Abstract
Objective:
To investigate the effect of low-frequency mechanical vibration (LFMV) on orthodontically induced root resorption.
Materials and Methods:
Forty male CD1, 12-week-old mice were used for the study. The mice were randomly divided into five groups: group 1 (baseline)—no spring and no mechanical vibration, group 2—orthodontic spring but no vibration, group 3—orthodontic spring and 5 Hz of vibration applied to the maxillary first molar, group 4—orthodontic spring and 10 Hz of vibration applied to maxillary first molar, and group 5—orthodontic spring and 20 Hz of vibration applied to maxillary first molar. In the different experimental groups, the first molar was moved mesially for 2 weeks using a nickel-titanium coil spring delivering 10 g of force. LFMVs were applied at 5 Hz, 10 Hz, and 20 Hz. Microfocus X-ray computed tomography imaging was used to analyze root resorption. Additionally, to understand the mechanism, we applied LFMV to MC3T3 cells, and gene expression analyses were done for receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG).
Results:
Orthodontic tooth movement leads to decreased root volume (increased root resorption craters). Our in vivo experiments showed a trend toward increase in root volume with different frequencies of mechanical vibration. In vitro gene expression analyses showed that with 20 Hz of mechanical vibration, there was a significant decrease in RANKL and a significant increase in OPG expression.
Conclusion:
There was a trend toward decreased root resorption with different LFMVs (5 Hz, 10 Hz, and 20 Hz); however, it was not more statistically significant than the orthodontic-spring-only group.
Keywords: Root resorption, Mechanical vibration, Orthodontic tooth movement
INTRODUCTION
Orthodontic tooth movement (OTM) involves modeling and remodeling of the alveolar bone in response to an external load.1 Numerous noninvasive approaches have been studied to decrease total treatment time.2,3 Longer duration of orthodontic treatment is associated with increased chances of inflammatory root resorption.2
Orthodontically induced root resorption (RR) is an inevitable sequela of OTM. RR is a physiological or pathological process that involves breakdown of root structure, specifically mineralized cementum and dentin and nonmineralized dentin.2,4,5 Physiological RR occurs naturally and is responsible for the RR of deciduous teeth, whereas pathological RR is usually inflammatory in nature.
A number of in vitro and in vivo studies have suggested that mechanical vibration can affect bone metabolism.6,7 Low-frequency mechanical vibration (LFMV) is believed to accelerate fracture healing by anabolic modeling of bone. Further, LFMV has been shown to improve bony architecture in the postmenopausal female.6
Osteoprotegerin (OPG) and receptor activator of nuclear factor-kappaB ligand (RANKL) are paracrine regulators of osteoclastogenesis and cementoclastogenesis.8–10 Among the many signals released in response to mechanical vibration, RANKL and OPG are regarded as two critical molecules regulating osteoclasts.7,11,12 RANKL is expressed as a transmembrane protein in osteoblasts, whereas OPG is a protein secreted by the osteoblasts acting as a decoy receptor blocking the RANKL-RANK ligand interaction, thus antagonizing the formation of mature osteoclasts.7,13,14
Our null hypothesis is that there is no difference between the root volumes resulting from the effect of different LFMVs. We sought to study the effects of different LFMVs on orthodontically induced RR and to understand the underlying cellular mechanism of LFMV. Our study had two specific aims: (1) to quantify the amount of RR between different control and experimental groups and (2) to quantify the expression of OPG and RANKL in the cells to understand the mechanism behind RR. We understand that this is the first mechanistic study (both in vivo and in vitro) looking at the effects of LFMV on RR.
MATERIALS AND METHODS
In Vitro Experiments
To stimulate the MC3T3 cells with LFMV, six-well plates cultured with MC3T3 were mounted on a platform. A feedback-loop-controlled, electromechanical actuator (Model 3230, Bose/EnduraTec, Minnetonka, Minn) was used to apply the LFMV. The loading protocol for individual cell culture plates consisted of 30 minutes of LFMV at 1 g of force through an electromechanical actuator at a frequency of 5, 10, or 20 Hz (cycles/s), depending on the group the cells were assigned to. LFMV was applied at 3-day intervals (days 1, 4, and 7).
