Abstract
Background
To investigate the correlation between vertebral Hounsfield unit (HU) values and cage subsidence in patients treated with stand-alone (SA) OLIF.
Methods
A retrospective review of collected data was performed on 76 patients who underwent SA OLIF. We utilized the HU value for lumbar bone mineral density (BMD) obtained on preoperative CT. The vertebral HU values of patients with subsidence were compared to those without subsidence. The correlation between cage subsidence and clinical score was investigated.
Results
Sixteen patients (21.1%) had at least radiographic evidence of interbody cage subsidence. The average cage subsidence was 2.5 ± 1.3 mm (range 0.9-4.8 mm). There were no significant differences in sex, BMI, preoperative diagnoses, or fused level (p > 0.05); however, there were significant differences between the cage subsidence group and the nonsubsidence group in age, average of the lowest T-score, and average HU value, including for the L1 vertebrae, L1-L4 horizontal plane, and L1-L4 sagittal plane (p < 0.05). The average HU value of the L1-L4 horizontal plane showed a more predictable AUC of 0.909 (95% CI, 0.834–0.984; P < 0.001) compared with the average of the lowest T-score following an AUC of 0.791 (95% CI, 0.674–0.909; P < 0.001). Based on logistic regression analysis, the average HU value of the L1-L4 horizontal plane (OR, 0.912; 95% CI, 0.861–0.966; P = 0.002) was an independent factor influencing cage subsidence.
Conclusions
Patients with lower average HU values of the lumbar vertebrae are at a much higher risk of developing cage subsidence after SA OLIF. Measurement of preoperative HU values on preexisting CT scans could be rapid, simple and feasible.
Keywords: Oblique lumbar interbody fusion, Hounsfield units, Osteoporosis, Cage subsidence, Degenerative lumbar diseases
Background
Recently, oblique lumbar interbody fusion (OLIF) has been introduced, providing a novel corridor to access the lumbar disc space [1, 2]. This approach uses the anatomic space between the anterior vessels and psoas muscles, allowing for efficient clearance of disc space and application of a large interbody cage to afford distraction for indirect decompression and endplate preparation for fusion. The OLIF technique is increasingly employed to treat structural degenerative conditions of the lumbar spine. Although OLIF combined with bilateral pedicle screw fixation is a widely performed procedure that provides a variety of advantages, such as excellent fixation intensity and a high fusion rate, it can be used as a stand-alone procedure to manage isolated degenerative disc diseases or spondylolisthesis [3–5]. More recently, it has been used as a part of reconstructive surgery in cases of degenerative deformity therapy [6]. Stand-alone OLIF (SA OLIF) has become a popular method of treatment of lumbar degenerative disease. Indirect decompression of the neural constructures is obtained by the restoration of the disk height and the reduction of slipped vertebra with the insertion of a proportionally sized cage. Additional posterior instrumentation has been suspected to result in more extensive dissection and blood loss, longer duration of surgery, higher risk of implant-related complications, and greater medical costs.
Cage subsidence is a potentially devastating complication after lumbar interbody fusion and is also an important issue in SA OLIF procedures. The development of cage subsidence is presumed to be a multifactorial process. Several potential risk factors have been examined in the literature, including overdistraction, insufficient cage width, poor construct length, lateral plating, and endplate violation [7, 8]. Bone quality is believed to be one of the important factors that cause cage subsidence associated with the lumbar interbody. Dual-energy X-ray absorptiometry (DXA) is cost effective, widely used clinically to diagnosis osteoporosis and represents the current gold standard for bone mineral density (BMD) assessment. However, various limitations of DXA have been described, such as distortion of estimated bone mineral mass values caused by overlying soft tissue, vascular calcifications, bowel content and degenerative spine changes. BMD measurement by vertebral body Hounsfield units (HU) as a predictor of cage subsidence should be included in preoperative planning for surgeons to determine surgical options. To date, few studies have attempted to assess BMD and cage subsidence following SA OLIF for degenerative lumbar diseases. The purposes of this work were to assess the relationship between BMD and cage subsidence following SA OLIF and to determine whether the vertebral body HU value should influence the operative plan in patients undergoing SA OLIF.
Materials and methods
Patients
After obtaining our institutional review board approval, a retrospective review of collected data was performed on all patients who underwent SA OLIF at a single institution between February 2015 and April 2020. All patients were followed up with 28.2 ± 9.3 months. The inclusion criteria for this study were as follows: (1) degenerative spondylolisthesis grade 1° according to the Meyerding classification; (2) lumbar instability; (3) discogenic back pain; (4) adjacent segmental diseases; (5) mild adult degenerative scoliosis; (6) chronic lower back pain and/or leg pain unresponsive to conservative therapy for at least 6 weeks; and (7) no experience of endplate damage during the procedure. The exclusion criteria for the study were as follows: (1) lumbar spondylolisthesis grade > 1°; (2) lumbar spondylolysis; (3) spinal infection; (4) spinal tumor pathologies; (5) spinal trauma; and (6) diseases involving the L5–S1 vertebrae.
