Abstract
The prevalence of toxin types and colonization factors (CFs) of enterotoxigenic Escherichia coli (ETEC) was prospectively studied with fresh samples (n = 4,662) obtained from a 2% routine surveillance of diarrheal stool samples over 2 years, from September 1996 to August 1998. Stool samples were tested by enzyme-linked immunoassay techniques and with specific monoclonal antibodies for the toxins and CFs. The prevalence of ETEC was 14% (n = 662), with over 70% of the strains isolated from children 0 to 5 years of age, of whom 93% were in the 0- to 3-year-old age range. Of the total ETEC isolates, 49.4% were positive for the heat-stable toxin (ST), 25.4% were positive for the heat-labile toxin (LT) only, and 25.2% were positive for both LT and ST. The rate of ETEC isolation peaked in the hot summer months of May to September and decreased in winter. About 56% of the samples were positive for 1 or more of the 12 CFs that were screened for. The coli surface antigens CS4, CS5, and/or CS6 of the colonization factor antigen (CFA)/IV complex were most prevalent (incidence, 31%), followed by CFA/I (23.5%) and coli surface antigens CS1, CS2, and CS3 of CFA/II (21%). In addition, other CFs detected in decreasing order were CS7 (8%), CS14 (PCFO166) (7%), CS12 (PCFO159) (4%), CS17 (3%), and CS8 (CFA/III) (2.7%). The ST- or LT- and ST-positive ETEC isolates expressed the CFs known to be the most prevalent (i.e., CFA/I, CFA/II, and CFA/IV), while the strains positive for LT only did not. Among children who were infected with ETEC as the single pathogen, a trend of relatively more severe disease in children infected with ST-positive (P < 0.001) or LT- and ST-positive (P < 0.001) ETEC isolates compared to the severity of the disease in children infected with LT only-positive ETEC isolates was seen. This study supports the fact that ETEC is still a major cause of childhood diarrhea in Bangladesh, especially in children up to 3 years of age, and that measures to prevent such infections are needed in developing countries.
Enterotoxigenic Escherichia coli (ETEC) is an important cause of diarrheal disease in humans, affecting children but also adults, in whom these bacteria were first described (19). In particular, ETEC is a cause of morbidity and mortality in children up to 5 years of age in developing countries (3). Of the seven to eight episodes of diarrhea per child every year, one to three attacks may be caused by ETEC infections. ETEC strains have two major virulence determinants: the enterotoxins (the heat-labile toxin [LT] and the heat-stable toxin [ST]) and the colonization factors (CFs) (9). To cause disease, ETEC must first adhere to the epithelium of the small intestine by means of the CFs and then produce secretory diarrhea due to the effects of the enterotoxin(s). Although at least 20 different CFs are known in human ETEC infections (9), there is still a high proportion of strains on which CFs have not been identified, and additional CFs may thus exist. Moreover, the CFs and the toxins often encoded by the same plasmids may be lost on passage as well as on storage (18). Against this background, it is best to screen for the enterotoxins and CFs as soon as possible after isolation of E. coli from stool specimens and after as few passages of the E. coli strains as possible. Reports on ETEC toxins and CFs that have accumulated in the literature over the past 15 years or more are based on a variety of techniques, with the CFs and toxins generally not detected simultaneously (25). The prevalence of the CFs has also been observed to vary from one geographic region to another (9). In this study we have used a combination of specific enzyme immunoassay techniques, using a variety of mouse monoclonal antibodies against LT, ST, and 12 different CFs, to determine prospectively the toxin types and CFs on fresh isolates of E. coli. The isolates of E. coli were obtained from a systematic sampling of diarrheal stool samples collected in Bangladesh over a 2-year period, and the seasonal occurrence of ETEC infection was analyzed. In addition, the data obtained have been analyzed for a possible association between toxin types and CFs on ETEC isolates and the severity of diarrhea in patients.
MATERIALS AND METHODS
Stool samples from diarrheal patients.
Stool samples were collected from patients enrolled in the 2% systematic routine surveillance system at the Clinical Research and Service Centre of the International Centre for Diarrhoeal Disease Research, Bangladesh. In the surveillance system every 50th patient attending the hospital is screened for major enteric pathogens. In addition, information regarding age, sex, and clinical picture (fever, vomiting, dehydration status) as well as data on the duration of diarrhea, etc., is also collected from the patients. Such stool samples were tested for ETEC over a 2-year period, from September 1996 to August 1998.
