organic Synthesis. Part 4. o-Iodoxybenzoic Acid as a Chemospecific Tool for Single Electron Transfer-Based Oxidation Processes. J. Am. Chem. Soc. 2002, 124, 2245–2258. 10.1021/ja012127+. [DOI] [PubMed] [Google Scholar]
- a Fujieda S.; Tomita M.; Fuhsuku K.-i.; Ohba S.; Nishiyama S.; Sugai T. Chemoenzymatic Route to Both Enantiomers of a 1-Isopropyl-3a-methyloctahydroinden-4-one Derivative: A Synthetic Intermediate for Sesqui- and Diterpenoids. Adv. Synth. Catal. 2005, 347, 1099–1109. 10.1002/adsc.200505034. [DOI] [Google Scholar]; b Cheng H. M.; Tian W.; Peixoto P. A.; Dhudshia B.; Chen D. Y.-K. Synthesis of ent-Nanolobatolide. Angew. Chem., Int. Ed. 2011, 50, 4165–4168. 10.1002/anie.201100926. [DOI] [PubMed] [Google Scholar]; c Jiang Y.-L.; Yu H.-X.; Li Y.; Qu P.; Han Y.-X.; Chen J.-H.; Yang Z. Asymmetric Total Synthesis of Pre-schisanartanin C. J. Am. Chem. Soc. 2020, 142, 573–580. 10.1021/jacs.9b11872. [DOI] [PubMed] [Google Scholar]
- a Behenna D. C.; Stoltz B. M. The Enantioselective Tsuji Allylation. J. Am. Chem. Soc. 2004, 126, 15044–15045. 10.1021/ja044812x. [DOI] [PubMed] [Google Scholar]; b White D. E.; Stewart I. C.; Grubbs R. H.; Stoltz B. M. The Catalytic Asymmetric Total Synthesis of Elatol. J. Am. Chem. Soc. 2008, 130, 810–811. 10.1021/ja710294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The difficulties encountered in its preparation explain why to date ketone 1 is not broadly employed in synthesis despite its apparent potential as a building block.
- Ponaras A. A. 3,3-Ethylenedioxybutylmagnesiumbromide — a nucleophilic 3-ketobutyl equivalent. Tetrahedron Lett. 1976, 17, 3105–3108. (without reporting any spectroscopic data for 1) 10.1016/S0040-4039(00)93853-X. [DOI] [Google Scholar]
- Brown H. C.; Rao B. C. S. Hydroboration. I. The Reaction of Olefins with Sodium Borohydride-Aluminum Chloride. A Convenient Route to Organoboranes and the Anti-Markownikoff Hydration of Olefins. J. Am. Chem. Soc. 1959, 81, 6423–6428. 10.1021/ja01533a023. [DOI] [Google Scholar]
- Collins J. C.; Hess W. W.; Frank F. J. Dipyridine-chromium(VI) oxide oxidation of alcohols in dichloromethane. Tetrahedron Lett. 1968, 9, 3363–3366. 10.1016/S0040-4039(00)89494-0. [DOI] [Google Scholar]
- Moeller K. D.; Tinao L. V. Intramolecular anodic olefin coupling reactions: the use of bis enol ether substrates. J. Am. Chem. Soc. 1992, 114, 1033–1041. (compound 13b therein, with experimental details given on p. 1040) 10.1021/ja00029a036. [DOI] [Google Scholar]
- a Larson G. L.; Klesse R. An Improved Synthesis of the Ethylene Acetal of 3-Iodopropanal and the Ethylene Ketal of 4-Iodo-2-butanone. J. Org. Chem. 1985, 50, 3627–3631. (the original report, 78% yield) 10.1021/jo00219a039. [DOI] [Google Scholar]; b Yalavac I.; Lyons S. E.; Webb M. R.; Procter D. J. SmI2-H2O-mediated 5-exo/6-exo lactone radical cyclisation cascades. Chem. Commun. 2014, 50, 12863–12866. (an application, the compound S13 therein, 15% yield, with the preparation shown on p. S29 of the Supporting Information of that article) 10.1039/C4CC05404K. [DOI] [PubMed] [Google Scholar]; c Nazef N.; Davies R. D.; Greaney M. F. Formal Synthesis of Merrilactone A Using a Domino Cyanide 1,4-Addition—Aldol Cyclization. Org. Lett. 2012, 14, 3720–3723. (an application, compound 11 therein, 49% yield, with the preparation shown on p. S4 of the Supporting Information of that article) 10.1021/ol301513h. [DOI] [PubMed] [Google Scholar]
- The corresponding overall yield of 1 from the starting material of iodide 10 would be much lower. For instance, the overall yield from 11 would be only 5–15%.
