Abstract
Immunoglobulin E (IgE)‐mediated allergy is the most common hypersensitivity disease affecting more than 30% of the population. Exposure to even minute quantities of allergens can lead to the production of IgE antibodies in atopic individuals. This is termed allergic sensitization, which occurs mainly in early childhood. Allergen‐specific IgE then binds to the high (FcεRI) and low‐affinity receptors (FcεRII, also called CD23) for IgE on effector cells and antigen‐presenting cells. Subsequent and repeated allergen exposure increases allergen‐specific IgE levels and, by receptor cross‐linking, triggers immediate release of inflammatory mediators from mast cells and basophils whereas IgE‐facilitated allergen presentation perpetuates T cell–mediated allergic inflammation. Due to engagement of receptors which are highly selective for IgE, even tiny amounts of allergens can induce massive inflammation. Naturally occurring allergen‐specific IgG and IgA antibodies usually recognize different epitopes on allergens compared with IgE and do not efficiently interfere with allergen‐induced inflammation. However, IgG and IgA antibodies to these important IgE epitopes can be induced by allergen‐specific immunotherapy or by passive immunization. These will lead to competition with IgE for binding with the allergen and prevent allergic responses. Similarly, anti‐IgE treatment does the same by preventing IgE from binding to its receptor on mast cells and basophils. Here, we review the complex interplay of allergen‐specific IgE, IgG and IgA and the corresponding cell receptors in allergic diseases and its relevance for diagnosis, treatment and prevention of allergy.
Keywords: allergy treatment, basic mechanisms in allergy, biologics, biomarkers, IgE, immunotherapy, tolerance induction, vaccines and mechanisms
Abbreviations
- AD
Atopic Dermatitis (AD)
- AIT
Allergen immunotherapy
- APC
Antigen‐Presenting Cells; β‐LG, β‐lactoglobulin
- CD23
Cluster of Differentiation 23 (the low‐affinity IgE receptor)
- CIU
Chronic Idiopathic Urticaria
- CSU
Chronic spontaneous urticaria
- DARPINs
Designed Ankyrin Repeat Proteins
- DCs
Dendritic Cells
- Fab
Antigen binding fraction of an antibody
- Fc
Constant region fraction of an antibody
- FcεRI
The high‐affinity IgE receptor
- FcγR
Fc receptor for IgG
- FcRN
Neonatal Fc Receptor
- FOXP3
Forkhead box protein P3
- FRET
Fluorescence Resonance Energy Transfer
- HDM
House Dust Mite
- Ig(A,D,G,M)
Immunoglobulin A,D,G,M
- IgE‐FAB
IgE‐facilitated antigen binding to CD23
- IL‐
Interleukin‐
- ITAMs
Immunoreceptor Tyrosine‐based Activation Motifs
- OIT
Oral Immunotherapy
- OVA
Ovalbumin
- sCD23
soluble CD23
- SCIT
Subcutaeous Immunotherapy
- SLIT
Sublingual Immunotherapy
- Tregs
Regulatory T cells
- Tfh
Follicular T helper cells
- Th2
T helper 2
1. INTRODUCTION
The discovery of immunoglobulin (Ig) E in the mid‐1960s by two independent groups led by Kimishige Ishizaka in the United States of America and S.G.O Johansson in Sweden resulted in a significant impact on the diagnosis and management of allergic diseases. 1 , 2 Since then, IgE has been shown to play an essential role in type I immediate allergic responses. 3 , 4 Antibody isotype class switching in favour of IgE can occur locally in the nasal mucosa, 5 bronchial tissues 4 , 6 , 7 and also in the intestinal mucosa. 8 Dendritic cells (DCs) present in the upper layers of the epithelium and lamina propria of the airways, 9 , 10 gut and the skin are well disposed to capture allergens and drive T‐cell polarization towards a pro‐allergic‐type II immune response. DCs migrate to the draining lymph nodes, where they prime and activate naïve T cells to differentiate, proliferate and clonally expand into T helper 2 (Th2), and also the recently identified follicular T helper cells 13(Tfh13) 11 that produce interleukin‐4 (IL‐4) and interleukin‐13 (IL‐13) and IL‐21, which lead to the differentiation and clonal expansion of naïve T cells to Th2 cells. However, earlier studies demonstrating that also B cells may be important antigen‐presenting cells (APCs) in the initiation of IgE sensitisation 12 , 13 are supported by more recently published studies. 14 , 15 Moreover, the enhanced expression of Th2 cytokines such as IL‐4 and IL‐13 produced by mast cells and basophils 6 , 7 , 16 in the nasal mucosa can promote tissue mast cells to induce IgE synthesis in B cells in an indirect manner, resulting in local IgE synthesis by B cells. 5 , 17 In turn, after sensitization, IgE can also enhance Th2‐cell response in a FcεRI and CD23‐dependent manner. 18 , 19 , 20 , 21 It is noteworthy that there is evidence that (non‐IgE) allergen‐specific antibodies in early life can modulate allergic sensitization. During pregnancy and through breast milk, maternal immunoglobulins are transferred to offspring and it seems that maternal allergen‐specific IgG may protect the offspring from allergic sensitization. 22 , 23 , 24 Birth cohorts and studies in animal models have revealed a long‐term influence on offspring allergy susceptibility. 23 , 25 Restoration of immune tolerance following long‐term allergen immunotherapy is associated with the induction of local and systemic IgG and IgA associated neutralizing antibodies. 26 , 27 , 28 , 29
This article reviews the role of IgE, IgG and IgA in allergic inflammation and induction of immune tolerance in early life as well as after allergen immunotherapy. IgE, IgG and IgA result from class switching events after IgM‐bearing B cells undergo activation and differentiation in lymph nodes in response to antigen or allergen. IgG antibodies accumulate to the highest levels in lymph and blood, providing broad systemic protection. IgA is primarily secreted across mucosal barriers or into breast milk, providing an immune barrier at these interfaces. IgE is found at the lowest concentration systemically as it becomes sequestered at cell surfaces through binding to its high‐affinity receptor. Furthermore, targeting of IgE with anti‐IgE antibodies as well as the effects of passive immunization with allergen‐specific IgG is considered and discussed.
2. IgE AND ITS RECEPTORS
2.1. Immunoglobulin E (IgE)
Structurally, in agreement with other antibody classes, IgE antibody comprises two identical light and heavy chains (Figure 1). Each chain is formed of 110 amino acid ‘immunoglobulin domains’. Disulphide bonds covalently link the light and heavy chains. Unlike IgD, IgG and IgA, which have three constant region domains, the heavy chain is structurally similar to the μ heavy chain of IgM as it has four constant region domains (Cε1‐Cε4). Cε3 and Cε4 domains are homologous in both sequence and quaternary structure to the Cγ2 and Cγ3 domains of IgG antibody isotype. 3 IgE can be distinguished from IgG by the position of its Cε2 domains, which are inserted into the corresponding Fab‐Fc hinge region of IgG. The two antigen‐binding sites are formed by pairing of the variable region of light and heavy chains.
FIGURE 1.
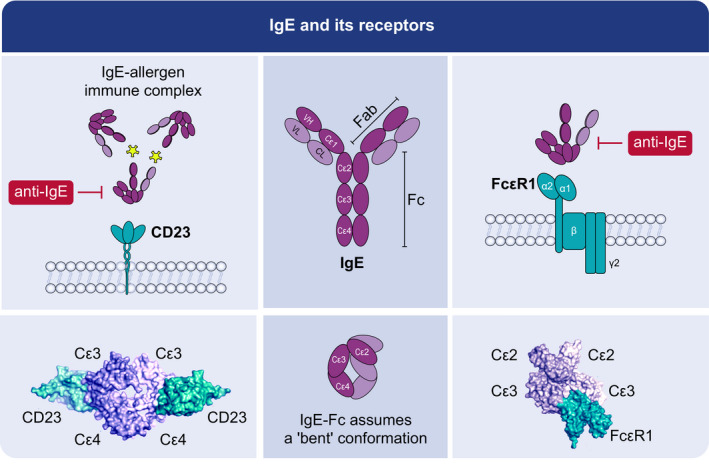
IgE and its receptors. IgE antibody uses two identical light and heavy chains and the constant region has four domains (Cε1‐Cε4). The two antigen‐binding sites are formed by pairing of the variable region of light and heavy chains. IgE is asymmetrically bent at the Cε2‐3 linker and folds on itself with the two Cε2 domains folded back and almost touching the Cε4 domains. IgE interacts with the high‐affinity IgE‐receptor FcεRI with the Cε2 and Cε3 domains, and with the low‐affinity IgE‐receptor CD23 with the Cε3 and Cε4 domains. Anti‐IgE antibodies like omalizumab bind to the Cε3 domain of IgE and can therefore inhibit the binding of IgE to both FcεRI as well as to CD23. IgE binding to FcεRI occurs in a 1;1 stoichiometry of 1:1, and IgE binding to CD23 in a stoichiometry of 2:1 or larger
IgE is asymmetrically bent at the Cε2‐3 linker and folds on itself with the two Cε2 domains folded back and almost touching the Cε4 domains 30 , 31 , 32 (Figure 1.2). Fluorescence resonance energy transfer (FRET) analysis has revealed that the distance between N‐ and C‐terminal ends of IgE in solution are consistent with a predominantly bent conformation, and that binding of IgE with its receptor induces conformational changes that further increase this bending, consistent with prior structural studies. 33
Immunoglobulin E is central to type I immediate allergic responses. 1 , 3 Several studies have illustrated that antibody isotype class switching in favour of IgE may occur locally in the nasal and bronchial mucosa in allergic patients and in lymphatic tissues adjacent to sites of allergen contact, but the precise sites for IgE production are not yet known. 5 , 6 , 34 , 35 Elevated concentrations of IgE antibodies and FcεRI have been demonstrated in target organs, reaching over ten times more in atopic and allergic individuals than non‐atopics. 5 , 7 , 36 IgE antibodies bind with high affinity to FcεRI (dissociation constant, Kd =10–10 M) on mast cell and basophil surfaces.
