INTRODUCTION
Across the United States, the incidence of herpes zoster (HZ) has been on the rise for decades by as much as 3.1% per year since 1994 [1–4]. Recent estimates of overall HZ incidence in the US range from 580 to 720 cases per 100,000 person-years. HZ presents as a painful dermatomal rash caused by reactivation of latent varicella zoster virus from the dorsal root ganglion [5]. The risk of HZ is strongly associated with increasing age [5]. The disease burden of HZ in the United States is large, affecting 1 million annually. Additionally, HZ is associated with an annual $2.4 billion in direct medical costs and productivity losses [6]. Approximately 30% of all Americans will contract HZ in their lifetime, with the potential for significant long-term sequelae including post-herpetic neuralgia, increased risk of stroke or heart attack, and herpes zoster ophthalmicus (HZO)-related vision loss [4].
Two vaccines have been developed to protect against HZ in older adults. Zoster vaccine live (ZVL; Zostavax, Merck Sharp & Dohme) was licensed by the Food and Drug Administration (FDA) in 2006 for adults aged 60 and older, and then approved for adults aged 50 and older in 2011. Both clinical and real-world studies of ZVL showed approximately 50% protection against HZ [7–9]. The recombinant zoster vaccine (RZV; Shingrix, GlaxoSmithKline) was approved by the FDA in late 2017 for adults aged 50 and older [10–12]. The first clinical trial investigating RZV in adults aged 50 or older (ZOE-50), and a subsequent study in individuals aged 70 and older (ZOE-70), demonstrated a 97.2% and 89.9% reduction in HZ, respectively, making RZV the preferred vaccine for HZ prevention in immunocompetent adults [11,13,14]. As a result, the sale and use of ZVL in the U.S. was discontinued on November 18, 2020 [11,15].
The results from ZOE-50 and ZOE-70 are promising, but significant differences exist between clinical trials and real-world healthcare settings, highlighting the need for further investigation of RZV effectiveness outside of clinical trials. To address this, our research team recently published a claims-based retrospective cohort study using OptumLabs® Data Warehouse (OLDW), providing the first evidence of RZV effectiveness among commercial and Medicare Advantage enrollees aged 50 and older in the United States [16]. In this cohort with a median age of 65 years old (IQR: 56–73), overall RZV effectiveness was 85.5% (95% CI: 83.5% to 87.3%), which, along with age-stratified estimates, was similar to those of ZOE-50 and ZOE-70.
Although these results strongly support RZV effectiveness in Americans aged 50 and older, further investigation of RZV effectiveness among different populations is of public health importance. Populations have differing underlying health conditions, racial and ethnic profiles, genetic predispositions, and socioeconomic circumstances, which have the potential to affect vaccine effectiveness. Hawaii is a geographically remote and racially distinct region of the US with unique characteristics including but not limited to a larger population of Asian and Native Hawaiian/Pacific Islanders. To our knowledge, no studies exist investigating RZV effectiveness among this population. The study aimed to assess RZV effectiveness among a population in Hawaii [16].
METHODS
Setting
A retrospective cohort study was conducted using de-identified electronic health records (EHRs) from Kaiser Permanente Hawaii (KPH) from January 1, 2018 through December 31, 2019. During this study period from 2018–2019, KPH was comprised of 1 hospital and 27 medical offices serving approximately 18% of the population of Hawaii.
Inclusion and Exclusion Criteria
KPH patients became eligible for inclusion in this study based on two criteria: (1) turned 50 or were 50 years of age or older in 2018 or 2019, meeting age eligibility for RZV based on recommendations from the Advisory Committee on Immunization Practices (ACIP) [11]; (2) had at least 365 days of continuous enrollment in KPH prior to becoming age-eligible for the RZV vaccine. The date on which these two criteria were met was defined as a patient’s index date. A patient’s age was estimated by birth year to protect patient confidentiality. Patients aged 50 or older who joined KPH on or after January 1, 2018 were excluded from the final cohort because of the possibility that they may have received RZV prior to joining KPH.
A total of 44 individuals who received their first dose of RZV prior to January 1, 2018 (0.05% of the total vaccinated cohort) were excluded. Patients who had only received a single dose of RZV were also excluded. Patients with HZ occurring between the first and second dose of RZV and up to 30 days after the second dose of RZV were excluded due to inadequate time for a protective immune response to develop after RZV. Patients who received their second dose of RZV less than 30 days or greater than 210 days after the first dose were excluded because the second dose fell outside of the recommended time frame for vaccination by the ACIP [11]. Further selection details of the final cohort (N = 78,358) are demonstrated in Figure 1.
Figure 1. Flow diagram of inclusion and exclusion criteria for study cohort.
