Abstract
STUDY QUESTION
Is intake of sugar-sweetened beverages (SSB) or artificially sweetened beverages (ASB) associated with testicular function in young men?
SUMMARY ANSWER
Among young men unaware of their semen quality and reproductive hormone levels, intake of SSBs was associated with lower sperm concentration, lower total sperm count, and a lower ratio of serum inhibin-B/FSH.
WHAT IS KNOWN ALREADY
SSBs may adversely impact testicular function, but results are not consistent across studies. Moreover, the associations of ASB, energy-drinks or fruit juices with testicular function are unclear.
STUDY DESIGN, SIZE, DURATION
Young healthy men and unselected for fertility status men enrolled in a cross-sectional study between 2008 and 2017.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A total of 2935 young (median age: 19 years) men enrolled in the study. Intake of SSBs, ASBs, fruit juices, and energy-drinks was assessed with a validated food frequency questionnaire. Testicular function was assessed through conventional semen quality parameters (semen volume, sperm concentration, total count, motility and morphology), testicular volume assessed with ultrasound, and serum reproductive hormone concentrations (total testosterone, free testosterone, E2, inhibin-B, LH, FSH, sex hormone-binding globulin) were measured.
MAIN RESULTS AND THE ROLE OF CHANCE
In multivariable-adjusted analyses, men in the highest category of SSB intake (median: 1.1 servings (∼220 ml)/day) had a 13.2 million/ml lower median sperm concentration (95% CI: –21.0, –5.5) than non-consumers. A similar pattern was observed with total sperm count (–28 million (95% CI: –48, –9)), serum inhibin-B (–12 pg/ml (95% CI: –21, –4)), and inhibin-B/FSH ratio (–9 (95% CI: –18, 0)). The adjusted median difference in sperm concentration and inhibin-B associated with increasing SSB intake by 1 serving (∼200ml)/day at the expense of water was –3.4 million sperm/ml (95% CI: –5.8, –1.0) and –7 pg/ml (95% CI: –11, –3), respectively.
LIMITATIONS, REASONS FOR CAUTION
Inferring causality is limited owing to the cross-sectional design. We adjusted for a number of potential confounders but cannot exclude that unmeasured lifestyle and behavior associated with soft drink intake is associated with testicular function in these young men.
WIDER IMPLICATIONS OF THE FINDINGS
In the largest study to date, intake of SSBs was associated with lower sperm concentration, total sperm count, and serum inhibin-B/FSH ratio, consistent with a direct suppressive effect of SSB intake on testicular function among otherwise healthy men, potentially affecting fertility. However, the observed association between higher SSB intake and lower semen quality does not necessarily imply a decrease in fertility.
STUDY FUNDING/COMPETING INTEREST(S)
Supported by research from the Danish Council for Strategic Research (2101-08-0058), Independent Research Fund Denmark (8020-00218B), European Union (212844), the Kirsten and Freddy Johansen’s Foundation (95-103-72087), the Research Fund of the Capital Region of Denmark (A6176), and the NIH (P30DK046200). The authors report no conflict of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: sugar-sweetened beverages / artificially sweetened beverages / energy-drinks / testosterone / male fertility
Introduction
One in six couples trying to conceive experience infertility—the inability to conceive after 12 months of unprotected sexual intercourse (Louis et al., 2013; Thoma et al., 2013), with major financial and psychological implications (Macaluso et al., 2010). Male factors are implicated in as many as 58% of such cases, including one third of cases attributed to a combination of male and female factors (Thonneau et al., 1991). Genetics contribute only to 10–15% of male infertility (Dohle et al., 2002), highlighting the growing evidence that testicular function, including spermatogenesis, is sensitive and responsive to environmental exposures such as environmental chemicals, air pollution and diet (Minguez-Alarcon et al., 2018).
The intake of energy-dense foods, added sugars and sugar-sweetened beverages (SSBs) has increased worldwide (Malik et al., 2010), and a large part of the Danish population exceeds the national dietary recommendation for intake of added sugars (Pedersen et al., 2010). While numerous adverse health effects of SSB intake have been documented, including higher rates of obesity, diabetes, and heart disease (Malik et al., 2010), whether SSBs can also negatively impact reproductive function generally and male reproductive function specifically is less clear. Three previous studies have examined the association between SSB intake and testicular function (Jensen et al., 2010; Chiu et al., 2014; Yang et al., 2015). Although they reported inverse associations with one or more semen parameters, the associations were different across studies. An American study (Chiu et al., 2014) found an inverse relation with progressive sperm motility and a Chinese study (Yang et al., 2015) reported lower semen volume with increased SSB intake. Also, in a previous report focused on intake of caffeinated beverages, we reported an inverse association of intake of cola soft drinks, i.e. SSB, but not other caffeine-containing drinks, with sperm concentration and total sperm count (Jensen et al., 2010). In addition, a study of couples attempting conception in North America, found that men’s intake of SSBs was associated with longer time to pregnancy, independent of their partners’ SSB intake (Hatch et al., 2018).
Although the harmful health effects of SSB have received substantial attention in recent years, the health effects of other beverages that could be used to substitute SSB intake, such as artificially sweetened beverages (ASB) and fruit juices, have received less attention. However, nutritionally, some fruit juices are not very different form SSB. Also, if health effects of SSB can be partially attributed to contamination with environmental chemicals, such as plasticizers used to cover the inner lining of cans, rather than their nutritional properties, similar associations could be expected with intake of ASBs. Moreover, in the last decade, many energy drinks have become widely available, some of which have sugar contents equivalent to SSBs but are substantially different in other regards such as their caffeine content. It is, therefore, important to characterize the health risk profile of beverages beyond that of SSBs. To address this important knowledge gap as it relates to male reproductive health, we examined the association of intakes of SSB, ASB, energy-drinks, and fruit juices among young Danish men with markers of testicular function including semen quality, testicular volume, and serum reproductive hormones. We hypothesized that higher intake of SSBs, but not other soft drinks (ASB, fruit juice, and energy drinks), would be associated with lower testicular function.
Materials and methods
Participants
Eighteen-year-old men in Denmark undergo a compulsory physical examination to determine their fitness for military service. Since 1996, the research team at the University Department of Growth and Reproduction at Rigshospitalet (Copenhagen, Denmark) has invited men attending this physical examination to participate in a study of determinants of male fertility (Priskorn et al., 2018). The local ethical committee approved the study (journal no. H-KF-289428). After providing informed consent, participants answered questionnaires on demographics, lifestyle, and medical history, underwent a physical exam, and provided a semen and a blood sample. Participants received 500 DKK (∼$85USD) after completing the study procedures. Since April 2008, a food frequency questionnaire (FFQ) was administered to participants. The current analysis includes men who answered the FFQ (April 2008–May 2017) and provided a non-azoospermic semen sample. The final sample was 2935 men including 2798 men with complete data on testicular volume and semen quality parameters, and 2734 men with complete data on serum reproductive hormone concentrations (Supplementary Fig. S1) (Nassan et al., 2020).
Soft drink assessment
Diet was assessed using a self-administered previously validated FFQ (Tjonneland et al., 1991; Mikkelsen et al., 2006). The questionnaire included questions regarding intake of SSBs (cola drinks with sugar, other soft drink with sugar, and fruit flavored beverage with sugar), ASBs (diet/light drinks and light fruit flavored beverages), energy-drinks (e.g. Red Bull), and fruit juices. Participants also answered questions about water intake (glasses of regular water and mineral water e.g. club soda). Questions related to consumption of soft drinks and energy drinks referred to the consumption in the past week, while questions regarding water, fruit juices and fruit flavored beverages referred to the average intake within the past 3 months. Beverage intakes were scaled to represent total servings per day (1 serving being equivalent to ∼200 ml). Total energy and nutrient intake was quantified using the Danish food Composition tables (National Food Institute, 2019).