Quantitative Real-Time Polymerase Chain Reaction
The total RNA from the cultured cells under different LFMVs was isolated 3 hours after the last mechanical vibration using Trizol reagent (Invitrogen, Carlsbad, Calif). Total RNA was converted to cDNA by an ABI High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, Calif) following the manufacturer’s protocol. Real-time polymerase chain reaction was performed to express OPG and RANKL, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. Three technical and biological replicates of each sample were amplified using predesigned, unlabeled, gene-specific PCR primers and TaqMan MGB FAM dye-labeled probe. The primer sequences used for OPG, RANKL, and GAPDH were based on the published literature. Gene expression labels were calculated and are presented as a fold change relative to the control samples.
In Vivo Experiments
Experimental design
The animal care committee at the University of Connecticut approved this study. Data were obtained from 40 male CD1 mice (Charles River Laboratories, Wilmington, Mass; body weight, 26–30 g). The 12-week-old mice were randomly divided into five groups: group 1 (baseline)—no spring and no mechanical vibration, group 2—orthodontic spring but no vibration, group 3—orthodontic spring and 5 Hz of vibration applied to the maxillary first molar, group 4—orthodontic spring and 10 Hz of vibration applied to the maxillary first molar, and group 5—orthodontic spring and 20 Hz of vibration applied to the maxillary first molar. The animals were allowed a week to acclimatize before the experimental procedure at the Health Center to compensate for their different origins.
Appliance delivery
The mice were anesthetized with xylazine (13 mg/kg) and ketamine (87 mg/kg). A custom mouth prop was fabricated from 0.036-mm stainless steel (SS) wire and placed between the maxillary and mandibular incisors to hold the mouth open. A 0.004-inch SS ligature wire was passed beneath the contact between the maxillary first and second molars and threaded to the first molar (Figure 1A). A nickel-titanium coil spring (Ultimate Arch Wires, Bristol, Conn) delivering 10 g of force was attached to the ligature wire around the first molar and the other end of the spring was attached to the incisors using the ligature wire.
Figure 1.

(A) Inserted NiTi coil spring. (B) Application of low-frequency mechanical vibration on maxillary right first molar.
To prevent dislodging the spring from the incisors due to their curvature, 0.5-mm deep grooves were prepared on the facial, lingual, and distal surfaces of the incisors. Self-etching primer (Transbond Plus, 3M Unitek, St Paul, Minn) and light-cured adhesive resin cement (Transbond, 3M Unitek) were applied to the lingual surface of the first molar and incisors to secure the ligature wire. In order to minimize distal movement of the left incisor and reinforce anterior anchorage, the right and left incisors were joined to act as a unit (Figure 1A). The appliance was checked every other day and additional bonding material added if necessary.
Mechanical vibration for OTM
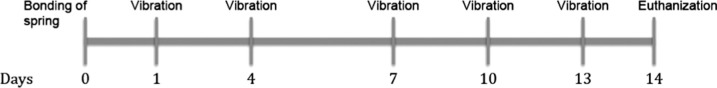
A feedback-loop-controlled, electromechanical actuator (Model 3230, Bose/EnduraTec) was used to apply unilateral LFMV to the occlusal surface of the maxillary right first molar along the tooth’s long axis (Figures 1B and 2). The mechanical vibrations in the different experimental groups were applied after the induction of anesthesia. A custom mouth prop fabricated from 0.017 × 0.025-inch titanium-molybdenum alloy wire was placed between the maxillary and mandibular incisors in order to hold the mouth open. The loading protocol for mechanical vibrations for individual animals consisted of 15 minutes of LFMV at 1 g of force through an electromechanical actuator at a frequency of 5, 10, or 20 Hz, depending on the experimental group the mouse was assigned to. The vibration was applied to the occlusal surface of the maxillary first molar in an occulusogingival direction. The vibrations were applied at 3-day intervals (days 1, 4, 7, 10, and 13) (Figure 2).