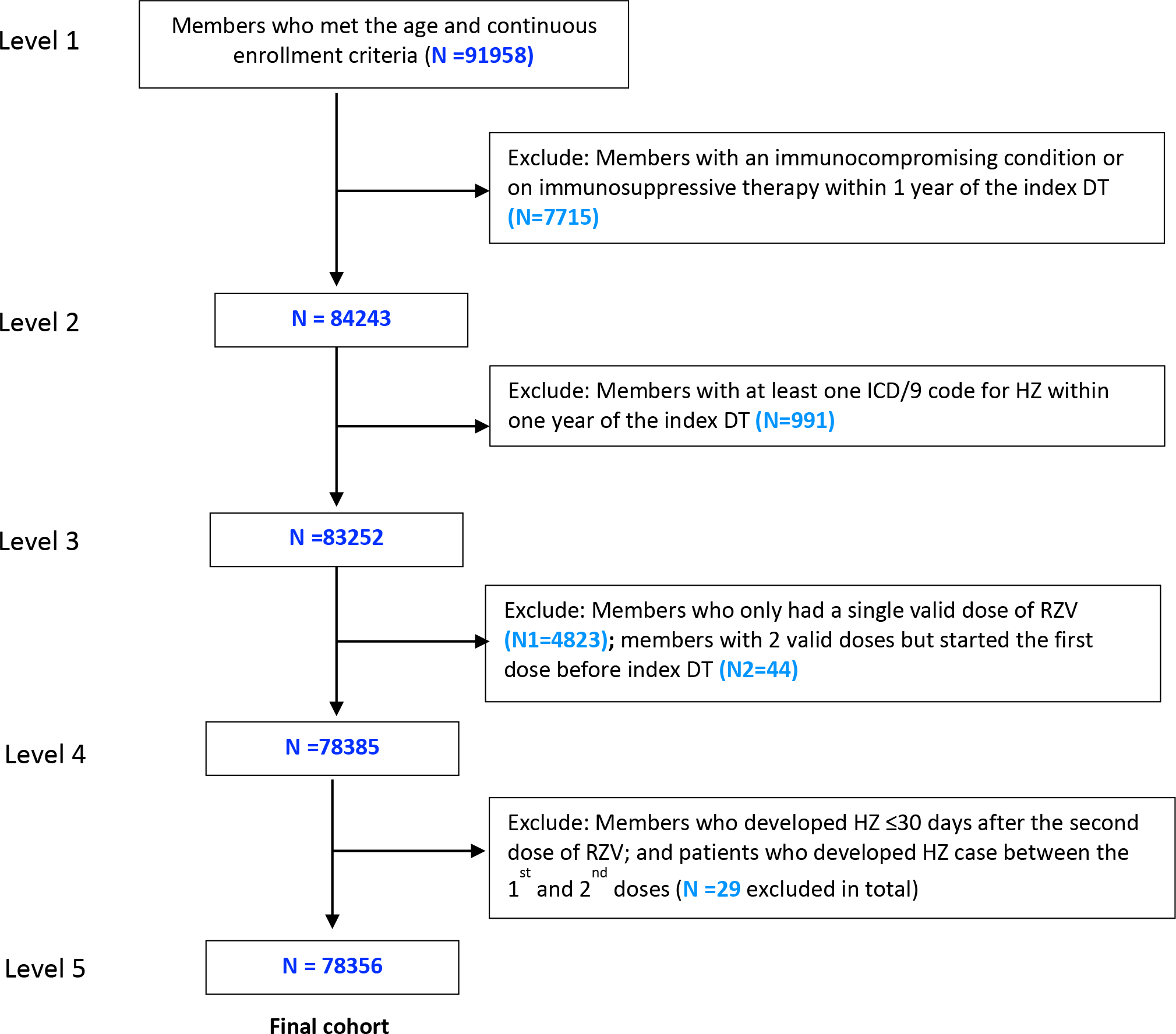
OLDW = OptumLabs Data Warehouse; ICD = International Classification of Disease; HZ = Herpes zoster; RZV = Recombinant Zoster Vaccine
a Index date was defined as the date at which an individual was eligible for study inclusion.
b Two valid doses of recombinant zoster vaccine were defined as receiving the second dose between 30 and 210 days after the first dose.
Patients who were diagnosed with HZ, as determined by an International Classification of Disease (ICD) 10th revision code (ICD-10 B02.xx), and individuals who were immunocompromised within 1 year prior to the index date were excluded. Immunocompromised status was defined as an ICD-10 code for human immunodeficiency virus, acquired immunodeficiency syndrome, leukemia, lymphoma, or a prescription for immunosuppressive medications (Table 5 and Table 6) [16].
Table 5.
ICD-10 codes for herpes zoster and immunocompromising conditions
| Condition | ICD version | ICD Code |
| Herpes zoster | ICD-10 | B02.xx |
| Leukemia/Lymphoma | ICD-10 | C81.xx, C82.xx, C83.xx, C84.xx, C85.xx, C86.xx, C87.xx, C88.xx, C89.xx, C90.xx, C91.xx, C92.xx, C93.xx, C94.xx, C95.xx, C96.xx |
| HIV/AIDS | ICD-10 | B20.xx, B21.xx, B22.xx, B23.xx, B24.xx, Z21.xx |
Table 6.
