Abstract
Background:
The soluble isoform of FcɛRI, the high affinity IgE receptor (sFcεRI), is a protein of the human IgE network with poorly defined functions.
Objective:
Define cellular sources and signals that result in the production of human sFcεRI and study its in vivo functions.
Methods:
FcεRI-transfected human cell lines (MelJuso), human monocyte-derived dendritic cells (moDCs), and murine bone marrow-derived mast cells (MC) were stimulated by FcεRI crosslinking and release of sFcεRI was analyzed in supernatants (ELISA, Western Blot). Murine LAMP-1 (lysosomal-associated membrane protein 1) degranulation assays and human basophil activation tests (BAT) were used to study IgE-dependent activation. Recombinant sFcεRI (rsFcεRI) was used to assess the role of this soluble IgE receptor in murine models of anaphylaxis with WT (wild type) and IgE−/− mice
Results:
Antigen-specific crosslinking of IgE-loaded FcɛRI on cell lines that express the trimeric or tetrameric receptor isoform induced the production of human sFcεRI. Using MCs and moDCs, we confirmed that IgE/FcɛRI activation induces sFcɛRI release. We demonstrated that generation of sFcɛRI requires Src phosphorylation and endo/lysosomal acidification. In experimental mouse models, sFcɛRI diminishes the severity of IgE-mediated anaphylaxis in vivo. BATs confirmed that observation, comparable to the humanized anti-IgE monoclonal antibody omalizumab, sFcɛRI is an inhibitor of the human innate IgE effector axis, implying that sFcɛRI and omalizumab potentially inhibit each other in vivo.
Conclusion:
sFcɛRI is produced after antigen-specific IgE/FcɛRI-mediated activation signals and functions as an endogenous inhibitor of IgE-loading to FcɛRI and IgE-mediated MC activation. Our results imply, therefore, that sFcɛRI contributes to a negative regulatory feedback loop that aims at preventing overshooting responses after IgE-mediated immune activation.
Keywords: FcεRI, IgE receptor, allergy, mast cell, omalizumab
INTRODUCTION
Allergen-specific crosslinking of IgE-loaded FcεRI on mast cells (MCs) and peripheral blood basophils is a central activation event in many types of allergies. Activation of this IgE/FcεRI effector axis is also pivotal to IgE-hypersensitivity reactions which, when elicited by food, range from oral tingling and swelling to life-threatening anaphylaxis (1, 2). For food allergy, no treatments other than allergen avoidance and emergency management with epinephrine, corticosteroids, and antihistamines are currently available to the general public. Therefore, there is a high priority to study the IgE/FcεRI pathway because such research offers opportunities to discover new therapeutic intervention targets for allergy and anaphylaxis.
FcεRI is a multimeric immune recognition receptor of the IgE network and has been studied in great detail due to its crucial role in allergy (3, 4). The tetrameric isoform consists of the IgE-binding alpha chain, the beta chain, and the gamma chain dimer; the latter two subunits serving as signaling proteins (1). Tetrameric FcεRI is constitutively expressed on the surface of immune cells of the innate IgE effector axis, such as MCs and basophils, in rodents and humans. During the acute phase of an allergic response, the cell-bound fraction of antigen-specific IgE pool gets crosslinked and induces an activation cascade that results in the immediate release of preformed intracellular mediators such as histamine. Additionally, the innate cells start to produce inflammatory Th2-type cytokines that perpetuate allergic inflammation (4, 5).
In humans, dendritic cells (DCs) constitutively express a trimeric isoform of FcεRI which has been described to have a regulatory function by contributing to the clearance of serum IgE (6) and by providing a feedback mechanism that restrains allergic tissue inflammation (7, 8). The trimeric isoform of FcεRI can be induced by virus infections on murine DCs (9) but is not constitutively expressed in wild type (WT) mice. In addition to the two transmembrane FcεRI isoforms, a truncated version of the alpha chain, further referred to as soluble FcεRI (sFcεRI) has been described in human serum (10, 11). Currently, the cellular source of sFcεRI in humans or the signaling requirements for its generation are poorly defined. Previous studies have shown that sFcεRI is released after IgE-crosslinking and this soluble IgE receptor prevents IgE-binding to different FcεRI-expressing cell lines (MelJuso (10) and RBL-2H3), and interferes with PCA (passive cutaneous anaphylaxis) response in rats (12). Several open questions with regards to the biology of sFcεRI and its role within the IgE-network (3) remain to be addressed. Specifically, the signaling requirements for sFcεRI production have not been defined and, furthermore, it remains to be tested how sFcεRI affects IgE-mediated anaphylaxis in vivo.
Omalizumab, a recombinant humanized anti-IgE monoclonal antibody, has been established as a treatment for uncontrolled allergic asthma and later for CSU, Chronic Spontaneous Urticaria (13). Omalizumab binds to the Fc portion of IgE and has been shown to interact with free IgE that circulates in serum (14). Initially, this type of anti-IgE therapy leads to an increase in IgE levels followed by a decrease of IgE and FcεRI expression on DCs, MCs, and basophils (15–19). Since omalizumab and sFcεRI can both interact with IgE in human serum, it is important to address if the anti-IgE antibody and the soluble IgE Fc receptor potentially overlap in their function.
Here we show that crosslinking of tetrameric and trimeric surface FcɛRI induces the production of the soluble receptor isoform in cell line models and primary human cells. In addition to IgE-mediated receptor activation, generation of sFcεRI depends on Src signals and requires endo/lysosomal acidification. Comparable to omalizumab, sFcεRI inhibits FcɛRI-mediated basophil activation, and experimental models of murine allergy showed that sFcεRI ameliorate antigen-induced anaphylaxis in vivo.
MATERIALS & METHODS
sFcεRI ELISA
Levels of soluble FcεRI (sFcεRI) from supernatants were measured by a commercial ELISA according to manufacturer’s protocol (BMS2101, Thermo Fisher Scientific,Waltham, MA, USA).
Cells and culture conditions
Human melanoma-derived cell line MelJuso were transfected with FcεRIαγ, FcεRIαβγ, or empty vector (MelJuso-αγ, MelJuso-αβγ, and MelJusoØØ, respectively), cultured as previously described (10, 20), and used to study production of sFcεRI and its function in vitro.
For the generation of monocyte-derived dendritic cells (moDCs), monocyte isolation from heparinized blood was performed with CD14 microbeads by MACS separation according to manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocytes were cultured in RPMI-medium supplemented with 10% FBS (Gibco), 10000 U/mL Pen-Strep (Gibco), 2 mM L-Glutamine and 0.1 mM non-essential amino acids, with the addition of IL-4 (Interleukin-4) and GM-CSF (granulocyte-macrophage colony-stimulating factor) (1000U/mL, PeproTech, Rocky Hill, USA).