Microbiological studies.
All stool samples obtained from patients enrolled in the 2% surveillance system are routinely screened for enteric pathogens including Vibrio cholerae O1 and O139, Salmonella spp., Shigella spp., Campylobacter jejuni, and rotavirus by standard techniques (26). For the detection of ETEC, fresh stool samples collected every day were plated onto MacConkey agar, and the plates were incubated at 37°C for 18 h. Six lactose-fermenting individual colonies morphologically resembling E. coli were tested immediately after isolation for the presence of toxins and CFs as described below.
Detection of toxin types and CFs.
The detection of LT and ST was carried out by previously described ganglioside GM1 enzyme-linked immunosorbent assays (ELISAs) (22, 23). For these purposes E. coli colonies were inoculated into separate wells of GM1-coated microtiter plates containing Luria-Bertani broth with lincomycin and glucose, and the plates were incubated overnight at 37°C with agitation (150 rpm). After incubation, the culture medium was transferred to fresh GM1-coated plates and was tested for ST by an inhibition ELISA procedure with the ST-cholera toxin B subunit (CTB) conjugate (ST-CTB) as described earlier (23). The original plate used for culture was analyzed for GM1-bound LT by using a specific mouse monoclonal antibody to the toxin (22). After the addition of rabbit anti-mouse immunoglobulin conjugated to horseradish peroxidase (Dako, Roskilde, Denmark), the enzyme substrates and hydrogen peroxide together with ortho-phenylenediamine were added and the optical density was measured at 450 nm. ETEC strains 286C2 and ST 64111 were used in the assays as LT- and ST-positive controls, respectively.
The colonies tested for toxin production were also plated onto colonization factor antigen (CFA) agar plates with and without bile salts (7, 17), and the plates were incubated overnight at 37°C. Enterotoxin-positive E. coli colonies from CFA agar plates were tested for the expression of CFA/1, CSI, CS2, CS3, CS4, and CS6; and such colonies from CFA agar plus bile plates were tested for the expression of CS5, CS7, CS17, CFA/III (CS8), CS12 (PCFO159) and CS14 (PCFO166) by monoclonal antibody-based dot blot assays (2, 14, 15, 24). Briefly, strips of nitrocellulose filter paper (pore size, 0.45 μm; Sigma, St. Louis, Mo.) were soaked in phosphate-buffered saline (10 mM; pH 7.2), 2 μl of bacterial suspension (corresponding to a 4 to 10 McFarland standard) was applied to the strips, and the strips were left at room temperature for about 15 min. The paper was washed several times with gentle agitation. Monoclonal antibodies against specific CFs, followed by rabbit anti-mouse immunoglobulin conjugated to horseradish peroxidase (Dako), were used to probe for the CFs expressed by the bacteria, and the CFs were visualized by using 4-chloro-1-naphthol and hydrogen peroxide as the substrate (14). In each strip, both CF-positive and CF-negative control strains were included. Only dots that were clearly stained (dark stain on a whitish background) were regarded as positive. The following ETEC strains were used as reference strains in the dot blot assay: E1392-75 (CS1 plus CS3), 258909-3 (CFA/I), E-20738A (CS17), VM 75688 (CS5 plus CS6), 350CIA (CS12 or PCFO159), E34420A (CS8 or CFA/III), 278485 (CS2, CS3), E11881/9 (CS4 plus CS6), E29101A (CS7), and E7474A (CS14 or PCFO166).
Analyses.
Chi-square for trends was used for statistical analyses with the Epi Info statistical package (version 6.0; USD, Stone Mountain, Ga.).