- With the corresponding overall yield of 1 from 11 being 9–28%.
- Presumably is caused by the great tendency of methyl vinyl ketone (MVK) to polymerize. Another problem associated with MVK is the accessibility: In China, MVK is a strictly regulated substance, which is practically impossible to purchase and definitely not permitted to store in ordinary laboratories.
- a Yin G.; Fan L.; Ren T.; Zheng C.; Tao Q.; Wu A.; She N. Synthesis of functionalized 2-aryl-4-(indol-3-yl)-4H-chromenes via iodine-catalyzed domino Michael addition–intramolecular cyclization reaction. Org. Biomol. Chem. 2012, 10, 8877–8883. (compound 3g therein, 90% yield, m.p. 138–139 °C) 10.1039/c2ob26642c. [DOI] [PubMed] [Google Scholar]; b Liu X.-H.; Lv P.-C.; Li B.; Zhu H.-L.; Song B.-A. Synthesis, Structure, and Antibacterial Activity of Novel 5-Arylpyrazole Derivatives. Aust. J. Chem. 2008, 61, 223–230. (compound 4a therein, 80% yield, m.p. 77–78 °C) 10.1071/CH07253. [DOI] [Google Scholar]; c Saito N.; Ryoda A.; Nakanishi W.; Kumamoto T.; Ishikawa T. Guanidine-Catalyzed Asymmetric Synthesis of 2,2-Disubstituted Chromane Skeletons by Intramolecular Oxa-Michael Addition. Eur. J. Org. Chem. 2008, 2759–2766. (compound 7 therein, 58% yield, m.p. 139–140 °C) 10.1002/ejoc.200800089. [DOI] [Google Scholar]
- Further acidification led to hydrolysis of substantial amounts of 15 back to 14.
- As compound 15 has never been reported before, for complete documentation, its physical and spectroscopic data are also collected on a small purified sample.
- For safety reasons, in our institute. performing hydrogenation in ordinary laboratories is prohibited; such experiments must be carried out in a special laboratory shared by the whole institute, and booking a space there prior to every use is required.
- a Tsukinoki T.; Kanda T.; Liu G.-B.; Tsuzuki H.; Tashiro M. Organic reaction in water. Part 3: A facile method for reduction of aromatic rings using a Raney Ni-Al alloy in dilute aqueous alkaline solution under mild conditions. Tetrahedron Lett. 2000, 41, 5865–5868. (the original report) 10.1016/S0040-4039(00)00636-5. [DOI] [Google Scholar]; b Tan S.-L.; Liu G.-B.; Gao X.; Thiemann T. Raney Ni–Al alloy-mediated reduction of alkylated phenols in water. J. Chem. Res. 2009, 5–7. 10.3184/030823409X393637. [DOI] [Google Scholar]
- a Rayhan U.; Kowser Z.; Islam M. N.; Redshaw C.; Yamato T. Reduction of phenylacetylenes using Raney Ni-Al alloy, Al powder in the presence of noble metal catalysts in water. ARKIVOC 2018, 241–251. (in the presence of Al powder and Pt/C in sealed tubes) 10.24820/ark.5550190.p010.277. [DOI] [Google Scholar]; b Simion C.; Mitona Y.; Katayama Y.; Simion A. M. Reduction of α,β-unsaturated carbonyl compounds and 1,3-diketones in aqueous media, using a Raney Ni-Al alloy. Rev. Roum. Chim. 2020, 65, 51–55. (benzene rings were not saturated) 10.33224/rrch.2020.65.1.05. [DOI] [Google Scholar]
- For related reduction of simple aromatics using Al–Ni alloy in neutral water in the presence of Al powder and co-catalysts, cf:; a Rayhan U.; Kwon H.; Yamato T. Reduction of aromatic compounds with Al powder using noble metal catalysts in water under mild reaction conditions. C. R. Chim. 2014, 17, 952–957. 10.1016/j.crci.2013.09.013. [DOI] [Google Scholar]; b Rayhan U.; Do J.-H.; Arimura T.; Yamato T. Reduction of carbonyl compounds by Raney Ni-Al alloy and Al powder in the presence of noble metal catalysts in water. C. R. Chim. 2015, 18, 685–692. 10.1016/j.crci.2014.10.011. [DOI] [Google Scholar]
- There have been a large number of reports on the Al-Ni alloy based reduction of phenyl rings in water, but to date, almost all the studies are methodological rather than synthetic in nature, involving only simple monofunctional substrates. Perhaps this is why such a nice inexpensive/practical reduction method appears to be largely overlooked by the synthetic community.
