Summary
The pharmaceutical industry faces a growing demand and recurrent shortages in many anticancer plant drugs given their extensive use in human chemotherapy. Efficient alternative strategies of supply of these natural products such as bioproduction by microorganisms are needed to ensure stable and massive manufacturing. Here, we developed and optimized yeast cell factories efficiently converting tabersonine to vindoline, a precursor of the major anticancer alkaloids vinblastine and vincristine. First, fine‐tuning of heterologous gene copies restrained side metabolites synthesis towards vindoline production. Tabersonine to vindoline bioconversion was further enhanced through a rational medium optimization (pH, composition) and a sequential feeding strategy. Finally, a vindoline titre of 266 mg l−1 (88% yield) was reached in an optimized fed‐batch bioreactor. This precursor‐directed synthesis of vindoline thus paves the way towards future industrial bioproduction through the valorization of abundant tabersonine resources.
Saccharomyces was engineered for the precursor directed synthesis of vindoline by integrating the Catharanthus roseus vindoline biosynthetic genes into hotspots of the yeast genome. Fine‐tuning of genes copy numbers as well as optimization of culture conditions (medium composition, pH) improved tabersonine to vindoline conversion up to reaching a 266 mg l−1 vindoline production (88% yield).
![]()
Introduction
Vinblastine and vincristine are two major anticancer monoterpene indole alkaloids (MIAs) accumulated in the leaves of Catharanthus roseus and extensively used in human chemotherapies. Due to a constantly growing demand, both vinblastine and vincristine are suffering from recurrent shortages caused by socio‐economic (e.g. demand pressure) and/or geopolitical and climatic constraints (Rabin, 2019). Additionally, the intensive culture of C. roseus conducted for the supply of these two drugs has a dramatic negative impact on the environment (e.g. water resource, pollution…) driving to the necessity of alternative methods for more sustainable production according to the Sustainable Development Goals (SDGs) of the United Nations (https://www.un.org/sustainabledevelopment/sustainable‐development‐goals). In such a perspective, the bioproduction of MIAs in heterologous hosts constitutes an attractive option to ensure a stable supply to the pharmaceutical industry (Courdavault et al., 2020). Among all generally regarded as safe (GRAS) microorganisms, Saccharomyces cerevisiae (baker yeast) displays many advantages such as a well‐known sequenced genome, as well as numerous molecular tools for genetic manipulation and constitutes a highly valuable platform for industrial bioproduction of plant natural products (Guirimand et al., 2020). Since both vinblastine and vincristine derive from the condensation of catharanthine and vindoline, a stable supply of these two anticancer compounds could rely on the development of yeast cell factories by metabolic engineering of S. cerevisiae to produce vindoline and catharanthine as precursors. Conceptually, the vindoline synthesis can be performed either de novo (i.e. from glucose as original carbon source) or through precursor‐directed synthesis by feeding engineered yeast with tabersonine, extractible at multigram scale from the highly abundant seeds of Voacanga africana (Kikura‐Hanajiri et al., 2009; Koroch et al., 2009). In this case, seven enzymatic steps are required for the biotransformation of tabersonine (Fig. 1A; Qu et al., 2015). Although the bioproduction of vindoline by yeast cell factories has been previously reported, the synthesis remains at a scarce level and thus requires optimization to reach industrial scale (Qu et al., 2015).
Fig. 1.
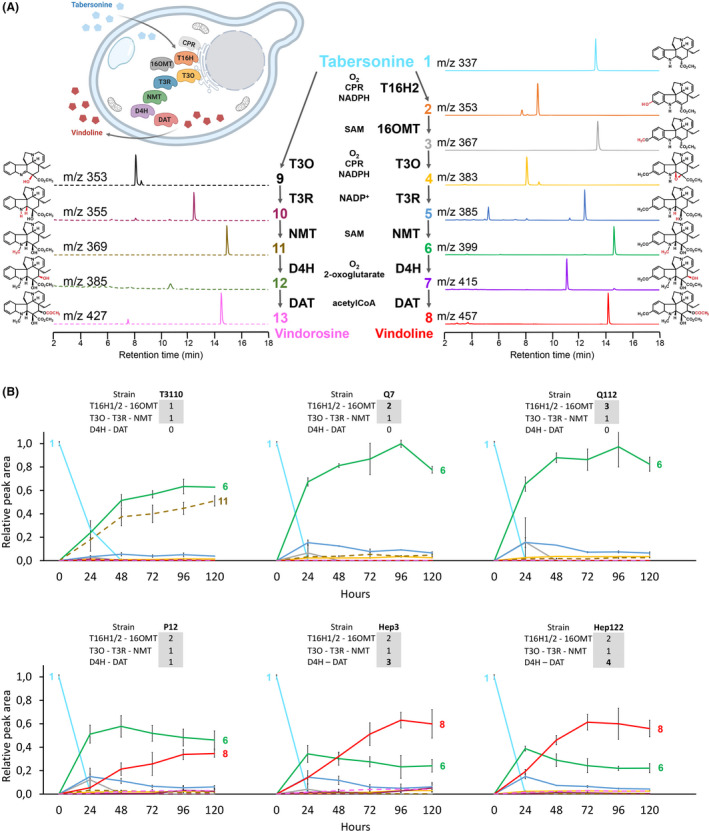
Tailoring yeast cell factories for vindoline bioproduction.