Semen analysis and testicular volume
Participants provided one semen sample by masturbation in a room close to the andrology laboratory. Men were asked to abstain from ejaculation for at least 48 h before their visit. Participants reported time of previous and the current ejaculation and the actual abstinence duration was calculated. Semen samples were analyzed in compliance with the current World Health Organization guidelines (WHO, 2010) as described before (Jorgensen et al., 2012) and semen volume, sperm concentration, sperm motility, and sperm morphology were assessed (Kruger et al., 1988; Menkveld et al., 1990; Jorgensen et al., 1997; WHO, 2010; Jorgensen et al., 2012). In brief, ejaculate volume was estimated by weight. Samples were diluted in a solution of 0.6 mol NaHCO3/l and 0.4% formaldehyde in distilled water. Sperm concentration was then assessed by using a Bürker-Türk hemocytometer (Paul Marienfeld GmbH & Co KG, Lauda-Königshofen, Germany). Only sperm with tails were counted. We calculated the total sperm count (million/ejaculate) as semen volume × sperm concentration. For sperm motility assessment, 10 µl well-mixed semen was placed on a clean glass slide, at 37°C, and covered with a 22 × 22 mm2 coverslip. The slide was placed on the heated stage of a microscope at 37°C and immediately examined at ×400 magnification. Sperm were classified as immotile, non-progressive, or progressively motile. Smears were prepared for morphologic evaluation, Papanicolaou stained, and assessed according to strict Kruger criteria (Kruger et al., 1988) by two experienced technicians (Menkveld et al., 1990; Jorgensen et al., 1997). For all assessments, counts were done in duplicates and the average was used. Since 1996, our laboratory has conducted a quality assurance/quality control program to assess sperm concentrations and to ensure that inter-laboratory differences remained unchanged in comparison with two other laboratories (Jorgensen et al., 1997; Jorgensen et al., 2012). During the physical examination, testicular volume was measured by ultrasonography and the average of both testicular volumes was calculated.
Serum reproductive hormones
In the morning of the visit day, a venous blood sample was drawn from each participant. Serum was isolated and stored at −20°C. Total testosterone (TT) concentrations were measured using time-resolved fluoroimmunoassay (DELFIA, Wallac, Turku, Finland) up to 2013 and with ELISA (Access2, Beckman Coultier Ltd, High Wycombe, UK) thereafter. We calculated free-testosterone (cFT) from TT concentrations and sex hormone-binding globulin (SHBG) assuming a fixed albumin concentration (Vermeulen et al., 1999). Inhibin-B concentrations were measured by a specific two-sided enzyme-immunometric assay (Inhibin-B gen II, Beckman Coulter Ltd, High Wycombe, UK). FSH, LH, SHBG, and estradiol (E2) concentrations were measured using a time-resolved immunofluorometric assay (Delfia, Wallac, Turku, Finland). From 2014, the concentration of E2 was measured using a RIA (Pantex, Panhandle, TX, USA). To ensure comparability over time, all hormone concentrations were measured in the same laboratory and were analyzed in batches yearly along with re-analysis of control samples from the year before. We also calculated the ratios of inhibin-B/FSH, TT/LH, cFT/LH, E2/TT, and (E2/cFT) ×100.
Statistical analysis
Non-consumers for each of the four beverage groups of interest (SSBs, ASBs, fruit juice, and energy drinks) were used as the reference group. All other men were divided into quartiles of intake for each beverage resulting in five intake categories. We examined the descriptive statistics of demographic characteristics and dietary intake for participants. Due to the skewed distribution of the outcomes, we used quantile (median) regression whose effect estimates can be interpreted as differences in medians of the outcomes and 95% CI compared to the reference category (non-consumers). We conducted tests for trend across all soft drink categories using the median value of each quartile as a continuous variable.
We also estimated the effect of substituting specific beverages with water. To do this, we included all beverages of interest (SSB, ASBs, fruit juice, and energy-drinks) along with water in the same model as continuous variables, while adjusting for the same covariates as in the main analysis. We estimated the substitution effect as the difference between the regression coefficient for a specific beverage and the regression coefficient for water; the corresponding 95% CI were estimated using the covariance matrix for the regression model (Willett and Stampfer, 1986).
We selected the covariates based on prior knowledge using directed acyclic graphs (Supplementary Fig. S2). The final models adjusted for age, BMI, height, smoking, use of marijuana and other recreational drugs, moderate-to-vigorous physical activities (hours/week), history of reproductive diseases, reproductive surgeries, history of sexually transmitted diseases, season and calendar year of the sample, mother’s education level (as indication of the young men’s socioeconomic status), total energy intake, and four dietary patterns derived using principal component analysis (Nassan et al., 2020) based on intake of 36 pre-defined food groups excluding the beverages of interest in this analysis, to account for potential confounding by overall dietary behavior related to beverage choices. For the semen variables, we further adjusted for abstinence duration, and for sperm motility also for time elapsed between semen collection and semen analysis. For the serum reproductive hormones, we further adjusted for time of the day of the sample collection. We conducted sensitivity analyses where: total energy intake and BMI were excluded from the models, as these may be causal intermediates of the association; and coffee was included in the models, as the main source of caffeine intake that could be considered as a potential confounder. We also conducted analyses restricted to men with normal BMI as defined by WHO categories (between 18.5 and 24.99 kg/m2). We conducted all statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
The 2,935 men included in this analysis (Supplementary Fig. S1) had a median (interquartile range) age of 19 (19, 20) years and BMI of 22 (21, 24) kg/m2. A total of 346 (12%) men did not consume SSBs, 1891 (64%) men did not consume ASBs, 419 (14%) men did not consume fruit juice, and 2065 (70%) men did not consume energy-drinks (Table I and Supplementary Table SI). The median intake, in servings (equivalent volumes)/day, for men in the highest intake category for each of the beverages of interest was 1.1 (∼220 ml) for SSBs, 0.9 (∼180 ml) of ASBs, 1.0 (∼200 ml) of fruit juice, and 0.4 (∼80 ml) of energy-drinks. Men within the highest category of SSB and energy-drink intake were more likely to be smokers, and to use marijuana and other drugs than men who did not consume these beverages (Table I and Supplementary Table SI). The median (95% CI) of the total sperm count was 140 (133, 146) million, 3.2 (3.2, 3.3) ml for semen volume, and 18 (18, 18) nmol/l for the TT concentration in the total population (Table II).
Table I.
Demographic characteristics according to categories of sugar-sweetened and artificially sweetened beverages consumption among 2935 young Danish men (2008–2017).
| Sugar-sweetened beverages |
Artificially sweetened beverages |
||||
|---|---|---|---|---|---|
| Total | Non-consumers | Highest consumers, Q4 | Non-consumers | Highest consumers, Q4 | |
| Men, N (%) | 2935 | 346 (12%) | 641 (22%) | 1891 (64%) | 280 (10%) |
| Median (range) (serving/day) | 0 | 1.1 (0.7, 8.6) | 0 | 0.9 (0.5, 7.6) | |
| Age, years, median (IQR) | 19 (19, 20) | 19 (19, 20) | 19 (19, 20) | 19 (19, 20) | 19 (19, 20) |
| BMI, kg/m2, median (IQR) | 22 (21, 24) | 23 (21, 24) | 22 (20, 24) | 22 (20, 24) | 23 (21, 25) |
| Height, m, median (IQR) | 1.82 (1.77, 1.87) | 1.82 (1.78, 1.86) | 1.81 (1.77, 1.86) | 1.82 (1.77, 1.87) | 1.82 (1.77, 1.87) |
| Cigarettes smoking, % | |||||
| Daily | 27 | 12 | 40 | 27 | 28 |
| Occasionally | 23 | 23 | 17 | 22 | 20 |
| Never | 50 | 65 | 43 | 51 | 52 |
| Marijuana smoking, % | |||||
| Daily | 4 | 2 | 8 | 4 | 4 |
| Occasionally | 34 | 24 | 38 | 33 | 32 |
| Never | 63 | 73 | 54 | 63 | 64 |
| Other recreational drug use, % | 12 | 7 | 15 | 12 | 11 |
| Education of the mother, % | |||||
| < 9 years | 3 | 3 | 4 | 3 | 5 |
| 9–10 years | 25 | 25 | 22 | 24 | 23 |
| > 11 years | 57 | 57 | 60 | 58 | 54 |
| Other | 6 | 8 | 4 | 5 | 6 |
| Missing/do not know | 9 | 8 | 11 | 9 | 12 |
| Physical activities, hr/wk, median (IQR)a | 8 (4, 14) | 9 (6, 15) | 7 (4, 13) | 8 (4, 13) | 8 (5, 15) |
| Fever in last 3 months, % | 8 | 7 | 8 | 8 | 9 |
| History of reproductive diseases, %b | 21 | 20 | 21 | 21 | 20 |
| History of reproductive surgery, %c | 11 | 14 | 10 | 12 | 10 |
| History of STDs, %d | 11 | 8 | 15 | 11 | 13 |
| Use of muscle-enhancing products in the last 3 months, % | 26 | 37 | 17 | 23 | 27 |
| Abstinence time, hours, median (IQR) | 62 (57, 84) | 62 (57, 85) | 62 (58, 84) | 62 (57, 84) | 62 (58, 84) |
| Sample collected during warm season, %e | 32 | 36 | 34 | 32 | 31 |
| Time of day of sample collection, hour, median (IQR) | 10 (9, 11) | 10 (9, 11) | 10 (10, 11) | 10 (9, 11) | 10 (10, 11) |
| Time to sperm motility analysis, min, median (IQR) | 30 (20, 45) | 30 (21, 49) | 30 (20, 50) | 30 (20, 45) | 30 (20, 50) |
| Total energy intake, kcal/day, median (IQR) | 1990 (1536, 2547) | 1890 (1437, 2423) | 2194 (1740, 2848) | 1982 (1538, 2497) | 2164 (1605, 2896) |
| ‘Western’ pattern, median (IQR) | −0.17 (−0.64, 0.4) | −0.60 (−1.02, −0.12) | 0.29 (−0.22, 0.93) | −0.23 (−0.69, 0.35) | 0.01 (−0.46, 0.65) |
| ‘Prudent’ pattern, median (IQR) | −0.13 (−0.69, 0.55) | 0.44 (−0.34, 1.24) | −0.41 (−0.97, 0.23) | −0.18 (−0.74, 0.5) | −0.09 (−0.67, 0.56) |
| ‘Open-Sandwich’ pattern, median (IQR) | −0.17 (−0.62, 0.39) | −0.08 (−0.59, 0.46) | −0.14 (−0.62, 0.5) | −0.16 (−0.62, 0.39) | −0.05 (−0.52, 0.61) |
| ‘Vegetarian-like’ pattern, median (IQR) | −0.13 (−0.57, 0.37) | −0.08 (−0.57, 0.40) | −0.15 (−0.58, 0.34) | −0.10 (−0.53, 0.39) | −0.28 (−0.62, 0.24) |
Numbers shown are median and interquartile range for the continuous variables unless noted as % for the categorical variables.
