Abstract
Objectives
Sickle cell disease (SCD) leads to chronic and acute complications that require specialised care to manage symptoms and optimise clinical results. The National Heart Lung and Blood Institute (NHLBI) evidence-based guidelines assist providers in caring for individuals with SCD, but adoption of these guidelines by providers has not been optimal. The objective of this study was to identify barriers to treating individuals with SCD.
Methods
The SCD Implementation Consortium aimed to investigate the perception and level of comfort of providers regarding evidence-based care by surveying providers in the regions of six clinical centres across the USA, focusing on non-emergency care from the providers’ perspective.
Results
Respondents included 105 providers delivering clinical care for individuals with SCD. Areas of practice were most frequently paediatrics (24%) or haematology/SCD specialist (24%). The majority (77%) reported that they were comfortable managing acute pain episodes while 63% expressed comfort with managing chronic pain. Haematologists and SCD specialists showed higher comfort levels prescribing opioids (100% vs 67%, p=0.004) and managing care with hydroxyurea (90% vs 51%, p=0.005) compared with non-haematology providers. Approximately 33% of providers were unaware of the 2014 NHLBI guidelines. Nearly 63% of providers felt patients’ medical needs were addressed while only 22% felt their mental health needs were met.
Conclusions
A substantial number of providers did not know about NHLBI’s SCD care guidelines. Barriers to providing care for patients with SCD were influenced by providers’ specialty, training and practice setting. Increasing provider knowledge could improve hydroxyurea utilisation, pain management and mental health support.
Keywords: mental health, haematology, paediatrics
Strengths and limitations of this study.
The Sickle Cell Disease Implementation Consortium surveyed providers in the regions of six clinical centres across the USA, focusing on non-emergency care from the providers’ perspective.
The providers were selected by convenience sampling and may not be representative of all SCD care in the USA, however, respondents were included from diverse regions.
This is one of the largest surveys of non-emergency SCD providers conducted.
Introduction
Sickle cell disease (SCD) is a genetic, multisystem disorder with chronic and acute complications.1 End-organ damage is cumulative and leads to organ dysfunction (eg, renal insufficiency or joint damage) as patients age.1 2 Acute events (eg, vaso-occlusive pain and acute chest syndrome) are often unpredictable and lead to frequent acute care visits.3 The complexity of SCD requires specialised, multidisciplinary care to mitigate disease complications and promote optimal clinical results. In the USA, SCD primarily affects people of colour who are often socioeconomically disadvantaged; therefore, clinical management requires not only specific medical expertise but cultural responsiveness from the providers.4 5
In 2014, the National Heart Lung and Blood Institute (NHLBI) published evidence-based guidelines to assist providers in caring for individuals with SCD.6 The guidelines appear not to be widely adopted in practice,7–9 which poses an important opportunity to improve care delivery for persons living with SCD. Barriers to guideline-based care include healthcare provider unpreparedness, lack of knowledge and stigma related to pain episodes and pain medication.10–12 Across the USA, providers caring for persons with SCD have a wide range of training and expertise, potentially leading to large discrepancies in provider knowledge and preparedness. Additionally, there is evidence that some bias and negative perceptions toward the SCD population exists among medical professionals, negatively affecting the quality of care received by patients.13 14
Discrepancies in provider knowledge and preparedness towards individuals with SCD likely impact care across specialties and settings but are not fully understood. This may affect multiple aspects of evidence-based sickle cell care, such as prescribing of opioids, prescribing the disease modifier hydroxyurea, treating acute pain or treating chronic complications of the disease. A more comprehensive understanding of provider perceptions and level of comfort caring for SCD is needed.
To address this research gap, the SCD Implementation Consortium (SCDIC) surveyed potential care providers for individuals with SCD, to identify likely modifiable barriers to optimal care for patients with SCD across the USA, with the ultimate goal of fostering adoption of national guidelines for SCD care. The SCDIC previously reported results from a separate survey to assess barriers to care within emergency departments (EDs) that did not include non-ED providers.15 In this exploratory study, we surveyed a wide range of providers from different regions of the country and in different areas of medicine, focusing on non-emergency care and assessing the perceptions and factors that influenced their level of comfort in prescribing opioids and hydroxyurea as well as their knowledge and perceptions of managing complications of the disease. Our goal was to identify barriers in treating patients with SCD, with a specific focus on hydroxyurea and opioid prescriptions, and to inform the development of specific intervention strategies to be employed in planned implementation science studies within the SCDIC.
