Abstract
Background:
Research on cannabis use among those with a history of cancer is limited.
Methods:
Prevalence of past-year cannabis use among individuals with and without a cancer history and predictors of use within these two groups were determined using data from the Population Assessment of Tobacco and Health study, a nationally representative, longitudinal survey conducted in the United States (Waves 1–4; 2013–2018). Discrete time survival analyses were used to estimate baseline (Wave 1) predictors (physical health status, mental health status, pain, and demographic variables) on past-year engagement with cannabis within individuals who reported a cancer diagnosis at Wave 1 (n=1,022) and individuals who reported never having cancer at any wave (n=19,702).
Results:
At the most recent survey, 8% of cancer survivors reported past-year cannabis use, compared to 15% of those without a cancer history. Across four timepoints, an estimated 3.8% of cancer survivors engaged with cannabis, as compared to 6.5% of those without a cancer history. Across both groups, older age and having health insurance were associated with lower likelihood of engaging with cannabis, while greater levels of pain were associated with higher likelihood of engaging with cannabis. Among those without a cancer history, being female, White, and having better mental health status were associated with lower likelihood of engaging with cannabis.
Conclusions:
Although cannabis use prevalence is lower among cancer survivors, the reasons for use are not markedly different from those without a cancer history. Continued monitoring of use, reasons for use, and harms or benefits is warranted.
Keywords: Cancer, Cannabis, Population Assessment of Tobacco and Health, PATH
Lay Summary:
Results from this study which uses data from the Population Assessment of Tobacco and Health Study indicate that cannabis use is generally increasing across cancer survivors and those without a history of cancer. Cancer survivors are using cannabis at slightly lower rates than those without a history of cancer, though the difference in prevalence between these rates seems to be decreasing over time. Factors related to pain seem to be more prevalent in cancer populations, relative to the general population and could be contributing to cannabis use within cancer survivor populations.
Precise:
Although cannabis use prevalence is lower among cancer survivors, reasons for use are not markedly different from those without a cancer history. Continued monitoring of use, reasons for use, and harms or benefits of cannabis use is warranted.
INTRODUCTION
Cancer survivors, or those who have been diagnosed with cancer and still living, are a rapidly growing population. It is estimated that there will be 26 million cancer survivors in the United States (US) by 20401. Advances in cancer screening and early detection, as well as improvements in treatment and supportive care have contributed to decreasing cancer-related mortality and increasing cancer survivorship2. With the increasing number of cancer survivors, there is a critical need to address cancer-related symptoms, such as chronic pain, fatigue, anxiety, and depression3.
To alleviate these symptoms, some cancer patients have looked towards alternative medicine, either in addition to conventional cancer therapies, or as a substitute for adjuvant therapies4. Qualitative data suggest that cancer patients generally have favorable attitudes toward use of medical cannabis (or marijuana) for cancer symptom and side-effect management5,6. Cannabis has been shown to demonstrate varying levels of benefit in symptom relief7,8 among cancer patients actively undergoing treatment, including that from: nausea and vomiting9,10, insomnia, anxiety, and depression6, and loss of appetite11, and cachexia12. Cannabis may also help to enhance relaxation, decrease stress, and improve quality of life; though, existing evidence is mixed13–15. While research is increasing in this area, there remains many unanswered questions ranging from the prevalence of cannabis use among cancer survivors to questions about the reasons for use and when it is being used during the cancer journey. The focus of this paper is on the prevalence of cannabis use and general sociodemographic and health-related factors that may be related to cannabis use.
There is increasing public support for medical cannabis use. Approximately 60% of the current US population reside in states with legalized use of medicinal cannabis16. Added to this, a recent report from Pew Research Center suggests that two-thirds of Americans support cannabis legalization17. The estimated prevalence of past-year cannabis in the US varies depending on the data source. For example, national prevalence estimates, such as those reported by the National Survey on Drug Use and Health (NSDUH), suggests that cannabis use increased from 10.5% in 2002 to 12.5% in 201318. These estimates are higher than those reported from the National Epidemiologic Survey of Alcohol and Related Conditions (NESARC) which were 4.1% in 2001–2002 and 9.5% in 2011–2012. Differences between these estimates may be due to differences in sampling methods and survey procedures: the NSDUH used audio-computer administered self-interview which enhances privacy, whereas participants in the NESARC were interviewed face-to-face19.
Prevalence estimates of use among cancer survivors also vary. In a cross-sectional, non-probabilistic sample of cancer survivors in Washington State (n=926) who varied with respect to treatment status (n=926; 5% newly diagnosed, 66% currently undergoing treatment, 21% finished therapy, and 9% not currently receiving treatment), investigators found that 24% of patients surveyed over a 6-week period between 2015 and 2016 reported using cannabis in the past year (termed “active users”). Active users were more likely to be younger and have a lower level of education, and less likely to have received hematopoietic cell transplants, in comparison with prior or never cannabis users. Cancer type was not related to cannabis use20. The investigators did not report on difference in cannabis use by cancer treatment status. Of note, the study was conducted in a state where, at the time, both medicinal and recreational cannabis use was legalized.
To date, only two studies have estimated the prevalence of cannabis use among cancer survivors using nationally representative population-based samples21,22. Utilizing data from the US National Health and Nutrition Examination Survey (NHANES, 2005–2014), Tringale et al. (2019) found that the past-year cannabis estimate among cancer survivors (40.3%) was not statistically different from past-year cannabis use estimates among those without a cancer history (38.1%)22. Cousins et al. (2021) utilized data from the National Survey of Drug Use and Health (NSDUH, 2015–2019) and reported much lower prevalence of cannabis use among those with a history of cancer. According to Cousins et al. (2021), 8.9% of those who had been diagnosed with cancer but not in the past year and 9.9% of those who had been diagnosed with cancer within the past year had also reported cannabis use in the past year21.
