Highlights
-
•
Sensitive (0.02 ng/mL LLOQ) serum Tg measures by LC–MS/MS at μL/min flow rates.
-
•
Tissue-derived Tg can circulate below 0.1 ng/mL in DTC patients with autoantibodies.
-
•
Feasibility of Interference-free Tg monitoring below 0.15 ng/mL before recurrence.
Keywords: Thyroglobulin, Mass spectrometry, Liquid chromatography, Micro-flow, Thyroid cancer, Autoantibody
Abstract
Although liquid chromatography–tandem mass spectrometry (LC–MS/MS) assays for thyroglobulin (Tg) are resistant to autoantibody (TgAb) interference, recent studies have demonstrated approximately 40% of TgAb-positive individuals with recurrent thyroid cancer have Tg concentrations below the lower limit of quantification (LLOQ) of the LC–MS/MS assays described to date (i.e., <0.5 ng/mL), resulting in false-negative findings during post-thyroidectomy monitoring. To reduce false negative results due to insufficient analytical sensitivity, a new Tg assay was developed on a commercially available LC–MS/MS system operating at microliter/minute flow-rates (i.e., µLC–MS/MS) to maximize the analytical sensitivity and achieve a LLOQ of 0.02 ng/mL. When applied to the measurement of TgAb-negative and TgAb-positive patient serum samples previously measuring below the LLOQ of current immunometric and LC–MS/MS assays (LLOQ, 0.1–0.2 ng/mL), concentrations were measurable by µLC–MS/MS in 66% and 44% of samples, respectively – possibly explaining the persistence of TgAb in those patients. Patients with low Tg concentrations measured by µLC–MS/MS (<0.1 ng/mL) also exhibited elevation in their Tg concentrations upon hormone stimulation, indicating the detected Tg was produced from remnant thyroid tissue and would be suitable as a tissue biomarker. Forty-eight TgAb-positive patient specimens with undetectable Tg by both conventional LC–MS/MS (<0.15 ng/mL) and immunometric (<0.1 ng/mL) assays demonstrated measureable Tg concentrations by the new µLC–MS/MS assay in approximately one-third of the specimens, despite all patients being disease free at the time of collection, suggesting interference-free monitoring of low Tg levels may be feasible prior to the on-set of recurrent disease using a sensitive LC–MS/MS assay.
1. Introduction
Serum thyroglobulin (Tg) measurements are used in detecting recurrent differentiated thyroid cancer (DTC) – specifically after total thyroidectomy with or without radioiodine ablation [1]. The premise of such measurements is that without thyroid tissue, the concentrations of circulating Tg should be undetectable within a few months after surgery and, thus, detection or marked increase of Tg in serum following a total thyroidectomy would be indicative of biochemical recurrence (i.e., a recurrent tumor releasing Tg into circulation). However, the threshold indicating biochemical recurrence has inherently been limited by the analytical sensitivity of the Tg assay utilized and has led to discrepant interpretations [2]. For instance, prior to the advent of 2nd generation immunoassays having a lower limit of quantification (LLOQ) below 0.5 ng/mL, serum Tg levels were stimulated (roughly 10-fold) by cessation of thyroid hormone replacement therapy or by treatment with recombinant human thyroid stimulating hormone (rhTSH) because unstimulated levels were routinely undetectable by 1st generation assays, irrespective of disease status [3]. Although increasing Tg levels are most informative, current consensus indicates undetectable suppressed Tg levels, defined as less than 0.2 ng/mL, following total thyroidectomy and ablation therapy are associated with a low risk of recurrent disease in the absence of autoantibodies [1].
No such consensus exists for biochemical monitoring in patients with Tg autoantibodies (TgAb), which are present in roughly 20–30% of patients with DTC and can interfere with immunometric measurements [4], [5]. When using non-competitive immunoassays, TgAb can result in under-recovery and falsely undetectable Tg measurements and, consequently, a false-negative indication of recurrent disease [6], [7]. Conversely, false-positive results can arise when using competitive radioimmunoassay due to displacement of the radio-labeled Tg by TgAb resulting in an erroneously high Tg measurement [8], [9], [10], [11]. Several laboratories have developed assays using liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) that are resistant to autoantibody interference (as well as heterophilic antibody interference that can result in false positives) through the use of protein denaturation and enzymatic digestion as part of the analytical workflow [12], [13], [14], [15], [16], [17]. Nonetheless, two recent studies have indicated suppressed Tg levels are undetectable (<0.5 ng/mL) by LC–MS/MS in approximately 40% of TgAb-positive, total thyroidectomy patients with recurrent thyroid cancer. Although these results are on par with those observed in TgAb-positive patients using 2nd generation immunoassays having a 5-fold lower reporting limit of (0.1 ng/mL) [18], [19], the findings exemplify the need for a more sensitive, interference-free assay to better enable biochemical monitoring for recurrent disease in TgAb-positive patients.