Methods
The SCDIC includes eight clinical centres across the USA, funded by NHLBI and coordinated through Research Triangle Institute International. The goal of the SCDIC is to improve care for patients with SCD by assessing current needs and implementing multilevel interventions. Consortium details have been previously described.16 Six of the eight sites from the consortium contributed to the provider needs assessment survey and the summary results are detailed in this report.
Study design
SCDIC investigators conducted an anonymous multisite, cross-sectional survey of healthcare providers. In this purposive sample, each SCDIC site offered the survey to multiple clinics and healthcare providers in their region through email, phone or in-person administration.
Patient and public involvement
The SCDIC includes a diverse, multidisciplinary group of clinicians, scientists, patients and patient advocates.16 All SCDIC members provided guidance and feedback for consortium projects. Results from this needs assessment were shared with the entire group, to inform future consortium initiatives.
Provider recruitment and procedures
Participants were eligible if they were healthcare professionals who practised at any type of healthcare facility in the same region as one of the six participating SCDIC sites (listed in the ethics section below). A multimodal recruitment strategy was used, recruiting participants through mail invitation, email invitation (online survey) and a face-to-face approach. Methods for identifying eligible providers included assessing Continuing Medical Education (CME) databases, board certification databases, medical professional organisations, the California SCD surveillance project, practices known to investigators, and professional networks. The current analysis includes all respondents who reported that they cared for patients with SCD. Due to the multimodal recruitment strategy, it was not feasible to track the total number of providers approached to take the survey, and no response rate can be calculated. Participants completed the survey online with responses recorded directly in a Research Electronic Data Capture17 database; responses were anonymous. Expected time to complete the survey was 10 min.
Measures/survey elements
A 44-question survey was designed by the SCDIC needs assessment committee and included four primary domains: (1) Provider Demographics, (2) Experiences and comfort providing care to patients with SCD, (3) CME in SCD, (4) Open-ended comments about the care and management of patients with SCD (online supplemental files 12). The survey consisted of sets of items compiled from existing provider surveys.11 18 19 Providers indicated their level of experience with caring for patients with SCD; care provided, such as routine screening; and comfort level with providing preventive care, managing comorbidities, and managing acute and chronic pain. Providers were asked what potential facilitators might improve care for patients with SCD, including higher reimbursement, case management services, access to pain management specialists and access to clinical decision support tools. Providers rated a list of 16 barriers to using opioids on a five-point scale from ‘not a barrier’ to ‘complete barrier’. Providers responded to specific questions about management with hydroxyurea (eg, criteria for, barriers to and comfort level with prescribing).8 Questions were evaluated individually without scaling or creating composite measures.
bmjopen-2021-050880supp001.pdf (846.4KB, pdf)
bmjopen-2021-050880supp002.pdf (246.7KB, pdf)
Statistical analysis
Analyses included descriptive and comparative statistics. Categorical variables are reported with frequencies and percentages overall and by groups; subjects with missing values were excluded from percentage calculation. Associations were evaluated with χ2 tests or, when appropriate, Fisher’s exact test. Two-sided p<0.05 was considered indicative of statistical significance. All provider demographics (including age, race, and gender of provider, provider type, area of practice, practice setting, number of years in clinical practice and age range of patients) were compared with responses to questions about experience and comfort providing care to patients with SCD as well as their experience with and perception of opioid and hydroxyurea treatments. No adjustment for multiple comparisons were done due to the exploratory nature of this analysis.20 Analyses were conducted in SAS V.9.4.
Results
Demographics
A total of 105 providers who cared for patients with SCD responded to the survey. Respondents were 53% female, 65% white and most (37%) were between 31 and 50 years of age (table 1). Seventy-one percent of the respondents were physicians, 18% nurse practitioners or physician’s assistants and 4% registered nurses (table 1). The providers’ primary areas of practice were most frequently paediatrics (24%), or haematology/SCD-specialty (24%). Respondents most frequently reported practising in an urban setting (83%), followed by rural (10%) and suburban (8%) (table 1).
Table 1.
