Abstract
Objective:
To evaluate three-dimensional (3D) condylar and mandibular growth in patients with juvenile idiopathic arthritis (JIA) with unilateral temporomandibular joint involvement treated with a distraction splint.
Materials and Methods:
Cone-beam computed tomography (CBCT) scans were taken for 16 patients with JIA with unilateral TMJ involvement before treatment (T0) and 2 years after treatment (T1). All patients received orthopedic treatment with a distraction splint. Eleven patients without JIA who were undergoing orthodontic treatment without a functional appliance or Class II mechanics and who had taken CBCT scans before and after treatment, served as controls. Reconstructed 3D models of the mandibles at T0 and T1 were superimposed on stable structures. Intra- and intergroup growth differences in condylar and mandibular ramus modifications and growth vector direction of the mandibular ramus were evaluated.
Results:
In all patients with JIA there were asymmetric condylar volume, distal and vertical condylar displacement, and ramus length differences that were smaller on the affected side. Condylar displacement was more distal and less vertical in the JIA group than in the control group. A larger distal growth of the condylar head and a more medial rotation of the ramus on the affected side were found in the JIA group.
Conclusion:
The orthopedic functional treatment for patients with JIA allows for condylar adaptation and modeling, thereby hindering, although with a widely variable response, a further worsening of the asymmetry. Unilateral affection has a possible influence on the growth of the nonaffected side.
Keywords: Juvenile arthritis, CBCT, Distraction splint, Mandible, Asymmetry
INTRODUCTION
The involvement of the temporomandibular joint (TMJ) in patients affected with juvenile idiopathic arthritis (JIA) is a known phenomenon and has been reported by several authors.1–4 Condylar lesions have been regarded as the primary contributory cause to mandibular growth deviations in patients with JIA and TMJ arthritis. The facial morphology of children with JIA and condylar lesions might become increasingly abnormal during growth, reflecting a decelerated vertical development of the posterior face height, malocclusion with anterior open bite, and altered muscular function.5–7 The prognosis of mandibular growth when TMJ arthritis has been diagnosed is based on the presence of condylar deformities as well as the onset, the severity and the activity of the disease.4,8,9
TMJ arthritis seems to affect long-term growth as well as growth during the subacute and chronic phases. Condylar growth takes place in periods, although subnormal in nature and with related dentoalveolar compensations.7
Initial medication has a certain effect on the inflammation8 and is therefore of vital importance, but its effect on dentofacial growth and development is still an open question. Functional/orthopedic treatment aims to support normal growth and control the dentoalveolar development. Thus, the current literature recommends early functional/orthopedic treatment.2,10,11
In the past, morphologic skeletal changes were usually analyzed by means of conventional two-dimensional radiologic images, but the development of three-dimensional (3D) technologies has improved the possibility of following the effects of disease and treatment in three planes of space, thus increasing the understanding of the clinical value.12–14 Anatomic reconstructions can be used to analyze a specific model of a patient and to measure the size and shape of a particular area of interest, such as the condylar head. These image analysis procedures include reconstruction of 3D objects, superimposition of the objects at various time points, and quantification of measurements between the 3D surfaces.
The aim of the present study was to evaluate condylar morphology and mandibular growth changes using 3D reconstructions and superimpositions of the mandible based on cone-beam computed tomography (CBCT) scans of patients with JIA and unilateral TMJ affection treated with a distraction splint.