To improve the clinical utility of Tg measurements in TgAb-positive patients with low (<0.5 ng/mL) circulating levels of Tg, a new LC–MS/MS assay was developed with a lower reporting limit of 0.02 ng/mL by using microliter/min chromatographic flow rates in combination with smaller column diameters (i.e., µLC–MS/MS) to maximize analytical sensitivity through improved chromatographic focusing and ionization efficiency [20], [21]. This µLC–MS/MS assay was derived from the validated, in-house developed test performed at Laboratory Corporation of America (LabCorp) with minor modifications to enable the analytical measurements on a µLC–MS/MS system rather than the predicate standard-flow LC–MS/MS system. After evaluating the analytical performance, the resulting µLC–MS/MS assay was used in the analysis of serum specimens from patients with previously “undetectable” Tg by interference prone immunometric and less sensitive LC–MS/MS platforms, which currently serve as the standard-of-care.
2. Materials & methods
2.1. µLC–MS/MS assay
External calibrators were prepared by reconstituting and diluting Access Thyroglobulin calibrators (Beckman-Coulter) in SigMatrix (MilliporeSigma) at values spanning 0.02 to 20 ng/mL. Accuracy controls were prepared likewise at values of 0.02, 0.04, 0.08, and 20 ng/mL. Three levels of matrix controls were prepared by pooling/mixing remnant TgAb-negative serum to produce true serum controls with approximate Tg concentrations of 0.1, 0.5, and 4 ng/mL. The lower two concentrations for serum matrix controls were selected because they have been previously considered as clinically relevant thresholds for biochemical monitoring of Tg [18]. Although desirable, lower concentrations of true matrix controls nearer the target LLOQ of 0.02 ng/mL could not be prepared in a practical fashion at the required volumes due to the prevalence of variable low levels of Tg (0.02 to 0.1 ng/mL) in the remnant specimens available to create the necessary pools (see below).
The sample preparation for the µLC–MS/MS assay utilized LabCorp’s proprietary, validated in-house LC–MS/MS methodology ostensibly without change. Similar methods from other labs have been described previously [12], [13], [14]. Briefly, 400 µL of serum was heat denatured in the presence of deoxycholate and dithiothreitol, followed by digestion with TPCK-treated bovine trypsin to produce the surrogate peptide for thyroglobulin (FSPDDSAGASALLR) and internal standard peptide derived from digestion of the stable isotopically-labeled cleavable peptide (FYQRRRFSPDDSAGASA[13C6,15N]LLRSGPYMP). Subsequently, the surrogate peptide and its internal standard were selectively enriched from the digested serum prior to injection on the µLC–MS/MS system. The final processed sample volume was 100 µL and the total sample preparation time was 4.5 hours.
Processed samples were analyzed on the ionKey/MS system (Waters Corporation) plumbed in a dual pump, reverse trap-forward elute configuration on an ACQUITY UPLC® M-Class system coupled with a Xevo® TQ-S tandem quadrupole (Waters) via an ionKey source (Fig. 1). Samples (45 µL) were loaded onto the trapping column for 1.6 min at 0.5% mobile phase B at 50 µL/min, then eluted onto and through the analytical column (iKey) at 3 µL/min by linear ramping from 5 to 31.5% mobile phase B over 0.53 min (see Fig. 1 in Supplemental Data). Mobile phase A and B were 0.1% formic acid (v/v) in water and acetonitrile, respectively. Analyte and internal standard peptides were detected by Selected Reaction Monitoring in positive electrospray mode (see Table 1 in Supplemental Data). Additional details of the µLC–MS/MS method parameters may be found in Supplemental Data.
Fig. 1.
Shown is the dual pump, trap-elute µLC–MS/MS configuration as implemented on the ionKey/MS system. The chromatographic run starts in the loading position (A) where sample is loaded through the guard column and onto the trapping column. Next, the valve switches to the elution position (B) and a gradient elutes the trapped surrogate peptide while the guard column is washed. After the surrogate peptide elutes the valve returns to the load configuration (A) removing the trapping column out-of-line with the analytical column for independent washing/equilibration.
2.2. Human specimens
Three sets of human serum specimens were used for these studies with Institutional Review Board approval at the respective institutions from which they were obtained. These included, LabCorp’s Center for Esoteric Testing in Burlington, North Carolina; the University of Southern California (USC) Endocrine Laboratories in Pasadena, California; and the University of Washington Medical Center (UWMC) in Seattle, Washington. All specimens were de-identified and frozen at <−70 °C prior to analysis on the µLC–MS/MS assay. Disease status of the LabCorp and USC specimens were unknown. For the UWMC specimens, charts were reviewed by ANH and disease status was determined from chart notes and radiological reports. Patients were classified as disease-free, indeterminate (e.g., unclear findings on imaging), or stable (e.g., mass identified on imaging, but no growth since the last imaging study). Patients were included without patient consent as approved by the University of Washington Human Subjects Division (approval # STUDY00002450).