Figure 2.
Schematic of experimental procedure and application of low-frequency mechanical vibration.
Euthanasia
After 14 days, the animals were euthanized by inhalation of carbon dioxide, followed by cervical dislocation.
Micro-CT
RR was measured on the mesiobuccal root of the maxillary first molar. The customized software segmented the mesiobuccal root, and the root volume was measured by contouring the root surfaces on the two-dimensional slices; the crown was not included in the measurement. The root volume was analyzed using microcomputed tomography (micro-CT) (Scanco Medical AG, Bruttisellen, Switzerland). The samples were scanned in liquid, one at a time, with a high resolution of 16 μm. The X-ray tube potential (peak) was 55 kVp at an intensity of 145 Joules-Ampere. There were 1000 projections for each sample, with a scanning time of 300,000 seconds for each sample.
The root volume measurement (including cementum and root dentin) was used to quantify RR. Changes in root volume were quantified by analyzing the mesiobuccal root of the maxillary first molar.
Statistical Analysis
Simple descriptive statistics were used to summarize the data. Outcomes examined were root volume and gene expression of RANKL and OPG. Statistical analyses were carried out using Graph Pad Prism Version 7 (GraphPad Software Inc, La Jolla, Calif). Statistical significance of differences among means was determined by one-way analysis of variance with a Bonferroni post hoc test. All statistical tests were two sided, and a P value of < .05 was deemed to be statistically significant.
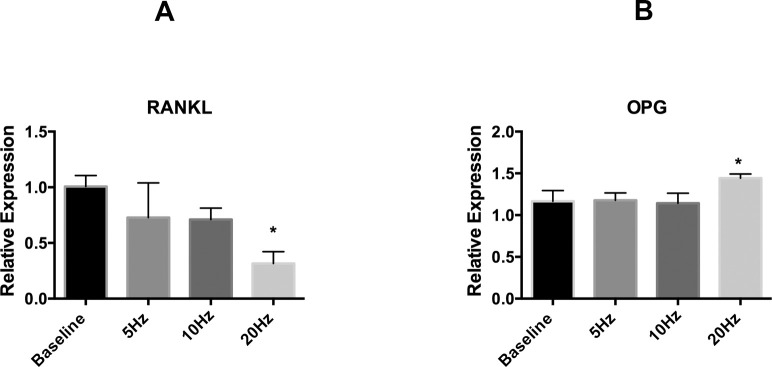
Our analysis of gene expression on the MC3T3 cell population showed that the 20-Hz vibration caused significantly decreased expression of RANKL (P < .05) compared with baseline (Figure 3A). Moreover, there was a tendency to decrease RANKL expression with increased frequency of vibration, but it was not statistically significant among the various vibration groups (Figure 3A). The transcript expression of OPG was significantly (P < .05) higher at 20 Hz compared with the other groups (baseline, 5 Hz, and 10 Hz) (Figure 3B).
Figure 3.
Gene expression of RANKL and OPG with different low-frequency mechanical vibrations in MC3T3 cells. Real-time PCR analysis was performed for gene expression of RANKL and OPG. Points are the mean ± SD for each group. (A) Relative gene expression analysis for RANKL transcript. * Significant difference between 20-Hz vibration and baseline (P < .05). (B) Relative gene expression analysis for OPG transcript.
* Significant difference between the 20-Hz vibration and other experimental groups (P < .05).
