Abstract
Objective:
To investigate the cytotoxicity of nickel-titanium (NiTi) esthetic orthodontic archwires with different surface coatings.
Materials and Methods:
Three fully coated, tooth-colored NiTi wires (BioCosmetic, Titanol Cosmetic, EverWhite), two ion-implanted wires (TMA Purple, Sentalloy High Aesthetic), five uncoated NiTi wires (BioStarter, BioTorque, Titanol Superelastic, Memory Wire Superelastic, and Sentalloy), one β-titanium wire (TMA), and one stainless steel wire (Stainless Steel) were considered for this study. The wire samples were placed at 37°C in airtight test tubes containing Dulbecco’s Modified Eagle’s Medium (0.1 mg/mL) for 1, 7, 14, and 30 days. The cell viability of human gingival fibroblasts (HGFs) cultured with this medium was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data were analyzed by a two-way analysis of variance (α = .05).
Results:
The highest cytotoxic effect was reached on day 30 for all samples. The archwires exhibited a cytotoxicity on HGFs ranging from “none” to “slight,” with the exception of the BioTorque, which resulted in moderate cytotoxicity on day 30. Significant differences were found between esthetic archwires and their uncoated pairs only for BioCosmetic (P = .001) and EverWhite (P < .001).
Conclusions:
Under the experimental conditions, all of the NiTi esthetic archwires resulted in slight cytotoxicity, as did the respective uncoated wires. For this reason their clinical use may be considered to have similar risks to the uncoated archwires.
Keywords: Esthetic archwires, Biocompatibility, Coated wires, Orthodontics
INTRODUCTION
The increasing number of adult patients seeking orthodontic treatment has been a main contributor to the growing availability of different appliances, such as lingual orthodontics, clear aligners, and esthetic labial fixed appliances, which are well-accepted solutions by these patients who demand a high esthetic requirement.1–3 Esthetic wires and esthetic brackets are the principal components of esthetic labial orthodontics. Ion implantation and surface coating are two surface treatments used to improve the esthetic characteristics of an orthodontic archwire; these processes cover the metallic archwires with a superficial layer, changing their optical properties.4
From a clinical point of view, these hybrid materials have a physical behavior that is different from that of the traditional metallic archwires. Even though there is still a debate in the literature, essentially esthetic archwires deliver less force when compared to regular nickel-titanium (NiTi) wires of the same dimension and manufacturer,5 they produce the same friction as their metallic counterpart,6 and they result in unsatisfactory coating stability and durability.7
The biocompatibility of an orthodontic appliance relies on its susceptibility to corrosion, which means its stability in the oral cavity. Many factors can influence the amount of substances released by a dental alloy since the oral environment operates as a complex and dynamic system. For instance, pH fluctuations due to the degradation and decomposition of food and biofilms can considerably change the corrosion rate of biomaterials.8,9 Regarding esthetic appliances, it has been shown that monocrystalline ceramic brackets have a similar level of toxicity as the traditional metallic ones,10,11 while polycrystalline brackets show a slightly lower percentage of cell viability; the highest cytotoxicity is expressed by polycarbonate brackets, likely as a result of the bisphenol-A release associated with these brackets.11 On the other hand, there has been no extensive research examining the biocompatibility of esthetic orthodontic archwires with different surface treatments on human gingival fibroblasts (HGFs). Thus, the aim of this study was to determine the cytotoxicity of esthetic archwires together with their uncoated metallic counterparts under in vitro conditions.
MATERIALS AND METHODS
Materials, Chemicals, and Cells
Primary HGFs were obtained from oral surgical interventions in healthy 20- to 30-year-old patients, after informed consent approved by the tnstitutional review board (University of Napoli “Federico II” No. 226/14) was obtained. Tissue fragments were washed twice in phosphate-buffered saline (PBS; Carlo Erba Reagents, Milan, Italy) and transferred into tissue culture dishes in Dulbecco’s Modified Eagle’s Medium (DMEM; Carlo Erba Reagents) supplemented with 10% fetal bovine serum (Carlo Erba Reagents), 2 mM glutamine, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C in a humidified 5% CO2 incubator. After 10 days, fragments were removed, and released fibroblasts started proliferating. Once confluence was obtained, cells were washed with PBS and detached from culture dishes using a brief treatment with trypsin/ethylenediamine tetraacetic acid for 5 minutes and were then recultured until a confluent monolayer was again obtained.