2.2. FcεRI—structure and function on effector cells and APCs
The high‐affinity IgE receptor (FcεRI) is a member of the immunoglobulin (Ig) superfamily. It is highly expressed as an αβγ2 tetramer (~200,000 molecules/cell) on the surface of mast cells and basophils. 37 , 38
It consists of four polypeptide chains, an α chain, a β chain and two disulphide‐linked γ chains. 39 In human monocytes, Langerhans’ cells and peripheral blood DCs, eosinophils, platelets and smooth‐muscle cells, FcεRI is expressed as a αγ2 trimer, consisting of one α and two γ chains. 3 , 40 The α chain consists of an extracellular domain, a single transmembrane helix domain and a short cytoplasmic sequence. The IgE binding function of the high‐affinity IgE receptor is confined to the two extracellular domains of the α chain, with a 1:1 binding stoichiometry. Both intracellular sequences of the β and γ chains consist of immunoreceptor tyrosine‐based activation motifs (ITAMs). The β subunit chain functions as an amplifier of downstream events after the initial activation of surface FcεRI. 39 The lack of a β chain may account for the variable expression of this receptor on certain cells. The trimeric expression of FcεRI on monocytes, DCs and Langerhans cells has been shown to facilitate allergen presentation to CD4+ T cells. The efficiency of FcεRI‐mediated allergen uptake by APCs is 100 to 1000‐fold more effective than any endocytosis or pinocytosis. 19 , 41 Cross‐linking of tetrameric FcεRI on the surface of mast cells and basophils leads to cellular activation, resulting in degranulation and the release of preformed mediators, synthesis of lipid mediators and the release of inflammatory cytokines, leading to the recruitment of leukocytes which further enhance the allergic response. 42 IgE enhances the expression of FcεRI by stabilization of the receptor, 43 , 44 and occupancy of FcεRI can also prolong mast cell survival by IgE. 45 Interestingly, IgE binding does not prolong basophil survival. 46 FcεRI is up‐regulated by mast cells and basophils in seasonal allergic rhinitis and its expression correlates with serum IgE concentrations. 47 , 48 , 49 , 50
2.3. CD23: structure and function on B cells
The low‐affinity IgE‐receptor FcεRII, also known as CD23, is a single chain type II integral membrane protein of 45 kD and belongs to the C‐type (Calcium‐dependent) lectin superfamily. 3 , 51 , 52 , 53 CD23 is an important regulator of IgE production by B cells and also contributes to antigen trafficking and presentation by IgE immune complexes. The membrane‐bound CD23 consists of a lectin ‘head’ domain spaced from the membrane by an N‐terminal stalk domain that is thought to form leucine‐zipper type oligomers, which have generally been represented as trimers. The lectin head domains of CD23 in the human form contain a C‐terminal tail sequence that is involved in CD21 binding. The CD23 stalk region is susceptible to proteolytic cleavage. ADAM10, a disintegrin and a metalloproteinase, has been shown to release soluble CD23 (sCD23) and this release is enhanced in the absence of IgE. ADAM10 proteolytic cleavage produces fragments of CD23 (37 kD) containing a portion of the stalk or smaller fragments (25 kD, 16 kD) lacking the stalk, 54 which can positively upregulate IgE production by B cells. ADAM10 knockout mice show an increase in membrane CD23 expression on B cells and reductions in both soluble CD23 and allergic inflammation.
Although the CD23 head domain fold is in the C‐type lectin family, the interaction with IgE does not involve carbohydrate. CD23 binds to the IgE at the junction of Cε3‐Cε4 domains and restrains the IgE‐Fc conformation, competing allosterically for FcεRI binding. A single lectin head fragment can bind to the IgE‐Fc portion with a Kd of ~10−6–10−7 M, while the membrane‐expressed CD23 binds IgE‐Fc with a significantly higher apparent affinity binding (Kd=10−8 −10−9 M), due to avidity affects. 54 , 55 Two isoforms of CD23 which differ by seven (CD23a) or six amino acids (CD23b) have been defined. CD23a is constitutively expressed primarily on antigen‐activated B cells, while CD23b seems to be the pre‐dominant isoform on monocyte‐derived DCs. 56 , 57 IL‐4 induces CD23b expression in several cell types, including B cells and epithelial cells. 3 , 58 , 59
3. PHYSIOLOGICAL ROLE OF IgE IN ALLERGIC INFLAMMATION
3.1. Role of IgE on mast and basophils responses
Mast cells and basophils were identified in tissue and blood, respectively, by Ehrlich almost 150 years ago, and their function was anticipated during this period. 60 However, the functional relationship between these cells was not described in detail until after the discovery of IgE. Follow‐up experiments by T. and K. Ishizaka revealed that both cells were activated through the high‐affinity IgE receptor in the presence of IgE. Allergen‐induced cross‐linking of IgE bound to FcεRIs on the surface of mast cells or basophils induce aggregation of the receptor and intracellular signalling events 61 that leads to Ca2+‐dependent release of preformed mediators and de novo synthesis and secretion of lipid mediators and cytokines (eg IL‐4 and IL‐13) 62 , 63 (Figure 2). The concentration of serum IgE regulates the FcεRI surface expression on these effector cells, and this feedback mechanism can reduce the allergen concentration needed for activation. 64 Moreover, recent findings suggest that IgE's glycosylation (sialylation) may be critical for the activation of mast cells in a mouse model of anaphylaxis based on IgE. 65 The essential role of IgE cross‐linking that leads to activation of effector cells has obtained less attention but was described in detail for basophils by Christensen et al. 66 and later similar observations were made for mast cells. 67 These studies confirmed that the concentration of allergen‐specific IgE, IgE affinity and the ratio of allergen‐specific to total IgE are key elements determining the strength of effector cell release. The effect of specific to total IgE ratio and the observation that one high‐affinity IgE in a mixture overrides the difference between high, medium, and low‐affinity IgE may explain why correlations between concentrations of allergen‐specific IgE and clinical symptoms have been difficult to establish. 68 Detailed studies with model antigens demonstrate that the spacing between the epitopes is critical 69 and the number as well as relative positioning of IgE epitopes on allergens 70 , 71 may also be critical for the ability to cross‐link the receptors, which adds to the complexity of IgE‐mediated effector cell activation.
FIGURE 2.
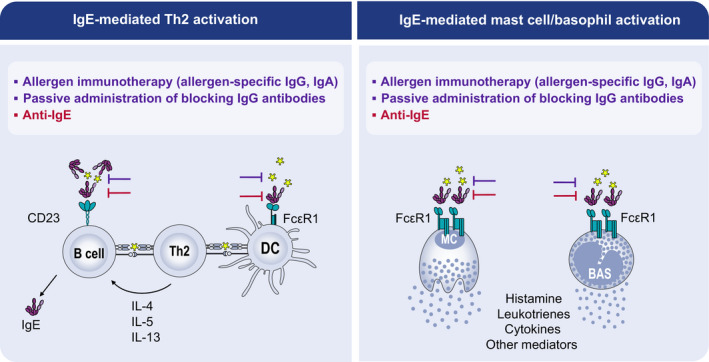
IgE‐mediated Th2 and Mast cell/basophil activation and inhibitory effects of allergen‐specific IgG and IgA as well as anti‐IgE. Inhibition of IgE‐mediated Th2‐cell activation (left panel) and basophil/mast cell degranulation (right panel) by allergen‐specific IgG and ‐IgA (purple), and anti‐IgE (red) treatment. Whereas allergen‐specific IgG and IgA compete with IgE for binding to allergens, anti‐IgE antibodies bind to IgE and block binding of IgE to both the high‐affinity (FcεRI) and low‐affinity (CD23) receptors for IgE expressed on antigen‐presenting cells and basophils/mast cells. In this way, they can inhibit IgE‐mediated activation of allergen‐specific T cells as well as the release of inflammatory mediators by basophils/mast cells induced by IgE‐mediated cross‐linking of FcεRI after allergen exposure
3.2. Role of IgE in enhancing T‐cell responses
The concept of IgE‐facilitated allergen presentation was first elucidated in studies showing that complexes of specific IgE with allergens could significantly enhance the responses of allergen‐specific T cells at low allergen concentrations. 18 , 21 , 72 This IgE‐mediated allergen presentation, or facilitated allergen presentation, involved binding the IgE‐allergen complexes to CD23 on antigen‐presenting B cells(Figure 2).
Around the same time, it became apparent that dendritic cells and monocytes from peripheral blood express the high‐affinity IgE receptor (FcεRI) and could also activate allergen‐specific T cells in an IgE‐facilitated manner. 19 , 55
These findings are relevant because allergen levels in the respiratory tract are extremely low upon natural allergen exposure. The IgE‐facilitated presentation of allergens to T cells enables T‐cell activation at these low allergen exposures. 19 , 21
The binding of allergen‐IgE complexes to antigen‐presenting cells is dependent on several parameters, like antigen specificity and affinity of the IgE antibodies, levels of receptor expression, and clonality of B cells. In principle and in a model system, monoclonal IgE is sufficient to present allergen. 73 Furthermore, the complexity of IgE binding to multiple epitopes on allergens and their affinity has been shown to correlate with the facilitation of T‐cell responses. 74 These findings suggest that the number of IgE molecules bound per allergen may play an essential role in this complex formation and binding. This was confirmed recently in a study by Villazala‐Merino et al. 75 where non‐FcεRI cross‐linking monoclonal IgE‐monomeric allergen complexes (ie one IgE molecule binding two Bet v1 molecules) could enhance T‐cell activation. However, this activation was further enhanced by 100‐fold when cross‐linking IgE‐allergen oligomer complexes were used (multimeric complexes). Finally, the heterogeneity of allergen epitopes recognized by IgE, the presence of competing IgG(4) antibodies, the density of CD23 on the surface of B cells in peripheral blood of allergic patients correlates with the ability to enhance T‐cell activation by allergen‐IgE complexes. 76
4. THE ROLE OF IgG AND IgA IN TOLERIZATION AND TREATMENT OF IgE‐MEDIATED ALLERGIES
4.1. The role of IgG and IgA in preventing sensitization in early life
Maternal IgA and IgG antibodies from breast milk or transferred over the placenta during pregnancy play an important role in the development of allergy in the offspring (summarized in Figure 3). Higher primates including humans and rodents have a hemochorial placenta. 77 In this type of placenta, the transfer of nutrients and antibodies from maternal blood to the foetal circulation is facilitated through direct contact of the blood with the foetal chorion. During the third trimester of pregnancy, IgG immunoglobulins are transferred from the placenta into the serum of the foetus using the non‐classical neonatal Fc Receptor (FcRN). These IgG antibodies are thought to be important for providing protection to infants from infectious disease. 24 , 78
FIGURE 3.
