Abstract
Purpose.
Although laparoscopic hyperthermic intraperitoneal chemotherapy (LS-HIPEC) has been proven safe in patients with gastric adenocarcinoma and carcinomatosis or positive cytology, patient selection criteria remain unclear. Thus, we performed a retrospective analysis to identify factors associated with improved survival and resection rates.
Methods.
Data for all patients undergoing LS-HIPEC for stage IV gastric adenocarcinoma between June 2014 and November 2018 were collected prospectively and analyzed for associations with survival and resection using uni- and multivariate logistic regression, Cox proportional hazards models, and Kaplan-Meier survival functions.
Results.
Of 70 patients who underwent LS-HIPEC, 43(61%) received 2 drugs (mitomycin C and cisplatin), and 27(39%) received 3 drugs (mitomycin C, cisplatin, and paclitaxel). The 2 groups’ demographic and oncologic differences were not significant, although the 3-drug group had a significantly lower rate of radiation therapy use (58% vs 15%;p<0.01). Univariate analysis revealed that poor differentiation (Cox hazard ratio[HR], 2.75; 95% confidence interval[CI], 1.34–5.63;p<0.01), gross carcinomatosis (HR,3.10; 95% CI,1.52–6.30;p=0.03), and ascites (HR,3.43; 95% CI,1.88–6.26;p<0.01) were associated with shorter median survival. Gastrectomy was associated with improved overall survival (HR,0.32; 95% CI,0.15–0.70;p<0.01). The resection rate of the 45 patients without ascites (38%) was significantly higher than that of the 25 patients with ascites (0%; p<0.01)
Conclusion.
Our findings identify ascites as a significant prognostic factor for gastric cancer patients with peritoneal metastases undergoing LS-HIPEC. Our findings can be used to help identify patients who are unlikely to proceed to resection after LS-HIPEC and are good candidates for novel therapeutic approaches or clinical trials.
Keywords: Gastric Cancer, Carcinomatosis, HIPEC
INTRODUCTION
At the time of staging laparoscopy, 4−41% of patients with clinically localized gastric cancer are found to have malignant cells on peritoneal cytology or gross peritoneal carcinomatosis1. Such findings indicate stage IV disease, which the National Comprehensive Cancer Network deems unresectable2. Owing to the unresectability of their disease, gastric cancer patients with peritoneal metastasis have a median survival duration of less than 1 year3. Regional therapies may offer a pathway to resection, but their role in the treatment of gastric cancer and the patients whom they would benefit most remain unclear.
The most studied regional therapy in gastric adenocarcinoma is heated intraperitoneal chemotherapy (HIPEC), a well-established modality for treating relatively low-grade histologies, such as appendiceal neoplasms, that have spread to the peritoneum. The use of HIPEC arose from the realization that the delivery of systemic chemotherapy to peritoneal metastases is limited and that the application of heated, high-dose chemotherapy directly to the peritoneum after cytoreductive surgery could be more efficacious 4–7. Moreover, there has been increased recognition that the peritoneum is a distinct organ and that peritoneal metastases, although visually diffuse, can be resected with negative margins. Given the success of HIPEC in treating low-grade malignancies, interest in its application to high-grade malignancies, such as gastric cancer, has increased8.
No randomized studies have shown that LS-HIPEC provides a greater benefit than systemic chemotherapy in patients with positive cytology and/or carcinomatosis from gastric adenocarcinoma. Previous studies have demonstrated that LS-HIPEC, given as part of a multidisciplinary approach, is safe for the treatment of select gastric cancer patients presenting with positive cytology and/or carcinomatosis and is associated with improved survival rates9,10. LS-HIPEC has been performed using several different regimens11. To date, most studies of HIPEC have aimed to identify the most effective and safe regimen. While these regimens have been shown to be safe with some efficacy, little is known about which patients are likely to benefit.
The purpose of this study was to identify factors associated with improved outcomes among gastric cancer patients undergoing LS-HIPEC for peritoneal metastasis. Identifying such factors will help refine treatment algorithms for these patients; improve the selection of patients for LS-HIPEC followed by gastrectomy or for the combination of cytoreduction, HIPEC, and gastrectomy. Similarly, this work will identify patients who are unlikely to benefit from LS-HIPEC, enabling them to consider other treatment modalities.