Sample Preparation
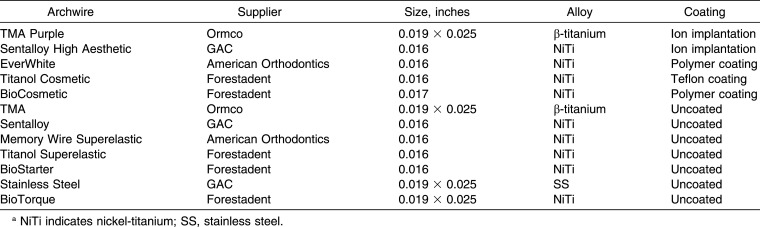
Five coated and seven uncoated metallic orthodontic archwires were investigated in this study (Table 1). The samples were sterilized following the protocol defined by the International Standard Organization (ISO) 10993-5 norm. The samples were immersed in DMEM and stored under stationary conditions at 37°C in airtight test tubes. The ratio between the weight of the samples and the volume of the dilutions was 0.1 mg/mL, as recommended by the ISO parameters. The aging periods selected comprised four (1, 7, 14, and 30 days). The four immersion period were independent of each other. For each aging period, new wire samples were immersed in the DMEM, and after each release interval, the extracts were sterile-filtered to eliminate solid particles and stored at −20°C until further use.
Table 1.
Description of Tested Archwiresa
Cell Viability Assessment
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Sigma Chemical Co, Milan, Italy) was used to evaluate cell viability. HGFs were planted into 96-well flat-bottomed tissue culture plates with a density of 104 cells/well. After 24 hours of incubation, the culture medium was replaced with 200 μL/well of archwire extract. After a further 24 hours, the medium was replaced with 100 μL/well of MTT solution (1 mg/mL) in PBS and the cells were incubated for an additional hour at 37°C in a 5% CO2 atmosphere. After the solution was removed, 100 μL/well of Dimethyl Sulfoxide (DMSO) was added and the plates were swirled gently for 10 minutes. The optical density of each well was immediately measured in a spectrophotometer (Sunrise™, Team, Mannederf/Zurich, Switzerland) at 590 nm. The optical density of the cells cultured in the DMEM medium without archwire sample extracts was used as a control for 100% cell viability and as a reference for the determination of the level of cytotoxicity in the assay.
According to Vande Vannet et al.,12 the following formula was used to calculate the cell viability:
Cell viability (%) = (optical density of test group ÷ optical desity of cellular control group) × 100.
Cell viability was then scored according to the classification of Ahrari et al.,13 as follows:
More than 90% cell viability = no cytotoxicity (none);
60–90% cell viability = slight cytotoxicity;
30–59% cell viability = moderate cytotoxicity;
Less than 30% cell viability = severe cytotoxicity.
Three independent experiments were performed in triplicate.
Statistical Analysis
Descriptive statistics and statistical analysis were performed using the Statistical Package for the Social Sciences (SPSS 19.0; SPSS IBM, New York, NY). The normal distribution of the data was confirmed by the Shapiro-Wilk test. Differences between mean values were determined by two-way analysis of variance (ANOVA), and the level of significance was set at α = .05.
RESULTS
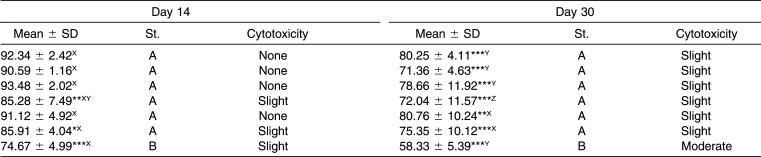
The MTT results and the level of cytotoxicity at different time points (1, 7, 14, and 30 days) of the archwires evaluated are shown in Tables 2 and 3.
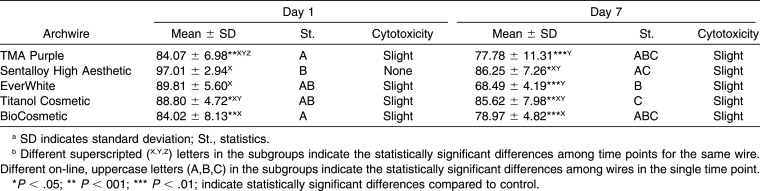
Table 2.
