Highlights
-
•
The assessment of urinary VOCs using MS has potential for early cancer diagnosis.
-
•
Hexanal, dimethyl disulfide and phenol are the most reported urinary VOC cancer biomarkers.
-
•
Elevated levels of phenol in urine appear correlated with breast cancer.
-
•
Urinary VOC models have slightly greater accuracy than PSA in diagnosis of prostate cancer.
-
•
Standardization and research with larger cohorts are needed before urinary VOC MS techniques can be applied clinically.
Abbreviations: CAS, chemical abstracts service; CYP450, cytochrome P450; eNose, electronic nose; FAIMS, high-field asymmetric waveform ion mobility spectrometry; GC, gas chromatography; HS, headspace; IMS, ion mobility spectrometry; LC, liquid chromatography; MS, mass spectrometry or mass spectrometric; NT, needle trap; PSA, prostate-specific antigen; PTR, proton transfer reaction; PTV, programed temperature vaporizer; ROS, reactive oxygen species; SBSE, stir bar sorptive extraction; SIFT, selected ion flow tube; SPME, solid phase microextraction; VOCs, volatile organic compounds
Keywords: Cancer, VOCs, Mass Spectrometry, Metabolomics, Biomarkers, Urine
Abstract
The development of non-invasive screening techniques for early cancer detection is one of the greatest scientific challenges of the 21st century. One promising emerging method is the analysis of volatile organic compounds (VOCs). VOCs are low molecular weight substances generated as final products of cellular metabolism and emitted through a variety of biological matrices, such as breath, blood, saliva and urine. Urine stands out for its non-invasive nature, availability in large volumes, and the high concentration of VOCs in the kidneys. This review provides an overview of the available data on urinary VOCs that have been investigated in cancer-focused clinical studies using mass spectrometric (MS) techniques. A literature search was conducted in ScienceDirect, Pubmed and Web of Science, using the keywords “Urinary VOCs”, “VOCs biomarkers” and “Volatile cancer biomarkers” in combination with the term “Mass spectrometry”. Only studies in English published between January 2011 and May 2020 were selected. The three most evaluated types of cancers in the reviewed studies were lung, breast and prostate, and the most frequently identified urinary VOC biomarkers were hexanal, dimethyl disulfide and phenol; with the latter seeming to be closely related to breast cancer. Additionally, the challenges of analyzing urinary VOCs using MS-based techniques and translation to clinical utility are discussed. The outcome of this review may provide valuable information to future studies regarding cancer urinary VOCs.
1. Introduction
Cancer is a major public health problem, leading to a huge number of mortalities each year. According to the World Health Organization (WHO), cancer is the second leading cause of death worldwide, responsible for approximately 9.6 million deaths in 2018; equating to about 1 in 6 deaths [1]. Furthermore, the incidence has been increasing. In 2018 approximately 18.1 million new cases were reported, and in 2040 it is estimated it will be near 29.4 million [2]. The earlier cancer is detected, the better the chances are for recovery, and the sooner an appropriate treatment can be implemented, the more likely it is to be effective. However, there are two major obstacles to identification and early treatment: first, there is a general paucity of easily identifiable signs and symptoms in the early stages of cancer, and even when there are signs, diagnosis frequently requires many expensive, invasive and time demanding procedures [3], [4], [5]. For these reasons, there is a global need for the research and development of low cost, rapid and non-invasive methodologies for early diagnosis, to reduce the time spent in different stages of health care systems and improve the chances of recovery [6], [7].
Metabolomics is an emerging field that has significant untapped potential for biomarker discovery and translation to cancer screening and early diagnosis [8], [9]. A recent and promising metabolomic approach is “volatilomics”, the study of volatile organic compounds (VOCs) produced in human body and emitted through breath, blood, urine, saliva, sweat, feces and other biological matrices [6], [10], [11], [12]. VOCs are low molecular weight substances that are generated as final products of cellular metabolism, exhibiting a high-vapor pressure and low boiling point (below 250 °C) [11]. Compared to other types of metabolites, which must be extracted from tissues or body fluids before analysis, VOCs are directly accessible in the gaseous phase (headspace), thus requiring minimal sample preparation and allowing non-invasive and real-time monitoring [13]. It is well-known that diseases alter the physiological and metabolic status of an individual; therefore, in pathological conditions it would not be unexpected if the concentrations of VOCs changed and/or new VOCs were generated. Consequently, a number of studies have been performing headspace analyses in clinical settings for the diagnosis of diabetes [14], ulcerative colitis [15], asthma [16], chronic obstructive pulmonary disease [17], irritable bowel syndrome [18], and, especially, cancer [19], [20], [21], [22].
As mentioned previously, VOCs are released through a variety of biological matrices, including bodily fluids and cell lines [10], [23], [24]. Therefore, numerous matrices may be explored in the context of VOC analysis for cancer biomarker discovery. The majority of the studies in the literature are related to breath analysis, with a special focus on lung cancer [25]. Reviews on VOC breath biomarkers in cancer can be found elsewhere [26], [27], [28]. Urine is another non-invasive specimen-type which is available in large volumes and the analytes excreted are already concentrated by the kidney. Hence, it is a well-suited source of VOCs for metabolic profiling, and has been extensively explored using metabolomics approaches to identify cancer biomarkers [8], [29], [30], [31]. After breath, urine is the main biological matrix used to detect VOCs in various types of cancers [25]. Regardless of the matrix, VOCs differ between individuals due to a number of uncontrolled variables, such as genetics, environment, therapeutics, diet, and smoking habits, making VOC assessment analytically challenging [3].
VOC analysis can be performed using a number of different tools. Gas chromatography coupled to mass spectrometry (GC–MS) is the gold standard technique employed for the chemical characterization of VOCs as cancer biomarkers [32]. However, other MS-based techniques, such as selected ion flow tube mass spectrometry (SIFT-MS) and proton transfer reaction mass spectrometry (PTR-MS) have also been successfully used for this purpose [33], [34], [35]. In addition, pattern recognition sensor arrays, such as electronic noses (eNose), and ion mobility spectrometry (IMS) based techniques are also employed to create specific “odor fingerprints” of VOC profiles. [36], [37], [38]. Finally, studies have shown that sniffer dogs can be trained to detect the presence of cancer, especially in urine samples [39], [40], [41]. There are a number of reviews that describe the techniques used to detect VOC cancer biomarkers in the literature. However, they are usually focused on breath analysis [27], on one specific type of cancer (mainly lung) [42], on canine and eNose methods [43], [44], or they approach only the non-separative mass spectrometric (MS) techniques [45].
In contrast, here we focus on mass spectrometry (MS)-based clinical studies of urinary VOCs as cancer biomarkers. Firstly, we provide a brief overview of the VOCs found in urine; then we review the most recent decade of published evidence covering the use of MS to detect urinary VOCs for cancer diagnosis, highlighting the methods performed and possible biomarkers; and finally we focus on the three most reported cancer types: lung, breast and prostate.
2. Literature search
A literature search was carried out in ScienceDirect, Pubmed and Web of Science, using the keywords “Urinary VOCs”, “VOCs biomarkers” and “Volatile cancer biomarkers” in combination with the term “Mass spectrometry”. Additionally, the search was supplemented by further checking the references present in pertinent articles. Only studies published in English and appearing in peer-reviewed journals between January 2011 and May 2020 were evaluated. In total, 72 papers were analyzed and 25 were included for full-text review. Only papers that conducted cancer clinical studies analyzing the urinary VOC profiles using MS-based methods were considered fit for our evaluation. It is worth mentioning that most of the excluded studies were related to the analysis of other types of biological matrices (mainly breath) [46], or used other techniques to analyze VOCs present in urine, such as sniffing dogs [47] or pattern recognition sensor methods [48].
3. VOCs in urine
The exact biochemical mechanisms by which VOCs are generated in our body is not yet fully understood. However, studies indicate that the reactive oxygen species (ROS) generated as part of the cellular respiration process may react with many structures, including cell membranes, proteins, DNA and RNA, and can generate small volatile molecules that are emitted by different body fluids [10], [11], [49]. In addition, these VOCs may be converted into different compounds by enzymatic reactions, which take place mainly in the liver via cytochrome P450 (CYP450) enzymes [10], [11]. For example, alkenes may be generated by oxidative stress and then converted into alcohols via CYP450 activity [49]. More details about VOC origin can be found elsewhere [11], [49].
The VOCs are mainly transported in our body through the blood and pass into urine after renal filtration [11]. In total, 279 urinary volatile compounds were identified and compiled in a review by de Lacy Costello and collaborators [50]. The VOCs in urine are considered intermediate or end products of metabolic pathways, and cover a range of chemical classes, such as ketones, alcohols, aldehydes, carboxylic acids, amines, furans, pyrroles, hydrocarbons and sulfur compounds [50], [51]. Compared to other bodily fluids, urine contains a larger number of ketones, which arise from enzymatic liver function and/or bacterial action in the gut [11], [50]; in addition, very low amounts of esters have been reported [50]. Finally, a large number of terpenes are described (also in saliva) and are hypothesized to originate from the diet [50].