PATIENTS AND METHODS
This study was approved by MD Anderson’s Institutional Review Board. The study included patients enrolled in either a phase 2 trial of a 2-drug LS-HIPEC protocol (October 2013−May 2016) or a phase 1 trial of a 3-drug LS-HIPEC protocol (January 2017–March 2018). The study also included patients who underwent LS-HIPEC off-protocol during these periods. These trials’ details including patient selection have been reported previously9,12,13. Patients had to be greater than 18 years old with confirmed gastric cancer and carcinomatosis or peritoneal cytology. Patients had to have completed systemic chemotherapy followed by restaging before enrollment. If no distant disease was identified on restaging following systemic chemotherapy, patients were then able to undergo diagnostic laparoscopy with LS-HIPEC. Early in our experience of investigating HIPEC for patients with gastric cancer, patients would undergo iterative LS HIPEC and only undergo gastrectomy if the peritoneal disease was undetectable. Later in our experience, patients that had disease amenable to complete resection at the first LS HIPEC would then be offered cytoreduction, gastrectomy, and HIPEC. Any patients developing distant solid organ, progressive, or who were found to have unresectable disease were referred for further systemic treatment options after multidisciplinary discussion. Clinical factors studied here were not included in the decision to proceed to gastrectomy. Patient demographic data along with tumor characteristics were prospectively collected and follow-up data collected by research coordinators per study protocol.
We analyzed demographic differences between the 2- and 3-drug LS-HIPEC groups using the Wilcoxon rank-sum test, t-test, or chi-squared test as appropriate. We used Cox hazards ratios (HRs) and the Kaplan−Meier method to analyze overall survival. To identify factors that were correlated with either resection or survival, we performed univariate and multivariate analyses; for both resection and survival, all independent variables showing a significant association (p<0.05) in the univariate analysis were included in the multivariate analysis. Final models were selected using the Akaike information criterion to compare quality between fitted multivariable Cox Proportional Hazards models.
To account for collinearity, we used a tree-based machine learning approach to examine the relationship between independent variables and outcomes. This modeling framework (recursively partitioned classification trees) is tolerant of collinear variables and also provides transparent logic for variable importance with if-then rules to aid clinical decision-making.14,15. All trees were grown without restriction and pruned using the following