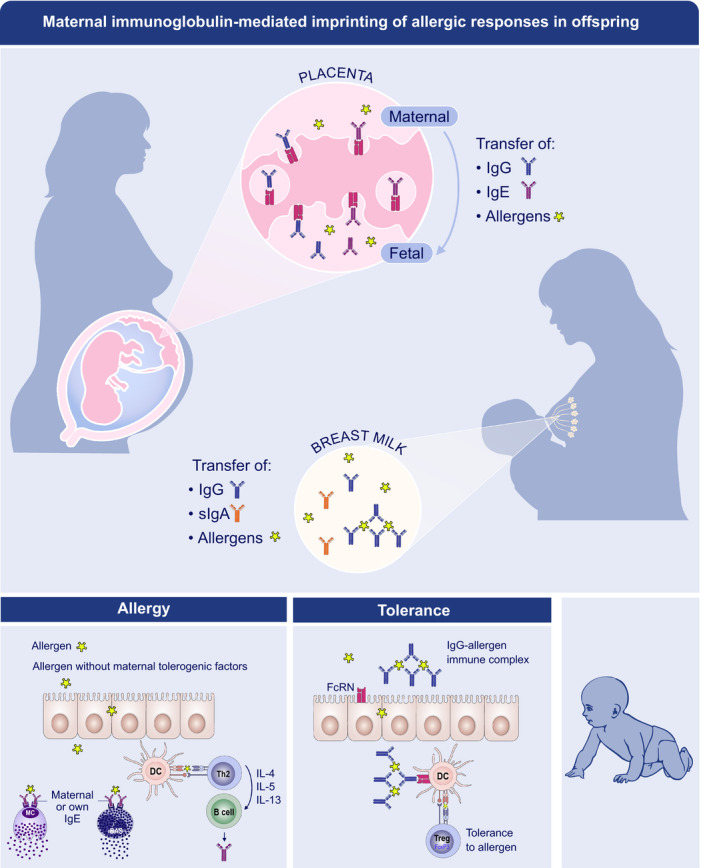
Maternal immunoglobulin‐mediated imprinting of allergic responses in the offspring. Maternal IgG (blue) to airborne allergens and food allergens reach the offspring in utero by a transfer across the placenta and after birth through breast milk and transfer across the gut. The neonatal Fc receptor (FcRn) carries maternal IgG either free or bound to allergen. Free IgG can inhibit allergic sensitization in offspring by modulating B‐cell reactivity. Allergen‐IgG immune complexes can induce immune tolerance by promoting allergen‐specific Treg expansion. Maternal IgE (purple) might also be transported across the placenta by FcRn. Foetal mast cells bear the IgE receptor (FcεR1) and bind maternal IgE. In mice, these IgE‐loaded foetal mast cells are functionally competent, degranulate upon exposure to allergen and persist in neonates, in whom they may mediate allergic disease in early life. Maternal secretary IgA (orange) are also found in human breast milk and might decrease allergic sensitization by controlling allergen transfer across offspring gut. Evidence in mice also suggests they might control the expansion of Tregs in offspring
These serum antibodies decline over time (with IgG having a T1/2 of approximately 21 days, and IgA of approximately 7 days), resulting in decreased protection against infections before the infant can produce enough antibodies itself. 79 Maternal IgG to airborne allergens (ie House dust mite, Birch pollen, cat) and food allergens (egg, cow milk) were also found to be transferred in utero in birth cohorts. 80 , 81 High levels of cord blood IgG antibodies to cat and birch, but not to food allergens, were associated with less atopic symptoms in the children during the first eight years of life. 23 , 80 Maternal allergen immunotherapy has also resulted in the induction of allergen‐specific IgG in the serum of the offspring, further confirming they are passively transferred across the placenta into the fetus. 82 , 83 Although this IgG transfer suggests it may reduce sensitization in the offspring, a review of five studies of allergen‐specific immunotherapy during pregnancy did not show any clear evidence of allergy reduction in the offspring. 84
In addition to IgG, also maternal IgE can be transported over the placenta via FcRN, resulting in IgE binding to already competent mast cells in the fetus. 85
Antibody levels change during lactation, with colostrum, the first milk, having increased IgA (>10 fold) and IgG (2–3 fold) levels compared with mature milk. 86 Several studies have reported that following birth, mothers continue to transfer IgG in addition to secretory IgA to their offspring through breast milk. 24 , 87 Antibodies to both airborne and food allergens have been detected in human milk. 81 , 88 , 89 Maternal allergen‐specific IgG can be detected in children's serum up to 6 months of age, and the specificity to the allergen in plasma, breast milk and cord blood is quite similar. 23 It is noteworthy that infants of mothers with high concentrations of allergen‐specific IgG in serum and breast milk did not show sensitization to the allergen at five years. More importantly, sensitized children had mothers with low concentrations of allergen‐specific IgG. 23
For four decades, rodent experiments have explored the impact of in utero and of milk transfer of IgG to offspring on allergy sensitization and their mechanisms of action. 90 Neutralization of the allergen and allergen‐specific modulation of B‐ and T‐cell regulatory properties of maternal IgG antibodies have been described. 90 In addition to possible immune regulation induced by the sole presence of maternal IgG, maternally derived immune complexes made of allergen bound to IgG may also be critical for regulation of long‐term allergy susceptibility. Allergen‐IgG immune complexes have been detected in cord blood 91 , 92 and human milk. 89 , 92 There is strong evidence from rodent experiments that allergen‐IgG immune complexes in breast milk are very potent in eliciting an immune response in offspring. Oral exposure to ovalbumin (OVA)‐IgG immune complexes through breast milk resulted in the induction of OVA‐specific Forkhead box protein P3 (FOXP3) regulatory T cells (Tregs) responsible for prolonged tolerance to OVA in offspring subsequently leading to respiratory and food allergy prevention. 22 , 89 This appeared to result from a protected transport of OVA across the gut barrier and an enhanced presentation by dendritic cells, both depending on the use of the neonatal Fc Receptor (FcRn).
A recent report analysed the influence of maternal immune status on the induction of protection against cow milk allergic sensitization upon β‐lactoglobulin (β‐LG) transfer through breast milk. Using two different protocols for maternal immunization, the study showed that the transfer of the antigen without antibody did not lead to protection and that levels of antibodies in breast milk positively correlated with the inhibition of allergic sensitization in offspring. 93
Similarly, maternal exposure to peanut during breastfeeding inhibited allergic response to peanut in offspring only when mothers had been immunized but not if naïve to peanut. 94 , 95 However, allergen transfer to offspring in the presence of maternal antibodies does not systematically result in tolerance induction, as shown for House dust mite (HDM) allergen. 96 A study in mice showed that mice nursed by HDM‐exposed mothers developed a gut immunity imbalance associated with the expansion of Th2 cells and a refractory state to oral tolerance. Importantly, when neutralizing HDM protease activity, this deleterious effect on gut immune ontogeny in offspring was abolished. 97 This observation highlights the importance of the biological properties of the allergen itself, as in the case of HDM, the proteolytic activity of the allergens was responsible for immune priming. 97
In addition to human breast milk, allergen‐specific IgG (bIgG) has been detected in cow's milk. 98 Several epidemiological studies have shown that consumption of raw farm milk is associated with a lowered occurrence of asthma, allergic rhinitis and atopic sensitization. 99 , 100 , 101
It is not clear whether allergen‐specific IgG is complexed to allergens in raw milk, or whether they bind allergens after swallowing of nasal secretions containing inhaled allergens. However, the bovine IgG can theoretically bind to allergens that are swallowed, and thereby play a role in tolerisation to the allergens.
In addition to allergen‐specific IgG, there is some evidence that allergen‐specific IgA in breast milk is associated with protection as shown for infants’ cow's milk allergy. 102 , 103 , 104 , 105 The total levels of IgA in breast milk are inversely associated with atopic dermatitis (AD) in early life. 106 A recent study reported that maternal milk IgA in mice might play an important role in establishing a gut regulatory T‐cell set point in offspring gut and thereby tuning gut immune responses and inflammatory disease susceptibility. 107
4.2. Induction and function of allergen‐specific IgG and IgA by allergen immunotherapy
Allergen immunotherapy involves the repeated administration of allergens or allergen products to IgE‐sensitized allergic individuals to induce long‐term tolerance on subsequent exposure to the offending allergen(s). 108 It is indicated in patients with symptoms on exposure to relevant allergens and failure to respond to regular use of anti‐allergic drugs. Allergen immunotherapy (AIT) has been shown to be effective for allergic rhinoconjunctivitis, allergic asthma and anaphylaxis due to venom of stinging insects. AIT traditionally involves subcutaneous injections of allergen extracts weekly then monthly for 3 years. Daily administration via the sublingual route has been shown to be an effective and safer alternative. 109 Strategies to improve efficacy, reduce side effects and enable shorter more convenient immunotherapy protocols are desirable. 110 These have included alternative routes (eg epicutaneous, intralymphatic) use of short T‐cell peptides, medium chain length hydrolysed or synthetic peptides, combination products of allergen with Toll‐like receptor agonists or biologics and recombinant major allergen mixtures or hypoallergenic variants. So far, these strategies have failed to deliver outcomes over and above currently available products. 111
Intralymphatic and epicutaneous immunotherapy are currently being investigated as an alternative route of administration of immunotherapy, with epicutaneous immunotherapy being more easy to administer and more acceptable for patients. Intralymphatic administration would shorten the immunotherapy regimen, resulting in fewer clinic visits. Their efficacy and advantages still need to be evaluated in larger studies. 112
Allergen immunotherapy has been shown to be accompanied by increases in allergen‐specific antibodies. Cooke originally identified passive transfer of a serum factor that provided protective immunity to ragweed following successful ragweed immunotherapy. 27 This was subsequently shown to reside within the immunoglobulin IgG fraction, long before IgE was discovered.
For pollen AIT, an initial transient rise in specific IgE is followed by blunting of seasonal IgE increases and a gradual decline over several years. 113 Both subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) result in increases in allergen‐specific IgG1/IgG4 and specific IgA1/IgA2. 114 These antibodies increase at 2–6 months and are detectable both in blood and in local target organ secretions, for example in nasal fluid. 115 Whereas SCIT induces largely IgG responses, a recent head‐to‐head trial showed that SLIT induces allergen‐specific 29 IgA1 and IgA2 in addition to IgG.
A major advance has been the availability of recombinant major and minor allergenic components that enable an accurate molecular diagnosis. There is a strong case that measurements of allergen‐specific antibodies to standardized whole extracts could be supplemented by molecular diagnosis using individual allergen molecules to discriminate between antibodies binding to allergens and non‐allergenic extract components. 116 , 117 Whether standardized allergen extracts will be replaced or supplemented by tailor‐made recombinant mixtures/hypoallergenic variants based on individual molecular profiles remains to be tested.
IgG4 and other human IgG subclasses are similar in structure but have differences in binding to accessory molecules and receptors, altering their functionality. IgG4, in particular, induced following chronic antigen responses co‐exist as two isomers diverging in their disulphide bonds of hinge cysteines. There is clear evidence that in vivo, half‐molecules of IgG4 can recombine randomly with other half‐molecules of IgG4, resulting in monovalent‐bispecific antibodies. 118 , 119 As a consequence, IgG4 is unable to efficiently cross‐link target allergen and form immune complexes. It is unable to bind with both Fab arms to a multivalent antigen, leading to a lower avidity. IgG4 has low affinity for activating Fc receptor for IgG (FcγR) while retaining high affinity for the FcγRIIb. These characteristics enable IgG4 to be an efficient inhibitor of IgE‐dependent reactions without untoward inflammation associated with IgG immune complex formation and complement activation.
In addition to the allergen neutralizing capacity of allergen‐specific IgG to inhibit basophil FcεRI‐mediated responses, studies have also shown that IgG can interact with FcγRIIB and inhibit downstream signalling through FcεRI, thus preventing release of histamine and other mediators. 120 , 121 This is also induced after oral IT, 122 , 123 which might be explained by the presence of IgG in IgE‐allergen complexes. 124
Allergen‐specific IgA2 and polymeric IgA2 has also been shown to be elevated following grass pollen SCIT. Polymeric IgA2 was purified from post‐immunotherapy serum and used to passively sensitize autologous monocytes. Subsequent cross‐linking in vitro of IgA on monocytes by antigen or anti‐IgA resulted in IL‐10 production, supporting an alternative role for IgA antibodies in inducing tolerance following AIT. 125
Immunoreactive IgG and IgA antibodies are elevated after AIT but have correlated poorly with the clinical response to treatment. This may be explained in part by responses to non‐allergenic proteins or to irrelevant minor or cross‐reactive allergens, and this can be addressed by measuring major allergen components. 114 , 126 However, at least as relevant, immunoreactive antibodies relate largely to allergen exposure during AIT and may have no bearing on the affinity and/or avidity of these antibodies in blocking the formation of allergen‐IgE complexes and hence blocking IgE responses. This highlights the importance of using functional antibody assays to supplement immunoreactive IgG and IgA assays, like the basophil activation test, FcεRI‐transfected RBL release assays, IgE‐facilitated antigen binding to CD23 (IgE‐FAB), and T‐cell activation (see also Box 1).