3. Results
3.1. LC–MS/MS vs µLC–MS/MS sensitivity comparison
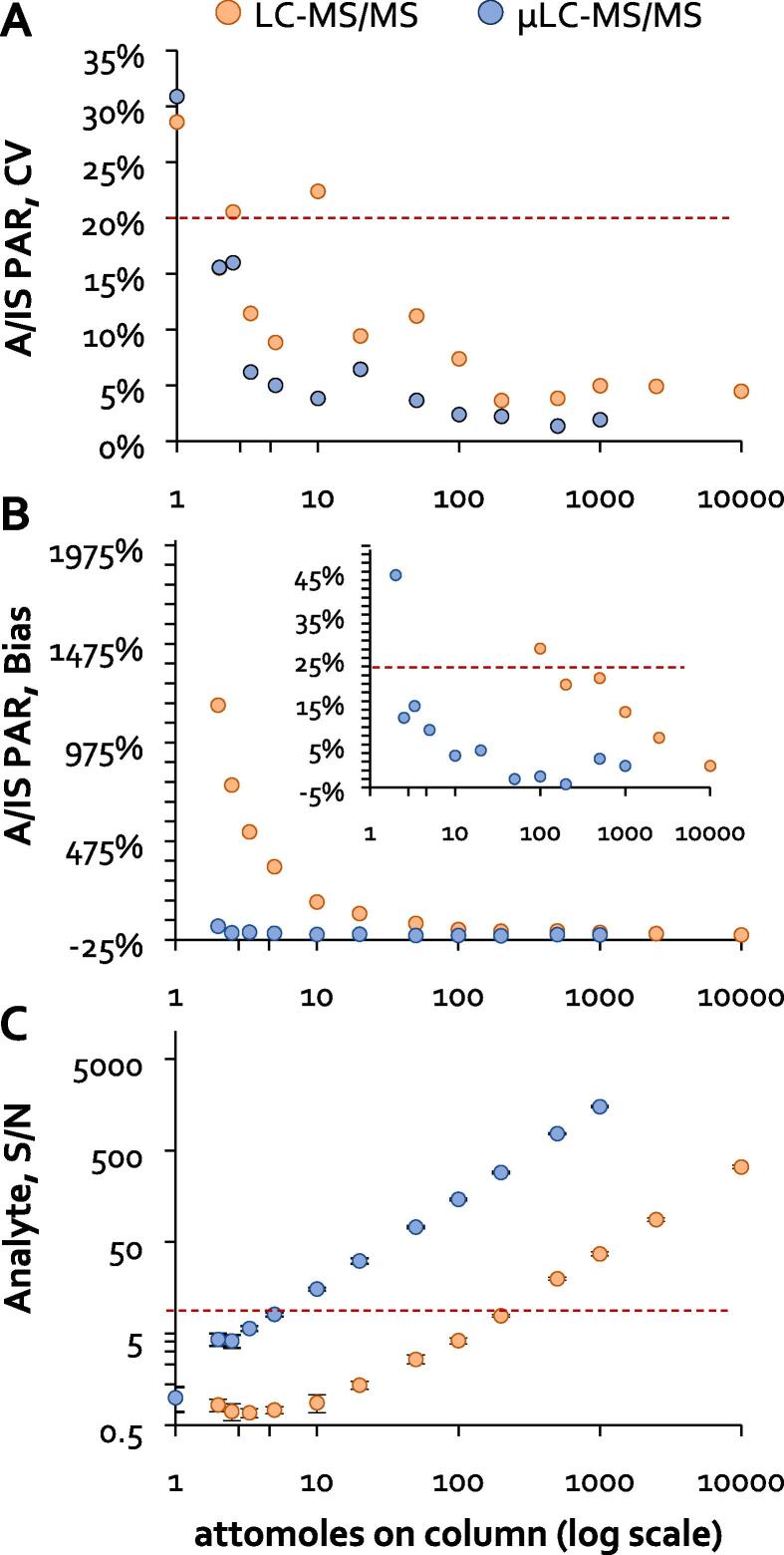
Development of the new µLC–MS/MS assay was accomplished using LabCorp’s in-house LC–MS/MS assay as a template. As such, the raw analytical sensitivity of the µLC–MS/MS system was first benchmarked relative to the LC–MS/MS configuration used for LabCorp’s in-house assay. Using matched aliquots of neat synthetic peptides, the on-column LLOQ of each system was defined as the lowest absolute amount of surrogate peptide loaded that met the pre-defined acceptance criteria for signal-to-noise, precision, and linearity of the normalized analytical response (Fig. 2). Based on this study, the on-column LLOQ of the µLC–MS/MS system (5 attomoles) was estimated to be 40-fold lower than that of the LC–MS/MS configuration used for LabCorp’s in-house assay (200 attomoles). Given the validated LLOQ of LabCorp’s in-house LC–MS/MS assay is 0.2 ng/mL, this suggested an assay LLOQ of 0.005 ng/mL could be feasible on the µLC–MS/MS system using the same procedure.