To refine population-based estimates of cannabis use among the general population and among cancer survivors, additional studies reporting a representative sample of recent and longitudinal data are needed. The objectives of this study were to: (1) determine the prevalence of cannabis use in cancer survivors, (2) describe factors that may be related to cannabis use among cancer survivors, and (3) determine to what degree these factors are unique to cancer survivors, relative to individuals without a history of cancer. We hypothesized that with the increasing availability of cannabis for medical use across many states, cannabis use among cancer survivors would increase over time. We also hypothesized that sociodemographic factors, such as age, gender, race/ethnicity, education, income, insurance status and health-related factors, such as measures of pain, physical health, and mental health status would be associated with cannabis use, and that these factors would be more related to use among cancer survivors than those without a history of cancer.
METHODS
Data Source and Study Sample
Data were obtained from the Population Assessment of Tobacco and Health (PATH) Study, a household-based, nationally representative, longitudinal cohort study of adults and youth in the US (N=32,320) that assesses cancer status and tobacco and other substance use. The methods and conceptual framework for the PATH study are described in more detail elsewhere23. Briefly, participants were recruited via an address-based, area-probability sampling approach. Adult tobacco users, young adults (18–24 years), and African Americans were oversampled relative to population proportions. Applied survey weights adjust for non-response bias and oversampling and yield representative estimates of the non-institutionalized, civilian US population. Audio-Computer Assisted Self-Interviews (CASI) available in English and Spanish were used to collect data. Data were collected in four annual waves beginning in 2013 to 2014 and proceeding annually in 2014 – 2015, 2015 – 2016, and 2016 – 201823–25.
There were 26,072 adult individuals with longitudinal weights available for Wave 1 to Wave 4. This includes participants that provided responses in each of the four waves1. As such, the longitudinal weights were calibrated to provide estimates representative of the population. Because there was not a perfect overlap between participants who reported cancer status and individual weights, the final weighted analytic sample included N = 20,724 participants. This analytic sample was divided into two groups of participants: those who indicated that they ever had cancer at the baseline (at Wave 1), excluding those who may have developed cancer from baseline through Wave 4 and including those who are likely to have already completed treatment by Wave 4 (Wave 1: n = 1,022), and participants who indicated that they never had cancer in any of the four waves (n = 19,702). This secondary data analysis of deidentified data was deemed exempt by the Institutional Review Board at Virginia Commonwealth University.
Measures
Cancer status.
Cancer status was derived from the following two questions: “Have you ever been told by a doctor or other health professional that you had cancer?” (at baseline, or Wave 1). Cancer status was categorized into cancer survivors (i.e., “ever had cancer” at Wave 1) and those without a cancer history (i.e., “never had cancer” at any time from Wave 1 to Wave 4).
Cannabis use.
This outcome variable was measured at each wave and derived from questions measuring past-year use (i.e. “In the past 12 months, have you smoked part or all of a traditional cigar, cigarillo, or filtered cigar with marijuana in it?”, “In the past 12 months, have you used marijuana, hash, THC, grass, pot, or weed?”). Past-year use was categorized into “used within past 12-months” (coded as 1), and “not used within the past 12-months” (coded as 0) at each wave.
A set of additional questions were available at wave 4. Those who had indicated past 12-month use of marijuana, hash, THC, grass, pot, or weed, were asked whether they had used the substance(s) weekly or more often. Those answering this question were also asked if they had used marijuana, hash, THC, grass, pot or weed in the past 30 days. Only those who had reported use of any electronic nicotine products were asked whether they had ever used marijuana concentrates, marijuana waxes, THC, or hash oils in an electronic nicotine product. Those who reported ever using an electronic nicotine product to ingest a marijuana byproduct(s) were asked to indicate the number of puffs taken from the electronic product either today, yesterday or the day before yesterday. Frequencies and weighted percentages or mean scores and standard errors for these variables were calculated and presented (see Table 2).
Table 2.
Cannabis Related Measures
| Full sample | No cancer history | Cancer Survivors | |||||
|---|---|---|---|---|---|---|---|
| N | weighted % | n | weighted % | n | weighted % | p | |
| Past-Year Cannabis Use | |||||||
| Wave 1 (N = 18,516) | 4511 | 15% | 4364 | 15% | 147 | 8% | <.001 |
| Wave 2 (N = 20,719) | 4705 | 13% | 4586 | 13% | 119 | 5% | <.001 |
| Wave 3 (N = 20,720) | 4766 | 13% | 4632 | 14% | 134 | 6% | <.001 |
| Wave 4 (N = 20,720) | 4958 | 14% | 4805 | 15% | 153 | 8% | <.001 |
| Cannabis Use Measures at Wave IV | |||||||
| Ever smoked marijuana from a hookah (N = 20,702)a | 3007 | 9% | 2883 | 10% | 124 | 7% | .011 |
| In past 12 months, smoked part or all of a traditional cigar, cigarillo or filtered cigar with marijuana (N = 20,705)a | 2593 | 6% | 2547 | 7% | 46 | 2% | <.001 |
| In past 12 months, used marijuana, hash, THC, grass, pot, or weed (N = 18,089)b | 2365 | 8% | 2258 | 8% | 107 | 6% | 0.009 |
| Marijuana, hash, THC or grass, pot or weed used weekly or more often (N = 9,427)c | 3321 | 27% | 3207 | 27% | 114 | 18% | <.001 |
| Ever used marijuana concentrates, marijuana waxes, THC or hash oils in electronic nicotine product (N = 6,803)d | 2702 | 34% | 2619 | 34% | 83 | 26% | .026 |
| In past 30 days, used marijuana, hash, THC, grass, pot or weed (N = 4,940)e | 3764 | 74% | 3647 | 74% | 117 | 72% | .666 |
| M | SE | M | SE | M | SE | ||
| n=446 | n=430 | n=16 | |||||
| Number of puffs of marijuana taken from an electronic nicotine product today/yesterday/day before yesterday (N = 446)f | 8.94 | 1.13 | 8.93 | 1.11 | 9.20 | 5.06 | .955 |
This item was asked of all participants who reported ever using marijuana (i.e., as a part of a traditional cigar, cigarillo, or filtered cigar; as marijuana, hash, THC, grass, pot, or weed)
This item was asked of participants who had not reported smoking cigars as blunts in the past 12 months
This item was asked of participants who reported ever using two or more substances (i.e., alcohol, other drugs) and who report using alcohol or other drugs weekly or more often in the past month, 2 to 12 months ago, or over a year ago
This item was asked of participants who reported using an electronic nicotine product
This item was asked of participants who reported using marijuana in the past 12 months
This item was asked of participants who reported ever using marijuana in an electronic nicotine product and who reported last used marijuana in an electronic nicotine product in the past hour, sometime today, yesterday, or the day before yesterday
Predictors.