Descriptive and Inferential Statistics of Cell Viability for Esthetic Wiresab
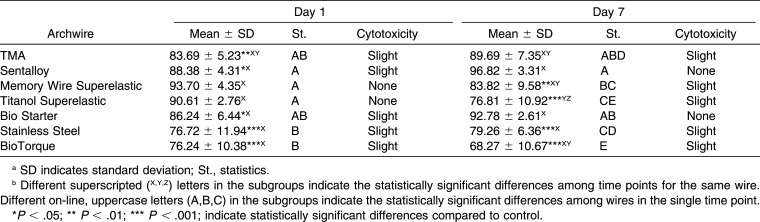
Table 3.
Descriptive and Inferential Statistics of Cell Viability for Metallic Wires
Table 2.
Extended
Table 3.
Extended
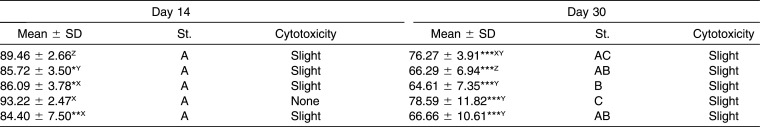
Aging considerably influenced the cytotoxicity of the samples by exhibiting variable patterns from day 1 to 14; however, all wires determined the lowest cell viability on day 30. Sentalloy High Aesthetic showed the highest increase in cytotoxicity (31%) between day 1 and day 30 (P < .001), while Stainless Steel was the most stable, with a variation in cytotoxicity between day 1 and day 30 of 1.4% (P = .801).
On day 1 the archwires showed a range from “none” to “slight” cytotoxicity on HGFs, and a statistically significant difference with respect to the control was found for three out of the five coated archwires (TMA Purple, Titanol Cosmetic, and BioCosmetic) and for five out of the seven metallic archwires (TMA, Sentalloy, BioStarter, Stainless Steel, and BioTorque).
On day 7 the archwires exhibited no cytotoxicity to slight cytotoxicity on HGFs, and all of the coated archwires were showed statistically significant higher cytotoxcity than the control.
On day 14 the archwires showed from no cytotoxicity to slight cytotoxicity on HGFs, and the wire that showed the highest cytotoxicity was BioTorque; moreover, three coated archwires and three metallic archwires presented a statistically significant difference compared to the control.
Finally, on day 30 the archwires revealed from no cytotoxicity to slight cytotoxicity on HGFs, with the exception of BioTorque, which had a moderately cytotoxic effect. All of the wires presented a statistically significant difference compared to the control.
Among NiTi esthetic wires, while Sentalloy High Aesthetic had the biggest decrease in cell viability, Titanol Cosmetic was the most stable; in fact, at day 30, it was the wire with the highest cell viability. TMA Purple, however, had a behavior similar to Titanol Cosmetic, showing higher biocompatibility than the other coated wires.
With regard to the metallic archwires, the rectangular wires, TMA, and Stainless Steel never showed a statistically significant difference among them, while BioTorque (NiTi) was more cytotoxic than were the other two rectangular wires on day 7, 14, and 30. On the other hand, the round NiTi archwires were never statistically significantly different.
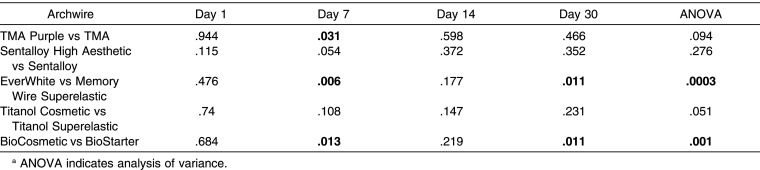
Comparing the esthetic and metallic NiTi wires, the MTT results for Sentalloy High Aesthetic and Titanol Cosmetic did not show any significant differences at each aging time considered in the experiment; for this reason they exhibited a similar cytotoxic effect on HGFs. On the other hand, EverWhite and BioCosmetic showed a statistically significant higher cytotoxicity than did Memory Wire Superelastic and the BioStarter, respectively, on days 7 and 30. Finally, TMA purple showed lower cell viability than did TMA only on day 7, as shown in Table 4.
Table 4.
P Value from Statistical Comparison Between Coated and Uncoated Archwires Bold Text Indicates Significant Valuesa
DISCUSSION
The present study evaluated the biocompatibility of various esthetic orthodontic archwires produced by ion implantation and polymer coating. As has been shown in in vivo studies,14 orthodontic wires and brackets show a slight cytotoxicity.
In order to assess the cytotoxicity on cell cultures of the media containing sample extractions HGFs were selected because they are the principal cell line in the oral tissues—along with epithelial keratinocytes—clinically exposed to the potential toxic effects of orthodontic materials.