Provider demographics and practice characteristics (N=105); SCD Implementation Consortium, USA, 2021
| Frequency n (%) | |
| Age group | |
| 18–30 years | 9 (8.6) |
| 31–50 years | 39 (37.1) |
| 51–70 years | 22 (21.0) |
| Don’t know/prefer not to respond | 35 (33.3) |
| Sex | |
| Male | 39 (45.2) |
| Female | 33 (53.4) |
| Don’t know/prefer not to respond | 1 (1.4) |
| (32 missing) | |
| Race | |
| American Indian or Alaskan native | 2 (2.6) |
| Asian | 9 (11.5) |
| Black or African American | 8 (10.3) |
| Native Hawaiian or Other Pacific Islander | 0 (0) |
| White | 51 (65.4) |
| Other | 1 (1.3) |
| Don’t know/prefer not to respond | 7 (9.0) |
| (27 missing) | |
| Years in practice | |
| <1 year | 2 (2.6) |
| 1–5 years | 25 (32.9) |
| 6–10 years | 13 (17.1) |
| 11–20 years | 15 (19.7) |
| 21–30 years | 14 (18.4) |
| 31–40 years | 5 (6.6) |
| 41–50 years | 0 (0.0) |
| Don’t know/prefer not to respond | 2 (2.6) |
| (29 missing) | |
| Provider licensure | |
| Medical doctor | 55 (71.4) |
| Nurse practitioner | 11 (14.3) |
| Physician’s assistant | 3 (3.9) |
| Registered nurse | 3 (3.9) |
| Other | 5 (6.5) |
| (28 Missing) | |
| Primary area of practice | |
| Family medicine | 15 (19.2) |
| Haematology/SCD specific | 19 (24.4) |
| Internal medicine | 8 (10.3) |
| Paediatrics | 19 (24.4) |
| Other/prefer not to provide | 17 (21.8) |
| (27 missing) | |
| Main practice setting | |
| Rural | 6 (9.5) |
| Urban | 52 (82.5) |
| Suburban | 5 (7.9) |
| (42 missing) | |
| Age of the patients with SCD cared for | |
| Infancy through young adult | 55 (59.1) |
| Adults (ages ≥18 years) | 38 (40.9) |
| (12 missing) |
SCD, sickle cell disease.
Comfort level with pain management and opioid prescriptions
The majority (76%) of the providers reported that they were either ‘somewhat comfortable’ or ‘very comfortable’ with their ability to manage acute pain episodes experienced by patients with SCD (table 2). A smaller proportion (63%) reported they were either ‘somewhat comfortable’ or ‘very comfortable’ managing chronic pain (table 2).
Table 2.
Comfort level with pain management in sickle cell disease (N=105); Sickle Cell Disease Implementation Consortium, USA, 2021
| Frequency n (%) | |
| Managing acute pain episodes | |
| Very uncomfortable | 9 (9.2) |
| Somewhat uncomfortable | 10 (10.2) |
| Neither comfortable or uncomfortable | 3 (3.1) |
| Somewhat comfortable | 30 (30.6) |
| Very comfortable | 45 (45.9) |
| Don’t know/prefer not to respond | 1 (1.0) |
| (7 missing) | |
| Managing chronic pain | |
| Very uncomfortable | 7 (7.4) |
| Somewhat uncomfortable | 20 (21.3) |
| Neither comfortable or uncomfortable | 5 (5.3) |
| Somewhat comfortable | 44 (46.8) |
| Very comfortable | 15 (16.0) |
| Don’t know/prefer not to respond | 3 (3.2) |
| (11 missing) | |
| Prescribe opioids | |
| Yes | 73 (73.7) |
| No | 23 (23.2) |
| Don’t know/prefer not to respond | 3 (3.0) |
| (6 missing) |
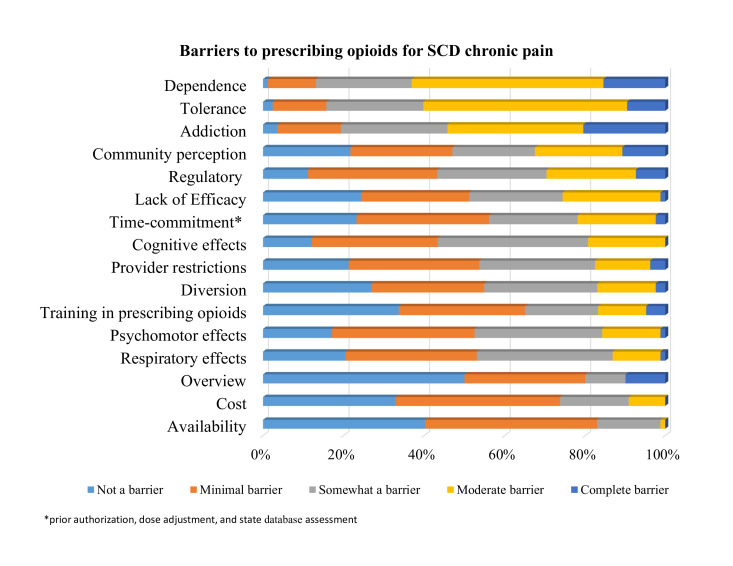
Approximately 74% of providers reported prescribing opioids to patients with SCD (table 2). The most commonly reported barriers (rated as ‘moderate’ or ‘complete’) to prescribing opioids to patients with SCD were drug dependence (63%), tolerance (60%) and addiction (54%) (figure 1, online supplemental file 2). No statistically significant associations were found between providers’ characteristics and comfort with managing acute pain.