As mentioned previously, urine has been used for VOC analysis in a number of studies since it is a non-invasive matrix that is easy to collect and can be obtained in large volumes [3]. In addition, urinary VOCs may be detected in higher concentrations since urine is relatively less complex, presenting fewer matrix interferences compared to blood, for instance, and VOCs are also concentrated by the kidneys before excretion [3], [10]. However, a drawback is that urinary VOCs may be affected by the ingestion of medications, food and/or drink, which must be taken into consideration when a VOC is considered as a candidate for a disease biomarker [51]. Alterations in urinary VOC patterns have been observed in a series of metabolic disorders. For instance, in maple syrup urine disease, the strong smell of maple syrup in urine is due to high levels of keto acids (keto acidosis), and in diabetes mellitus, acetones and ketones (mainly 4-heptanone) are also present in higher amounts [10], [52]. Urinary VOC patterns in cancer patients are often different from those found in the urine samples of control subjects, and these differences depend also on cancer type and stage [51]. Thus, possible cancer biomarkers and the MS-based methods used to assess urinary VOCs will be discussed in the following section.
4. Diagnosing cancer via determination of urinary VOCs using MS techniques
4.1. Analytical methods overview
The main MS-based technique that has been employed in urinary VOC cancer biomarker research is GC–MS. This method was used in 21 of the 25 evaluated studies (84%). VOC analyses using GC–MS usually require a preconcentration step to increase detectability [53]. Fifteen studies used the solid phase microextraction (SPME) method for this purpose. SPME is a solventless technique based on the sorption of analytes by an extracting phase immobilized over the surface of a fused-silica fiber which can be immersed in the sample or placed within its headspace [54]. SPME is a well-established procedure, which has been performed in VOC analysis since the early 90 s [55]; thus, it was expected that this technique would be the most employed procedure. However, other preconcentration techniques have been used, such as Needle Trap (NT) devices, which were employed in three studies [56], [57], [58]. NT involves a stainless-steel needle packed with a sorbent bed that is used for the extraction of gaseous samples [59]. The claimed main advantage of NT over SPME is that the sensitivity of an NT method can be improved by increasing the sample volume, since it is an exhaustive technique [60]. In addition, one study performed a Stir Bar Sorptive Extraction (SBSE) [61], which is a similar approach to SPME, where a stir bar coated with a sorbent is used to extract the VOCs from a liquid sample [62].
GC–MS is an extremely useful tool; however, it is expensive, requires highly trained personnel and it is not easy to implement in clinical settings, mainly due to its lack of portability [3], [63]. Therefore, simpler and less expensive MS-based techniques have been used for VOCs analysis, such as SIFT-MS [64]. SIFT-MS is a technique that exploits a fast flow tube reactor combined with chemical ionization to analyze trace amounts of VOCs in air samples [65]. SIFT-MS does not require a preconcentration step and can be performed in real-time (online) for the quantification of VOCs; hence, it is a method that is widely used in breath analysis for VOC biomarkers [65]. More details on SIFT-MS principles and applications can be found elsewhere [65], [66]. Urine is less frequently analyzed using SIFT-MS than breath samples [45], with only two of the evaluated studies using this technique to detect urinary VOC biomarkers [67], [68]. Thus, it is a method that may be further explored in future studies.
It is important to mention that one study performed an unusual method to asses urinary VOCs: they used high-field asymmetric waveform ion mobility spectrometry (FAIMS) coupled with Liquid Chromatography Mass Spectrometry (LC-MS) to assess VOC patterns in colorectal cancer [69]. This work demonstrated that cancer patients presented different urinary VOC profiles from non-cancer controls, although the authors did not discuss compound identification. Finally, one study performed urine analysis coupling a headspace sampler, a programed temperature vaporizer and a mass-spectrometer (HS-PTV-MS) [70]. In this way, the analytes were introduced directly into the MS, which allows one to obtain a volatile fingerprint of the samples, although no biomarkers were identified [70].
An overview of the reported studies and their respective detection methods and possible biomarkers are listed in alphabetical order of first authors in Table 1. Details on biomarkers will be discussed in the following section.
Table 1.
Summary of the analytical methods and the possible urinary volatile biomarkers (alphabetical order).
| First author (Year) | Cancer type | Method | Possible biomarkers (Number of VOCs) | Ref |
|---|---|---|---|---|
| Arasaradnam (2014) | Colorectal | FAIMS and GC–MS | Not reported | [71] |
| Cauchi (2016) | Bladder | SPME-GC–MS | (16): 2,3-Butanedione; 2-Butanone; 2-Pentanone; 2-Propanol; 3-Hydroxyanthranilic acid; 4-Heptanone; Acetic acid; Benzaldehyde; Benzoic acid; Butyrophenone; cis-3-Hexanoic acid; Dimethyl disulfide; Hexanal; Piperitone; Thujone; trans-3-Hexanoic acid. | [72] |
| Gao (2019) | Prostate | SBSE-GC–MS | (11): 1-(2,4-Dimethylphenyl)-3-(tetrahydrofuryl-2)propane;1,1,1,5,5,5-hexamethyl-3,3-bis[(trimethylsilyl)oxy]-Trisiloxane; 1,1,3,3,5,5,7,7,9,9-decamethyl-pentasiloxane; 1-Propylpentachlorotriphosphazene; 2,6-Di-t-butyl-4-hydroxymethylene-2,3,5,6-detetrahydrocyclohexanone; 2-Amino-Imidazole-5-carboxylic acid; 4-(3,4-dihydro-2,2,4-trimethyl-2H-1-benzopyran-4-yl)-phenol; 4-Nitro-4′-chlorodiphenylsulfoxide; Estradiol; Ethyl à-hydroxymyristate trisiloxane; Phthalic acid, bis(7-methyloctyl) ester. | [61] |
| Guadagni (2011) | Lung | SPME-GC–MS | Hexanal | [73] |
| Hanai (2012) | Lung | SPME-GC–MS | (4): 2-Ethyl-1-hexanol; 2-Methylpyrazine; 2-Pentanone; Tetrahydrofuran | [74] |
| Hua (2018) | Lymphoma | SPME-GC–MS | (5): 2,6-Dimethyl-7-octen-2-ol; 2-Methylbutanal; 2-Methylpyrazine; 4-Heptanone; Decanoic acid. | [75] |
| Huang (2013) | Gastroesophageal | SIFT-MS | (7): Acetaldehyde; Acetic Acid; Acetone; Hexanoic Acid; Hydrogen Sulfide; Methanol; Phenol | [67] |
| Jiménez-Pacheco (2018) | Prostate | SPME-GC–MS | (9): 2,6-Dimethyl-7-octen-2-ol; 2-Butanone; 2-Ethylhexanol; 3,5-Dimethylbenzaldehyde; 3-Methylphenol; Furan; Phenol; P-xylene; Santolina Triene. | [76] |
| Jobu (2012) | Bladder | NTME-GC–MS | (6): Ethylbenzene; Nonanoyl chloride; Dodecanal; 2-Nonenal; 5-Dimethyl-3(2H)- Isoxazolone. | [58] |
| Khalid (2015) | Prostate | SPME-GC–MS | (4): 2,6-Dimethyl-7-octen-2-ol; 2-Octanone; 3-Octanone; Pentanal. | [77] |
| Lima (2019) | Prostate | SPME-GC–MS | (6): 2,5-Dimethylbenzaldehyde; 3-Phenylpropionaldehyde; 4-Methylhexan-3-one; Dihydroedulan IA; Hexanal; Methylglyoxal. | [78] |
| McFarlane (2019) | Colorectal | LC-FAIMS-MS | Not reported | [69] |
| Monteiro (2017) | Renal Cell Carcinoma | SPME-GC–MS | (2): 2-Oxopropanal; 2,5,8-Trimethyl-1,2,3,4-tetrahydronaphthalene-1-o. | [79] |