All of the patients included had preoperative evaluations with detailed neurologic examination and radiologic imaging, which involved static (anterior-posterior and lateral) and dynamic (flexion and extension) plain lumbar radiographs, magnetic resonance imaging (MRI), and computed tomography (CT) scans.
Preoperative CT was routinely used to calculate HU values. The vertebral body HU values were measured according to the technique described by Schreiber [9]. Using standard picture archiving and communication system (PACS) software, an elliptical area of interest (AOI) was drawn on an axial image of the lumbar vertebral body, including the largest possible cancellous bone and excluding cortical edges, osteophytes, and osseous abnormalities, such as apparent sclerotic areas. Special attention was paid not to allow the AOI to include intervertebral spaces, which might cause inaccurate measurement of trabecular HU values in the vertebral body. Three measurements were obtained: just superior to the inferior endplate; the mid-vertebral body; and just inferior to the superior endplate. The picture system calculates the HU value of the area within the ellipse. The vertebral body BMD was defined as the average HU value of the three AOIs. A mid-sagittal CT image of the lumbar spine provided measurement of HU values. One major challenge of endplate CT assessments is the reproducibility of measurements because the methodology is not as standardized as a routine assessment. Therefore, the vertebral body HU values were measured in this series. DXA scans were performed routinely. The lowest T-score of the hips and lumbar spine was recorded based on preoperative DXA scans because the criterion for osteoporosis was the lowest T-score ≤ − 2.5 [10]. Disc height was measured on the midsagittal image of CT scans at the midway point of the vertebral bodies.
The average patient age at the time of surgery was 56.1 ± 10.4 years old (29–81 yr), and the study group comprised 46 women and 30 men. The most frequent diagnosis was degenerative spondylolisthesis grade 1° (32 cases, 42.1%), followed by lumbar instability (17 cases, 22.4%), adjacent segmental disease (18 cases, 23.7%), adult degenerative scoliosis (6 cases, 7.9%) and discogenic low back pain (3 cases, 3.9%). A total of 84 levels were treated: 92.1% one level, 5.3% two level, and 2.6% three level surgeries. The patients’ demographic characteristics are listed in Table 1.
Table 1.
Demographics and treatment data for 76 patients
| Characteristics | All (n = 76) |
|---|---|
| Number of patients | 76 |
| Mean age (years) | 56.1 ± 10.4 |
| BMI (kg/m2) | 24.7 ± 2.2 |
| Sex (M/F) (% male) | 30/46 (39.5) |
| Diagnoses [no.(%) of patients] | |
| Degenerative spondylolisthesis | 32 (42.1) |
| Lumbar instability | 17 (22.4) |
| Adjacent segmental disease | 18 (23.7) |
| Adult degenerative scoliosis | 6 (7.9) |
| Discogenic back pain | 3 (3.9) |
| Fused level [no.(%)of patients] | |
| One level | 70 (92.1) |
| Two level | 4 (5.3) |
| Three level | 2 (2.6) |
| Operative segment | |
| L2-L3 | 4 (4.8) |
| L3-L4 | 24 (28.6) |
| L4-L5 | 56 (66.7) |
Abbreviations: BMI Body Mass Index
Surgical technique
All of the patients underwent OLIF only through a left-side approach without neurophysiologic monitoring. The procedures were performed utilizing expandable retractors (OLIF 25 system; Medtronic, Memphis, TN, USA) according to the procedure described previously [3]. Polyetheretherketone (PEEK) intervertebral cages (OLIF25 Clydesdale Spinal System; Medtronic SofamorDanek, MN, USA) filled with allograft bone were used to achieve fusion. Special care was taken to avoid endplate fracturing during the disc space preparation and to span the apophyseal rings of both end plates during cage insertion. No supplementary anterior or posterior instrumentation or direct decompression was performed for 76 patients.
Clinical and radiographic measures
Clinical parameters included visual analog scores (VAS) and the Oswestry Disability Index (ODI). The ODI, version 2.0, was used both before surgery and after surgery. Considering that the sex question (Section 8) was omitted, the total possible score was 45. After discharge from the hospital, the patients had regular follow-ups conducted by the corresponding author. Plain lumbar radiographs and, if uncertain, computed tomography scans were ordered to detect cage subsidence. Cage subsidence was defined as cage vertical protrusion through the cephalad or caudal (or both) endplate of the vertebral body. Subsidence was measured by gauging the maximal migration of the cage into the endplate on lateral images of standing lumbar radiographs at a minimum of 6 months postoperatively. If a patient had multiple radiographs during the period, the last radiograph was used for subsidence assessment. Cage subsidence was assessed by two researchers after a training session and under the supervision of a board-certified orthopedic spine surgeon. Furthermore, we evaluated the correlation between cage subsidence and clinical scores.