RESULTS
A total of 4,662 diarrheal stool samples were analyzed in the 2-year study period. Of these samples, 56% were from children up to 5 years of age. About 14% of all stool samples were positive for ETEC. In the first 12 months of the study, the prevalence of ETEC was 17% (381 of 2,251 stool samples tested), while in the second half of the period it was 11% (281 of 2,411 stool samples tested). The overall prevalence of ETEC was 18% in children 0 to 5 years of age (470 of 2,612); further analysis of the younger subjects (ages 0 to 3 years) also showed a similar prevalence (435 of 2,364). ETEC was the only diarrheal pathogen isolated from 59% of the subjects. The frequency of mixed infections generally increased with the age of the patients in the following order: less than 6 months, 23%; >6 months to less than 1 year, 27%; >1 to 2 years, 42%; >2 to 5 years, 44%; above 5 years, 70%. Thus, in the 0- to 1-year-old age group, ETEC was the sole diarrheal pathogen in approximately 75% of the children, and in the 0- to 3-year-old age group, ETEC was the sole diarrheal pathogen in about 70% of the children. In patients with mixed infections, rotavirus was most common copathogen with ETEC (15.6%), followed by V. cholerae serogroups O1 and O139 (13%), Campylobacter jejuni (8.4%), Shigella spp. (2.2%), and Salmonella spp. (1.5%). The detection of ETEC together with rotavirus occurred mostly in younger children (peaking at 6 to 12 months of age), whereas mixtures of V. cholerae and ETEC were common mostly in older children and adults.
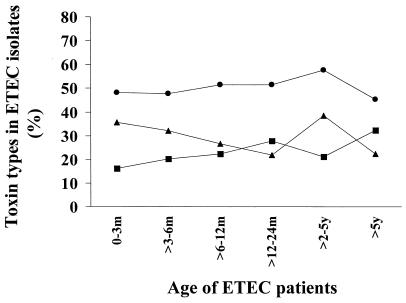
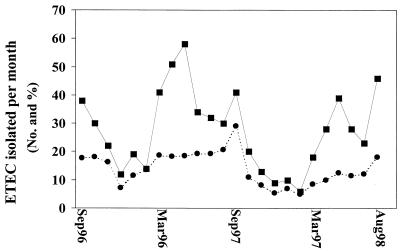
Of the total number of ETEC isolates, about 71% (470 of 662) were found in children who ranged in age from 0 to 5 years (median age, 10 months). Among these children 93% (435 of 470) were in the 0- to 3-year-old age group. Among the ETEC isolates, 49.4% (n = 327) were only ST producers, 25.2% (n = 167) were LT and ST producers, and 25.4% (n = 168) were only LT producers. In all the age groups, the ST only-producing isolates were the most prevalent, followed by LT- and ST-producing ETEC isolates and LT only-producing ETEC isolates (Fig. 1). In both years of surveillance, the highest rate of isolation of ETEC was in the hot summer months (Fig. 2). In the first 12 months (September 1996 to August 1997), the largest numbers were isolated in May 1997 (n = 58; 18.5% of all isolates), while the smallest numbers of ETEC isolates and also the lowest frequency of ETEC isolation were in the month of December 1997 (n = 12; 7.1%). In the second year of the study (September 1997 to August 1998), the largest numbers were isolated in August 1998 (n = 46; 18%) and the smallest numbers were isolated in December 1998 (n = 9; 5.4%). Thus, the total number of ETEC strains isolated showed a downward trend from October to January and showed a rise from about February, peaking from March to September.
FIG. 1.
Distribution of enterotoxins in ETEC strains isolated from stool samples of diarrheal patients in different age groups. The percentage of the isolates positive for ST (●), LT (■) and both LT and ST (▴) are shown. Ages are given in months (m) or years (y).
FIG. 2.
Monthly isolation of ETEC over the 2-year surveillance period. The total numbers of ETEC isolates (■) as well as the percentage (●) of ETEC isolates detected per total number of diarrheal stool samples screened are shown.