| Navaneethan (2015) | Biliary Duct | SIFT-MS | (3): 2-Propanol; Carbon disulfide; Trimethyl amine | [68] |
| Opitz (2018) | Head and Neck | SPME-GC–MS | (35): 2-Methyl-5-(methylthio) furan; 2-Methylbutanal; 2-Methyl-butyric acid; 2-Methylthiophene; 3,4-Dehydro-β-ionone; 3,4-Dimethyl-2, 5-furanedione; 3-Heptanone; 3-Methyl-2-heptanone; 4-Methyl-2-heptanone; 4-Tert-butylphenolpheno; Acetone; Benzene; Dimethyl disulfide; Dimethyl trisulfide; Ethanoic acid; Ethylbenzene; Furan; Heptanal; Hexanal; Linalool; m-Cresol; Nonanal; Phenol; Styrene; Tetrahydro-2, 2-dimethyl-5-(1-methyl −1-propenyl) furan; Tetrahydro2,2,5,5-tetramethylfuran; Thiophene; α-Terpineol. | [80] |
| Porto-Figueira (2018)b | Lung | NTME-GC–MS | (29): 1,1,3-Trimethyl-1H-indene; 1,2,3-Trimethylbenzene; 1,4-Cineole; 1,6-Dimethylhepta-1,3,5-triene; 1-Ethyl-3-methylbenzene; 2,2,6-Trimethyl-6-vinyltetrahydropyran; 2,3-dihydro-1,1,5,6-tetramethyl-1H-indene; 2,4-Dimethyl-3-pentanone; 2-Butanone; 2-Ethyl-5-methylfuran; 2-Heptanone; 3,3-Dimethyl-6-methylenecyclohexene; 3,5-Di-t-butylphenol; 3-Hexanone; 4-tert-Butylphenol; Acetaldehyde; Acetone; Carbon disulfide; Carvacrol; Dimethyl sulfde; Hexanal; Isoterpinolene; Methyl chloride; p-Cresol; Tiophene; α-Calacorene; α-Curcumene; α-Phellandrene; α-Terpinene. | [57] |
| Porto-Figueira (2018)a | Breast | NTME-GC–MS | (53):1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one; 1-(4-methoxyphenyl)-1,3-butanedione; 1,2,3,4-tetrahydro-1,4,6-trimethylnaphthalene; 1,2,3,4-tetrahydro-1,5,8-trimethylnaphthalene; 1,2,3-Trimethylbenzene; 1,2,5,5,6,7-Hexamethylbicyclo[4.1.0]hept-2-en-4-one; 1,2,5,5-Tetramethyl-1,3-cyclopentadiene; 1,2-Dihydro-1,5,8-trimethylnaphthalene; 1,4-Cineole; 1,5,5-Trimethyl-6-methylene-cyclohexene; 1,6,7-Trimethylnaphthalene; 1,6-Dimethylhepta-1,3,5-triene; 2,20-Ethylidenebis(5-methylfuran); 2-Acetyl-6-methoxynaphthalene; 2-Acetylfuran; 2-Bromophenol; 2-Methylbutanal; 2-Methylfuran; 2-Pentylfuran; 3,3-Dimethyl-6-methylenecyclohexene; 3-Hexanone; 3-Methylfuran; 4-Heptanone; 4-tert-Butyl-2-Bromophenol; 4-tert-Butylphenol; 6-methyllilolidine; 7,7-Dimethyl-9-oxatricyclo[6.2.2.0(1,6)]dodecan-10-one; 9-Methyl-S-octahydrophenanathracene; Benzene, 1,2,3,4-tetramethyl-5-(1-methylethyl)-; Benzoic Acid; Butanal; Carbon disulfide; Cyclohexene, 5-methyl-3-(1-methylethenyl)-, trans-(-)-; Dehydro-Ar-ionene; Dehydro-b-ionone; Dihydromyrcenol; Dimethyl disulfide; Ethanone, 1-(2,4,5-triethylphenyl)-; Ethyl ether; Furan; Guaiacol; Lavender lactone; m-Anisalcohol; Octanoic Acid; o-Cymene; p-Cresol; Pentane; Phenol; Thiophene; Trans-2-Methyl-1,3-pentadiene; α–Curcumene; α–Terpinene; γ-Terpinene. | [56] |
| Colon | NTME-GC–MS | (41): 1-(4-methoxyphenyl)-1,3-butanedione; 1,2,3-Trimethylbenzene; 1,2,5,5,6,7-Hexamethylbicyclo[4.1.0]hept-2-en-4-one; 1,2,5,5-Tetramethyl-1,3-cyclopentadiene; 1,3-Dimethyl-1-cyclohexene; 1,5,5-Trimethyl-6-methylene-cyclohexene; 1,6,7-Trimethylnaphthalenel; 1,6-Dimethylhepta-1,3,5-triene; 2,2,6-Trimethylcyclohexanone; 2,4-Dimethyl-3-pentanone; 2-Acetylfuran; 2-Ethyl-5-methylfuran; 2-methoxy-5-methyl-Thiophene; 2-methyl-5-(methylthio)furan; 2-Methylfuran; 2-Pentylfuran; 3,3-Dimethyl-6-methylenecyclohexene; 3,5-Di-t-butylphenol; 3-Hexanone; 3-Methylfuran; 4-tert-Butyl-2-Bromophenol; 4-tert-Butylphenol; 5-Methylfurfural; 7,7-Dimethyl-9-oxatricyclo[6.2.2.0(1,6)]dodecan-10-one; Butanal; Carbon disulphide; Dehydro-Ar-ionene; Dimethyl disulfide; Dimethyl sulphide; Ethanone, 1-(2,4,5-triethylphenyl)-; Ethyl ether; Furan; Guaiacol; Isoprene; m-Anisalcohol; Methanethiol; Methyl allyl disulphide; p-Cresol; Pentane; Phenol; Thiophene. | ||
| Ramos (2017) | Lung | HS-PTV-MS | Not reported | [70] |
| Santos (2017) | Lung | HS-GC–MS | (3): 3-Heptanone; 3-Octanone; Ethyl acetate. | [81] |
| Silva (2011) | Colorectal, Leukemia, Lymphoma | SPME-GC–MS | (17):1,2,4-Trimethylbenzene; 1,2-Dihydro-1,1,6-trimethyl-naphthalene; 1,4,5-Trimethyl-naphthalene; 1-Octanol; 2,7-Dimethyl-quinoline; 2-Methoxythiophene; 2-Methyl-3-phenyl-2-propenal; 3-Heptanone; 4-Methyl-pheno; 4-Methyl-phenol; Anisole; Bornylene; Dimethyl disulfide; Heptanal; Hexanal; p-Cymene γ -Terpinene | [82] |
| Silva (2012) | Breast | SPME-GC–MS | (6): 4-Carene; 1,2,4-Trimethylbenzene; 2-Methoxytiophene; 3-Heptanone; Dimethyl disulfide; Phenol. | [83] |
| Silva (2019) | Breast | SPME-GC–MS | (10): 2-Methyl-3-phenyl-2-propenal; 3-Methyl-thiophene; 1,2-Dihydro-1,1,6-trimethylnaphthalene; 1-Methyl-4-(1- methylethenyl)-benzene; 2-pentylfuran; 4-Heptanone; Acetic acid; p-Cymene; Trimethyl trisulfde; α-Terpinene. | [84] |
| Taunk (2018) | Breast | SPME-GC–MS | (14): 1,4-Dimethylpent-2-enylbenzene; 1–4-Hydroxy-3,5-di-tert-butylphenyl-2- methyl-3-morpholinopropan-1-one; 2,2,7,7-Tetramethyltricyclo[6.2.1.0 (1,6)]undec-4-en-3-one; 2-Ethyl-1-hexanol; Acetic acid; Dimethyl trisufide; Dodecanoic acid; Furan; Guaiacol; Isolongifolenone; m-Cresol; p-Cresol; Phenol; Ylangene | [85] |
| Taware (2017) | Head and Neck | SPME-GC–MS | (4): 4-methyl-2-heptanone; 1-butanol; 2,6-dimethyl-7-octen-2-ol; p-xylene | [86] |
| Wang (2016) | Renal Cell Carcinoma | SPME-GC–MS | (14): 1,6‑Dioxacyclododecane‑7,12‑dione; 1‑bromo‑1‑(3‑methyl‑1‑pentenylidene)‑2,2,3,3‑tetramethyl-cyclopropane; 2,5‑Cyclohexadiene‑1,4‑dione, 2,6‑bis(1,1‑dimethylethyl)‑; 2,6,10,14‑Tetramethyl‑pentadecane; 3‑Ethyl‑3‑methylheptane; 4-heptanone; Aniline; Decanal; Dimethyl‑silanediol; Isolongifolene‑5‑ol; Nonanal; Phenol; Styrene; Tetradecane. | [87] |
4.2. Urinary VOCs cancer biomarkers
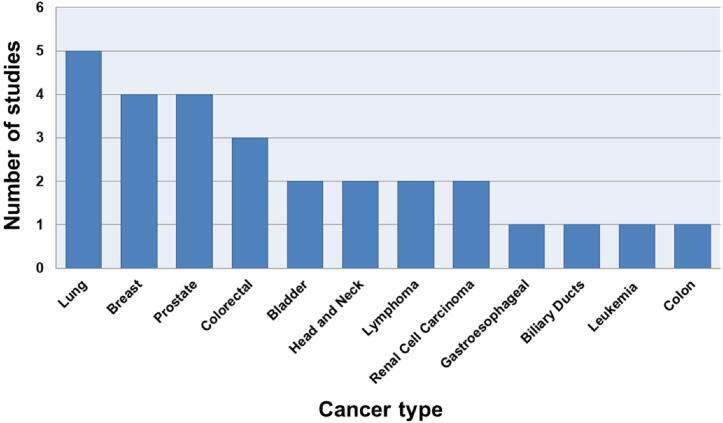
As shown in Table 1, 12 different types of cancer were evaluated in clinical studies through the analysis of urinary VOCs by MS-based techniques between January 2011 and May 2020. The most studied type of cancer was lung (5), followed by breast (4) and prostate (4).
These results are consistent with reports from the World Health Organization indicating that lung and breast are the two most common types of cancer, and prostate is the fourth [1]. However, other high frequency types of cancers, such as ovarian, endometrial and thyroid were not reported. In order to better visualize the data, Fig. 1 presents the types of cancer and the number of studies in which they were reported. It is important to mention that two works evaluated more than one type of cancer: Porto-Figueira et al. (breast and colon) [56] and Silva et al. (colorectal, leukemia and lymphoma) [82]. Additionally, colon and colorectal were considered different types of cancer, according to Lee et al. [98].
Fig. 1.
Number of published cancer studies using VOCs between January 2011 and May 2020 according to cancer type.
A total of 188 different urinary VOCs were reported as possible cancer biomarkers, largely comprising ketones, aldehydes, carboxylic acids, alcohols, furans, phenols, sulfur compounds and hydrocarbons (mainly monoterpenes and benzene derivates). Twenty-two of the twenty-five evaluated studies contributed to the identification of biomarkers, with an average of approximately 14 clinically relevant substances per study. These substances were chosen as possible biomarkers because they were present in significantly different levels between the cancer patient group and control group. The statistical methods and criteria to identify the biomarkers can be found in the original studies.