All predictors were measured at the baseline (Wave 1). Predictors included demographic variables, such as: sex (male, female), age (18 to 24 years, 25 to 34 years, 35 to 44 years, 45 to 54 years, 55 to 64 years, and 65 years and older), highest level of education attained (less than high school, high school graduate/GED, some college or associate degree, and bachelor’s degree or higher), health insurance status (has health insurance, does not have health insurance), and annual household income (less than $10,000; $10,000 to $24,999; $25,000 to $49,9999; $50,000 to $99,999; and $100,000 or more annually). Other predictors included self-rated measures of physical and mental health (each rated on a 5-point Likert scale from poor to excellent, in response to “How would you rate your [physical/mental] health?”) and pain in the past 7 days (rated on a scale from 0 to 10, where 0 is no pain and 10 is the worst pain).
Statistical Methods
First, we computed descriptive statistics to compare baseline characteristics (at Wave 1) among the group of cancer survivors and among those without a history of cancer. In addition, we computed the prevalence estimates of past-year cannabis use for each group for each of the four waves. Prevalence estimates on the additional cannabis-related questions related to the ways they used cannabis were also computed.
For the main part of the analysis, we used discrete time survival analysis to provide an estimate of time to engagement with cannabis over the period of observation. The outcome in the analysis was defined as a latent variable with four dichotomous variables, reflecting past-year cannabis use for Wave 1 to Wave 4 as its indicators, with equal loadings (i.e., all loadings equal to 1). The predictors in this model included health factors (i.e., self-rated physical health, self-rated mental health, and pain) and background variables (i.e., age, education, health insurance status, and annual household income) assessed at baseline (Wave 1). The model was estimated as a multigroup model with separate estimates for the cancer survivor group and for those without a history of cancer. The results are reported as adjusted odds ratios (ORs), reflecting the probability of engaging with cannabis over the subsequent three waves (Waves 2, 3, and 4), following the initial baseline (Wave 1). These ORs are adjusted for all health factor and background variables in the model.
To compute proportions and pairwise comparisons, we used longitudinal weights with 100 replicate weights for precisely estimating the standard errors. For the discrete time survival analysis, we used longitudinal weights in conjunction with modeling the complex, longitudinal structure of the data using Taylor series linearization for adjusting standard errors. All analyses were done in Mplus 826.
RESULTS
Table 1 shows sample characteristics for the full sample and separated by cancer history status, in efforts to determine whether sociodemographic characteristics differed between those with and without a history of cancer. Most of the sample was female (52%, n=10831), White (78%, n=14834), and had at least some college education (59%, n=11936). Approximately 17% (n=1807) of the sample was 65 years and older. Participants in the cancer survivor group were significantly older, more likely to have a health insurance, and more likely to be White, as opposed to other racial/ethnic groups (all ps < 0.05). Cancer survivors also rated their pain levels as significantly higher than those without a history of cancer, and their self-ratings of physical health as significantly lower (ps < 0.05). No significant differences were found for self-ratings of mental health.
Table 1.
Descriptive Statistics of the Study Variables
| Full Sample | No cancer history | Cancer Survivors | ||||||
|---|---|---|---|---|---|---|---|---|
| N | weighted % | n | weighted % | n | weighted % | χ2 / t p | ||
| Age | 18 to 24 years | 5659 | 13% | 5625 | 14% | 34 | 1% | <.001 |
| 25 to 34 years | 4095 | 18% | 4029 | 19% | 66 | 4% | ||
| 35 to 44 years | 3271 | 17% | 3158 | 18% | 113 | 6% | ||
| 45 to 54 years | 3250 | 18% | 3052 | 18% | 198 | 17% | ||
| 55 to 64 years | 2638 | 17% | 2382 | 16% | 256 | 21% | ||
| 65 years or older | 1807 | 17% | 1452 | 14% | 355 | 51% | ||
| Education | Less than high school | 2636 | 11% | 2520 | 11% | 116 | 10% | .182 |
| GED/ High school graduate | 6072 | 29% | 5812 | 29% | 260 | 27% | ||
| Some college or associates degree | 7332 | 31% | 6974 | 31% | 358 | 31% | ||
| Bachelor’s degree / Advanced degree | 4604 | 28% | 4317 | 28% | 287 | 32% | ||
| Income | <$10K | 3666 | 14% | 3535 | 14% | 131 | 9% | .021 |
| $10K to $24,999 | 4398 | 20% | 4197 | 20% | 201 | 19% | ||
| $25K to $49,999 | 4351 | 23% | 4129 | 23% | 222 | 26% | ||
| $50K to $99,999 | 4068 | 26% | 3857 | 26% | 211 | 27% | ||
| >=$100,000 | 2505 | 18% | 2352 | 18% | 153 | 19% | ||
| Insurance | No health insurance | 3790 | 15% | 3714 | 15% | 76 | 4% | <.001 |
| Has health insurance | 16753 | 85% | 15812 | 85% | 941 | 96% | ||
| Race | White | 14834 | 78% | 13960 | 77% | 874 | 91% | <.001 |
| Black | 3335 | 12% | 3255 | 13% | 80 | 5% | ||
| Other | 2027 | 10% | 1970 | 10% | 57 | 4% | ||
| Sex | Male | 9873 | 48% | 9485 | 48% | 388 | 41% | <.001 |
| Female | 10831 | 52% | 10199 | 52% | 632 | 59% | ||
| M | SE | M | SE | M | SE | |||
| Pain rating over past 7 days | (Range: 0 – no pain to 10 – worst pain) | 1.91 | 0.03 | 1.86 | 0.03 | 2.63 | 0.12 | <.001 |
| Mental health | (Range: 0 – poor to 5 – excellent) | 3.67 | 0.01 | 3.66 | 0.01 | 3.70 | 0.04 | .336 |
| Physical health | (Range: 0 – poor to 5 – excellent) | 3.52 | 0.01 | 3.54 | 0.01 | 3.26 | 0.04 | <.001 |
Overall, 15% (n=4511) of the full sample had reported past-year cannabis use at Wave 1. Table 2 shows how patterns of cannabis use differed between cancer survivors and those without a history of cancer. Group comparisons demonstrated that past-year cannabis use was much higher among those without a cancer history at baseline, as compared to the cancer survivor group (Wave 1: 15% or n=4364 vs 8% or n=147) and at subsequent waves (Wave 2: 13% or n=4586 vs 5% or n=119; Wave 3: 14% or n=4632 vs 6% or n=134; Wave 4: 15% or n=4805 vs 8% or n=153; all ps <.001).
