Abstract
Selected ion flow tube mass spectrometry, SIFT-MS, is a non-separative method for direct quantitative analyses of volatile compounds, VOCs, in air and humid breath based on chemical ionization. Selected reagent ions, either H3O+, NO+ or O2+ (non-reactive with major components of air), ionize analyte molecules during a defined time in a flow tube by ion-molecule reactions thus producing analyte ions that are characteristic of the neutral analyte VOCs. Concentrations can be calculated in real-time from the ion count rates. Direct on-line analysis of single or multiple breath exhalations or off-line analysis of breath samples collected into bags can be performed. Several volatile breath metabolites have been quantified by SIFT-MS, including ammonia, acetone, hydrogen cyanide, alcohols, pentane, acetic acid, methane, and sulphur compounds. Their potential as biomarkers is discussed.
Keywords: SIFT-MS, Breath VOCs, Metabolites, Biomarkers
1. Introduction
The accurate quantification of trace metabolites in exhaled breath, mostly being volatile organic compounds, VOCs, can provide a meaningful addition to the clinical diagnostics already available to clinicians if they can be identified as biomarkers. A biomarker is defined by the National Institute of Health as ‘a characteristic that is reliably and accurately measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to an intervention’. A good example is the concentration of blood cholesterol (non-volatile). A great deal of research is now being carried out by scientists and clinicians to identify breath VOC biomarkers. Unfortunately, absolute quantification of identified metabolites is too often ignored, principally because it can be challenging and time-consuming and the adopted analytical method is not suited to accurate VOC quantification in the humid media that is exhaled breath [1].
Selected ion flow tube mass spectrometry, SIFT-MS, was conceived and developed for the direct, real-time quantification of metabolites in the humid breath, obviating sample collection into bags or onto some form of trap that can pre-concentrate the trace VOCs, but which may compromise the breath sample. SIFT-MS has been used to detect and quantify, to acceptable accuracy, several VOCs in single breath exhalations, directly to the patient/volunteer, and identified single metabolites related to specific pathological and physiological conditions. From a clinical perspective, it is easy to use and patient friendly. If direct sampling is not convenient, bag or trap sampling can be achieved with off-line SIFT-MS analysis, but data analysis must be approached with circumspection. Note that, besides SIFT-MS, other non-separative chemical ionisation techniques have been developed and are used for trace gas analyses of air, including those of volatile breath metabolites [2]. Of these, most widely used are proton transfer reaction mass spectrometry, PTR-MS [1], [3], and secondary electrospray ionisation mass spectrometry, SESI-MS [4]. For the sake of brevity, the scope of this review is limited to SIFT-MS breath analyses.
2. SIFT-MS analytical method
Detailed overviews of the SIFT-MS technique have been given previously in several reviews [5], [6], [7]. Briefly, it is based on the chemical ionization by selected reagent ions, H3O+, NO+ and O2+•, that ionise gaseous analytes present in air/breath samples in trace amounts. The range of neutral analytes accessible using the SIFT-MS technique has been extended recently by the exploitation of five additional negative reagent ions (O−, OH−, O2−, NO2−, and NO3−) [8]. Reactions occur between the selected reagent ion and the neutral analyte molecules present in sample that continuously enters the flow tube at a known flow rate, producing characteristic analyte ions, the signal intensities of which are recorded by a downstream analytical mass spectrometer. Identification of the neutral trace compounds from the analyte ions generated by different selected reagent ions is based on knowledge of the relevant ion-molecule chemistry. Concentrations of the trace analyte molecules in the sample are calculated from the reagent and analyte ion count rates and accounting for several measured physical parameters, including the flow tube gas pressure, temperature and carrier gas (usually helium) and sample gas (air/breath) flow rates. [9], [10]. An understanding of the ion chemistry occurring in SIFT-MS, especially when humid samples are being analysed, is needed to achieve reliable and accurate trace gas analysis [11], [12], [13]. Thus, an associated ion kinetics library has been built based on extensive studies of the reactions of the individual reagent ions with a wide range of VOCs that are present at trace concentrations in exhaled breath and other gaseous media, such as the headspace of blood serum, urine and cell cultures. The typical limits of detection achieved using the current SIFT-MS instrumentation are below one part-per-billion by volume in the sample (ppbv) [14].
The raw data from SIFT-MS analysis of a breath/air sample is usually in the form of a mass spectrum acquired by the downstream analytical mass spectrometer (usually a quadrupole) over a chosen mass-to-charge ratio, m/z. The peaks on the spectrum are those of the reagent ions and the analyte ions. The kinetics library relates the analyte ions to the trace compounds (VOCs) present in the sample (breath) and treatment of the ion counts (signals) and the known kinetics of the reagent ion/VOC reactions allows the quantification of the VOCs. This is called the full scan (FS) mode of analysis. For more accurate analysis of chosen neutral compounds, the count rates of their specific analyte ions are measured, which allows longer ion integration times and, hence, a greater precision. This is called the multiple ion monitoring (MIM) or selected ion monitoring (SIM) mode of analysis and is chosen when analysing single breath exhalations for chosen metabolites. By this method, several metabolites can be quantified simultaneously in single breath exhalations. A further valuable feature of SIFT-MS is that analysis of both mouth-exhaled and nose-exhaled breath can be accomplished on-line and in real-time using the MIM mode [15], [16], [17], [18].
3. Quantified exhaled breath metabolites
Much breath analysis research has been directed toward identifying the ranges of concentrations of the common metabolites in healthy people, distinguishing endogenous from exogenous compounds and the search for and quantification of VOCs that could be biomarkers of disease. As the concentrations of many volatile metabolites in exhaled breath are low (ranging from sub-ppbv to 20 ppbv), it is important that analytical methods and instruments provide adequate analytical accuracy and precision to be clinically useful [19]. Much effort has been made in comparing the analytical FS spectra obtained for the breath of patients with specific diseases to those obtained for healthy controls, using statistical models to differentiate them without identifying the neutral VOCs present in the breath samples. This is just a first step in biomarker discovery. Its main value is that it can provide the rationale for the more important targeted analyses. In this short article, the focus is on those few breath metabolites that have been identified and quantified (providing approximate reference ranges) and on their potential as biomarkers.