A. Vindoline biosynthetic pathway and parallel branch vindorosine pathway. Each colour and number correspond to enzyme product, represented by molecular structure, chromatogram, m/z and retention time: 1 tabersonine, 2 16‐hydroxytabersonine, 3 16‐methoxytabersonine, 4 16‐methoxytabersonine epoxide, 5 16‐methoxy‐2,3‐dihydro‐3‐hydroxytabersonine, 6 desacetoxyvindoline, 7 deacetylvindoline, 8 vindoline and the by‐products 9 tabersonine imine alcohol, 10 2,3‐dihydro‐3‐hydroxytabersonine, 11 desacetoxyvindorosine, 12 deacetylvindorosine and 13 vindorosine. MS/MS fragmentation patterns of compounds are presented in Table S1. Cofactors are indicated for each enzyme.
B. Time‐course monitoring of extracellular metabolite content in generated S. cerevisiae strains. The feedings were performed with 125 µM of tabersonine in the initial 200 µl of YPD/strain/time point. The names and the number of gene copies integrated into each strain are written on the top of each graphic. The curves represent the means of peak areas relative to tabersonine. Error bars: standard deviation (n = 3 biological replicates). By‐products are shown in dashed lines.
In the present study, the tabersonine‐to‐vindoline biosynthetic pathway from C. roseus was stably integrated within hotspots of the genome of S. cerevisiae, using CRISPR‐Cas9 molecular tools (Mikkelsen et al., 2012). The fine‐tuning of the copy numbers of the pathway genes and the optimization of the fermentation conditions allowed us to synthesize an unprecedented amount of vindoline, thus paving the way for a complementary production process of anticancer MIAs through metabolic engineering combined with the valorization of a renewable natural resource.
Results and discussion
Engineering of a vindoline‐producing yeast strain
The bioconversion of tabersonine to vindoline requires seven enzymatic steps starting with T16H hydroxylating tabersonine (1) at carbon 16 to form 16‐hydroxytabersonine (2), which is further methylated by 16OMT to 16‐methoxytabersonine (3) Downstream conversion to 16‐methoxytabersonine epoxide (4) is catalysed by T3O, which in turn is reduced to 16‐methoxy‐2,3‐dihydro‐3‐hydroxytabersonine (5) by T3R. In the following steps, the T3R product is converted to desacetoxyvindoline (6) by NMT, then hydroxylated to deacetylvindoline (7) by D4H and finally converted to vindoline (8) by DAT (Fig. 1A; Courdavault et al., 2014). However, substrate promiscuity enables T3O using tabersonine directly to form tabersonine imine alcohol (9) (Kellner et al., 2015). This marks the onset of a parallel branch pathway leading to the production of undesirable by‐products including 2,3‐dihydro‐3‐hydroxytabersonine (10), desacetoxyvindorosine (11), deacetylvindorosine (12) and vindorosine (13), whose in planta amounts depend on T16H expression level (Besseau et al., 2013). Consequently, the heterologous production of vindoline may face suboptimal flux according to the initial decoration of tabersonine by T16H or T3O.
In order to create yeast cell factories producing vindoline from tabersonine, we selected the prototrophic S. cerevisiae strain CEN.PK113‐7D, commonly used in metabolic engineering for industrial purposes. We initially implemented the five first genes of the tabersonine‐to‐vindoline biosynthetic pathway to produce desacetoxyvindoline through tabersonine transformation (strain T3110). T16H2, 16OMT, T3O, T3R, NMT coding sequences and ATR2 (required for T16H and T3O reduction) were integrated into three distinct hotspots of the S. cerevisiae genome, selected for high transcription activity (Mikkelsen et al., 2012), under strong glycolytic promoters (Table S2). To assess the production of desacetoxyvindoline, the T3110 strain was fed with 125 µM of tabersonine and grown for 120 h. Culture medium was collected every 24 h, and extracellular metabolite content was analysed by liquid chromatography coupled with mass spectrometry (LC‐MS) since the majority of alkaloids are accumulated in the extracellular medium (Qu et al., 2015; Fig. S1A). Although tabersonine was not fully consumed after 24 h, the T3110 strain produced both desacetoxyvindoline and desacetoxyvindorosine in similar proportions (Fig. 1B). This confirms that the metabolic flux is simultaneously directed towards vindoline and vindorosine pathways as a result of T16H and T3O competition for tabersonine consumption. Therefore, in order to limit the vindorosine accumulation and improve the efficiency of tabersonine bioconversion, one additional copy of both T16H and 16OMT genes was introduced into the T3110 strain. The resulting daughter strain, named Q7, showed an 11‐fold decrease of desacetoxyvindorosine accumulation, while desacetoxyvindoline drastically increased and the tabersonine was almost completely consumed after 24 h. Due to this improved metabolism and given that further increase in T16H/16OMT gene copy number did not impact desacetoxyvindoline production (Q112 strain), the Q7 strain was used as a background for the downstream integration of the final vindoline biosynthesis steps D4H and DAT. The resulting P12 strain, containing a single copy of D4H and DAT, produced 14.7 mg l‐1 of vindoline (Table S3). However, an important accumulation of desacetoxyvindoline was also observed, highlighting a D4H‐dependent bottleneck. To circumvent this lack of activity, a total of three copies of both D4H and DAT genes were introduced into the Q7 strain to generate the Hep3 strain. As a consequence, the addition of two supplemental copies of D4H and DAT increased vindoline production up to 26.8 mg l−1 after 120 h (i.e. two times higher than P12 strain) and halved the accumulation of desacetoxyvindoline. Interestingly, adding a fourth copy of both D4H and DAT genes did not improve vindoline titre (Hep122 strain), suggesting that other parameters such as culture conditions could be limiting rather than the number of gene copies. Moreover, after 72–96 h the bioconversion was reaching a plateau, which is probably due to the depletion of nutrients in the medium and an increasing competition with native metabolism for cofactors. Thus, Hep3 strain was selected for further optimization of vindoline production.