All the archwires had the greatest cytotoxic effect on day 30; however, significant differences among cell viability levels at different time points were found for several wires. It has been reported15 that NiTi archwires immersed in artificial saliva released increasing amounts of Ni and Ti ions with longer immersion periods, evidence that supports our findings. Ni ions are considered the principal cytotoxic agents released by orthodontic alloys16; higher concentrations of this element likely cause toxic effects and might play a role in the innate immune response to metal biomaterials.17
Nevertheless, the time-dependent cytotoxicity observed for Sentalloy High Aesthetic, BioCosmetic, and EverWhite might depend on two distinct factors: an increasing concentration of metallic ions in the medium over time or a continuous release of substances as a result of the biodegradation of the esthetic coating.
Moreover, the different behavior in terms of cytotoxicity in the different time points exhibited by archwires with a similar surface treatment—such as Titanol Cosmetic with respect to BioCosmetic (polymer coated) and Sentalloy High Aesthetic with respect to TMA Purple (ion implanted)—might depend on the different surface stability of the coating material, which influences the corrosion tendency.
Sentalloy High Aesthetic (NiTi) and TMA Purple (β-titanium) are the two ion-implanted wires that had divergent results: this different response in terms of cytotoxicity on HGFs might depend on the alloy of the metallic inner core or the different material implanted. Kao et al.18 found that titanium nitride (TiN) ion-plated stainless steel brackets have a similar corrosion tendency and biocompatibility to non–TiN-plated brackets. Furthermore, NiTi archwires with a TiN surface exhibited higher corrosion resistance in artificial saliva than did their nonimplanted counterpart.19,20 Nevertheless, it has been reported21 that rhodium-coated NiTi wires showed lower cytotoxic effects and DNA damage than do uncoated NiTi wires. In our study, any difference in cell viability levels was found between ion-implanted and nonimplanted archwires, and these findings suggest that the rhodium layer does not limit the release of metal ions in solution, as was reported in a previous study.21
EverWhite, BioCosmetic, and Titanol Cosmetic were the polymer-coated NiTi archwires evaluated. Significantly lower cell viability levels were found for EverWhite and BioCosmetic compared to Titanol Cosmetic on day 30. Moreover, BioCosmetic and EverWhite showed lower cell viability on days 7 and 30 than did BioStarter and Memory Wire Superelastic, while Titanol Cosmetic and Titanol Superelastic did not present any differences. This should suggest that Titanol Cosmetic was the most stable esthetic archwire; this behavior might be due to the type of polymer and the manufacturing process employed to realize the superficial coating. In fact, although the same com-pany (Forestadent) manufactures BioCosmetic and Titanol Cosmetic, they undergo different methods of coating, and different esthetic materials are used. More specifically, Titanol Cosmetic archwires have a classical Teflon coating, whereas BioCosmetic archwires are manufactured through an innovative procedure: the metallic core is embedded in a flexible material instead of being sprayed by color particles, which usually happens for the majority of polymer-coated archwires present in the market. It has been shown22 that Teflon coating was able to improve the corrosion resistance of NiTi archwires better than does resin coating. Moreover, titanium discs with a Teflon coating had no toxic effects on MG-63 osteoblasts,23 shown by higher expression levels of the alkaline phosphatase (ALP), osteocalcin (OC), and OPG/RANKL ratios in cells grown on polytetrafluoroethylene (PTFE) surface in comparison to those cultured on an uncoated titanium surface. Teflon-coated platinum-iridium wires, which are medical devices placed in the vitreous as electrodes in visual prosthetic systems, resulted in no toxic reactions toward intraocular tissues.24 These studies clearly indicate a low cytotoxicity of Teflon as well as its favorable interaction with biological systems, and these studies are in agreement with the good biocompatibility that Titanol Cosmetic expressed in our study. Hence, NiTi archwires coated with Teflon, which has a high molecular stability, may have a better biocompatibility than do those coated with other polymers. In spite of this, it has to be considered that the Teflon surface coating exhibits a higher delamination than do other surface coatings when used intraorally.6,7 Therefore, it should be emphasized that the technique employed and the materials used in the manufacturing of esthetic archwires can influence not only their mechanical properties but also their biocompatibility.