settings: minimal split=1, minimum bucket=5, complexity parameter=0. These criteria were selected to decrease the bias-variance ratio and intentionally overfit the models to maximize their description of the data. No test or training cohorts were used, and all validation was performed in-sample. Tree-based models were assembled using the rpart package (Recursive Partitioning and Regression Trees, version 4.1–15) in R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
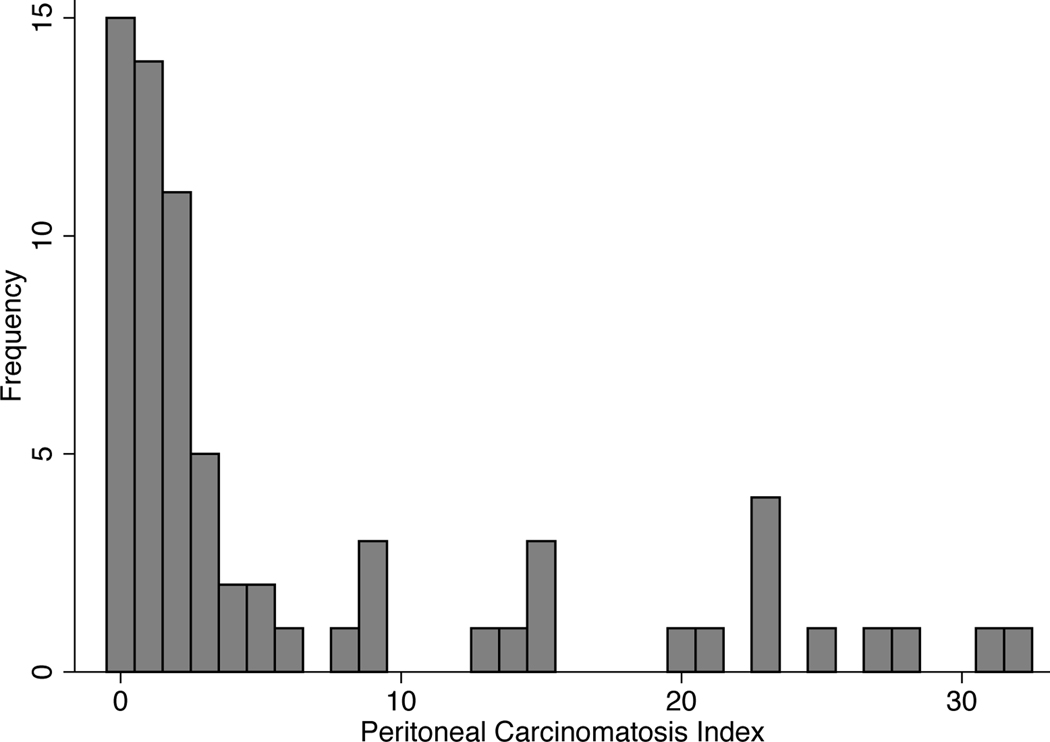
The study included 70 patients (43 men and 27 women), whose demographic, histologic, and treatment characteristics are summarized by LS-HIPEC group in Table 1. The mean age for all patients was 54.8 years (standard deviation, 12.8 years). Most patients had poorly differentiated malignancies (77%). Most patients had signet-ring cell histology (70%), and 18 patients (26%) had linitis plastica; significant treatment differences did not exist between these histologic groups. For all patients, the median number of lines of prior systemic chemotherapy was 1 (range, 1–4 lines) and the median number of cycles was 8 (range, 3–53). Twenty-five patients (36%) had ascites. Fifty-seven patients (81%) had gross carcinomatosis; these patients’ median peritoneal cancer index (PCI) score was 3 (range, 0–32) (Fig. 1). Twenty-two (31%) of the patients with gross carcinomatosis were noted to have ascites as well.
TABLE 1.
Patients’ demographic, histologic, and treatment characteristics by LS-HIPEC regimen
| Characteristic | Two-drug regimen (n=43) | Three-drug regimen (n=27) | p |
|---|---|---|---|
| Mean age ± SD, years | 54.3 ± 13.2 | 55.6 ± 12.3 | 0.70 |
| Female sex | 15 (35) | 12 (44%) | 0.42 |
| Signet-ring cell pathology | 27 (63) | 22 (81%) | 0.10 |
| Poor differentiation | 29 (67%) | 25 (93) | 0.05 |
| Median PCI score | 2 | 6 | 0.01 |
| Carcinomatosis | 32 (74) | 25 (93) | 0.01 |
| Ascites | 14 (33) | 11 (41) | 0.49 |
| Radiation therapy | 25 (58) | 4 (15) | <0.01 |
| Median no. of lines of prior systemic chemotherapy | 1 | 1 | 0.03 |
| Readmission | 4 (9) | 1 (4) | 0.37 |
| 30 Day Mortality | 0 (0) | 1 (4) | 0.81 |
| Resection | 10 (23) | 7 (26) | 0.80 |
Note: All data are no. of patients (%) unless otherwise noted.
Abbreviations: SD, standard deviation; PCI, peritoneal cancer index.
FIG. 1.
Histogram of peritoneal carcinomatosis index scores for all patients (n=70) included in this analysis.
Of the 70 patients, 27 (39%) were in the 3-drug trial, and 43 (61%) were in the 2-drug trial. The 2 groups’ histologic differences were not statistically significant. However, the radiation therapy rate of the 2-drug group (58%) was significantly higher than that of the 3-drug group (15%; p<0.01), which reflects treatment trends at our institution.