MATERIALS AND METHODS
In a retrospective longitudinal study, condylar morphology was analyzed by comparing affected and nonaffected condyles within the same patient with JIA. Furthermore, the morphology of the nonaffected condyles was compared with that of a control group. All patients were referred to the Clinic for Dento- and Craniofacial Anomalies, Section of Orthodontics, Institute of Odontology, Aarhus University, Denmark, for management of the TMJ arthritis and dentofacial growth deviation. The use of the database with patients’ information was approved by the national Danish committee for data protection (Datatilsynet j.nr. 2011-54-1291). According to the treatment protocol this group had been treated with an orthopedic functional appliance, a so-called distraction splint.11 Inclusion criteria for the study group were as follows: (1) diagnosis of JIA according to the International League of Associations for Rheumatology criteria,15 (2) unilateral radiographically diagnosed deformity of the condyle, (3) number of activations of the functional orthopedic appliance more than two, and (4) CBCT scans taken before the start of treatment (T0) and 2 years after treatment (T1). The diagnosis of involvement was made according to the following findings: reduced chewing function, decreased maximum-opening capacity, reduced condylar translation and mandibular range of mobility, and clear sign of asymmetric growth. Exclusion criteria were: (1) no confirmed JIA diagnosis; (2) bilateral TMJ affection developing during treatment; and (3) changes in treatment due to other types of functional/orthopedic appliances, fixed orthodontic appliances, and/or bone distraction. Sixteen patients were included in the study group; they had a mean age of 11.9 years (range = 6.6 to 15.6 years), and the male to female ratio was 7:9.
The control group was selected among patients referred for orthodontic treatment at the Section of Orthodontics, Institute of Odontology, Aarhus University. The inclusion criteria in this control group were as follows: (1) absence of temporomandibular disorders, (2) no treatment with functional/orthopedic appliance, and (3) CBCT scans taken similarly to the study group. Eleven individuals were chosen with a mean age of 11.7 years, (range = 7.7 to 14.5 years) and a male to female ratio of 3:8.
Image Acquisition
Two sets of full-head CBCT scans were obtained using a NewTom 3G CBCT scanner (3G, QR, Verona, Italy) available at the Section of Radiology, Institute of Odontology, University of Aarhus, Aarhus, Denmark. A single 360° rotation, 36-second scan, comprising 360 single projections, using the 12-inch field of view, was taken for each patient, resulting in data sets with a diameter and height of 20 cm. All CBCT scans were reconstructed with a voxel dimension of 0.3 × 0.3 × 0.36 mm3, which is the native resolution of the scanner. The images were saved in a DICOM (Digital Imaging and Communications in Medicine) format.
Mandibular Segmentation and 3D Reconstruction
The DICOM images were imported in the Mimics (Mimics 13.1, Materialise, Leuven, Belgium) software for 3D reconstruction. In the single-slice images bone and soft tissues were separated from each other by setting a threshold value between 500 and 700 Hounsfield units (HU) (It should be noted that the HUs used here are not the original HUs for computed tomography. However, it is an adapted gray value scale for CBCT), resulting in a so-called bone mask containing those pixels representing bone. Any pixels connecting the condyle to the fossa were manually removed and from this cleaned bone mask of the mandible a 3D object was generated.
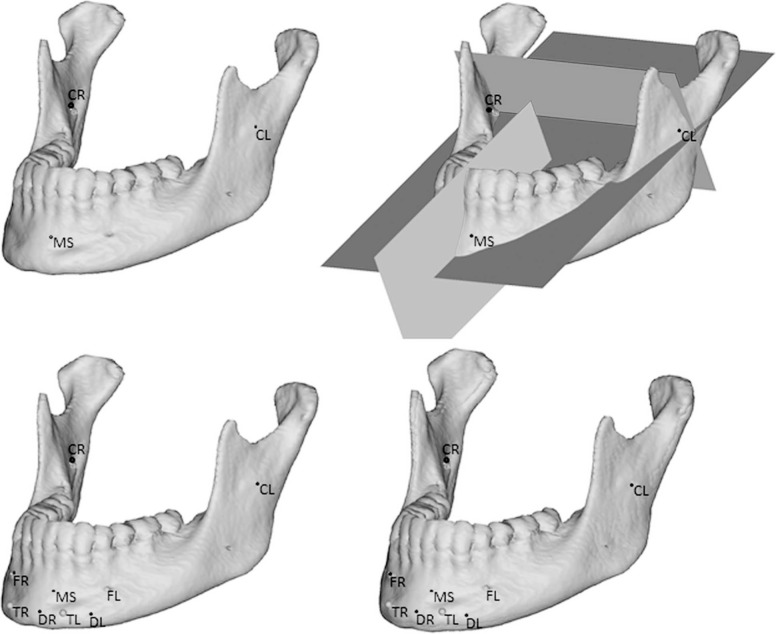
The following coordinate system was defined (Figure 1):
Figure 1.