In this study five uncoated NiTi wires were analyzed, and although they were manufactured with the same alloy, they showed different behaviors in terms of cytotoxicity on HGFs; actually, BioTorque exhibited the lowest levels of cell viability during all of the study time points. This variable cytotoxicity among NiTi archwires might be due to the different surface stability of the alloy, which can influence the corrosion tendency and thus the release of Ni ions in solution. The presence and the integrity of a superficial titanium oxide layer is, in fact, a key factor in limiting the biodegradation of NiTi biomaterials.25 It should be noted that BioTorque was the only rectangular NiTi wire considered in our study (0.019 × 0.025 inch), and this characteristic, together with the different manufacturing process employed for its production, could explain its higher cytotoxicity expressed compared to all the other NiTi wires having a round section.
TMA and TMA Purple, an uncoated and an ion-implanted β-titanium archwire, respectively, exhibited in general good levels of cell viability. The low cytotoxicity of TMA observed in our investigation agreed with the findings of Spalj et al.21 and Mockers et al.,10 and this peculiar behavior is likely due to the absence of Ni in the alloy as well as to its high corrosion resistance.26
Considering that both the coated and the uncoated wires exhibited roughly the same behavior on HGFs, it can be concluded that the ion-implanted layer does not alter the good biocompatibility of the β-titanium alloy.
In this study, a comparison among archwires of various dimensions was performed. With respect to the ISO protocol for the sample preparation, the total surface area among samples was different. The variation in the surface area could be considered a confounding factor, which was not investigated in the study.
CONCLUSIONS
In conclusion, the data suggest that the esthetic treatment—with both the Teflon coating and the ion-implantation method—to the NiTi wires did not modify the biocompatibility of orthodontic archwires.
Aging considerably influenced the cytotoxic effect on HGFs, and all of the archwires tested exhibited a higher cytotoxicity on day 30; however, the cytotoxicity on HGFs was between “none” and “slight,” with the exception of BioTorque, which exhibited moderate cytotoxicity on day 30.
Since esthetic archwires have shown nearly the same level of biocompatibility as metallic wires, their clinical use may be considered safe. Nevertheless, the manufacturer should take care when selecting the coating material, since it may cause more toxic effects on oral tissues.
ACKNOWLEDGMENT
The authors thank Dr Vincenzo De Simone for his contribution to the research.
REFERENCES
- 1.Washington B, Evans CA, Viana G, Bedran-Russo A, Megremis S. Contemporary esthetic nickel-titanium wires: do they deliver the same forces. Angle Orthod. 2015;85:95–101. doi: 10.2319/092513-701.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziuchkovski JP, Fields HW, Johnston WM, Lindsey DT. Assessment of perceived orthodontic appliance attractiveness. Am J Orthod Dentofacial Orthop. 2008;133:S68–S78. doi: 10.1016/j.ajodo.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Walton DK, Fields HW, Johnston WM, Rosenstiel SF, Firestone AR, Christensen JC. Orthodontic appliance preferences of children and adolescents. Am J Orthod Dentofacial Orthop. 2010;138:698.e1–698.e12. doi: 10.1016/j.ajodo.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 4.D’Antò V, Rongo R, Ametrano G, et al. Evaluation of surface roughness of orthodontic wires by means of atomic force microscopy. Angle Orthod. 2012;82:922–928. doi: 10.2319/100211-620.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaphoor AA, Sundareswaran S. Aesthetic nickel titanium wires—how much do they deliver. Eur J Orthod. 2012;34:603–609. doi: 10.1093/ejo/cjr089. [DOI] [PubMed] [Google Scholar]
- 6.Rongo R, Ametrano G, Gloria A, et al. Effects of intraoral aging on surface properties of coated nickel-titanium archwires. Angle Orthod. 2014;84:665–672. doi: 10.2319/081213-593.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva DL, Mattos CT, Sant'Anna EF, Ruellas AC, Elias CN. Cross-section dimensions and mechanical properties of esthetic orthodontic coated archwires. Am J Orthod Dentofacial Orthop. 2013;143:S85–S91. doi: 10.1016/j.ajodo.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Galeotti A, Uomo R, Spagnuolo G, et al. Effect of pH on in vitro biocompatibility of orthodontic miniscrew implants. Prog Orthod. 2013;1:14–15. doi: 10.1186/2196-1042-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadopoulou K, Eliades T. Microbiologically-influenced corrosion of orthodontic alloys: a review of proposed mechanisms and effects. Aust Orthod J. 2009;25:63–75. [PubMed] [Google Scholar]