For the generation of murine bone marrow-derived mast cells (MCs), femurs of hFcεRIα+/mFcεRIα−/− and mFcεRIα−/− mice were flushed an progenitor cells were cultured as described (21) in RPMI-medium supplemented with 10% FBS (Gibco, Thermo Fisher Scientific,Waltham, MA, USA), 10000 U/mL Pen-Strep (Gibco, Thermo Fisher Scientific,Waltham, MA, USA), 2 mM L-Glutamine, 50 μM β-mercaptoethanol (Gibco, Thermo Fisher Scientific,Waltham, MA, USA), 10 ng/mL recombinant murine IL-3 (Interleukin-3) and SCF (stem cell factor) (PeproTech, Rocky Hill, USA).
Flow cytometry analysis
Cells were harvested, washed and centrifuged at 330 g at 4ºC with staining-buffer (DPBS 1X supplemented with 2% FBS and 0.1% NaN3). Cells were stained for 15 minutes at 4°C with NP-PE (4-Hydroxy-3-nitrophenylacetyl hapten conjugated to PE (Phycoerythrin) protein through lysine by amide bonds) (Biosearch Technologies, Petaluma, CA, USA) for cIgE (chimeric humanized anti-NP IgE, MCA333S clone JW8/1 BioRad, Serotec) detection, CRA-1-FITC or -APC (mouse IgG2b,K anti-human FcεRIα, clone AER-37) and MAR-1-APC (hamster IgG anti-murine FcεRIα) (BioLegend, San Diego, CA, USA) for human and murine FcεRIα detection respectively, IgE-PE ((BioLegend, San Diego, CA, USA) for murine IgE detection, CD49b-Pacific Blue™ (BioLegend, San Diego, CA, USA) for murine basophil characterization, CD1a-APC (mouse IgG1, K anti-human CD1a, clone HI149) (BioLegend, San Diego, CA, USA) for dendritic cell characterization, c-Kit-FITC (BioLegend, San Diego, CA, USA) for mast cell characterization, CD107a-PE/Cy7 (BioLegend, San Diego, CA, USA), and Fixable Viability Dye eFlour™ 660 (eBioscience, Thermo Fisher Scientific, Waltham, MA, USA) for LAMP-1 assays. Basophils (50 μL whole blood) were IgE-stripped with lactic acid (pH=3.9) for 5 minutes and loaded with cIgE in presence or absence of recombinant sFcεRI (rsFcεRI) or omalizumab for 1 hour at 37ºC. Basophil stimulation (NP-OVA (4-Hydroxy-3-nitrophenylacetyl ovalbumin), 2.85 μM) and staining with CD63-FITC and CCR3-PE were performed according to manufacturer’s protocol (Flow CAST, Bühlmann, Schönenbuch, Switzerland). Data was acquired on a FACS-Canto II flow cytometer and analysis was performed by FlowJo v10.
FcεRI crosslinking assays
MelJuso cells (5×105 cells), moDCs (1.7–2.5×106 cells), and MCs (5×105 cells) were loaded overnight with cIgE (from 0.1to10 μg/mL) and stimulated with NP-OVA or -BSA (4-Hydroxy-3-nitrophenylacetyl bovine serum albumin) (from 0.1 to 100 μg/mL) for 2–24 hours. MelJuso cells were incubated with TAPI-2 (18–72 μM) for 30 minutes prior to NP-OVA stimulation. Omalizumab (0.27–0.8 μM) and sFcεRI (25–500 nM) were incubated with cIgE for 30 minutes prior to loading on MelJuso and basophils. Supernatants from cell cultures were collected between 2–24 hours after FcεRI crosslinking and analyzed by ELISA or Western Blot.
LAMP-1 degranulation assays
Mature murine MCs (1×105 cells per condition), defined as at least 8 weeks old cells with a purity of greater than 90% assessed by cell surface coexpression of mast cell markers c-Kit and FcɛRIα by flow cytometry, were loaded overnight with cIgE (500 ng/mL). The next day, cells were washed twice with medium, plated in a 96-well tissue culture plate (105 cells in 100 μL per well), and challenged for 10 minutes with a cocktail containing NP-BSA (100 ng/mL) as antigen for IgE-specific receptor activation, an anti–mouse LAMP-1 antibody, and a viability dye (22). Following 2 washes with cold staining-buffer, cells were acquired on a FACS Canto II flow cytometer (BD Bioscience) and further analyzed using FlowJo v10.
Murine anaphylaxis and testing of inhibitory features of sFcεRI in vivo
Age- and sex-matched BALB/c wild type (WT) and IgE deficient (IgE−/−) mice (23) were allocated to experimental groups. Mice were passively (n=3, 3–6 mice per group; total of 19 mice) sensitized with IgE-anti-DNP (dinitrophenyl) (5 μg) in presence or absence of human rsFcεRI (3 μg) by retro-orbital (r.o.) injections. Mice sensitized with heat-denatured IgE-anti-DNP (3 hours at 65°C) were used as a control. Active (n=2, 3–4 mice per group; total of 24 mice) sensitization was performed by intra-peritoneal (i.p.) injections of OVA/Alum (ovalbumin/aluminum hydroxide and magnesium hydroxide) (100 μg) once a week for three weeks. Experimental groups received additionally rsFcεRI (3 μg) by i.p. injection 24 hours prior challenge. Mice were challenged with DNP-OVA (100–200 μg) or OVA (500 μg) by i.p. injections for passive and active sensitization models respectively. Mice challenged with PBS were used as a control. Drop of core body temperature was used as a readout for systemic anaphylaxis and was recorded up to one hour after challenge (24, 25).
Inhibition of IgE/FcεRI signaling
Murine MCs (1×105 and 5×105 cells per condition) were loaded overnight with cIgE (500 ng/mL) followed by 30 minutes incubation with 5–50 μM PP2 (Sigma-Aldrich, Merck, St. Louis, MO, USA) or 20–50 μM chloroquine (Sigma-Aldrich, Merck, St. Louis, MO, USA). NP-BSA (100 ng/mL) was added for 10 minutes and LAMP-1 degranulation assays were performed. IgG anti-human FcεRIα antibodies 19–1 and 15–1 (26) were used (1 and 10 ng/mL) in absence of cIgE and NP-BSA for 2 hours. Same stimulation conditions were performed in parallel and supernatant was collected after 2–24 hours for ELISA analysis.