The defined coordinate system used for superimposition.
The mandibular axial plane was defined as the plane passing through the three most stable structures of the mandibular body; the upper contour of the posterior mandibular foramen on the right (CR) and the left (CL) and the inner part of the posterior cortical plate of the symphysis on the midline (MS).
The coronal plane was defined as being perpendicular to the mandibular axial plane while passing through CR and CL.
The sagittal plane was defined as being perpendicular to both the mandibular axial plane and the coronal plane while passing through MS.
Mandibular Superimpositions
Each 3D mandibular T0 object was superimposed on the corresponding T1 object. These superimpositions were performed using the point-to-point registration routine of Mimics for nine anatomic landmarks consisting of the aforementioned CR, CL, and MS plus the left and right anterior mental foramen and the anterior insertion of the muscles in the mental protuberance (triangularis and platysma in the external part and digastric in the internal part) (Figure 1). The repositioning of the pretreatment mandible was then calculated based on the least-squares difference between these nine corresponding points. Potential errors caused by placement of the landmark points are an integral part of the overall method error and the magnitude of these errors was assessed by double measurements.
Measurements
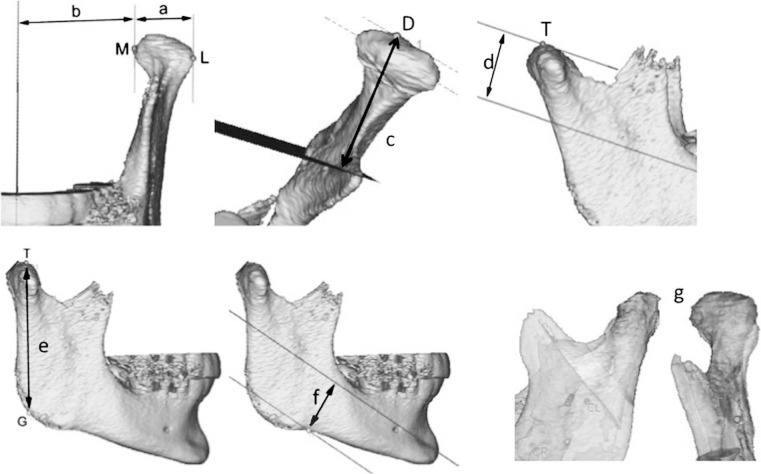
The CBCT scans were used for the evaluation of (1) condylar modifications with both linear and volumetric measurements (Figure 2), (2) mandibular ramus modifications, and (3) calculation of the growth vector direction of the mandibular ramus.
Figure 2.
Overview of the measured distances between: (a) the most lateral (L) and most medial (M) points of the condylar head (CW); (b) M and sagittal plane (TD); (c) the most distal (d) point of the condylar head and transverse plane (DD); (d) the most coronal (t) point of the condylar head and axial plane (VD); (e) T and the gonion (g) point (RL); (f) the deepest point of the mandibular body and axial plane (ND). (g) In addition, the differences in condylar volume at T0 and T1 were measured (CV).
Ad 1. The condylar modifications consisted of condylar width (CW): transverse, distal, and vertical displacement (TD, DD, and VD, respectively): and volume (CV). Before the CV was calculated, a flood-fill operation was applied to the bone mask, so that the total volume was calculated with no regard of trabecular porosity in the condyles.
Ad 2. The ramus modifications consisted of ramus length (RL) and notch depth (ND).
Ad 3. The magnitude and direction of the growth vector were determined by finding the differences over time of the aforementioned variables expressed in the aforementioned coordinate system.
Statistics
The method error was calculated using the Dahlberg formula. Validity was assumed if this error was smaller than the standard deviation of the double measurements. Evaluation of the intraobserver variance of the manual segmentation and superimposition procedures was performed by duplicating 10 randomly chosen segmentations.