3.1. Ammonia (NH3)
Interest in breath ammonia stems from the anticipation that its analysis will be useful in clinical practice, especially in nephrology [20]. A detailed study of mouth-exhaled and nose-exhaled ammonia shows that most of it is generated in the oral cavity by the action of enzymes on salivary urea [21], [22], and therefore it is concluded that mouth-exhaled ammonia is always elevated above the alveolar concentration that would be equilibrated with blood ammonia. Nose-exhaled ammonia (typically below 100 ppbv) largely originates at the alveolar interface and so its concentration more closely relates to the expected alveolar blood ammonia concentration [21]. Mouth-exhaled ammonia concentration in healthy people ranges from about 50 ppbv to 3000 ppbv [23]. Ingestion of proteins results in increased blood/saliva urea [24], causing an increase in mouth-exhaled ammonia. The presence of H. pylori infection [21], [25] also leads to elevated breath ammonia. So elevated mouth-exhaled ammonia may be due to either abnormally high blood urea, high pH of the saliva/mouth/airways mucosa, poor oral hygiene or a combination of these. Breath ammonia concentrations in patients with chronic kidney disease can exceed 10,000 ppbv and is thus a potential estimator of the severity of uraemia [20], [26]. A further point is that laboratory air often contains appreciable concentrations of ammonia (typically 20–100 ppbv), which is inhaled during the normal breathing cycle and partially exhaled, apparently contributing to “endogenous” ammonia. A careful study of the retention coefficients of several inhaled compounds, including ammonia, which can enhance their concentrations in exhaled breath, has been carried out using on-line SIFT-MS [27]. It showed that the retention coefficient describing the effect of inhaled background on exhaled breath ammonia is 0.5 for nose-exhaled and 0.7 for mouth-exhaled breath.
3.2. Acetone (CH3COCH3)
Exhaled breath acetone (one of the ketone bodies produced during gluconeogenesis or lipolysis [28]) is interesting because of its suggested association with blood glucose and diabetes, yet there is no convincing experimental evidence that this association is clinically sound [29], [30]. In healthy people, breath acetone concentrations range from about 100 ppbv to 2500 ppbv [19], [23]. A confounding influence is diet, well demonstrated by the fact that a ketogenic diet can greatly increase breath acetone [31]. The wider effects of dietary nutrients on breath metabolites include a direct impact on metabolism or an alteration of the gastrointestinal flora [32]. Breath acetone, as related to diabetes, is discussed in some detail in [1], [33]. Breath acetone, as measured by SIFT-MS and by single mixed metal oxide sensors in a group of Type 1 diabetes patients during hypoglycaemic glucose clamps, was found to correlate linearly with blood glucose [30]. However, since there were widely differing concentrations for patients with the same blood glucose, it is doubtful that breath analysis can entirely replace blood glucose testing.
A very interesting recent SIFT-MS study on the restriction of diet by bariatric surgery [34] showed that exhaled acetone increased significantly after surgery, whereas some other breath ketones actually decreased after dieting and the surgery. It was concluded that breath acetone measurement could be potentially clinically useful as a non-invasive nutritional assessment in obese patients.
3.3. Hydrogen cyanide (HCN)
Since the first discovery of hydrogen cyanide above cultures of the bacterium Pseudomonas aeruginosa, PA, using SIFT-MS direct analysis [35], much work has been carried out to detect and quantify HCN in exhaled breath of cystic fibrosis (CF) patients, whose airways can be infected by PA [36]. Following the early seminal work [37], which showed that HCN was at a very low concentration in both mouth-exhaled and nose-exhaled breath of healthy non-smokers (typically 1–2 ppbv), a concerted effort has been carried out involving analysis of the breath of cohorts of adult patients with and without CF [38]. This study showed elevated concentrations of HCN in the exhaled breath of the CF patients. Additionally, much in vitro work has been carried out on genotypically different strains of PA, which showed that HCN and other volatile compounds, including 2-aminoacetophenone, are produced by most strains of PA in highly variable amounts [39]. A separate detailed study using SIFT-MS showed that methyl thiocyanate was emitted by 36 strains of PA and was also present in the exhaled breath of 28 CF patients [40]. Further to these studies, HCN and 2-aminoacetophenone emitted by PA cultured under planktonic and biofilm conditions have been studied using SIFT-MS analysis [41]. This collective major effort, on both in vitro PA cultures and on HCN in exhaled breath, amounted to more than a decade of work, which is summarized in a short review [36].
To investigate if breath HCN is a clinically valuable biomarker of PA infection in CF, a multicentre study involving 8 hospitals in Central England was carried out [42] in which 233 children with CF, but initially free from PA infection, were followed for 2 years. Breath HCN concentrations were compared to microbiology surveillance for PA (71 positive patients at the end of the study) with a conclusion that exhaled breath HCN is a specific (99%) biomarker of new PA infection in children with CF. However, it was deduced that the genetic variability of PA strains [43] can mean that in some patients the elevation in breath HCN is less pronounced leading to lower diagnostic sensitivity (more than a half false negatives). Hence, breath HCN should be complemented by other biomarker compounds to avoid false negatives for those patients infected with PA strains that produce less HCN.