Of the 70 patients, 17 (24%) were able to proceed to resection of their malignancy; all underwent gastrectomy. As expected, the median survival duration of these patients was significantly longer than that of patients who did not proceed to resection (Cox hazard ratio [HR], 0.37; 95% confidence interval [CI], 0.18–0.76; p<0.01). Four (24%) of patients undergoing gastrectomy and CRS had a CC-1 resection while the remaining 13 (76%) were cytoreduced to CC-0. Univariate and multivariate analyses revealed that the association between ascites and resection was colinear (Table 2). No patient with ascites at the time of staging laparoscopy was able to proceed to resection. This included 3 patients without carcinomatosis but with ascites and positive cytology. These 3 patients were unable to be resected due to development of carcinomatosis despite iterative LS-HIPEC in two patients and invasion into the celiac axis precluding resection in a third.
TABLE 2.
Results of the univariate and multivariate analyses for factors associated with resection
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
||||
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Age | 0.01 (−0.04, 0.06) | 0.59 | - | - |
| Female sex | −1.35 (−2.71, 0.01) | 0.05 | - | - |
| Signet ring pathology | −1.35 (−2.50, −0.20) | 0.02 | −0.84 (−2.26, 0.57) | 0.24 |
| Poor differentiation | −1.47 (−2.66, 0.28) | 0.02 | −0.52 (−2.21, 1.17) | 0.55 |
| PCI score | −0.12 (−0.25, 0.00) | 0.06 | - | - |
| Carcinomatosis | −1.34 (−2.52, −0.17) | 0.03 | −0.79 (−2.25, 0.67) | 0.29 |
| Ascites | Colinear | - | Colinear | - |
| Radiation therapy | −0.01 (−1.12, 1.10) | 0.98 | - | - |
| No. of lines of prior systemic chemotherapy | −1.1 (−2.2, 0.11) | 0.08 | - | - |
Abbreviations: HR, hazard ratio; CI, confidence interval; PCI, peritoneal cancer index.
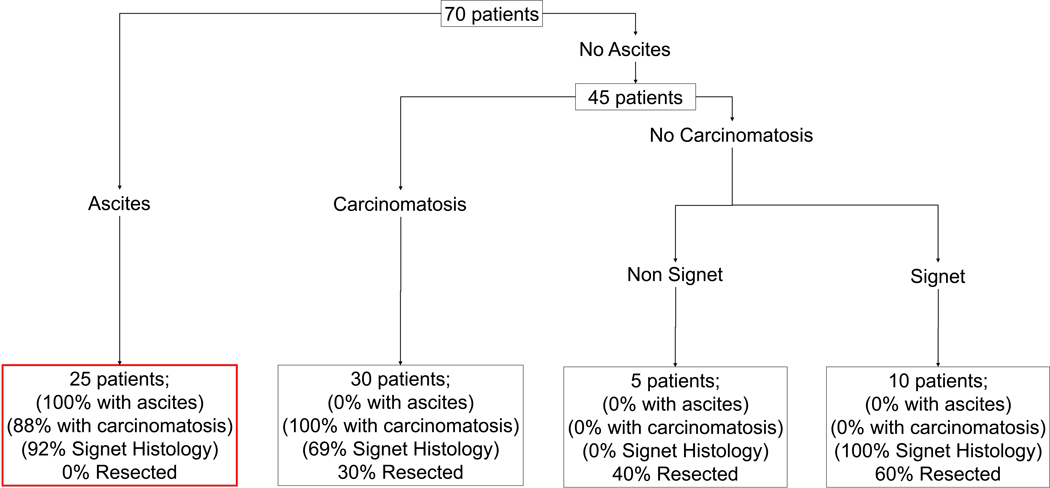
Similar to the multivariate analyses, recursively partitioned classification trees demonstrated that for patients with ascites, the probability of undergoing resection was 0.0%. Among patients without ascites, those who had carcinomatosis, as well as those who did not have carcinomatosis but who had non−signet-ring cell carcinoma, had a <50.0% probability of undergoing resection (Fig. 2). Patients without ascites or carcinomatosis, but with signet-ring cell carcinoma had a 60% probability of undergoing surgical resection after LS-HIPEC.
FIG. 2.
A recursively partitioned classification tree showing probabilities of surgical resection. Percentage of patients by partitioning factors listed in final bins.