For ease of data handling, all affected JIA condyles were considered as left, the nonaffected JIA condyles as right. Since no difference between right and left condyle was found in the control group, the left condyle was used for intergroup comparison.
The measured variables were compared over time within the same condyle and within the same patient. Paired t-tests were performed after verifying that the data were normally distributed. A independent-samples 2-tailed t-test was used to perform the comparison between groups. Statistical analysis was performed using SPSS software version 13 (SPSS Inc, Chicago, Ill). A significance level of 5% was chosen.
RESULTS
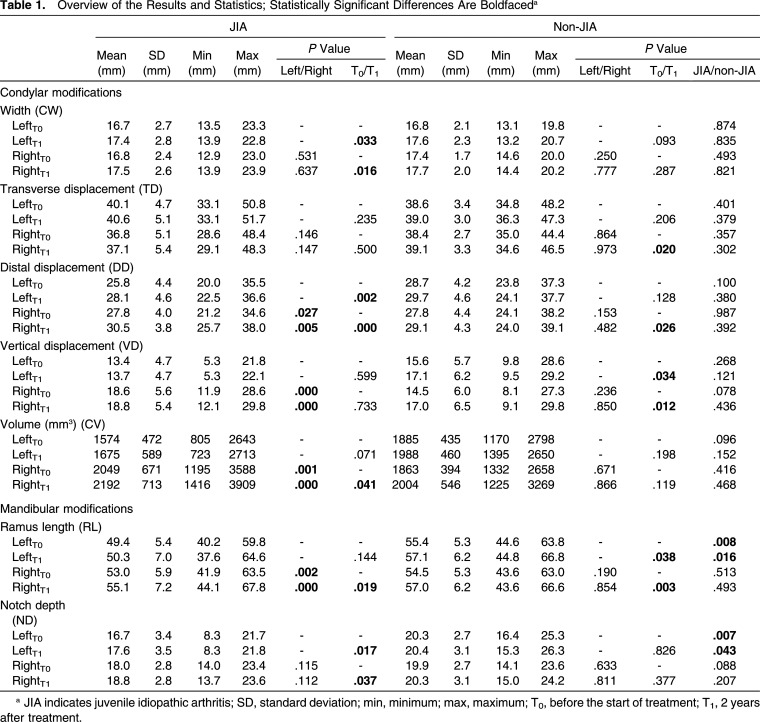
All the measurements were valid and are shown in Table 1.
Table 1.
Overview of the Results and Statistics; Statistically Significant Differences Are Boldfaceda
Condylar Modifications
CW and DD increased significantly during treatment in the study group. Furthermore, DD in the study group showed a significant difference between sides both at T0 and T1, the affected side being smaller than the nonaffected.
VD was significantly smaller on the affected side, and a clear intergroup difference was observed: it did not change over time on the affected or the nonaffected side in the study group, whereas it increased significantly on both sides in the control group.
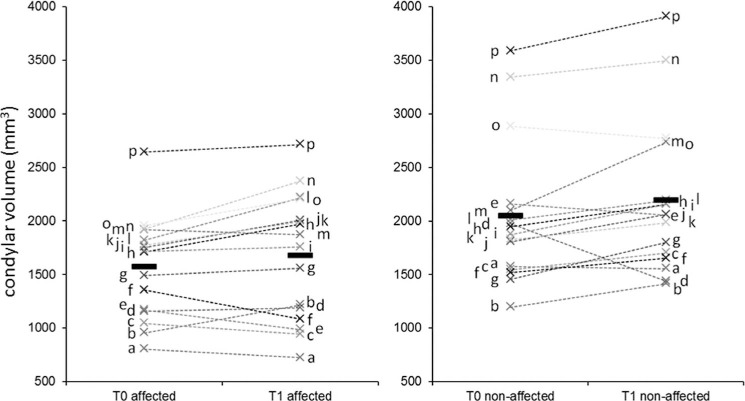
Increased volume was observed in 11 affected condyles and in 12 nonaffected condyles. Decreased CV was observed in two affected condyles and in one nonaffected condyle (Figure 3).
Figure 3.
