Abstract
Background
We evaluated clinical outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19) in the second wave of the pandemic in a national COVID-19 treatment unit (CTU) in Uganda.
Methods
We conducted a retrospective cohort study of COVID-19 patients hospitalized at the Mulago National Referral Hospital CTU between May 1 and July 11, 2021. We performed Kaplan-Meier analysis to evaluate all-cause in-hospital mortality.
Results
Of the 477 participants, 247 (52%) were female, 15 (3%) had received at least 1 dose of the COVID-19 vaccine, and 223 (46%) had at least 1 comorbidity. The median age was 52 (interquartile range, 41–65) years. More than 80% of the patients presented with severe (19%, n=91) or critical (66%, n=315) COVID-19 illness. Overall, 174 (37%) patients died. Predictors of all-cause in-hospital mortality were as follows; age ≥50 years (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.2–3.2; P=.011), oxygen saturation at admission of ≥92% (aOR, 0.97; 95% CI, 0.91–0.95; P<.001), and admission pulse rate of ≥100 beats per minute (aOR, 1.01; 95% CI, 1.00–1.02; P=.042). The risk of death was 1.4-fold higher in female participants compared with their male counterparts (hazards ratio, 1.4; 95% CI, 1.0–2.0; P=.025).
Conclusions
In this cohort, where the majority of the patients presented with severe or critical illness, more than one third of the patients hospitalized with COVID-19 at a national CTU died of the illness.
Keywords: COVID-19, mortality, high-dependency unit, second wave, Uganda
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is a life-threatening illness associated with significant morbidity and mortality [1]. Initially described as a cluster of pneumonia cases in Wuhan city, Hubei Province in China, SARS CoV-2 has rapidly spread globally, infecting over 223 million people and resulting into more than 4.6 million deaths as of September 8, 2021 [2, 3].
The majority of people infected with SARS-CoV-2 are asymptomatic [3, 4]. However, 15% to 20% of symptomatic patients present with severe illness, manifesting as the need for hospitalization, supplemental oxygenation, mechanical ventilation, and eventually death [5, 6]. Although severe COVID-19 can occur in otherwise healthy individuals of any age, the risk of severe illness is more marked in adults with advanced age or underlying medical comorbidities, including diabetes mellitus, obesity, malignancies, and hypertension [7–9].
At the present, there is no optimal treatment for COVID-19, and mass administration of vaccine and other public health interventions remain the only hope for successful control of the pandemic [10–12]. This is further complicated by the emerging variants of SARS-CoV-2, which are more transmissible, possibly more pathogenic, and show a reduced sensitivity to neutralizing antibody [13, 14]. How these emerging variants affects clinical presentation, clinical outcomes, and vaccine response remains an area of interest.
In mid-April 2021, Uganda started facing the second wave of the COVID-19 pandemic, mainly driven by the delta (B.1.617.2) variant [15]. Since the emergence of this variant in the country, we have seen an exponential increase in the number of COVID-19 cases, with a substantial proportion of cases requiring hospitalization and some dying of the disease. In the first wave in Uganda, the vast majority of COVID-19 cases were mild and mortality rate was extremely low [2, 16, 17].
In this retrospective study, we evaluated outcomes of patients hospitalized with COVID-19 pneumonia during the second wave of the COVID-19 pandemic at a national referral COVID-19 Treatment Unit (CTU) in Uganda.
METHODS
Study Design and Setting
This retrospective cohort study was conducted at the High Dependency Unit (HDU) of Mulago National Referral Hospital (MNRH) CTU. The MNRH CTU is the largest public facility in Uganda with a total bed capacity of 900 beds inclusive of 15 intensive care unit (ICU) beds and 300 HDU beds.
Study Population
Charts of all patients hospitalized with COVID-19 at the HDU CTU and outcome documented between May 1, 2021 and July 11, 2021 were reviewed by 3 trained research assistants, and data were extracted using a pretested data abstraction tool. During this period, Uganda experienced its second wave of the COVID-19 pandemic, predominantly of the delta variant of SARS-CoV-2 virus. Patients without microbiological evidence of SARS-CoV-2 infection or radiological evidence of COVID-19 pneumonia were excluded from the study. In addition, charts missing more than 75% of the predefined items in the data abstraction tool were excluded.