Interbody fusion was determined by lumbar radiographic examination and/or CT scans if needed at the final follow-up. The criterion for pseudarthrosis was the presence of regional motion of more than 3° or intervertebral translation of more than 3 mm on lateral dynamic X-ray images. The criterion of fusion status from the CT scan was the presence of bony bridge in the sagittal and coronal reconstruction planes and its connections to the lower and upper endplates. If there were any defects in any position, the fusion status was classified as pseuarthrosis.
Statistical analyses
The values are shown as the mean ± SD. Statistical analyses were performed using SPSS software (version 23, USA). P values < 0.05 were accepted for significance. The Shapiro-Wilk test was used to verify the normal distribution of continuous variables. The independent sample t-test was used for variables that followed a normal distribution. The Mann-Whitney U test was used for those not following normal distribution. The paired t-test was used for intragroup comparison. The chi-square test or Fisher’s exact test was used for categorical data. Intra-class correlation coefcient (ICC) was used to evaluate inter-observer and intra-observer reliability of HU and cage subsidence measurements. (ICC ≥ 0.8 was considered to indicate excellent reliability.) Receiver operating characteristic (ROC) curves were used to establish a separation criterion between the two groups. The areas under the ROC curve (AUCs) were calculated for regional assessment. Logistic regression analysis was used to identify the independent factors of cage subsidence, and the results are presented as odds ratios (ORs) with 95% confidence intervals. Spearman’s correlation analysis was used to identify the correlation between the distance of cage subsidence and improvement in pain VAS or ODI scores.
Results
A total of 84 segments in 76 patients were included in the final analysis (30 male and 46 female patients; mean age 56.1 ± 10.4 years old), with a mean BMI of 24.7 ± 2.2 kg/m2. The patients were divided into a cage subsidence group (n = 16) and a nonsubsidence group (n = 60) based on the occurrence of cage subsidence.
HU value and cage subsidence
The intra-observer and inter-observer reliability in measuring HU value was excellent with ICCs of 0.989 and 0.972, respectively. The ICCs of the intra-observer and inter-observer reliability were 0.986 and 0.963 in measuring the distance of cage subsidence. Sixteen of 76 (21.1%) patients developed at least radiographic evidence of subsidence. The mean subsidence measured by the last follow-up lateral radiographs was 2.5 ± 1.3 mm (range, 0.9-4.8 mm). Cage subsidence of < 2 mm was found in 9 patients, and subsidence of ≥2 mm was found in 7 patients. The comparison of demographics and clinical data between the cage subsidence and nonsubsidence groups is shown in Table 2. The patients with cage subsidence of ≥2 mm had significantly lower HU values on the L1-L4 horizontal plane (81.2 ± 10.4) than the patients with subsidence of < 2 mm (106.5 ± 13.7) (P = 0.007). There were no significant differences in sex, BMI, diagnoses, or fused level (p > 0.05); however, there were significant differences between the two groups in age, lowest T-score, and HU value, including L1 vertebrae, L1-L4 horizontal plane, and L1-L4 sagittal plane (p < 0.05). We recorded the disc heights at both the operating and suprajacent levels in each patient. The preoperative and postoperative disc heights showed no significant difference between the two groups (p > 0.05) (Table 3).
Table 2.
Comparison of demographic and clinical data between the two groups
| Subsidence group (n = 16) |
Nonsubsidence group (n = 60) |
P value | |
|---|---|---|---|
| Mean age (years) | 64.4 ± 7.1 | 54.0 ± 10.1 | < 0.001 |
| Sex (M/F) (% male) | 6/10 (37.5) | 24/36 (40.0) | 0.856 |
| BMI (kg/m2) | 24.6 ± 1.8 | 24.7 ± 2.3 | 0.892 |
| Lowest T-score | − 2.8 ± 0.8 | − 1.6 ± 1.3 | < 0.001 |
| HU value | |||
| L1 vertebrae | 98.5 ± 18.8 | 140.8 ± 28.6 | < 0.001 |
| L1-L4 horizontal plane | 95.4 ± 17.6 | 136.8 ± 28.3 | < 0.001 |
| L1-L4 sagittal plane | 90.7 ± 22.4 | 135.2 ± 31.3 | < 0.001 |
| Operated segment | 96.3 ± 19.2 | 134.4 ± 29.7 | < 0.001 |
| Upper vertebrae of operated segment | 94.7 ± 20.4 | 133.2 ± 28.8 | < 0.001 |
| Lower vertebrae of operated segment | 97.8 ± 20.5 | 135.6 ± 32.4 | < 0.001 |
| Diagnoses [no.(%) of patients] | |||
| Degenerative spondylolisthesis | 8 (50) | 24 (40) | 0.472 |
| Lumbar instability | 5 (31.3) | 12 (20) | 0.534 |
| Adjacent segmental disease | 3 (18.8) | 15 (25) | 0.848 |
| Adult degenerative scoliosis | 0 (0) | 6 (10.0) | 0.426 |
| Discogenic back pain | 0 (0) | 3 (5.0) | 1.000 |
| Fused level [no.(%)of patients] | |||
| One level | 16 (100) | 54 (90) | 0.426 |
| Two level | 0 (0) | 4 (6.7) | 0.573 |
| Three level | 0 (0) | 2 (3.3) | 1.000 |
Abbreviations: BMI Body Mass Index
Table 3.