There was a higher proportion of participants with no cancer history having had ever experienced smoking marijuana from a hookah (10% or n=2883 vs 7% or n=124, p = .011). Regarding past-year cannabis use, a lower percentage of cancer survivors reported smoking cigars with marijuana (2% or n=46 vs. 7% or n=2547, p <.001) and using marijuana, hash, THC, grass, pot or weed (6% or n=107 vs. 8% or n=2258, p =.009). A lower percentage of cancer survivors reported weekly cannabis use relative to those without a cancer history (18% or n=114 vs 27% or n=3207, p <.001). However, among ever cannabis users who reported past-year use, no differences were found between cancer survivors and those without a cancer history for past 30-day cannabis use (72% or n=117 vs. 74% or n=3647, p=.666). Among participants that indicated having used cannabis, cancer survivors reported a lower prevalence of ever using cannabis from an electronic nicotine product (26% or n=83 vs. 34% or n=2702, p = .026), when compared to those without a cancer history. No group difference was found for the number of puffs of marijuana taken by participants who said they used an electronic nicotine product (cancer survivors: n=16, mean = = 9.20, se = 5.06; no cancer history: n=430, mean = 8.93, se = 1.11; p = .955).
Discrete time survival analysis was used to determine risk factors for cannabis engagement among those with and without a history of cancer. Results showed that across the four timepoints, an estimated 6.5% of those without a cancer history started using cannabis as compared to 3.8% of cancer survivors. Figure 1 shows the survival curves for both groups. As shown in Table 3, for both groups, having health insurance (those without a cancer history OR = 0.75, 95% CI: 0.68, 0.83; cancer survivors OR = 0.50, 95% CI: 0.28, 0.89) and older age (those without a cancer history OR = 0.65; 95% CI: 0.63, 0.68; cancer survivors OR = 0.55, 95% CI: 0.49, 0.60) were associated with lower likelihood of engaging with cannabis use. Specifically, compared to those without health insurance, those with health insurance had a 50% decrease in the odds of engaging with cannabis use among cancer survivors and a 25% decrease in odds among individuals without cancer. For every 1-year age increase at baseline (Wave 1), there was 45% decrease in odds of engaging with cannabis use over the subsequent waves for cancer survivors and 35% decrease in odds of engaging with cannabis use for individuals without cancer history. Higher self-reported levels of perceived pain at baseline was associated with a higher likelihood of engaging in cannabis use for both those without a cancer history (OR = 1.07, 95% CI: 1.06, 1.09) and for cancer survivors (OR = 1.14, 95% CI: 1.06, 1.23). Compared to males, females were less likely to engage with cannabis, but only in the group with no cancer history (OR = 0.66, 95% CI: 0.61, 0.72). Among those without a cancer history, Black participants were more likely to engage with cannabis relative to White participants (OR = 1.27, 95% CI: 1.13, 1.43). A similar pattern was observed among those with a history of cancer, though this was not statistically significant - likely due to the lower numbers of Black participants in this group. Lower income was associated with higher likelihood of engaging in cannabis use for those without a cancer history (OR = 0.89, 95% CI: 0.86, 0.93). A similar pattern was observed for those with a cancer history, but this was not statistically significant. Better self-rated mental health was associated with lower likelihood of engaging with cannabis in those without a cancer history (OR = 0.82, 95% CI: 0.79, 0.85). Similar, though not statistically significant, trends were found among those with a cancer history. Higher self-rated pain at baseline for both groups was related to a higher likelihood of engaging with cannabis over the subsequent follow-up assessments (OR= 1.05, 95% CI: 1.06, 1.09 among those without a history of cancer; OR= 1.14, 95% CI: 1.06, 1.23 among cancer survivors).
Figure 1.