The disparity in results between the groups can most likely be explained by two reasons. First, the lack of standardized procedures for VOC analysis and statistical treatment of data, which is a common problem and debated subject in the field of VOC assessment [26], [28], [32], [88]. Second, as already mentioned, urinary VOCs may vary according to the patients’ type and stage of cancer, and also according to their genetics, lifestyle and environmental exposures [51], [89].
However, despite a lack of standardization leading to divergent results, some VOCs consistently appear in several cancer types. To better interpret the biomarkers present in Table 1, we filtered the clinically-relevant substances that were reported in four or more types of cancer, or in four or more studies. These compounds are listed in Table 2 along with their Chemical Abstracts Service (CAS) number, and any cited concentration change (up for increased and down for decreased). Of the 188 different urinary VOCs reported as possible biomarkers, 16 were categorized as highly relevant. The three most frequent biomarkers were hexanal, dimethyl disulfide and phenol. As they are the most reported substances, they will be discussed later in more detail. However, it is relevant to mention that for obtaining an unambiguous diagnosis, it is also important to set a chemometric fingerprint consisting of multiple of substances, not just one compound [26].
Table 2.
Biomarkers identified in four or more types of cancer or in at least four studies.
| Substance | CAS | N° of cancer types | N° of studies | Cancer types | Concentration change | Reference |
|---|---|---|---|---|---|---|
| Hexanal | 66-25-1 | 7 | 6 | Bladder, Lung(2), Colorectal, Head and Neck, Leukemia, Lymphoma, Prostate, | Up 72,73,80 Down 78,82 |
[57], [72], [73], [78], [80], [82] |
| Dimethyl disulfide | 624-92-0 | 7 | 5 | Breast (2), Colon, Colorectal, Bladder, Head and Neck, Lymphoma, Leukemia | Up 80 Down 72,82,83 |
[56], [72], [80], [82], [83] |
| Phenol | 108-95-2 | 6 | 7 | Breast (3), Colon, Gastroesophageal, Head and Neck, Renal Cell Carcinoma, Prostate | Up 76, 80,83,85,87 Down 67 |
[56], [67], [83], [85], [87] |
| 3-Heptanone | 106-35-4 | 6 | 4 | Breast, Colorectal, Head and Neck Leukemia, Lymphoma , Lung | Up 80–83 | [80], [81], [82], [83] |
| 4-Heptanone | 123-19-3. | 4 | 5 | Bladder, Breast(2), Lymphoma, Renal Cell Carcinoma | Up 75,84 Down 72,87 |
[56], [72], [5], [84], [87] |
| 2-Butanone | 78-93-3 | 4 | 4 | Bladder, Head and Neck, Lung, Prostate | Up 72,76 Down 80 |
[57], [72], [76], [80] |
| Furan | 110-00-9 | 4 | 4 | Breast(2), Colon, Head and Neck, Prostate | Up 76,80,85 | [56], [76], [80], [85] |
| Carbon disulfide | 75-15-0 | 4 | 3 | Biliary Ducts, Breast, Colon, Lung | Down 68 | [56], [57], [68] |
| 4-tert-Butylphenol | 98-54-4 | 4 | 3 | Breast, Colon, Head and Neck, Lung | Up 80 | [56], [57], [80] |
| p-Cymene | 99-87-6 | 4 | 2 | Breast, Colorectal, Leukemia, Lymphoma | Up 82 Down 84 |
[82], [84] |
| Heptanal | 111-71-7 | 4 | 2 | Colorectal, Head and Neck, Leukemia, Lymphoma, | Up 80 Down 82 |
[80], [82] |
| 2-Methyl-3-phenyl-2-propenal | 101-39-3 | 4 | 2 | Breast, Leukemia, Lymphoma, Colorectal | Up 82 Down 84 |
[82], [84] |
| 1,2,4-Trimethylbenzene | 95-63-6 | 4 | 2 | Breast, Colorectal, Leukemia, Lymphoma | Up 82,83 | [82], [83] |
| γ-Terpinene | 99-85-4 | 4 | 2 | Breast, Colorectal, Leukemia, Lymphoma | Up 82 | [56], [82] |
| 2,6-Dimethyl-7-octen-2-ol | 18479-58-8 | 3 | 4 | Head and Neck, Lymphoma, Prostate (2) | Down 76,77,86 | [86], [75], [76], [77] |
| Acetic acid | 64-19-7 | 3 | 4 | Breast(2), Bladder, Gastroesophageal | Up 67,85 Down 72,84 |
[67], [72], [84], [85] |
Hexanal is a possible biomarker reported in 6 articles and related to 7 types of cancer. This compound is one of the main targets in lung cancer studies and is reported at significantly higher levels in different biological matrices, such as blood and breath when comparing cancer patients and healthy controls [26], [90]. Increased levels of hexanal and other aldehydes, in general, may be related to the elevated activity of ROS originating from cancer cells and their surrounding environment [11], [90]. Cauchi et al. [72], Guadagni et al. [73] and Opitz et al. [80], who evaluated bladder, lung, and head and neck cancer, respectively, presented statistically higher levels of hexanal in urine. However, Lima et al. [78] and Silva et al. [82], who evaluated prostate, and colorectal, lymphoma and leukemia, respectively, reported that hexanal was actually presented in significantly lower levels in cancer patients. Interestingly, the other aldehydes present in Table 2, follow the same trend: heptanal is reported in significantly increased levels in one study [80] and in decreased levels in another [82]; and the same with 2-Methyl-3-phenyl-2-propenal: increased in Silva et al. 2011 [82] and decreased in Silva et al. 2019 [84]. Hence, hexanal may be considered as a urinary VOC related to cancer, but more studies focused on pathway dysregulation are needed in order to investigate what is potentially causing the disparity between the levels of volatile aldehydes present in urine.
Dimethyl disulfide was presented as a possible biomarker in 7 of the 12 types of cancer reviewed and it was reported in 5 different studies. This substance is an example of a sulfur compound, a class that is highly present in urine and one of the main factors responsible for its odor [91], [92]. Volatile sulfur metabolites are produced mainly by incomplete metabolism of methionine in the transamination pathway, which is often down-regulated in cancer patients [83]. In this sense, according to Table 2, dimethyl disulfide was presented in statistically significantly lower levels in five cancer types (bladder, breast, colorectal, leukemia and lymphoma) [72], [82], [83] and increased in only one (head and neck) [80]. In addition, carbon disulfide, another sulfur volatile compound considered as a relevant biomarker [56], [57], [68], was reported to be present in lower levels in one study [68]. Thus, down-regulation of sulfur metabolites in urine may be a common feature of tumor growth. In this respect, dimethyl disulfide, as a VOC urinary cancer biomarker, is a good candidate for further study.
Finally, phenol was the most reported biomarker, being present in 7 studies [56], [67], [76], [80], [83], [84], [87] and related to 6 different types of cancer (breast, colon, gastroesophageal, head and neck, renal cell carcinoma and prostate). Phenols are one of the major chemical families identified in urine from oncologic groups [84] and their formation is considered to be related to the metabolism of tyrosine by the gut microbiota [50]. In addition, alterations in urinary levels of phenol may correlate with breast cancer, since three of the four reviewed articles mention this compound as a possible VOC biomarker [56], [83], [84].
In the next section, the three most reported cancer types presented in this review, lung, breast and prostate, will be discussed in greater depth.
4.2.1. Lung cancer
Four of the five reviewed lung cancer studies suggested possible biomarkers. In total, these four studies reported 36 different urinary VOC biomarkers and only hexanal was present in more than one report [57], [73]. High levels of this compound have been related to lung cancer in other matrices, such as blood [93] and breath [94], and also in studies that evaluated urinary VOCs through non MS-based analysis, such as Liu et al. [95]. Therefore, it is likely that significantly higher levels of hexanal may be related to lung cancer. However, elucidating the exact mechanism causing this elevation should be further studied, as in other types of cancers the levels of this aldehyde are significantly decreased [78], [82].
As hexanal was the only biomarker reported in more than one study, the results regarding urinary VOCs related to lung cancer are clearly inconsistent, despite it being the most studied type of cancer among those reviewed. This inconsistency could be related to the lack of standardized analysis, and/or limitations in the cohort size. The four works that suggested potential biomarkers utilized three different techniques: SPME-GC-MS [73], [74], NTME-GC-MS [57] and HS-GC-MS [81]. Only Santos et al. [81] used creatinine measurement to normalize dilution factors. Regarding the cohort size, the studies evaluated between 10 and 20 cancer patients, a relatively low number that may have contributed to the variance in results.
Regarding the methods’ ability to distinguish between lung cancer and control samples, the study that presented the best results was that of Ramos et al. [70]. Interestingly, this is the only work that did not suggest any biomarkers since they used a non-separative analysis based on HS-PTV-MS combined with pattern recognition techniques to obtain a “urinary odor fingerprint” of lung cancer. Their predictive model achieved 100% sensitivity and specificity.
The analytical method, cohort, sensitivity and specificity (if mentioned in the article) of the reviewed lung cancer studies have been summarized in Table 3 in alphabetical order.
Table 3.