Data Collection
Routine patient files used at Mulago CTU were retrieved by the study team, and each file was evaluated for eligibility. We collected data on demographic characteristics (age, sex, religion, residence, and occupation) as well as clinical presentation, underlying comorbidities (hypertension, diabetes mellitus, previous or current chronic heart disease, smoking, and obesity), treatment received, the need for ICU admission (from the clinical notes), oxygenation and oxygen administration modality, referral from peripheral facility, date of admission, and date of discharge or death. Our outcomes of interests were duration of hospitalization, the need for ICU admission, and all-cause mortality at hospital discharge. We collected baseline vitals such as random blood sugar, respiratory rate, temperature, and pulse rate.
Classification of Disease Severity
Severity of COVID-19 was assessed using the World Health Organization ordinal scale with values ranging from 0 (uninfected, no viral ribonucleic acid detected) to 10 (dead) [18].
Data Analysis
Anonymized data were cleaned in Excel and exported to STATA software version 16 for analysis. Normality testing of continuous data was achieved using Shapiro-Wilk test and normal QQ plot. Parametric data were presented as mean and standard deviation, and nonparametric data were presented as median and interquartile range (IQR). Categorical data were summarized as frequencies and percentages. Numerical variables were compared using the Mann-Whitney U test or Wilcoxon sign-ranked sum test, and categorical variables were compared using either the χ2 or Fischer’s exact tests as appropriate. A multivariable logistic regression model was constructed to investigate predictors of mortality and/or the need for ICU admission. All variables with a P<.2 at univariate analysis were introduced in the final logistic regression analysis to determine independent predictors. We conducted survival analysis using Kaplan-Meier curves to estimate mortality during the first 28 days after admission. Time to outcome was calculated by subtracting the date of admission from the date of endpoint (death while at the hospital, or discharge from the hospital). Censorship was done for all patients who died or were discharged before 28 days of admission. Log-rank test and Cox proportional hazard regression were used to determine the factors associated with all-cause in-hospital mortality. All statistical tests were 2-tailed and a P<.05 was considered statistically significant.
Patient Consent Statement
Before commencement of the study, the protocol was approved by the Mulago Hospital Research and Ethics Committee (MHREC) (Reference number MHREC 2030). Because this is a retrospective study, informed consent was not required. However, a waiver of consent to review charts of patients was provided by MHREC. All records were deidentified and handled anonymously. We adhered to all principles of research involving human subjects outlined in the Declaration of Helsinki.
RESULTS
Baseline Characteristics of Patients
A total of 480 patient charts were retrieved and data from 477 unique patients were analyzed after removing duplicates. The majority (68.1%, n=325) were referrals from peripheral health facilities from all over Uganda. The median age was 52 years (IQR, 41–65 years) and the majority (57.9%, n=192) were aged 50 years or older. There were slightly more female patients than males (52% vs 48%). Only 15 (3%) participants had received at least 1 dose of the AstraZeneca COVID-19 vaccine. Up to 46.8% (n=223) of the participants had at least 1 comorbidity, with hypertension (29.6%, n=141) and diabetes mellitus (19.5%, n=43) being the most common underlying conditions (Table 1).
Table 1.
Baseline Characteristics of Participants
| Variables | Frequency | % |
|---|---|---|
| Age (median, IQR) | 52 | 41–65 |
| <18 | 9 | 1.9 |
| 18–50 | 192 | 40.3 |
| >50 | 276 | 57.9 |
| Sex | ||
| Female | 247 | 51.9 |
| Male | 229 | 48.1 |
| COVID-19 Vaccination Status | ||
| None | 462 | 96.9 |
| First dose | 10 | 2.1 |
| Two complete doses | 5 | 1.0 |
| Any comorbidity | 223 | 46.8 |
| Hypertension | 141 | 29.6 |
| Diabetes mellitus | 93 | 19.5 |
| Human immunodeficiency virus | 31 | 6.5 |
| Chronic obstructive pulmonary disease | 3 | 0.6 |
| Malignancies | 2 | 0.4 |
| Heart failure | 2 | 0.4 |
| Chronic kidney disease | 2 | 0.4 |
Abbreviations: COVID-19, coronavirus disease 2019; IQR, interquartile range.