Physical activity was moderate and vigorous.
Reproductive diseases include self-reported history of varicocele, cryptorchidism, testicular mumps, inguinal hernia, testicular injury (hit, kicked or otherwise injured so it caused swelling of the scrotum), hydrocele, testicular torsion, hypospadias, epididymo-orchitis, cystitis or prostatitis.
Reproductive surgeries include self-reported history of surgery for inguinal hernia, varicocele, hydrocele, testicular torsion, hypospadias, testicular cancer, phimosis, testicular biopsy, vasectomy, re-fertilization, and other reproductive surgeries.
Sexually transmitted diseases (STDs) include self-reported history of gonorrhea, chlamydia, and other venereal diseases.
Warm season: April through September.
N; number of men, m; meters, hr; hours, wk; week.
Table II.
Summary of the distribution of reproductive parameters in young men.
| Reproductive parameter | Median (95% CI) | Mean (SD) |
|---|---|---|
| Total population, N: 2798 | ||
| Semen volume, ml | 3.2 (3.2, 3.3) | 3.4 (1.5) |
| Sperm concentration, million/ml | 44 (42, 46) | 57 (50) |
| Total sperm count, million | 140 (133, 146) | 183 (165) |
| Motile spermatozoa, % | 69 (68, 70) | 66 (16) |
| Progressively motile spermatozoa, % | 61 (60, 62) | 58 (17) |
| Normal sperm morphology, % | 6.5 (6.5, 7.0) | 7.2 (4.6) |
| Testicular volume by ultrasound, ml | 13.5 (13.3, 13.7) | 13.8 (3.8) |
| Total population, N: 2734 | ||
| TT, nmol/l | 18 (18, 18) | 19 (6) |
| cFT, pmol/l | 445 (441, 453) | 468 (154) |
| Estradiol, pmol/l | 82 (81, 83) | 85 (30) |
| LH, IU/l | 3.3 (3.3, 3.4) | 3.6 (1.6) |
| FSH, IU/l | 2.6 (2.5, 2.7) | 3.0 (1.9) |
| SHBG, Nmol/l | 28 (28, 29) | 30 (11) |
| Inhibin-B, pg/ml | 168 (166, 170) | 174 (61) |
| Inhibin-B/FSH | 65 (63, 69) | 99 (507) |
| TT/LH | 6 (5, 6) | 6 (10) |
| cFT/LH | 135 (133, 137) | 159 (463) |
| Estradiol/TT | 4 (4, 4) | 5 (2) |
| (Estradiol/cFT) *100 | 18 (18, 18) | 19 (7) |
TT, total testosterone; cFT, calculated free testosterone; SHBG, sex hormone-binding globulin.
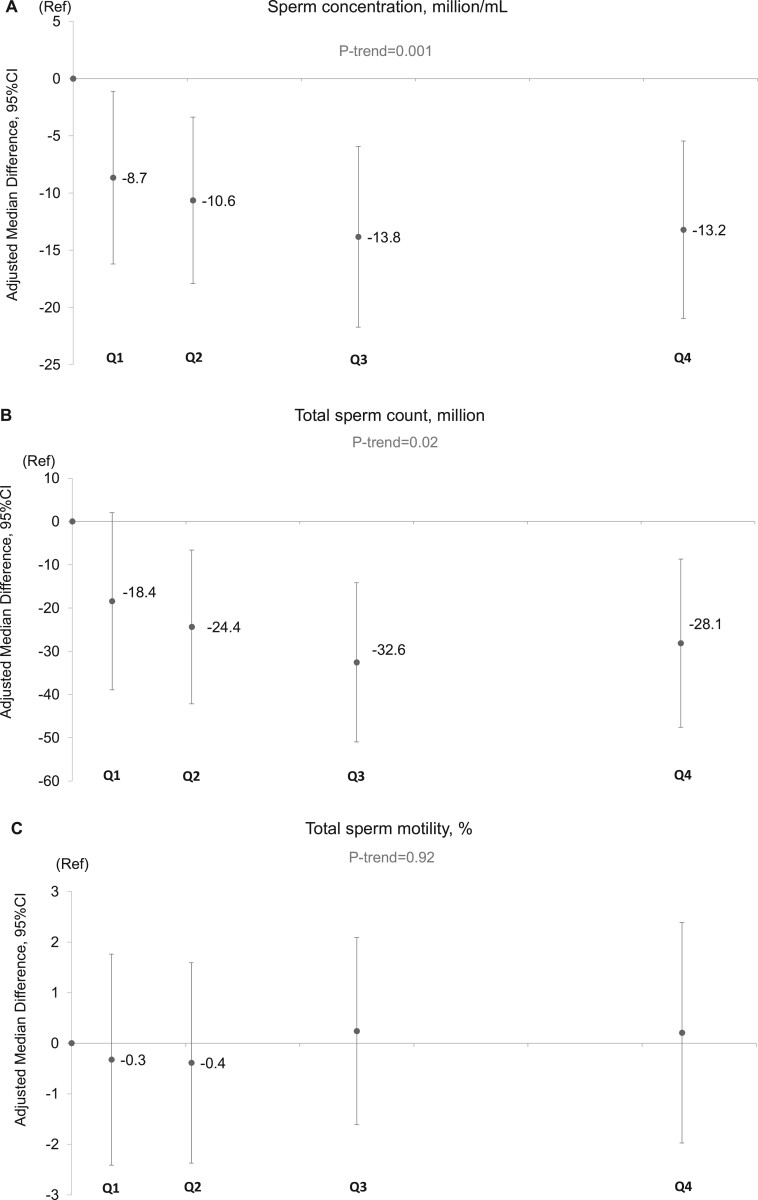
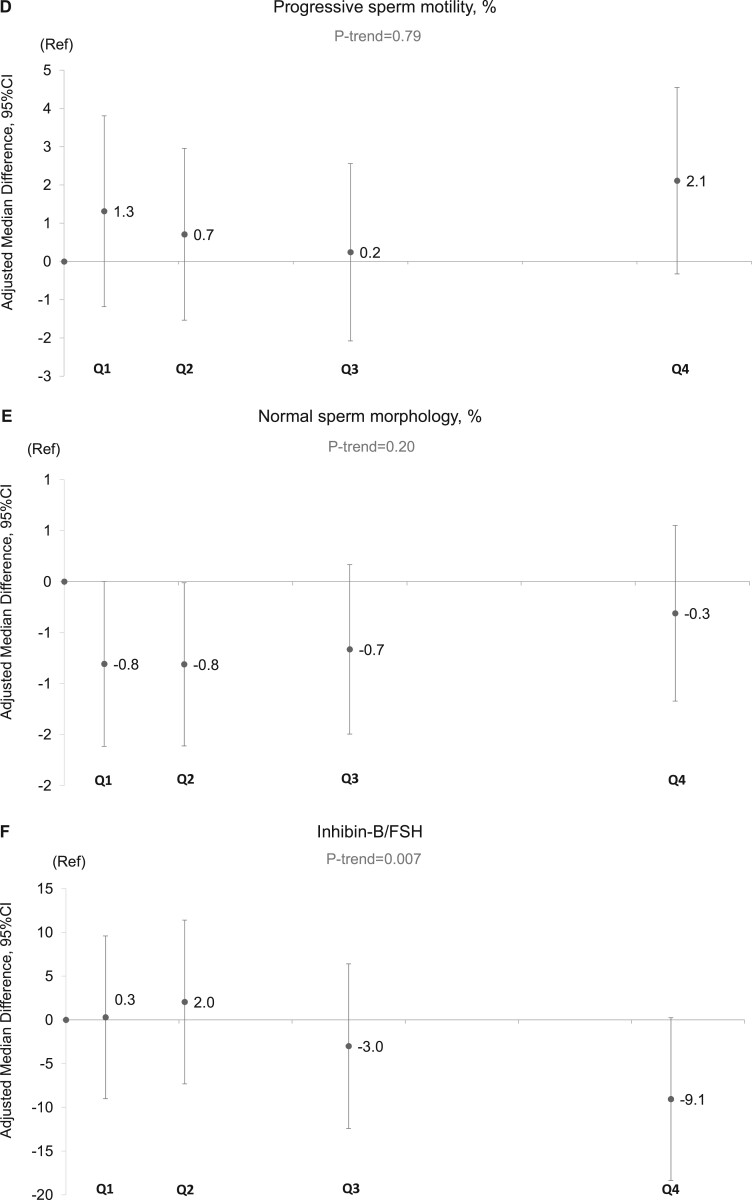
In unadjusted analyses, intake of SSBs was inversely related to sperm concentration, total sperm count, serum inhibin B, and the inhibin B/FSH ratio; ASBs intake was associated with lower total and progressive sperm motility, and serum SHBG; and energy drinks were positively related to normal sperm morphology, serum E2 and serum LH (Supplementary Tables SII and SIII). The same associations persisted after adjusting for potential confounders, although the magnitude of the associations changed in some cases. In multivariable adjusted analyses, the median sperm concentration for men in the highest category of SSB intake (median intake: 220, range: 140 to 1720 ml/day) was 13.0 (95% CI: –21.0, –5.5) million/ml lower than non-consumers (P-trend = 0.001) (Fig. 1, Supplementary Table SIV). A similar pattern was observed for total sperm count (–28 million (95% CI: –48, –9)), serum inhibin-B (–12 pg/ml (95% CI: –21, –4)), and inhibin-B/FSH ratio (–9 (95% CI: –18, 0)) (Fig. 1, Supplementary Table SIV, and Table III). The association with total sperm count was weaker in multivariable adjusted analyses than in crude analyses, whereas the association with the inhibin-B/FSH ratio was stronger. Of note, although visual assessment of the relation between SSB with sperm concentration and total sperm count suggested a non-linear relation, formal testing for non-linearity suggested that these associations did not depart significantly from linearity (sperm concentration P for non-linearity = 0.75; sperm count P for non-linearity = 0.32).
Figure 1.
Adjusted median differences in reproductive parameters in young men according to intake categories of sugar-sweetened beverages (SSBs). Data are presented for (A) sperm concentration, (B) total sperm count, (C) total sperm motility, (D) progressive sperm motility, (E) normal sperm morphology, and (F) inhibin B/FSH ratio. The X-axis represents the categories spaced according to the median intake of each category of the SSB intake. The median and range of intakes (serving/day) of the categories of SSB were in ascending manner: non-consumers: 0 (0, 0), Q1: 0.07 (0.01, 0.14), Q2: 0.21 (0.14, 0.32), Q3: 0.50 (0.32, 0.65), and Q4: 1.07 (0.67, 8.64). Intakes were scaled to represent total servings per day (1 serving being equivalent to ∼200 ml). The number of men (%) in the categories are: non-consumers: 346 (12%), Q1: 558 (19%), Q2: 740 (25%), Q3: 650 (22%), and Q4: 641 (22%). The final models adjusted for age, BMI, height, smoking, use of