Analytical method, cohort, sensitivity and specificity of the five reviewed lung cancer studies.
| First Author (year) | Method | Cohort | Sensitivity | Specificity | Reference |
|---|---|---|---|---|---|
| Guadagni (2011) | SPME-GC–MS | 10 cancer − 25 control | – | – | [73] |
| Hanai (2012) | SPME-GC–MS | 20 cancer − 20 control | 0.95–1 | 0.70–1 | [74] |
| Porto-Figueira (2018) | NTME-GC–MS | 17 cancer − 30 control | – | – | [57] |
| Ramos (2017) | HS-PTV-MS | 14 cancer − 24 control | 1 | 1 | [70] |
| Santos (2017) | HS-GC–MS | 12 cancer − 12 control | 0.75–1 | 0.80–1 | [81] |
4.2.2. Breast cancer
Breast cancer had the highest number of possible VOC biomarkers, with 73. Among them, 9 VOCs were reported in at least two studies: 2-pentylfuran [56], [84], 4-heptanone [56], [84], acetic acid [84], [85], dimethyl disulfide [56], [83], furan [56], [85], guaiacol [56], [85], p-cresol [56], [85] α-terpinene [56], [84] and phenol [56], [83], [85]. Phenol seems to be the most promising biomarker, since 3 of the 4 studies reported statistically higher levels of this compound in breast cancer samples. Additionally, in the only study where this substance was not considered a biomarker, phenol was also reported at elevated levels in the urine of cancer patients compared to controls [84]. The mechanism that leads to phenol production by cancer cells and/or its environment is not yet fully understood, but some studies indicate that it may be related to alterations in aromatic amino acid (mainly tyrosine) metabolism [50], [96], [97]. As mentioned previously, p-cresol, which is a methyl phenol produced mainly via gut microbiota degradation of tyrosine [99], was reported in significantly higher levels in two breast cancer studies [56], [85]. Therefore, there might be a link between breast cancer, tyrosine metabolism and higher levels of phenol and p-cresol in urine. However, the exact mechanisms that lead to these changes are still not well known.
The breast cancer articles reviewed appear to be from the same research group, with the only difference being the techniques utilized: NTME-GC–MS versus SPME-GC–MS. This may explain why it has more possible VOC biomarkers in common than the lung cancer studies. Yet, some inconsistencies are still present, such as acid acetic being up-regulated in Taunk et al. [85] and down-regulated in Silva et al. [84]. This may be due to the fact that in Taunk et al. [85] the samples were collected in India, and in Silva et al. [84] they were from Portugal, which reinforces that life habits and genetic factors influence the VOC profile and this should be further studied in depth with larger cohorts. Although the number of patients and controls in the breast cancer studies were larger than that of lung, they are still far from ideal.
The breast cancer articles reviewed do not focus their discussions on method sensitivity and specificity, but rather on which metabolic pathways were dysregulated in cancer samples. In fact, there are common metabolic pathways that are altered in two or more studies. For instance, the pyruvate pathway was reported to be up-regulated in two studies [84], [85]. Both studies cite acetic acid as a metabolite that has a central influence on this dysregulation; however, as mentioned previously, this VOC is reported as down-regulated in Silva et al. [84] and up-regulated in Taunk et al. [85], which emphasizes the need for further studies on the influence of genetics and lifestyle on the urinary VOC levels, and also further investigation between the relation of acetic acid levels and breast cancer. The synthesis and degradation of ketone bodies were also altered in two studies [83], [85], and the authors hypothesized this is due to the fact that cancer cells prefer to use ketone bodies as an energy source under hypoxic conditions. In addition, sulfur metabolism requires special attention since it was reported as up-regulated in 3 of the 4 reviewed studies [83], [84], [85]. Regarding the urinary sulfur compounds, dimethyl disulfide [83] and trimethyl trisulfide [84] were present in significantly lower levels in cancer patients, while dimethyl trisulfide was reported as up-regulated [85].
The analytical method, cohort, and the main metabolic alterations of the reviewed breast cancer studies are summarized in Table 4 in alphabetical order.
Table 4.
Analytical method, cohort, main metabolic alterations of the four reviewed breast cancer studies.
| First Author (year) | Method | Cohort | Main metabolic alterations | Reference |
|---|---|---|---|---|
| Porto-Figueira (2018)a | NTME-GC–MS | 30 cancer − 60 control | Dysregulation in phenylalanine pathway, butanoate metabolism, and xenobiotics metabolism by cytochrome P450 | [56] |
| Silva (2012) | SPME-GC–MS | 26 cancer − 21 control | Dysregulation in ketones and sulfur compounds metabolism | [83] |
| Silva (2019) | SPME-GC–MS | 31 cancer − 40 control | Dysregulation in pyruvate pathway and sulfur compounds metabolism | [84] |
| Taunk | SPME-GC–MS | 65 cancer − 70 control | Dysregulation in pyruvate pathway, sulfur, ketones and tyrosine metabolism, and fatty acid biosynthesis | [85] |
4.2.3. Prostate cancer
In total, 29 urinary VOCs were reported as possible biomarkers in the four reviewed prostate cancer articles. As with the lung cancer studies, a lack of standardization may have been a primary main reason why only one biomarker was replicated between the prostate cancer reports. For instance, in a study carried out by Jiménez-Pacheco et al. [76] urine samples were collected from patients at different periods of the day, which could lead to differing patterns of VOCs, while also increasing the number of confounding factors.
The only compound repeated between them (i.e., 2,6-Dimethyl-7-octen-2-ol) appears to be an interesting biomarker for further evaluation, since in both studies [76], [77] this VOC is present in significantly lower levels in cancer patients. A possible explanation for this pattern is that cancerous cells may be using this metabolite as an energy source [77]. However, the presence of 2,6-Dimethyl-7-octen-2-ol may be also due to a contamination since this compound is a common surface cleaner [100].
Currently, prostate-specific antigen (PSA) is considered the most important biomarker for prostate cancer diagnosis, despite its low specificity [76]. There is a need to identify a more suitable biomarker for diagnosis of this disorder, and metabolomics is one of the most promising approaches [78]. With this in mind, the reviewed studies are generally focused on urinary VOC metabolites and the comparison of their models to the PSA results. Three of the four studies developed VOC-based models with this purpose: Khalid et al. [77], Lima et al. [78] and Gao et al. [61].
The method of Khalid et al. was based on 4 VOCs (i.e., 2,6-dimethyl-7-octen-2-ol, 3-octanone, 2-octanone and pentanal), obtaining an accuracy of 63% to 65%; a value slightly better than when they using PSA alone (62–64%). When combining PSA levels and the four VOCs, the accuracy was increased (65–74%). The method of Lima et al. [78] was able to identify prostate cancer with a sensitivity of 89%, specificity of 83%, and accuracy of 86%, and was based on 6 VOCs (i.e., 2,5-dimethylbenzaldehyde, 3-phenylpropionaldehyde, 4-methylhexan-3-one, dihydroedulan IA, hexanal and methyloxal). Gao et al. [61] developed a urinary VOC model based on 11 VOCs (see Table 1) to detect prostate cancer with a higher accuracy (87%) than PSA alone (59%).
Finally, Jiménez-Pacheco et al. [76] did not compare their VOC-based method with PSA results, but compared prostate cancer patients samples with benign prostatic hyperplasia patients, and reported significant differences between urinary furan and p-xylene levels from both groups before and after prostate massage, supporting the proposal that VOCs may serve as prostate cancer biomarkers.
The summary of the reviewed prostate cancer studies are presented in alphabetical order in Table 5.
Table 5.
Analytical method, cohort, sensitivity, specificity and accuracy of the five reviewed prostate cancer studies.
| First Author (year) | Method | Cohort | Sensitivity | Specificity | Accuracy | Reference |
|---|---|---|---|---|---|---|
| Gao (2019) | SBSE-GC–MS | 55 cancer − 53 control | 0.96 | 0.8 | 87% | [61] |
| Jiménez-Pacheco (2018) | SPME-GC–MS | 29 cancer − 21 control | – | – | – | [76] |
| Khalid (2015) | SPME-GC–MS | 59 cancer − 43 control | 0.8 | 0.57 | 63–65% | [77] |
| Lima (2019) | SPME-GC–MS | 40 cancer − 42 control | 0.89 | 0.93 | 86% | [78] |
5. Conclusion and perspectives
The assessment of urinary VOCs using MS-based methods has the potential to be applied in cancer diagnosis. Over the past 10 years, 25 articles that conducted these types of analyses were reported. In total, 12 different types of cancer were evaluated, with lung (5 studies), breast (4 studies) and prostate (4 studies) being the most studied. There are a variety of other prevalent cancers that were not evaluated, such as ovarian, endometrial and thyroid. Thus, there is a coverage gap that other future studies may explore.
Regarding cancer diagnosis, a total of 188 different urinary VOCs were reported as possible biomarkers, and 16 were considered most relevant since they appeared in four or more types of cancer, or in at least four studies. Among these 16 compounds, the most frequent were hexanal, dimethyl disulfide and phenol. It is very likely that significantly higher levels of hexanal may be related to lung cancer. Phenol seems to be closely related to breast cancer. Additionally, when evaluating the alterations in metabolic pathways related to breast cancer, sulfur metabolism requires special attention. Regarding prostate cancer studies, it is interesting to note that all VOC models presented accuracy that was moderately better than PSA alone; it is clear that these results could be further improved, given that Cornu et al. used the olfactory detection power of trained dogs to distinguish between 33 urine samples of prostate cancer volunteers and 33 controls, and obtained 91% of specificity and sensitivity [39]. In another similar study with a cohort of 902 urine samples of prostate cancer patients and 540 controls, trained dogs achieved a sensitivity of 98.6–100% and specificity of 97.6–98.7% [47].