Clinical Presentation and Disease Severity
Most patients presented to the hospital with difficulty in breathing (80.9%, n=386) and cough (79.9%, n=381), and only 4 patients (0.8%) presented with altered mental state. At admission, the median SPO2 was 89% (IQR, 80%–95%), blood pressure 133/80 mmHg, pulse rate of 100 (86–112) beats per minute, and a respiratory rate of 28 breaths per minute (22–36 breaths/minute). More than 80% of the patients were diagnosed with severe (19.1%, n=91) and critical (66%, n=315) COVID-19 (Table 2).
Table 2.
Presenting Complaints and Vital Signs at Admission Among the Patients
| Presenting Complaints | Frequency or Median | Percent or Interquartile Range |
|---|---|---|
| Difficulty in breathing | 386 | 80.9 |
| Cough | 381 | 79.9 |
| Chest pain | 154 | 32.3 |
| Fever | 126 | 26.4 |
| General body weakness | 97 | 20.3 |
| Headaches | 34 | 7.1 |
| Runny nose | 26 | 5.5 |
| Abdominal pain | 13 | 2.7 |
| Loss of appetite | 12 | 2.5 |
| Loss or reduced smell | 11 | 2.3 |
| Diarrhea | 7 | 1.5 |
| Altered mental status | 4 | 0.8 |
| Baseline Vitals (Median, IQR) | ||
| SPO2 | 89 | 80–95 |
| Systolic blood pressure | 133 | 120–147 |
| Diastolic blood pressure | 80 | 70–90 |
| Pulse rate | 100 | 86–112 |
| Respiratory rate | 28 | 22–36 |
| Temperature | 36.5 | 36.2–36.8 |
Abbreviations: IQR, interquartile range.
Treatment
Oxygen therapy, dexamethasone, anticoagulants, antibiotics, and micronutrient supplementation (vitamin C, vitamin D, or zinc) were the most frequent treatment modalities received by the patients. Up to 396 (83%) patients received dexamethasone and 361 (75.7%) received enoxaparin. Approximately 73% (n=348) were given oxygen therapy: 80% using non-rebreather masks. A total of 19.9% (n=95) of the patients were nebulized using salbutamol, ipratropium, or budesonide (Table 3).
Table 3.
Disease Severity and Treatment Outcomes
| Treatment Outcomes | Frequency | Percent |
|---|---|---|
| Disease severity (n=467) | ||
| Mild | 27 | 5.8 |
| Moderate | 34 | 7.1 |
| Severe | 91 | 19.1 |
| Critical | 315 | 66.0 |
| Treatment Modalities (n=477) | ||
| Dexamethasone | 396 | 83.0 |
| Enoxaparin | 361 | 75.7 |
| Oxygen | 348 | 73.0 |
| Non-rebreather mask | 272 | 80.0 |
| Nasal prong | 92 | 30.0 |
| High-flow nasal canula | 19 | 10.0 |
| CPAP | 3 | 1.0 |
| Ceftriaxone | 333 | 69.8 |
| Zinc | 282 | 59.1 |
| Azithromycin | 162 | 34.0 |
| Nebulization | 95 | 19.9 |
| Antihypertensives | 63 | 13.2 |
| N-acetyl cysteine | 42 | 8.8 |
| Piperacillin/tazobactam | 27 | 5.7 |
| Vitamin C | 19 | 4.0 |
| Amoxycillin/clavulanic acid | 6 | 1.3 |
| Ivermectin | 2 | 0.4 |
| Remdesivir | 1 | 0.2 |
Patient Outcomes