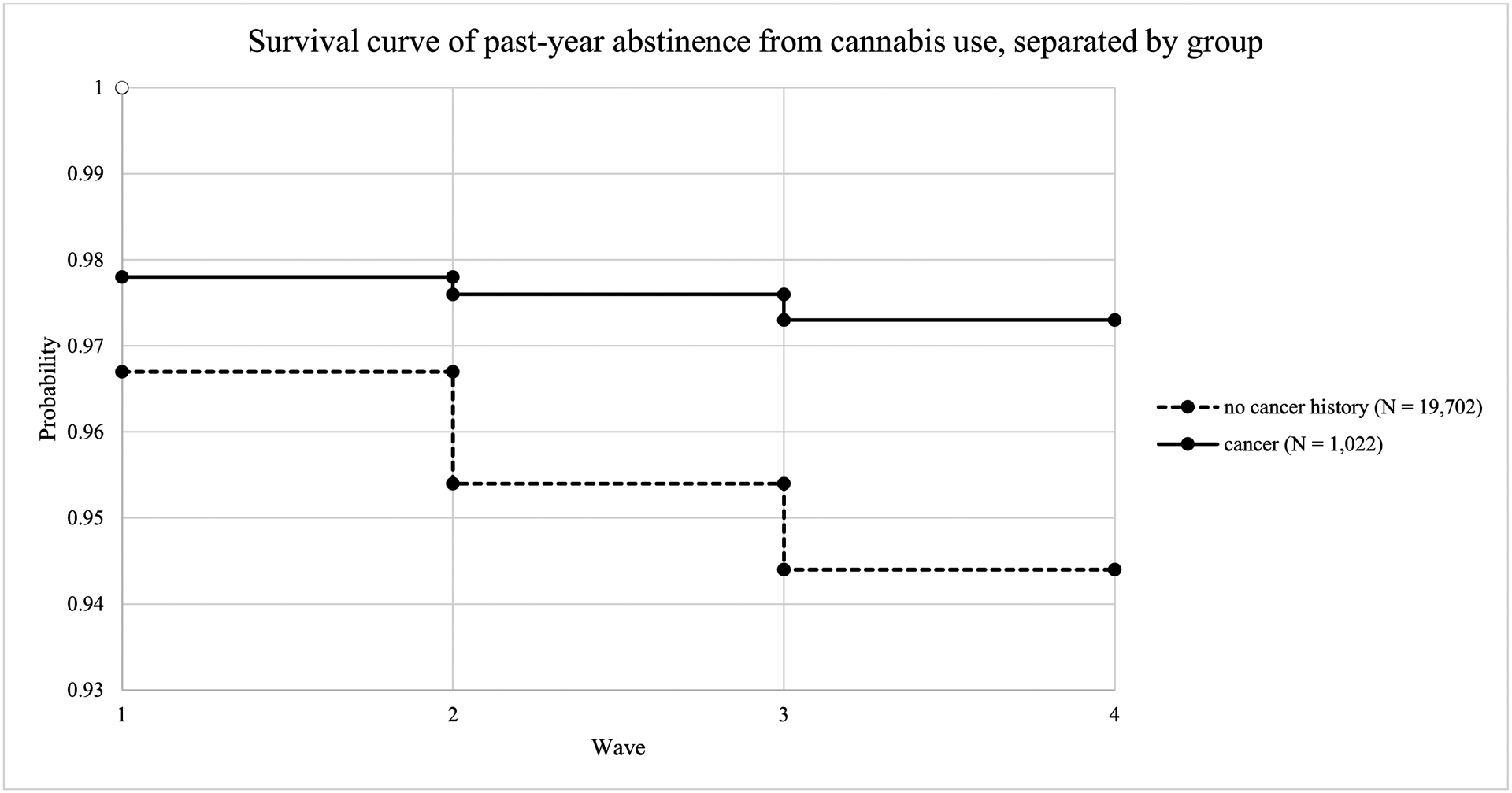
Survival curves for past-year abstinence from cannabis use for cancer survivors and those without a history of cancer
Table 3.
Results from Discrete Time Survival Analysis Predicting Cannabis Engagement
| No cancer history | Cancer Survivors | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 2.5% OR CI |
97.5% OR CI | OR | 2.5% OR CI |
97.5% OR CI | |||
| Female a | 0.66 | 0.61 | 0.72 | Femalea | 0.70 | 0.47 | 1.05 | |
| Age | 0.65 | 0.63 | 0.68 | Age | 0.55 | 0.49 | 0.60 | |
| Black b | 1.27 | 1.13 | 1.43 | Blackb | 1.48 | 0.76 | 2.88 | |
| Otherb | 0.93 | 0.80 | 1.08 | Otherb | 0.81 | 0.41 | 1.60 | |
| Education W1 | 1.05 | 0.99 | 1.11 | Education W1 | 0.92 | 0.73 | 1.17 | |
| Income W1 | 0.89 | 0.86 | 0.93 | Income W1 | 0.85 | 0.71 | 1.01 | |
| Has insurance W1 c | 0.75 | 0.68 | 0.83 | Has insurance W1 c | 0.50 | 0.28 | 0.89 | |
| Pain past 7 days W1 | 1.07 | 1.06 | 1.09 | Pain past 7 days W1 | 1.14 | 1.06 | 1.23 | |
| Physical health status W1 | 1.03 | 0.98 | 1.08 | Physical health W1 | 1.03 | 0.83 | 1.29 | |
| Mental health status W1 | 0.82 | 0.79 | 0.85 | Mental health W1 | 0.84 | 0.70 | 1.01 | |
Note. W1 = answered at Wave 1. Bold parameters are statistically significant at p <.05.
reference group is male
reference group is White
reference group is not having an insurance
DISCUSSION
The overall prevalence of cannabis use in the general population within PATH is within the range provided by other prevalence estimates at the national level (i.e., between 13% and 15% in PATH vs. 2.8% to 12.9% in other studies18,27). However, unlike other studies of cancer survivors, a much lower prevalence of cannabis use was reported among cancer survivors in the PATH survey (6% to 8% across Waves 1–4 vs. 8% to 40% in other studies20–22). Other nationally representative studies21,22 find that younger individuals are more likely to use cannabis. Comparably, our analysis showed that older individuals at baseline were less likely to use cannabis in subsequent years – regardless of whether they were cancer survivors or those without a cancer history20. Across both groups, greater levels of perceived pain were associated with a higher likelihood of engaging with cannabis. The finding on perceived pain aligns with another study examining cannabis use for the management of pain, which found that average past 7-day pain intensity score was significantly higher among users of cannabis within the past year, relative to controls28.