BOX 1. Methods for measuring (effects of) blocking antibodies.
Serological assays
The induction of blocking antibodies in allergic patients during AIT can be studied using serological assays demonstrating their ability to inhibit IgE binding to the allergen 128 , 167 . The allergen neutralizing effect of IgG can not be measured when the amount of allergen on the solid phase is in excess to allergen‐specific antibodies as it occurs for example in the Immuno CAP system (Thermofisher, Uppsala, Sweden) 168 . However, micro‐arrays such as the Immuno CAPISAC (Thermofisher, Uppsala, Sweden) and the Me DALL allergen chip 169 contain approximately only 100 fg of allergen per spot. Therefore, it is possible to visualize with such assays the competition of IgG with IgE for allergen binding 126 , 170 , 171 , 172 . Therefore, one can compare allergen‐specific IgE binding in serum samples obtained before AIT and after AIT, when blocking allergen‐specific IgG has developed. In the case that blocking antibodies have developed the IgE signal will be strongly reduced in the post‐treatment samples.
Basophils and mast cells
Measuring the effects of blocking antibodies on allergen‐induced basophil activation Shortly after developing the allergen‐specific basophil histaminerelease assay, Lichtenstein and colleagues used this test to study the effects of desensitization during AIT 173 . The effects of blocking antibodies induced by AIT or even of purified human monoclonal allergen‐specific IgG antibodies on allergen‐induced basophilde granulation can be visualized by pre‐incubation of the allergen before exposure to IgE‐loaded basophils from allergic patients when the cells had been isolated and washed tore move serum (i.e.,basophil activation with washed cells) 141 , 174 . Alternatively, basophil activation can be performed in blood samples obtained from patients in the presence of serum and blocking antibodies. In this setting, the effects of blocking antibodies become visible due to addition of all ergen to the full blood sample containing already the blocking antibodies (i.e.,full blood assay) 175 .
More recently, rat basophil and mast cell lines transfected with the human Fce can be cultivated and loaded with sera obtained before AIT to represent the patient's sensitivity before the treatment. The cells are then exposed to allergen pre‐incubated with serum samples obtained before and after treatment to investigate the development of blocking antibodies in the post‐treatment serum 117 . These experiments can be performed with sera as such, purified IgG fractions or sera that had been heat‐in activated at 56°C to remove IgE effects. The advantage of using the transfected cell lines is that the experiments can be conducted with all sera simultaneously with cells having comparable sensitivity. In contrast, experiments performed with fresh basophils from patients at different time points can be subject to variations due to general differences of basophil sensitivity occurring in subjects at different time points.
IgE‐facilitated allergen presentation
It is known that IgE‐facilitated allergen presentation via CD23 on Bcells is akeymechanism in allergen presentation to Tcells in allergic patients because it allows tiny amounts of allergens to be presented by an efficient pick‐upmechanism. This is of particular relevance in allergy because one has to consider that only minute amounts of allergens can enter the systemic circulation of allergic subjects due to the presence of epithelial barriers. The first study investigated if AIT‐induced blocking antibodies can inhibit IgE‐facilitated allergen presentation via CD23 was published in 1999 127 . In this study, it could be shown that AIT‐induced blocking antibodies inhibited allergen‐specific Tcell proliferation and secretion of inflammatory cytokine responses. This result was remarkable because it indicated that the reduction of Tcell activity during AIT is mediated by blocking antibodies and not or not only by Tcell‐mediated immunological tolerance mechanisms 176 .
To simplify the assay, a CD23‐expressing Bcell line was developed which can be loaded with serum IgE from a patient allergic to the given allergen and one can then measure the binding of labelled allergen and its inhibition by AIT‐induced blocking antibodies 129 . Although this FAB assay133 can be easily performed for extensive screening of sera it has the disadvantage that the cells are usually loaded only with one IgE‐containing serum (i.e.,indicator serum) and hence one can not assess the blocking of allergen binding to CD23‐bound IgE of each of the patients to be tested. However, one can also perform this assay with APCs obtained from each patient to be tested and add allergen in the presence of pre‐and post‐treatment sera to measure the development of blocking antibodies in each of the tested patients 175 .
A question which still needs to be investigated is if the inhibition of IgE‐facilitated allergen presentation and its effects on subsequent Tcell activation is related to a certainty pe of allergic symptoms. By intuition, one would expect that inhibition of Tcell activation by blocking antibodies would be related to are duction of late‐phase allergic reactions. However, to study this, it is not sufficient to measure only the effects of blocking antibodies on IgE‐facilitated presentation and their effects on Tcell activation and relate the latter parameters with clinical effects such as late‐phase skin reactions eventually atopy patch test results. It has been challenging to relate allergen‐specific Tcell proliferation and cytokine secretion in blood‐derived cells with atopy patch test results 177 .
When comparing serological assays, basophil activation tests and FAB assays a good correlation was observed among the different assays, and it may therefore be sufficient to perform serological assays for the assessment of AIT‐induced blocking antibodies 128 .
In vivo methods
The classic experiment demonstration that AIT‐induced blocking antibodies inhibit allergen‐induced skin test reactions by Prausnitz‐Kuestnerreaction 27 , 178 in humans 27 can not be performed any more due to ethical reasons. One, therefore, can only compare results of in vivo provocation testing such as skin testing 179 , 180 , Conjunctival provocation testing 181 (and allergen exposure testing 175 with the development of potentially blocking non‐IgE antibodies in patients during AIT and calculate correlations. A direct demonstration of the effects of blocking antibodies on in vivo test results is hence not possible.
Allergen‐specific IgG4 (and likely other antibody isotypes) compete with IgE for allergen and prevents the formation of allergen‐IgE complexes from binding to FcεRI on effector cells (mast cells, basophils and dendritic cells) and to FcεRII (CD23) on B cells (Figure 2). van Neerven originally demonstrated that serum obtained after birch pollen immunotherapy inhibited IgE‐facilitated allergen presentation by B cells to an allergen‐specific T‐cell clone, with decreased specific clonal T‐cell proliferation and cytokine production. 127 This was confirmed by further studies of birch immunotherapy. 128 , 129 Confirmed increases in serum IgG‐associated blocking activity for IgE‐facilitated antigen binding to CD23 (IgE‐FAB) in grass pollen immunotherapy. 130 That persisted for years after discontinuation along with clinical benefit and by affinity chromatography showed that the inhibitory factor resided largely but not exclusively within the IgG4 fraction. 131 Recent data support a putative role for allergen‐specific IgG2 132 as a blocking factor for IgE‐mediated reactions. Shamji validated the IgE‐FAB assay and showed that serum IgE‐FAB increased in a time‐ and dose‐dependent fashion after grass pollen AIT 133 , 134 and correlated more closely with clinical response than accompanying elevated IgG4 levels. This raised the possibility for IgE‐FAB inhibition to predict individual responses to AIT. 114 Such blocking antibodies could also prevent captured allergen from stimulating IgE‐producing cells thereby reducing boosts of IgE production caused by allergen exposure. 75 , 135 , 136
The functional role of serum‐blocking antibodies after AIT has also been illustrated by inhibition of IgE‐mediated basophil activation (Figure 2). After grass pollen AIT, post‐immunotherapy serum inhibited basophil histamine release with a time‐course that paralleled inhibition of IgE‐FAB and correlated with inhibition of the immediate skin response to grass pollen at 8–16 weeks. 137 This was also shown using Bet v 1‐specific IgG1 and IgG4 antibodies after birch pollen AIT. 138 In a murine model, this inhibitory effect of IgG was mediated via the FcγRIIB receptor. However, antibodies directed against FcγRII did not prevent serum IgG‐mediated inhibition of basophil activation following birch AIT, implying that direct competition with IgE for allergen rather than activation of FcγRII‐mediated inhibition of downstream IgE‐receptor signalling pathways was responsible. 139
During grass pollen AIT, inhibition of basophil activation has been shown by suppression of surface CD63 expression and by increases in intracellular Diamine Oxidase as detected by whole blood flow cytometry. 140 Suppression of basophil activation has also been shown for birch pollen immunotherapy, 141 as well as following venom immunotherapy. 142
The therapeutic potential of blocking antibodies following AIT is highlighted by a recent study of passive immunotherapy in cat allergic individuals who received a single dose of two synthetic anti‐Fel d1‐specific IgG4 antibodies that resulted in inhibition of the nasal response to a standardized cat whole allergen extract that persisted for twelve weeks. 143 , 144
5. IgE AND IgE RECEPTOR‐TARGETING THERAPIES FOR TREATING ALLERGIES
Another group of antibodies that prevent histamine release by basophils and mast cells are the anti‐IgE antibodies. They exert their effect by preventing IgE from binding to FcεRI and CD23 (Figures 1 and 2). Binding of IgE to CD23 may involve different portions of CD23 and interestingly can be blocked with omailizumab which also blocks IgE binding to the high‐affinity receptor for IgE. 76 In addition, anti‐IgE has a similar inhibitory effect as AIT‐induced IgG and IgA antibodies that block IgE‐mediated T‐cell activation. 145
The structures of the ectodomain regions of FcεRI and CD23 in complexes with IgE‐Fc have revealed how these two distinct receptors interact with IgE. 146 , 147 IgE binding to its two receptors is regulated through unique conformational changes in the IgE‐Fc domain that enable an allosteric competition between low and high‐affinity receptors. 146 , 148 IgE binding to FcεRI occurs through the tips of the two IgE Cε3 domains, engaging both antibody heavy chains in an asymmetric ‘open’ conformation. 147 , 148 In contrast, CD23 binding occurs to a distinct surface of the IgE‐Fc at the junction between Cε3‐Cε4 domains and favours a ‘closed’ conformation that inhibits FcεRI binding. 146 High‐affinity binding to FcεRI leads to the prebinding of serum IgE to receptor‐expressing cells, sensitizing them to respond upon allergen exposure and cross‐linking. In contrast, IgE binding to CD23 is of lower affinity and is stabilized through avidity effects, most notably by IgE‐allergen complex formation. Strikingly, IgE bound to FcεRI is incredibly stable, persisting on peripheral mast cells for weeks‐months and impacting the safety and speed of AIT/OIT approaches.
Two anti‐IgE antibodies, omalizumab and ligelizumab 149 , 150 have been advanced as therapeutics for the treatment of allergic diseases, including allergic asthma, chronic spontaneous urticaria, chronic rhinosinusitis and food allergies. However, other anti‐IgE antibodies are in clinical development (eg Xmab7195/UB221/omalizumab biosimilars). Omalizumab and ligelizumab highlight the impressive impact that anti‐IgE can have in allergy treatment. 151 Omalizumab was the first anti‐IgE developed as a therapeutic, initially for the treatment of severe allergic asthma in 2003. Since then, omalizumab has shown efficacy in treating chronic spontaneous urticaria (CSU), food allergy and chronic rhinosinusitis. 152 As discussed elsewhere in this review, omalizumab enhanced OIT treatment in food allergy clinical trials, reducing allergen challenge reactions and enabling a more rapid increase in allergen dosing and simultaneous tolerization for multiple allergens. 153 Ligelizumab is a next‐generation, higher affinity anti‐IgE that shows an improved ability to suppress free IgE in patients. 135 Despite having an ~100‐fold higher affinity for IgE, ligelizumab surprisingly did not show improved efficacy in treating allergic asthma patients. 154 , 155 However, in phase II clinical studies, ligelizumab showed improved efficacy over omalizumab for the treatment of chronic idiopathic urticaria (CIU). 156 It remains to be established whether ligelizumab will have a significant benefit in oral immunotherapy (OIT) or AIT relative to omalizumab.