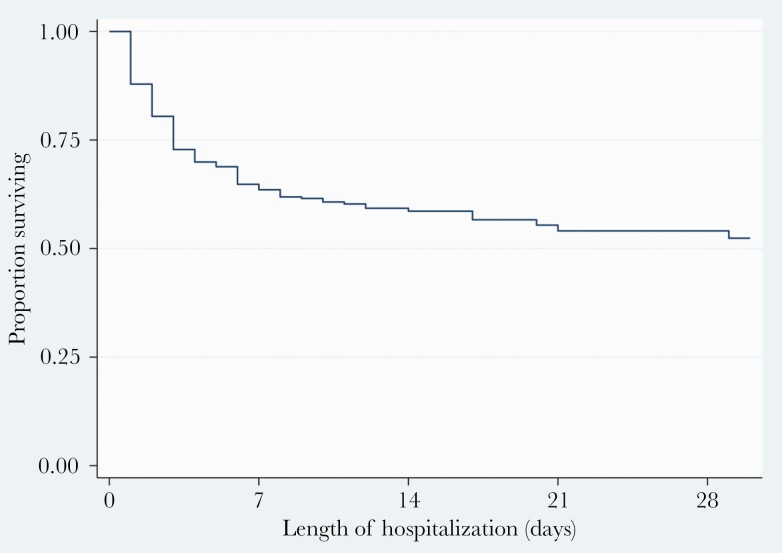
Overall, 174 (36.5%) patients died (Figure 1, Table 4). The median duration of hospitalization was 6 (IQR, 3–12) days; 10 (IQR, 6–15) days for patients discharged alive, and 3 (IQR, 1–5) days for patients who died at the hospital. At bivariate analysis (Table 4), all-cause mortality was associated with older age; 50 years or older (crude odds ratio [COR], 2.5; 95% CI, 1.7–3.7; P<.001), female sex (COR, 1.5; 95% CI, 1.1–2.2; P=.026), having at least 1 comorbidity (COR, 1.5; 95% CI, 1.0–2.1; P=.042), and hypertension (COR, 1.5; 95% CI, 1.0–2.3; P=.046). Presenting with difficulty in breathing (P=.014), abdominal pain (P=.038), altered mental state (P=.017), SPO2>92% (P<.001), pulse rate >100 beats per minute (P=.001), and higher respiratory rate (P<.001) were also associated with mortality. At multivariable logistic regression (Table 5), the odds of death were approximately 2-fold higher in patients aged 50 years or older than in those aged <50 years (adjusted odds ratio [aOR], 1.9; 95% CI, 1.2–3.2; P=.011). A SPO2 >92% at admission was associated with a 3% decrease in the likelihood of death (aOR, 0.97; 95% CI, 0.91–0.95; P<.001). Likewise, a pulse rate >100 beats per minute was marginally associated with an increased likelihood of death (aOR, 1.01; 95% CI, 1.00–1.02; P=.042).
Figure 1.
Kaplan-Meier curve showing survival after admission with coronavirus disease 2019.
Table 4.
Differences in Patient Characteristics and Mortality Among Hospitalized Patients
| Variables (N=477) | Survived (n=303) Frequency (%) | Died (n=174) Frequency (%) | χ2 or Fisher’s P Value |
|---|---|---|---|
| Age, median (IQR) | 50 (39–60) | 60 (47–72) | <.001 |
| <18 | 5 (55.6) | 4 (44.4) | <.001 |
| 18–50 | 146 (76) | 46 (24) | |
| >50 | 152 (55.1) | 124 (44.9) | |
| Sex (n=476) | |||
| Female | 145 (58.7) | 102 (41.3) | .026 |
| Male | 157 (68.6) | 72 (31.4) | |
| COVID-19 Vaccination Status | |||
| Completed | 3 (60) | 2 (40) | .214 |
| First dose | 9 (90) | 1 (10) | |
| None | 291 (63) | 171 (37) | |
| Any comorbidity | 131 (58.7) | 92 (41.3) | .042 |
| Hypertension | 80 (56.7) | 61 (43.3) | .046 |
| Diabetes mellitus | 54 (58.1) | 39 (41.9) | .223 |
| HIV | 17 (54.8) | 14 (45.2) | .299 |
| Pregnancy | 6 (75) | 2 (25) | .716 |
| COPD | 2 (66.7) | 1 (33.3) | 1.000 |
| Malignancies | 2 (100) | 0 (0) | .536 |
| Heart failure | 2 (100) | 0 (0) | .536 |
| Chronic kidney disease | 1 (50) | 1 (50) | 1.000 |
| Presenting complaints | |||
| Difficulty in breathing | 235 (60.9) | 151 (39.1) | .014 |
| Cough | 244 (64) | 137 (36) | .638 |
| Chest pain | 102 (66.2) | 52 (33.8) | .396 |
| Fever | 83 (65.9) | 43 (34.1) | .523 |