The different CFs studied were detected on only 56% of the total ETEC isolates tested (370 of 662). Of these CFs, the CFA/IV coli surface antigens in different combinations of CS4, CS5, and CS6 were the most common ones and were detected on 31% of all CF-positive isolates. This was followed by CFA/I (23.5%) and the CFA/II coli surface antigens (21%; in different combinations of CS1, CS2, and CS3). Of the other CFs that were tested, CS7 was found at a frequency of 7.8% (29 of 370 isolates), followed by CS14 (6.7%) and CS12 (4.2%). All 12 CFs studied were detected on the ETEC isolates, although at different frequencies. Analysis of the data showed no seasonality or restriction to age in the prevalence of the CFs on ETEC. However, some CFs were detected more frequently in children, e.g., 21 of the 29 CS7-positive ETEC isolates (79%) had been isolated from the stools of children 0 to 3 years of age. Similarly, CS17-expressing ETEC isolates were also observed at the highest frequency (8 of 11) in this age group. It was observed that in comparison to 78% of the LT- and ST-producing ETEC isolates and 61% of the ST-producing ETEC isolates, a significantly smaller number of the LT-producing isolates (24%; P < 0.001) expressed CFs (Table 1). A comparison of the toxin pattern and the expression of those CFs that are included in an oral inactivated ETEC vaccine currently in phase III studies (this vaccine contains CFA/I and coli surface antigens of CFA/II and CFA/IV) was also carried out (27). These CFs constituted, on average, about 75% of the total CFs expressed on ETEC (Table 2). It was observed that although 59% (99 of 167) of the LT- and ST-producing ETEC isolates and 55% (181 of 327) of the ST only-producing ETEC isolates expressed these CFs, none of the LT only-producing isolates expressed these CF antigens. These vaccine-specific CFs were present on 91% of the ST-expressing, CF-positive ETEC isolates and 76% of the ST- and LT-expressing, CF-positive ETEC isolates.
TABLE 1.
Association of enterotoxins with CF expressed on ETEC isolates from Bangladeshi diarrheal patients
| Toxin produced | Total no. of ETEC strainsa | No. (%) of isolatesb
|
|
|---|---|---|---|
| CF positive | Vaccine CF positive | ||
| ST | 327 | 200 (61) | 181 (55.4) |
| LT and ST | 167 | 130 (78) | 99 (59.3) |
| LT | 168 | 40 (24) | 0 |
Total number of strains in each toxin type isolated.
ETEC strains positive for any of the 12 CFs or vaccine-specific CFs (CFA/1 and coli surface antigens of CFA/II or CFA/IV).
TABLE 2.
Occurrence and association of toxins and CFs in ETEC isolates
| Toxin produced | Total no. of isolatesa | CF type(s) produced | No. (%) of isolates |
|---|---|---|---|
| ST | 200 | CFA/I | 48 (24) |
| CS1 + CS3 | 18 (9.0) | ||
| CS2 + CS3 | 22 (11.0) | ||
| CS4 + CS6 | 19 (9.5) | ||
| CS5 + CS6 | 39 (19.5) | ||
| CS6 | 35 (17.5) | ||
| CS7 | 5 (2.5) | ||
| CS14 | 9 (4.5) | ||
| CS12 | 5 (2.5) | ||
| LT and ST | 130 | CFA/I | 39 (30.0) |
| CS1 + CS3 | 16 (12.3) | ||
| CS2 + CS3 | 14 (10.7) | ||
| CS3 | 9 (7.0) | ||
| CS4 + CS6 | 21 (16.0) | ||
| CS7 | 5 (3.8) | ||
| CS14 | 16 (12.3) | ||
| CS12 | 10 (7.7) | ||
| LT | 40 | CS7 | 19 (47.5) |
| CS17 | 11 (27.5) | ||
| CS8 | 10 (25.0) |
ETEC isolates of each toxin type positive for the different CFs.
Moreover, in most cases the expression of the CFs in relation to the toxin types was found to agree well with that shown in earlier studies (9). Some differences were, however, observed. Thus, we found that some CS7-expressing isolates produced only ST (CS7-expressing strains have earlier been shown [9rsqb; to produce both LT and ST), while 64% of the PCFO166-expressing isolates produced both LT and ST, although PCFO166-expressing strains have been known to produce only ST (9).
A comparison of the ages of the patients and the CFs expressed on the ETEC isolates from those patients showed that the vaccine-specific CFs constituted an average of about 75% of the total CFs expressed by ETEC strains isolated from each of the different age groups. In the younger age group, other CFs were detected at a somewhat higher frequency (e.g., on about 20% of the CF-positive ETEC isolates from the group of patients ages 0 to 3 months but on only 3% of such strains from the group of patients >5 years of age).
A comparison was also carried out to find associations between the presence of CFs, the toxin pattern, and the clinical severity of disease in children (ages 0 to 5 years) with diarrhea from whom only ETEC and no other pathogen was isolated (Table 3). No significant difference in the degree of severity of disease with the presence or absence of CFs on ETEC was seen. However, a trend of the occurrence of more severe disease following infection with ST- or LT- and ST-positive ETEC strains was seen. Thus, significantly fewer patients with infection due to LT-positive isolates had moderate or severe disease compared to the number of patients infected with ST-expressing (P < 0.001) or LT- and ST-expressing ETEC (P < 0.001) who had moderate or severe disease. Similarly, when this comparison was carried out for children from whose stools ETEC and some other copathogen were isolated, a trend of more severe disease caused by ST-expressing (P = 0.002) or LT- and ST-expressing (P = 0.0002) ETEC isolates in comparison to the severity of disease caused by isolates that expressed only LT was also seen.