marijuana and other recreational drug, moderate-to-vigorous physical activities (hours/week), history of reproductive diseases, reproductive surgeries, and sexually transmitted diseases (STDs), season and calendar year of the sample, mother’s education level, data-driven dietary patterns, total energy consumption, and abstinence time. In addition, for sperm motility models, we further adjusted for time elapsed between specimen collection and analysis. For the serum reproductive hormone models (here inhibin-B/FSH), we further adjusted for time of the day of the sample collection. The estimates shown are adjusted estimates of the absolute difference in the medians of each category compared to the reference category (non-consumers). Tests for trend were conducted across categories using the median value in each category of the beverage consumptions as a continuous variable in the regression models and P value was based on Wald test. N, number of men; Ref, reference category (non-consumers); Q, quartile.
Table III.
Adjusted median differences (95% CI) in serum reproductive hormone concentrations in young men on the scale for the individual hormones as stated in the table according to categories of soft drink consumption.
| Reproductive parameters | TT, nmol/l | cFT, pmol/l | Estradiol, pg/ml | Inhibin-B, pg/ml | LH, IU/l | FSH, IU/l | SHBG, nmol/l | Inhibin-B/FSH | TT/LH | cFT/LH | Estradiol/ TT | (Estradiol/ cFT)*100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugar- sweetened beveragesa | Non-consumers, 12% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q1, 19% | 0.2 (−0.6, 1.0) | 13 (−6, 32) | 2 (−2, 6) | 0 (−9, 9) | 0.1 (−0.1, 0.4) | −0.1 (−0.3, 0.1) | −0.7 (−2.3, 1) | 0 (−9, 10) | −0.1 (−0.5, 0.3) | −3 (−13, 7) | 0 (−0.2, 0.3) | −0.6 (−1.5, 0.3) | |
| Q2, 25% | 0.6 (−0.2, 1.4) | 23 (5, 41) | 6 (3, 10) | 1 (−6, 9) | 0.1 (−0.1, 0.4) | −0.3 (−0.5, 0) | −0.1 (−1.7, 1.4) | 2 (−7, 11) | −0.1 (−0.5, 0.2) | 4 (−5, 13) | 0.2 (−0.1, 0.4) | 0.4 (−0.4, 1.2) | |
| Q3, 22% | 0.2 (−0.6, 1.0) | 11 (−9, 31) | 6 (3, 10) | −3 (−11, 5) | 0.1 (−0.1, 0.4) | −0.1 (−0.3, 0.1) | 0.2 (−1.5, 1.9) | −3 (−12, 6) | −0.1 (−0.5, 0.3) | 2 (−8, 12) | 0.1 (−0.1, 0.3) | 0.3 (−0.5, 1.2) | |
| Q4, 22 % | 0.7 (−0.2, 1.5) | 18 (−5, 41) | 5 (1, 9) | −12 (−21, −4) | 0.1 (−0.1, 0.4) | 0 (−0.3, 0.2) | 0.1 (−1.6, 1.8) | −9 (−18, 0) | −0.3 (−0.7, 0.1) | 0 (−11, 10) | 0 (−0.2, 0.3) | −0.1 (−1, 0.8) | |
| P trend | 0.15 | 0.28 | 0.07 | 0.0001 | 0.54 | 0.63 | 0.28 | 0.007 | 0.32 | 0.65 | 0.59 | 0.71 | |
|
Artificially− sweetened beveragesb |
Non-consumers, 64% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q1, 10% | −0.4 (−1.0, 0.2) | −13 (−33, 7) | −3 (−7, 1) | 1 (−8, 11) | 0 (−0.1, 0.2) | 0.1 (−0.2, 0.3) | 0 (−1.3, 1.3) | 4 (−5, 13) | −0.1 (−0.5, 0.2) | −1 (−12, 10) | 0.1 (−0.1, 0.3) | 0.5 (−0.2, 1.2) | |
| Q2, 8% | −0.4 (−1.2, 0.5) | −13 (−33, 8) | −3 (−7, 1) | −2 (−13, 9) | 0 (−0.2, 0.2) | −0.1 (−0.4, 0.2) | −0.6 (−2.5, 1.3) | 5 (−2, 13) | −0.1 (−0.5, 0.3) | 2 (−8, 11) | −0.2 (−0.4, 0.1) | −0.1 (−0.9, 0.8) | |
| Q3, 8% | 0.1 (−0.8, 0.9) | −16 (−37, 6) | −5 (−9, 0) | 2 (−5, 8) | −0.1 (−0.4, 0.1) | 0 (−0.2, 0.2) | 2.0 (0.6, 3.5) | 0 (−5, 4) | 0 (−0.4, 0.5) | −3 (−13, 8) | 0.1 (−0.1, 0.3) | 0.6 (−0.4, 1.5) | |
| Q4, 10% | −0.7 (−1.6, 0.2) | −12 (−34, 11) | −1 (−4, 2) | 3 (−6, 12) | 0 (−0.2, 0.2) | 0.1 (−0.2, 0.3) | −2.2 (−3.2, −1.2) | −2 (−10, 6) | −0.3 (−0.6, −0.1) | −6 (−13, 1) | 0 (−0.2, 0.2) | 0.1 (−0.9, 1.2) | |
| P trend | 0.25 | 0.33 | 0.21 | 0.45 | 0.94 | 0.43 | 0.004 | 0.38 | 0.12 | 0.09 | 0.99 | 0.70 | |
|
Fruit juicec |
Non-consumers, 14% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q1, 26% | −0.2 (−0.9, 0.5) | −13 (−30, 3) | −1 (−5, 2) | 7 (−1, 15) | −0.1 (−0.3, 0.2) | −0.1 (−0.3, 0.1) | 0.6 (−0.7, 1.9) | 1 (−5, 7) | 0.4 (0, 0.7) | 5 (−3, 13) | 0 (−0.2, 0.2) | −0.2 (−1, 0.6) | |
| Q2, 24% | 0.1 (−0.6, 0.7) | 1 (−17, 18) | 2 (−1, 5) | 10 (1, 19) | 0 (−0.3, 0.2) | −0.1 (−0.3, 0.1) | 1.2 (0, 2.4) | 4 (−2, 11) | 0.2 (−0.1, 0.5) | 4 (−4, 11) | −0.1 (−0.3, 0.1) | 0.2 (−0.6, 1.0) | |
| Q3, 15% | 0.1 (−0.7, 0.9) | −1 (−19, 16) | −1 (−5, 3) | 7 (−3, 16) | 0 (−0.3, 0.2) | 0 (−0.3, 0.2) | 0.1 (−1.1, 1.4) | 0 (−8, 9) | 0.2 (−0.2, 0.6) | −1 (−9, 7) | −0.1 (−0.3, 0.2) | −0.5 (−1.5, 0.5) | |
| Q4, 20% | −0.1 (−0.9, 0.6) | 3 (−14, 20) | −3 (−6, 0) | 5 (−3, 14) | −0.1 (−0.3, 0.1) | 0 (−0.2, 0.3) | 1.2 (−0.1, 2.4) | −3 (−10, 4) | 0.4 (0.1, 0.8) | 3 (−5, 12) | −0.2 (−0.4, 0) | −0.8 (−1.6, 0.1) | |
| P trend | 0.99 | 0.28 | 0.08 | 0.78 | 0.61 | 0.25 | 0.21 | 0.22 | 0.16 | 0.90 | 0.05 | 0.19 | |