There are still some points that need to be improved so that MS-based techniques can be applied, with confidence, at the clinical level. First, GC–MS is still the gold standard method to analyze VOC biomarkers. However, it is not an easy technique to implement in the clinical setting because it is expensive and not portable. Thus, more studies need to be done concerning MS-based techniques that are less expensive and allow real-time monitoring, such as SIFT-MS. In addition, methods must be standardized from collection to data processing, and performed with larger cohorts, preferably with patients from different countries and/or ethnicities, in order to better evaluate the influence of confounding factors, such as diet, medication and genetics. Finally, the pathways affecting VOC production and consumption are not clear and could use elucidation in order to provide more detailed information on the potential mechanisms of cancers, which could be useful in development of treatments.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), process number 88882.332037/2019-01.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization, WHO, Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer, 2018 (Accessed 09 May 2020).
- 2.World Health Organization. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. Technical Report. World Health Organization: Geneva, Switzerland, 2020.
- 3.Schmidt K., Podmore I. Current challenges in volatile organic compounds analysis as potential biomarkers of cancer. J. Biomarker. 2015 doi: 10.1155/2015/981458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou B., Xu J.W., Cheng Y.G., Gao J.Y., Hu S.Y., Wang L., Zhan H.X. Early detection of pancreatic cancer: Where are we now and where are we going? Int. J. Cancer. 2017;141(2):231–241. doi: 10.1002/ijc.30670. [DOI] [PubMed] [Google Scholar]
- 5.Nardi-Agmon I., Peled N. Exhaled breath analysis for the early detection of lung cancer: recent developments and future prospects. Lung Cancer. Targets Ther. 2017;8:31. doi: 10.2147/LCTT.S104205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monedeiro F., dos Reis R.B., Peria F.M., Sares C.T.G., De Martinis B.S. Investigation of sweat VOC profiles in assessment of cancer biomarkers using HS-GC-MS. J. Breath Res. 2020;14(2):026009. doi: 10.1088/1752-7163/ab5b3c. [DOI] [PubMed] [Google Scholar]
- 7.Dixit C.K., Kadimisetty K., Otieno B.A., Tang C., Malla S., Krause C.E., Rusling J.F. Electrochemistry-based approaches to low cost, high sensitivity, automated, multiplexed protein immunoassays for cancer diagnostics. Analyst. 2016;141(2):536–547. doi: 10.1039/C5AN01829C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton C., Ma Y. Current trends in cancer biomarker discovery using urinary metabolomics: achievements and new challenges. Curr. Med. Chem. 2019;26(1):5–28. doi: 10.2174/0929867324666170914102236. [DOI] [PubMed] [Google Scholar]
- 9.Patel S., Ahmed S. Emerging field of metabolomics: big promise for cancer biomarker identification and drug discovery. J. Pharm. Biomed. Anal. 2015;107:63–74. doi: 10.1016/j.jpba.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Amann A., de Lacy Costello B., Miekisch W., Schubert J., Buszewski B., Pleil J., Ratcliffe N., Risby T. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014;8(3) doi: 10.1088/1752-7155/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 11.Broza Y.Y., Mochalski P., Ruzsanyi V., Amann A., Haick H. Hybrid volatolomics and disease detection. Angew. Chem. Int. Ed. 2015;54(38):11036–11048. doi: 10.1002/anie.201500153. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa J.M.G., Pereira N.Z., David L.C., et al. Cerumenogram: a new frontier in cancer diagnosis in humans. Sci. Rep. 2019;9:11722. doi: 10.1038/s41598-019-48121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansurova M., Ebert B.E., Blank L.M., Ibáñez A.J. A breath of information: the volatilome. Curr. Genet. 2018;64(4):959–964. doi: 10.1007/s00294-017-0800-x. [DOI] [PubMed] [Google Scholar]
- 14.Behera B., Joshi R., Vishnu G.K.A., Bhalerao S., Pandya H.J. Electronic-nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019;13:024001. doi: 10.1088/1752-7163/aafc77. [DOI] [PubMed] [Google Scholar]
- 15.Smolinska A., Bodelier A.G.L., Dallinga J.W., Masclee A.A.M., Jonkers D.M., van Schooten F.J., Pierik M.J. The potential of volatile organic compounds for the detection of active disease in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2017;45(9):1244–1254. doi: 10.1111/apt.14004. [DOI] [PubMed] [Google Scholar]
- 16.van Vliet D., Smolinska A., Jöbsis Q., Rosias P., Muris J., Dallinga J., Dompeling E., van Schooten F.-J. Can exhaled volatile organic compounds predict asthma exacerbations in children? J. Breath Res. 2017;11(1):016016. doi: 10.1088/1752-7163/aa5a8b. [DOI] [PubMed] [Google Scholar]
- 17.Allers M., Langejuergen J., Gaida A., Holz O., Schuchardt S., Hohlfeld J.M., Zimmermann S. Measurement of exhaled volatile organic compounds from patients with chronic obstructive pulmonary disease (COPD) using closed gas loop GC-IMS and GC-APCI-MS. J. Breath Res. 2016;10(2) doi: 10.1088/1752-7155/10/2/026004. [DOI] [PubMed] [Google Scholar]
- 18.Baranska A., Mujagic Z., Smolinska A., Dallinga J.W., Jonkers D.M.A.E., Tigchelaar E.F., Wijmenga C.J., Dekens A., Zhernakova T., Ludwig A.A.M., Masclee C., Wijmenga F.J. van Schooten, Volatile organic compounds in breath as markers for irritable bowel syndrome: a metabolomic approach. Aliment. Pharmacol. Ther. 2016;44(1):45–56. doi: 10.1111/apt.13654. [DOI] [PubMed] [Google Scholar]
- 19.Rudnicka J., Kowalkowski T., Buszewski B. Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer. 2019;135:123–129. doi: 10.1016/j.lungcan.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Markar S.R., Chin S.T., Romano A., Wiggins T., Antonowicz S., Paraskeva P., Ziprin P., Darzi A., Hanna G.B. Breath volatile organic compound profiling of colorectal cancer using selected ion flow-tube mass spectrometry. Ann. Surg. 2019;269(5):903–910. doi: 10.1097/SLA.0000000000002539. [DOI] [PubMed] [Google Scholar]
- 21.Shigeyama H., Wang T., Ichinose M., Ansai T., Lee S.W. Identification of volatile metabolites in human saliva from patients with oral squamous cell carcinoma via zeolite-based thin-film microextraction coupled with GC–MS. J. Chromatogr. B. 2019;1104:49–58. doi: 10.1016/j.jchromb.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Bond A., Greenwood R., Lewis S., Corfe B., Sarkar S., O'Toole P., Probert C. Volatile organic compounds emitted from faeces as a biomarker for colorectal cancer. Aliment. Pharmacol. Ther. 2019;49(8):1005–1012. doi: 10.1111/apt.15140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filipiak W., Mochalski P., Filipiak A., Ager C., et al. A compendium of volatile organic compounds (VOCs) released by human cell lines. Curr. Med. Chem. 2016;23(20):2112–2131. doi: 10.2174/0929867323666160510122913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusano M., Mendez E., Furton K.G. Comparison of the volatile organic compounds from different biological specimens for profiling potential. J. Forensic. Sci. 2013;58(1):29–39. doi: 10.1111/j.1556-4029.2012.02215.x. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal S.M. VOCC: a database of volatile organic compounds in cancer. RSC Adv. 2016;6(115):114783–114789. doi: 10.1039/C6RA24414A. [DOI] [Google Scholar]
- 26.Saalberg Y., Wolff M. VOC breath biomarkers in lung cancer. Clin. Chim. Acta. 2016;459:5–9. doi: 10.1016/j.cca.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Krilaviciute A., Heiss J.A., Leja M., Kupcinskas J., Haick H., Brenner H. Detection of cancer through exhaled breath: a systematic review. Oncotarget. 2015;6(36):38643. doi: 10.18632/oncotarget.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanna G.B., Boshier P.R., Markar S.R., Romano A. Accuracy and methodologic challenges of volatile organic compound–based exhaled breath tests for cancer diagnosis: A systematic review and meta-analysis. JAMA Oncol. 2019;5(1) doi: 10.1001/jamaoncol.2018.2815. e182815-e182815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pérez-Rambla C., Puchades-Carrasco L., García-Flores M., Rubio-Briones J., López-Guerrero J.A., Pineda-Lucena A. Non-invasive urinary metabolomic profiling discriminates prostate cancer from benign prostatic hyperplasia. Metabolomics. 2017;13(5):52. doi: 10.1007/s11306-017-1194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan A.W., Mercier P., Schiller D., Bailey R., Robbins S., Eurich D.T., Broadhurst D. 1 H-NMR urinary metabolomic profiling for diagnosis of gastric cancer. Br. J. Cancer. 2016;114(1):59–62. doi: 10.1038/bjc.2015.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen C., Sun Z., Chen D., Su X., Jiang J., Li G., Yan J. Developing urinary metabolomic signatures as early bladder cancer diagnostic markers. OMICS J. Integr. Biol. 2015;19(1):1–11. doi: 10.1089/omi.2014.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Lena M., Porcelli F., Altomare D.F. Volatile organic compounds as new biomarkers for colorectal cancer: a review. Colorectal Dis. 2016;18(7):654–663. doi: 10.1111/codi.13271. [DOI] [PubMed] [Google Scholar]