3.4. Alcohols (CH3OH, C2H5OH, C3H7OH …)
Methanol (CH3OH) and ethanol (C2H5OH) are present in the exhaled breath of all healthy people at concentrations of typically 100 to 400 ppbv [23]. Concentrations of these alcohols can readily be measured by SIFT-MS and it is seen that mouth-exhaled and nose-exhaled breath have very similar methanol concentrations [37]. However, ethanol is often at a much higher concentration in mouth-exhaled breath when sugary foods are ingested due to bacterial and enzymatic activity in the oral cavity [44], [45]. While ethanol has received much attention for obvious reasons (see below), methanol has received much less attention than other common breath VOCs. It is contained in some foods and drinks, such as ripe fruits and fruit juices, ingestion of which can increase the methanol in the blood circulation and, hence, in the exhaled breath. It is also formed in the hydrolysis of the artificial sweetener Aspartame (methyl-L-α-aspartyl-L-phenylalaninate), which is used in foods and drinks and is considered to be safe at an ADI (acceptable daily intake) of 40 mg per kg of body weight. Aspartame is hydrolysed in the gastrointestinal tract to aspartic acid, phenylalanine and methanol, all being toxic at high concentrations. So a study was carried out of the exhaled breath of ten healthy volunteers following the ingestion of a single ADI dose of aspartame scaled according to their body weight [46] to quantify the volatile methanol component. Methanol concentrations were measured by SIFT-MS on-line and in real-time in single mouth-exhaled and nose-breath exhalations, before aspartame ingestion to establish individual pre-dose baseline and then for two hours post-ingestion to follow the increase and subsequent decrease of methanol. It was shown that the methanol concentration increased in all volunteers by 1082 ± 205 ppbv from their pre-ingestion values, which ranged from 193 to 436 ppbv to peak values ranging from 981 to 1622 ppbv and slowly decreased with time. So, for theses few healthy volunteers, a single ADI dose of aspartame resulted in a 3- to 6-fold increase of breath methanol concentration above the baseline values.
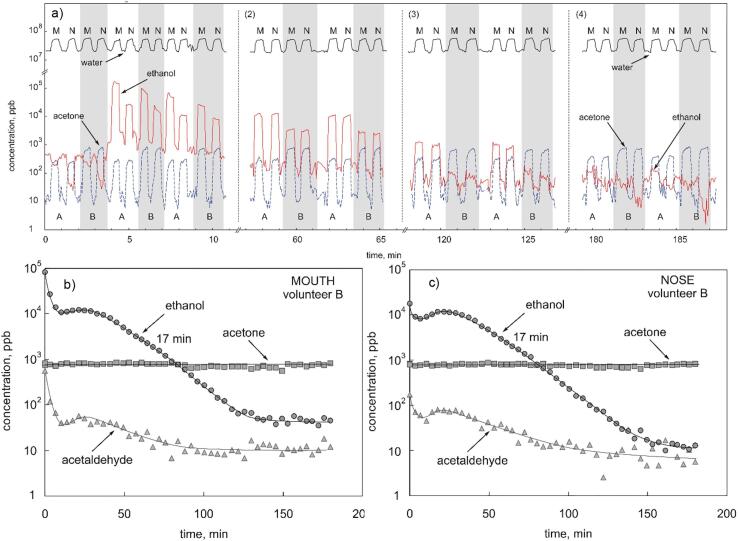
Physiological and psychological effects of excessive alcohol (ethanol) ingestion have been intensively researched and are well understood, largely because of its social consequences. A specific interest lies in the speed at which it is removed from the body, that is in the pharmacokinetics of its metabolism. The speed and precision of SIFT-MS for on-line direct analysis of mouth-exhaled and nose-exhaled breath provides an excellent way to monitor ethanol decay in the body. So the decay of ethanol in single mouth-exhalations and nose-exhalations of healthy volunteers was studied following the ingestion of varying doses of alcohol in aqueous solutions at different dilutions [47]. SIFT-MS measurements were made continuously over some 200 min following each alcohol dose. The time interval between breath exhalations was only a few seconds (Fig. 1a), and this results in well-defined decay curves for breath ethanol over 2–3 orders-of-magnitude for mouth-exhaled (Fig. 1b) and nose-exhaled (Fig. 1c) breath. The data show that ethanol mouth contamination rapidly diminished in just a few minutes post ingestion. As expected, the peak concentration of breath ethanol and its decay rate depend on the aqueous solution concentration and volume of the solution ingested. Both the efficiency of the first-pass metabolism of ethanol and the gastric emptying rates at the various doses and ingested volumes can be estimated [47]. Simultaneous measurements of acetaldehyde, acetic acid and acetone breath concentrations were also measured in each single breath exhalation. Thus, acetaldehyde, which is the primary product of ethanol metabolism, tracks the breath ethanol. Acetic acid, which is a secondary product of this metabolism, was detected at low concentrations, but was shown to largely originate in the oral cavity. Interestingly, breath acetone was seen to increase over the 3-hour period of measurement due to fasting of the volunteers.
Fig. 1.
SIFT-MS MIM time profiles of water vapour, acetone and ethanol concentrations (in ppbv) measured in alternating mouth (M) and nose (N) exhalations of two volunteers, labelled A and B, after ingestion of 15 mL (by A) and 7.5 mL (by B) of ethanol diluted in 250 mL of water. a) raw data showing concentration in single exhalations, b) variation of concentration with time, including acetaldehyde, for these compounds in mouth exhalations (by B), c) the same for the nose exhalations. The exponential decay rates are both indicated as 17 min. Reproduced from [47] with permission from Wiley.
The alcohols propanol (C3H7OH) and ethylene glycol (CH2OH)2 can also be analysed in gaseous samples using SIFT-MS [48], [49]. A growing societal problem is poisoning by oral ingestion of these compounds [50]. Whilst breath analysis has not yet been trialled as a systemic detector of these compounds, it may be a ready, immediate and more rapid diagnostic than blood analysis in the emergency clinic.
3.5. n-pentane (C5H12)
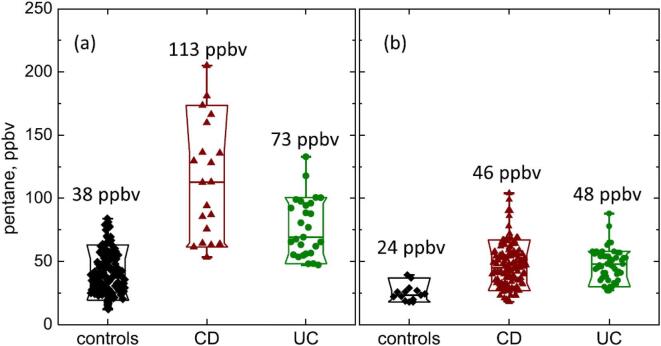
The alkane, n-pentane, is thought to be an indicator of oxidative stress, lipid peroxidation and inflammation in the body, including inflammatory bowel disease, IBD [2], [51], [52], [53]. If present in exhaled breath at concentrations accessible to real-time SIFT-MS analysis it could be a very valuable addition to medical diagnosis. A pilot study of pentane in the exhaled breath of a small cohort of patients with Crohn’s disease (CD), ulcerative colitis (UC) and a few healthy volunteers, by real-time, on-line SIFT-MS analysis [54] revealed that it was significantly elevated in the exhaled breath of both the CD patients (mean 114 ppbv), and the UC (mean 84 ppbv) patients relative to a healthy cohort (mean 40 ppbv), as seen in Fig. 2a. This suggests that SIFT-MS can be used to quantify pentane in human breath in and might form the basis of non-invasive screening for inflammatory processes, including IBD.