| General body weakness | 64 (66) | 33 (34) | .573 |
| Headaches | 26 (76.5) | 8 (23.5) | .104 |
| Runny nose | 16 (61.5) | 10 (38.5) | .829 |
| Abdominal pain | 12 (92.3) | 1 (7.7) | .038 |
| Loss of appetite | 8 (66.7) | 4 (33.3) | 1.000 |
| Loss or reduced smell | 7 (63.6) | 4 (36.4) | 1.000 |
| Diarrhea | 2 (28.6) | 5 (71.4) | .105 |
| Altered mental status | 0 (0) | 4 (100) | .017 |
| Vitals at admission | |||
| SPO2, median (IQR) | 92 (85–95) | 81 (63–89) | <.001 |
| Systolic blood pressure, median (IQR) | 132 (120–145) | 136 (120–149) | .149 |
| Diastolic blood pressure, median (IQR) | 81 (71–90) | 78 (69–89) | .169 |
| Pulse rate, median (IQR) | 99 (83–110) | 104 (91–116) | .001 |
| Respiratory rate, median (IQR) | 25 (20–30) | 32 (28–41) | .000 |
| Temperature, median (IQR) | 36.6 (36.1–37.2) | 36.4 (36.2–36.8) | .850 |
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; IQR, interquartile range.
Table 5.
A Multivariable Logistic Regression Model Showing Factors Associated With Mortality Among COVID-19 Patients
| Variables | AOR (95% CI) | P Value |
|---|---|---|
| Age, years | ||
| <50 | Reference | |
| ≥50 | 1.9 (1.2–3.2) | .011 |
| Sex | ||
| Male | Reference | |
| Female | 1.6 (0.7–3.7) | .309 |
| Difficulty in breathing at presentation | 1.0 (0.5–1.9) | .969 |
| Any comorbidity | 1.7 (0.9–3.8) | .092 |
| Hypertension | 0.9 (0.5–1.8) | .762 |
| Diabetes mellitus | 1.0 (0.5–2.0) | .932 |
| Admission SpO2>92% | 0.93 (0.91–0.95) | <.001 |
| Admission pulse rate (beats/minute) | 1.01 (1.00–1.02) | .042 |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; COVID-19, coronavirus disease 2019 (COVID-19); IQR, interquartile range.
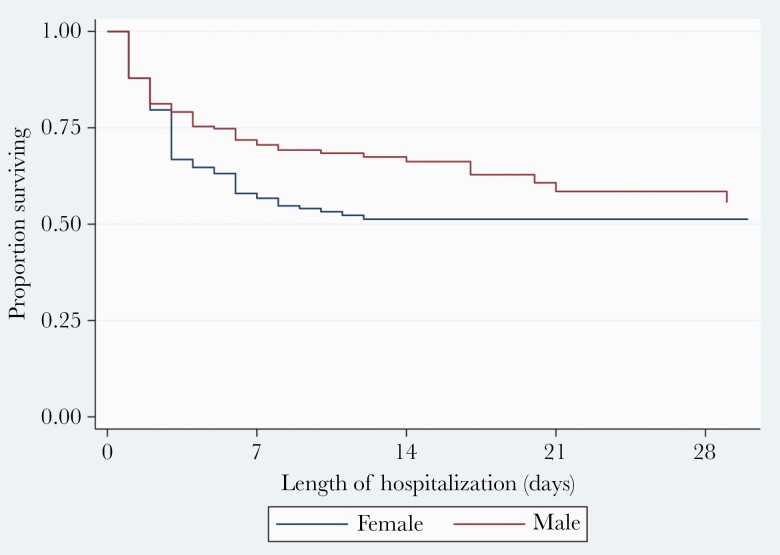
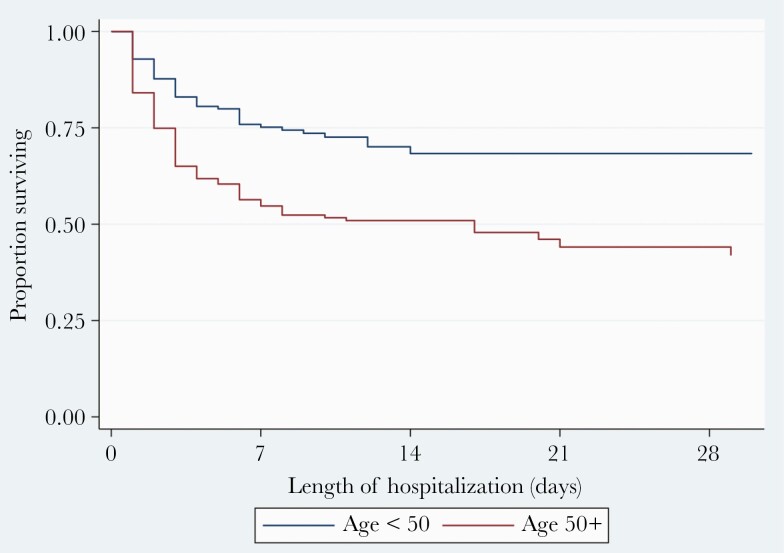
Overall, survival was over 50% throughout the time of observation (duration 62 days) (Figure 1). Survival at 7 days, 14 days, 21 days, and 28 days was 64%, 57%, 54%, and 52%. Survival differed significantly by sex (log-rank P=.019) (Figure 2) and age (<50 vs ≥50 years, log-rank P<.001) (Figure 3). The risk of death was 1.4-fold higher in female patients compared to their male counterparts (hazards ratio, 1.4; 95% CI, 1.0–2.0; P=.025). Likewise, patients aged 50 years or older had 2.1-fold higher odds of dying that those <50 years of age (adjusted hazards ratio, 2.1; 95% CI, 1.5–2.9; P<.001).
Figure 2.
Kaplan-Meier curve showing survival after admission with coronavirus disease 2019 stratified by sex.
Figure 3.
Kaplan-Meier curve showing survival after admission with coronavirus disease 2019 stratified by age.
DISCUSSION