- 33.Chin S.T., Romano A., Doran S.L., Hanna G.B. Cross-platform mass spectrometry annotation in breathomics of oesophageal-gastric cancer. Sci. Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-22890-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Španěl P., Smith D. Quantification of volatile metabolites in exhaled breath by selected ion flow tube mass spectrometry, SIFT-MS. Clin. Mass Spectrom. 2020;16:18–24. doi: 10.1016/j.clinms.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou W., Huang C., Zou X., Lu Y., Shen C., Ding X., Chu Y. Exhaled breath online measurement for cervical cancer patients and healthy subjects by proton transfer reaction mass spectrometry. Anal. Bioanal. Chem. 2017;409(23):5603–5612. doi: 10.1007/s00216-017-0631-0. [DOI] [PubMed] [Google Scholar]
- 36.Westenbrink E., Arasaradnam R.P., O'Connell N., Bailey C., Nwokolo C., Bardhan K.D., Covington J.A. Development and application of a new electronic nose instrument for the detection of colorectal cancer. Biosens. Bioelectron. 2015;67:733–738. doi: 10.1016/j.bios.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 37.Zhu S., Corsetti S., Wang Q., Li C., Huang Z., Nabi G. Optical sensory arrays for the detection of urinary bladder cancer-related volatile organic compounds. J. Biophotonics. 2019;12(10) doi: 10.1002/jbio.201800165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Goor R.M., Hardy J.C., van Hooren M.R., Kremer B., Kross K.W. Detecting recurrent head and neck cancer using electronic nose technology: A feasibility study. Head Neck. 2019;41(9):2983–2990. doi: 10.1002/hed.25787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornu J.N., Cancel-Tassin G., Ondet V., Girardet C., Cussenot O. Olfactory detection of prostate cancer by dogs sniffing urine: a step forward in early diagnosis. Eur. Urol. 2011;59(2):197–201. doi: 10.1016/j.eururo.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Rudnicka J., Walczak M., Kowalkowski T., Jezierski T., Buszewski B. Determination of volatile organic compounds as potential markers of lung cancer by gas chromatography–mass spectrometry versus trained dogs. Sens. Actuators, B. 2014;202:615–621. doi: 10.1016/j.snb.2014.06.006. [DOI] [Google Scholar]
- 41.Willis C.M., Britton L.E., Harris R., Wallace J., Guest C.M. Volatile organic compounds as biomarkers of bladder cancer: Sensitivity and specificity using trained sniffer dogs. Cancer Biomarkers. 2011;8(3):145–153. doi: 10.3233/CBM-2011-0208. [DOI] [PubMed] [Google Scholar]
- 42.Dent A.G., Sutedja T.G., Zimmerman P.V. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 2013;5(Suppl 5):S540. doi: 10.3978/j.issn.2072-1439.2013.08.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks S.W., Moore D.R., Marzouk E.B., Glenn F.R., Hallock R.M. Canine olfaction and electronic nose detection of volatile organic compounds in the detection of cancer: a review. Cancer Invest. 2015;33(9):411–419. doi: 10.3109/07357907.2015.1047510. [DOI] [PubMed] [Google Scholar]
- 44.D'Amico A., Di Natale C., Falconi C., Martinelli E., Paolesse R., Pennazza G., Sterk P.J. Detection and identification of cancers by the electronic nose. Expert Opin. Med. Diagn. 2012;6(3):175–185. doi: 10.1517/17530059.2012.665870. [DOI] [PubMed] [Google Scholar]
- 45.Casas-Ferreira A.M., del Nogal-Sánchez M., Pérez-Pavón J.L., Moreno-Cordero B. Non-separative mass spectrometry methods for non-invasive medical diagnostics based on volatile organic compounds: A review. Anal. Chim. Acta. 2019;1045:10–22. doi: 10.1016/j.aca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Phillips M., Cataneo R.N., Cruz-Ramos J.A., Huston J., Ornelas O., Pappas N., Pathak S. Prediction of breast cancer risk with volatile biomarkers in breath. Breast Cancer Res. Treat. 2018;170(2):343–350. doi: 10.1007/s10549-018-4764-4. [DOI] [PubMed] [Google Scholar]
- 47.Taverna G., Tidu L., Grizzi F., Torri V., Mandressi A., Sardella P., Hurle R. Olfactory system of highly trained dogs detects prostate cancer in urine samples. J. Urol. 2015;193(4):1382–1387. doi: 10.1016/j.juro.2014.09.099. [DOI] [PubMed] [Google Scholar]
- 48.Niemi R.J., Roine A.N., Eräviita E., Kumpulainen P.S., Mäenpää J.U., Oksala N. FAIMS analysis of urine gaseous headspace is capable of differentiating ovarian cancer. Gynecol. Oncol. 2018;151(3):519–524. doi: 10.1016/j.ygyno.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Haick H., Broza Y.Y., Mochalski P., Ruzsanyi V., Amann A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014;43(5):1423–1449. doi: 10.1039/C3CS60329F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Lacy Costello B., Amann A., Al-Kateb H., Flynn C., Filipiak W., Khalid T., Ratcliffe N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014;8(1) doi: 10.1088/1752-7155/8/1/014001. [DOI] [PubMed] [Google Scholar]
- 51.Shirasu M., Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011;150(3):257–266. doi: 10.1093/jb/mvr090. [DOI] [PubMed] [Google Scholar]
- 52.Liebich H.M. Gas chromatographic-mass spectrometric determination of total 4-heptanone, a new marker in diabetes mellitus. J. Chromatogr. B Biomed. Sci. Appl. 1983;273(1):67–75. doi: 10.1016/S0378-4347(00)80923-8. [DOI] [PubMed] [Google Scholar]
- 53.Bravo M., Valenzuela A., Fuentes E. Critical evaluation of fiber coatings for organotin determination by using solid phase microextraction in headspace mode. J. Chromatogr. A. 2012;1223:9–14. doi: 10.1016/j.chroma.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 54.Augusto F., Valente A.L.P. Applications of solid-phase microextraction to chemical analysis of live biological samples. TrAC. Trends Anal. Chem. 2002;21(6–7):428–438. doi: 10.1016/S0165-9936(02)00602-7. [DOI] [Google Scholar]
- 55.Arthur C.L., Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990;62(19):2145–2148. [Google Scholar]
- 56.Porto-Figueiraa P., Pereira J.A., Câmara J.S. Exploring the potential of needle trap microextraction combined with chromatographic and statistical data to discriminate different types of cancer based on urinary volatomic biosignature. Anal. Chim. Acta. 2018;1023:53–63. doi: 10.1016/j.aca.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 57.Porto-Figueirab P., Pereira J., Miekisch W., Câmara J.S. Exploring the potential of NTME/GC-MS, in the establishment of urinary volatomic profiles. Lung cancer patients as case study. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-31380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jobu K., Sun C., Yoshioka S., Yokota J., Onogawa M., Kawada C., Miyamura M. Metabolomics study on the biochemical profiles of odor elements in urine of human with bladder cancer. Biol. Pharm. Bull. 2012;35(4):639–642. doi: 10.1248/bpb.35.639. [DOI] [PubMed] [Google Scholar]
- 59.Lord H.L., Zhan W., Pawliszyn J. Fundamentals and applications of needle trap devices. Anal. Chim. Acta. 2010;677:3–18. doi: 10.1016/j.aca.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 60.Trefz P., Kischkel S., Hein D., James E.S., Schubert J.K., Miekisch W. Needle trap micro-extraction for VOC analysis: effects of packing materials and desorption parameters. J. Chromatogr. A. 2012;1219:29–38. doi: 10.1016/j.chroma.2011.10.077. [DOI] [PubMed] [Google Scholar]
- 61.Gao Q., Su X., Annabi M.H., Schreiter B.R., Prince T., Ackerman A., Lee W.Y. Application of urinary volatile organic compounds (VOCs) for the diagnosis of prostate cancer. Clin. Genitourin. Cancer. 2019;17(3):183–190. doi: 10.1016/j.clgc.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Kawaguchi M., Takatsu A., Ito R., Nakazawa H. Applications of stir-bar sorptive extraction to food analysis. TrAC, Trends Anal. Chem. 2013;45:280–293. doi: 10.1016/j.trac.2013.01.007. [DOI] [Google Scholar]
- 63.A.D, Wilson, Recent applications of electronic-nose technologies for the noninvasive early diagnosis of gastrointestinal diseases. Proceedings (2018), 2, 147. 10.3390/ecsa-4-04918 [DOI] [PMC free article] [PubMed]
- 64.Scotter J.M., Langford V.S., Wilson P.F., McEwan M.J., Chambers S.T. Real-time detection of common microbial volatile organic compounds from medically important fungi by Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS) J. Microbiol. Methods. 2005;63(2):127–134. doi: 10.1016/j.mimet.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 65.Smith D., Španěl P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005;24(5):661–700. doi: 10.1002/mas.20033. [DOI] [PubMed] [Google Scholar]
- 66.Smith D., Španěl P. Progress in SIFT-MS: Breath analysis and other applications. Mass Spectrom. Rev. 2011;30(2):236–267. doi: 10.1002/mas.20303. [DOI] [PubMed] [Google Scholar]
- 67.Huang J., Kumar S., Abbassi-Ghadi N., Španěl P., Smith D., Hanna G.B. Selected ion flow tube mass spectrometry analysis of volatile metabolites in urine headspace for the profiling of gastro-esophageal cancer. Anal. Chem. 2013;85(6):3409–3416. doi: 10.1021/ac4000656. [DOI] [PubMed] [Google Scholar]