Fig. 2.
Box-and-whisker plot of breath pentane concentrations, in ppbv, for healthy controls, CD and UC cohorts: (a) data obtained from direct exhalations [54], (b) data obtained from bag samples of breath [55]. Median values are as indicated. Reproduced from [55] with permission from IOP Publishing.
So, recently, a more detailed study has been carried out involving 136 CD patients and 51 UC patients and 14 controls [55]. Breath samples were collected in Nalophan bags for off-line SIFT-MS analysis of pentane and several VOCs, which confirmed that the median concentration of breath pentane was elevated in IBD patients compared to healthy controls. However, the absolute median pentane concentrations were about a factor of two lower in the bag samples compared to those in the real-time directly analysed single exhalation (Fig. 2b). This is a good illustration of the dilution of VOCs in bag samples (due to diffusive loss through the bag material and surface adsorption) and the real value of on-line SIFT-MS analysis. The breath concentrations of hydrogen sulphide, acetic acid, propionic acid and butanoic acid collected into the bag samples were more widely spread in the IBD patient samples compared to those for the healthy controls. The relative concentrations of the last VOCs and pentane weakly correlated with simple clinical activity indices. It was suggested that hydrogen sulphide and these carboxylic acids might be breath biomarkers of intestinal bacterial overgrowth, and their quantification might assist therapeutic intervention to alleviate the symptoms of IBD.
3.6. Acetic acid (CH3COOH)
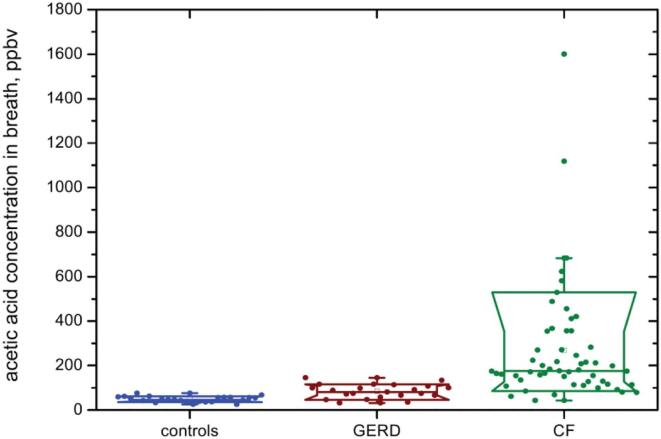
Gastro-oesophageal reflux disease, GERD, is a common cause of chronic cough [56]. A study of the mouth-exhaled breath of 22 GERD patients using direct SIFT-MS analyses revealed that end-expiratory median concentration of acetic acid (85 ppbv) was significantly higher than that in the breath of a control cohort (48 ppbv; see Fig. 3) [57]. It was concluded that elevated breath acetic acid results from a lowering of the pH of the lining of the airways by reflux acid (essentially HCl). Thus, breath acetic acid may be useful as a non-invasive diagnostic of GERD.
Fig. 3.
Exhaled breath concentrations of acetic acid measured by SIFT-MS in healthy controls and in patients with GERD and CF. Reproduced from [58] with permission from IOP publishing.
Subsequently, a study of the VOCs present in the exhaled breath of 58 CF patients, carried out by collecting breath into Nalophan bags and analysed within 20 min of collection [58], revealed that the acetic acid vapor concentration was significantly elevated in their breath (Fig. 3), and was independent of their Pseudomonas aeruginosa (PA) infection status. Thus, the median acetic acid concentrations were: PA-infected (170 ppbv); PA-negative (182 ppbv); healthy controls (48 ppbv). This elevation cause may again be due to a decreased pH of the mucosa of the CF airways (like that for the GERD patients), but with the acetic acid concentration reaching much higher values in CF, as can be seen in the comparison shown in Fig. 3 [59]. Thus, breath acetic acid concentration could be an indicator of the acidity of the CF airways mucosa.
3.7. Methane (CH4)
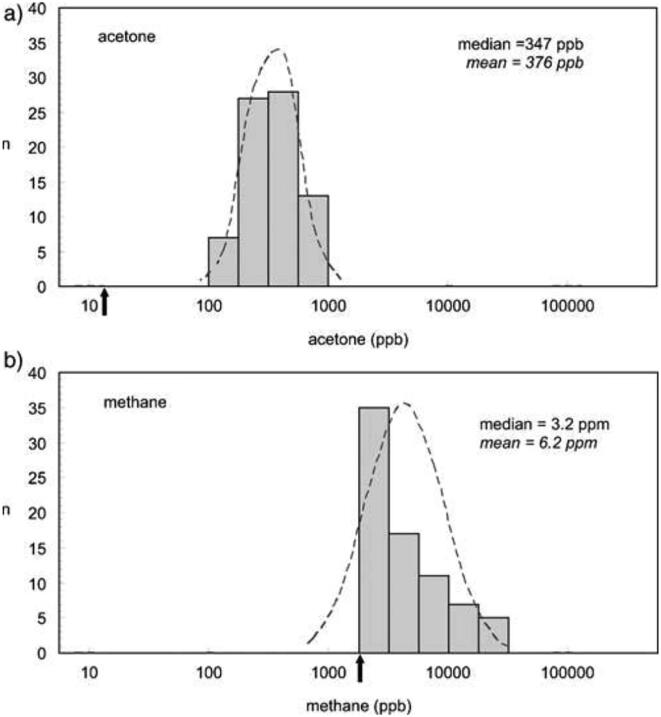
Methane (CH4) is a component of air that is a major contributor to climate change. A significant fraction is produced by ruminants and decaying plant material. It is also present in exhaled breath of a significant fraction of human beings, its concentration being promoted by gastrointestinal diseases, bowel cleansing and, to a lesser degree, antibiotic therapy. Thus, breath methane has some clinical relevance. So a SIFT-MS study of methane in the exhaled breath of some 75 volunteers, was carried out on-line and in real-time, simultaneously with breath acetone, all within the short period of 3 h. Whereas the acetone population distribution was approximately log normal, Fig. 4a, as are most common breath VOCs [23], that for methane was found to range continuously (monomodal distribution, Fig. 4b) from the ambient tropospheric concentration of 2 ppmv to the high value 30 ppmv, with no significant variation with age and gender. This remarkable data flow rate well demonstrates what is possible using on-line, real-time analyses by SIFT-MS.