RESULTS
Antigen-mediated IgE-crosslinking of tetrameric and trimeric FcεRI results in production of sFcεRI
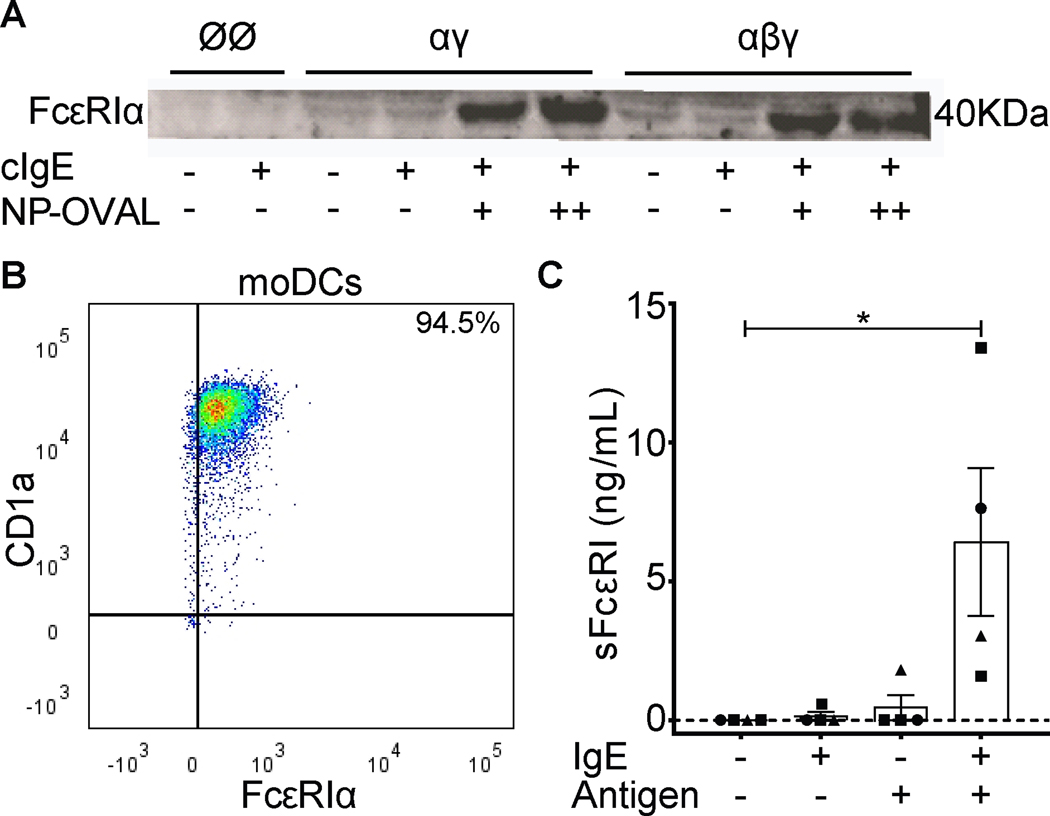
To test if sFcεRI was released by the tetrameric and/or trimeric isoform of FcεRI, we first used cell lines (MelJuso-αγ and MelJuso-αβγ (10)). MelJuso cell lines that stably express trimeric or tetrameric FcεRI have been published as suitable model to study IgE binding to different FcεRI isoforms and receptor activation via antigen-specific IgE-mediated crosslinking (10, 20). Cell lines were loaded with hapten NP-specific (4-Hydroxy-3-nitrophenylacetyl) chimeric IgE (cIgE) overnight. After removal of excess cIgE, cells were treated with haptenized bovine serum albumin (NP-BSA) or ovalbumin (NP-OVA) to activate IgE-loaded FcεRI. Supernatants from cell cultures were harvested and analyzed by Western Blot (Figure 1A). Supernatants from MelJusoØØ cells served as negative controls as they neither express nor release sFcεRI. Low sFcεRI levels were detected in supernatants from unloaded and cIgE-loaded MelJuso-αγ and MelJuso-αβγ cells and were significantly increased after crosslinking, which demonstrates that activation of both human receptor isoforms induces production of sFcεRI.
Figure 1. FcεRI-crosslinking on moDCs induces sFcεRI production.
Panel A shows detection of sFcεRI by Western Blot analysis from MelJusoØØ/-αγ/-αβγ cell cultures. Panel B shows a representative dot plot of mature monocyte-derived DCs. Panel C shows detection sFcεRI by ELISA analysis from monocyte-derived DCs given by mean ± SEM. cIgE (5–10 μg/mL) and NP-OVA (50–100 μg/mL). Individual points represent each donor where squares are males, circles females and triangle unknown. Kruskal-Wallis test plus Dunn’s multiple correction was performed where *p<0.05.
To confirm out findings with human primary cells, we generated monocyte-derived DCs (moDCs) defined as CD1a+/FcεRIα+ cells by FACS analysis (Figure 1B). moDCs were loaded with cIgE and activated as described for MelJuso cells earlier in the manuscript. After receptor crosslinking, sFcεRI was found in supernatants from moDC cultures of four individual donors. Low amounts of sFcεRI were released from moDC from 1 of 4 donors in the absence of IgE-mediated activation. IgE-mediated cell activation induced or significantly increased production of sFcεRI (p<0.05) in all cultures (Figure 1C).
Production of sFcεRI requires Src kinase activation and endo/lysosomal acidification
In order to understand the process of sFcεRI release, we used MelJuso-αγ cells in presence of a potent inhibitor of matrix metalloproteinases (MMP); a class of proteases that has been described as being responsible for the shedding of several surface proteins (27–29). Cells were loaded with cIgE overnight and incubated with the inhibitor TAPI-2 for 30 minutes before receptor activation with NP-OVA (Supplementary Figure 1A). Supernatants were harvested after 2 hours of activation and analyzed by ELISA. TAPI-2 treatment resulted in a non-significant reduction of sFcεRI production (Supplementary Figure 1B). Our results indicate that MMPs might be partially involved but not solely responsible for the release of sFcεRI.
To further investigate signaling requirements for the production of sFcεRI, we generated bone marrow-derived MCs from mice that were humanized for the expression of the human alpha chain of FcεRI (21, 24, 30). These animals were generated on the murine FcεRIα−/− background and, therefore, express FcεRI on MCs as a chimeric tetrameric receptor of the human alpha chain with the murine signal transducing receptor units. MCs, according to literature (31) and defined as c-Kit+/FcεRIα+, were loaded with cIgE on the chimeric FcεRI and the receptor internalized after NP-BSA stimulation (Supplementary Figure 2).