Univariate analysis revealed no significant difference in overall survival between patients in the 2-drug group and those in the 3-drug group (Cox HR, 1.61; 95% CI, 0.85–3.03; p=0.14). Even after controlling for significant differences in disease and treatment factors, including PCI score, the number of prior chemotherapy lines, and radiation therapy; we found no significant difference in survival (Cox HR, 0.79; 95% CI, 0.39–1.57; p=0.50). Although uncommon, 4 patients who underwent gastrectomy and cytoreduction had a CC-1 cytoreduction. For these patients, overall survival was not significantly improved from those not undergoing cytoreduction (13.6 versus 10.9 months, p=0.81).
The results of the univariate and multivariate analyses for factors associated with survival are summarized in Table 3. Univariate analysis revealed that tumor grade, PCI score, carcinomatosis, ascites, and resection were significantly associated with survival. A multivariate model comprising these factors demonstrated that only PCI score was significantly associated with overall survival. The association between survival and PCI was linear. However, a PCI score of 1–7 (HR,2.94; 95% CI, 1.26–6.84; p=0.01), carried a lower risk of death than did a PCI score of >7 (HR,13.3; 95% CI, 4.94–36.2; p<0.01) (Fig. 3,4).
TABLE 3.
Results of the univariate and multivariate analyses for factors associated with survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
||||
| Variable | Cox HR (95% CI) | p | Cox HR (95% CI) | p |
| Age | 0.99 (0.97–1.02) | 0.62 | - | - |
| Female sex | 1.44 (0.80–2.59) | 0.22 | - | - |
| Signet-ring cell pathology | 1.83 (0.96–3.45) | 0.06 | - | - |
| Poor differentiation | 2.75 (1.34–5.63) | 0.01 | 1.18 (0.447–2.98) | 0.73 |
| Median PCI score | 1.12 (1.08–1.16) | <0.01 | 1.09 (1.04–1.14) | <0.01 |
| Carcinomatosis | 3.10 (1.52, 6.30) | <0.01 | 1.74 (0.67, 4.53) | 0.26 |
| Ascites | 3.43 (1.88–6.26) | <0.01 | 1.50 (0.65–3.46) | 0.34 |
| Radiation therapy | 0.81 (0.46–1.43) | 0.48 | - | - |
| No. of lines of prior systemic chemotherapy | 0.84 (0.57–1.23) | 0.36 | - | - |
| Resection | 0.37 (0.18–0.76) | 0.01 | 0.63 (0.28–1.42) | 0.27 |
| Three-drug regimen | 1.61 (0.85–3.03) | 0.14 | - | - |
Abbreviations: HR, hazard ratio; CI, confidence interval; PCI, peritoneal cancer index.
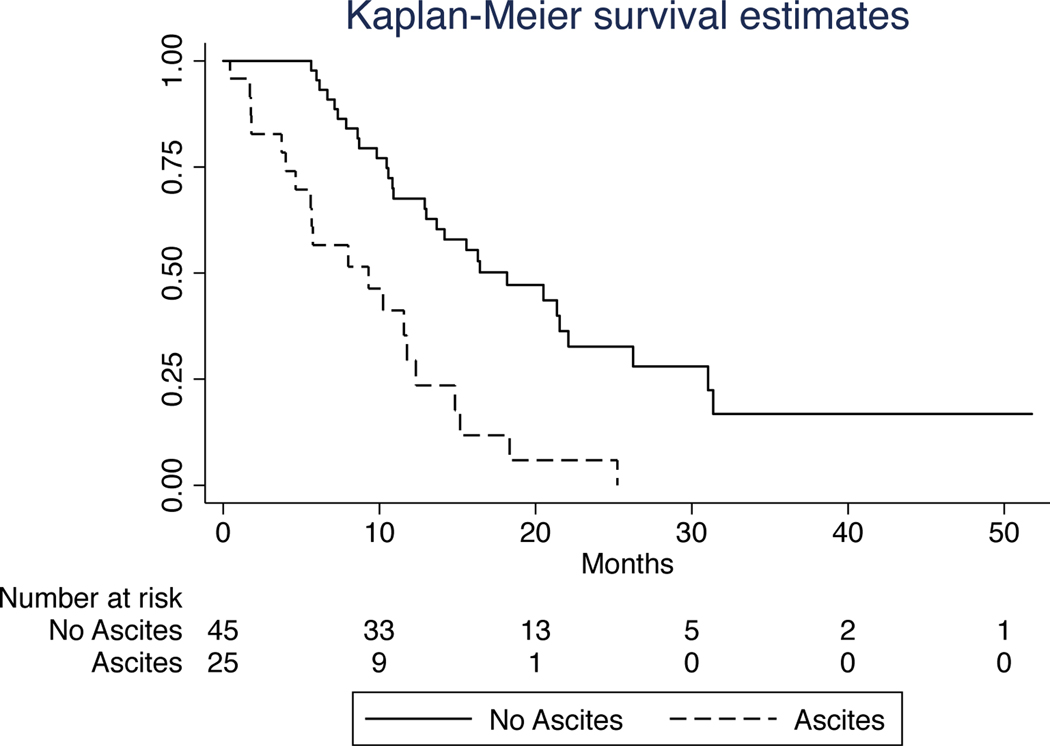
FIG. 3.