TABLE 3.
Association of severity of disease with CFs on toxin types produced by ETEC strains isolated as the only diarrheal pathogen from children up to 5 years of age during the 2 years of surveillance
| CF types or toxin produceda | No. (%) of patients with the following severity of diseaseb:
|
|||
|---|---|---|---|---|
| Mild | Moderate | Severe | Total | |
| Vaccine CF positivec | 66 (55) | 47 (39) | 7 (6) | 120 |
| Other CF positive | 25 (66) | 12 (31) | 1 (3) | 38 |
| CF negative | 78 (56) | 55 (39) | 6 (5) | 139 |
| ST | 90 (53) | 66 (39) | 12 (8) | 168 |
| LT and ST | 32 (43) | 41 (54) | 2 (3) | 75 |
| LT | 47 (87) | 7 (13) | 0 (0) | 54 |
A significant difference was not seen (P > 0.05) between the presence or absence of CFs and severity of disease. ETEC strains positive for ST (P < 0.001) or LT and ST (P < 0.001) were isolated at significantly higher frequencies from children with moderate or severe disease than the frequency of isolation of ETEC strains positive for LT only. Chi-square for trends was used for statistical analysis (P > 0.05 was not significant).
Degree of dehydration based on World Health Organization guidelines.
Vaccine-specific CFs indicate CFA/I and coli surface antigens of CFA/II and CFA/IV.
A comparison was also carried out to see if there was an association between the severity of disease and the age of children with diarrhea caused by ETEC. This analysis showed that those in the 0- to 3-month-old age group (n = 30) more often suffered from moderate rather than mild (P < 0.001) or severe (P = 0.022) dehydration. In the older children (6- to 12-month age range; n = 198), the children suffered from mild rather than moderate or severe disease (P < 0.001). This trend was also observed in the older children (2 to 5 years of age; n = 57) (P < 0.001); however, in children 12 to 24 months of age (n = 101), similar frequencies of mild, moderate, or severe episodes of disease were observed (P was not significant).
DISCUSSION
This is the first prospective study in which a systematically obtained collection of fresh diarrheal stool samples obtained from patients over a 24-month period has been used to determine the prevalence of both the toxin and the CF types expressed on ETEC. The stool specimens studied were from patients who had mild to severe forms of the disease and who were ill enough to be brought to the hospital for treatment. ETEC was isolated from patients of different age groups (from a minimum of less than 1 month to over 60 years of age), although the highest incidence, in terms of both total number and relative distribution, was in children up to 3 years of age (median age, 9 months). We also observed that mixed infections were very frequent, particularly in patients over 5 years in age. In approximately 75% of infants and small children up to 1 year of age, ETEC was seen at the highest frequencies and was the only pathogen that was isolated. This information supports earlier studies which have shown that in developing countries, it is the very young children who are at risk of diarrhea due to ETEC and, hence, who suffer most from its consequences (5).
As expected from data from earlier studies conducted in Bangladesh (1, 16), ETEC disease peaked in the hot summer months, from about May to September, decreasing thereafter in the winter months. Each year an increase in the number of ETEC cases was observed in the spring, from about March to April. The previously reported peak of diarrhea in Dhaka, Bangladesh, on the basis of the surveillance stool samples has also been shown to be due to more cases of ETEC infection than cases of V. cholerae O1 infection, suggesting that the traditional spring peaks of diarrhea in Bangladesh may also be contributed by ETEC (8).