Our in vivo experiments showed that the mice in all the experimental groups tolerated the appliance well, there was no net loss in weight, and the application of orthodontic force led to a significant decrease (P < .05) in root volume (spring + no vibration <baseline) (Figures 4 and 5). Moreover, we saw an increase in root volume (fewer resorption craters) at different low-frequency mechanical vibrations. However, it was not significantly different from baseline (P > .05) and spring + no vibration group (P > .05) (Figures 4 and 5).
Figure 4.
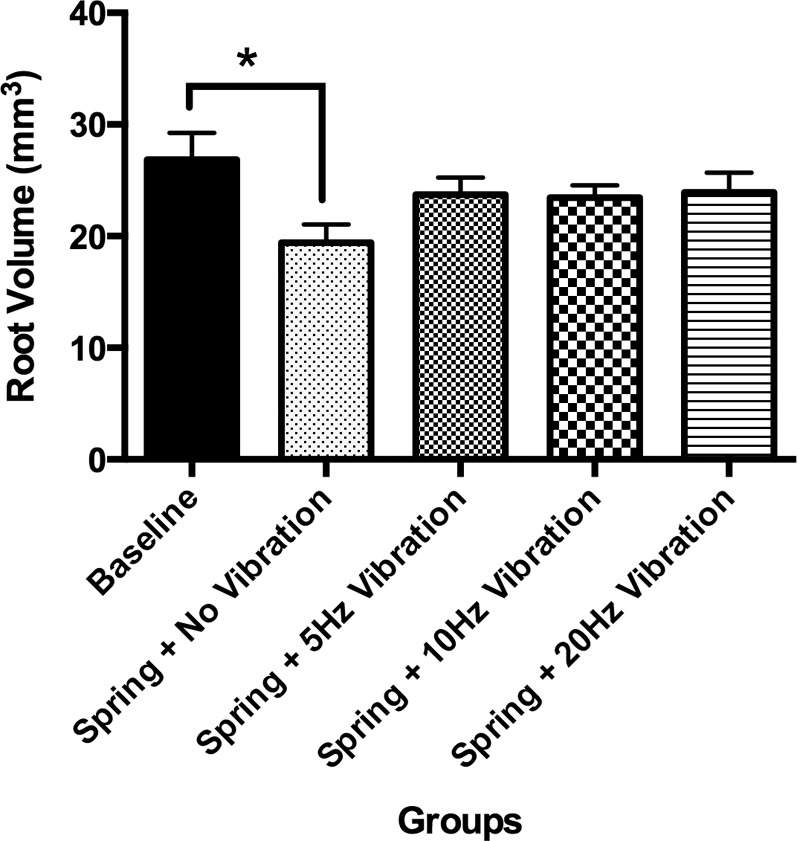
Micro-CT data showing root volume of mesiobuccal root in different groups. Note significantly (*) decreased root volume in the spring + no vibration group (group 2) compared with baseline group.
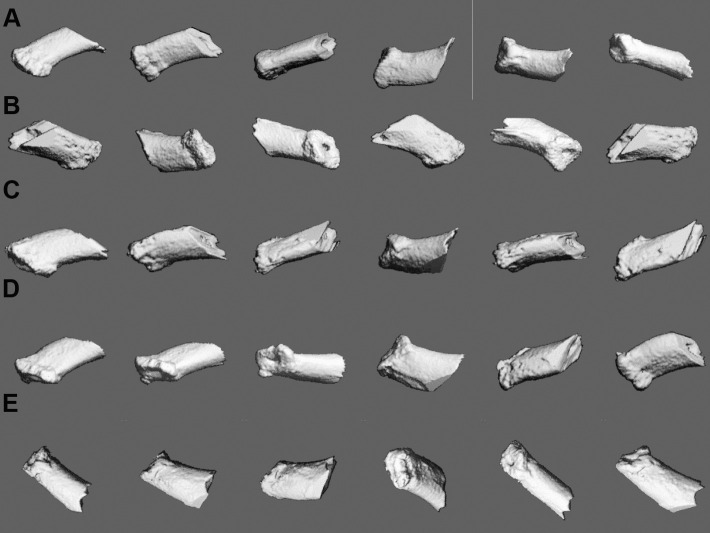
Figure 5.