- 10.Mockers O, Deroze D, Camps J. Cytotoxicity of orthodontic bands, brackets and archwires in vitro. Dent Mater. 2002;18:311–317. doi: 10.1016/s0109-5641(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 11.Retamoso LB, Luz TB, Marinowic DR, et al. Cytotoxicity of esthetic, metallic, and nickel-free orthodontic brackets: cellular behavior and viability. Am J Orthod Dentofacial Orthop. 2012;142:70–74. doi: 10.1016/j.ajodo.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Vande Vannet B, Mohebbian N, Wehrbein H. Toxicity of used orthodontic archwires assessed by three-dimensional cell culture. Eur J Orthod. 2006;28:426–432. doi: 10.1093/ejo/cjl002. [DOI] [PubMed] [Google Scholar]
- 13.Ahrari F, Tavakkol Afshari J, Poosti M, Brook A. Cytotoxicity of orthodontic bonding adhesive resins on human oral fibroblasts. Eur J Orthod. 2010;32:688–692. doi: 10.1093/ejo/cjq019. [DOI] [PubMed] [Google Scholar]
- 14.House K, Sernetz F, Dymock D, Sandy JR, Ireland AJ. Corrosion of orthodontic appliances—should we care. Am J Orthod Dentofacial Orthop. 2008;133:584–592. doi: 10.1016/j.ajodo.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 15.Huang HH, Chiu YH, Lee TH, et al. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials. 2003;24:3585–3592. doi: 10.1016/s0142-9612(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 16.D'Antò V, Valletta R, Amato M, et al. Effect of nickel chloride on cell proliferation. Open Dent J. 2012;6:177–181. doi: 10.2174/1874210601206010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Antò V, Eckhardt A, Hiller KA, et al. The influence of Ni(II) on surface antigen expression in murine macrophages. Biomaterials. 2009;30:1492–1501. doi: 10.1016/j.biomaterials.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Kao CT, Ding SJ, Chen YC, Huang TH. The anticorrosion ability of titanium nitride (TiN) plating on an orthodontic metal bracket and its biocompatibility. J Biomed Mater Res. 2002;63:786–792. doi: 10.1002/jbm.10484. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Johnson JW. Corrosion of stainless steel, nickel-titanium, coated nickel-titanium, and titanium orthodontic wires. Angle Orthod. 1999;69:39–44. doi: 10.1043/0003-3219(1999)069<0039:COSSNT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Iijima M, Yuasa T, Endo K, Muguruma T, Ohno H, Mizoguchi I. Corrosion behavior of ion implanted nickel-titanium orthodontic wire in fluoride mouth rinse solutions. Dent Mater J. 2010;29:53–58. doi: 10.4012/dmj.2009-069. [DOI] [PubMed] [Google Scholar]
- 21.Spalj S, Mlacovic Zrinski M, Tudor Spalj V, Ivankovic Buljan Z. In-vitro assessment of oxidative stress generated by orthodontic archwires. Am J Orthod Dentofacial Orthop. 2012;141:583–589. doi: 10.1016/j.ajodo.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan M, Seema S, Kumar AV, et al. Corrosion resistance of surface modified nickel titanium archwires. Angle Orthod. 2014;84:358–367. doi: 10.2319/021813-140.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleischmann L, Crismani A, Falkensammer F, Bantleon HP, Rausch-Fan X, Andrukhov O. Behavior of osteoblasts on TI surface with two different coating designed for orthodontic devices. J Mater Sci Mater Med. 2015;26:5335. doi: 10.1007/s10856-014-5335-9. [DOI] [PubMed] [Google Scholar]
- 24.Nishida K, Sakaguchi H, Xie P, et al. Biocompatibility and durability of Teflon-coated platinum-iridium wires implanted in the vitreous cavity. J Artif Organs. 2011;14:357–363. doi: 10.1007/s10047-011-0591-7. [DOI] [PubMed] [Google Scholar]
- 25.Espinar E, Llamas JM, Michiardi A, Ginebra MP, Gil FJ. Reduction of Ni release and improvement of the friction behaviour of NiTi orthodontic archwires by oxidation treatments. J Mater Sci Mater Med. 2011;22:1119–1125. doi: 10.1007/s10856-011-4292-9. [DOI] [PubMed] [Google Scholar]
- 26.Gopikrishnan S, Melath A, Ajith VV, Mathews NB. A comparative study of bio degradation of various orthodontic arch wires: an in vitro study. J Int Oral Health. 2015;7:12–17. [PMC free article] [PubMed] [Google Scholar]