Condylar volume at T0 and T1 in the affected and nonaffected side (patients coded by letters). Please note the individual variability of the response of the condyles on both sides.
Mandibular Modifications
The RL was significantly smaller on the affected side in the study group. The RL on the nonaffected side as well as in the control group increased significantly during treatment. These two findings are expressed as significantly smaller RL between the study and control groups at T0 and T1.
ND increased significantly on both the affected and nonaffected sides during treatment in the study group. Despite this increase the values were significantly smaller than in the control group both at T0 and T1.
Growth Vectors
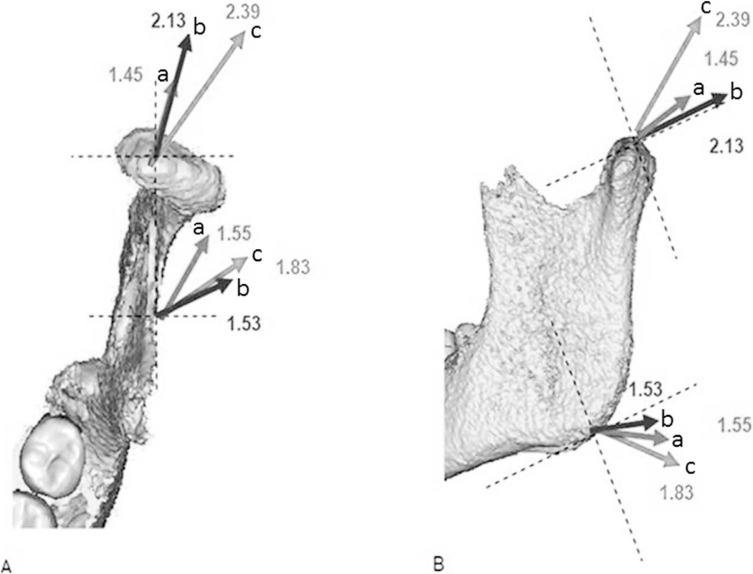
The growth vectors are illustrated in Figure 4. The most interesting observation was the more distal displacement of the gonion in the mandible from the affected condyle compared with the nonaffected contralateral condyle; also, the JIA-affected hemimandible tended to be less lateral than the nonaffected and non-JIA condyles. Intergroup differences showed that the lower point of the rami in the patients with JIA moved slightly more distal and less vertical compared with the patients without JIA.
Figure 4.
Growth vectors for the top of the condyle and the gonion. (a) JIA affected side, (b) JIA nonaffected side, and (c) non-JIA.
VD in the patients with JIA was smaller compared with the patients without JIA. In the study group both the affected and the nonaffected condyles showed a more posterior and less vertical displacement compared with the control condyles.
In the patients with JIA the ratio between the nonaffected and affected side was evaluated in terms of interside differences in ramus height and its displacement during treatment, and significant changes were found in ramus height in the nonaffected side, as well as changes at the condylar level in both sides, yet these were not significant.
DISCUSSION
The present study resulted in three main findings: (1) the asymmetry in patients with JIA was expressed in condylar volume, distal condylar displacement, vertical displacement, and ramus length, all being smaller on the affected side: (2) the affected patients showed more distal condylar growth on both sides: and (3) the affected side showed always the smallest displacement and a more medial growth of the gonion angle.
Asymmetry
In the patients with JIA we confirmed the occurrence of asymmetry. The differences between affected and nonaffected sides in CV, DD and VD, and RL were maintained over time and the asymmetry did not worsen. In relation to the CV, both groups did not show any significant increase in condylar volume in time. In our study, we calculated the entire volume of the condyle; the changes in total volume of the condyle would be a measure of growth and/or remodeling during treatment. For patients with JIA, there is no consensus in the literature.14 A recent animal study carried out in our section concluded that changes in growth did not result from a mineralization defect, meaning that the bone produced is of normal quality and structure, but endochondral growth velocity may have been slowed down and may worsen the process of bone and growth deformities in times of active inflammation.16
Growth Directions
Our results indicated more DD than VD, as from linear measurement, in the JIA study group than in the control group. The RL increased as expected in the control group17 and in the nonaffected hemimandible within the JIA group. The affected side showed a slower growth pattern. In the JIA group ND became less pronounced in both affected and nonaffected sides, while it remained unchanged in the control group. This may be the result of the overall impaired function in the patients affected by JIA.