Figure 1.
Barriers to prescribing opioids: the extent of providers caring for persons with SCD who report each reason as a moderate or complete barrier to opioid prescription for chronic pain. SCD, sickle cell disease.
Prescribing opioids for SCD pain was also associated with providers’ area of practice with haematologists and SCD specialists more likely to prescribe opioids than those of different specialties (p=0.004, table 3). Barriers to prescribing opioids were explored for potential differences by practice specialty, area of practice, provider type and years in clinical practice. The only significant association found suggested that providers from rural areas may have more moderate or complete regulatory barriers (4/6 (66.7%)) compared with those in urban settings (11/49 (29%)) (p=0.0213).
Table 3.
Associations between provider area of practice and opioid and hydroxyurea utilisation; Sickle Cell Disease (SCD) Implementation Consortium, USA, 2021
| Main area of practice | |||
| Haematology/SCD | Other | P value* | |
| Prescribe opioids to patients with SCD | |||
| No | 0 (0) | 18 (32.7) | 0.004 |
| Yes | 17 (100) | 37 (67.3) | |
| Comfortable managing hydroxyurea therapy for SCD | |||
| Neutral, somewhat or very uncomfortable | 2 (10.5) | 25 (49.0) | 0.0049 |
| Somewhat or very comfortable | 17 (89.5) | 26 (51.0) | |
*Fisher’s exact test.
Comfort level in prescribing hydroxyurea
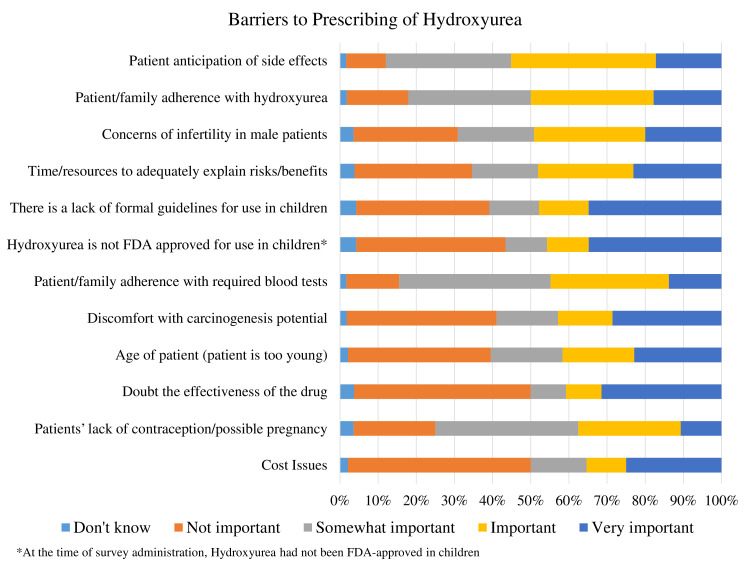
Nearly 85% of providers indicated that they managed hydroxyurea therapy for their patients with SCD with 35% of them managing it for more than half of their patients. The most common criteria for placing patients on hydroxyurea therapy included episodes of acute chest syndrome (43%), at least three painful episodes per year requiring hospitalisation (42%), at least three painful episodes per year at home (31%), chronic pain requiring excessive or frequent opioid use (35%) or a history of stroke (31%) (online supplemental file 2). Providers’ frequently reported that their decision to prescribe hydroxyurea was influenced by patient anticipation of side effects, concerns for infertility in male patients and patient/family adherence with hydroxyurea (figure 2, online supplemental file 2). Additionally, 59% of providers reported some level of discomfort prescribing hydroxyurea based on a perceived carcinogenesis potential. Providers’ comfort level in managing hydroxyurea for patients with SCD was significantly associated with their practice specialty, with 90% of haematology or SCD providers reporting they were ‘somewhat’ or ‘very comfortable’, compared with 51% other providers (p=0.0049, table 3).