Fig. 4.
Vertical bar charts of the population distributions of the log-transformed concentrations (in ppbv) of a) acetone and b) methane. The dashed curves are fitted log-normal distributions, indicating a good match for acetone, as is usual, and a poor match for methane. The ambient air concentrations are indicated by the black arrows. Reproduced from [23] with permission from IOP publishing.
3.8. Sulphur-containing compounds (H2S, CH3SCH3…)
Oral malodour is an unfortunate condition, expressed occasionally even in healthy people. Often this is due to poor oral hygiene when the odorous volatile compounds, which are usually sulphur atom-containing, are generated by the action of oral flora and/or salivary enzymes. However, they may be produced systemically and be an indicator of some underlying disease. Thus, a SIFT-MS study of some sulphur-containing compounds, specifically hydrogen sulphide (H2S), methanethiol (CH3SH), dimethyl sulphide ((CH3)2S), dimethyl disulphide ((CH3)2S2) and carbon disulphide (CS2), was carried out in mouth- and nose-exhaled breath and in the oral cavity of several healthy volunteers [60]. This revealed the concentrations of these compounds to be in the range below 10 ppbv, except for H2S that reached a few tens of ppbv in the oral cavity. This study demonstrated the ease by which SIFT-MS can study these compounds in humid exhaled breath, opening the way to wider studies of halitosis and to investigating liver disease for which these compounds are also associated [61]. To quantify H2S at such low concentrations with good precision and accuracy, measurements have been made by combining thermal desorption with SIFT-MS [16].The mean (±SD) H2S concentrations in ppbv in ambient air, and in nose-exhaled and mouth-exhaled breath of 10 healthy volunteers were measured as 0.12 ± 0.02 0.40 ± 0.11 and 3.1 ± 2.5 respectively. The H2S concentration in the oral cavity was 13.5 ± 8.6 ppbv and correlated well with oral, but not nasal, breath concentrations. This suggests that mouth exhaled H2S derives mainly from the oral cavity, but nasal breath is more likely of systemic in origin. Interestingly, H2S acts as a gasotransmitter when present in the blood stream at very low concentrations (sub-ppbv) [16].
3.9. Deuterated water vapour for measurement of total body water (TBW)
Determination of total body water (TBW) in patients suffering from kidney disease is challenging. The failure of homeostatic mechanisms controlling fluid status, the chronic condition and associated comorbidity collectively disturb body composition, which can result in muscle wasting or obesity. Measurement of fluid status at the bedside could inform better clinical decision making. To investigate this potential assist to diagnosis, a near patient method for determining TBW has been developed. Following ingestion of a known amount of heavy water (D2O), which equilibrates as HDO within the TBW, HDO content of exhaled breath is elevated. On-line, real-time analysis of single breath exhalations using flowing afterglow mass spectrometry (FAMS) or SIFT-MS can accurately measure the concentration of breath HDO, thus allowing calculations of TBW without further intervention. Several clinical studies along these lines were undertaken [20], [62], [63] to check the validity of this minimally intervention method, especially for longitudinal measurements, and in comparison with other bed-side TBW methods, such as bio-impedance analysis. By these concerted studies, the feasibility and acceptability of TBW determinations using breath analysis in the dialysis clinic was established.
4. Summary
The extensive work on the development of the SIFT-MS analytical method and its use for on-line, real-time quantification of the volatile metabolites in single breath exhalations to an acceptable precision and accuracy has revealed its enormous potential as a non-invasive technique to assist clinical diagnosis. The examples given in this review of the measurements of individual breath VOC concentrations, and their value as potential biomarkers of normal and adverse clinical status, are just a prelude to the wider and more comprehensive studies that are needed. The current SIFT-MS instrumentation, which can quantify volatile compounds over a wide molecular mass range, is larger and heavier (about 110 kg) than is desirable. In-process developments are seeing significant reductions in size and weight of similar instrumentation [64], which could be exploited for breath analysis in restricted spaces, such as general practitioner surgeries and intensive care units. Further to this, even smaller specialised instruments and sensors are being developed to detect and quantify individual metabolites in exhaled breath [65]. The premise is that when suitable instrumentation is available then clinical use will follow.
Disclosures
The authors have no disclosures.
Declaration of competing interest
The authors have no conflicts to declare.