List of immunocompromising medications [16]
| I. Antineoplastics | CYTADREN | HYCAMTIN |
| ABARELIX | CYTARABINE | HYDREA |
| ABRAXANE | CYTOSAR | HYDROXYUREA |
| ACTIMMUNE | CYTOXAN | INDIUM |
| ADRIAMYCIN | DACARBAZINE | YTTRIUM |
| ADRUCIL | DACOGEN | IDAMYCIN |
| AFINITOR | DACTINOMYCIN | IDARUBICIN |
| ALDESLEUKIN | DASATINIB | IFEX |
| ALEMTUZUMAB | DAUNORUBICIN | MESNEX |
| ALFERON | DAUNOXOME | IFOSFAMIDE |
| ALIMTA | DECITABINE | IMATINIB |
| ALITRETINOIN | DEGARELIX | INTERFERON |
| ALKERAN | DENILEUKIN DIFTITOX | INTRON |
| ALTRETAMINE | DICLOFENAC | IRESSA |
| AMINOGLUTETHIMIDE | DOCETAXEL | IRINOTECAN |
| AMINOLEVULINIC | DOXIL | IXABEPILONE |
| ANASTROZOLE | DOXORUBICIN | IXEMPRA |
| ARIMIDEX | DROXIA | LAPATINIB |
| AROMASIN | DTIC | LENALIDOMIDE |
| ARRANON | EFUDEX | LETROZOLE |
| ARSENIC | ELIGARD | LEUKERAN |
| ASPARAGINASE | ELLENCE | LEUPROLIDE |
| AVASTIN | ELOXATIN | LEUSTATIN |
| AZACITIDINE | ELSPAR | LEVAMISOLE |
| BCG | EMCYT | LEVULAN |
| BENDAMUSTINE | EPIRUBICIN | LOMUSTINE |
| BEVACIZUMAB | ERBITUX | LUPRON |
| BEXAROTENE | ERLOTINIB | LYSODREN |
| BEXXAR | ESTRAMUSTINE | MATULANE |
| BICALUTAMIDE | ETOPOPHOS | MECHLORETHAMINE |
| BICNU | ETOPOSIDE | MEGESTROL |
| BLENOXANE | EVEROLIMUS | MELPHALAN |
| BLEOMYCIN | EXEMESTANE | MERCAPTOPURINE |
| BORTEZOMIB | FARESTON | METHOTREXATE |
| BUSULFAN | FASLODEX | METHOXSALEN |
| BUSULFEX | FEMARA | MITOMYCIN |
| CAMPATH | FLOXURIDINE | MITOTANE |
| CAMPTOSAR | FLUDARA | MITOXANTRONE |
| CAPECITABINE | FLUDARABINE | MUSTARGEN |
| CARAC | FLUOROPLEX | MUTAMYCIN |
| CARBOPLATIN | FLUOROURACIL | MYLERAN |
| CARMUSTINE | FLUTAMIDE | MYLOCEL |
| CASODEX | FUDR | MYLOTARG |
| CEENU | FULVESTRANT | NAVELBINE |
| CERUBIDINE | GEFITINIB | NELARABINE |
| CETUXIMAB | GEMCITABINE | NEOSAR |
| CHLORAMBUCIL | GEMTUZUMAB | NEXAVAR |
| CISPLATIN | GEMZAR | NILANDRON |
| CLADRIBINE | GLEEVEC | NILOTINIB |
| CLOFARABINE | GLIADEL | NILUTAMIDE |
| CLOLAR | GOSERELIN | NIPENT |
| COSMEGEN | HERCEPTIN | NOLVADEX |
| CYCLOPHOSPHAMIDE | HEXALEN | NOVANTRONE |
| ONCASPAR | TORISEL | II. Antiarthritics |
| ONTAK | TOSITUMOMAB | CERTOLIZUMAB |
| ONXOL | TREANDA | CIMZIA |
| OXALIPLATIN | TRELSTAR | PENICILLAMINE |
| PACLITAXEL | TRETINOIN | ANAKINRA |
| PANITUMUMAB | TREXALL | KINERET |
| PANRETIN | TYKERB | ADALIMUMAB |
| PARAPLATIN | URACIL | ENBREL |
| PEGASPARGASE | UVADEX | ETANERCEPT |
| PEMETREXED | VALRUBICIN | HUMIRA |
| PENTOSTATIN | VALSTAR | LEFLUNOMIDE |
| PHOTOFRIN | VECTIBIX | ARAVA |
| PLATINOL | VELCADE | AURANOFIN |
| PLENAXIS | VEPESID | AUROTHIOGLUCOSE |
| PLICAMYCIN | VESANOID | THIOMALATE |
| PORFIMER | VIADUR | ABATACEPT |
| PROCARBAZINE | VIDAZA | ORENCIA |
| PROLEUKIN | VINBLASTINE | REMICADE |
| PURINETHOL | VINCASAR | RHEUMATREX |
| REVLIMID | VINCRISTINE | SULFASALAZINE |
| RITUXAN | VINORELBINE | AZULFIDINE |
| RITUXIMAB | VORINOSTAT | INFLIXIMAB |
| ROFERON | VUMON | TOCILIZUMAB |
| SOLARAZE | XELODA | ACTEMRA |
| SOLTAMOX | ZANOSAR | |
| SORAFENIB | ZEVALIN | |
| SPRYCEL | ZOLADEX | III. Other |
| STREPTOZOCIN | ZOLINZA | Immunosuppressants |
| SUNITINIB | AZASAN | |
| TRIPTORELIN | AZATHIOPRINE | |
| TRASTUZUMAB | BASILIXIMAB | |
| SUTENT | CELLCEPT | |
| TAMOXIFEN | CYCLOSPORINE | |
| TARABINE | DACLIZUMAB | |
| TARCEVA | GENGRAF | |
| TARGRETIN | GOLIMUMAB | |
| TASIGNA | SIMPONI | |
| TAXOL | HYDROXYCHLOROQUINE | |
| TAXOTERE | PLAQUENIL | |
| TEMODAR | IMURAN | |
| TEMOZOLOMIDE | MUROMONAB | |
| TEMSIROLIMUS | MYCOPHENOLATE | |
| TENIPOSIDE | MOFETIL | |
| TESLAC | NEORAL | |
| TESTOLACTONE | ORTHOCLONE | |
| THERACYS | PROGRAF | |
| THIOGUANINE | RAPAMUNE | |
| THIOPLEX | SANDIMMUNE | |
| THIOTEPA | SIMULECT | |
| TICE BCG | SIROLIMUS | |
| TOPOSAR | TACROLIMUS | |
| TOPOTECAN | ZENAPAX | |
| TOREMIFENE |
Exposure and Outcome
Receipt of RZV was identified by searching for the brand name for RZV, Shingrix (GlaxoSmithKline), within an individual’s vaccination record from their EHR. The outcome of interest was the first diagnosis of HZ that occurred during the study follow-up.