- Oxidation of a mixture of these side products (free from any methyl ketone 17 as shown by the 1H NMR) with NaClO/TEMPO/KBr gave the known dione 18 in 72% yield as the only observed product, indicating that the starting material must consist mainly of the diastereomers of diol 16. Note that before the C-3′ must have been reduced by Al-Ni alloy/aq. KOH at 90 °C, the ketal function was hydrolyzed to a free ketone under strongly basic conditions, an event somewhat unexpected and thus worthy of mentioning.
- For Al–Ni alloy reduction of simple ketones, see:; a Tomin A.; Lazarev A.; Bere M. P.; Redjeb H.; Török B. Selective reduction of ketones using water as a hydrogen source under high hydrostatic pressure. Org. Biomol. Chem. 2012, 10, 7321–7326. [DOI] [PubMed] [Google Scholar]; b Suceveanu M.; Raicopol M.; Enache R.; Fînaru A.; Roşca S. I. Selective reductions of the carbonyl compounds and aryl halides with Ni-Al alloy in aqueous alkali medium. Lett. Org. Chem. 2011, 8, 690–695. 10.2174/157017811799304115. [DOI] [Google Scholar]
- Anelli P. L.; Carlo Biffi C.; Montanari F.; Quici S. Fast and Selective Oxidation of Primary Alcohols to Aldehydes or to Carboxylic Acids and of Secondary Alcohols to Ketones Mediated by Oxoammonium Salts under Two-Phase Conditions. J. Org. Chem. 1987, 52, 2559–2562. 10.1021/jo00388a038. [DOI] [Google Scholar]
- For another scalable oxidation protocol (with CH2Cl2 as the solvent), which gave 1 in 82% yield, see:Taber D. F.; Amedio J. C. Jr.; Jung K.-Y. P2O5/DMSO/Triethylamine (PDT): A Convenient Procedure for Oxidation of Alcohols to Ketones and Aldehydes. J. Org. Chem. 1987, 52, 5621–5622. [Google Scholar]
- Li N.; Zhang X.; Xu X.; Chen Y.; Qiu R.; Chen J.; Wang X.; Yin S.-F. Synthesis and Structures of Air-Stable Binuclear Hafnocene Perfluorobutanesulfonate and Perfluorobenzenesulfonate and their Catalytic Application in C-C Bond-Forming Reactions. Adv. Synth. Catal. 2013, 355, 2430–2440. (compound 9b therein, with NMR data and spectra shown on pages S11 and S48, respectively, of the Supporting Information of that article). [Google Scholar]
- The crude product was rather pure, with 1H and 13C NMR essentially the same as those recorded after removal of the colored species by filtration through silica gel.
- Without this route, it would have been impossible for us to get enough quantity of ketone 1 for the total synthesis of panamonon B (ref 1c).
- It is possible to use a magnetic stirrer in sub-gram-scale experiments, though some of the Al–Ni and the Ra-Ni formed during the reaction might stick to the stirring bar. However, for larger-scale experiments, a mechanical stirrer proved necessary for effective suspension of the solids in the reaction mixture.
- CAS [7681-52-9].
- It was not possible to use pH test paper here because of the bleaching effect of NaClO; a pH-meter was used.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.