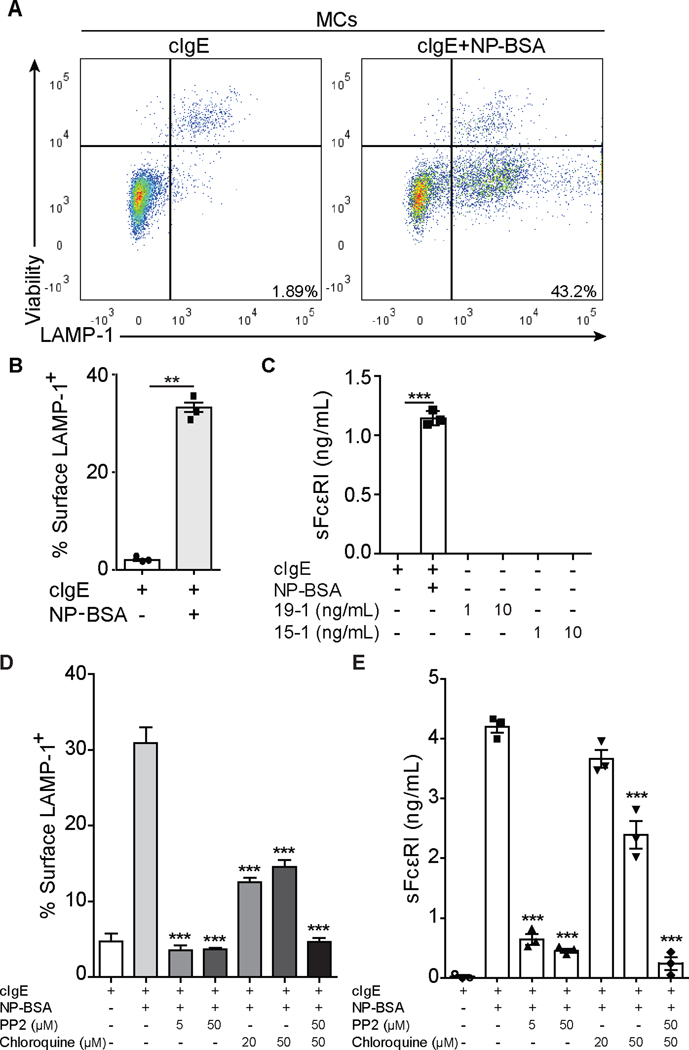
LAMP-1 (lysosomal-associated membrane protein 1) degranulation assays were used as readout for IgE/FcεRI-mediated MC activation (Figure 2A). In line with previous studies, FcεRI internalized, dependent on the used crosslinking conditions, with an average degranulation rate of 40% (Figure 2B). Comparable to human moDCs, human sFcεRI was detected in supernatants of murine humanized MCs 2 hours after antigen-specific crosslinking (Figure 2C). Release of sFcεRI in the supernatant was also correlated with β-hexosaminidase release (data not shown), another common marker of MCs activation (32). To further assess the production of sFcεRI after receptor activation, we crosslinked with the human alpha-chain specific antibodies, 19–1 and 15–1, that target two different epitopes of the alpha-chain of the FcεRI which are both located close to the IgE binding site and induce crosslinking in an IgE-independent manner (33). Antibody-mediated crosslinking of the receptor did not provide a signal that increased the production of sFcεRI, at least not at the concentration tested in our assay (Figure 2C).
Figure 2. Src kinase activation and endo/lysosomal acidification are required for sFcεRI production.
Detection of surface LAMP-1 expression on bone marrow MCs by flow cytometry (A, B, D) and sFcεRI in supernatants by ELISA (C, E). Panel A shows representative dot plots of MCs from LAMP-1 degranulation assays. Panels B and D show the quantification of percentage of surface LAMP-1+ cells. Panels C and E show total sFcεRI levels from supernatant of MCs cultures. MCs were loaded overnight with cIgE (500 ng/mL) and added NP-BSA (100 ng/mL) for 10 minutes for LAMP-1 degranulation assays. PP2 and chloroquine were added for 30 min prior to NP-BSA. In parallel, MCs were loaded overnight with cIgE and added NP-BSA, 19–1, or 15–1 for 2 (19–1 and 15–1) or 24 hours before supernatants were harvested. Graphs show mean ± SEM where paired t-test (B) or 1way ANOVA tests plus Tukey’s multiple correction (C-E) was performed; where **p<0.01 and ***p<0.001 compared to cells incubated with cIgE and NP-BSA.
The signaling cascade of tetrameric FcεRI is far better understood than that of the trimeric isoform. Therefore we further investigated the signaling requirements for sFcεRI production with MCs from FcεRIα-humanized mice. IgE-mediated activation of MCs triggers a series of FcεRI signaling events starting with phosphorylation of ITAM domains by Src kinases, specifically Lyn and Syk, followed by receptor internalization and degradation by the endo/lysosomal compartment (34, 35). Consequently, we tested the effects of the Src inhibitor PP2 and the endo/lysosomal acidification inhibitor chloroquine on the production of sFcεRI by MCs. By blocking Src kinases (36, 37), the ITAM-dependent signaling cascade after receptor crosslinking will be stopped. When receptor activation-crosslinking occurred in presence of PP2, degranulation and sFcεRI production (24 hours after activation) were significantly inhibited (Figure 2). A significant inhibition of sFcεRI production was also observed in the presence of chloroquine, an acidification inhibitor, which impairs receptor internalization and degradation (Figure 2D–E). When both inhibitors were combined, MC degranulation and sFcεRI production was completely abrogated.
Human sFcεRI blocks loading of IgE on cell surface FcεRI and inhibits basophil activation
The ability of sFcεRI to interfere with IgE-loading on the receptor was first tested on MelJuso cells. When MelJuso-αγ and MelJuso–αβγ cells were loaded with cIgE in the presence of either endogenously produced sFcεRI or recombinant sFcεRI (rsFcεRI), the binding of IgE to the cell surface was impaired in a dose dependent manner (Supplementary Figure 3). The effect of endogenously produced and recombinant sFcεRI on loading of surface receptor was comparable. MelJusoØØ cells were used as negative control and showed no binding of IgE.
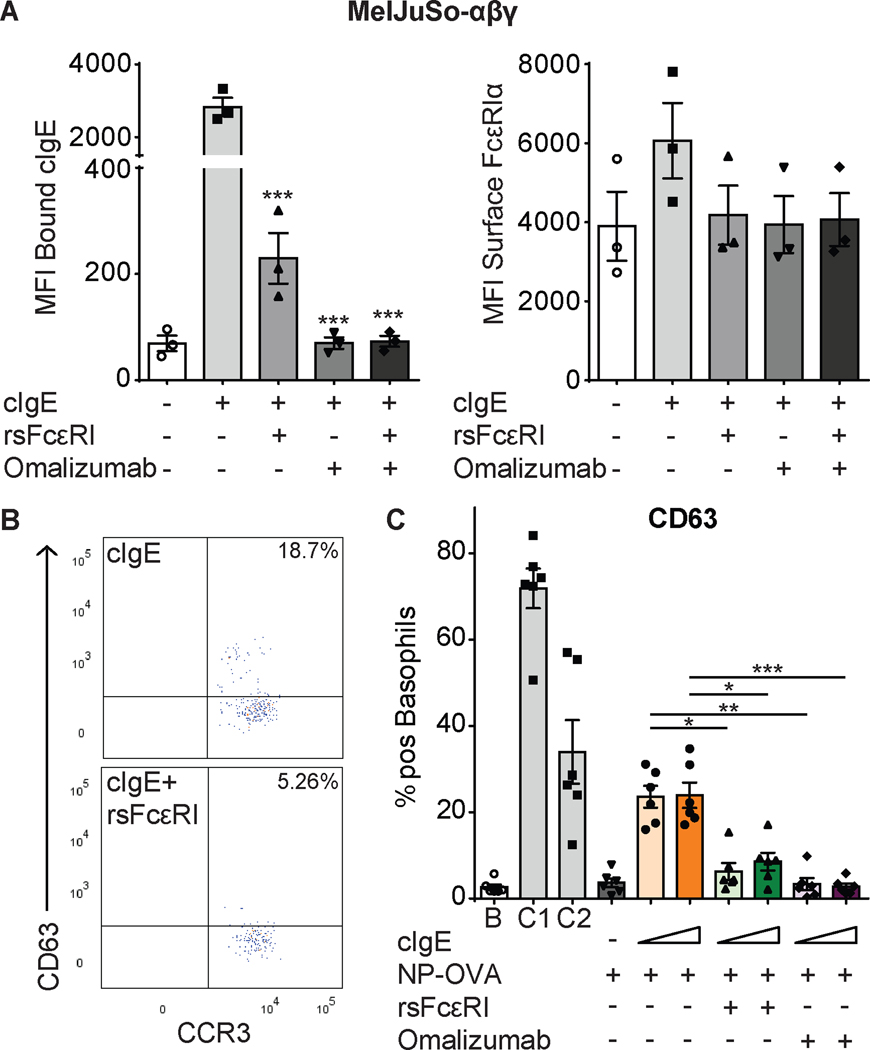
Since sFcεRI seemed to have a strong blocking effect on IgE-binding to the surface receptor, we compared its effect to widely studied humanized monoclonal antibody omalizumab, which binds to IgE-Fc and blocks the FcεRI-binding site (15, 17). Using the same setting, the inhibitory effect of rsFcεRI was comparable to omalizumab (Figure 3A and Supplementary Figure 4).