Covariates and Follow-Up Period
Time-fixed covariates that were identified as potential confounders included sex (female, male, unknown), race (Asian, White, Multiracial, Native Hawaiian/Other Pacific Islander, unknown, Black and American Indian/Alaska Native), ethnicity (Hispanic, non-Hispanic, unknown), and vaccination with ZVL in the 1 year before the index date.
Time-varying covariates included age, healthcare utilization (inpatient care (IP), institutional stay (IS), ambulatory care visits (AV), and emergency department outpatient hospital visits (ED)), Deyo Comorbidity Index, and systemic antiviral use [17]. These were updated for each 6-month period. Inpatient stays, institutional stays, emergency department visits, and antiviral use were categorized as binary variables for each 6-month period due to the low number of events among this cohort. Ambulatory care visits were treated as a continuous variable for each 6-month period. Antiviral medications included valacyclovir (Valtrex), acyclovir (Zovirax), and famciclovir (Famvir). Receipt of ZVL was identified by searching for the brand name for ZVL, Zostavax, or Zoster Vaccine Live (Merck Sharp & Dohme), within an individual’s vaccination record from their EHR. Patient-time was recorded in 6-month intervals. Patients contributed to unvaccinated person-time until they received two valid doses of RZV. Two doses were defined as valid if the second dose of RZV was administered between 30 days and 210 days after the first dose [11]. Patients began to contribute to vaccinated person-time after their second dose of RZV. Prior to this date, patients contributed to unvaccinated person-time. If vaccination occurred during a 6-month interval, the period was split into two periods on the vaccination date.
Patients were followed from the index date until HZ diagnosis, development of immunocompromised status, ZVL receipt, disenrollment from the KPH insurance plan, or the end of the follow-up study period, December 31, 2019.
Statistical Analysis
The statistical analysis plan of the present study was derived from the analysis that our group used to assess the effectiveness of RZV in the OptumLabs Data Warehouse for comparability (eMethod) [16]. Incidence rates of HZ and HZO for each year post-vaccination were computed as the number of HZ and HZO cases per 100 000 person-years. The corresponding 95% confidence intervals were estimated assuming occurrence of HZ and HZO followed a Poisson distribution. Cox proportional hazards regression models were used to estimate the hazard ratio of HZ and HZO associated with RZV, stratified by birth year using calendar time as the timescale. Inverse probability weighting was used to control for confounding. Models for both HZ and HZO were weighted by the product of the inverse probability of treatment weight and inverse probability of censoring weight to estimate adjusted hazard ratios. Covariate balance improvement was assessed through inverse weighting by comparing absolute standardized differences in the unweighted and weighted samples [18]. The 95% confidence intervals of these models were estimated using robust standard errors, which are conservative for inversely weighted estimators [18]. Vaccine effectiveness was estimated as: 1- hazard ratio x 100%. An E-value was calculated to estimate the effect of unmeasured confounding on RZV effectiveness [19–21].
All statistical analyses were conducted in R (Version 3.6.3 The R Project for Statistical Computing, Vienna, Austria; http://www.r-project.org). Only de-identified data were available for analysis. This study received approval from the Institutional Review Board of the University of California, San Francisco, and Kaiser Permanente Hawaii and was performed in accordance with the tenets of the Declaration of Helsinki.
RESULTS
A total of 78 356 individuals contributing 128 010 person-years were included in this study. The total number of individuals vaccinated with two valid doses of RZV was 11 864 (15.1%). The overall median age of all patients at index date was 61 years (interquartile range (IQR): 54 – 69), while the median age at the index date for patients who received RZV was 74 (IQR: 70 – 80), compared to 59 (IQR: 53 – 65) for unvaccinated individuals. The median follow-up time was 730 days (IQR: 730 – 730) for vaccinated individuals as well as for unvaccinated individuals (IQR: 430 – 730). Asians, whites and non-Hispanics were the most common racial and ethnic demographic groups within both the vaccinated and unvaccinated cohorts. Native Hawaiian/Other Pacific Islanders comprised 9.2% of the unvaccinated cohort and 4.7% of the vaccinated cohort. Table 1 presents demographic characteristics for the vaccinated and unvaccinated cohorts at their index date.
Table 1.