The seasonalities of ETEC during the corresponding seasons over the 2-year study period were similar, although different total numbers and relative frequencies of ETEC isolations were seen during the corresponding months in the 2 years in which the study was carried out. This to some extent reflects the various numbers of patients with diarrhea caused by ETEC coming to the treatment center and being enrolled in the 2% systemic surveillance system each year. The most prevalent of the toxins was ST, which was produced by approximately 75% of the total ETEC isolates (about 50% were positive for ST production and 25% were positive for LT and ST production). The ST only-positive and the LT- and ST-positive isolates expressed the prevalent CF antigens, i.e., CFA/I and CS1-CS6 (9), while the LT only-expressing isolates did not. We also found that the diarrhea caused by the ST- or LT- and ST-expressing isolates was significantly more often more severe disease than the diarrhea caused by the LT-expressing isolates. This is in agreement with the notion that LT only-producing strains of ETEC are less important as pathogens (9, 18).
There is a wide variation in the prevalence of ETEC isolates that express the different CFs (which varies from 33 to 69%), as described in reports (25) from different countries. It is, however, difficult to compare the prevalences since different procedures have been used. The enzyme immunoassay techniques used for detection of the enterotoxins and the CFs are dependent on the in vitro production of these factors, whereas molecular probing techniques (1, 8, 20) can detect the presence of the genes. In two earlier reports from Bangladesh it was shown that the prevalence of ETEC may vary from 64 to 75% (12, 18). Even though both of those studies used enzyme immunoassay techniques, it is difficult to compare the previous studies with the one carried out here, since the methods and the categories of patients that were studied were different. It is now appreciated that enzyme-linked immunoassay procedures are the most suitable for the detection of the CFs (25). However, using all possible precautions so that the isolates did not lose their virulence, we were not able to detect CFs in more than 56% of the ETEC isolates that were tested. The reasons for the relatively low prevalence of CFs on the ETEC strains isolated in this study may be that (i) the CF antigens were lost on subculture, even though only two to three passages were done; (ii) the CF antigens are only produced in vivo; (iii) the CF antigens are different from the 12 CFs screened for in the present study; or (iv) a certain proportion of strains do not produce CFs.
It was possible for us to screen for only 12 of the 20 or so of the CFs that are known to exist (9), since specific monoclonal antibodies only to these antigens were available. It may be interesting to screen for other CFs including, e.g., longus (CS21), which is a long pilus produced (11) and expressed in human ETEC isolates, in addition to the other CF antigens (10).
In this study we have analyzed the ETEC isolates only for the production of toxins and CFs, although other bacterial antigens may be important for conferring immunity to the disease. The O antigens (e.g., O157) of the E. coli strains may be considered putative protective bacterial antigens in other diarrheagenic E. coli categories; however, it may be difficult to consider these antigens as useful components in an ETEC vaccine, since a large number (about 78 serogroups) have been detected in ETEC strains isolated worldwide (25).
Successful prevention of ETEC diarrhea depends on the availability of an effective vaccine, and efforts to design such a vaccine are under way. Since both antibacterial and antitoxic immunities are believed to contribute to protection (6, 13, 21), the inclusion of CFs as components of a vaccine may prove to be useful since these are known to be critical somatic antigens that may give a broad range of protection. However, the effectiveness of a vaccine based on CFs will depend on the region in which it is to be tested since the expression of CFs on ETEC vary from one geographic region to another (9, 20). Studies have shown that CFA/I and the coli surface antigens of CFA/II and CFA/IV are the most common ones, and a review of the available data a couple of years ago showed that these were present on 50 to 80% of all ETEC isolates (9, 26). On the basis of this information, an oral inactivated ETEC vaccine containing a combination of five strains of formalin-killed ETEC strains expressing CFA/I and the different CS components of CFA/II and CFA/IV together with 1 mg of cholera toxin B subunit is being evaluated and is at various stages of clinical trials (21, 27). As a background for future vaccine studies in Asia, updated new information on the prevalence of the different CFs is important. More than 15 years ago such studies were carried out in Bangladesh by use of polyclonal antisera and immunodiffusion or ELISA techniques to screen for CFA/I, CFA/II, and CFA/IV antigens (12, 18). At that time, neither specific monoclonal antibodies to such a wide range of CFs nor sensitive techniques were available for the detection of specific CFs. Furthermore, such large numbers of isolates from diarrheal stool specimens were not studied prospectively. In the present study, we have shown that the prevalence of the CFs are found in the following decreasing order: CFA/IV (CS4 + CS6 > CS5 + CS6 > CS6) > CFA/I > CFA/II (CS2 + CS3 > CS1 + CS3 > CS3) > CS7 > CS14 > CS12 > CS17 > CS8.