References
- 1.Turner C. Techniques and issues in breath and clinical sample headspace analysis for disease diagnosis. Bioanalysis. 2016;8:677–690. doi: 10.4155/bio.16.22. [DOI] [PubMed] [Google Scholar]
- 2.Casas-Ferreira A.M., del Nogal-Sanchez M., Perez-Pavon J.L., Moreno-Cordero B. Non-separative mass spectrometry methods for non-invasive medical diagnostics based on volatile organic compounds: a review. Anal. Chim. Acta. 2019;1045:10–22. doi: 10.1016/j.aca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Smith D., Španěl P., Herbig J., Beauchamp J. Mass spectrometry for real-time quantitative breath analysis. J. Breath Res. 2014;8 doi: 10.1088/1752-7155/8/2/027101. [DOI] [PubMed] [Google Scholar]
- 4.Sinues P.M.L., Kohler M., Zenobi R. Monitoring diurnal changes in exhaled human breath. Anal. Chem. 2013;85:369–373. doi: 10.1021/ac3029097. [DOI] [PubMed] [Google Scholar]
- 5.Španěl P., Smith D. Progress in SIFT-MS; breath analysis and other applications. Mass Spectrom. Rev. 2011;30:236–267. doi: 10.1002/mas.20303. [DOI] [PubMed] [Google Scholar]
- 6.Smith D., Španěl P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 2005;24:661–700. doi: 10.1002/mas.20033. [DOI] [PubMed] [Google Scholar]
- 7.Smith D., Španěl P. Ambient analysis of trace compounds in gaseous media by SIFT-MS. Analyst. 2011;136:2009–2032. doi: 10.1039/c1an15082k. [DOI] [PubMed] [Google Scholar]
- 8.Hera D., Langford V.S., McEwan M.J., McKellar T.I., Milligan D.B. Negative reagent ions for real time detection using SIFT-MS. Environments. 2017;4:16. doi: 10.3390/environments4010016. [DOI] [Google Scholar]
- 9.Španěl P., Dryahina K., Smith D. A general method for the calculation of absolute trace gas concentrations in air and breath from selected ion flow tube mass spectrometry data. Int. J. Mass Spectrom. 2006;249:230–239. doi: 10.1016/j.ijms.2005.12.024. [DOI] [Google Scholar]
- 10.Španěl P., Smith D. Advances in on-line absolute trace gas analysis by SIFT-MS. Curr. Anal. Chem. 2013;9:525–539. [Google Scholar]
- 11.Spanel P., Zabka J., Zymak I., Smith D. Selected ion flow tube study of the reactions of H3O+ and NO+ with a series of primary alcohols in the presence of water vapour in support of selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 2017;31:437–446. doi: 10.1002/rcm.7811. [DOI] [PubMed] [Google Scholar]
- 12.Smith D., Chippendale T.W.E., Španěl P. Reactions of the selected ion flow tube mass spectrometry reagent ions H3O+ and NO+ with a series of volatile aldehydes of biogenic significance. Rapid Commun. Mass Spectrom. 2014;28:1917–1928. doi: 10.1002/Rcm.6977. [DOI] [PubMed] [Google Scholar]
- 13.Španěl P., Smith D. Influence of weakly bound adduct ions on breath trace gas analysis by selected ion flow tube mass spectrometry (SIFT-MS) Int. J. Mass Spectrom. 2009;280:128–135. doi: 10.1016/j.ijms.2008.07.021. [DOI] [Google Scholar]
- 14.Zhu J.H., Nones C., Li Y., Milligan D., Prince B., Polster M., Dearth M. Ultra-trace real time VOC measurements by SIFT-MS for VIAQ. SAE Int. J. Engines. 2017;10:1815–1819. doi: 10.4271/2017-01-0989. [DOI] [Google Scholar]
- 15.Smith D., Spanel P. Pitfalls in the analysis of volatile breath biomarkers: suggested solutions and SIFT-MS quantification of single metabolites. J. Breath Res. 2015;9:11. doi: 10.1088/1752-7155/9/2/022001. [DOI] [PubMed] [Google Scholar]
- 16.Wondimu T., Wang R., Ross B. Hydrogen sulphide in human nasal air quantified using thermal desorption and selected ion flow tube mass spectrometry. J. Breath Res. 2014;8:8. doi: 10.1088/1752-7155/8/3/036002. [DOI] [PubMed] [Google Scholar]
- 17.Dummer J., Storer M., Sturney S., Scott-Thomas A., Chambers S., Swanney M., Epton M. Quantification of hydrogen cyanide (HCN) in breath using selected ion flow tube mass spectrometry-HCN is not a biomarker of Pseudomonas in chronic suppurative lung disease. J. Breath Res. 2013;7 doi: 10.1088/1752-7155/7/1/017105. [DOI] [PubMed] [Google Scholar]
- 18.Smith D., Chippendale T.W.E., Dryahina K., Španěl P. SIFT-MS analysis of nose-exhaled breath; mouth contamination and the influence of exercise. Curr. Anal. Chem. 2013;9:565–575. [Google Scholar]
- 19.Smith D., Španěl P. Pitfalls in the analysis of volatile breath biomarkers; suggested solutions and SIFT-MS quantification of single metabolites. J. Breath Res. 2015;9 doi: 10.1088/1752-7155/9/2/022001. [DOI] [PubMed] [Google Scholar]
- 20.Davies S.J., Španěl P., Smith D. Breath analysis of ammonia, volatile organic compounds and deuterated water vapor in chronic kidney disease and during dialysis. Bioanalysis. 2014;6:843–857. doi: 10.4155/bio.14.26. [DOI] [PubMed] [Google Scholar]
- 21.Spanel P., Smith D. What is the real utility of breath ammonia concentration measurements in medicine and physiology? J. Breath Res. 2018;12 doi: 10.1088/1752-7163/aa907f. [DOI] [PubMed] [Google Scholar]
- 22.Ross B.M., Babgi R. Volatile compounds in blood headspace and nasal breath. J. Breath Res. 2017;11 doi: 10.1088/1752-7163/aa7d10. [DOI] [PubMed] [Google Scholar]
- 23.Smith D., Turner C., Španěl P. Volatile metabolites in the exhaled breath of healthy volunteers: their levels and distributions. J. Breath Res. 2007;1 doi: 10.1088/1752-7155/1/1/014004. [DOI] [PubMed] [Google Scholar]
- 24.Smith D., Španěl P., Davies S. Trace gases in breath of healthy volunteers when fasting and after a protein-calorie meal: a preliminary study. J. Appl. Physiol. 1999;87:1584–1588. doi: 10.1152/jappl.1999.87.5.1584. [DOI] [PubMed] [Google Scholar]
- 25.C. Penault, P. Španěl, D. Smith, Detection of H-pylori infection by breath ammonia following urea ingestion, in: Breath Analysis: for Clinical Diagnosis and Therapeutic Monitoring, A. Amann, D. Smith (Eds.), 2005, pp. 393–399.