| Energy− drinksd | Non-consumers, 70% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Q1, 8% | −0.5 (−1.1, 0.1) | 21 (0, 42) | −2 (−6, 2) | 0 (−10, 10) | 0 (−0.3, 0.3) | 0.2 (−0.1, 0.5) | −1.4 (−2.6, −0.1) | −5 (−12, 1) | 0 (−0.5, 0.6) | 2 (−8, 13) | 0 (−0.3, 0.2) | −0.8 (−1.9, 0.3) | |
| Q2, 6% | −0.3 (−1.1, 0.4) | 10 (−6, 25) | 2 (−2, 6) | 19 (5, 33) | 0.1 (−0.1, 0.2) | 0 (−0.2, 0.2) | −0.1 (−1.9, 1.7) | 6 (−2, 15) | −0.3 (−0.6, 0) | 0 (−10, 10) | 0.2 (−0.1, 0.4) | −0.3 (−1.4, 0.8) | |
| Q3, 8% | 0.5 (−0.5, 1.5) | 34 (9, 59) | 2 (−2, 6) | 0 (−9, 9) | 0.5 (0.2, 0.8) | 0.2 (−0.1, 0.4) | 0 (−2.1, 2) | 0 (−6, 5) | −0.5 (−0.9, −0.2) | −8 (−15, 0) | 0.1 (−0.2, 0.3) | −1.1 (−2, −0.3) | |
| Q4, 8% | 1.3 (−0.1, 2.7) | 2 (−27, 31) | 4 (0, 8) | 3 (−8, 13) | 0.4 (0.2, 0.5) | −0.1 (−0.3, 0.2) | 1.4 (−0.1, 2.9) | 1 (−8, 10) | −0.2 (−0.8, 0.3) | −9 (−19, 1) | 0.1 (−0.1, 0.3) | 0.4 (−0.5, 1.2) | |
| P trend | 0.05 | 0.55 | 0.01 | 0.61 | 0.004 | 0.49 | 0.34 | 0.99 | 0.18 | 0.05 | 0.44 | 0.89 | |
The final models adjusted for age, BMI, height, smoking, use of marijuana and other recreational drug, moderate-to-vigorous physical activities (hours/week), history of reproductive diseases, reproductive surgeries, and STDs, season and calendar year of the sample, mother’s education level, data-driven dietary patterns, total energy consumption, and time of the day of the sample collection.
The estimates shown are adjusted estimates of the absolute difference in the medians of each category compared to the reference category (non-consumers).
Intakes were scaled to represent total servings per day (1 serving being equivalent to ∼200 ml).
The median and range of intakes (serving/day) of the categories of the sugar-sweetened beverages were in ascending manner: non-consumers: 0 (0,0), Q1: 0.07 (0.01, 0.14), Q2: 0.21 (0.14, 0.32), Q3: 0.50 (0.32, 0.65), and Q4: 1.07 (0.67, 8.64).
The median and range of intakes (serving/day) of the categories of the artificially-sweetened beverages were in ascending manner: non-consumers: 0 (0,0), Q1: 0.03 (0.001, 0.07), Q2: 0.08 (0.08, 0.14), Q3: 0.10 (0.15, 0.50), and Q4: 0.86 (0.50, 7.64).
The median and range of intakes (serving/day) of the categories of the Fruit juices were in ascending manner: non-consumers: 0 (0, 0), Q1: 0.08 (0.03, 0.08), Q2: 0.21 (0.21, 0.21), Q3: 0.50 (0.50, 0.50), and Q4: 1.00 (0.79, 4.00).
The median and range of intakes (serving/day) of the categories of the energy-drinks were in ascending manner: non-consumers: 0 (0, 0), Q1: 0.02 (0.0004, 0.02), Q2: 0.04 (0.02, 0.05), Q3: 0.14 (0.07, 0.25), and Q4: 0.43 (0.29, 7.14).
Tests for trend were conducted across categories using the median value in each category of the beverage consumptions as a continuous variable in the regression models and P value was based on Wald test.
Figure 1.
Continued
The associations of ASBs and energy drink intake with markers of testicular function also persisted, without major changes in the magnitude of these relations, after statistical adjustment for potential confounders. Men in the highest category of ASB intake (median intake: 180, range: 100–1520 ml/day) had lower percentage total (progressive + non-progressive) sperm motility (–3.6 (95% CI: –5.8, –1.4)), percentage progressive motility (–3.5 (95% CI: –5.8, –1.2)), and serum SHBG concentration (–2.2 (95% CI:–3.2,–1.2)) nmol/L than non-consumers (Table III and Supplementary Table SIV). In addition, men in the highest category of energy-drink intake (median: 0.4, range: 0.3: 7.1) had a higher percentage of morphologically normal sperm (1.5 (95% CI: 0.7, 2.3)), E2 (4 (95% CI: 0, 8) pg/ml), and LH (0.4, 95% CI: 0.2, 0.5) IU/l) concentrations than men who did not consume energy drinks (Table III and Supplementary Table SIV). Intakes of SSBs, ASBs and energy drinks were unrelated to all the other outcomes examined. Fruit juice intake was not related to any of the outcomes evaluated. Similarly, drinking water was not associated with any of the testicular function parameters examined.
We then estimated the effect on markers of testicular function of consuming each of the beverages of intake instead of consuming water (Table IV). In these models, increasing SSB intake by one serving (∼200 ml)/day at the expense of water was associated with a lower sperm concentration (–3.4 (95% CI: –5.8, –1.0) million sperm/ml) and lower serum concentration of inhibin-B (–7 (95% CI: –11, –3) pg/ml) (Table IV). Similarly consuming ASBs instead of water was associated with a lower percentage of total motility (–1.8 (95% CI: –3.3, –0.3)), as well as higher concentrations of E2 (6 (95% CI: 2, 9) pmol/l) and LH (0.4 (95% CI: 0.1, 0.8) IU/l) (Table IV).