This shows that the formalin-inactivated ETEC vaccine currently being evaluated in different countries (21, 27) contains about 75% of the CF antigens prevalent in Bangladesh and thus should prove to be useful. However, for greater efficacy in Bangladesh, it may be useful to add additional components, i.e., CS7 and CS14, and perhaps also CS12 and CS17. It was interesting that some CFs (especially CS7 and CS17) were more prevalent in younger children than in children in the older age groups. This suggests that as they grow older these children develop immunity to these antigens and are protected from further infections.
Since ETEC is a major cause of disease burden in children in developing countries, leading to considerable growth retardation, malnutrition, and mortality, serious efforts should be made to design more effective vaccines for the prevention of these infections, especially in countries of the world where ETEC is endemic (27). The information obtained from this study may be useful for this purpose.
ACKNOWLEDGMENTS
This research was supported by the Swedish Agency for Research and Economic Cooperation (Sida-SAREC) and the Centre for Health and Population Research of the International Centre for Diarrhoeal Disease Research, Bangladesh. The Centre is supported by agencies and countries which share its concern for the health problems of developing countries. Current donors providing unrestricted support include the governments of Australia, Bangladesh, Belgium, Canada, Saudi Arabia, Sri Lanka, Sweden, Switzerland, the United Kingdom, and the United States; international organizations include the United Nations Children's Fund (UNICEF).
REFERENCES
- 1.Albert M J, Faruque S M, Faruque A S G, Neogi P K B, Ansaruzzaman M, Bhuiyan N A, Alam K, Akbar M S. Controlled study of Escherichia coli diarrheal infections in Bangladeshi children. J Clin Microbiol. 1995;33:973–977. doi: 10.1128/jcm.33.4.973-977.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binsztein N, Jouve M J, Viboud G I, Lopez Moral L, Rivas M, Orskov L I, Arhen C, Svennerholm A-M. Colonization factors of enterotoxigenic Escherichia coli isolated from children with diarrhea in Argentina. J Clin Microbiol. 1991;29:1893–1898. doi: 10.1128/jcm.29.9.1893-1898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black R E. Epidemiology of diarrheal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 4.Black R E, Brown K H, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 5.Black R E, Merson M H, Huq I, Alim A R M A, Yunus M. Incidence and severity of rotavirus and Escherichia coli diarrhoea in rural Bangladesh: implication for vaccine development. Lancet. 1981;i:141–143. doi: 10.1016/s0140-6736(81)90719-4. [DOI] [PubMed] [Google Scholar]
- 6.Clemens J D, Sack D A, Harris J R, Chakraborty J, Neogy P K, Stanton B, Huda N, Khan M U, Lay B A, Khan M R, Ansaruzzaman M, Yunus M, Rao M R, Svennerholm A-M, Holmgren J. Cross protection by B-subunit whole cell cholera vaccine against diarrhea associated with heat-labile toxin producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis. 1988;158:372–377. doi: 10.1093/infdis/158.2.372. [DOI] [PubMed] [Google Scholar]
- 7.Evans D G, Evans D J, Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977;18:330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faruque A S G, Salam M A, Faruque S M, Fuchs G J. Aetiological, clinical and epidemiological characteristics of a seasonal peak of diarrhoea in Dhaka, Bangladesh. Scand J Infect Dis. 1998;30:393–396. doi: 10.1080/00365549850160701. [DOI] [PubMed] [Google Scholar]
- 9.Gaastra W, Svennerholm A-M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 10.Giron J A, Levine M M, Kaper J B. Longus: a long pilus ultrastructure produced by human enterotoxigenic Escherichia coli. Mol Microbiol. 1994;12:71–82. doi: 10.1111/j.1365-2958.1994.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 11.Giron J A, Gomez-Duarate O G, Jarvis K G, Kaper J B. Longus pilus of enterotoxigenic Escherichia coli and its relatedness to other type-4 pili—a minireview. Gene. 1997;192:39–43. doi: 10.1016/s0378-1119(97)00039-5. [DOI] [PubMed] [Google Scholar]