Kaplan-Meier survival curves for patients with and without ascites. The median survival duration of patients with ascites (9.3 months) was substantially shorter than that of patients without ascites (18.2 months), p<0.01.
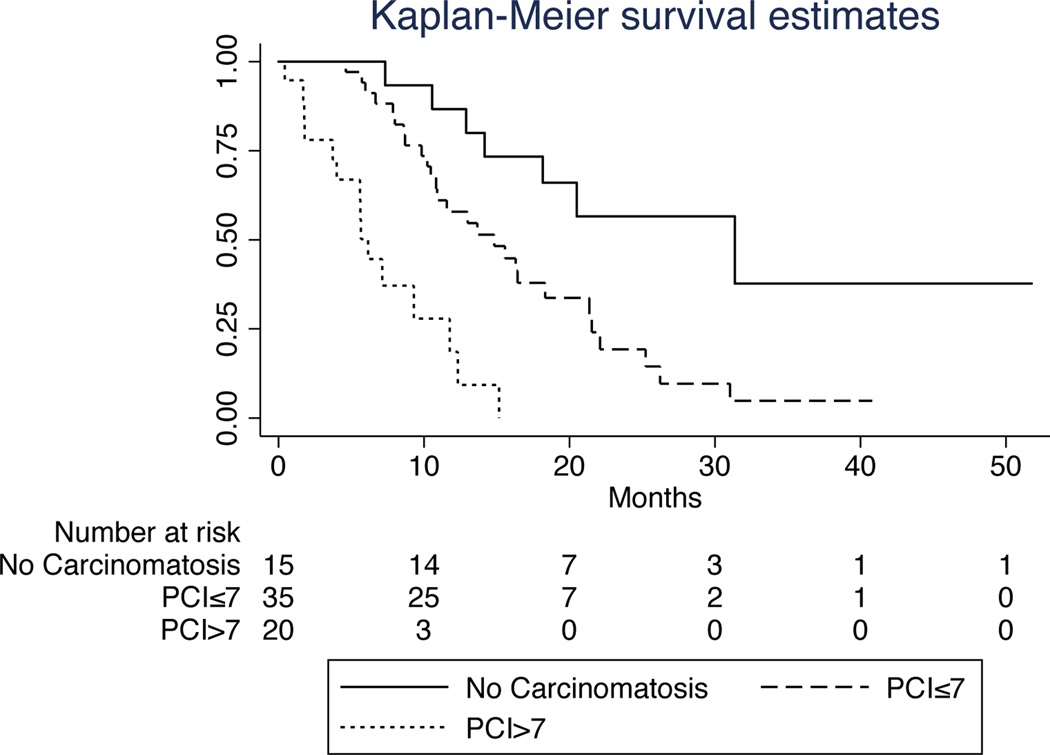
FIG. 4.
Kaplan-Meier survival curves for patients with and without carcinomatosis. The median survival durations of patients without gross carcinomatosis (PCI=0), patients with PCI scores of 1–7, and patients with PCI scores greater than 7 were 31.4, 14.8, and 5.7 months, respectively.