Table IV.
Estimated adjusted median difference in testicular function parameters for the substitution of water with soft drinks among 2935 young Danish men.
| Estimated substitutions (1 serving/day) | Sugar-sweetened beverages replacing water |
Artificially-sweetened beverages replacing water |
Fruit juice replacing water |
Energy-drink replacing water |
||||
|---|---|---|---|---|---|---|---|---|
| Estimated median difference (95% CI) | P-Value | Estimated median difference (95% CI) | P-Value | Estimated median difference (95% CI) | P-Value | Estimated median difference (95% CI) | P-Value | |
| Semen volume, ml | 0.1 (0.0, 0.1) | 0.11 | −0.1 (−0.2, 0.0) | 0.10 | 0 (−0.2, 0.1) | 0.54 | −0.1 (−0.5, 0.2) | 0.35 |
| Sperm concentration, million/ml | −3.4 (−5.8, −1.0) | 0.006 | −0.1 (−0.2, 0.0) | 0.86 | −0.9 (−4.1, 2.3) | 0.54 | 4.2 (−8.2, 16.5) | 0.51 |
| Total sperm count, million | −8 (−17, 0) | 0.048 | −2 (−17, 14) | 0.83 | −3 (−12, 5) | 0.45 | 7 (−36, 49) | 0.76 |
| Motile spermatozoa, % | 0.3 (−0.7, 1.4) | 0.53 | −1.8 (−3.3, −0.3) | 0.02 | 0.9 (0, 1.7) | 0.05 | 2.1 (−1.5, 5.7) | 0.25 |
| Progressively motile spermatozoa, % | 1 (0, 2) | 0.15 | −1 (−3, 0) | 0.14 | 1 (−1, 2) | 0.34 | 0 (−5, 5) | 0.99 |
| Normal sperm morphology, % | 0.1 (−0.2, 0.5) | 0.45 | 0 (−0.5, 0.6) | 0.92 | 0 (−0.5, 0.5) | 0.92 | 1.2 (0.3, 2.2) | 0.01 |
| Testicular volume by ultrasound, mean (ml) | −0.1 (−0.4, 0.2) | 0.38 | 0 (−0.3, 0.3) | 0.76 | 0.1 (−0.3, 0.4) | 0.68 | −0.3 (−1.4, 0.7) | 0.54 |
| Total testosterone, nmol/l | 0.3 (−0.1, 0.7) | 0.11 | −0.2 (−1.1, 0.8) | 0.72 | −0.2 (−0.8, 0.3) | 0.36 | 1.1 (−1.5, 3.8) | 0.39 |
| Free testosterone, pmol/l | 8 (−5, 22) | 0.23 | −1 (−10, 7) | 0.75 | 4 (−6, 14) | 0.44 | 9 (−41, 59) | 0.72 |
| Estradiol, pmol/l | 0 (−2, 2) | 0.90 | 0 (−1, 2) | 0.59 | −2 (−4, 1) | 0.14 | 6 (2, 9) | 0.001 |
| LH, IU/l | 0 (−0.1, 0.2) | 0.59 | 0 (−0.2, 0.2) | 0.85 | 0 (−0.1, 0.1) | 0.73 | 0.4 (0.1, 0.8) | 0.01 |
| FSH, IU/l | 0 (−0.2, 0.1) | 0.44 | 0.1 (−0.1, 0.2) | 0.32 | 0 (−0.1, 0.2) | 0.54 | −0.2 (−0.6, 0.2) | 0.29 |
| SHBG, Nmol/l | −0.3 (−1, 0.5) | 0.50 | −0.7 (−1.5, 0.2) | 0.12 | 0.4 (−0.5, 1.4) | 0.35 | 0.5 (−3.3, 4.3) | 0.80 |
| Inhibin-B, pg/ml | −7 (−11, −3) | 0.0002 | 6 (−1, 13) | 0.07 | −2 (−7, 3) | 0.48 | 3 (−16, 22) | 0.78 |
| Inhibin-B/FSH | −4 (−8, 1) | 0.09 | −1 (−6, 4) | 0.78 | −4 (−9, 2) | 0.20 | 10 (−15, 34) | 0.43 |
| Total testosterone/LH | −0.2 (−0.4, 0) | 0.12 | −0.1 (−0.3, 0) | 0.03 | 0.1 (−0.1, 0.3) | 0.21 | 0 (−1, 1) | 0.93 |
| Free testosterone/LH | −2 (−7, 3) | 0.39 | −3 (−8, 1) | 0.14 | 0 (0, 0) | 0.81 | −12 (−21, −3) | 0.01 |
| Estradiol/total testosterone | 0 (−0.1, 0) | 0.36 | 0 (−0.1, 0.1) | 0.87 | 0 (−0.1, 0.1) | 0.69 | 0.2 (−0.2, 0.5) | 0.34 |
| (Estradiol/free testosterone) *100 | −0.4 (−0.9, 0.1) | 0.12 | 0.2 (−0.4, 0.8) | 0.59 | −0.4 (−0.9, 0.1) | 0.08 | 0.7 (−1.4, 2.8) | 0.54 |
The final models adjusted for age, BMI, height, smoking, use of marijuana and other recreational drug, moderate-to-vigorous physical activities (hours/week), history of reproductive diseases, reproductive surgeries, and STDs, season and calendar year of the sample, mother’s education level, data-driven dietary patterns, and total energy consumption. For the semen variables, we further adjusted for abstinence time. In addition, for sperm motility models, we further adjusted for time elapsed between specimen collection and analysis. For the serum reproductive hormone models, we further adjusted for time of the day of the sample collection.
The substitution estimate was scaled to represent one serving/day. Intakes were scaled to represent total servings per day (1 serving being equivalent to ∼200 ml).
Last, we conducted a series of sensitivity analyses to examine the robustness of the results. The associations observed in the primary analysis remained in analyses where we did not adjust for total energy intake and BMI (Supplementary Tables SV and SVI), in analyses restricted to men with a BMI between 18.5 and 25 kg/m2 (Supplementary Tables SVII and SVIII), and in analyses with additional adjustment for coffee intake (Supplementary Tables SIX and SX).
Discussion
We examined the association of intakes of SSBs, ASBs, energy drinks and fruit juices with markers of testicular function among healthy young men who were unaware of their fertility status. Most saliently, we found a relation between increased intake of SSBs and reduced semen quality, although the affected semen parameters in our study (total sperm count and concentration) differed from those previously related to SSB intake (sperm motility and semen volume) (Chiu et al., 2014; Yang et al., 2015). We also observed associations with inhibin-B/FSH ratio, further suggesting a negative impact on spermatogenesis. Overall, these findings suggest that high intakes of SSB may adversely affect testicular function.
To our knowledge, only three other epidemiological studies have examined the association between SSB and testicular function (Jensen et al., 2010; Chiu et al., 2014; Yang et al., 2015). Among 189 young men (aged 18–22 years) in the USA, we previously reported that men who consumed more than 200 ml of SSBs per day had a lower percentage of progressive sperm motility, but no other associations with semen quality or reproductive hormones were found (Chiu et al., 2014). Similarly, a Chinese study, reported an inverse dose–response association between intake of colas and semen volume (Yang et al., 2015). Further, in a non-overlapping group of 2544 Danish young men, we reported that men who drank > 14 bottles (500 ml) of cola/week had a lower sperm concentration and total sperm count than non-consumers, which was not explained by caffeine intake (Jensen et al., 2010). Our findings are also consistent with those of Hatch and colleagues (Hatch et al., 2018) who reported that men’s intake of SSB was associated with lower fecundability among couples seeking conception independently of women’s SSB intake. Interestingly, unlike previous semen quality studies but similar to the study by Hatch and collaborators (Hatch et al., 2018), the association of SSB intake and low sperm counts was observed at low intake levels and expanded in magnitude with greater intake.
Jointly considering the associations of SSB intake with semen parameters and reproductive hormone levels suggests a biologically plausible mechanism. Specifically, assuming the associations we detected are reflective of underlying causal relations, the lower inhibin-B/FSH ratio indicates a primary reduction in spermatogenesis owing to the fact that inhibin B/FSH ratio is a well-established marker of Sertoli cell function. Moreover, the lack of increase in FSH could also point towards a central (secondary) contributing cause. Our results are consistent with an experimental study in which Ruff et al. (2013) fed male mice a fructose/glucose solution resembling SSBs resulting in decreased male fertility as observed by fewer offspring. Men who consumed fructose, compared with glucose or sucrose, had altered fatty acid synthesis and fatty acid composition that may affect semen quality (Esmaeili et al., 2015; Nassan et al., 2018). SSBs, but not diet-soda or fruit juice, have also been associated with shorter telomeres (Leung et al., 2014), indicating faster cellular aging and contributing to lower male fertility (Ferlin et al., 2013). The results could also be explained by higher insulin resistance, that leads to oxidative stress (Park et al., 2009) and in turn may cause testicular suppression and lower semen quality (Agarwal et al., 2014). In addition, sperm have glucose receptors that are important for sperm motility and post-ejaculation maturation (Williams and Ford, 2001). Furthermore, glucose and insulin metabolism can disrupt the hypothalamic–pituitary–testicular axis (Schoeller et al., 2012).