- 26.Davies S., Španěl P., Smith D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997;52:223–228. doi: 10.1038/ki.1997.324. [DOI] [PubMed] [Google Scholar]
- 27.Španěl P., Dryahina K., Smith D. A quantitative study of the influence of inhaled compounds on their concentrations in exhaled breath. J. Breath Res. 2013;7 doi: 10.1088/1752-7155/7/1/017106. [DOI] [PubMed] [Google Scholar]
- 28.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes-Metab. Res. Rev. 1999;15:412–426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Smith D., Španěl P., Fryer A.A., Hanna F., Ferns G.A.A. Can volatile compounds in exhaled breath be used to monitor control in diabetes mellitus? J. Breath Res. 2011;5 doi: 10.1088/1752-7155/5/2/022001. [DOI] [PubMed] [Google Scholar]
- 30.Walton C., Patel M., Pitts D., Knight P., Hoashi S., Evans M., Turner C. The use of a portable breath analysis device in monitoring type 1 diabetes patients in a hypoglycaemic clamp: validation with SIFT-MS data. J. Breath Res. 2014;8 doi: 10.1088/1752-7155/8/3/037108. [DOI] [PubMed] [Google Scholar]
- 31.Španěl P., Dryahina K., Rejskova A., Chippendale T.W.E., Smith D. Breath acetone concentration; biological variability and the influence of diet. Physiol. Meas. 2011;32:N23–N31. doi: 10.1088/0967-3334/32/8/n01. [DOI] [PubMed] [Google Scholar]
- 32.Ajibola O.A., Smith D., Španěl P., Ferns G.A.A. Effects of dietary nutrients on volatile breath metabolites. J. Nutr. Sci. 2013:e34. doi: 10.1017/jns.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner C. Potential of breath and skin analysis for monitoring blood glucose concentration in diabetes. Expert. Rev. Mol. Diagn. 2011;11:497–503. doi: 10.1586/erm.11.31. [DOI] [PubMed] [Google Scholar]
- 34.Boshier P.R., Fehervari M., Markar S.R., Purkayastha S., Spanel P., Smith D., Hanna G.B. Variation in exhaled acetone and other ketones in patients undergoing bariatric surgery: a prospective cross-sectional study. Obes. Surg. 2018;28:2439–2446. doi: 10.1007/s11695-018-3180-5. [DOI] [PubMed] [Google Scholar]
- 35.Carroll W., Lenney W., Wang T.S., Španěl P., Alcock A., Smith D. Detection of volatile compounds emitted by Pseudomonas aeruginosa using selected ion flow tube mass spectrometry. Pediatr. Pulmonol. 2005;39:452–456. doi: 10.1002/ppul.20170. [DOI] [PubMed] [Google Scholar]
- 36.Smith D., Španěl P., Gilchrist F.J., Lenney W. Hydrogen cyanide, a volatile biomarker of Pseudomonas aeruginosa infection. J. Breath Res. 2013;7 doi: 10.1088/1752-7155/7/4/044001. [DOI] [PubMed] [Google Scholar]
- 37.Wang T.S., Pysanenko A., Dryahina K., Španěl P., Smith D. Analysis of breath, exhaled via the mouth and nose, and the air in the oral cavity. J. Breath Res. 2008;2 doi: 10.1088/1752-7155/2/3/037013. [DOI] [PubMed] [Google Scholar]
- 38.Gilchrist F.J., Bright-Thomas R.J., Jones A.M., Smith D., Španěl P., Webb A.K., Lenney W. Hydrogen cyanide concentrations in the breath of adult cystic fibrosis patients with and without Pseudomonas aeruginosa infection. J. Breath Res. 2013;7 doi: 10.1088/1752-7155/7/2/026010. [DOI] [PubMed] [Google Scholar]
- 39.Gilchrist F.J., Alcock A., Belcher J., Brady M., Jones A., Smith D., Španěl P., Webb K., Lenney W. Variation in hydrogen cyanide production between different strains of Pseudomonas aeruginosa. Eur. Respir. J. 2011;38:409–414. doi: 10.1183/09031936.00166510. [DOI] [PubMed] [Google Scholar]
- 40.Shestivska V., Nemec A., Drevinek P., Sovová K., Dryahina K., Španěl P. Quantification of methyl thiocyanate in the headspace of Pseudomonas aeruginosa cultures and in the breath of cystic fibrosis patients by selected ion flow tube mass spectrometry. Rapid. Commun. Mass Spectrom. 2011;25:2459–2467. doi: 10.1002/rcm.5146. [DOI] [PubMed] [Google Scholar]
- 41.Gilchrist F.J., Sims H., Alcock A., Belcher J., Jones A.M., Smith D., Španěl P., Webb A.K., Lenney W. Quantification of hydrogen cyanide and 2-aminoacetophenone in the headspace of Pseudomonas aeruginosa cultured under biofilm and planktonic conditions. Anal. Methods. 2012;4:3661–3665. doi: 10.1039/c2ay25652e. [DOI] [Google Scholar]
- 42.Gilchrist F.J., Belcher J., Jones A.M., Smith D., Smyth A.R., Southern K.W., Španěl P., Webb A.K., Lenney W. Exhaled breath hydrogen cyanide as a marker of early Pseudomonas aeruginosa infection in children with cystic fibrosis. ERJ Open Res. 2015;1:00044–02015. doi: 10.1183/23120541.00044-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shestivska V., Španěl P., Dryahina K., Sovová K., Smith D., Musilek M., Nemec A. Variability in the concentrations of volatile metabolites emitted by genotypically different strains of Pseudomonas aeruginosa. J. Appl. Microbiol. 2012;113:701–713. doi: 10.1111/j.1365-2672.2012.05370.x. [DOI] [PubMed] [Google Scholar]
- 44.Turner C., Španěl P., Smith D. A longitudinal study of ethanol and acetaldehyde in the exhaled breath of healthy volunteers using selected-ion flow-tube mass spectrometry. Rapid. Commun. Mass Spectrom. 2006;20:61–68. doi: 10.1002/rcm.2275. [DOI] [PubMed] [Google Scholar]
- 45.Španěl P., Turner C., Wang T.S., Bloor R., Smith D. Generation of volatile compounds on mouth exposure to urea and sucrose: implications for exhaled breath analysis. Physiol. Meas. 2006;27:N7–N17. doi: 10.1088/0967-3334/27/2/n01. [DOI] [PubMed] [Google Scholar]