Uganda is currently facing the second wave of the COVID-19 pandemic with positivity rates ranging as high as 12%–20% [2]. In this retrospective cohort study to evaluate mortality and predictors of mortality among patients hospitalized with COVID-19 at a national referral CTU in Uganda, more than one third of the patients died of the illness. This is in contrast to the first wave of the COVID-19 pandemic in Uganda where mortality rates were extremely low [16, 17]. However, our findings are consistent with a recent study from South Africa that showed that patients in the second wave had a higher in-hospital mortality than during the first wave [19]. The in-hospital mortality in the South African cohort ranged between 18% and 27% [19].
The high mortality in the second wave of COVID-19 in Uganda is probably due to the emergence of the SARS-CoV-2 variants of concern in Uganda [15]. The delta variant, which is the predominant lineage driving the second wave of COVID-19 in Uganda, is probably more pathogenic and associated with high transmissibility, severe disease, and reduced efficacy of current vaccines [13, 20]. In fact, more than two thirds of the patients presented to our facility in critical condition. The high mortality rate in our study and that reported in the South Africa [19] is in contrast with published data from Western world where morbidity and mortality were much lower in the second wave than in the first wave [21–24]. The tremendous reduction in mortality in the second wave in these countries has been attributed to widespread diagnostic testing, which allows early identification of milder cases, early initiation of treatment, and better clinical care given the experience learned from the first wave [21]. Moreover, the roll out and access to COVID-19 vaccines has been more problematic in sub-Saharan Africa than in these countries.
To date, there is no known cure for COVID-19. Therapeutic advances in the management of COVID-19 have rapidly evolved over the past year, with corticosteroid, anticoagulants, remdesivir, and monoclonal antibodies playing an important role in the management of severe/critical illness [25, 26]. However, in our dataset, we did not observe any difference in mortality across treatment regimens, despite a high proportion of patients receiving dexamethasone and enoxaparin. Only 1 patient received remdesivir. In addition, approximately 20% of the patients received nebulized salbutamol, ipratropium, or budesonide in combinations. The STOIC trial showed that inhaled budesonide shortens time to recovery and reduces the likelihood of disease progression in early COVID-19 [27]. Optimal therapy for COVID-19 is still largely undefined across the spectrum of the disease.