The distal growth pattern of the condyle was observed, although in a smaller scale, also in the nonaffected condyles with a nonuniform response, leading to the hypothesis that the nonaffected side is influenced by the affected side. The distal growth pattern reflects the typical mandibular growth direction in patients with JIA and affected TMJ6,7,9,18 being, together with mandibular posterior rotation, one of the most pronounced clinical features. The rotational pattern of facial growth was not analyzed in this study. Araujo et al.19 reported that functional treatment in patients without JIA induces significantly greater posterior displacement of gonion and condylion, compensating for the mandibular advancement. This is in agreement with our results, although more accentuated at the condylar level. The fact that the amount of displacement of the control condyles was the largest is not surprising, as they were neither affected directly by the disease nor indirectly by a different functional demand.
The growth vector in the horizontal plane in the affected hemimandible is more medial than in the nonaffected and the control group (Figure 4). This growth pattern has not been reported in other studies.
A significant interindividual variability was observed. Using the nonaffected condyle as control for the same patient allowed control for interindividual variability. The lack of substantial changes in the asymmetric pattern could be due to the short observation time, together with the small sample size, the heterogeneity of the patients age, variation of cooperation, and pharmacologic treatment. The latter varied widely, according to the course of the disease. Some patients showed a clear positive response to the functional therapy. The question is whether healing is an expression of growth or of modeling toward a normal shape due to a functional therapy (distraction splint), taking into consideration the influence of medications over tissue response. A definitive answer to this question could not be provided by our results. The large number of variables potentially involved in the answer requires a much larger sample size and longer observation time in order to be able to identify the best combination of pharmacologic and functional treatment in future studies.
The distraction splint is constructed to provide distraction on both condyles of an asymmetric amount, that is, larger on the affected side. Therefore, both condyles were submitted to distraction forces. All measurements after 2 years were positive, meaning that both condyles responded to the therapy or displayed some degree of growth, although with a large individual variation. To be able to analyze the effect of therapy compared with growth, an untreated JIA control group should have been used. However, as previously mentioned, those data are not available, at least not as 3D data sets.
An advantage of using 3D technology is to have unbiased volumetric information of the joint components in their real anatomic size. The possibility of looking at the superimposed condyles from several viewpoints greatly improved our appreciation of the morphologic changes.
CONCLUSIONS
In patients with JIA, functional treatment allows for condylar adaptation and modeling, although the treatment response is characterized by a wide individual variability.
The distraction splint therapy is therefore considered an effective tool for the treatment of patients with JIA with unilateral TMJ arthritis.