Fig. 2.
Matched, neat solutions containing decreasing amounts of the unlabeled surrogate peptide (analyte, A) and a fixed amount of labeled surrogate peptide (internal standard, IS) were each injected in quadruplicate on the two instrument platforms along with a blank solution lacking the unlabeled peptide. The resulting data was used to determine the lowest amount of unlabeled peptide loaded on column that could be reliably measured based on: (A) imprecision of the A:IS peak area ratio (PAR) less than 20%, (B) linearity bias of the A:IS PAR less than +/- 20%, and (C) signal-to-noise of greater than 5. Signal-to-noise was calculated from the unlabeled peptide peak area in the respective sample (i.e., signal) divided by the average unlabeled peptide peak area integrated at the same retention time in the blank solution lacking the unlabeled peptide (i.e., noise).
3.2. µLC–MS/MS assay refinement
Despite the superior raw sensitivity of the µLC–MS/MS system, there were multiple challenges preventing simple translation of LabCorp’s LC–MS/MS assay procedure onto the µLC–MS/MS system and realization of a 0.005 ng/mL assay LLOQ. First, the additional sensitivity of the µLC–MS/MS system revealed low-level contamination/interferences in multiple surrogate matrices (e.g., animal serum and purified human serum albumin) that precluded their use for preparing external calibrators for the µLC-MS/MS assay despite having no detectable contamination or interference on the LC–MS/MS system (see Fig. 2 in Supplemental Data).
The only analyte/contaminant-free surrogate matrix tested on the µLC–MS/MS system was SigMatrix, which is prepared from recombinant human serum albumin expressed in rice (see Fig. 3 in Supplemental Data). Ultimately, parallelism with serum was demonstrated (see below) and, as such, SigMatrix was used for preparation of external calibrators for the µLC–MS/MS assay.
Furthermore, attempts to analyze fully-processed serum samples on the µLC–MS/MS system were initially hindered due to deleterious clogging of both the trapping and analytical columns, presumably due to accumulation/precipitation of undigested protein on the column beds and/or chemical frits. Addition of a protein-scavenging, guard column between the injector and trapping column markedly improved chromatographic ruggedness of the µLC–MS/MS system (Fig. 1). Although implementation of the guard column resulted in a 40% sensitivity loss (see Fig. 4 in Supplemental Data), no loss in chromatographic fidelity or further loss of sensitivity was observed during the course of over 1000 injections of fully-extracted samples, suggesting even greater longevity would be reasonably expected for this application (see Fig. 5 in Supplemental Data). Nonetheless, it was decided to prudently inject only half of the processed specimen volume on the µLC–MS/MS system in order to allow for re-injection in the event of a system failure. In summary, a roughly 4-fold sensitivity reduction was the compromise to achieve robust performance on µLC–MS/MS system, allowing a net 10-fold improvement in the assay LLOQ.
3.3. µLC–MS/MS performance evaluation
Following modification of the assay parameters, the analytical performance of the µLC–MS/MS assay was evaluated over the course of 10 batches, which were prepared and analyzed during an eight-day period. Studies were solely focused on assessing the imprecision, inaccuracy, and interference of the µLC–MS/MS assay at the target LLOQ of 0.02 ng/mL. A detailed description and summary of the performance evaluation studies may be found in Supplemental Data (see Tables 2–5 and Figs. 6–7 in Supplemental Data). All studies successfully met the pre-defined goals for acceptable performance, which were imprecision and inaccuracy < 15% throughout the analytically measurable range (up to 20 ng/mL), with 20% allowable at the target LLOQ. Additionally, sources of interference (e.g., carryover) were to be less than 25% at the LLOQ and relative accuracy to comparator methods were to be between 90 and 110%.
In particular, inter-assay imprecision and parallelism was assessed by repeat analysis of a serially diluted serum pool over the 10 batches to estimate the LLOQ, which was defined as the lowest concentration at which 20% imprecision and 20% inaccuracy was achieved. The precision profile across the 9 levels of diluted serum was used to create a model, which determined that 0.0204 ng/ml was the lowest concentration at which a coefficient of variation (CV) of 20% would be observed (Fig. 3A). Parallelism across the same 9 levels of diluted serum was determined by calculating the bias of the measured value relative to the expected value at each dilution, the latter being determined from the mean measured value in the undiluted serum sample and the applied dilution factor (Fig. 3B). By this method, parallelism (acceptable bias from linearity) was observed down to 0.00478 ng/mL, thereby confirming an LLOQ of 0.02 ng/mL.
Fig. 3.