Figure 3. sFcεRI blocks cell surface cIgE binding and basophil activation.
Detection of bound cIgE (A, left) and FcεRIα (A, right) on MelJuso-αβγ (A) cells by flow cytometry (sFcεRI (25 nM) and omalizumab (0.27 μM)). Graphs show mean ± SEM. Individual points represent means of independent experiments (n=3). Detection of surface CD63 and CCR3 on basophils by flow cytometry. Panel B shows representative dot plots for basophils loaded with cIgE in presence or absence of rsFcεRI. Panel C shows the percentage of positive CD63 basophils stimulated with NP-OVA (2.85 μM) and sFcεRI (0.52 μM) or omalizumab (0.8 μM). Graphs show mean ± SEM. Individual points represent each donor (n=6). 1way ANOVA tests plus Tukey’s multiple correction were performed where *p<0.05, **p<0.01, and ***p<0.001, compared to cells loaded with cIgE. B: background; C1: anti-FcεRI mAb; C2: fMLP.
To assess functional consequences of the inhibition of IgE-binding, we performed modified Basophil Activation Tests (BAT). Surface IgE was stripped from human basophils from healthy donors. Thereafter, basophils were re-loaded with cIgE in the presence or absence of rsFcεRI or omalizumab and stimulated with NP-OVA. CD63 expression was used as readout for basophil activation (Figure 3B–C). Compared to unstimulated basophils (Supplementary Figure 5), the expression levels of CD63 increased after activation of cIgE-loaded basophils (Figure 3C). Basophil activation was diminished by rsFcεRI and omalizumab (Figure 3C). A significant inhibition was achieved by sFcεRI (0.5μM), and the effect was comparable to omalizumab (0.8μM) despite the lower concentration. Two positive controls, FcεRI-dependent and -independent (C1 and C2 respectively) were used to ensure viability of basophils.
sFcεRI is an in vivo modulator of IgE-mediated immune responses
Our next aim was to investigate how the presence of sFcεRI in serum would affect IgE-mediated anaphylaxis. It is important to note here that murine IgE can bind to human FcεRIα and murine FcεRIα with comparable binding kinetics (30, 38). WT BALB/c mice were passively sensitized by retro-orbital injections using murine IgE-anti-DNP (dinitrophenyl) in the presence or absence of human rsFcεRI 24 hours before a challenge with DNP-OVA (Figure 4A and Supplementary Figure 6A for the characterization of rsFcεRI). Challenge-induced drop in core body temperature was observed in IgE-sensitized mice. Importantly, animals that were sensitized in the presence of rsFcεRI responded with a significantly diminished drop in temperature (Figure 4B). Mice that were injected with IgE and rsFcεRI, but not challenged and animals that received only rsFcεRI were used as specificity controls and did not show alterations of body temperature (Figure 4C). An experimental condition in which we used heat-denatured IgE (dIgE) for passive sensitization confirmed that our experimental settings specifically monitor IgE-mediated anaphylaxis (Figure 4C).
Figure 4. sFcεRI prevents systemic sensitization and improves recovery.
Analysis of core body temperature from passively (A-C) and systemically (D, E) sensitized mice. Panel A shows the passive sensitization and challenge strategy. Panel B shows the time course of core body temperature drop in sensitized mice that were challenged in the absence or presence of rsFcεRI. Representative experiment, average temperature of 3–6 mice per time point. Panel C shows the composite graph of temperature drops at the 20 min time point following DNP-OVA challenge, from three independent experiments (n= 3, total of 19 mice). Panel D shows the Th2-type sensitization and challenge strategy. Panel E shows the time course of body temperature drop in sensitized mice that were challenged with OVA in absence or presence of rsFcεRI 24 hours before challenge. rsFcεRI (3 μg), IgE-anti-DNP (5 μg), OVA/Alum (100 μg), and OVA (100–500 μg). Graphs show mean ± SEM with n=3–6/group where 1way ANOVA test and Tukey’s multiple correction (C), or 2way ANOVA tests and Tukey’s (B) or Bonferroni’s (E) multiple correction were performed; *p<0.05; **p<0.01; ***p<0.001, and ****p<0.0001. * represents statistics between IgE and dIgE (B), and PBS and OVA (E) groups, *§ represents statistics between IgE-anti-DNP and IgE-anti-DNP + rsFcεRI (B), and OVA and OVA+24h rsFcεRI (E) groups.
Symptoms of murine anaphylaxis observed in passive sensitization experiments are commonly rather moderate (39). As a complementary approach, we therefore addressed whether rsFcεRI would be able to improve symptoms of anaphylaxis after systemic immunization. WT BALB/c mice were sensitized with OVA/Alum (ovalbumin/aluminum hydroxide and magnesium hydroxide) injections once a week for three weeks. The experimental group received a single rsFcεRI injection 24 hours before antigen-challenge while the control group was injected with PBS alone (Figure 4D). Animals responded to antigen-challenge with a pronounced temperature drop from which they started to recover after 40 minutes (Figure 4E and Supplementary Figure 6). Temperature of mice that were injected with rsFcεRI 24 hours before challenge dropped significantly less and animals started to recover as early as 20 minutes after challenge.