- 68.Navaneethan U., Parsi M.A., Lourdusamy D., Grove D., Sanaka M.R., Hammel J.P., Dweik R.A. Volatile organic compounds in urine for noninvasive diagnosis of malignant biliary strictures: a pilot study. Dig. Dis. Sci. 2015;60(7):2150–2157. doi: 10.1007/s10620-015-3596-x. [DOI] [PubMed] [Google Scholar]
- 69.McFarlane M., Millard A., Hall H., Savage R., Constantinidou C., Arasaradnam R., Nwokolo C. Urinary volatile organic compounds and faecal microbiome profiles in colorectal cancer. Colorectal Dis. 2019;21(11):1259–1269. doi: 10.1111/codi.14739. [DOI] [PubMed] [Google Scholar]
- 70.Ramos Á.G., Antón A.P., del Nogal Sánchez M., Pavón J.L., Cordero B.M. Urinary volatile fingerprint based on mass spectrometry for the discrimination of patients with lung cancer and controls. Talanta. 2017;174:158–164. doi: 10.1016/j.talanta.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Arasaradnam R.P., McFarlane M.J., Ryan-Fisher C., Westenbrink E., Hodges P., Thomas M.G., Nwokolo C.U. Detection of colorectal cancer (CRC) by urinary volatile organic compound analysis. PLoS ONE. 2014;9(9) doi: 10.1371/journal.pone.0118975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cauchi M., Weber C.M., Bolt B.J., Spratt P.B., Bessant C., Turner D.C., Morgan G. Evaluation of gas chromatography mass spectrometry and pattern recognition for the identification of bladder cancer from urine headspace. Anal. Methods. 2016;8(20):4037–4046. doi: 10.1039/C6AY00400H. [DOI] [Google Scholar]
- 73.Guadagni R., Miraglia N., Simonelli A., Silvestre A., Lamberti M., Feola D., Sannolo N. Solid-phase microextraction–gas chromatography–mass spectrometry method validation for the determination of endogenous substances: urinary hexanal and heptanal as lung tumor biomarkers. Anal. Chim. Acta. 2011;701(1):29–36. doi: 10.1016/j.aca.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 74.Hanai Y., Shimono K., Matsumura K., Vachani A., Albelda S., Yamazaki K., Oka H. Urinary volatile compounds as biomarkers for lung cancer. Biosci. Biotechnol. Biochem. 2012;76(4):679–684. doi: 10.1271/bbb.110760. [DOI] [PubMed] [Google Scholar]
- 75.Hua Q., Wang L., Liu C., Han L., Zhang Y., Liu H. Volatile metabonomic profiling in urine to detect novel biomarkers for B–cell non–Hodgkin's lymphoma. Oncol. Lett. 2018;15(5):7806–7816. doi: 10.3892/ol.2018.8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiménez-Pacheco A., Salinero-Bachiller M., Iribar M.C., López-Luque A., Miján-Ortiz J.L., Peinado J.M. Furan and p-xylene as candidate biomarkers for prostate cancer. Urol. Oncol.: Semin. Orig. Invest. 2018;36:243. doi: 10.1016/j.urolonc.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 77.Khalid T., Aggio R., White P., Costello B.D.L., Persad R., Al-Kateb H., Ratcliffe N. Urinary volatile organic compounds for the detection of prostate cancer. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lima A.R., Pinto J., Azevedo A.I., Barros-Silva D., Jerónimo C., Henrique R., Carvalho M. Identification of a biomarker panel for improvement of prostate cancer diagnosis by volatile metabolic profiling of urine. Br. J. Cancer. 2019;121(10):857–868. doi: 10.1038/s41416-019-0585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monteiro M., Moreira N., Pinto J., Pires-Luís A.S., Henrique R., Jerónimo C., Guedes de Pinho P. GC-MS metabolomics-based approach for the identification of a potential VOC-biomarker panel in the urine of renal cell carcinoma patients. J. Cell Mol. Med. 2017;21(9):2092–2105. doi: 10.1111/jcmm.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Opitz P., Herbarth O. The volatilome–investigation of volatile organic metabolites (VOM) as potential tumor markers in patients with head and neck squamous cell carcinoma (HNSCC) J. Otolaryngol-head. N. 2018;47(1):42. doi: 10.1186/s40463-018-0288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santos P.M., del Nogal Sánchez M., Pozas Á.P.C., Pavón J.L.P., Cordero B.M. Determination of ketones and ethyl acetate—a preliminary study for the discrimination of patients with lung cancer. Anal. Bioanal. Chem. 2017;409(24):5689–5696. doi: 10.1007/s00216-017-0508-2. [DOI] [PubMed] [Google Scholar]
- 82.Silva C.L., Passos M., Camara J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer. 2011;105(12):1894–1904. doi: 10.1038/bjc.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silva C.L., Passos M., Camara J.S. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers—a powerful strategy for breast cancer diagnosis. Talanta. 2012;89:360–368. doi: 10.1016/j.talanta.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 84.Silva C.L., Perestrelo R., Silva P., Tomás H., Câmara J.S. Implementing a central composite design for the optimization of solid phase microextraction to establish the urinary volatomic expression: a first approach for breast cancer. Metabolomics. 2019;15(4):64. doi: 10.1007/s11306-019-1525-2. [DOI] [PubMed] [Google Scholar]
- 85.Taunk K., Taware R., More T.H., Porto-Figueira P., Pereira J.A., Mohapatra R., Rapole S. A non-invasive approach to explore the discriminatory potential of the urinary volatilome of invasive ductal carcinoma of the breast. RSC Adv. 2018;8(44):25040–25050. doi: 10.1039/C8RA02083C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taware R., Taunk K., Pereira J.A., Dhakne R., Kannan N., Soneji D., Rapole S. Investigation of urinary volatomic alterations in head and neck cancer: a non-invasive approach towards diagnosis and prognosis. Metabolomics. 2017;13(10):111. doi: 10.1007/s11306-017-1251-6. [DOI] [Google Scholar]
- 87.Wang D., Wang C., Pi X., Guo L., Wang Y., Li M., Li E. Urinary volatile organic compounds as potential biomarkers for renal cell carcinoma. Biomed. Rep. 2016;5(1):68–72. doi: 10.3892/br.2016.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Horváth I., Barnes P.J., Loukides S., Sterk P.J., Högman M., Olin A.C., Boots A.W. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur. Respir. J. 2017;49(4):1600965. doi: 10.1183/13993003.00965-2016. [DOI] [PubMed] [Google Scholar]
- 89.Zhou J., Huang Z.A., Kumar U., Chen D.D. Review of recent developments in determining volatile organic compounds in exhaled breath as biomarkers for lung cancer diagnosis. Anal. Chim. Acta. 2017;996:1–9. doi: 10.1016/j.aca.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 90.Janfaza S., Nojavani M.B., Nikkhah M., Alizadeh T., Esfandiar A., Ganjali M.R. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Microchim. Acta. 2019;186(3):137. doi: 10.1007/s00604-019-3241-z. [DOI] [PubMed] [Google Scholar]
- 91.Mochalski P., Unterkofler K. Quantification of selected volatile organic compounds in human urine by gas chromatography selective reagent ionization time of flight mass spectrometry (GC-SRI-TOF-MS) coupled with head-space solid-phase microextraction (HS-SPME) Analyst. 2016;141(15):4796–4803. doi: 10.1039/C6AN00825A. [DOI] [PubMed] [Google Scholar]
- 92.Mills G.A., Walker V. Headspace solid-phase microextraction profiling of volatile compounds in urine: application to metabolic investigations. J. Chromatogr. B Biomed. Sci. Appl. 2001;753(2):259–268. doi: 10.1016/S0378-4347(00)00554-5. [DOI] [PubMed] [Google Scholar]
- 93.Deng C., Zhang X., Li N. Investigation of volatile biomarkers in lung cancer blood using solid-phase microextraction and capillary gas chromatography–mass spectrometry. J. Chromatogr. B. 2004;808(2):269–277. doi: 10.1016/j.jchromb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 94.Jia Z., Patra A., Kutty V.K., Venkatesan T. Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer. Metabolites. 2019;9(3):52. doi: 10.3390/metabo9030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J.F., Yuan B.F., Feng Y.Q. Determination of hexanal and heptanal in human urine using magnetic solid phase extraction coupled with in-situ derivatization by high performance liquid chromatography. Talanta. 2015;136:54–59. doi: 10.1016/j.talanta.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Sovova K., Wiggins T., Markar S.R., Hanna G.B. Quantification of phenol in urine headspace using SIFT-MS and investigation of variability with respect to urinary concentration. Anal. Methods. 2015;7(12):5134–5141. doi: 10.1039/C5AY01051A. [DOI] [Google Scholar]
- 97.Miyagi Y., Higashiyama M., Gochi A., Akaike M., Ishikawa T., Miura T., Moriyama M. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0024143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee G.H., Malietzis G., Askari A., Bernardo D., Al-Hassi H.O., Clark S.K. Is right-sided colon cancer different to left-sided colorectal cancer?–a systematic review. Eur. J. Surg. Oncol. 2015;41(3):300–308. doi: 10.1016/j.ejso.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 99.Holmes E., Li J.V., Athanasiou T., Ashrafian H., Nicholson J.K. Understanding the role of gut microbiome–host metabolic signal disruption in health and disease. Trends Microbiol. 2011;19(7):349–359. doi: 10.1016/j.tim.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Ham J., Wells J.R. Surface chemistry of dihydromyrcenol (2,6-dimethyl-7-octen-2-ol) with ozone on silanized glass, glass, and vinyl flooring tiles. Atmos. Environ. 2009;43(26):4023–4032. doi: 10.1016/j.atmosenv.2009.05.007. [DOI] [Google Scholar]