The structures and mechanisms of omalizumab vs ligelizumab are revealing and provide insight into the possible differences in their therapeutic impact. Omalizumab and ligelizumab both engage epitopes in the IgE Cε3 domains adjacent to the binding site for FcεRI. 154 , 157 , 158 Despite the substantial overlap in their epitopes, ligelizumab binds across the IgE dimer engaging residues in both Cε3 domains and overlapping the space that would be occupied by FcεRI. In contrast, omalizumab engages an epitope towards an outer face of the Cε3 domains, does not bind across the IgE dimer and lies somewhat peripherally to FcεRI. One of the consequences of these distinct binding interactions is that omalizumab can effectively inhibit binding to FcεRI and CD23, while ligelizumab shows preferential inhibition of FcεRI. 154 The ability of ligelizumab to block CD23 binding is weaker than omalizumab, despite its much higher IgE affinity. The weaker inhibition of IgE:CD23 interactions exhibited by ligelizumab may account for its failure to outperform omalizumab in clinical trials for allergic asthma, 154 , 155 where CD23 is thought to play an essential role in disease through antigen presentation and or antigen transport. 159 , 160 CD23 has also been studied as a target in allergic diseases. However, although a phase 1/2 study with the anti‐CD23 mAb lumiliximab in asthma patients showed a good safety profile, anti‐CD23 has not been developed further in asthma or allergy. 161
It will be exciting and informative to compare the activities of omalizumab and ligelizumab in AIT, which may help assess the clinical importance of the inhibition of CD23 and FcεRI interactions during tolerization to food or other allergens.
The rationale of combining anti‐IgE with AIT or OIT is that the combination may prevent allergic side effects of AIT 145 and OIT, allow more rapid updosing of allergen and will provide immediate clinical benefit. Since 2007, several studies have addressed this combination treatment. These are reviewed in detail REFin. 153 , 162 , 163 Studies on the addition of anti‐IgE to OIT treatment for peanut, milk and allergen mixes have shown that a quicker updosing until maintenance treatment is possible because adverse events decrease significantly. Future studies are needed to further evaluate optimal dosing and long‐term efficacy for this type of combination treatment.
Finally, a class of ‘disruptive’ IgE inhibitors has been described based on Designed Ankyrin Repeat Proteins (DARPins), which can rapidly dissociate FcεRI‐bound IgE in vitro and in vivo. 164 , 165 Such kinetically active anti‐IgE inhibitors may have the potential to rapidly desensitize peripheral mast cells and significantly accelerate the timelines for AIT in the future.
6. FUTURE PERSPECTIVES
The important role of IgE in type 1 allergic diseases has been known for a very long time. The functional role of allergen‐specific IgG and IgA antibodies induced by AIT has shown their ability to interfere with the interaction of IgE with the allergen. In addition, transplacental or breastfeeding‐mediated transfer of immune complexes of maternal IgG with allergens to the foetus may protect against sensitization to allergens in early life.
The knowledge we have gained over the last two decades has been instrumental in developing novel therapeutic approaches by targeting IgE itself with anti‐IgE antibodies or receptor‐targeting antibodies, enhancing blocking antibodies by AIT or even passive transfer of allergen‐specific IgG to allergic patients (see Box 1 for methods used to measure these allergen‐specific antibodies and their function in more detail). This knowledge may help to further establish the relevance of blocking antibodies as a biomarker for clinical effects of AIT. 166
Finally, this may lead to future therapeutic approaches such as combination treatments with therapeutic antibodies and AIT or OIT (eg combination with anti‐IgE, allergen‐specific IgG or cytokine‐directed antibody therapies), as well as preventive approaches such as maternal allergen vaccination to enhance delivery of allergen‐specific IgG and IgA antibodies during pregnancy and early life to prevent sensitization to respiratory and food allergens.
CONFLICT OF INTEREST
Dr. Shamji has nothing to disclose; Dr. Valenta reports grants and personal fees from Viravaxx, Vienna, Austria, grants from HVD Biotech, Vienna, Austria, grants and personal fees from WORG Pharmaceuticals, Hangzhou, China, outside the submitted work; Dr. Jardetzky reports other from Excellergy, Inc., outside the submitted work; In addition, Dr. Jardetzky has a patent covering novel anti‐IgE agents pending; Dr. Verhasselt has nothing to disclose; Dr. Durham has nothing to disclose; Dr. Würtzen is an employee of and owns stocks in ALK, a medical company producing allergy vaccines; Dr van Neerven has nothing to disclose.
ACKNOWLEDGEMENTS
The authors would like to thank Didem Sanver Akbas for copy editing of the manuscript and Anna Głobińska for help with the figures.
Shamji MH, Valenta R, Jardetzky T, et al. The role of allergen‐specific IgE, IgG and IgA in allergic disease. Allergy. 2021;76:3627–3641. 10.1111/all.14908
Funding information
This research was supported in part by NIH grants AI115469 and HL141493 (to T.S.J.).
REFERENCES
- 1. Ishizaka K, Ishizaka T, Hornbrook MM. Physico‐chemical properties of human reaginic antibody: IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J Immunol. 1966;97(1):75‐85. [PubMed] [Google Scholar]
- 2. Johansson S, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967;13(4):381. [PMC free article] [PubMed] [Google Scholar]
- 3. Gould HJ, Sutton BJ, Beavil AJ, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21(1):579‐628. [DOI] [PubMed] [Google Scholar]
- 4. Pillai P, Fang C, Chan Y‐C, et al. Allergen‐specific IgE is not detectable in the bronchial mucosa of nonatopic asthmatic patients. Journal of Allergy and Clinical Immunology. 2014;133(6):1770–1772.e11. [DOI] [PubMed] [Google Scholar]
- 5. Durham SR, Gould HJ, Hamid QA. Local IgE production in nasal allergy. Int Arch Allergy Immunol. 1997;113(1–3):128‐130. [DOI] [PubMed] [Google Scholar]
- 6. Ying S, Humbert M, Meng Q, et al. Local expression of ϵ germline gene transcripts and RNA for the ϵ heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J Allergy Clin Immunol. 2001;107(4):686‐692. [DOI] [PubMed] [Google Scholar]
- 7. Wilson DR, Merrett TG, Varga EM, et al. Increases in allergen‐specific IgE in BAL after segmental allergen challenge in atopic asthmatics. Am J Respir Crit Care Med. 2002;165(1):22‐26. [DOI] [PubMed] [Google Scholar]
- 8. Hoh RA, Joshi SA, Lee JY, et al. Origins and clonal convergence of gastrointestinal IgE+ B cells in human peanut allergy. Sci Immunol. 2020;5(45):eaay4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lambrecht B, Peleman R, Bullock G, Pauwels R. Sensitization to inhaled antigen by intratracheal instillation of dendritic cells. Clin Exp allergy. 2000;30(2):214‐224. [DOI] [PubMed] [Google Scholar]
- 10. Deckers J, De Bosscher K, Lambrecht BN, Hammad H. Interplay between barrier epithelial cells and dendritic cells in allergic sensitization through the lung and the skin. Immunol Rev. 2017;278(1):131‐144. [DOI] [PubMed] [Google Scholar]
- 11. Gowthaman U, Chen JS, Zhang B, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. 2019;365(6456):eaaw6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen‐presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155(8):3734‐3741. [PubMed] [Google Scholar]
- 13. Rivera A, Chen C‐C, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen‐presenting cells in vivo revisited: antigen‐specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol. 2001;13(12):1583‐1593. [DOI] [PubMed] [Google Scholar]
- 14. Dullaers M, Schuijs MJ, Willart M, et al. House dust mite–driven asthma and allergen‐specific T cells depend on B cells when the amount of inhaled allergen is limiting. J Allergy Clin Immunol. 2017;140(1):76–88.e7. [DOI] [PubMed] [Google Scholar]
- 15. Wypych TP, Marzi R, Wu GF, Lanzavecchia A, Sallusto F. Role of B cells in T(H) cell responses in a mouse model of asthma. J Allergy Clin Immunol. 2018;141(4):1395‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scadding GW, Eifan A, Lao‐Araya M, et al. Effect of grass pollen immunotherapy on clinical and local immune response to nasal allergen challenge. Allergy. 2015;70(6):689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takhar P, Corrigan CJ, Smurthwaite L, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol. 2007;119(1):213‐218. [DOI] [PubMed] [Google Scholar]
- 18. Santamaria LF, Bheekha R, van Reijsen FC, et al. Antigen focusing by specific monomeric immunoglobulin E bound to CD23 on Epstein‐Barr virus‐transformed B cells. Hum Immunol. 1993;37(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 19. Maurer D, Ebner C, Reininger B, et al. The high‐affinity IgE receptor (Fc epsilon RI) mediates IgE‐dependent allergen presentation. J Immunol. 1995;154(12):6285‐6290. [PubMed] [Google Scholar]
- 20. Maurer D, Fiebiger S, Ebner C, et al. Peripheral blood dendritic cells express Fc epsilon RI as a complex composed of Fc epsilon RI alpha‐and Fc epsilon RI gamma‐chains and can use this receptor for IgE‐mediated allergen presentation. J Immunol. 1996;157(2):607‐616. [PubMed] [Google Scholar]
- 21. Van der Heijden F, Van Neerven RJ, Van Katwijk M, Bos J, Kapsenberg M. Serum‐IgE‐facilitated allergen presentation in atopic disease. J Immunol. 1993;150(8):3643‐3650. [PubMed] [Google Scholar]
- 22. Mosconi E, Rekima A, Seitz‐Polski B, et al. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol. 2010;3(5):461‐474. [DOI] [PubMed] [Google Scholar]
- 23. Lupinek C, Hochwallner H, Johansson C, et al. Maternal allergen‐specific IgG might protect th e child against allergic sensitization. J Allergy Clin Immunol. 2019;144(2):536‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marchant A, Sadarangani M, Garand M, et al. Maternal immunisation: collaborating with mother nature. Lancet Infect Dis. 2017;17(7):e197–e208. [DOI] [PubMed] [Google Scholar]
- 25. Uthoff H, Spenner A, Reckelkamm W, et al. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J Immunol. 2003;171(7):3485‐3492. [DOI] [PubMed] [Google Scholar]
- 26. Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen‐specific immunotherapy. Nat Rev Immunol. 2006;6(10):761‐771. [DOI] [PubMed] [Google Scholar]