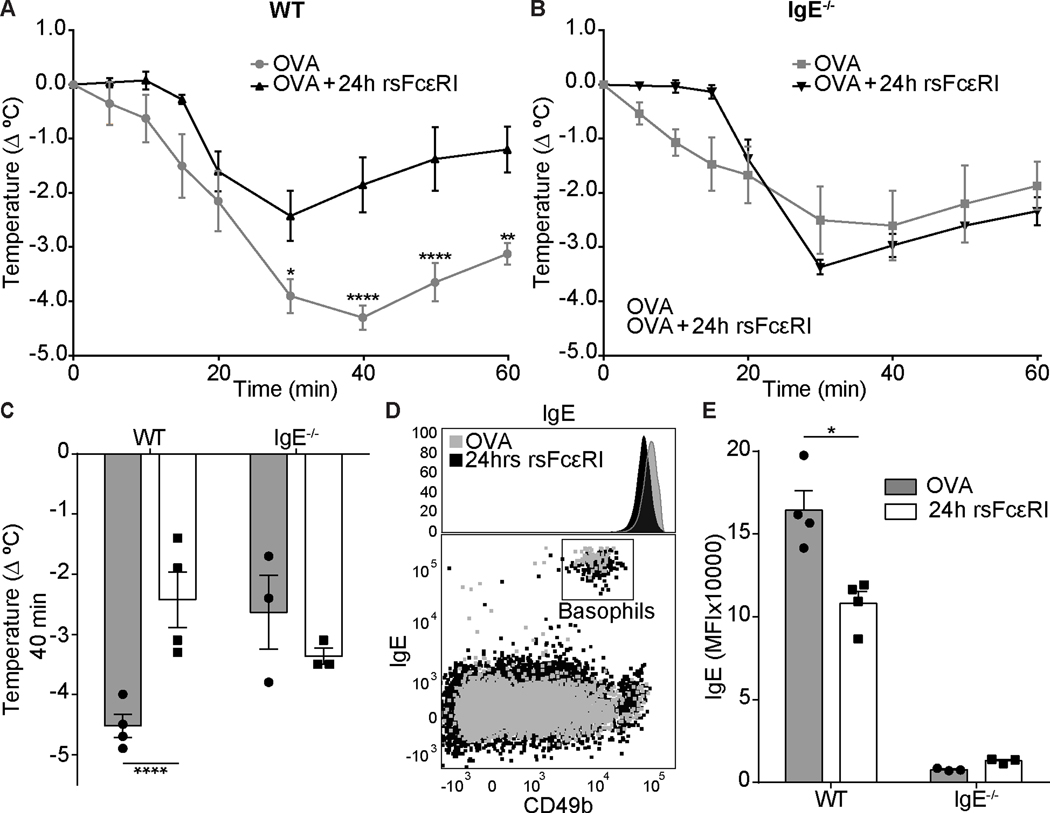
To assess if sFcεRI specifically impairs IgE-mediated anaphylaxis, we performed side-by-side experiments in WT and IgE-deficient (IgE−/−) animals. Both strains were sensitized and challenged using the experimental outline detailed in Figure 4D. Using a different aliquot of rsFcεRI, we confirmed that the temperature of WT mice that received a single dose of rsFcεRI 24 hours before challenge suffered from less pronounced anaphylaxis than untreated WT animals (Figure 5A and C). In IgE−/− animals, in which other pathways than IgE-mediated FcεRI crosslinking on MCs are responsible for the induction of anaphylaxis (40, 41), the protective effect of rsFcεRI was not observed (Figure 5B and C). When measuring IgE on peripheral blood basophils, we found that basophils from WT mice that were treated with rsFcεRI expressed lower levels of surface IgE than controls (Figure 5D and E). As expected, basophils from IgE−/− mice had no surface IgE (Figure 5E). In summary this set of experiments confirms that sFcεRI specifically affects anaphylaxis induced by the IgE/FcεRI-mediated activation of the innate effector axis of allergy (7, 34).
Figure 5. Recombinant sFcεRI prevents anaphylaxis and reduces FcεRI bound IgE on the surface of basophils.
Analysis of rectal temperature from systemically sensitized mice (A-C). Panels A and B compare the effect of rsFcεRI treatment in a time course of core temperature drop in WT (A) or IgE−/− (B) mice. Panel C shows the maximum temperature drop of WT and IgE−/− mice 40 min after OVA challenge. Panel D shows a representative histogram and a dot plot of peripheral blood basophils from WT mice with and without rsFcεRI treatment. Panel E shows the MFI of surface bound IgE on basophils with and without rsFcεRI treatment. rsFcεRI (3 μg), OVA/Alum (100 μg), and OVA (500 μg). Graphs show mean ± SEM with n=3–4/group where 2way ANOVA tests and Tukey’s multiple correction were performed; *p<0.05 and ****p<0.0001.
DISCUSSION
Here, we demonstrated that sFcεRI release occurs for the tetrameric isoform when expressed on MelJuso cells and from murine humanized MCs. Most importantly, this study describes sFcεRI release from human moDCs for the first time. We further delineate the requirements for sFcεRI release, such as antigen-specific receptor crosslinking, phosphorylation, internalization, and intracellular endo/lysosomal acidification. We cannot completely exclude the effect of proteinase-induced shedding, which might also contribute in the release of sFcεRI found in presence of Src kinase or endo/lysosomal acidification inhibitors in MCs. Furthermore, our data indicate that sFcεRI may prevent binding of IgE to FcεRI and thereby, impairs IgE-mediated effector cell activation. This effect was further assessed showing amelioration of anaphylaxis. Our findings, together with the similarities in effect between sFcεRI and omalizumab, provide evidence that sFcεRI is an endogenous modulator of IgE-mediated responses and the induction of its production might be a potential strategy to modulate allergic responses.
It has been previously described that sFcεRI can be found in serum, almost uniquely bound to IgE (42). Our results suggest that both DCs and MCs are potential sources of sFcεRI in serum. However, we cannot exclude that other cell types that express either trimeric or tetrameric FcεRI, such as basophils, eosinophils, macrophages, or Langerhans cells, additionally contribute to the serum levels of sFcεRI. Moreover, FcεRI expression can be induced on neutrophils (43) in allergic asthma and murine DCs after viral infection (9), which might also have an effect on sFcεRI in these particular conditions.
It has been previously shown that sFcεRI can prevent IgE-binding on MelJuso-αγ (10) and RBL-2H3 (12) cells. Here we have shown that, in vitro, sFcεRI binds to IgE leading to the formation of sFcεRI/IgE complexes, thus lowering the capacity of free IgE to bind to and subsequently activate cell surface FcεRI on MelJuso-αβγ cells and human basophils. We propose the process of sFcεRI release to be a protective mechanism against subsequent allergen-mediated FcεRI/IgE crosslinking and sustained cellular activation. This hypothesis is supported by in vivo experiments in mouse models of anaphylaxis in the current publication. When rsFcεRI was injected at the time of sensitization, it was sufficient to protect from anaphylaxis. If rsFcεRI was injected in a systemic sensitization model, it resulted in a diminished severity and improved recovery of IgE-mediated anaphylaxis, as well as a decrease in bound IgE to basophils. An additional argument for the specificity of the inhibitor activity of sFcεRI for the IgE/FcεRI-mediated pathway of anaphylaxis is derived from the observation that no amelioration of IgE-independent anaphylaxis (40, 41) was observed in IgE−/− animals. The strong immune response triggered in the active sensitization model, compared to the passive sensitization model, might explain why we still observe a temperature drop in presence of rsFcεRI, which suggests that the IgE:rsFcεRI ratio may important. These results imply that sFcεRI is an in vivo modulator of IgE-mediated responses, and support our proposed mechanism of a negative regulatory feedback loop. It is tempting to speculate that sFcεRI could be used as an adjuvant medication during oral immune therapy to prevent immediate responses during therapeutic antigen exposure.