Figure 2.
Barriers to prescribing hydroxyurea: the importance of barriers providers face to prescribing hydroxyurea in sickle cell disease
Physician resources and perception on patients needs
Over half of all respondents reported providing primary care for at least 10 patients with SCD in the past year (53%) (online supplemental file 2). Additionally, over half of the providers reported using resources such as the internet (60%), a colleague (57%) or a specialist (71%) when seeking information about the management of patients with SCD. Only 63% reported that they felt the medical needs of their patients with SCD were addressed and merely 22% felt that the behavioural or mental health needs of their patients with SCD were being met. No significant associations between provider demographics were found for physician resources or perception of patient needs.
Tools for SCD care
About 33% of the providers were unaware of the SCD Evidence-Based Guidelines published by the NHLBI (online supplemental file 2). Over half of the providers reported that a clinical decision support tool for SCD would be ‘useful’ or ‘very useful’ for diagnosis, treatment and preventing complications (50%, 74% and 75% respectively) (online supplemental file 2). There were no significant provider demographic associations with tools, guidelines or barriers to prescribing hydroxyurea.
Discussion
Although there are evidence-based guidelines for patients with SCD in the USA, they have not been fully adopted in practice.10 21 Providers’ perspectives on not fully adopting national SCD guidelines of care have not been systematically investigated and constitute important barriers to delivery of quality care. In this study, we surveyed physicians, advanced practice providers, psychologists and registered nurses from a variety of non-acute practice settings to better understand their perceptions toward barriers to care in SCD and non-adoption of national SCD guidelines. Our findings showed that providers who were not haematologists or SCD specialists were less comfortable prescribing hydroxyurea and cited concerns about side effects and patient adherence as potential barriers. Most providers felt comfortable managing pain in patients with SCD but reported substantial barriers to prescribing opioids. Additionally, providers of patients with SCD reported that the medical needs of the patients are not being sufficiently met and an overwhelming majority of them reported that the behavioural and mental health needs of patients with SCD are not being met.
The setting and type of practitioner were associated with the frequency of and barriers to prescribing opioids. Haematologists or SCD specialists more frequently prescribed opioids for patients with SCD and providers in urban settings cited fewer barriers. Frequently cited barriers to opioid use were addiction, dependence and tolerance. These barriers may be exacerbated by the current opioid crisis and related measures to restrict opioid use in the USA, which continues to receive national attention.22 However, it has been shown that addiction to opioids is not more common in SCD than the general population, despite higher opioid utilisation.23 Unconscious bias among physicians has been shown to be a factor that negatively impacts persons with SCD.13 Results from an SCDIC survey of ED physicians found similar barriers to opioid use in patients with SCD including the opioid epidemic, addiction and implicit bias.15 Additionally, the authors found that, although ED physicians felt very comfortable in their knowledge of caring for patients with SCD, 75% were unaware of NHLBI care recommendations for patients with SCD and vaso-occlusive crisis.15 In both non-emergency and emergency settings, these factors, coupled with suboptimal awareness of SCD care guidelines, create great dissatisfaction with care among patients,24 and suggest the need for more SCD specific training for physicians. Many of the providers in this survey were relatively early in their career or cared for fewer than 10 patients with SCD per year. Training should be developed specifically to target physicians who are not haematologists or SCD specialists, who likely see lower numbers of persons with SCD. This training could also be integrated into training programmes for general practitioners.