Mesiobuccal root in different experimental groups was imaged by micro-CT (VivaCT40, Scanco Medical AG, Bassersdorf, Switzerland). (A) Baseline. (B) Spring + no vibration. (C) Spring + 5-Hz vibration. (D) Spring + 10-Hz vibration. (E) Spring + 20-Hz vibration.
DISCUSSION
The null hypothesis of this study—that there would be no difference in the amount of root volume (RR) between different LFMVs—was accepted. To our knowledge, this is the first study reporting the effects of LFMV on orthodontically induced RR. Our results showed that there is a trend for decreased RR with different frequencies of mechanical vibration, but this was not statistically significant between the various experimental groups.
OTM in this study was induced using a light, continuous, 10-g force. Zhuang et al.15 have reported that in rodents, 10 g of force may lead to RR, having possibly exceeded the physiological limit. Micro-CT with a high resolution of 16 μm per pixel can be used to calculate the root volume and visualize the RR craters, as done in the present study. Similarly, other studies have used micro-CT to investigate RR.16,17 Our study showed a trend toward increase in root volume with different frequencies of mechanical vibration.
Rubin et al.6 reported that mechanical vibration could be used to stimulate bone formation. Studies have suggested that mechanical vibration can positively influence skeletal homeostasis.18,19 Moreover, it has been shown that low-magnitude, high-frequency vibrations decrease osteoclastic activity.20 Our results show that LFMV alters the levels of RANKL and OPG expression, thus altering osteoclastic activity and that the expression of RANKL significantly decreases (P < .05) with 20-Hz vibration compared with other experimental groups. Our results were similar to those of Lau et al.,7 who showed that low-magnitude, high-frequency vibrations decrease RANKL expression with increased frequency, but they applied the mechanical vibrations to osteocytes rather than to the osteoblastic cell line.7 Similarly, Dalla-Bona et al.21 treated cementoblasts with high-intensity (150 mW/cm2) and low-intensity (30 mW/cm2) ultrasonic radiation (low-intensity, pulsed ultrasound) and found that both high and low intensity increased the OPG expression but did not affect RANKL expression. Our results were similar to those of Dalla-Bona et al.21 with respect to OPG expression, but in our research there was a decrease in RANKL expression, and the observed difference may be due to differences in cell type and mechanical vibration applied.
Mechanical vibration has been shown to stimulate proliferation and differentiation of mesenchymal stem cells into osteogenic and chondrogenic lineage.22,23 These findings suggest that mechanical vibration has broad tissue-regeneration potential and in the near future might be used for hard tissue (bone and root) repair and regeneration (apical RR). Since this was an animal study, extrapolation of our findings to the clinical situation must be done with caution, as rodents lack the secondary remodeling present in humans and higher animals. Nevertheless, this study helped us understand the effect and mechanism of different mechanical vibration frequencies on root volume.
CONCLUSIONS
Mechanical vibration with frequencies of 5, 10, and 20 Hz for 15 minutes on days 1, 4, 7, 10, and 13 did not significantly affect root volume. However, there was a trend toward increasing root volume with different low-frequency mechanical vibrations.
Mechanical vibration with 20 Hz led to a significant decrease in RANKL expression and a significant increase in OPG expression.
ACKNOWLEDGMENTS
The American Association of Orthodontists Foundation supported this research.