Characteristics of the study population at the index datea by vaccination status
| Characteristicb | Unvaccinated (n = 66 492) | Vaccinated (n = 11 864) | Overall (n = 78 356) |
|---|---|---|---|
| Age, median (IQR), y | 59 (53 – 65) | 74 (70 – 80) | 61 (54 – 69) |
| Sex | |||
| Male | 32676 (49.1) | 5351 (45.1) | 38027 (48.5) |
| Female | 33816 (50.9) | 6513 (54.9) | 40329 (51.5) |
| Race | |||
| Asian | 23551 (35.4) | 5550 (46.8) | 29101 (37.1) |
| White | 19846 (29.8) | 3627 (30.6) | 23473 (30.0) |
| More Than One Race | 10586 (15.9) | 1735 (14.6) | 12321 (15.7) |
| Native Hawaiian/Other Pacific Islander | 6122 (9.2) | 575 (4.8) | 6697 (8.5) |
| Black | 641 (1.0) | 54 (0.5) | 695 (0.9) |
| American Indian/Alaska Native | 165 (0.2) | 13 (0.1) | 178 (0.2) |
| Unknownc | 5581 (8.4) | 310 (2.6) | 5891 (7.5) |
| Ethnicity | |||
| Hispanic/Latino | 3060 (4.6) | 351 (3.0) | 3411 (4.4) |
| Not Hispanic/Latino | 59341 (89.2) | 11339 (95.6) | 70680 (90.2) |
| Unknownd | 4091 (6.2) | 174 (1.5) | 4265 (5.4) |
| Inpatient Care (IC) e | |||
| ≥1 Visits | 3979 (6.0) | 1188 (10.0) | 5167 (6.6) |
| No Visits | 62513 (94.0) | 10676 (90.0) | 73189 (93.4) |
| Institutional Stay (IS) e | |||
| ≥1 Visits | 762 (1.1) | 168 (1.4) | 930 (1.2) |
| No Visits | 65730 (98.9) | 11696 (98.6) | 77426 (98.8) |
| Emergency Department Visit (ED) e | |||
| ≥1 Visits | 9977 (15.0) | 2221 (18.7) | 12198 (15.6) |
| No Visits | 56515 (85.0) | 9643 (81.3) | 66158 (84.4) |
| Ambulatory Visit (AV), median (IQR) e | 3 (1 – 7) | 6 (3 – 11) | 4 (1 – 8) |
| Deyo comorbidity index, median (IQR) f | 0 (0 – 1) | 1 (0 – 3) | 0 (0 −1) |
| Prior ZVL vaccination within 1 year of index date | |||
| Yes | 1981 (3.0) | 296 (2.5) | 2277 (2.9) |
| No | 64511 (97.0) | 11568 (97.5) | 76079 (97.1) |
| Follow up time (days), median (IQR) | 730 (430 – 730) | 730 (730 – 730) | 730 (480 – 730) |
IQR = Interquartile range.
The index date was defined as the date at which an individual was eligible for study inclusion.
Values are reported as No. (%) unless otherwise indicated.
The unknown race category includes individuals with either unknown or missing race.
The unknown ethnicity category includes individuals with either unknown or missing ethnicity.
Healthcare utilization was assessed in the 1 year prior to the index date.
Deyo comorbidity index was assessed in the 1 year prior to the index date
A total of 27 HZ cases were reported among patients who were fully vaccinated during a total of 8 291 vaccinated person-years. The incidence rate of HZ during vaccinated person-time was 325.6 cases per 100 000 person-years (95% CI: 217.7, 464.4). A total of 1 273 HZ cases occurred with 119 719 person-years of unvaccinated person-time. The incidence rate of HZ during unvaccinated person-time was 1063.3 HZ cases per 100 000 person-years (95% CI: 1006, 1122.8) (Table 2).
Table 2.
Incidence of herpes zoster per 100 000 person-years by baseline characteristics and RZV status from 2018 to 2019
| Unvaccinated | Vaccinated | ||||||
|---|---|---|---|---|---|---|---|
| Number of cases | Number of Person-Years | Incidence rate (95%CI) | Number of cases | Number of Person-Years | Incidence rate (95% CI) | Rate Ratioa (95% CI) | |
| Overall | 1273 | 119719 | 1063.3 (1006, 1122.8) | 27 | 8291 | 325.6 (217.7, 464.4) | 0.31 (0.21,0.45) |
| Age group | |||||||
| 50–59 | 467 | 49449 | 944.4 (861.3,1032.7) | 0 | 196 | 0 | 0 |
| 60–69 | 442 | 42592 | 1037.7 (944, 1137.5) | 4 | 717 | 557. 7 (173.1, 1295.3) | 0.54 (0.2,1.44) |
| 70–79 | 214 | 17914 | 1194.6 (1041.6, 1361.9) | 13 | 4537 | 286.5 (157.6, 471.7) | 0.24 (0.14,0.42) |
| 80+ | 150 | 9764 | 1536.2 (1303.3, 1795.4) | 10 | 2841 | 352.0 (176.4, 617.3) | 0.23 (0.12,0.43) |
| Sex | |||||||
| Female | 740 | 61386 | 1205.5 (1120.7, 1294.4) | 19 | 4492 | 423.0 (260.1, 642.6) | 0.35 (0.22,0.55) |
| Male | 533 | 58334 | 913.7 (838.3, 993.5) | 8 | 3799 | 210.6 (96.2, 391.9) | 0.23 (0.11,0.46) |
| Race | |||||||
| Asian | 534 | 45147 | 1182.8 (1085.3,1286) | 12 | 4107 | 292.2 (156.4, 490) | 0.25 (0.14,0.44) |
| White | 347 | 34475 | 1006.5 (904.3, 1116.2) | 7 | 2266 | 308.9 (132.7, 597.3) | 0.31 (0.15,0.65) |