- 27. Cooke RA, Barnard JH, Hebald S, Stull A. Serological evidence of immunity with coexisting sensitization in a type of human allergy (hay fever). J Exp Med. 1935;62(6):733‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Platts‐Mills TA, von Maur RK, Ishizaka K, Norman PS, Lichtenstein LM. IgA and IgG anti‐ragweed antibodies in nasal secretions. Quantitative measurements of antibodies and correlation with inhibition of histamine release. J Clin Invest. 1976;57(4):1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shamji MH, Larson D, Eifan A,et al. Differential Induction of Allergen‐specific IgA Responses following Timothy Grass Subcutaneous and Sublingual Immunotherapy. J Allergy Clin Immunol. 2021. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wan T, Beavil RL, Fabiane SM, et al. The crystal structure of IgE Fc reveals an asymmetrically bent conformation. Nat Immunol. 2002;3(7):681‐686. [DOI] [PubMed] [Google Scholar]
- 31. Zheng Y, Shopes B, Holowka D, Baird B. Dynamic conformations compared for IgE and IgG1 in solution and bound to receptors. Biochemistry. 1992;31(33):7446‐7456. [DOI] [PubMed] [Google Scholar]
- 32. Zheng Y, Shopes B, Holowka D, Baird B. Conformations of IgE Bound to its Receptor Fc. epsilon. RI and in Solution. Biochemistry. 1991;30(38):9125‐9132. [DOI] [PubMed] [Google Scholar]
- 33. Hunt J, Keeble AH, Dale RE, et al. A fluorescent biosensor reveals conformational changes in human immunoglobulin E Fc: implications for mechanisms of receptor binding, inhibition, and allergen recognition. J Biol Chem. 2012;287(21):17459‐17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilon RI, CD40L, IL‐4, and IL‐13, and can induce IgE synthesis in B cells. J Clin Investig. 1997;99(7):1492‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cameron L, Gounni AS, Frenkiel S, Lavigne F, Vercelli D, Hamid Q. SεSμ and SεSγ switch circles in human nasal mucosa following ex vivo allergen challenge: evidence for direct as well as sequential class switch recombination. J Immunol. 2003;171(7):3816‐3822. [DOI] [PubMed] [Google Scholar]
- 36. Humbert M, Grant JA, Taborda‐Barata L, et al. High‐affinity IgE receptor (Fc epsilon RI)‐bearing cells in bronchial biopsies from atopic and nonatopic asthma. Am J Respir Crit Care Med. 1996;153(6):1931‐1937. [DOI] [PubMed] [Google Scholar]
- 37. Daëron M. Fc receptor biology. Annu Rev Immunol. 1997;15(1):203‐234. [DOI] [PubMed] [Google Scholar]
- 38. Ravetch JV. Fc receptors. CurrOpin Immunol. 1997;9(1):121‐125. [DOI] [PubMed] [Google Scholar]
- 39. Saini SS, Richardson JJ, Wofsy C, Lavens‐Phillips S, Bochner BS, MacGlashan DW. Expression and modulation of FcϵRIα and FcϵRIβ in human blood basophils. J Allergy Clin Immunol. 2001;107(5):832‐841. [DOI] [PubMed] [Google Scholar]
- 40. Kraft S, Kinet J‐P. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7(5):365‐378. [DOI] [PubMed] [Google Scholar]
- 41. Kinet J‐P. The high‐affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17(1):931‐972. [DOI] [PubMed] [Google Scholar]
- 42. Siraganian RP. Mast cell signal transduction from the high‐affinity IgE receptor. Curr Opin Immunol. 2003;15(6):639‐646. [DOI] [PubMed] [Google Scholar]
- 43. Saini SS, MacGlashan DW, Sterbinsky SA, et al. Down‐regulation of human basophil IgE and FcεRIα surface densities and mediator release by anti‐IgE‐infusions is reversible in vitro and in vivo. J Immunol. 1999;162(9):5624‐5630. [PubMed] [Google Scholar]
- 44. MacGlashan DW, Bochner BS, Adelman DC, et al. Down‐regulation of Fc (epsilon) RI expression on human basophils during in vivo treatment of atopic patients with anti‐IgE antibody. J Immunol. 1997;158(3):1438‐1445. [PubMed] [Google Scholar]
- 45. Asai K, Kitaura J, Kawakami Y, et al. Regulation of mast cell survival by IgE. Immunity. 2001;14(6):791‐800. [DOI] [PubMed] [Google Scholar]
- 46. Zellweger F, Buschor P, Hobi G, et al. IL‐3 but not monomeric IgE regulates FcεRI levels and cell survival in primary human basophils. Cell Death Dis. 2018;9(5):510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rajakulasingam K, Durham SR, O'Brien F, et al. Enhanced expression of high‐affinity IgE receptor (FcϵRI) α chain in human allergen‐induced rhinitis with co‐localization to mast cells, macrophages, eosinophils, and dendritic cells. Journal of Allergy and Clinical Immunology. 1997;100(1):78‐86. [DOI] [PubMed] [Google Scholar]
- 48. Saini SS, Klion AD, Holland SM, Hamilton RG, Bochner BS, MacGlashan DW Jr. The relationship between serum IgE and surface levels of FcϵR on human leukocytes in various diseases: correlation of expression with FcϵRI on basophils but not on monocytes or eosinophils. J Allergy Clin Immunol. 2000;106(3):514‐520. [DOI] [PubMed] [Google Scholar]
- 49. Allam J‐P, Novak N, Fuchs C, et al. Characterization of dendritic cells from human oral mucosa: A new Langerhans’ cell type with high constitutive FcϵRI expression. J Allergy Clin Immunol. 2003;112(1):141‐148. [DOI] [PubMed] [Google Scholar]
- 50. Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcεRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112(6):1132‐1138. [DOI] [PubMed] [Google Scholar]
- 51. Kikutani H, Inui S, Sato R, et al. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986;47(5):657‐665. [DOI] [PubMed] [Google Scholar]
- 52. Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class‐switch recombination. Nat Rev Immunol. 2003;3(9):721‐732. [DOI] [PubMed] [Google Scholar]
- 53. Lüdin C, Hofstetter H, Sarfati M, et al. Cloning and expression of the cDNA coding for a human lymphocyte IgE receptor. The EMBO journal. 1987;6(1):109‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Letellier M, Sarfati M, Delespesse G. Mechanisms of formation of IgE‐binding factors (soluble CD23)—I. Fcϵ R II bearing B cells generate IgE‐binding factors of different molecular weights. Mol Immunol. 1989;26(12):1105‐1112. [DOI] [PubMed] [Google Scholar]
- 55. McCloskey N, Hunt J, Beavil RL, et al. Soluble CD23 Monomers Inhibit and Oligomers Stimulate IGE Synthesis in Human B Cells*. J Biol Chem. 2007;282(33):24083‐24091. [DOI] [PubMed] [Google Scholar]
- 56. Engeroff P, Vogel M. The role of CD23 in the regulation of allergic responses. Allergy. 2021;76(7):1981‐1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Engeroff P, Fellmann M, Yerly D, Bachmann MF, Vogel M. A novel recycling mechanism of native IgE‐antigen complexes in human B cells facilitates transfer of antigen to dendritic cells for antigen presentation. J Allergy Clin Immunol. 2018;142(2):557‐568.e6. [DOI] [PubMed] [Google Scholar]
- 58. Novak N, Kraft S, Bieber T. IgE receptors. Curr Opin Immunol. 2001;13(6):721‐726. [DOI] [PubMed] [Google Scholar]
- 59. Yokota A, Yukawa K, Yamamoto A, et al. Two forms of the low‐affinity Fc receptor for IgE differentially mediate endocytosis and phagocytosis: identification of the critical cytoplasmic domains. Proc Natl Acad Sci U S A. 1992;89(11):5030‐5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kay AB. Paul Ehrlich and the Early History of Granulocytes. MicrobiolSpectr. 2016;4(4).https://pubmed.ncbi.nlm.nih.gov/27726791/ [DOI] [PubMed] [Google Scholar]
- 61. Turner H, Kinet J‐P. Signalling through the high‐affinity IgE receptor FcεRI. Nature. 1999;402(6760):24‐30. [DOI] [PubMed] [Google Scholar]
- 62. Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12(6):624‐631. [DOI] [PubMed] [Google Scholar]
- 63. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2):S73‐S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim HS, Kawakami Y, Kasakura K, Kawakami T. Recent advances in mast cell activation and regulation. F1000Research. 2020;9:196. 10.12688/f1000research.22037.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shade K‐TC, Conroy ME, Washburn N, et al. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature. 2020;582(7811):265‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122(2):298‐304. [DOI] [PubMed] [Google Scholar]
- 67. Hjort C, Schiøtz PO, Ohlin M, Würtzen PA, Christensen LH, Hoffmann HJ. The number and affinity of productive IgE pairs determine allergen activation of mast cells. J Allergy Clin Immunol. 2017;140(4):1167. [DOI] [PubMed] [Google Scholar]
- 68. Hamilton RG, Saito H. IgE antibody concentration, specific activity, clonality, and affinity measures from future diagnostic confirmatory tests. J Allergy Clin Immunol. 2008;122(2):305‐306. [DOI] [PubMed] [Google Scholar]
- 69. Holowka D, Sil D, Torigoe C, Baird B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunol Rev. 2007;217(1):269‐279. [DOI] [PubMed] [Google Scholar]
- 70. Gieras A, Linhart B, Roux KH, et al. IgE epitope proximity determines immune complex shape and effector cell activation capacity. J Allergy Clin Immunol. 2016;137(5):1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gieras A, Focke‐Tejkl M, Ball T, et al. Molecular determinants of allergen‐induced effector cell degranulation. J Allergy Clin Immunol. 2007;119(2):384‐390. [DOI] [PubMed] [Google Scholar]
- 72. Mudde GC, Bheekha R, Bruijnzeel‐Koomen CA. Consequences of IgE/CD23‐mediated antigen presentation in allergy. Immunol Today. 1995;16(8):380‐383. [DOI] [PubMed] [Google Scholar]
- 73. Reginald K, Eckl‐Dorna J, Zafred D, et al. Different modes of IgE binding to CD23 revealed with major birch allergen, Bet v 1‐specific monoclonal IgE. Immunol Cell Biol. 2013;91(2):167‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Holm J, Willumsen N, Würtzen PA, Christensen LH, Lund K. Facilitated antigen presentation and its inhibition by blocking IgG antibodies depends on IgE repertoire complexity. J Allergy Clin Immunol. 2011;127(4):1029‐1037. [DOI] [PubMed] [Google Scholar]
- 75. Villazala‐Merino S, Rodriguez‐Dominguez A, Stanek V, et al. Allergen‐specific IgE levels and the ability of IgE‐allergen complexes to cross‐link determine the extent of CD23‐mediated T‐cell activation. J Allergy Clin Immunol. 2020;145(3):958‐967.e955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Selb R, Eckl‐Dorna J, Twaroch TE, et al. Critical and direct involvement of the CD23 stalk region in IgE binding. J Allergy Clin Immunol. 2017;139(1):281–289.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Soares MJ, Varberg KM, Iqbal K. Hemochorial placentation: development, function, and adaptations. BiolReprod. 2018;99(1):196‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715‐725. [DOI] [PubMed] [Google Scholar]
- 79. Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345(18):1331‐1335. [DOI] [PubMed] [Google Scholar]
- 80. Jenmalm M, Björkstén B. Cord blood levels of immunoglobulin G subclass antibodies to food and inhalant allergens in relation to maternal atopy and the development of atopic disease during the first 8 years of life. Clin Exp Allergy. 2000;30(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 81. Macchiaverni P, Arslanian C, Frazao J, et al. Mother to child transfer of IgG and IgA antibodies against Dermatophagoides pteronyssinus. Scand J Immunol. 2011;74(6):619‐627. [DOI] [PubMed] [Google Scholar]