Comparison of disc heights between the two groups
| Subsidence group (n = 16) |
Nonsubsidence group (n = 60) |
P value | |
|---|---|---|---|
| Preoperative operative levels | 9.0 ± 1.5 | 8.8 ± 1.5 | 0.463 |
| Preoperative suprajacent levels | 10.2 ± 1.2 | 10.3 ± 1.4 | 0.794 |
| Immediate postoperative operative levels | 11.9 ± 0.9 | 11.7 ± 1.4 | 0.541 |
On the basis of ROC analysis (Table 4), the lowest T-score had a significant ability to predict cage subsidence, with an AUC of 0.791 (95% CI, 0.674–0.909; P < 0.001) (Fig. 1), while the average HU value of the L1-L4 horizontal plane followed a more predictable AUC of 0.909 (95% CI, 0.834–0.984; P < 0.001) (Fig. 2). The average HU value of 115.7 on the L1-L4 horizontal plane with balanced sensitivity (93.8%) and specificity (81.7%) in predicting cage subsidence was chosen as the cutoff value for finding patients with a higher risk of subsidence. The cutoff value of the lowest T-score was − 2.55 with balanced sensitivity (87.5%) and specificity (65.0%) (P < 0.001).
Table 4.
Results of ROC analysis
| AUC (95%CI) | P value | Cutoff | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Average of the lowest T-score | 0.791 (0.674-0.909) | < 0.001 | −2.55 | 87.5% | 65% |
| Average HU value | |||||
| L1 | 0.907 (0.840-0.975) | < 0.001 | 124.6 | 93.8% | 78.3% |
| L1-L4 horizontal plane | 0.909 (0.834-0.984) | < 0.001 | 115.7 | 93.8% | 81.7% |
| L1-L4 sagittal plane | 0.891 (0.809-0.973) | < 0.001 | 114.9 | 87.5% | 81.7% |
| Operated segment | 0.880 (0.789-0.970) | < 0.001 | 116.1 | 93.8% | 80.0% |
| Upper vertebrae of operated segment | 0.885 (0.785-0.986) | < 0.001 | 111.8 | 93.8% | 81.7% |
| Lower vertebrae of operated segment | 0.866 (0.782-0.949) | < 0.001 | 121.0 | 93.8% | 73.3% |
Abbreviations: ROC Receiver operating characteristic curve
Fig. 1.
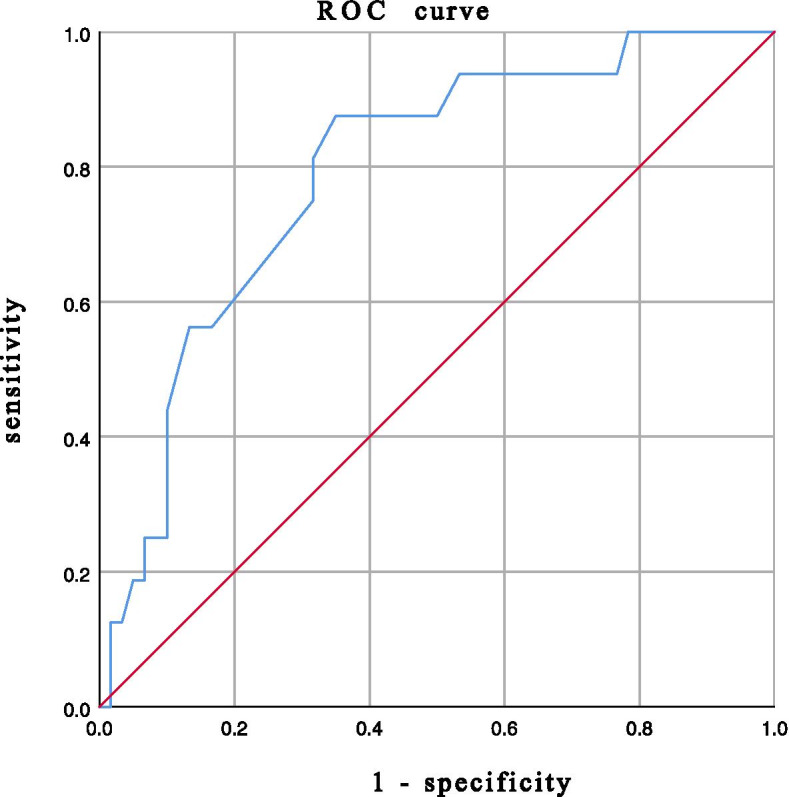
Receiver operating characteristic curve for the lowest T-score as a predictor of cage subsidence, with an area under the curve of 0.791, sensitivity of 87.5% and specificity of 65.0%
Fig. 2.