Differences in reported prevalence of cannabis use and associated factors might be attributed to differences in study design, sampling methods, and population composition. For example, the study by Pergram et al. which found that 24% of surveyed cancer patients currently undergoing treatment reported past-year use was conducted in Washington State, a state where cannabis is legal20. Respondents in PATH were sampled across the US where legalization laws vary. This is also the case for the studies conducted using NHANES22 and NSDUH21 data. Additionally, the time of diagnosis and stage of cancer were unknown among PATH respondents. As such, it is possible that those undergoing treatment may have very different attitudes and willingness of using cannabis to manage symptoms of the treatment or their disease from those who are just completing treatment, or from those who are no longer in treatment. Differences in reported prevalence of cannabis use across national data sets might also be attributed to differences in the age of the sample populations. For example, NHANES respondents were aged 20 to 60 years, while PATH data does not have a maximum age cap. Additionally, NHANES data is collected through a medical examination and biospecimens (i.e., urine sample), which, even though participants are told that the urine is not for drug testing, might nevertheless encourage respondents to report more truthfully.
To our knowledge, there are only two other studies that have employed a nationally representative sample to examine cannabis use among cancer survivors21,22. One is a recent study published by Cousins et al. (2021), which found that cannabis use was less common in those with past (8.9%) or recent (9.9%) cancer diagnosis, relative to those without a history of cancer (15.9%)21. The other is a study published by Tringale et al. (2019), using the US National Health and Nutrition Examination Survey29 (NHANES, 2005–2014) data. This study reported past-year cannabis use to be 40.3% (n=826) among cancer survivors and 38% among respondents without cancer22. The estimates provided by the NHANES study were more than triple that reported in other national prevalence studies, which range between 2.8% and 12.9%18,19,21,27,30. The peculiarly high prevalence estimate of past-year use in the NHANES data is also 130% to 140% higher than the estimate in a very similar national survey (National Survey on Drug Use and Health, NSDUH31) conducted over the same period21,32. Alshaarawy and Anthony (2017) speculated that the health context of the NHANES study, which was conducted similarly to a physical exam where blood and urine were collected, might have promoted more accurate reporting of cannabis use – especially if participants were told that the biospecimen is not being used for drug testing32.
If this is the case, the prevalence estimate obtained in our study using PATH data and in the Cousins et al. (2021) study using NSDUH data may be underestimating past-year cannabis use. Continued research on the extent of cannabis use among cancer survivors would help more accurately determine the point prevalence. Research on factors related to cannabis use in the subpopulation is also needed.
One of the factors related to use, as suggested by Cousins et al. (2021), is age.21 That study found that differences in recent cannabis use (which includes recent or past use anytime within the past year) between those with and without cancer were not seen in older adults (aged 50 years or older) or in the youngest age group (18–34 years) but were found for the middle age group (35 – 49 years). Similar to that study, we also found that age was a significant predictor of whether or not someone engaged with cannabis. In our study, which modeled the relationship between age at baseline and subsequent likelihood of engaging with cannabis over three annual waves of assessment, we observed that the older the participant was at the initial baseline assessment, the less likely they were to engage with cannabis over the subsequent waves of data collection.
In addition to finding similar relationships between age and cannabis use, our study and that of Cousins et al. (2021) were highly aligned with respect to the prevalence of cannabis use (i.e., 8.9% of those with a history of cancer vs. 15.9% of those without a history of cancer in NSDUH; 8% of those with a history of cancer vs. 15% of those without a history of cancer in the most recent wave of PATH). Our study expands the literature in that it capitalizes on the longitudinal design of PATH and complements the cross-sectional study designs of Tringale et al. (2019) and Cousins et al. (2021). Specifically, our study estimates the percent of respondents within each group who used cannabis over the approximate 4-year observational period and the factors that predicted this likelihood of use over this period.
Although cannabis may be used for several medical issues, prior literature suggests that cannabis use for pain is common, especially among those who experience chronic pain. Recent studies suggest that between 45% and 80% of individuals who receive medical cannabis do so for pain management33,34. Cannabis use has also been attributed to mitigating mental health challenges, such as anxiety or depression35,36. Though, we did not find evidence for this among the cancer survivor group in our analyses. Surprisingly, physical health status was also not found to be a significant predictor of cannabis use for either cancer survivors or those without a history of cancer in our study. This finding differs from other studies, which suggests that cannabis is used commonly for the relief of physical symptoms20,37,38.
Our study results should also be considered within the context of certain limitations. Cancer diagnosis was self-reported and not confirmed by a medical professional. Further, although the PATH dataset has a very large sample of cancer survivors, it is designed to primarily study tobacco use. As a result, some information that is unique to cancer survivors is not available as part of the PATH dataset, such as information on specific cancer type/stage, time since diagnosis, cancer re-occurrence, or prescribed cancer treatments. Also, the dearth of information on why cancer survivors use cannabis does not allow for the identification of unique predictors among cancer survivors. Having this information available in future studies would be useful for determining why someone with a history of cancer may use cannabis.
Although the PATH data does provide some information on how cannabis may have been used (i.e., from hookah, as a wax, THC/hash oil in electronic nicotine products, in a traditional cigar, cigarillo, or filtered cigar, or as hash, pot, or weed) and provides clues for how use might differ between cancer survivors and those without a history of cancer, there is a limited amount of information available on cannabis type, frequency, and reasons for use (i.e., recreation vs. managing symptoms). Two studies focused on a diverse group of patients seeking certification for medical cannabis in Michigan39,40 suggest that those with a history of cancer are: less likely to endorse daily or almost daily use of cannabis40, less frequently endorse smoking cannabis39, and more frequently endorse edible use39. Though, more studies are needed to validate these findings.
Also missing from the current analyses is information regarding whether participants reside in a state with legal recreational and/or medical cannabis use laws. The inclusion of this information in future studies would be especially informative for cancer treatment. Results from prior studies suggest that cancer patients seeking medical cannabis are different from those seeking medical cannabis without cancer and that the methods by which cannabis is used may also differ by cancer status39,40. In addition, more refined measurement of cancer and marijuana use is needed in future studies in order to address existing limitations to available survey items.