REFERENCES
- 1.Billiau AD, Hu Y, Verdonck A, Carels C, Wouters C. Temporomandibular joint arthritis in juvenile idiopathic arthritis: prevalence, clinical and radiological signs, and relation to dentofacial morphology. J Rheumatol. 2007;34:1925–1933. [PubMed] [Google Scholar]
- 2.Farronato G, Carletti V, Maspero C, Farronato D, Giannini L, Bellintani C. Craniofacial growth in children affected by juvenile idiopathic arthritis involving the temporomandibular joint: functional therapy management. J Clin Pediatr Dent. 2009;33:351–357. doi: 10.17796/jcpd.33.4.05287m400q508772. [DOI] [PubMed] [Google Scholar]
- 3.Martini A, Lovell DJ. Juvenile idiopathic arthritis: state of the art and future perspectives. Ann Rheum Dis. 2010;69:1260–1263. doi: 10.1136/ard.2010.133033. [DOI] [PubMed] [Google Scholar]
- 4.Twilt M, Schulten AJ, Verschure F, Wisse L, Prahl-Andersen B, Suijlekom-Smit LW. Long-term followup of temporomandibular joint involvement in juvenile idiopathic arthritis. Arthritis Rheum. 2008;59:546–552. doi: 10.1002/art.23532. [DOI] [PubMed] [Google Scholar]
- 5.Twilt M, Schulten AJ, Nicolaas P, Dulger A, van Suijlekom-Smit LW. Facioskeletal changes in children with juvenile idiopathic arthritis. Ann Rheum Dis. 2006;65:823–825. doi: 10.1136/ard.2005.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjellberg H, Fasth A, Kiliaridis S, Wenneberg B, Thilander B. Craniofacial structure in children with juvenile chronic arthritis (JCA) compared with healthy children with ideal or postnormal occlusion. Am J Orthod Dentofacial Orthop. 1995;107:67–78. doi: 10.1016/s0889-5406(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 7.Kjellberg H. Craniofacial growth in juvenile chronic arthritis. Acta Odontol Scand. 1998;56:360–365. doi: 10.1080/000163598428329. [DOI] [PubMed] [Google Scholar]
- 8.Ince DO, Ince A, Moore TL. Effect of methotrexate on the temporomandibular joint and facial morphology in juvenile rheumatoid arthritis patients. Am J Orthod Dentofacial Orthop. 2000;118:75–83. doi: 10.1067/mod.2000.104953. [DOI] [PubMed] [Google Scholar]
- 9.Fjeld MG, Arvidsson LZ, Stabrun AE, Birkeland K, Larheim TA, Ogaard B. Average craniofacial development from 6 to 35 years of age in a mixed group of patients with juvenile idiopathic arthritis. Acta Odontol Scand. 2009;67:153–160. doi: 10.1080/00016350902740506. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen TK, Gronhoj J, Melsen B, Herlin T. Condylar condition and mandibular growth during early functional treatment of children with juvenile chronic arthritis. Eur J Orthod. 1995;17:385–394. doi: 10.1093/ejo/17.5.385. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen TK, Verna C. Functional and orthopedic treatment in developing dentofacial growth deviation in juvenile idiopathic arthritis. Semin Orthod. 2015;21:134–139. [Google Scholar]
- 12.Cevidanes LH, Franco AA, Gerig G et al. Comparison of relative mandibular growth vectors with high-resolution 3-dimensional imaging. Am J Orthod Dentofacial Orthop. 2005;128:27–34. doi: 10.1016/j.ajodo.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Krarup S, Darvann TA, Larsen P, Marsh JL, Kreiborg S. Three-dimensional analysis of mandibular growth and tooth eruption. J Anat. 2005;207:669–682. doi: 10.1111/j.1469-7580.2005.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huntjens E, Kiss G, Wouters C, Carels C. Condylar asymmetry in children with juvenile idiopathic arthritis assessed by cone-beam computed tomography. Eur J Orthod. 2008;30:545–551. doi: 10.1093/ejo/cjn056. [DOI] [PubMed] [Google Scholar]
- 15.Petty RE, Southwood TR, Manners P et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 16.Stoustrup P, Kristensen KD, Kuseler A et al. Condylar lesions in relation to mandibular growth in untreated and intra-articular corticosteroid-treated experimental temporomandibular joint arthritis. Clin Exp Rheumatol. 2010;28:576–583. [PubMed] [Google Scholar]
- 17.Buschang PH, Gandini Junior LG. Mandibular skeletal growth and modelling between 10 and 15 years of age. Eur J Orthod. 2002;24:69–79. doi: 10.1093/ejo/24.1.69. [DOI] [PubMed] [Google Scholar]
- 18.Mericle PM, Wilson VK, Moore TL et al. Effects of polyarticular and pauciarticular onset juvenile rheumatoid arthritis on facial and mandibular growth. J Rheumatol. 1996;23:159–165. [PubMed] [Google Scholar]
- 19.Araujo AM, Buschang PH, Melo AC. Adaptive condylar growth and mandibular remodelling changes with bionator therapy—an implant study. Eur J Orthod. 2004;26:515–522. doi: 10.1093/ejo/26.5.515. [DOI] [PubMed] [Google Scholar]