Omalizumab, the humanized anti-IgE antibody, has proven to be a successful treatment for diverse IgE-mediated diseases such as severe allergic asthma, rhinitis or Chronic Spontaneous Urticaria (19, 44–49, 16, 50, 51). In addition, omalizumab has occasionally been used for the treatment of food allergy in combination with food-specific immunotherapy and successfully reduced IgE-mediated side effects (19, 52, 53). Furthermore, studies based on IgE-trapping molecules have shown potential to modulate IgE-mediated responses, such as anti-IgE Designed Ankyrin Repeat proteins (DARPins), which show inhibition of basophil activation and release of proinflammatory mediators (54), and PepE that prevents passive systemic anaphylaxis (25).
Based on the similarities between omalizumab and sFcεRI, and the emerging research on IgE-trapping strategies, we compared both molecules, and both could effectively inhibit IgE-binding to the receptor as well as IgE-mediated basophil activation. This effect was observed even at low concentrations of sFcεRI as compared to omalizumab, indicating a high affinity and inhibitory capacity of sFcεRI. Our data suggest that both molecules have a similar functional effect as they compete for the same binding domain on IgE, indicating that monitoring of sFcεRI prior to omalizumab therapy might be necessary to identify patients in which the serum IgE binding site is already occupied by an endogenous inhibitor. However, further research on circulating sFcεRI/IgE complexes and human data are needed to fully understand this mechanism.
In conclusion, although the possible implication of sFcεRI as an interference molecule in the IgE-network has been considered and partially investigated, we provide strong evidence that human sFcεRI is released from moDCs and MCs, and it might be part of a negative regulatory feedback loop to prevent detrimental IgE-mediated immune activation. The development of strategies to boost endogenous sFcεRI levels might be a potential therapeutic strategy for prevention and treatment of IgE-mediated allergies.
Supplementary Material
Acknowledgments
We thank all the members of the Szépfalusi, Fiebiger, Oettgen, Bohle, and Jardetzky laboratories for discussions and technical assistance. We thank Svetlana Tarchevskaya for her contribution in the generation of the recombinant protein.
This work was supported by the Austrian Science Fund (FWF): DK W 1248-B13 (ZS) and by the Harvard Digestive Diseases Center Grant P30DK034854, Cores B and C (EF). EF is supported by a Bridge Grant from the Research Council of Boston Children’s Hospital, an Emerging Investigator Award from FARE, a Senior research grant of the CCF, and an unrestricted gift from the Mead Johnson Nutrition Company.
Abbreviations:
- Alum
aluminum hydroxide and magnesium hydroxide
- BAT
Basophil Activation Test
- cIgE
chimeric humanized anti-NP immunoglobulin E
- CSU
Chronic Spontaneous Urticaria
- DARPins
anti-IgE Designed Ankyrin Repeat proteins
- DC
dendritic cell
- DNP
dinitrophenyl
- FcεRI
Fc epsilon Receptor I, high affinity IgE Fc receptor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- hFcεRI
human Fc epsilon Receptor I, high affinity IgE Fc receptor
- IgE
Immunoglobulin E
- IgE−/−
Immunoglobulin E deficiency
- IL-3
Interleukin-3
- IL-4
Interleukin-4
- i.p.
intra-peritoneal
- LAMP-1
lysosomal-associated membrane protein 1
- MC
mast cell
- mFcεRI
murine Fc epsilon Receptor I, high affinity IgE Fc receptor
- MMP
matrix metalloproteinase
- moDC
monocyte-derived dendritic cell
- NP-BSA
4-Hydroxy-3-nitrophenylacetyl bovine serum albumin
- NP-OVA
4-Hydroxy-3-nitrophenylacetyl ovalbumin
- OVA
ovalbumin
- PCA
passive cutaneous anaphylaxis
- r.o.
retro-orbital
- rsFcεRI
recombinant human sFcεRI
- SCF
stem cell factor
- sFcεRI
soluble isoform of FcεRI
- SPT
skin prick test
- WT
wild type
Footnotes
Conflicts of interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
REFERENCES
- 1.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nature reviews Immunology. 2007;7(5):365–78. [DOI] [PubMed] [Google Scholar]
- 2.von Bubnoff D, Novak N, Kraft S, Bieber T. The central role of FcepsilonRI in allergy. Clinical and experimental dermatology. 2003;28(2):184–7. [DOI] [PubMed] [Google Scholar]
- 3.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature reviews Immunology. 2008;8(3):205–17. [DOI] [PubMed] [Google Scholar]
- 4.Hogan SP, Wang YH, Strait R, Finkelman FD. Food-induced anaphylaxis: mast cells as modulators of anaphylactic severity. Semin Immunopathol. 2012;34(5):643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140(2):335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greer AM, Wu N, Putnam AL, Woodruff PG, Wolters P, Kinet JP, et al. Serum IgE clearance is facilitated by human FcεRI internalization. J Clin Invest. 2014;124(3):1187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platzer B, Stout M, Fiebiger E. Functions of dendritic-cell-bound IgE in allergy. Mol Immunol. 2015;68(2 Pt A):116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bieber T. The pro- and anti-inflammatory properties of human antigen-presenting cells expressing the high affinity receptor for IgE (Fc epsilon RI). Immunobiology. 2007;212(6):499–503. [DOI] [PubMed] [Google Scholar]
- 9.Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, et al. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med. 2007;204(11):2759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehlink E, Platzer B, Baker AH, Larosa J, Pardo M, Dwyer P, et al. A soluble form of the high affinity IgE receptor, Fc-epsilon-RI, circulates in human serum. PLoS ONE. 2011;6(4):e19098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platzer B, Ruiter F, van der Mee J, Fiebiger E. Soluble IgE receptors--elements of the IgE network. Immunology Letters. 2011;141(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ra C, Kuromitsu S, Hirose T, Yasuda S, Furuichi K, Okumura K. Soluble human high-affinity receptor for IgE abrogates the IgE-mediated allergic reaction. Int Immunol. 1993;5(1):47–54. [DOI] [PubMed] [Google Scholar]
- 13.Licari A, Marseglia A, Caimmi S, Castagnoli R, Foiadelli T, Barberi S, et al. Omalizumab in children. Paediatr Drugs. 2014;16(6):491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115(3):459–65. [DOI] [PubMed] [Google Scholar]
- 15.Pennington LF, Tarchevskaya S, Brigger D, Sathiyamoorthy K, Graham MT, Nadeau KC, et al. Structural basis of omalizumab therapy and omalizumab-mediated IgE exchange. Nat Commun. 2016;7:11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan AP, Giménez-Arnau AM, Saini SS. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72(4):519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano-Candelas E, Martinez-Aranguren R, Valero A, Bartra J, Gastaminza G, Goikoetxea MJ, et al. Comparable actions of omalizumab on mast cells and basophils. Clinical and Experimental Allergy. 2015. [DOI] [PubMed] [Google Scholar]