DISCUSSION
We identified clinical factors that are correlated with the outcomes of gastric cancer patients undergoing LS-HIPEC for peritoneal metastasis. Most notably, no patients who had ascites at the time of staging laparoscopy were able to proceed to gastrectomy. Using these findings to narrow the criteria used to select gastric cancer patients for LS-HIPEC could improve these patients’ rates of progression to gastrectomy as well as their survival. Similarly, our findings could be used to identify patients who are unlikely to proceed to resection and might instead benefit from novel systemic therapies and/or enrollment in clinical trials.
We are not the first to report a correlation between ascites and proceeding to resection in patients with metastatic gastric cancer. Benizri et al.16 reported that ascites was associated with a higher likelihood of incomplete cytoreduction. In the PHOENIX-GC trial, which compared intraperitoneal chemotherapy with systemic chemotherapy, ascites was noted as an adverse prognostic factor in both arms. The higher rate of ascites in the intraperitoneal arm was considered as a factor relating to the trial’17. Given our own finding that ascites is associated with decreased survival and proceeding to conversion surgery, a more in-depth investigation of this association could help validate this association.
Despite the findings described above, LS-HIPEC still has a potential role in the treatment of gastric adenocarcinoma patients with ascites. One previous study showed small groups of patients with symptomatic ascites benefited from LS-HIPEC18. This finding underscores the importance of defining the purpose and expectations of LS-HIPEC at the outset of treatment. For example, LS-HIPEC could be offered to a highly selected group of patients to control their symptoms; in contrast, LS-HIPEC was offered to the patients in the present study to prolong their survival. Patient and provider expectations need to be clearly defined prior to using this approach.
A valid criticism of the use of LS-HIPEC to treat gastric cancer is that the therapy has low rates of conversion to negative cytology, enabling relatively few patients to undergo resection. Using ascites alone as a contraindication to LS-HIPEC would increase this group’s current resection rate from 24.3% to 37.8%. The criteria used to select patients for LS-HIPEC could be refined further, but such refinement could affect the generalizability of existing treatment algorithms, leading to the exclusion of patients who would benefit from resection.
The present study had some limitations. First, although the study included patients enrolled in prospective trials, its analyses were retrospective; thus, the study had all the limitations inherent to a retrospective analysis. Second, although a single surgeon performed all LS-HIPEC procedures, the patients’ preoperative workup and treatment were not standardized prior to referral and subject to temporal treatment trends within the institution. Similarly, the number of patients included and their inherent heterogeneity may obscure our identification of more nuanced selection criteria. For example, the presence of carcinomatosis was a significant predictor of survival and progression to resection while an individual PCI cutoff was unable to be identified. During the study time period additional outcomes data, such as the CYTOCHIP study, was published and, in combination with our own increasing expertise in performing gastrectomy and HIPEC, allowed us to expand the indications for offering LS HIPEC. Further work will be needed among a larger group of patients to further refine ideal selection criteria. Finally, among patients with high-volume disease only a few elected to proceed to LS-HIPEC. Therefore, the study sample largely comprised patients with low-volume disease, whose functional statuses were such that the surgeon believed the patients could undergo LS-HIPEC.
In conclusion, the findings of the present study can be used to more accurately identify patients with metastatic gastric cancer who will benefit from LS-HIPEC. Given improved patient selection, patients who undergo LS-HIPEC should have increased rates of conversion to resection, and other treatment algorithms could be considered earlier for patients who would not benefit from LS-HIPEC. Currently, our group offers patients with stage IV peritoneal disease LS HIPEC with either a two- or three-drug regimen (based on NCT02092298 and NCT03330028, respectively) followed by cytoreduction, gastrectomy, and HIPEC with a two-drug regimen (NCT02891447) for patients with disease that appears amenable to a CC0 resection and following an excellent response to systemic therapy. Alternately, and especially in patients that are reluctant to consider cytoreduction, gastrectomy, and HIPEC, we offer enrollment into an iterative intraperitoneal Paclitaxel trial via an intraperitoneal port NCT04220827. Moving forward, additional studies are needed to not only refine patient selection criteria and standard treatment algorithms, but also investigate novel diagnostic and therapeutic technologies.