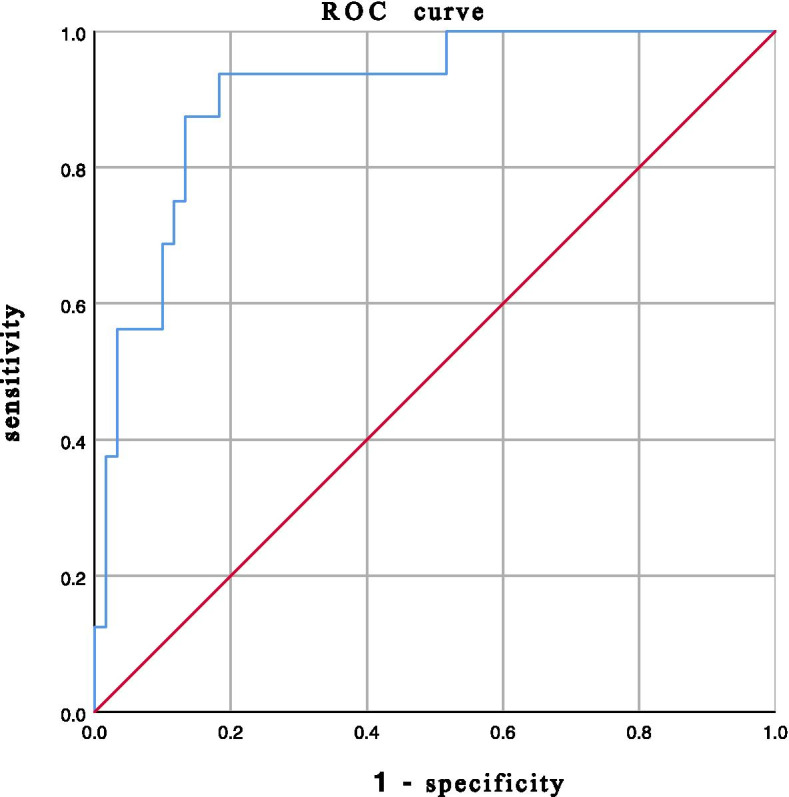
Receiver operating characteristic curve for Hounsfield units of the L1-L4 horizontal plane as a predictor of cage subsidence, with an area under the curve of 0.909, sensitivity of 93.8% and specificity of 81.7%
The factors with a P value of < 0.1 in Table 2 were chosen as potential cage subsidence influencing factors and entered into the logistic regression, including age, the average HU value of the L1-L4 horizontal plane with the highest AUC, and the lowest T-score. The other average HU values were not included in the logistic regression because they were highly correlated with the average HU value of the L1-L4 horizontal plane. Further logistic regression analysis showed that the average HU value of the L1-L4 horizontal plane (OR, 0.912; 95% CI 0.861–0.966; P = 0.002) was an independent factor influencing cage subsidence. The OR of the lowest T-score was 1.302 (95% CI, 0.534–3.179; P = 0.562), and the OR of age was 1.017 (95% CI, 0.908-1.139; P = 0.770).
Clinical outcomes
A total of 76 patients, including 84 segments, were available for review. Both the ODI and VAS scores significantly decreased at the last follow-up compared to the preoperative scores, not only in the subsidence group but also in the nonsubsidence group (P < 0.05). However, the preoperative and postoperative VAS and ODI scores showed no significant differences between the two groups (P > 0.05) (Table 5).
Table 5.
Comparison of clinical outcomes between the subsidence group and the nonsubsidence group
| Clinical outcomes |
Subsidence group |
Nonsubsidence group | P value | |
|---|---|---|---|---|
| Preoperatively | VAS (Back) | 5.4 ± 0.8 | 5.3 ± 0.9 | 0.615 |
| VAS (Leg) | 6.4 ± 1.0 | 6.1 ± 0.7 | 0.086 | |
| ODI | 27.4 ± 4.2 | 27.3 ± 5.7 | 0.614 | |
| Postoperative at 3 months | VAS (Back) | 2.3 ± 0.7 | 2.0 ± 0.6 | 0.108 |
| VAS (Leg) | 2.9 ± 0.6 | 2.6 ± 0.5 | 0.155 | |
| ODI | 15.4 ± 2.6 | 14.2 ± 2.8 | 0.071 | |
| Postoperative at 6 months | VAS (Back) | 1.9 ± 0.6 | 1.6 ± 0.7 | 0.123 |
| VAS (Leg) | 2.1 ± 0.6 | 1.9 ± 0.6 | 0.206 | |
| ODI | 13.5 ± 2.3 | 12.7 ± 2.8 | 0.228 | |
| Last follow-up | VAS (Back) | 1.4 ± 0.5 | 1.3 ± 0.7 | 0.473 |
| VAS (Leg) | 1.9 ± 0.4 | 1.8 ± 0.6 | 0.196 | |
| ODI | 12.2 ± 2.4 | 12.0 ± 3.0 | 0.723 |
Abbreviations: VAS Visual Analog Scale, ODI Oswestry Disability Index
Of the 16 patients with cage subsidence, 7 patients suffered from debilitating back pain within 3 months after SA OLIF surgery. After these patients wore a waist brace and underwent conservative treatment, the symptoms of low back pain were significantly reduced, and no patients required revision surgery. No recurrent lower extremity radiculopathy occurred. Transient symptoms, including left groin and/or thigh dysesthesia, in six patients disappeared within 2 months after conservative therapy. When comparing the clinical score of last follow-up between cage subsidence of < 2 mm group and subsidence of ≥2 mm group,there were no significant differences in VAS back pain scores(1.6 ± 0.5 VS 1.3 ± 0.5 P = 0.296), VAS leg pain scores (2.0 ± 0.5 VS 1.9 ± 0.4 P = 0.534), or ODI scores (11.8 ± 2.8 VS 12.7 ± 2.0 P = 0.463). The distance of cage subsidence at the last follow-up showed no significant correlation with improvements in pain VAS (rs = − 0.123, P = 0.649) or ODI scores (rs = 0.438, P = 0.09).