Approximately 3% of patients in our study had received at least 1 dose of the AstraZeneca vaccine. However, there was no difference in mortality rates across vaccine status among these patients, probably due to the small number of participants. As of July 23, 2021, vaccination coverage in Uganda was less than 2% of the population [28]. Therefore, we may need some time to truly evaluate the impact of vaccination on disease severity and mortality. However, in countries with high vaccine coverage, the proportion of vaccinated patients presenting with severe disease requiring hospitalization or resulting into deaths have remarkably decreased [10, 21], which is evidence that the vaccine works. There is an urgent need to increase vaccination coverage in Uganda.
Approximately half of our study participants had at least 1 comorbidity, especially hypertension and diabetes mellitus. These underlying diseases are common in patients with COVID-19 [5, 8, 29] and are independently associated with severe disease and mortality [8]. From our analysis, hypertension and having any comorbidity were associated with mortality at bivariate analysis. However, it was not apparent why comorbidities were not associated with mortality multivariate analysis. It is likely that older age (>50 years) was a confounder at bivariate analysis because older people have more comorbidities than people aged <50 years. We note that the proportion of patients with hypertension and diabetes mellitus in this cohort was much higher than the burden in the general Ugandan population. This reflects the disproportionate effect of COVID-19 in these group of patients. It is interesting to note that the proportion of participants with human immunodeficiency virus and COVID-19 was comparable with the general population.
The association of COVID-19 mortality and older age (>50 years) has been consistently reported in literature [6]. This could be attributed to immune senescence that comes with older age, alongside prevalent comorbidities [19]. The increased risk of mortality among women was surprising and is contrary to several reports in which mortality is observed more in men [9]. However, similar to our findings, mortality has been reported to be higher in women men than men in India, Nepal, Vietnam, and Slovenia as well [30]. More studies are needed to explore gender differences in COVID-19 mortality especially with the advent of new SARS-C0V-2 variants. In Uganda, the second wave overwhelmed the healthcare services and lack of ICU facilities and oxygen was rampant. Men traditionally have better access to specialized health services in Uganda, and therefore women could have been preferentially referred only if they had severe disease.
Our study has some important limitations. First, the retrospective study design, which may have introduced significant bias, has to be accounted for while interpreting our findings. As such, data on markers of organ dysfunction and immune inflammation, including important scores such as qSOFA, were unavailable to us, yet these are known predictors of COVID-19 mortality. Furthermore, these are findings from an HDU of a national referral CTU, which handles mostly severe and critical cases of COVID-19. Therefore, these findings are not generalized to other centers across the country that may be caring for mainly mild cases. We extracted data of patients only admitted in the HDU; therefore, we may have underestimated mortality, which is much higher among patients in the ICU. However, this study provides important baseline data to inform clinicians on predictors of poor outcomes to guide patient-centered care. We recommend a large, multicentered study across CTUs in the country to provide further insights into this evolving viral illness.
CONCLUSIONS
In conclusion, in this second wave of the COVID-19 pandemic, we report a very high mortality rate among patients hospitalized with COVID-19 pneumonia in Uganda. Approximately 4 in 5 of the patients presented with severe or critical illness and vaccination coverage was very low. Expanded access to the COVID-19 vaccine is recommended to diminish the number of patients presenting with critical illness and decrease deaths from COVID-19.
Acknowledgments
We are indebted to our study participants and to clinicians, nurses, and administration of Mulago National Referral Hospital for making this study a success.