Unexpectedly, we also found associations of intake of ASBs with lower sperm motility and SHBG levels and of energy-drinks with a higher percentage of morphologically normal sperm, E2, and LH concentration. These results should be interpreted with caution for various reasons. First, our prior hypothesis was that these beverages would be unrelated to markers of testicular function, and ASB intake specifically was thought of as a negative control for SSB intake. For the association pattern observed for SSBs, the findings for ASBs are still consistent with our prior hypothesis of no effect of this type of beverage specifically. Second, the findings for ASBs and energy drinks are not consistent either with alternate hypotheses for an association between this type of beverage and testicular function. For example, it has been suggested that canned beverages as a whole may be associated with adverse reproductive outcomes owing to contamination of the beverages with plasticizers in the inner lining of cans, which can leach into the can. If this were the case, we would have expected a similar association pattern across SSBs, ASBs, and energy drinks. This was not the case. Even though we found associations with all three beverages, the associations were not with the same outcomes and were not in the same direction. Importantly, the associations with energy drinks were in the opposite direction to that predicted by the hypothesis that chemicals in packaging would be responsible for an association, especially given that energy drinks are exclusively packaged in cans, whereas SSBs and ASBs are not. Moreover, to our knowledge, there are no studies that have previously examined the associations of ASB or energy drink intake with markers of testicular function to compare our results with. Clearly, additional research evaluating whether these beverages have an impact on testicular function is needed.
It is critically important to consider the clinical implications of the observed associations. Even though semen quality is undoubtedly important for fertility and remains the cornerstone for the clinical diagnosis of male factor infertility, its ability to predict the chance of pregnancy is limited both among couples trying naturally and in couples attempting to conceive with medical assistance (Slama et al., 2002; Jedrzejczak et al., 2008, Harris et al., 2019, Romero Herrera et al., 2021). Therefore, the observed association between higher SSB intake and lower semen quality does not necessarily imply a decrease in fertility. For example, among couples undergoing infertility treatment with ART, we have previously found that dietary and lifestyle factors related to semen quality are unrelated to the probability of achieving a live birth, including physical activity and intakes of processed meats, dairy, and soy foods (Chavarro et al., 2008; Afeiche et al., 2013, 2014a,b,c; Minguez-Alarcon et al., 2014, 2015; Gaskins et al., 2015; Xia et al., 2015, 2016). Conversely, we have found that dietary factors that have been consistently found to be unrelated to semen quality, such as intakes of alcohol and caffeine (Li et al., 2011; Ricci et al., 2017), were, paradoxically, related to live birth rates in ART (Karmon et al., 2017). On the other hand, there is also evidence of concordance in associations with semen quality and with fertility for various nutritional and lifestyle factors. We and others have reported associations between intake of fish, fish oil, or marine fatty acids with better semen quality and other markers of testicular function (Attaman et al., 2012; Salas-Huetos et al., 2017; Jensen et al., 2020) and with higher fertility (Gaskins et al., 2018). Also, and of greatest relevance to our findings, previous research in an independent study suggests that men’s intake of SSBs is associated with delayed conception in couples without a history of infertility who are attempting natural conception (Hatch et al., 2018). It is evident that the implications of our findings for fertility deserve additional evaluation.
Our study had a few limitations. First, because men provided one semen sample and one blood sample, potential misclassification of reproductive parameters cannot be excluded. However, we and others have reported previously that one semen sample suffices for studies aiming to identify the average differences in semen quality between men (Stokes-Riner et al., 2007; Chiu et al., 2017). Second, our ability to infer causality is limited owing to the cross-sectional design. However, the men were unaware of their testicular function when responding to the FFQ. Therefore systematic differences in dietary behavior conditional on testicular function are unlikely to arise in this study and the most likely type of error is non-differential misclassification leading to attenuation of effect estimates. Finally, while we adjusted for a number of potential confounders, we cannot exclude that unmeasured lifestyle and behavior associated with soft drink intake is associated with testicular function in these young men. Strengths include the study size which, to our knowledge, is the largest study to date to have examined the association between SSB and testicular function in men. Second, because the men in this study are generally healthy and unaware of their fertility, selection bias is unlikely and the generalizability of the results extend to men in the general population. Furthermore, as concentrations of the reproductive hormones were similar in study participants and non-participants (Andersen et al., 2000; Nassan et al., 2020), our results may be generalizable to men in the general population.
In conclusion, findings from this large study of healthy young men support the hypothesis that intake of sugary drinks impairs testicular function, especially sperm concentration and count. We also found unexpected relations of intakes of ASBs and energy drinks with sperm motility, whose clinical relevance is uncertain given the lack of previous literature and an a priori hypothesis linking these two beverage types with testicular function. As our study was cross-sectional, we cannot assess causality and we cannot exclude confounding by unmeasured lifestyle or behavioral factors associated with intake of SSBs. While additional research is necessary, the consistency of our findings for SSBs with the existing literature suggest that replacing SSBs with water may be advisable to protect testicular function.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of the individuals that participated in the study. The data will be shared on reasonable request to the corresponding author provided that ethical permission can be obtained for the specific request.
Authors' roles
F.L.N. performed the statistical analysis and wrote the manuscript; A.S.H. reviewed the statistical analysis and output; T.K.J., L.P., A.S.H., T.I.H., J.E.C., and N.J. reviewed and edited the manuscript; N.J. and T.K.J. designed research; F.L.N., N.J., and J.E.C. had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This work was supported by the Danish Council for Strategic Research, Program Commission on Health, Food and Welfare (Project no. 2101-08-0058), Independent Research Fund Denmark (Grant 8020-00218B), European Union, DEER (Grant 212844), the Danish Ministry of Health and the Danish Environmental Protection Agency and Kirsten and Freddy Johansen’s Foundation (Grant 95-103-72087), the Research Fund of the Capital Region of Denmark (A6176), and NIH Grant P30DK046200.
Conflict of interest
The authors report no conflict of interest.