Fusion rates
The overall fusion rate was 97.4% in our series. At the final follow-up, nonfusion status was observed in one patient with cage subsidence of < 2 mm. A higher fusion rate of 98.3% was found in the nonsubsidence group compared to the subsidence group (93.8%). However, there was no significant difference between the groups (P = 0.379).
Discussion
Previous studies demonstrated that osteoporosis is one of the major risk factors for cage subsidence after lumbar interbody fusion. In patients with poor bone quality, consideration could be made to supplement the lateral lumbar interbody fusion cage with posterior instrumentation [11]. In a large series of patients, Le et al [7] reported a subsidence rate of 14.3%, while Marchi et al [8] reported a subsidence rate of 10-30% depending on graft size. The development of cage subsidence is presumed to be a multifactorial process. Several potential risk factors have been examined in the literature, consisting of overdistraction, smaller cage width, construct length, lateral plating, and endplate violation, which are directly related to surgical technique and intraoperative decision making [7, 8, 12–14].
Patients with cage subsidence might suffer from axial pain and recurrent neurological symptoms, although SA OLIF could be as effective as OLIF aided by different fixation devices with less blood loss, shorter operative time, and lower operative costs for implants. The contact area between the cage and the endplate would most likely be directly related to cage width, which in turn would be associated with cage subsidence. Larger diameter cages have been shown to have a lower risk of cage subsidence. Therefore, theoretically, the use of a larger cage in the SA OLIF procedure would improve spinal stabilization and decrease the likelihood of cage subsidence. Low bone mineral density (BMD) has been previously tied to higher rates of postoperative interbody cage subsidence in patients undergoing OLIF. Osteoporosis could play a more important role in the occurrence of cage subsidence during SA-OLIF than cage size. For this reason, accurate preoperative assessment of a patient’s underlying bone quality in the operated vertebral body is paramount for OLIF, especially SA OLIF.
Dual-energy X-ray absorptiometry (DXA) is the current “gold standard” to assess BMD and is widely used clinically to diagnose osteopenia or osteoporosis. However, a growing body of research literature has suggested that in vivo assessments of BMD using DXA are inaccurate and imperfect. Alternative means of preoperative bone quality assessment have been investigated for patients being considered for spinal fusion [9, 15, 16]. The HU value is less affected by lumbar degenerative conditions than DXA by avoiding cortical bone, vascular calcification, and degenerative structures. Considering that lumbar CT is a routine preoperative examination for patients requiring lumbar fusion, the HU value can be measured on preexisting CT images at no additional cost and without radiation. Another advantage of a CT-based technique over DXA is that CT-based techniques can selectively measure detailed site-specific bone density, such as the pedicles and endplates [17].
The BMD of the endplate region was highly reliable and more predictive of vertebral fracture and cage subsidence [18]. Modic changes and endplate sclerosis with higher HU values are potential predictors for preventing cage subsidence, making the endplate with MCs or sclerosis a potentially suitable condition that can be submitted to SA OLIF [19]. Microstructural and/or material property changes associated with modic type 2 changes, which are independent of regional endplate BMD values, however, might have a protective effect on cage subsidence after stand-alone OLIF [20]. However, few studies have comprehensively evaluated the specific relationship between vertebral body HU and interbody graft failure during SA OLIF. It is worth assessing the influence of site-specific BMD on the risk of cage subsidence since the contact surfaces between cage and bone only include the implant and endplates in SA OLIF. However, it is difficult to determine the area of interest (AOI) and measure the HU value of the endplate because of its irregular shape. Therefore, the mean HU value of the three AOIs was measured as the vertebral body HU value to investigate the correlation between BMD and cage subsidence in our series.