Despite these limitations, there are several strengths to this study. Our study characterizes cannabis use in cancer survivors and those without a cancer history and describes trends over time within these two groups. Further, data obtained for these analyses come from a large national survey conducted in the US, is weighted to adjust for its complex sampling design, and utilizes statistical models that take advantage of the longitudinal nature of the data. It represents one of the largest nationally representative studies to date to compare cannabis use among cancer survivors and those without a cancer history. However, to get more precise estimates of cannabis use within the general population and among cancer survivors and to determine factors that might be predictive of use over time, more research is needed in this area.
Our results indicated that cancer survivors are using cannabis at slightly lower rates than those without a history of cancer. However, the specific causes for why this trend may be occurring remains unknown. It is possible that cancer survivors who have more frequent follow-up with medical practitioners might not need to self-medicate for cancer-related symptoms. Physicians might also be choosing to not prescribe patients cannabis due to limited evidence for the effectiveness of cannabis alleviating cancer-related symptoms41.
Under many state laws, cannabis is becoming increasingly available for medical use. Yet, there is a paucity of evidence to guide clinical management of cannabis use among patients. Given that patients, regardless of cancer status, may elect to use cannabis for pain, other symptom management, or recreational purposes, clinicians will need to be able to counsel patients on cannabis use in clinical contexts, particularly related to the efficacy and harms of cannabis as a symptom management tool42. Clinicians will also need to work with researchers to consider how best to expand cannabis research to fill the gaps of knowledge regarding clinical and public health effects of expanded use.
Acknowledgements:
The authors would like to thank the funders (NIDA, NIH, and the FDA) and participants of the Population Assessment of Tobacco and Health (PATH) Study and acknowledge Kennedy C. Bradley for her assistance in conducting the literature review for this work.
Funding Statement:
The authors received no financial support for the research, authorship, and/or publication of this manuscript. The Population Assessment of Tobacco and Health Study is supported with federal funds from the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH), and the U.S. Food and Drug Administration (FDA), DHHS, under a contract to Westat (Contract No. HHSN271201100027C). NIDA and FDA contributed to the study design, but not the collection or analysis of the data.
Footnotes
Conflict of Interest Statement: The authors declare no potential conflicts of interest.
There was one exception to how the groups were coded. A new skip logic was introduced at Wave 4, such that only individuals who said they had visited a doctor in the past 12 months were asked about new diagnoses of cancer. The introduction of this skip logic pattern created a large number of missing participants (N ~ 6,000). To align with previous waves that did not use this skip logic pattern, we decided to remove those who had indicated that they had been diagnosed with cancer in the past 12 months at Wave 4 and coded missing values resulting from the skip logic pattern in Wave 4 to be a part of the non-cancer group.
REFERENCES
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. doi: 10.1158/1055-9965.EPI-16-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro CL. Cancer Survivorship. New England Journal of Medicine. 2018;379(25):2438–2450. doi: 10.1056/NEJMra1712502 [DOI] [PubMed] [Google Scholar]
- 3.Dulaney C, Wallace AS, Everett AS, Dover L, McDonald A, Kropp L. Defining Health Across the Cancer Continuum. Cureus. 2017;9(2). doi: 10.7759/cureus.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson SB, Park HS, Gross CP, Yu JB. Complementary Medicine, Refusal of Conventional Cancer Therapy, and Survival Among Patients With Curable Cancers. JAMA Oncol. 2018;4(10):1375–1381. doi: 10.1001/jamaoncol.2018.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birdsall SM, Birdsall TC, Tims LA. The Use of Medical Marijuana in Cancer. Curr Oncol Rep. 2016;18(7):40. doi: 10.1007/s11912-016-0530-0 [DOI] [PubMed] [Google Scholar]
- 6.Victorson D, McMahon M, Horowitz B, Glickson S, Parker B, Mendoza-Temple L. Exploring cancer survivors’ attitudes, perceptions, and concerns about using medical cannabis for symptom and side effect management: A qualitative focus group study. Complementary Therapies in Medicine. 2019;47:102204. doi: 10.1016/j.ctim.2019.102204 [DOI] [PubMed] [Google Scholar]
- 7.Wilkie G, Sakr B, Rizack T. Medical Marijuana Use in Oncology: A Review. JAMA Oncol. 2016;2(5):670. doi: 10.1001/jamaoncol.2016.0155 [DOI] [PubMed] [Google Scholar]
- 8.Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735–744. doi: 10.1111/j.1365-2125.2011.03970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todaro B Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting. J Natl Compr Canc Netw. 2012;10(4):487–492. doi: 10.6004/jnccn.2012.0048 [DOI] [PubMed] [Google Scholar]
- 10.Tramèr MR, Carroll D, Campbell FA, Reynolds DJM, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mousa A, Petrovic M, Fleshner NE. Prevalence and predictors of cannabis use among men receiving androgen-deprivation therapy for advanced prostate cancer. Can Urol Assoc J. 2020;14(1):E20–E26. doi: 10.5489/cuaj.5911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer JL. Medical marijuana for cancer. CA: A Cancer Journal for Clinicians. 2015;65(2):109–122. doi: 10.3322/caac.21260 [DOI] [PubMed] [Google Scholar]
- 13.Elliott DA, Nabavizadeh N, Romer JL, Chen Y, Holland JM. Medical marijuana use in head and neck squamous cell carcinoma patients treated with radiotherapy. Support Care Cancer. 2016;24(8):3517–3524. doi: 10.1007/s00520-016-3180-8 [DOI] [PubMed] [Google Scholar]