Nearly 80% of providers surveyed felt the mental health needs of patients with SCD were not met, highlighting an important gap in care. Mental health has an important impact on health-related quality of life (HRQOL)25 and influences pain management for persons with SCD.26 Furthermore, worse HRQOL has been associated with low adherence to hydroxyurea27 as well as increased healthcare utilisation.28 29 Mental health training should be incorporated into all SCD-specific education. Additionally, there has been growing evidence to support the feasibility, acceptability and preliminary efficacy of digital health interventions in SCD.30 In particular, recent work in mobile health has demonstrated the feasibility of more innovative approaches to address the mental health needs in SCD.31 Moreover, there are ongoing efforts to leverage mobile health as an approach to optimise patients’ adherence to hydroxyurea.32
The potential impact of hydroxyurea therapy remains diminished by underprescription and non-adherence. In this survey, providers reported reasons for initiation of treatment with hydroxyurea that are consistent with guidelines, with a higher frequency of disease complications (or higher disease severity) influencing the provider to offer this treatment. However, providers also reported that less than half of patients are treated with hydroxyurea, citing patient anticipation of side effects and concerns about adherence. Although hydroxyurea has not been linked to causing cancer,33 more than half of providers endorsed some level of importance to the perception of carcinogenesis as a deterrent to prescribing this medication.34 At the time of the survey, hydroxyurea prescribing may have been negatively influenced by the lack of US Food and Drug Adminstration (FDA) approval for use in children, a lack of formal guidelines for use in children, and concerns about medication effectiveness. With the recent FDA approval of hydroxyurea in children, a major barrier has been removed. Dissemination of user-friendly, evidence-based guidelines is a logical next step to improve provider knowledge in prescribing hydroxyurea, potentially addressing the remaining barriers. Physicians indicated frequent consultation with other providers and external sources, highlighting the need for additional support mechanisms for non-SCD specialists who care for individuals with SCD. Ideally, hydroxyurea can be prescribed within a co-management model where the SCD specialist prescribes and adjusts dosing in combination with a primary care provider that could assist with lab value monitoring. However, providers across all specialty types and practice settings reported that they were unaware of the NHLBI primary care management guidelines and felt a clinical decision support tool would be useful for diagnosis, treatments, and avoiding complications. These guidelines may be of the most benefit to non-SCD specialty providers. This is currently being tested within the SCDIC in a prospective multicenter study utilizing implementation science to investigate the acceptability, perceived usefulness, and usability of a hydroxyurea toolbox for providers.35
Limitations
Although our study is one of the few to systematically survey the barriers to care in prescribing opioids and hydroxyurea (the two most prescribed medications in SCD) in different areas of the country, there were notable limitations. The convenience sampling of participants recruited by various methods across sites might have introduced bias but could arguably have improved the generalisability of the responses across regions of the country. Providers who chose to participate may have different perspectives on SCD care than those who did not participate. Specifically, many providers may have been affiliated with the SCDIC study sites and, therefore, provide more evidence-based SCD care because of their affiliation with an SCD centre. This limitation would tend to underestimate the barriers and overestimate comfort level with prescribing hydroxyurea and opioids as well as treating acute and chronic pain. We were also unable to report a response rate due to our decision to increase the number of responses using anonymous data collection. Despite limitations, this is one of the largest surveys of non-emergency SCD providers conducted, and important barriers have been identified for further study.
Conclusions
Knowledge of the NHLBI guidelines for SCD among providers in the USA is low. Barriers to care among providers of patients with SCD exist and may be influenced by providers’ specialty, prior training and practice setting. We have identified areas for improvement in pain management and hydroxyurea utilisation. Specifically, we found that perceptions of tolerance, addiction and dependence influence providers’ comfort level in prescribing opioids and concerns about potential side effects and adherence are barriers to hydroxyurea utilisation. Additionally, we identified that improving mental health support for patients is an important need. Strategies to increase adoption of evidence-based guidelines should be tested to increase widespread use of best practices for individuals with SCD. In particular, future implementation studies are needed to improve provider knowledge and reduce misperceptions of therapies known to reduce or treat SCD complications and improve patients’ health outcomes.
Supplementary Material
Acknowledgments
The authors would like to thank all members of the SCDIC, survey respondents, and patient advocates who provided feedback.
Footnotes
Twitter: @mattsmeltzer
Collaborators: Sickle Cell Disease Implementation Consortium
Contributors: MPS, KH, LK, SB and JSH drafted the article. MPS and LP conducted data analysis and interpretation. MPS, MT, AK, JG, PT, LD, RG, JK, LK and JSH contributed to conception or design of the work. All authors gave critical revision of the article and Final approval of the version to be published. MPS is the guarantor.