- 46.Spanel P., Dryahina K., Vicherková P., Smith D. Increase of methanol in exhaled breath quantified by SIFT-MS following aspartame ingestion. J. Breath Res. 2015;9 doi: 10.1088/1752-7155/9/4/047104. [DOI] [PubMed] [Google Scholar]
- 47.Smith D., Pysanenko A., Španěl P. Kinetics of ethanol decay in mouth- and nose-exhaled breath measured on-line by selected ion flow tube mass spectrometry following varying doses of alcohol. Rapid. Commun. Mass Spectrom. 2010;24:1066–1074. doi: 10.1002/rcm.4481. [DOI] [PubMed] [Google Scholar]
- 48.Pysanenko A., Španěl P., Smith D. Analysis of the isobaric compounds propanol, acetic acid and methyl formate in humid air and breath by selected ion flow tube mass spectrometry SIFT-MS. Int. J. Mass Spectrom. 2009;285:42–48. doi: 10.1016/j.ijms.2009.04.002. [DOI] [Google Scholar]
- 49.Španěl P., Wang T.S., Smith D. A selected ion flow tube, SIFT, study of the reactions of H3O+, NO+ and O2+ ions with a series of diols. Int. J. Mass Spectrom. 2002;218:227–236. [Google Scholar]
- 50.Krautt J.A., Kurtztt I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin. J. Am. Soc. Nephrol. 2008;3:208–225. doi: 10.2215/cjn.03220807. [DOI] [PubMed] [Google Scholar]
- 51.Kurada S., Alkhouri N., Fiocchi C., Dweik R., Rieder F. Review article: breath analysis in inflammatory bowel diseases. Aliment Pharmacol. Ther. 2015;41:329–341. doi: 10.1111/apt.13050. [DOI] [PubMed] [Google Scholar]
- 52.Patel N., Alkhouri N., Eng K., Cikach F., Mahajan L., Yan C., Grove D., Rome E.S., Lopez R., Dweik R.A. Metabolomic analysis of breath volatile organic compounds reveals unique breathprints in children with inflammatory bowel disease: a pilot study. Aliment Pharmacol. Ther. 2014;40:498–507. doi: 10.1111/apt.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strober W., Fuss I., Mannon P. The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dryahina K., Španěl P., Pospisilova V., Sovova K., Hrdlicka L., Machkova N., Lukas M., Smith D. Quantification of pentane in exhaled breath, a potential biomarker of bowel disease, using selected ion flow tube mass spectrometry. Rapid. Commun. Mass Spectrom. 2013;27:1983–1992. doi: 10.1002/rcm.6660. [DOI] [PubMed] [Google Scholar]
- 55.Dryahina K., Smith D., Bortlík M., Machková N., Lukáš M., Španěl P. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn’s disease and ulcerative colitis. J. Breath Res. 2017;12 doi: 10.1088/1752-7163/aa8468. [DOI] [PubMed] [Google Scholar]
- 56.Shaker M., Hunt J. An economic analysis of an acid-reflux breath test in the evaluation of chronic cough. J. Breath Res. 2008;2 doi: 10.1088/1752-7155/2/3/037006. [DOI] [PubMed] [Google Scholar]
- 57.Dryahina K., Pospisilova V., Sovova K., Shestivska V., Kubista J., Spesyvyi A., Pehal F., Turzikova J., Votruba J., Spanel P. Exhaled breath concentrations of acetic acid vapour in gastro-esophageal reflux disease. J. Breath Res. 2014;8 doi: 10.1088/1752-7155/8/3/037109. [DOI] [PubMed] [Google Scholar]
- 58.Smith D., Sovová K., Dryahina K., Doušová T., Dřevínek P., Španěl P. Breath concentration of acetic acid vapour is elevated in patients with cystic fibrosis. J. Breath Res. 2016;10 doi: 10.1088/1752-7155/10/2/021002. [DOI] [PubMed] [Google Scholar]
- 59.Španěl P., Sovová K., Dryahina K., Doušová T., Dřevínek P., Smith D. Acetic acid is elevated in the exhaled breath of cystic fibrosis patients. J. Cyst. Fibros. 2017;16:e17–e18. doi: 10.1016/j.jcf.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Pysanenko A., Španěl P., Smith D. A study of sulfur-containing compounds in mouth- and nose-exhaled breath and in the oral cavity using selected ion flow tube mass spectrometry. J. Breath Res. 2008;2 doi: 10.1088/1752-7155/2/4/046004. [DOI] [PubMed] [Google Scholar]
- 61.Alkhouri N., Cikach F., Eng K., Moses J., Patel N., Yan C., Hanouneh I., Grove D., Lopez R., Dweik R. Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur. J. Gastroenterol. Hepatol. 2014;26:82–87. doi: 10.1097/MEG.0b013e3283650669. [DOI] [PubMed] [Google Scholar]
- 62.Chan C., Smith D., Španěl P., McIntyre C.W., Davies S.J. A non-invasive, on-line deuterium dilution technique for the measurement of total body water in haemodialysis patients. Nephrol. Dial. Transplant. 2008;23:2064–2070. doi: 10.1093/ndt/gfn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies S.J., Engel B., Chan C., Tan B.K., Yu Z.Z., Asghar R., John B., Španěl P., Smith D. Breath analysis and the measurement of total body water using isotope dilution - applications in the dialysis clinic. Curr. Anal. Chem. 2013;9:593–599. [Google Scholar]
- 64.Spesyvyi A., Smith D., Spanel P. Selected ion flow-drift tube mass spectrometry: quantification of volatile compounds in air and breath. Anal. Chem. 2015;87:12151–12160. doi: 10.1021/acs.analchem.5b02994. [DOI] [PubMed] [Google Scholar]
- 65.Guntner A.T., Abegg S., Konigstein K., Gerber P.A., Schmidt-Trucksass A., Pratsinis S.E. Breath sensors for health monitoring. ACS Sensors. 2019;4:268–280. doi: 10.1021/acssensors.8b00937. [DOI] [PubMed] [Google Scholar]