REFERENCES
- 1.Huang H, Williams RC, Kyrkanides S. Accelerated orthodontic tooth movement: molecular mechanisms. Am J Orthod Dentofacial Orthop. 2014;146:620–632. doi: 10.1016/j.ajodo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Nimeri G, Kau CH, Abou-Kheir NS, Corona R. Acceleration of tooth movement during orthodontic treatment—a frontier in orthodontics. Prog Orthod. 2013;14:42. doi: 10.1186/2196-1042-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung SE, Tompson B, Gong SG. The effect of light emitting diode phototherapy on rate of orthodontic tooth movement: a split mouth, controlled clinical trial. J Orthod. 2015 doi: 10.1179/1465313315Y.0000000013. 1465313315Y0000000013 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Tieu LD, Saltaji H, Normando D, Flores-Mir C. Radiologically determined orthodontically induced external apical root resorption in incisors after non-surgical orthodontic treatment of Class II division 1 malocclusion: a systematic review. Prog Orthod. 2014;15:48. doi: 10.1186/s40510-014-0048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzahawi K, Faerovig E, Brudvik P, Boe O, Mavragani M. Root resorption after leveling with super-elastic and conventional steel arch wires: a prospective study. Prog Orthod. 2014;15:35. doi: 10.1186/s40510-014-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin J, Murphy TC, Fan X, Goldschmidt M, Taylor WR. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res. 2002;17:1452–1460. doi: 10.1359/jbmr.2002.17.8.1452. [DOI] [PubMed] [Google Scholar]
- 7.Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–1515. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–159. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz MC, Xi Y, Wilson K, Kacena MA. Control of osteoclastogenesis and bone resorption by members of the TNF family of receptors and ligands. Cytokine Growth Factor Rev. 2001;12:9–18. doi: 10.1016/s1359-6101(00)00030-7. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor P, Kharbanda OP, Monga N, Miglani R, Kapila S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: a systematic review. Prog Orthod. 2014;15:65. doi: 10.1186/s40510-014-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis–inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, et al. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun. 2000;275:768–775. doi: 10.1006/bbrc.2000.3379. [DOI] [PubMed] [Google Scholar]
- 14.Hikita A, Yana I, Wakeyama H, Nakamura M, Kadono Y, Oshima Y, et al. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-kappaB ligand. J Biol Chem. 2006;281:36846–36855. doi: 10.1074/jbc.M606656200. [DOI] [PubMed] [Google Scholar]
- 15.Zhuang L, Bai Y, Meng X. Three-dimensional morphology of root and alveolar trabecular bone during tooth movement using micro-computed tomography. Angle Orthod. 2011;81:420–425. doi: 10.2319/071910-418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomizuka R, Shimizu Y, Kanetaka H, Suzuki A, Urayama S, Kikuchi M, et al. Histological evaluation of the effects of initially light and gradually increasing force on orthodontic tooth movement. Angle Orthod. 2007;77:410–416. doi: 10.2319/0003-3219(2007)077[0410:HEOTEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Kohno T, Matsumoto Y, Kanno Z, Warita H, Soma K. Experimental tooth movement under light orthodontic forces: rates of tooth movement and changes of the periodontium. J Orthod. 2002;29:129–135. doi: 10.1093/ortho/29.2.129. [DOI] [PubMed] [Google Scholar]
- 18.Garman R, Gaudette G, Donahue LR, Rubin C, Judex S. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732–740. doi: 10.1002/jor.20354. [DOI] [PubMed] [Google Scholar]
- 19.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Dalla-Bona DA, Tanaka E, Inubushi T, Oka H, Ohta A, Okada H, et al. Cementoblast response to low- and high-intensity ultrasound. Arch Oral Biol. 2008;53:318–323. doi: 10.1016/j.archoralbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Li J, Zhang L, Zhou Y, Hou W, Quan H, et al. Effects of mechanical vibration on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Arch Oral Biol. 2012;57:1395–1407. doi: 10.1016/j.archoralbio.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Lu Y, Zhang L, Liu Y, Zhou Y, Chen Y, et al. Influence of different intensities of vibration on proliferation and differentiation of human periodontal ligament stem cells. Arch Med Sci. 2015;11:638–646. doi: 10.5114/aoms.2015.52370. [DOI] [PMC free article] [PubMed] [Google Scholar]