Evaluation data for the µLC–MS/MS assay sensitivity are shown. (A) Imprecision, expressed as the coefficient of variation (CV) in the measured Tg concentration across 10 batches (2 replicates/level/batch), is plotted and modeled for serum serially diluted with SigMatrix, with the Tg concentration interpolating at 20% CV indicated. (B) Inaccuracy in the serum dilution series, with the mean bias from linearity plotted, bars showing the maximum and minimum bias across all replicates in the 10 batches (2 replicates/level/batch), and dotted lines indicating ±20% bias. (C) The measured analyte:internal standard peak area ratio (A:IS PAR) in the zero and 0.02 ng/mL calibrator are plotted for the 10 batches (1 replicate/level/batch), with the response curve fitted to the mean A:IS PAR and the LOD indicated as the Tg concentration interpolating at 3 standard deviations above the mean A:IS PAR of the zero calibrator.
The limit of detection (LOD) was also estimated in these 10 batches using the mean and standard deviation (SD) of the measured response of the blank (zero) standard (Fig. 3C). The response LOD was calculated as the mean plus 3SD, which was then interpolated through a response curve generated from the mean response of both the blank (zero) and 0.02 ng/mL standards over the 10 batches to provide an estimated concentration LOD of 0.0057 ng/mL. Consequently, a measured value at or above 0.0057 ng/mL in these 10 batches can be distinguished from a zero value with 99% confidence.
3.4. Evidence for remnant thyroid tissue and sub-clinical Tg
Potential utility of the µLC–MS/MS assay with an LLOQ of 0.02 ng/mL was first gauged by measuring two sets of de-identified serum specimens during the 10 performance evaluation batches (Fig. 4). The first set of specimens comprised 53 TgAb-negative (<1 IU/mL) specimens measuring below the LLOQ of the Beckman Access Tg Immunoassay (<0.1 ng/mL) and the second set of specimens comprised 57 TgAb-positive (median: 8.7 IU/mL, range: 1.0 to 1376.0 IU/mL) specimens measuring below the LLOQ of LabCorp’s in-house LC–MS/MS Tg assay (<0.2 ng/mL). When measured on the µLC–MS/MS assay, 35 of 53 TgAb-negative samples and 25 of 57 TgAb-positive samples had Tg values greater than 0.02 ng/mL (i.e., quantifiable values). Furthermore, 50 of 53 TgAb-negative samples and 42 of 57 TgAb-positive samples had Tg concentrations above the LOD established for this study (0.0057 ng/mL, Fig. 3C). Although the clinical status of these individuals was unknown, it is unlikely the Tg measured in these specimens was a true indication of biochemical recurrence given the relative low prevalence for recurrent DTC following thyroidectomy [1]. Nonetheless, these results indicate a significant portion of both TgAb-negative and TgAb-positive patients classified as having “undetectable” Tg by conventional measurements will have sub-clinical levels of Tg in circulation, which explains how TgAb may persist in TgAb-positive patients despite the apparent absence of circulating Tg determined by less sensitive Tg assays. Further, the lower prevalence of measurable Tg by the µLC–MS/MS assay in TgAb-positive specimens as compared to TgAb-negative specimens in this study (χ2 = 8.562, p = 0.003) supports the hypothesis that the presence of TgAb reduces the circulating concentrations of Tg by an increased rate of clearance.
Fig. 4.
Remnant serum specimens received for Tg and TgAb testing at LabCorp in Burlington, North Carolina were selected for analysis by the µLC–MS/MS assay. Serum Tg measurements made by the µLC–MS/MS assay are plotted for (left) 53 TgAb-negative individuals with corresponding Tg measurements less than 0.1 ng/mL by the Beckman Access Immunoassay and (right) 57 TgAb-positive individuals with corresponding Tg measurements less than 0.2 ng/mL by LabCorp’s in-house LC–MS/MS assay. The LLOQ for the three Tg assays are indicated along with the estimated LOD for the µLC-MS/MS during these studies. TgAb measurements for this specimen cohort were performed on the Beckman Access Immunoassay from Beckman-Coulter (functional sensitivity = 1 IU/mL).
To determine whether or not Tg circulating at sub-clinical levels was derived from remnant thyroid tissue, and could still serve as a marker for DTC, matched specimens were obtained pre- and post-thyroid stimulation for 7 patients with low/undetectable TgAb (≤2.1 IU/mL by KRONUS RIA) and with pre-stimulated Tg values <0.1 ng/mL as measured by the Beckman Access Immunoassay. When analyzed by the µLC–MS/MS assay (Fig. 5), pre- and post-stimulation specimens for 2 of 7 patients showed no quantifiable Tg in any specimen, indicating an insufficient remnant thyroid tissue mass to secrete measurable Tg by the µLC–MS/MS assay. Increasing Tg measurements were observed post-stimulation by the µLC–MS/MS assay in 4 of 7 patients relative to low (<0.1 ng/mL) pre-stimulated measured values, indicating the Tg measured pre- and post-stimulation in these patient specimens was derived from remnant thyroid tissue and, thus, may be suitable as a marker for thyroid tumor growth.
Fig. 5.