| More Than One Race | 199 | 19273 | 1032.5 (895.6, 1182.7) | 8 | 1247 | 641.8 (293.3, 1194.5) | 0.62 (0.31,1.26) |
| Native Hawaiian/Other Pacific Islander | 110 | 10525 | 1045.1 (861.8, 1252.8) | 0 | 420 | 0 | 0 |
| Black | 8 | 1056 | 757.4 (346.1, 1409.7) | 0 | 37 | 0 | 0 |
| American Indian/Alaska Native | 6 | 282 | 2125.8 (844.8, 4307.2) | 0 | 8 | 0 | 0 |
| Unknownb | 69 | 8960 | 770.1 (602.4, 966.4) | 0 | 207 | 0 | 0 |
| Ethnicity | |||||||
| Non-Hispanic | 1161 | 108059 | 1074.4 (1013.8, 1137.4) | 27 | 7922 | 340.8 (227.9, 486) | 0.32 (0.22,0.46) |
| Hispanic | 66 | 5237 | 1260.3 (980.2, 1589.3) | 0 | 251 | 0 | 0 |
| Unknownc | 46 | 6424 | 716.1 (528.6, 943.4) | 0 | 118 | 0 | 0 |
| Prior ZVL vaccination within one year of index date | |||||||
| Yes | 26 | 3695 | 703.6 (466.6, 1009.7) | 1 | 184 | 543.8 (31, 2392.2) | 0.77 (0.1,5.7) |
| No | 1247 | 116024 | 1074.8 (1016.2, 1135.5) | 26 | 8107 | 320.7 (212.7, 460.2) | 0.3 (0.2,0.44) |
Rate ratio was computed as incidence rate in vaccinated group/incidence rate in unvaccinated group.
The unknown race category includes individuals with either unknown or missing race.
The unknown ethnicity category includes individuals with either unknown or missing ethnicity
Only 1 HZO case occurred during vaccinated person-time, with a total of 8 404 person-years. The incidence rate of HZO for vaccinated person-time was 11.9 cases per 100 000 person-years (95% CI: 0.7, 52.3). 87 HZO cases occurred during unvaccinated person-time, with a total of 120 739 person-years. The incidence rate of HZO for unvaccinated person-time was 72.1 cases per 100 000 person-years (95% CI: 58.0, 88.3) (Table 3).
Table 3.
Incidence of herpes zoster ophthalmicus per 100 000 person-years by RZV status from 2018 to 2019
| Unvaccinated | Vaccinated | ||||||
|---|---|---|---|---|---|---|---|
| Number of Cases | Number of Person-Years | Incidence Rate (95% CI) | Number of Cases | Number of Person-Years | Incidence Rate (95% CI) | Rate Ratio (95% CI) | |
| Overall | 87 | 120 739 | 72.1 (58.0, 88.3) | 1 | 8 404 | 11.9 (0.7, 52.3) | 0.17 (0.02, 1.19) |
Inverse probability weighting significantly improved covariate balance between the vaccinated and unvaccinated cohorts (eTable). Overall adjusted vaccine effectiveness in preventing HZ was 83.5% (95% CI: 74.9, 89.2), with an effectiveness of 67.7% (95% CI: 11.8, 88.1) for individuals aged 60 to 69, 83.8% (95% CI: 70.1, 90.7) for individuals aged 70 to 79, and 86.4% (95% CI: 73.5, 93.0) for individuals aged 80 and above (Table 4). Vaccine effectiveness was 100% for the following subgroups: 50–59-year-olds, Native Hawaiian/Other Pacific Islander, Black, American Indian/Alaska Native races and Hispanic ethnicity. However, the confidence intervals could not be calculated for these subgroups because there were no cases of HZ in the vaccinated cohort. The null E-value to assess for unmeasured confounding was 11.6.
Table 4.
Unadjusted and adjusted vaccine effectiveness of RZV by subgroups from 2018 to 2019
| Unadjusted VE point estimate (95%CI) | Adjusted VE point estimate (95%CI) | |
|---|---|---|
| Overall a | ||
| 73.8 (60.7, 82.5) | 83.5 (74.9, 89.2) | |
| Age group b | ||
| 50–59 | 100 | 100 |
| 60–69 | 48.0 (- 40.7, 80.8) | 67.7 (11.8, 88.1) |
| 70–79 | 73.0 (51.8, 84.9) | 83.3 (70.1, 90.7) |
| 80+ | 78.3 (58.1, 88.8) | 86.4 (73.5, 93.0) |
| Sex | ||
| Female | 68.5 (48.5, 80.8) | 79.5 (66.0, 87.7) |
| Male | 80.6 (60.8, 90.4) | 89.3 (77.9, 94.8) |
| Race c | ||
| Asian | 80.0 (63.8, 89.0) | 88.1 (77.5, 93.7) |
| White | 73.7 (39.6, 88.5) | 82.0 (58.4, 92.2) |
| Multiple | 17.9 (- 81.4, 62.9) | 43.7 (- 22.2, 74.0) |
| Native Hawaiian/Other Pacific Islander | 100 | 100 |
| American Indian/Alaska Native | 100 | 100 |
| Black | 100 | 100 |
| Unknown | 100 | 100 |
| Ethnicity d | ||
| Hispanic | 100 | 100 |
| Non-Hispanic | 72.4 (58.6, 81.6) | 82.7 (73.6, 88.7) |
| Unknown | 100 | 100 |
| Prior ZVL | ||
| Yes_ZVL | 18.9 (- 309.4, 83.9) | 61.1 (- 124.9, 93.3) |
| No_ZVL | 74.4 (61.3, 83.0) | 83.9 (75.2, 89.5) |
Values are reported as %.