- 12.Gothefors L, Ahren C, Stoll B, Barua D K, Orskov F, Salek M K, Svennerholm A-M. Presence of colonization factor antigens on fresh isolates of fecal Escherichia coli: a prospective study. J Infect Dis. 1985;152:1128–1133. doi: 10.1093/infdis/152.6.1128. [DOI] [PubMed] [Google Scholar]
- 13.Levine M M, Ristaino P, Marley G, Symth C, Knutton S, Boedeker E, Black R E, Young C, Clements M L, Cheney C, Patnaik R. Coli surface antigen 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification and immune responses in humans. Infect Immun. 1984;44:409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Vidal Y, Klemm P, Svennerholm A-M. Monoclonal antibodies against different epitopes on colonization factor antigen I of enterotoxin producing Escherichia coli. J Clin Microbiol. 1988;26:1967–1972. doi: 10.1128/jcm.26.10.1967-1972.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Vidal Y, Svennerholm A-M. Monoclonal antibodies against the different subcomponents of colonization factor antigen II of enterotoxigenic Escherichia coli. J Clin Microbiol. 1990;28:1906–1912. doi: 10.1128/jcm.28.9.1906-1912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merson M H, Sack R B, Islam S, Saklayen G, Huda N, Huq I, Zulich A W, Kapikian A Z. Disease due to enterotoxigenic Escherichia coli in Bangladeshi adults: clinical aspects and a controlled trial of tetracycline. J Infect Dis. 1980;141:702–711. doi: 10.1093/infdis/141.6.702. [DOI] [PubMed] [Google Scholar]
- 17.McConnell M M, Chart H, Field A M, Hibberd M, Rowe B. Characterization of a putative colonization factor (PCFO166) of enterotoxigenic Escherichia coli of serogroup O166. J Gen Microbiol. 1989;135:1135–1144. doi: 10.1099/00221287-135-5-1135. [DOI] [PubMed] [Google Scholar]
- 18.McConnell M M, Thomas L V, Day N P, Rowe B. Enzyme linked immunosorbent assays for the detection of adhesion factor antigens of enterotoxigenic Escherichia coli. J Infect Dis. 1985;152:1120–1127. doi: 10.1093/infdis/152.6.1120. [DOI] [PubMed] [Google Scholar]
- 19.Sack R B, Gorbach S L, Banwell J G, Jacobs B, Chatterjee B D, Mitra R C. Enterotoxigenic Escherichia coli isolated from patients with severe cholera-like disease. J Infect Dis. 1971;123:378–385. doi: 10.1093/infdis/123.4.378. [DOI] [PubMed] [Google Scholar]
- 20.Sommerfelt H, Steinsland H, Grewal H M S, Viboud G I, Bhandari N, Gastra W, Svennerholm A-M, Bhan M K. Colonization factors of enterotoxigenic Escherichia coli isolated from children in North India. J Infect Dis. 1996;174:768–776. doi: 10.1093/infdis/174.4.768. [DOI] [PubMed] [Google Scholar]
- 21.Svennerholm A-M, Ahren C, Jertborn M. Oral inactivated vaccines against enterotoxigenic Escherichia coli. In: Levine M M, Woodrow G C, Kaper J B, Gaborn G S, editors. New generation vaccines. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 865–874. [Google Scholar]
- 22.Svennerholm A-M, Wiklund G. Rapid GM1-enzyme-linked immunosorbent assay with visual reading for identification of Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1983;17:596–600. doi: 10.1128/jcm.17.4.596-600.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svennerholm A-M, Wikstrom M, Lindblad M, Holmgren J. Monoclonal antibodies against Escherichia coli heat-stabile toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J Clin Microbiol. 1986;24:585–590. doi: 10.1128/jcm.24.4.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viboud G I, Binsztein N, Svennerholm A-M. Characterization of monoclonal antibodies against putative colonization factors of enterotoxigenic Escherichia coli and their use in an epidemiological study. J Clin Microbiol. 1993;31:558–564. doi: 10.1128/jcm.31.3.558-564.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf M K. Occurrence, distribution and association of O and H serogroup, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev. 1997;10:569–584. doi: 10.1128/cmr.10.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Manual for laboratory investigation of acute enteric infections. Geneva, Switzerland: World Health Organization; 1987. Programme for control of diarrhoeal disease (CDD/93.3 Rev 1) pp. 9–20. [Google Scholar]
- 27.World Health Organization. New frontiers in the development of vaccines against enterotoxigenic (ETEC) and enterohaemorrhagic (EHEC) E. coli infections. Weekly Epidemiol Rec. 1999;13:98–100. [PubMed] [Google Scholar]