Archived samples received for Thyroid Stimulating Hormone (TSH), Tg, and TgAb testing at the USC Endocrine Laboratories were assayed by the µLC–MS/MS assay. These low TgAb samples comprised matched specimens received 3 to 5 days apart with a marked increase in TSH results in subsequent specimens, which are indicative of TSH stimulation and/or cessation of suppression therapy. Four individuals (A-D) had baseline Tg measurements below the µLC–MS/MS assay LLOQ, but two who showed measureable Tg after stimulation. Three individuals (E-G) had measureable Tg at baseline, with increasing Tg measurements post-stimulation. The high baseline Tg measurement for patient E is unexplained, but increasing trends between days 2 and 4 are suggestive of remnant thyroid tissue. TSH measurements for this specimen cohort were performed on the Roche Cobas Immunoassay, while the TgAb measurements were on the RIA from KRONUS (functional sensitivity = 0.4 IU/mL).
3.5. Sub-clinical Tg in the absence of disease
To determine if the µLC–MS/MS assay could improve the ability to detect recurrent disease in TgAb-positive patients with low (<0.5 ng/mL) Tg concentrations, 48 TgAb-positive serum specimens (median 11.95 IU/mL; range 1–654.5 IU/mL by the Beckman Immunoassay) archived at UWMC from 44 total thyroidectomy patients were analyzed by the µLC–MS/MS assay based on prior negative indications for biochemical recurrence. Specifically, TgAb-positive patient samples were blindly selected for analysis by the µLC–MS/MS assay based on low Tg results by both the Beckman Access Immunoassay (Tg < 0.1 ng/mL) and UWMC LC–MS/MS assay (Tg < 0.15 ng/mL), but without prior knowledge of the patient’s disease status. When analyzed by the new µLC–MS/MS assay, 35% of these samples (17 of 48) had quantifiable levels of Tg, suggesting biochemical recurrence that was missed by the Beckman Access Immunoassay and UWMC LC–MS/MS assays [19]. However, no patient in this cohort was classified as having recurrent disease on follow-up (median follow-up >1 year, Table 1). These results do suggest that Tg concentrations of 0.15 ng/mL and lower in TgAb-positive DTC patients may indicate the absence of disease (or at least stable findings by imaging) over a long duration.
Table 1.
Tg measurement summary for TgAb-positive DTC patients.
| Disease Classification at Follow-up | Median (range) Follow-up, Days | µLC–MS/MS (LLOQ = 0.02 ng/mL) |
UWMC LC–MS/MS (LLOQ = 0.15 ng/mL) |
Beckman Immunoassay (LLOQ = 0.1 ng/mL) |
|||
|---|---|---|---|---|---|---|---|
| <0.15 ng/mL | >0.15 ng/mL | <0.15 ng/mL | >0.15 ng/mL | <0.15 ng/mL | >0.15 ng/mL | ||
| Free | 403.5 (6–577) | 40 | 0 | 40 | 0 | 40 | 0 |
| Indeterminate | 375 (0–496) | 5 | 0 | 5 | 0 | 5 | 0 |
| Stable | 532 (403–637) | 3 | 0 | 3 | 0 | 3 | 0 |
| Recurrent | – | 0 | 0 | 0 | 0 | 0 | 0 |
4. Discussion
While no single serum Tg measurement is expected to be diagnostic/prognostic of recurrent thyroid cancer, increasing longitudinal Tg measurements are considered critical information to the management of DTC patients [1]. Unfortunately, LC–MS/MS and immunometric Tg assays described to date have been shown ineffective in monitoring for recurrent disease in a large portion of TgAb-positive, total thyroidectomy patients due to insufficient analytical sensitivity and autoantibody interference, respectively [18], [19]. To that end, a highly sensitive LC–MS/MS assay was developed to allow for interference-free longitudinal monitoring of low serum Tg levels as a marker for recurrent DTC in both TgAb-negative and TgAb-positive patients.
The sensitive bottom-up protein assay for the measurement of serum Tg was developed using a commercially available µLC–MS/MS system to achieve an estimated LLOQ of 0.02 ng/mL. While the analytical performance of the µLC–MS/MS assay needs to be verified over a long period of time (>6 months) to prove clinical reliability [22], a 0.02 ng/mL LLOQ constitutes over an order of magnitude improvement in analytical sensitivity relative to other LC–MS/MS assays described to date. Although µLC–MS/MS platforms have not been broadly deployed in clinical diagnostic settings at least in part due to poor reliability, the commercially available system utilized herein demonstrated robust performance after the inclusion of a protein-scavenging guard column up-stream of the trapping and analytical columns to capture insoluble and/or undigested material. As such, this µLC–MS/MS system afforded a reliable and marked improvement in the analytical sensitivity for the measurement of serum Tg.