Funding: This project was supported by grant number 1U01HL133996-01 from National Institute of Health, National Heart, Lung and Blood Institute. The Sickle Cell Disease Implementation Consortium has been supported by US Federal Government cooperative agreements HL133948, HL133964, HL133990, HL133996, HL133994, HL133997, HL134004, HL134007 and HL134042 from the National Heart Lung and Blood Institute and the National Institute on Minority Health and Health Disparities (Bethesda, MD).
Competing interests: JSH receives research funding support from Global Blood Therapeutics and is received consultancy fees from Bluebird Bio, Forma Therapeutics, and Global Blood Therapeutics; JG receives research funding support from Pfizer; AK owns stock in Magenta Therapeutics.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Data from the NHLBI Sickle Cell Disease Implementation Consortium are maintained by RTI International, Research Triangle Park, NC.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Data collection occurred from October 2017 to March 2018. The study was conducted with the oversight of the Institutional Review Boards at St. Jude Children’s Research Hospital (IRB NR17-060), University of California San Francisco Benioff Children’s Hospital Oakland (IRB 2017-027), Augusta University (IRB 1104776), Washington University School of Medicine (IRB 201706016), Duke University (IRB Pro00073506), and Medical University of South Carolina (IRB Pro00066242) with a waiver of informed consent from the participants.
References
- 1. Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. life expectancy and risk factors for early death. N Engl J Med 1994;330:1639–44. 10.1056/NEJM199406093302303 [DOI] [PubMed] [Google Scholar]
- 2. Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers 2018;4:18010. 10.1038/nrdp.2018.10 [DOI] [PubMed] [Google Scholar]
- 3. Lanzkron S, Little J, Field J, et al. Increased acute care utilization in a prospective cohort of adults with sickle cell disease. Blood Adv 2018;2:2412–7. 10.1182/bloodadvances.2018018382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panepinto JA, Pajewski NM, Foerster LM, et al. Impact of family income and sickle cell disease on the health-related quality of life of children. Qual Life Res 2009;18:5. 10.1007/s11136-008-9412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar V, Chaudhary N, Achebe MM. Epidemiology and predictors of all-cause 30-day readmission in patients with sickle cell crisis. Sci Rep 2020;10:2082. 10.1038/s41598-020-58934-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Heart L, and Blood Institute . Evidence-Based management of sickle cell disease: expert panel report, 2014, 2014. [Google Scholar]
- 7. Cabana MD, Kanter J, Marsh AM, et al. Barriers to pediatric sickle cell disease guideline recommendations. Glob Pediatr Health 2019;6:2333794X1984702. 10.1177/2333794X19847026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol 2010;3:255–60. 10.1586/ehm.10.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA 2014;312:1033–48. 10.1001/jama.2014.10517 [DOI] [PubMed] [Google Scholar]
- 10. Tanabe P, Myers R, Zosel A, et al. Emergency department management of acute pain episodes in sickle cell disease. Acad Emerg Med 2007;14:419–25. 10.1111/j.1553-2712.2007.tb01801.x [DOI] [PubMed] [Google Scholar]
- 11. Whiteman LN, Haywood C, Lanzkron S, et al. Primary care providers' comfort levels in caring for patients with sickle cell disease. South Med J 2015;108:531–6. 10.14423/SMJ.0000000000000331 [DOI] [PubMed] [Google Scholar]
- 12. Adams-Graves P, Bronte-Jordan L. Recent treatment guidelines for managing adult patients with sickle cell disease: challenges in access to care, social issues, and adherence. Expert Rev Hematol 2016;9:541–52. 10.1080/17474086.2016.1180242 [DOI] [PubMed] [Google Scholar]
- 13. Haywood C, Diener-West M, Strouse J, et al. Perceived discrimination in health care is associated with a greater burden of pain in sickle cell disease. J Pain Symptom Manage 2014;48:934–43. 10.1016/j.jpainsymman.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wakefield EO, Pantaleao A, Popp JM, et al. Describing perceived racial bias among youth with sickle cell disease. J Pediatr Psychol 2018;43:779–88. 10.1093/jpepsy/jsy015 [DOI] [PubMed] [Google Scholar]