- 82. Glovsky MM, Ghekiere L, Rejzek E. Effect of maternal immunotherapy on immediate skin test reactivity, specific rye I IgG and IgE antibody, and total IgE of the children. Annals of allergy. 1991;67(1):21‐24. [PubMed] [Google Scholar]
- 83. Flicker S, Marth K, Kofler H, Valenta R. Placental transfer of allergen‐specific IgG but not IgE from a specific immunotherapy‐treated mother. J Allergy Clin Immunol. 2009;124(6):1358. [DOI] [PubMed] [Google Scholar]
- 84. Oykhman P, Kim HL, Ellis AK. Allergen immunotherapy in pregnancy. Allergy, Asthma Clin Immunol. 2015;11(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Msallam R, Balla J, Rathore AP, et al. Fetal mast cells mediate postnatal allergic responses dependent on maternal IgE. Science. 2020;370(6519):941‐950. [DOI] [PubMed] [Google Scholar]
- 86. Mickleson KN, Moriarty KM. Immunoglobulin levels in human colostrum and milk.JPediatr Gastroenterol. Nutr. 1982;1(3):381‐384. [DOI] [PubMed] [Google Scholar]
- 87. Brandtzaeg P. Mucosal immunity: integration between mother and the breast‐fed infant. Vaccine. 2003;21(24):3382‐3388. [DOI] [PubMed] [Google Scholar]
- 88. Rekima A, Macchiaverni P, Turfkruyer M, et al. Long‐term reduction in food allergy susceptibility in mice by combining breastfeeding‐induced tolerance and TGF‐β‐enriched formula after weaning. Clin Exp Allergy. 2017;47(4):565‐576. [DOI] [PubMed] [Google Scholar]
- 89. Ohsaki A, Venturelli N, Buccigrosso TM, et al. Maternal IgG immune complexes induce food allergen–specific tolerance in offspring. J Exp Med. 2018;215(1):91‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Victor JR. Allergen‐specific IgG as a mediator of allergy inhibition: Lessons from mother to child. Hum Vaccin Immunother. 2017;13(3):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Casas R, Björkstén B. Detection of Fel d 1–immunoglobulin G immune complexes in cord blood and sera from allergic and non‐allergic mothers. Pediatr Allergy Immunol. 2001;12(2):59‐64. [DOI] [PubMed] [Google Scholar]
- 92. Bernard H, Ah‐Leung S, Drumare MF, et al. Peanut allergens are rapidly transferred in human breast milk and can prevent sensitization in mice. Allergy. 2014;69(7):888‐897. [DOI] [PubMed] [Google Scholar]
- 93. Adel‐Patient K, Bernard H, Fenaille F, Hazebrouck S, Junot C, Verhasselt V. Prevention of allergy to a major cow's milk allergen by breastfeeding in mice depends on maternal immune status and oral exposure during lactation. Front Immunol. 2020;11:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. López‐Expósito I, Song Y, Järvinen KM, Srivastava K, Li X‐M. Maternal peanut exposure during pregnancy and lactation reduces peanut allergy risk in offspring. J Allergy Clin Immunol. 2009;124(5):1039‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Järvinen KM, Westfall J, De Jesus M, et al. Role of maternal dietary peanut exposure in development of food allergy and oral tolerance. PLoS One. 2015;10(12):e0143855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Macchiaverni P, Rekima A, Turfkruyer M, et al. Respiratory allergen from house dust mite is present in human milk and primes for allergic sensitization in a mouse model of asthma. Allergy. 2014;69(3):395‐398. [DOI] [PubMed] [Google Scholar]
- 97. Rekima A, Bonnart C, Macchiaverni P, et al. A role for early oral exposure to house dust mite allergens through breast milk in IgE‐mediated food allergy susceptibility. J Allergy Clin Immunol. 2020;145(5):1416–1429.e11. [DOI] [PubMed] [Google Scholar]
- 98. Collins A, Roberton D, Hosking C, Flannery G. Bovine milk, including pasteurised milk, contains antibodies directed against allergens of clinical importance to man. Int Arch Allergy Immunol. 1991;96(4):362‐367. [DOI] [PubMed] [Google Scholar]
- 99. Von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10(12):861‐868. [DOI] [PubMed] [Google Scholar]
- 100. Brick T, Hettinga K, Kirchner B, Pfaffl MW, Ege MJ. The beneficial effect of farm milk consumption on asthma, allergies, and infections: from meta‐analysis of evidence to clinical trial. J Allergy Clin Immunol: In Practice. 2020;8(3):878–889.e3. [DOI] [PubMed] [Google Scholar]
- 101. Sozańska B. Raw cow’s milk and its protective effect on allergies and asthma. Nutrients. 2019;11(2):469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Machtinger S, Moss R. Cow's milk allergy in breast‐fed infants: the role of allergen and maternal secretory IgA antibody. J Allergy Clin Immunol. 1986;77(2):341‐347. [DOI] [PubMed] [Google Scholar]
- 103. Savilahti E, Tainio VM, Salmenperä L, et al. Low colostral IgA associated with cow's milk allergy. Acta Pædiatrica. 1991;80(12):1207‐1213. [DOI] [PubMed] [Google Scholar]
- 104. Järvinen KM, Westfall JE, Seppo MS, et al. Role of maternal elimination diets and human milk IgA in the development of cow's milk allergy in the infants. Clin Exp Allergy. 2014;44(1):69‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. JärvinenKM STL, Laine ST, Jêvenpêê A‐L, Suomalainen HK. Does low IgA in human milk predispose the infant to development of cow's milk allergy?. Pediatr Res. 2000;48(4):457‐462. [DOI] [PubMed] [Google Scholar]
- 106. Orivuori L, Loss G, Roduit C, et al. Soluble immunoglobulin A in breast milk is inversely associated with atopic dermatitis at early age: the PASTURE cohort study. Clin Exp Allergy. 2014;44(1):102‐112. [DOI] [PubMed] [Google Scholar]
- 107. Ramanan D, Sefik E, Galván‐Peña S, et al. An immunologic mode of multigenerational transmission governs a gut Treg setpoint. Cell. 2020;181(6):1276–1290.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bousquet J, Lockey R, Malling H‐J. Allergen immunotherapy: therapeutic vaccines for allergic diseases A WHO position paper. J Allergy Clin Immunol. 1998;102(4):558‐562. [DOI] [PubMed] [Google Scholar]
- 109. Passalacqua G, Compalati E, Canonica GW. Sublingual immunotherapy: clinical indications in the WAO‐SLIT position paper. World Allergy Organ J. 2010;3(7):216‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hoffmann H, Valovirta E, Pfaar O, et al. Novel approaches and perspectives in allergen immunotherapy. Allergy. 2017;72(7):1022‐1034. [DOI] [PubMed] [Google Scholar]
- 111. Gunawardana NC, Durham SR. New approaches to allergen immunotherapy. Ann Allergy Asthma Immunol. 2018;121(3):293‐305. [DOI] [PubMed] [Google Scholar]
- 112. Dorofeeva Y, Shilovskiy I, Tulaeva I, et al. Past, present, and future of allergen immunotherapy vaccines. Allergy. 2021;76(1):131‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six‐year prospective study. J Allergy Clin Immunol. 1982;70(4):261‐271. [DOI] [PubMed] [Google Scholar]
- 114. Shamji M, Kappen J, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72(8):1156‐1173. [DOI] [PubMed] [Google Scholar]
- 115. Shamji MH, Kappen J, Abubakar‐Waziri H, et al. Nasal allergen‐neutralizing IgG4 antibodies block IgE‐mediated responses: Novel biomarker of subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2019;143(3):1067‐1076. [DOI] [PubMed] [Google Scholar]
- 116. Matricardi PM, Dramburg S, Potapova E, Skevaki C, Renz H. Molecular diagnosis for allergen immunotherapy. J Allergy Clin Immunol. 2019;143(3):831‐843. [DOI] [PubMed] [Google Scholar]
- 117. Rodríguez‐Domínguez A, Berings M, Rohrbach A, et al. Molecular profiling of allergen‐specific antibody responses may enhance success of specific immunotherapy. J Allergy Clin Immunol. 2020;146(5):1097‐1108. [DOI] [PubMed] [Google Scholar]
- 118. Van Der Zee JS, Van Swieten P, Aalberse R. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137(11):3566‐3571. [PubMed] [Google Scholar]
- 119. van der Velden VH, te Marvelde JG, Hoogeveen PG, et al. Targeting of the CD33‐calicheamicin immunoconjugate Mylotarg (CMA‐676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97(10):3197‐3204. [DOI] [PubMed] [Google Scholar]
- 120. Daëron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high‐affinity IgE receptor‐mediated mast cell activation by murine low‐affinity IgG receptors. J Clin Invest. 1995;95(2):577‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bruhns P, Frémont S, Daëron M. Regulation of allergy by Fc receptors. Curr Opin Immunol. 2005;17(6):662‐669. [DOI] [PubMed] [Google Scholar]
- 122. Burton OT, Tamayo JM, Stranks AJ, Koleoglou KJ, Oettgen HC. Allergen‐specific IgG antibody signaling through FcgammaRIIb promotes food tolerance. J Allergy Clin Immunol. 2018;141(1):189‐201.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Burton OT, Logsdon SL, Zhou JS, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE‐mediated hypersensitivity. J Allergy Clin Immunol. 2014;134(6):1310‐1317.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Meulenbroek LA, de Jong RJ, den Hartog Jager CF, et al. IgG antibodies in food allergy influence allergen‐antibody complex formation and binding to B cells: a role for complement receptors. J Immunol. 2013;191(7):3526‐3533. [DOI] [PubMed] [Google Scholar]
- 125. Pilette C, Nouri‐Aria KT, Jacobson MR, et al. Grass pollen immunotherapy induces an allergen‐specific IgA2 antibody response associated with mucosal TGF‐β expression. J Immunol. 2007;178(7):4658‐4666. [DOI] [PubMed] [Google Scholar]
- 126. Lupinek C, Wollmann E, Valenta R. Monitoring allergen immunotherapy effects by microarray. Curr Treat Options Allergy. 2016;3(2):189‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Van Neerven R, Wikborg T, Lund G, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum‐IgE‐facilitated allergen presentation. J Immunol. 1999;163(5):2944‐2952. [PubMed] [Google Scholar]
- 128. Würtzen PA, Lund G, Lund K, Arvidsson M, Rak S, Ipsen H. A double‐blind placebo‐controlled birch allergy vaccination study II: correlation between inhibition of IgE binding, histamine release and facilitated allergen presentation. Clin Exp Allergy. 2008;38(8):1290‐1301. [DOI] [PubMed] [Google Scholar]
- 129. Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen‐IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112(5):915‐922. [DOI] [PubMed] [Google Scholar]
- 130. James LK, Shamji MH, Walker SM, et al. Long‐term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. J Allergy Clin Immunol. 2011;127(2):509–516.e5. [DOI] [PubMed] [Google Scholar]
- 131. Nouri‐Aria KT, Wachholz PA, Francis JN, et al. Grass pollen immunotherapy induces mucosal and peripheral IL‐10 responses and blocking IgG activity. J Immunol. 2004;172(5):3252‐3259. [DOI] [PubMed] [Google Scholar]