Utilizing the µLC–MS/MS assay to measure TgAb-negative and TgAb-positive specimens revealed a significant proportion of individuals that had Tg in circulation despite previously undetectable Tg measurements by other methods that were not attributable to TgAb interference. Confirmation that Tg can remain in circulation at sub-clinical levels (<0.1 ng/mL) can explain the persistence of TgAb in TgAb-positive individuals despite the apparent lack of Tg as measured by less sensitive methods or methods prone to TgAb interference. An increase in measured Tg concentrations was observed using the µLC–MS/MS assay in a subset of individuals following TSH stimulation, indicating the low levels of Tg measured by the new assay were indeed derived from remnant thyroid tissue and, thus, may be suitable in monitoring for the biochemical recurrence of DTC.
To that end, specimens from TgAb-positive, total thyroidectomy patients with negative indications for biochemical recurrence by other Tg measurements (Tg < 0.15 ng/mL) were retrospectively measured by this µLC–MS/MS assay without a priori knowledge of their disease status. Although a significant portion (35%) of specimens tested had quantifiable levels of Tg by the µLC–MS/MS assay (>0.02 ng/mL), follow-up confirmed the absence of recurrent disease in all patients tested (Table 1). Although these results do not demonstrate an improved ability of the µLC–MS/MS assay to detect recurrent disease, they do suggest that a Tg result below 0.15 ng/mL is indicative of a low risk of recurrent disease in TgAb-positive individuals following total thyroidectomy.
Most importantly, the prevalence of low, but measurable Tg levels in these disease-free patients demonstrates the potential for interference-free monitoring of low serum Tg levels (<0.5 ng/mL) by µLC–MS/MS prior to the on-set of recurrent disease in TgAb-positive patients, which has been a limitation for both less sensitive LC–MS/MS assays and immunoassays prone to autoantibody interference [18], [19]. Moreover, monitoring Tg levels by equally sensitive LC–MS/MS assays, irrespective of TgAb status, may be most appropriate given the discordance between TgAb assays [18] and the possibility that reliable (interference-free) monitoring of low Tg levels could allow for earlier detection of recurrent disease – something that may be particularly critical for high-risk patients with more aggressive forms of disease. However, it is also possible that earlier detection of recurrent disease by sensitive biochemical monitoring will not improve clinical outcomes if ablation therapy is not warranted (based on individualized risk) and/or if the imaging techniques performed as follow-up are insensitive to locate the cancer and, thus, do not allow for surgical intervention. Obviously these clinical implications are unsubstantiated as of yet and will need to be verified in future clinical studies using a sensitive LC–MS/MS assay. Indeed, following a full analytical validation, the µLC–MS/MS assay described herein is intended as the enabling technology for those future clinical studies.
Acknowledgments
Acknowledgements
We thank the Duke Proteomics and Metabolomics Shared Resource, in particular Professors Arthur Moseley and Erik Soderblom, for use of the ionKey/MS system. Also, special thanks to Dr. Larissa Fenn and Dr. James Murphy of Waters Corporation for facilitating the pilot studies on the ionKey/MS system and to Dr. Andre Valcour of LabCorp for input on the manuscript. The work performed at the University of Washington was partially funded by NIDDK/NIH (grant P30DK035816, ANH).
Declaration of Competing Interest
Dr. Shuford, Mr. Johnson, Dr. Thompson, Ms. Holland, and Dr. Grant have nothing to disclose. Dr. Hoofnagle reports grants from Waters, grants from NIH, during the conducted studies, personal fees from Kilpatrick Townsend & Stockton LLP, outside the submitted work. In addition, Dr. Hoofnagle has a patent Anti-thyroglobulin peptide antibodies licensed to SAT, Inc..