Supplementary Material
References
- Afeiche M, Williams PL, Mendiola J, Gaskins AJ, Jorgensen N, Swan SH, Chavarro JE.. Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod 2013;28:2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, Petrozza JC, Hauser R, Chavarro JE.. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril 2014a;101:1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, Tanrikut C, Hauser R, Chavarro JE.. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr 2014b;144:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeiche MC, Williams PL, Gaskins AJ, Mendiola J, Jorgensen N, Swan SH, Chavarro JE.. Meat intake and reproductive parameters among young men. Epidemiology 2014c;25:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Virk G, Ong C, Du Plessis SS.. Effect of oxidative stress on male reproduction. World J Mens Health 2014;32:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AG, Jensen TK, Carlsen E, Jorgensen N, Andersson AM, Krarup T, Keiding N, Skakkebaek NE.. High frequency of sub-optimal semen quality in an unselected population of young men. Hum Reprod 2000;15:366–372. [DOI] [PubMed] [Google Scholar]
- Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, Chavarro JE.. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod 2012;27:1466–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarro JE, Toth TL, Sadio SM, Hauser R.. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod 2008;23:2584–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Mendiola J, Jorgensen N, Swan SH, Chavarro JE.. Sugar-sweetened beverage intake in relation to semen quality and reproductive hormone levels in young men. Hum Reprod 2014;29:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Edifor R, Rosner BA, Nassan FL, Gaskins AJ, Minguez-Alarcon L, Williams PL, Tanrikut C, Hauser R, Chavarro JE.. What does a single semen sample tell you? Implications for male factor infertility research. Am J Epidemiol 2017; 186:918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohle GR, Halley DJ, Van Hemel JO, van den Ouwel AM, Pieters MH, Weber RF, Govaerts LC.. Genetic risk factors in infertile men with severe oligozoospermia and azoospermia. Hum Reprod 2002;17:13–16. [DOI] [PubMed] [Google Scholar]
- Esmaeili V, Shahverdi AH, Moghadasian MH, Alizadeh AR.. Dietary fatty acids affect semen quality: a review. Andrology 2015;3:450–461. [DOI] [PubMed] [Google Scholar]
- Ferlin A, Rampazzo E, Rocca MS, Keppel S, Frigo AC, De Rossi A, Foresta C.. In young men sperm telomere length is related to sperm number and parental age. Hum Reprod 2013;28:3370–3376. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Mendiola J, Afeiche M, Jorgensen N, Swan SH, Chavarro JE.. Physical activity and television watching in relation to semen quality in young men. Br J Sports Med 2015;49:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Sundaram R, Buck Louis GM, Chavarro JE.. Seafood intake, sexual activity, and time to pregnancy. J Clin Endocrinol Metab 2018;103:2680–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL, Vanegas JC, Hariton E, Bortoletto P, Palmor M, Humphries LA, Tanrikut C, Chavarro JE, Styer AK.. Semen parameters on the day of oocyte retrieval predict low fertilization during conventional insemination IVF cycles. J Assist Reprod Genet 2019;36:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, Wesselink AK, Hahn KA, Michiel JJ, Mikkelsen EM, Sorensen HT, Rothman KJ, Wise LA.. Intake of sugar-sweetened beverages and fecundability in a North American Preconception Cohort. Epidemiology 2018;29:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrzejczak P, Taszarek-Hauke G, Hauke J, Pawelczyk L, Duleba AJ.. Prediction of spontaneous conception based on semen parameters. Int J Androl 2008;31:499–507. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Priskorn L, Holmboe SA, Nassan FL, Andersson AM, Dalgård C, Petersen JH, Chavarro JE, Jørgensen N.. Associations of fish oil supplement use with testicular function in young men. JAMA Netw Open 2020;3:e1919462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Swan SH, Skakkebaek NE, Rasmussen S, Jorgensen N.. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol 2010;171:883–891. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Auger J, Giwercman A, Irvine DS, Jensen TK, Jouannet P, Keiding N, Le Bon C, MacDonald E, Pekuri AM. et al. Semen analysis performed by different laboratory teams: an intervariation study. Int J Androl 1997;20:201–208. [DOI] [PubMed] [Google Scholar]
- Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH. et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2012;2:e000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmon AE, Toth TL, Chiu YH, Gaskins AJ, Tanrikut C, Wright DL, Hauser R, Chavarro JE, The Earth Study Team. Male caffeine and alcohol intake in relation to semen parameters and in vitro fertilization outcomes among fertility patients. Andrology 2017;5:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S.. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril 1988;49:112–117. [DOI] [PubMed] [Google Scholar]
- Leung CW, Laraia BA, Needham BL, Rehkopf DH, Adler NE, Lin J, Blackburn EH, Epel ES.. Soda and cell aging: associations between sugar-sweetened beverage consumption and leukocyte telomere length in healthy adults from the National Health and Nutrition Examination Surveys. Am J Public Health 2014;104:2425–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lin H, Li Y, Cao J.. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril 2011;95:116–123. [DOI] [PubMed] [Google Scholar]
- Louis JF, Thoma ME, Sørensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM.. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 2013;1:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso M, Wright-Schnapp TJ, Chandra A, Johnson R, Satterwhite CL, Pulver A, Berman SM, Wang RY, Farr SL, Pollack LA.. A public health focus on infertility prevention, detection, and management. Fertil Steril 2010;93:16.e11–16.e10. [DOI] [PubMed] [Google Scholar]
- Malik VS, Popkin BM, Bray GA, Després J-P, Hu FB.. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010;121:1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA.. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 1990;5:586–592. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TB, Osler M, Olsen SF.. Validity of protein, retinol, folic acid and n-3 fatty acid intakes estimated from the food-frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutr 2006;9:771–778. [DOI] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Afeiche MC, Chiu YH, Vanegas JC, Williams PL, Tanrikut C, Toth TL, Hauser R, Chavarro JE.. Male soy food intake was not associated with in vitro fertilization outcomes among couples attending a fertility center. Andrology 2015;3:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Chavarro JE, Mendiola J, Gaskins AJ, Torres-Cantero AM.. Physical activity is not related to semen quality in young healthy men. Fertil Steril 2014;102:1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Williams PL, Chiu YH, Gaskins AJ, Nassan FL, Dadd R, Petrozza J, Hauser R, Chavarro JE; Earth Study Team. Secular trends in semen parameters among men attending a fertility center between 2000 and 2017: Identifying potential predictors. Environ Int 2018;121:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassan FL, Chavarro JE, Tanrikut C.. Diet and men's fertility: does diet affect sperm quality? Fertil Steril 2018;110:570–577. [DOI] [PubMed] [Google Scholar]
- Nassan FL, Jensen TK, Priskorn L, Halldorsson TI, Chavarro JE, Jørgensen N.. Association of dietary patterns with testicular function in young Danish men. JAMA Netw Open 2020;3:e1921610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Food Institute TUoD. Danish food composition databank; 2019.
- Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, Jacobs DR. Jr.,. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care 2009;32:1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen AN, Fagt S, Groth MV, Christensen T, Biltoft-Jensen AP, Matthiessen J, Andersen NL, Kørup K, Hartkopp HB, Ygil KH.. Danskernes kostvaner 2003–2008: hovedresultater. Fødevareinstituttet: Danmarks Tekniske Universitet, 2010. [Google Scholar]
- Priskorn L, Nordkap L, Bang AK, Krause M, Holmboe SA, Egeberg Palme DL, Winge SB, Morup N, Carlsen E, Joensen UN.. et al. Average sperm count remains unchanged despite reduction in maternal smoking: results from a large cross-sectional study with annual investigations over 21 years. Hum Reprod 2018;33:998–1008. [DOI] [PubMed] [Google Scholar]
- Ricci E, Al Beitawi S, Cipriani S, Candiani M, Chiaffarino F, Vigano P, Noli S, Parazzini F.. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online 2017;34:38–47. [DOI] [PubMed] [Google Scholar]
- Romero Herrera JA, Bang AK, Priskorn L, Izarzugaza JMG, Brunak S, Jørgensen N.. Semen quality and waiting time to pregnancy explored using association mining. Andrology 2021;9:577–587. [DOI] [PubMed] [Google Scholar]
- Ruff JS, Suchy AK, Hugentobler SA, Sosa MM, Schwartz BL, Morrison LC, Gieng SH, Shigenaga MK, Potts WK.. Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nat Commun 2013;4:2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Huetos A, Bullo M, Salas-Salvado J.. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update 2017;1–19. [DOI] [PubMed] [Google Scholar]
- Schoeller EL, Schon S, Moley KH.. The effects of type 1 diabetes on the hypothalamic, pituitary and testes axis. Cell Tissue Res 2012;349:839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Eustache F, Ducot B, Jensen TK, Jørgensen N, Horte A, Irvine S, Suominen J, Andersen AG, Auger J.. et al. Time to pregnancy and semen parameters: a cross-sectional study among fertile couples from four European cities. Hum Reprod 2002;17:503–515. [DOI] [PubMed] [Google Scholar]
- Stokes-Riner A, Thurston SW, Brazil C, Guzick D, Liu F, Overstreet JW, Wang C, Sparks A, Redmon JB, Swan SH.. One semen sample or 2? Insights from a study of fertile men. J Androl 2007;28:638–643. [DOI] [PubMed] [Google Scholar]
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM.. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331. e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, Lopes P, Tabaste JM, Spira A.. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991;6:811–816. [DOI] [PubMed] [Google Scholar]
- Tjonneland A, Overvad K, Haraldsdottir J, Bang S, Ewertz M, Jensen OM.. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int J Epidemiol 1991;20:906–912. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM.. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th edn. Geneva, Switzerland: World Health Organization Department of Reproductive Health and Research, 2010. [Google Scholar]
- Willett W, Stampfer MJ.. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- Williams AC, Ford WC.. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 2001;22:680–695. [PubMed] [Google Scholar]
- Xia W, Chiu YH, Afeiche MC, Williams PL, Ford JB, Tanrikut C, Souter I, Hauser R, Chavarro JE, EARTH Study Team. Impact of men's dairy intake on assisted reproductive technology outcomes among couples attending a fertility clinic. Andrology 2016;4:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Chiu YH, Williams PL, Gaskins AJ, Toth TL, Tanrikut C, Hauser R, Chavarro JE.. Men's meat intake and treatment outcomes among couples undergoing assisted reproduction. Fertil Steril 2015;104:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Chen Q, Zhou N, Sun L, Bao H, Tan L, Chen H, Zhang G, Ling X, Huang L.. et al. Lifestyles associated with human semen quality: results from MARHCS Cohort Study in Chongqing, China. Medicine (Baltimore )2015;94:e1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of the individuals that participated in the study. The data will be shared on reasonable request to the corresponding author provided that ethical permission can be obtained for the specific request.