- 15. Linton EA, Goodin DA, Hankins JS, et al. A survey-based needs assessment of barriers to optimal sickle cell disease care in the emergency department. Ann Emerg Med 2020;76:S64–72. 10.1016/j.annemergmed.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DiMartino LD, Baumann AA, Hsu LL, et al. The sickle cell disease implementation Consortium: translating evidence-based guidelines into practice for sickle cell disease. Am J Hematol 2018;93:E391–5. 10.1002/ajh.25282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grahmann PH, Jackson KC, Lipman AG. Clinician beliefs about opioid use and barriers in chronic nonmalignant pain. J Pain Palliat Care Pharmacother 2004;18:7–28. 10.1080/J354v18n02_02 [DOI] [PubMed] [Google Scholar]
- 19. Mainous AG, Tanner RJ, Harle CA, et al. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia 2015;2015:1–6. 10.1155/2015/853835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–6. 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 21. Lanzkron S, Haywood C, Hassell KL, et al. Provider barriers to hydroxyurea use in adults with sickle cell disease: a survey of the sickle cell disease adult provider network. J Natl Med Assoc 2008;100:968–74. 10.1016/S0027-9684(15)31419-X [DOI] [PubMed] [Google Scholar]
- 22. U.S. Departmetn of Health and Human Services . What is the U.S. opioid epidemic? 2019. Available: https://www.hhs.gov/opioids/about-the-epidemic/index.html
- 23. Ballas SK, Kanter J, Agodoa I, et al. Opioid utilization patterns in United States individuals with sickle cell disease. Am J Hematol 2018;93:E345–7. 10.1002/ajh.25233 [DOI] [PubMed] [Google Scholar]
- 24. Kanter J, Gibson R, Lawrence RH, et al. Perceptions of US adolescents and adults with sickle cell disease on their quality of care. JAMA Netw Open 2020;3:e206016. 10.1001/jamanetworkopen.2020.6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Panepinto JA, Bonner M. Health-Related quality of life in sickle cell disease: past, present, and future. Pediatr Blood Cancer 2012;59:377–85. 10.1002/pbc.24176 [DOI] [PubMed] [Google Scholar]
- 26. Myrvik MP, Burks LM, Hoffman RG, et al. Mental health disorders influence admission rates for pain in children with sickle cell disease. Pediatr Blood Cancer 2013;60:1211–4. 10.1002/pbc.24394 [DOI] [PubMed] [Google Scholar]
- 27. Badawy SM, Thompson AA, Lai J-S, et al. Health-Related quality of life and adherence to hydroxyurea in adolescents and young adults with sickle cell disease. Pediatr Blood Cancer 2017;64:e26369. 10.1002/pbc.26369 [DOI] [PubMed] [Google Scholar]
- 28. Jonassaint CR, Jones VL, Leong S, et al. A systematic review of the association between depression and health care utilization in children and adults with sickle cell disease. Br J Haematol 2016;174:136–47. 10.1111/bjh.14023 [DOI] [PubMed] [Google Scholar]
- 29. Badawy SM, Thompson AA, Holl JL, et al. Healthcare utilization and hydroxyurea adherence in youth with sickle cell disease. Pediatr Hematol Oncol 2018;35:297–308. 10.1080/08880018.2018.1505988 [DOI] [PubMed] [Google Scholar]
- 30. Badawy SM, Cronin RM, Hankins J, et al. Patient-Centered eHealth interventions for children, adolescents, and adults with sickle cell disease: systematic review. J Med Internet Res 2018;20:e10940. 10.2196/10940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jonassaint CR, Kang C, Prussien KV, et al. Feasibility of implementing mobile technology-delivered mental health treatment in routine adult sickle cell disease care. Transl Behav Med 2020;10:58–67. 10.1093/tbm/iby107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alberts NM, Badawy SM, Hodges J, et al. Development of the InCharge health mobile APP to improve adherence to hydroxyurea in patients with sickle cell disease: User-Centered design approach. JMIR Mhealth Uhealth 2020;8:e14884. 10.2196/14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5 year follow-up. Am J Hematol 2010;85:403–8. 10.1002/ajh.21699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:10.1182/blood-2009-04-146852:5300–11. 10.1182/blood-2009-04-146852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hankins JS, Shah N, DiMartino L, et al. Integration of mobile health into sickle cell disease care to increase hydroxyurea utilization: protocol for an efficacy and implementation study. JMIR Res Protoc 2020;9:e16319. 10.2196/16319 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-050880supp001.pdf (846.4KB, pdf)
bmjopen-2021-050880supp002.pdf (246.7KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Data from the NHLBI Sickle Cell Disease Implementation Consortium are maintained by RTI International, Research Triangle Park, NC.