Synopsis.
While LS-HIPEC for stage IV gastric cancer has been shown safe, little is known about optimal patient selection. Here we retrospectively examine patients from our single institution to identify factors associated with survival and the ability to proceed to resection.
ACKNOWLEDGEMENTS.
The authors thank Joseph Munch of the Department of Scientific Publications for editorial assistance. This trial was supported by the Holly Clegg Gastric Cancer Research Fund and the No Stomach for Cancer Award for Gastric Cancer Research.
Footnotes
Author Disclosure Statement: The authors have no competing interests to report.
REFERENCES
- 1.Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Annals of surgical oncology. October 2008;15(10):2684–2691. [DOI] [PubMed] [Google Scholar]
- 2.Ajani JA, D’Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. October 2016;14(10):1286–1312. [DOI] [PubMed] [Google Scholar]
- 3.Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. International journal of cancer. Journal international du cancer. February 1 2014;134(3):622–628. [DOI] [PubMed] [Google Scholar]
- 4.Neuwirth MG, Alexander HR, Karakousis GC. Then and now: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), a historical perspective. J Gastrointest Oncol. February 2016;7(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yonemura Y, Canbay E, Endou Y, et al. Peritoneal cancer treatment. Expert Opin Pharmacother. Apr 2014;15(5):623–636. [DOI] [PubMed] [Google Scholar]
- 6.Sugarbaker PH, Stuart OA, Vidal-Jove J, Pessagno AM, DeBruijn EA. Pharmacokinetics of the peritoneal-plasma barrier after systemic mitomycin C administration. Cancer Treat Res. 1996;82:41–52. [DOI] [PubMed] [Google Scholar]
- 7.Flessner MF. The transport barrier in intraperitoneal therapy. Am J Physiol Renal Physiol. March 2005;288(3):F433–442. [DOI] [PubMed] [Google Scholar]
- 8.Bonnot PE, Piessen G, Kepenekian V, et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. August 10 2019;37(23):2028–2040. [DOI] [PubMed] [Google Scholar]
- 9.Badgwell B, Blum M, Das P, et al. Lessons learned from a phase II clinical trial of laparoscopic HIPEC for gastric cancer. Surg Endosc. Jan 2018;32(1):512. [DOI] [PubMed] [Google Scholar]
- 10.Newhook TE, Agnes A, Blum M, et al. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy is Safe for Patients with Peritoneal Metastases from Gastric Cancer and May Lead to Gastrectomy. Annals of surgical oncology. May 2019;26(5):1394–1400. [DOI] [PubMed] [Google Scholar]
- 11.Desiderio J, Chao J, Melstrom L, et al. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. July 2017;79:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badgwell B, Blum M, Das P, et al. Phase II Trial of Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Carcinomatosis or Positive Peritoneal Cytology in Patients with Gastric Adenocarcinoma. Annals of surgical oncology. October 2017;24(11):3338–3344. [DOI] [PubMed] [Google Scholar]
- 13.Blum Murphy M, Ikoma N, Wang X, et al. Phase I Trial of Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) with Cisplatin, Mitomycin, and Paclitaxel in Patients with Gastric Adenocarcinoma and Associated Carcinomatosis or Positive Cytology. Annals of surgical oncology. January 23 2020. [DOI] [PubMed] [Google Scholar]
- 14.Dormann CF, Elith J, Bacher S, et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36(1):27–46. [Google Scholar]
- 15.Hastie T, Tibshirani R, Friedman JH. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer; 2009. [Google Scholar]
- 16.Benizri EI, Bereder JM, Rahili A, Bernard JL, Benchimol D. Ascites and malnutrition are predictive factors for incomplete cytoreductive surgery for peritoneal carcinomatosis from gastric cancer. Am J Surg. Jun 2013;205(6):668–673. [DOI] [PubMed] [Google Scholar]
- 17.Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol. Jul 1 2018;36(19):1922–1929. [DOI] [PubMed] [Google Scholar]
- 18.Randle RW, Swett KR, Swords DS, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Annals of surgical oncology. May 2014;21(5):1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]