According to a systematic review of studies that specified the subsidence rate, the pooled patient population equaled 1362 patients, 141 of whom experienced cage settling after LLIF. Therefore, the estimated incidence of subsidence reached 10.3% [21]. The present study demonstrated that there was a relatively high rate of cage subsidence (21.1%) in 76 patients compared to that (18.7%) reported by Abe et al. [22] There were significantly lower vertebral body HU values in 16 patients with subsidence than in 60 patients without subsidence. Our data demonstrated that the average HU value of the L1-L4 horizontal plane was more predictive of cage subsidence, with an AUC of 0.909 (95% CI, 0.834–0.984; P < 0.001), than DXA t-scores, with an AUC of 0.791 (95% CI, 0.674–0.909 P < 0.001). Unlike DXA t-scores, accepted standard cutoff values for HU measurement have not been determined. Using 115.7 HU in the L1-L4 horizontal plane as the cutoff value provided 93.8% sensitivity and 81.7% specificity in our study. Patients with HU values less than 115.7 who underwent stand-alone OLIF were at greater risk for developing cage subsidence. Our findings suggested that a significant association exists between lower HU values of the lumbar vertebral body and the likelihood of cage subsidence after SA OLIF. Therefore, we recommend routinely measuring the HU value on preoperative CT scans when planning SA OLIF for lumbar degenerative conditions. If spine surgeons decide to perform SA OLIF and avoid the occurrence of cage subsidence, patients with HU values greater than 110 should be chosen as candidates.
Considering that no apparent loss of postoperative disc height was observed when local and mild-scale cage migration in the endplates occurred, we did not use the grading system of Marchi et al. [8] Our results demonstrated that patients with cage subsidence of ≥2 mm had a significantly lower average HU value in the lumbar spine than the patients with subsidence of < 2 mm, which was comparable to that reported by Oh et al. [23]
The clinical outcomes were comparable between the subsidence group and the nonsubsidence group, although 16 cases (21.1%) of cage subsidence were found in this study. We recommend a longer time for the patient to wear the waist brace to prevent further subsidence. It is questionable whether mild or moderate cage subsidence is clinically significant. Cho et al [24] concluded that cage subsidence did not significantly affect clinical outcomes. Tempel et al [25] reported that severe cage subsidence was significantly associated with revision surgery after SA-OLIF, in contrast to Cho et al.
Our patient population was relatively heterogeneous with regard to surgical indication, which included spondylolisthesis, lumbar instability, kyphoscoliosis, adjacent segmental diseases, and discogenic pain, despite the patients undergoing the same surgical procedure. There were other limitations, such as the relatively small sample size, short follow-up time, and retrospective nature of this study. Additionally, we were not able to definitively determine endplate injury during endplate preparation and cage insertion, which might have played a role in cage subsidence. We did not investigate the correlations among cage size, cage positioning, excessive disc space correction and subsidence. Finally, we did not evaluate any preventive measures for cage subsidence.
Conclusion
In summary, SA OLIF has many advantages that render it attractive; however, there is a risk of cage subsidence. Patients with lower vertebral body HU values are at significantly higher risk of experiencing cage subsidence. Accurate assessment of preoperative HU values on preexisting CT scans is rapid, simple and reliable when planning the procedure. SA OLIF should be performed in patients with good bone quality. However, our results showed that cage subsidence is not related to clinical deterioration.
Acknowledgements
Not applicable.
Abbreviations
- AOI
Area of interest
- AUC
Areas under the ROC curve
- BMD
Bone mineral density
- BMI
Body Mass Index
- DXA
Dual-energy x-ray absorptiometry
- HU
Hounsfield Units
- ODI
Oswestry Disability Index
- PACS
Picture archiving and communication system
- ROC
Receiver operating characteristic curve
- SA OLIF
Stand-alone Oblique Lumbar Interbody Fusion
- VAS
Visual Analog Scale
Authors’ contributions
JZ and JW designed the work and made substantial contribution to its conception and development. JZ and LZ developed the theory and collected the data. CY and CL verified the analytical methods. JZ, CY and CL drafted the paper. JW critically revised the manuscript and helped to realize the final draft. All authors discussed the results and contributed to the final manuscript. The author(s) read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics board of Xinqiao Hospital and all participants signed written informed consent forms.
Consent for publication
All patients provided consent to participate in the present study, and the consent specified that personal identifiable date and personal images may be used for publication.