Author contributions. All authors have made substantial contributions to the publication. F.B. conceptualized the study. F. B., B. F., R. O., B. N., S. K., P. B. K., J. B. B., and F. N. N. contributed to design of the study and acquisition of the data, drafted the manuscript, revised it critically for important intellectual content, and approved the final version for submission. R. O. contributed to statistical analysis. F. B. and S. K. sought sources for funding.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Research reported in this publication was funded by the Fogarty International Center of the National Institutes of Health, US Department of State’s Office of the US Global AIDS Coordinator and Health Diplomacy (S/GAC), and President’s Emergency Plan for AIDS Relief (PEPFAR) under Award Number 1R25TW011213.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 19 January 2021. [Google Scholar]
- 2. Worldometer. COVID-19 Coronavirus pandemic. Available at: https://www.worldometers.info/coronavirus/. Accessed 11 September 2021. [Google Scholar]
- 3. Chi WY, Sung CC, Jiun CY.. The outbreak of COVID-19: an overview. J Chinese Med Assoc 2020. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koichi Y, Miho F, Sophia K.. COVID-19 pathophysiology: a review. Clin Immunol 2020. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajen P, Xian Z, Ungaro RC, et al. . Presence of comorbidities associated with severe coronavirus infection in patients with inflammatory bowel disease. Dig Dis Sci 2021. doi: 10.1007/s10620-021-07104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geehan S, Fadel RA, Malette KM, et al. . Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open 2020; 3: e2012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fei Z, Ting Y, Ronghui D, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angelo S, Marco DM, Rodolfo C, et al. . Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovasc Disord 2021; 21:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giacomo G, Massimiliano G, Alberto Z, et al. . Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180:1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noa D, Noam B, Eldad K, et al. . BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fauci AS. The story behind COVID-19 vaccines. Science 2021; 372:109. [DOI] [PubMed] [Google Scholar]
- 12. Kim PS, Read SW, Fauci AS.. Therapy for early COVID-19. JAMA 2020; 324:2149. [DOI] [PubMed] [Google Scholar]
- 13. Delphine P, David V, Artem B, et al. . Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 14. Yaniv L, Neta Z, Ital N, et al. . Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Eurosurveillance 2021; 26. doi: 10.2807/1560-7917.ES.2021.26.26.2100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uganda Virus Research Institute. Kampala registers Indian Covid-19 variant. Available at: https://www.uvri.go.ug/news/kampala-registers-indian-covid-19-varriant. Accessed 12 July 2021. [Google Scholar]
- 16. Ronald O, Felix B.. Uganda’s first 100 COVID-19 cases: trends and lessons. Int J Infect Dis 2020; 96:517–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruce K, Winters M, Alex K, et al. . Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Respir Res 2020; 7:e000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marshall JC, Srinivas M, Janet D, et al. . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waasila J, Caroline M, Lovelyn O, et al. . Difference in mortality among individuals admitted to hospital with COVID-19 during the first and second waves in South Africa: a cohort study. Lancet Glob Heal 2021. doi: 10.1016/S2214-109X00289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. WHO reports delta plus variant. WHO, 2021. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 20 July 2021. [Google Scholar]
- 21. Bernard L, Anne-Françoise R, Laurence S, et al. . Outcome improvement between the first two waves of the coronavirus disease 2019 pandemic in a single tertiary-care hospital in Belgium. Crit Care Explor 2021; 3:e0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simona I, López-Azcona AF, Immaculada V, et al. . First and second waves of coronavirus disease-19: a comparative study in hospitalized patients in Reus, Spain. PLoS One 2021; 16:e0248029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navaratnam AV, Gray WK, Jamie D, Julia W, Briggs TWR.. Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: an observational study using administrative data. Lancet Respir Med 2021; 9:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farzad JS, Mostafa G, Simin M, et al. . Epidemiologic comparison of the first and second waves of coronavirus disease in Babol, North of Iran. Casp J Intern Med 2020; 11(Suppl 1):544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Massa Z, Eduardo DC, Gangemi AJ, et al. . Anakinra and intravenous IgG versus tocilizumab in the treatment of COVID-19 pneumonia [preprint]. MedRxiv 2020; 2020.09.11.20192401. doi: 10.1101/2020.09.11.20192401. [DOI] [Google Scholar]
- 26. Peter H, Shen LW, Emberson JR, et al. ; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med 2020; NEJMoa2021436. doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 27. Sanjay R, Nicolau DV, Beverly L, et al. . Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med 2021; 9:763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ministry of Health, Republic of Uganda. Update on COVID-19 vaccination in Uganda. Available at: https://www.health.go.ug/cause/update-on-covid-19-vaccination-in-uganda/. Accessed 2 June 2021. [Google Scholar]
- 29. Kunihiro M, Ning D, Minghao K, et al. . The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. Glob Heart 2020; 15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nabamallika D, Anita R.. Sex differences in COVID-19 case fatality: do we know enough? Lancet Glob Heal 2021; 9:e14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]