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinms.2020.01.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., et al. American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovanella L., Suriano S., Ceriani L., Verburg F.A. Undetectable thyroglobulin in patients with differentiated thyroid carcinoma and residual radioiodine uptake on a postablation whole-body scan. Clin. Nucl. Med. 2011;36:109–112. doi: 10.1097/RLU.0b013e318203bb84. [DOI] [PubMed] [Google Scholar]
- 3.Spencer C., LoPresti J., Fatemi S. How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21:394–404. doi: 10.1097/MED.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer C.A. Clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC) J. Clin. Endocrinol. Metab. 2011;96:3615–3627. doi: 10.1210/jc.2011-1740. [DOI] [PubMed] [Google Scholar]
- 5.Spencer C.A. Challenges of serum thyroglobulin (Tg) measurement in the presence of Tg autoantibodies. J. Clin. Endocrinol. Metab. 2004;89:3702–3704. doi: 10.1210/jc.2004-0986. [DOI] [PubMed] [Google Scholar]
- 6.Mariotti S., Barbesino G., Caturegli P., Marino M., Manetti L., Pacini F., et al. Assay of thyroglobulin in serum with thyroglobulin autoantibodies: an unobtainable goal? J. Clin. Endocrinol. Metab. 1995;80:468–472. doi: 10.1210/jcem.80.2.7852506. [DOI] [PubMed] [Google Scholar]
- 7.Bayer M.F., Kriss Joseph P. Immunoradiometric assay for serum thyroglobulin: semiquantitative measurement of thyroglobulin in antithyroglobulin-positive sera. J. Clin. Endocrinol. Metab. 1979;49:557–564. doi: 10.1210/jcem-49-4-557. [DOI] [PubMed] [Google Scholar]
- 8.Crane M.S., Strachan M.W.J., Toft A.D., Beckett G.J. Discordance in thyroglobulin measurements by radioimmunoassay and immunometric assay: a useful means of identifying thyroglobulin assay interference. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2013;50:421–432. doi: 10.1177/0004563213480492. [DOI] [PubMed] [Google Scholar]
- 9.Schneider A.B., Pervos R. Radioimmunoassay of human thyroglobulin: effect of antithyroglobulin autoantibodies. J. Clin. Endocrinol. Metab. 1978;47:126–137. doi: 10.1210/jcem-47-1-126. [DOI] [PubMed] [Google Scholar]
- 10.Mariotti S., Cupini C., Giani C., Lari R., Rolled E., Falco A., et al. Evaluation of a solid-phase immunoradiometric assay (IRMA) for serum thyroglobulin: effect of anti-thyroglobulin autoantibody. Clin. Chim. Acta. 1982;123:347–355. doi: 10.1016/0009-8981(82)90181-4. [DOI] [PubMed] [Google Scholar]
- 11.Black E.G., Sheppard M.C. Serum thyroglobulin measurements in thyroid cancer: evaluation of ‘false’ positive results. Clin. Endocrinol. (Oxf). 1991;35:519–520. doi: 10.1111/j.1365-2265.1991.tb00937.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoofnagle A.N., Becker J.O., Wener M.H., Heinecke J.W. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin. Chem. 2008;54:1796–1804. doi: 10.1373/clinchem.2008.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke N.J., Zhang Y., Reitz R.E. A novel mass spectrometry-based assay for the accurate measurement of thyroglobulin from patient samples containing antithyroglobulin autoantibodies. J. Invest. Med. 2012;60:1157–1163. doi: 10.2310/JIM.0b013e318276deb4. [DOI] [PubMed] [Google Scholar]
- 14.Kushnir M.M., Rockwood A.L., Roberts W.L., Abraham D., Hoofnagle A.N., Meikle A.W. Measurement of thyroglobulin by liquid chromatography-tandem mass spectrometry in serum and plasma in the presence of antithyroglobulin autoantibodies. Clin. Chem. 2013;59:982–990. doi: 10.1373/clinchem.2012.195594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netzel B.C., Grebe S.K.G., Algeciras-Schimnich A. Usefulness of a thyroglobulin liquid chromatography-tandem mass spectrometry assay for evaluation of suspected heterophile interference. Clin. Chem. 2014;60:1016–1018. doi: 10.1373/clinchem.2014.224816. [DOI] [PubMed] [Google Scholar]
- 16.Hoofnagle A.N., Roth M.Y. Improving the measurement of serum thyroglobulin with mass spectrometry. J. Clin. Endocrinol. Metab. 2013;98:1343–1352. doi: 10.1210/jc.2012-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler S.E., Liu L., Blair H.C., Sivak R., Longo N., Tischler J., et al. Clinical laboratory verification of thyroglobulin concentrations in the presence of autoantibodies to thyroglobulin: comparison of EIA, radioimmunoassay and LC MS/MS measurements in an Urban Hospital. BMC Res. Notes BioMed Central. 2017;10:725. doi: 10.1186/s13104-017-3050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netzel B.C., Grebe S.K.G., Carranza Leon B.G., Castro M.R., Clark P.M., Hoofnagle A.N., et al. Thyroglobulin (Tg) testing revisited: Tg assays, TgAb assays, and correlation of results with clinical outcomes. J. Clin. Endocrinol. Metab. 2015;100:E1074–E1083. doi: 10.1210/jc.2015-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azmat U., Porter K., Senter L., Ringel M.D., Nabhan F. Thyroglobulin liquid chromatography-tandem mass spectrometry has a low sensitivity for detecting structural disease in patients with antithyroglobulin antibodies. Thyroid. 2017;27:74–80. doi: 10.1089/thy.2016.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Needham S.R., Valaskovic G.A. Microspray and microflow LC–MS/MS: the perfect fit for bioanalysis. Bioanalysis. 2015;7:1061–1064. doi: 10.4155/bio.15.42. [DOI] [PubMed] [Google Scholar]
- 21.T.R. Covey, B.B. Schneider, H. Javaheri, J.C.Y. LeBlanc, G. Ivosev, J.J. Corr, et al. ESI, APCI, and MALDI a Comparison of the Central Analytical Figures of Merit: Sensitivity, Reproducibility, and Speed. Electrospray MALDI Mass Spectrom. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2012. pp. 441–490.
- 22.Laboratory Medicine Practice Guidelines: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Thyroid, 2003, 13, 3–126. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.