Competing interests
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997;22:691–700. doi: 10.1097/00007632-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 2.Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J. 2012;6:89–97. doi: 10.4184/asj.2012.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J. 2017;26:671–678. doi: 10.1007/s00586-015-4170-0. [DOI] [PubMed] [Google Scholar]
- 4.Fujibayashi S, Hynes RA, Otsuki B, et al. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976) 2015;40:E175–E182. doi: 10.1097/BRS.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 5.Ohtori S, Orita S, Yamauchi K, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for lumbar spinal degeneration disease. Yonsei Med J. 2015;56:1051–1059. doi: 10.3349/ymj.2015.56.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohtori S, Mannoji C, Orita S, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spinal kyphoscoliosis. Asian spine J. 2015;9:565–572. doi: 10.4184/asj.2015.9.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le T, Baaj A, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37:1268–1273. doi: 10.1097/BRS.0b013e3182458b2f. [DOI] [PubMed] [Google Scholar]
- 8.Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine. 2013;19(1):110–118. doi: 10.3171/2013.4.SPINE12319. [DOI] [PubMed] [Google Scholar]
- 9.Schreiber JJ, Anderson PA, Rosas HG, et al. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93:1057–1063. doi: 10.2106/JBJS.J.00160. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd JA, Schousboe JT, Broy SB, et al. Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom. 2015;18(3):274–286. doi: 10.1016/j.jocd.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Tempel ZJ, Gandhoke GS, Okonkwo DO, et al. Impaired bone mineral density as a predictor of graft subsidence following minimally invasive transpsoas lateral lumbar interbody fusion. Eur Spine J. 2015;24(Suppl 3):414–419. doi: 10.1007/s00586-015-3844-y. [DOI] [PubMed] [Google Scholar]
- 12.Dua K, Kepler CK, Huang RC, et al. Vertebral body fracture after anterolateral instrumentation and interbody fusion in two osteoporotic patients. Spine J. 2010;10:E11–E15. doi: 10.1016/j.spinee.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Brier-Jones JE, Palmer DK, Inceoglu S, et al. Vertebral body fractures after transpsoas interbody fusion procedures. Spine J. 2011;11:1068–1072. doi: 10.1016/j.spinee.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Le TV, Smith DA, Greenberg MS, et al. Complications of lateral plating in the minimally invasive lateral transpsoas approach. J Neurosurg Spine. 2012;16:302–307. doi: 10.3171/2011.11.SPINE11653. [DOI] [PubMed] [Google Scholar]
- 15.McCoy S, Tundo F, Chidambaram S, et al. Clinical considerations for spinal surgery in the osteoporotic patient: a comprehensive review. Clin Neurol Neurosurg. 2019;180:40–47. doi: 10.1016/j.clineuro.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Pennington Z, Ehresman J, Lubelski D, et al. Assessing underlying bone quality in spine surgery patients:a narrative review of dual-energy X-ray absorptiometry (DXA) and alternatives. Spine J. 2021;21:321–331. doi: 10.1016/j.spinee.2020.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Sakai Y, Takenaka S, Matsuo Y, et al. Hounsfield unit of screw trajectory as a predictor of pedicle screw loosening after single level lumbar interbody fusion. J Orthop Sci. 2018;23:734–738. doi: 10.1016/j.jos.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Okano I, Jones C, Salzmann S, et al. Endplate volumetric bone mineral density measured by quantitative computed tomography as a novel predictive measure of severe cage subsidence after standalone lateral lumbar fusion. Eur Spine J. 2020;29:1131–1140. doi: 10.1007/s00586-020-06348-0. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Ding W, Yang D, et al. Modic changes (MCs) associated with endplate sclerosis can prevent cage subsidence in oblique lumbar interbody fusion (OLIF) stand-alone. World Neurosurg. 2020;138:e160–e168. doi: 10.1016/j.wneu.2020.02.047. [DOI] [PubMed] [Google Scholar]
- 20.Okano I, MD. Jones C, Rentenberger C, et al. The association between endplate changes and risk for early severe cage subsidence among standalone lateral lumbar Interbody fusion patients. Spine (Phila Pa 1976) 2020;45:E1580–E1587. doi: 10.1097/BRS.0000000000003668. [DOI] [PubMed] [Google Scholar]
- 21.Macki M, Anand SK, Surapaneni A, et al. Subsidence rates after lateral lumbar interbody fusion:a systematic review. World Neurosurg. 2019;122:599–606. doi: 10.1016/j.wneu.2018.11.121. [DOI] [PubMed] [Google Scholar]
- 22.Abe K, Orita S, Mannoji C, et al. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery: perspectives and indications from a retrospective, multicenter survey. Spine (Phila Pa 1976) 2017;42:55–62. doi: 10.1097/BRS.0000000000001650. [DOI] [PubMed] [Google Scholar]
- 23.Oh KW, Lee JH, Lee JH, et al. The correlation between cage subsidence, bone mineral density, and clinical results in posterior lumbar interbody fusion. Clin Spine Surg. 2017;30:E683–E689. doi: 10.1097/BSD.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 24.Cho JH, Hwang CJ, Kim H, et al. Effect of osteoporosis on the clinical and radiological outcomes following one-level posterior lumbar interbody fusion. J Orthop Sci. 2018;23:870–877. doi: 10.1016/j.jos.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Tempel ZJ, McDowell MM, Panczykowski DM, et al. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J Neurosurg Spin. 2018;28:50–56. doi: 10.3171/2017.5.SPINE16427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