RZV effectiveness confidence intervals could not be computed for the age subgroup 50–59 due to zero reported HZ cases in the vaccinated cohort.
RZV effectiveness confidence intervals could not be computed for Native Hawaiian/Other Pacific Islander, American Indian/Alaska Native, Black, and Unknown racial subgroups due to zero reported cases of HZ in the vaccinated cohorts.
RZV effectiveness confidence intervals could not be computed for Hispanic and unknown ethnic subgroups due to zero reported cases of HZ in the vaccinated cohorts.
The overall adjusted vaccine effectiveness in preventing HZO was 93.3% (95% CI: 48.7, 99.1). We did not estimate RZV effectiveness for HZO prevention among subgroups due to the small number of HZO cases.
DISCUSSION
In a managed care setting in Hawaii, overall adjusted RZV effectiveness against HZ was 83.5%. This is consistent with findings from our OLDW claims-based study, which reported an overall adjusted RZV effectiveness of 85.5% among enrollees in commercial insurance, Medicare Advantage, or Medicare Part D in the United States [16]. The current results from a different population and setting provide further real-world evidence of high RZV effectiveness outside of a clinical trial setting.
We found no evidence for age-specific differences in vaccine effectiveness among age strata where there were a sufficient number of events to estimate vaccine effectiveness with precision. Our prior study using OLDW suggested that RZV may have lower effectiveness in individuals aged 80 and older, but the results from KPH support comparable efficacy in this oldest age group compared with younger age groups. [13,14, 16]. There were no HZ cases reported in the vaccinated cohort for the 50–59-year-old age group, perhaps due to relatively small sample size, short follow-up time since vaccination, and the strong protection the vaccine provided. Thus, vaccine effectiveness was reported as 100% for this subgroup, however, we were not able to calculate a confidence interval for this estimate.
The results from this study support comparable effectiveness of RZV among different races. The relatively high number of patients of Asian race in this study allowed for a more precise estimate of vaccine effectiveness for this racial subgroup compared to our previous study using a claims database. In the OLDW claims-based study, vaccine effectiveness in Asians was 75.3% with a 95% CI of 60.2% to 84.7%. In the current study, vaccine effectiveness was 88.1% with a tighter confidence interval of 77.5% to 93.7%. Our estimated vaccine effectiveness (88.1%, 95% CI: 77.5–93.7) for Asian KPH enrollees aged 50 years and older is in-line with our previous OLDW claims-based study and post-hoc analyses of ZOE-50 randomized trials (add citations). This finding is reassuring in terms of vaccine effectiveness in Asians being comparable to other racial groups. Given that both clinical trials and real-world studies have demonstrated the performance of RZV for Asian people, clinicians could provide strong recommendations to this racial minority group. The confidence intervals of RZV effectiveness could not be calculated for the following four racial subcategories due to zero cases of HZ reported within the vaccinated cohorts: Native Hawaiian/Other Pacific Islander, American Indian/Alaska Native, Black, and Unknown. Additionally, confidence intervals could not be calculated for Hispanic ethnicity for the same reason.
RZV proved effective in preventing HZO among this study’s patient population. The incidence rate of HZO was 72.1 cases per 100 000 person-years among those unvaccinated and 11.1 cases per 100 000 person-years among those vaccinated, with only 1 case of HZO reported in the vaccinated cohort. The incidence rate among the unvaccinated in the present study is higher than the overall incidence rate (30.9 cases per 100 000 person-years) estimated from the Pacific Ocular Inflammation Study conducted with KPH patients from January 1, 2006 to December 31, 2007. That study was conducted prior to widespread availability of ZVL, so the assumption was that KPH patients in that study had not yet been vaccinated to prevent zoster [22]. Our data suggest an increase in HZO incidence among unvaccinated KPH patients when compared to this previous study, which is consistent with studies showing a continued rise in the incidence of HZ and HZO across the United States [4,23]. Overall adjusted RZV effectiveness against HZO was 93.3%, further supporting the benefit of this vaccine. Further research should be conducted to determine RZV effectiveness against HZO across differing populations for comparability and generalizability.
Vaccine coverage was 15.1% in the Kaiser Hawaii network compared to 3.6% in the OLDW claims-based study. Kaiser is known for health promotion efforts including vaccinations, so the higher rate of coverage within this system compared to commercial insurance and Medicare is expected. However, there is significant room for improvement in terms of vaccine coverage in both populations. Further research is needed to assess barriers to RZV uptake and provide public health guidance to increase vaccine coverage to reduce the disease burden of HZ and HZO.