- 14.Rotermann M, Langlois K. Prevalence and correlates of marijuana use in Canada, 2012. Health reports. 2015;26:10–15. [PubMed] [Google Scholar]
- 15.Tanco K, Dumlao D, Kreis R, et al. Attitudes and Beliefs About Medical Usefulness and Legalization of Marijuana among Cancer Patients in a Legalized and a Nonlegalized State. Journal of Palliative Medicine. 2019;22(10):1213–1220. doi: 10.1089/jpm.2019.0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y Medical marijuana policies and hospitalizations related to marijuana and opioid pain reliever. Drug and Alcohol Dependence. 2017;173:144–150. doi: 10.1016/j.drugalcdep.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Two-thirds of Americans support marijuana legalization | Pew Research Center. Accessed December 12, 2020. https://www.pewresearch.org/fact-tank/2019/11/14/americans-support-marijuana-legalization/ [Google Scholar]
- 18.Grucza RA, Agrawal A, Krauss MJ, Cavazos-Rehg PA, Bierut LJ. Recent Trends in the Prevalence of Marijuana Use and Associated Disorders in the United States. JAMA Psychiatry. 2016;73(3):300–301. doi: 10.1001/jamapsychiatry.2015.3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasin DS, Saha TD, Kerridge BT, et al. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015;72(12):1235. doi: 10.1001/jamapsychiatry.2015.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pergam SA, Woodfield MC, Lee CM, et al. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer. 2017;123(22):4488–4497. doi: 10.1002/cncr.30879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cousins MM, Jannausch ML, Coughlin LN, Jagsi R, Ilgen MA. Prevalence of cannabis use among individuals with a history of cancer in the United States. Cancer. Published online June 3, 2021. doi: 10.1002/cncr.33646 [DOI] [PubMed] [Google Scholar]
- 22.Tringale KR, Huynh‐Le M-P, Salans M, Marshall DC, Shi Y, Hattangadi‐Gluth JA. The role of cancer in marijuana and prescription opioid use in the United States: A population-based analysis from 2005 to 2014. Cancer. 2019;125(13):2242–2251. doi: 10.1002/cncr.32059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tobacco Control. 2017;26(4):371–378. doi: 10.1136/tobaccocontrol-2016-052934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tourangeau R, Yan T, Sun H, Hyland A, Stanton CA. Population Assessment of Tobacco and Health (PATH) reliability and validity study: selected reliability and validity estimates. Tobacco Control. 2019;28(6):663–668. doi: 10.1136/tobaccocontrol-2018-054561 [DOI] [PubMed] [Google Scholar]
- 25.Population Assessment of Tobacco and Health (PATH) Study Series. Accessed December 12, 2020. https://www.icpsr.umich.edu/web/NAHDAP/series/606
- 26.Muthén LK, Muthén BO. Mplus User’s Guide. 8th Edition. Muthén and Muthén; 1998. [Google Scholar]
- 27.Pacula RL, Smart R. Medical Marijuana and Marijuana Legalization. Annu Rev Clin Psychol. 2017;13:397–419. doi: 10.1146/annurev-clinpsy-032816-045128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ware MA, Wang T, Shapiro S, et al. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). The Journal of Pain. 2015;16(12):1233–1242. doi: 10.1016/j.jpain.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 29.NHANES - National Health and Nutrition Examination Survey Homepage. Published December 10, 2020. Accessed December 12, 2020. https://www.cdc.gov/nchs/nhanes/index.htm
- 30.DiNitto D Marijuana use among older adults in the U.S.A.: User characteristics, patterns of use, and implications for intervention. International Psychogeriatrics. 2011;23:732–741. doi: 10.1017/S1041610210002176 [DOI] [PubMed] [Google Scholar]
- 31.National Survey on Drug Use and Health. Accessed December 12, 2020. https://nsduhweb.rti.org/respweb/homepage.cfm
- 32.Alshaarawy O, Anthony JC. The replicability of cannabis use prevalence estimates in the United States. International Journal of Methods in Psychiatric Research. 2017;26(2):e1524. doi: 10.1002/mpr.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse. 2014;40(1):23–30. doi: 10.3109/00952990.2013.821477 [DOI] [PubMed] [Google Scholar]
- 34.Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug and Alcohol Dependence. 2013;132(3):654–659. doi: 10.1016/j.drugalcdep.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 35.Martell K, Fairchild A, LeGerrier B, et al. Rates of cannabis use in patients with cancer. Curr Oncol. 2018;25(3):219–225. doi: 10.3747/co.25.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: Systematic review and meta-analysis. Social Science & Medicine. 2019;233:181–192. doi: 10.1016/j.socscimed.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 37.Boden MT, Babson KA, Vujanovic AA, Short NA, Bonn‐Miller MO. Posttraumatic Stress Disorder and Cannabis Use Characteristics among Military Veterans with Cannabis Dependence. The American Journal on Addictions. 2013;22(3):277–284. doi: 10.1111/j.1521-0391.2012.12018.x [DOI] [PubMed] [Google Scholar]
- 38.Schafer J, Brown SA. Marijuana and Cocaine Effect Expectancies and Drug Use Patterns. Journal of Consulting and Clinical Psychology. 1991;59(4):558–565. [DOI] [PubMed] [Google Scholar]
- 39.Cousins MM, Jannausch M, Jagsi R, Ilgen M. Differences between cancer patients and others who use medicinal Cannabis. PLOS ONE. 2021;16(3):e0248227. doi: 10.1371/journal.pone.0248227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cousins MM, Jagsi R, Ilgen M. How Do Cancer Patients Differ from Others Who Use Medicinal Cannabis? International Journal of Radiation Oncology, Biology, Physics. 2020;108(3):e176. doi: 10.1016/j.ijrobp.2020.07.1381 [DOI] [Google Scholar]
- 41.Campbell G, Hall WD, Peacock A, et al. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: findings from a 4-year prospective cohort study. Lancet Public Health. 2018;3(7):e341–e350. doi: 10.1016/S2468-2667(18)30110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savage SR, Romero-Sandoval A, Schatman M, et al. Cannabis in Pain Treatment: Clinical and Research Considerations. The Journal of Pain. 2016;17(6):654–668. doi: 10.1016/j.jpain.2016.02.007 [DOI] [PubMed] [Google Scholar]