- 18.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112(6):1147–54. [DOI] [PubMed] [Google Scholar]
- 19.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol. 2016;137(4):1103–10.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platzer B, Fiebiger E. The signal peptide of the IgE receptor alpha-chain prevents surface expression of an immunoreceptor tyrosine-based activation motif-free receptor pool. J Biol Chem. 2010;285(20):15314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157(4):1645–51. [PubMed] [Google Scholar]
- 22.Lexmond WS, Goettel JA, Lyons JJ, Jacobse J, Deken MM, Lawrence MG, et al. FOXP3+ Tregs require WASP to restrain Th2-mediated food allergy. J Clin Invest. 2016;126(10):4030–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370(6488):367–70. [DOI] [PubMed] [Google Scholar]
- 24.Shade KT, Platzer B, Washburn N, Mani V, Bartsch YC, Conroy M, et al. A single glycan on IgE is indispensable for initiation of anaphylaxis. J Exp Med. 2015;212(4):457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou JS, Sandomenico A, Severino V, Burton OT, Darling A, Oettgen HC, et al. An IgE receptor mimetic peptide (PepE) protects mice from IgE mediated anaphylaxis. Mol Biosyst. 2013;9(11):2853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiebiger E, Tortorella D, Jouvin MH, Kinet JP, Ploegh HL. Cotranslational endoplasmic reticulum assembly of FcepsilonRI controls the formation of functional IgE-binding receptors. J Exp Med. 2005;201(2):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemieux GA, Blumenkron F, Yeung N, Zhou P, Williams J, Grammer AC, et al. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J Biol Chem. 2007;282(20):14836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–701. [DOI] [PubMed] [Google Scholar]
- 29.Shiomi T, Lemaitre V, D’Armiento J, Okada Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol Int. 2010;60(7):477–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platzer B, Baker K, Vera MP, Singer K, Panduro M, Lexmond WS, et al. Dendritic cell-bound IgE functions to restrain allergic inflammation at mucosal sites. Mucosal Immunol. 2015;8(3):516–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinet JP. The essential role of mast cells in orchestrating inflammation. Immunol Rev. 2007;217:5–7. [DOI] [PubMed] [Google Scholar]
- 32.de Las Vecillas Sanchez L, Alenazy LA, Garcia-Neuer M, Castells MC. Drug Hypersensitivity and Desensitizations: Mechanisms and New Approaches. Int J Mol Sci. 2017;18(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B, Rieger A, Kilgus O, Ochiai K, Maurer D, Fodinger D, et al. Epidermal Langerhans cells from normal human skin bind monomeric IgE via Fc epsilon RI. J Exp Med. 1992;175(5):1353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. [DOI] [PubMed] [Google Scholar]
- 35.Castells M. Drug Hypersensitivity and Anaphylaxis in Cancer and Chronic Inflammatory Diseases: The Role of Desensitizations. Front Immunol. 2017;8:1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulimenko V, Draberova E, Sulimenko T, Macurek L, Richterova V, Draber P. Regulation of microtubule formation in activated mast cells by complexes of gamma-tubulin with Fyn and Syk kinases. J Immunol. 2006;176(12):7243–53. [DOI] [PubMed] [Google Scholar]
- 37.Chung SC, Limnander A, Kurosaki T, Weiss A, Korenbrot JI. Coupling Ca2+ store release to Icrac channel activation in B lymphocytes requires the activity of Lyn and Syk kinases. J Cell Biol. 2007;177(2):317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallmann E, Reininger B, Brandt S, Duschek N, Hoflehner E, Garner-Spitzer E, et al. High-affinity IgE receptors on dendritic cells exacerbate Th2-dependent inflammation. J Immunol. 2011;187(1):164–71. [DOI] [PubMed] [Google Scholar]
- 39.Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: Insights into etiology, prevention, and treatment provided by murine models. J Allergy Clin Immunol. 2014;133(2):309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clay CD, Strait RT, Mahler A, Khodoun MV, Finkelman FD. Anti-FcγRIIB mAb suppresses murine IgG-dependent anaphylaxis by Fc domain targeting of FcγRIII. J Allergy Clin Immunol. 2018;141(4):1373–81.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016;137(6):1674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lexmond W, der Mee J, Ruiter F, Platzer B, Stary G, Yen EH, et al. Development and validation of a standardized ELISA for the detection of soluble Fc-epsilon-RI in human serum. Journal of immunological methods. 2011;373(1–2):192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mora J, Riggs EK, Fu J, MacGlashan DW, Fox SA, Yu B, et al. Expression of the high affinity IgE receptor by neutrophils of individuals with allergic asthma is both minimal and insensitive to regulation by serum IgE. Clin Immunol. 2009;132(1):132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holgate ST, Djukanović R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408–16. [DOI] [PubMed] [Google Scholar]
- 45.Vignola AM, Humbert M, Bousquet J, Boulet LP, Hedgecock S, Blogg M, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59(7):709–17. [DOI] [PubMed] [Google Scholar]
- 46.Iyengar SR, Hoyte EG, Loza A, Bonaccorso S, Chiang D, Umetsu DT, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162(1):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramírez del Pozo ME, Contreras Contreras E, López Tiro J, Gómez Vera J. Omalizumab (an anti-IgE antibody) in the treatment of severe atopic eczema. J Investig Allergol Clin Immunol. 2011;21(5):416–7. [PubMed] [Google Scholar]
- 48.Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, et al. A phase II, randomized, double‑blind, parallel‑group, placebo‑controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127(5):1309–10.e1. [DOI] [PubMed] [Google Scholar]
- 49.Niven RM, Saralaya D, Chaudhuri R, Masoli M, Clifton I, Mansur AH, et al. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study). BMJ Open. 2016;6(8):e011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaplan A, Ferrer M, Bernstein JA, Antonova E, Trzaskoma B, Raimundo K, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. Journal of Allergy and Clinical Immunology. 2015. [DOI] [PubMed] [Google Scholar]
- 51.Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368(10):924–35. [DOI] [PubMed] [Google Scholar]
- 52.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127(6):1622–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gernez Y, Nowak-Wegrzyn A. Immunotherapy for Food Allergy: Are We There Yet? J Allergy Clin Immunol Pract. 2017;5(2):250–72. [DOI] [PubMed] [Google Scholar]
- 54.Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P, et al. Accelerated dissociation of IgE-FcepsilonRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. Journal of Allergy and Clinical Immunology. 2014;133(6):1709–19 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.