Strengths and Limitations
This study applied the same robust methodology to a Health Maintenance Organization (HMO) EHR database that our group previously used for a large insurance claims database. By applying comparable methodology to different sources of data, we can compare generalizability of RZV effectiveness across diverse real-world settings. KPH provides an ideal setting for this population-based cohort study, as patients generally receive all their medical care at Kaiser facilities, making exposure misclassification unlikely. Additionally, misclassification of vaccination status was unlikely due to the exclusion of patients who did not have one consecutive prior year of enrollment in KPH when they turned 50 years old in 2019. These results are likely generalizable within the patient population of KPH 50 years of age or older. However, KPH covers only 18% of the population of Hawaii, and therefore, further research is required to assess RZV effectiveness among this state’s general population. The application of inverse probability weighting controlled for confounding and selection bias, minimizing the differences between the unvaccinated and vaccinated cohorts. It is possible that residual bias could have still been present due to unmeasured confounders. We calculated an E-value to quantify the minimum strength of association on the risk ratio scale that an unmeasured confounder must have with both the treatment and outcome to shift the observed treatment-outcome association. In previous studies, the relative risk of factors such as gender, race, and chronic health conditions on HZ was between 1 to 3. The high E-value of 11.6 indicates that it is unlikely that there is an unmeasured confounder which could have a significant effect on RZV effectiveness in this study [19–21]. The study has several limitations. The relatively short follow-up time since vaccination (mean 0.7 years) limited our ability to track HZ outcomes in vaccinated people. With smaller overall sample size and fewer HZ cases within certain subgroups, the precision of point estimates was lower, limiting our ability to make comparisons. Future investigation is needed to assess long-term vaccine effectiveness in different subgroups as the vaccine uptake goes up and more data becomes available. RZV recipients contributed to the unvaccinated person-time until after their second dose of RZV. The protection of RZV after one dose could contribute to the lower RZV vaccine effectiveness observed in our study comparing to the ZOE trials [24].
CONCLUSION
RZV has demonstrated high effectiveness both in and outside of a clinical trial setting in the United States. Vaccine coverage is low, emphasizing the need for public health efforts to increase vaccination to reduce morbidity due to HZ and HZO. Areas for future research include assessing long-term vaccine effectiveness and waning and identifying barriers to RZV vaccination coverage.
Supplementary Material
REFERENCES
- 1.Harpaz R, Leung JW. The epidemiology of herpes zoster in the united states during the era of varicella and herpes zoster vaccines: Changing patterns among older adults. Clin Infect Dis 2019; 69:341–344. [DOI] [PubMed] [Google Scholar]
- 2.Yih WK, Brooks DR, Lett SM, et al. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003. BMC Public Health 2005; 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfson LJ, Daniels VJ, Altland A, Black W, Huang W, Ou W. The Impact of Varicella Vaccination on the Incidence of Varicella and Herpes Zoster in the United States: Updated Evidence from Observational Databases, 1991–2016. Clin Infect Dis 2020; 70:995–1002. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RR, Kong CL, Porco TC, Kim E, Ebert CD, Acharya NR. Herpes zoster and postherpetic neuralgia: changing incidence rates from 1994 to 2018 in the United States [manuscript published online ahead of print 23 August 2020]. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JI. Herpes Zoster. N Engl J Med 2013; 369:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey M, Prosser LA, Rose AM, Ortega-Sanchez IR, Harpaz R. Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain 2020; 161:361–368. [DOI] [PubMed] [Google Scholar]
- 7.Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA 2011; 305:160–166. [DOI] [PubMed] [Google Scholar]
- 8.Baxter R, Bartlett J, Fireman B, et al. Long-Term Effectiveness of the Live Zoster Vaccine in Preventing Shingles: A Cohort Study. Am J Epidemiol 2018; 187:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 10.Baylor NW; US Food and Drug Administration (FDA). Approval letter - Zostavax. 2006. Available at: http://wayback.archive-it.org/7993/20170723093336/https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm132873.htm. Accessed 10 March 2021. [Google Scholar]
- 11.Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018; 67:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration (FDA). BLA approval - zoster vaccine recombinant. 2017. Available at: https://www.fda.gov/media/108274/download. Accessed 10 March 2021.
- 13.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–1032. [DOI] [PubMed] [Google Scholar]
- 14.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–2096. [DOI] [PubMed] [Google Scholar]
- 15.U.S. CDC. What Everyone Should Know about Zostavax. Available at: https://www.cdc.gov/vaccines/vpd/shingles/public/zostavax/index.html. Accessed 10 March 2021.
- 16.Sun Y; Kim E; Kong CL; Arnold BF; Porco TC; Acharya NR. Effectiveness of the recombinant zoster vaccine in adults aged 50 and older in the United States: a claims-based cohort study. [manuscript published online ahead of print 13 February 2021]. Clin Infect Dis 2021. doi: 10.1093/cid/ciab121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613–619. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016; 35:5642–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haneuse S, Vanderweele TJ, Arterburn D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. JAMA 2019; 321:602–603. [DOI] [PubMed] [Google Scholar]
- 20.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web Site and R Package for Computing E-values. Epidemiology 2018; 29:e45–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Weele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-Value. Ann Intern Med 2017; 167:268–274. [DOI] [PubMed] [Google Scholar]
- 22.Borkar DS, Tham VM, Esterberg E, et al. Incidence of herpes zoster ophthalmicus: Results from the pacific ocular inflammation study. Ophthalmology 2013; 120:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong CL, Thompson RR, Porco TC, Kim E, Acharya NR. Incidence Rate of Herpes Zoster Ophthalmicus: A Retrospective Cohort Study from 1994 through 2018. Ophthalmology 2020; 127:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izurieta HS, Wu X, Forshee R, et al. Recombinant Zoster Vaccine (Shingrix) real-world effectiveness in the first two years post-licensure. [manuscript published online ahead of print 13 February 2021]. Clin Infect Dis 2021. doi: 10.1093/cid/ciab125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