- 132. Heeringa JJ, McKenzie CI, Varese N, et al. Induction of IgG2 and IgG4 B‐cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy. 2020;75(5):1121‐1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shamji MH, Wilcock LK, Wachholz PA, et al. The IgE‐facilitated allergen binding (FAB) assay: validation of a novel flow‐cytometric based method for the detection of inhibitory antibody responses. J Immunol Methods. 2006;317(1–2):71‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Shamji M, Ljørring C, Francis J, et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy. 2012;67(2):217‐226. [DOI] [PubMed] [Google Scholar]
- 135. Valenta R, Campana R, Niederberger V. Recombinant allergy vaccines based on allergen‐derived B cell epitopes. Immunol Lett. 2017;189:19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Valenta R, Karaulov A, Niederberger V, et al. Molecular aspects of allergens and allergy. Adv Immunol. 2018;138:195‐256. [DOI] [PubMed] [Google Scholar]
- 137. Francis JN, James LK, Paraskevopoulos G, et al. Grass pollen immunotherapy: IL‐10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121(5):1120–1125.e2. [DOI] [PubMed] [Google Scholar]
- 138. Rauber MM, Wu HK, Adams B, et al. Birch pollen allergen‐specific immunotherapy with glutaraldehyde‐modified allergoid induces IL‐10 secretion and protective antibody responses. Allergy. 2019;74(8):1575‐1579. [DOI] [PubMed] [Google Scholar]
- 139. Ejrnaes A, Svenson M, Lund G, Larsen J, Jacobi H. Inhibition of rBet v 1‐induced basophil histamine release with specific immunotherapy‐induced serum immunoglobulin G: no evidence that FcγRIIB signalling is important. Clin Exp Allergy. 2006;36(3):273‐282. [DOI] [PubMed] [Google Scholar]
- 140. Shamji MH, Layhadi JA, Scadding GW, et al. Basophil expression of diamine oxidase: a novel biomarker of allergen immunotherapy response. J Allergy Clin Immunol. 2015;135(4):913–921.e9. [DOI] [PubMed] [Google Scholar]
- 141. Mothes N, Heinzkill M, Drachenberg K, et al. Allergen‐specific immunotherapy with a monophosphoryl lipid A‐adjuvanted vaccine: reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy‐induced blocking antibodies. Clin Exp Allergy. 2003;33(9):1198‐1208. [DOI] [PubMed] [Google Scholar]
- 142. Korosec P, Erzen R, Silar M, Bajrovic N, Kopac P, Kosnik M. Basophil responsiveness in patients with insect sting allergies and negative venom‐specific immunoglobulin E and skin prick test results. Clin Exp Allergy. 2009;39(11):1730‐1737. [DOI] [PubMed] [Google Scholar]
- 143. Orengo J, Radin A, Kamat V, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun. 2018;9(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Shamji MH, Singh I, Layhadi JA, et al. Passive Prophylactic Administration with a Single Dose of Anti‐Fel d 1 Monoclonal Antibodies REGN1908‐1909 in Cat Allergen‐Induced Allergic Rhinitis: A Randomized, Double‐blind. Am J Respir Crit Care Med. 2021;1908–1909. 10.1164/rccm.202011-4107OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Van Neerven R, Van Roomen C, Thomas W, De Boer M, Knol E, Davis F. Humanized anti‐IgEmAb Hu‐901 prevents the activation of allergen‐specific T cells. Int Arch Allergy Immunol. 2001;124(1–3):400‐402. [DOI] [PubMed] [Google Scholar]
- 146. Dhaliwal B, Yuan D, Pang MO, et al. Crystal structure of IgE bound to its B‐cell receptor CD23 reveals a mechanism of reciprocal allosteric inhibition with high affinity receptor FcεRI. Proc Natl Acad Sci. 2012;109(31):12686‐12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. McDonnell JM, Calvert R, Beavil RL, et al. The structure of the IgE Cɛ2 domain and its role in stabilizing the complex with its high‐affinity receptor FcɛRIα. Nature structural biology. 2001;8(5):437‐441. [DOI] [PubMed] [Google Scholar]
- 148. Wurzburg BA, Garman SC, Jardetzky TS. Structure of the human IgE‐Fc Cε3‐Cε4 reveals conformational flexibility in the antibody effector domains. Immunity. 2000;13(3):375‐385. [DOI] [PubMed] [Google Scholar]
- 149. Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti‐IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunology. 2001;108(2):184‐190. [DOI] [PubMed] [Google Scholar]
- 150. Arm JP, Bottoli I, Skerjanec A, et al. Pharmacokinetics, pharmacodynamics and safety of QGE 031 (ligelizumab), a novel high‐affinity anti‐IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44(11):1371‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gasser P, Eggel A. Targeting IgE in allergic disease. Curr Opin Immunol. 2018;54:86‐92. [DOI] [PubMed] [Google Scholar]
- 152. Cox L. Biologics and Allergy Immunotherapy in the Treatment of Allergic Diseases. Immunol Allergy Clin. 2020;40(4):687‐700. [DOI] [PubMed] [Google Scholar]
- 153. Lin C, Lee IT, Sampath V, et al. Combining anti‐IgE with oral immunotherapy. Pediatr Allergy Immunol. 2017;28(7):619‐627. [DOI] [PubMed] [Google Scholar]
- 154. Gasser P, Tarchevskaya SS, Guntern P, et al. The mechanistic and functional profile of the therapeutic anti‐IgE antibody ligelizumab differs from omalizumab. Nat Commun. 2020;11(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Gauvreau GM, Arm JP, Boulet L‐P, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen‐induced early asthmatic responses. Journal of Allergy and Clinical Immunology. 2016;138(4):1051‐1059. [DOI] [PubMed] [Google Scholar]
- 156. Maurer M, Giménez‐Arnau AM, Sussman G, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med. 2019;381(14):1321‐1332. [DOI] [PubMed] [Google Scholar]
- 157. Pennington LF, Tarchevskaya S, Brigger D, et al. Structural basis of omalizumab therapy and omalizumab‐mediated IgE exchange. Nat Commun. 2016;7(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Davies AM, Allan EG, Keeble AH, et al. Allosteric mechanism of action of the therapeutic anti‐IgE antibody omalizumab. J Biol Chem. 2017;292(24):9975‐9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8(3):205‐217. [DOI] [PubMed] [Google Scholar]
- 160. Coyle AJ, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C. Central role of immunoglobulin (Ig) E in the induction of lung eosinophil infiltration and T helper 2 cell cytokine production: inhibition by a non‐anaphylactogenic anti‐IgE antibody. J Exp Med. 1996;183(4):1303‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Rosenwasser LJ, Busse WW, Lizambri RG, Olejnik TA, Totoritis MC. Allergic asthma and an anti‐CD23 mAb (IDEC‐152): results of a phase I, single‐dose, dose‐escalating clinical trial. J Allergy Clin Immunol. 2003;112(3):563‐570. [DOI] [PubMed] [Google Scholar]
- 162. Virkud YV, Wang J, Shreffler WG. Enhancing the safety and efficacy of food allergy immunotherapy: a review of adjunctive therapies. Clin Rev Allergy Immunol. 2018;55(2):172‐189. [DOI] [PubMed] [Google Scholar]
- 163. Dantzer JA, Wood RA. Omalizumab as an adjuvant in food allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2021;21(3):278‐285. [DOI] [PubMed] [Google Scholar]
- 164. Kim B, Eggel A, Tarchevskaya SS, Vogel M, Prinz H, Jardetzky TS. Accelerated disassembly of IgE‐receptor complexes by a disruptive macromolecular inhibitor. Nature. 2012;491(7425):613‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Eggel A, Baravalle G, Hobi G, et al. Accelerated dissociation of IgE‐FcεRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J Allergy Clin Immunol. 2014;133(6):1709–1719.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Breiteneder H, Peng YQ, Agache I, et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy. 2020;75(12):3039‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Campana R, Marth K, Zieglmayer P, et al. Vaccination of nonallergic individuals with recombinant hypoallergenic fragments of birch pollen allergen Bet v 1: safety, effects, and mechanisms. J Allergy Clin Immunol. 2019;143(3):1258‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. van Hage M, Hamsten C, Valenta R. ImmunoCAP assays: Pros and cons in allergology. J Allergy Clin Immunol. 2017;140(4):974‐977. [DOI] [PubMed] [Google Scholar]
- 169. Skrindo I, Lupinek C, Valenta R, et al. The use of the MeDALL‐chip to assess IgE sensitization: a new diagnostic tool for allergic disease? Pediatr Allergy Immunol. 2015;26(3):239‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lupinek C, Wollmann E, Baar A, et al. Advances in allergen‐microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen‐chip. Methods. 2014;66(1):106‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wollmann E, Lupinek C, Kundi M, Selb R, Niederberger V, Valenta R. Reduction in allergen‐specific IgE binding as measured by microarray: a possible surrogate marker for effects of specific immunotherapy. J Allergy Clin Immunol. 2015;136(3):806–809.e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Schmid JM, Würtzen PA, Dahl R, Hoffmann HJ. Pretreatment IgE sensitization patterns determine the molecular profile of the IgG4 response during updosing of subcutaneous immunotherapy with timothy grass pollen extract. J Allergy Clin Immunol. 2016;137(2):562‐570. [DOI] [PubMed] [Google Scholar]
- 173. Lichtenstein LM, Norman PS, Winkenwerder WL, Osler AG. In vitro studies of human ragweed allergy: changes in cellular and humoral activity associated with specific desensitization. J Clin Investig. 1966;45(7):1126‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Niederberger V, Horak F, Vrtala S, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Natl Acad Sci. 2004;101(suppl 2):14677‐14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zieglmayer P, Focke‐Tejkl M, Schmutz R, et al. Mechanisms, safety and efficacy of a B cell epitope‐based vaccine for immunotherapy of grass pollen allergy. EBioMedicine. 2016;11:43‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Investig. 1998;102(1):98‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Campana R, Moritz K, Marth K, et al. Frequent occurrence of T cell–mediated late reactions revealed by atopy patch testing with hypoallergenic rBet v 1 fragments. J Allergy Clin Immunol. 2016;137(2):601–609.e608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Prausnitz C, Küstner H. Studienüber die Überempfindlichkeit. ZentralblBakteriol. 1921;86:160‐169. [Google Scholar]
- 179. Pauli G, Larsen TH, Rak S, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch‐allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2008;122(5):951‐960. [DOI] [PubMed] [Google Scholar]
- 180. Scadding GW, Calderon MA, Shamji MH, et al. Effect of 2 Years of Treatment With Sublingual Grass Pollen Immunotherapy on Nasal Response to Allergen Challenge at 3 Years Among Patients With Moderate to Severe Seasonal Allergic Rhinitis: The GRASS Randomized Clinical Trial. JAMA. 2017;317(6):615‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Mösges R, Koch A, Raskopf E, et al. Loliumperenne peptide immunotherapy is well tolerated and elicits a protective B‐cell response in seasonal allergic rhinitis patients. Allergy. 2018;73(6):1254‐1262. [DOI] [PMC free article] [PubMed] [Google Scholar]