Optimization of culture conditions to enhance vindoline production
In order to evaluate the potential effect of culture medium and nutrient supply on the production of vindoline by the vindoline‐producing strain Hep3, several sources of peptone nutrients (Table S3) were assayed. Compared with the bovine peptone (BovMP) routinely used, another peptone produced from bovine tissues (BovP), a peptone obtained from a mix of bovine and porcine tissues (BovPrcP), as well as tryptone and polypeptone (PolyP), showed drastic negative effects on the activity of NMT and D4H leading to the accumulation of intermediates 5 and 6, along with the poor production of vindoline (Fig. S1B). Consequently, BovMP was selected for further optimization of culture conditions. Above all, these results demonstrate that growth media composition has an acute effect on bioproduction underlining the importance of a careful choice of peptone sources.
Similarly, the pH of the culture medium was found to drastically impact the production of vindoline, pH 6.0 being determined as optimal (Fig. S1C). As the acidification of 2 points of the culture medium pH was observed after 120 h (Table S3), the importance of maintaining a constant level of optimal pH was investigated. In parallel, to prevent any starvation‐related metabolic stress, the supply with fresh YP nutrients (yeast extract and peptone) every 24h after 48h of culture was assessed. The best vindoline production of 34.6 mg l−1‐1 was achieved by adding YP (condition YPt48h), which demonstrated an enhanced activity of D4H after 96h of culture and a 1.5‐fold increase in vindoline titre compared with standard conditions (condition BovMP) (Fig. 2A, Table S3). Interestingly, the sole addition of YP was sufficient to maintain pH close to 6.0, driving to a slightly higher vindoline production compared with additional buffering of culture medium (condition pH6YPt48h). Hence, using BovMP peptone as the best source of nutrients and supplementing the culture with YP every 24 h from 48 h post feeding was defined as the optimal conditions for vindoline production.
Fig. 2.
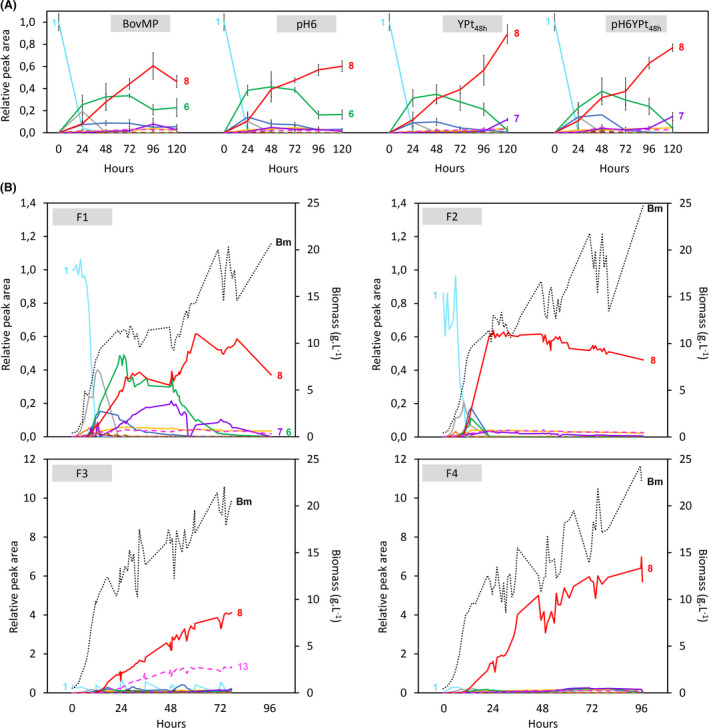
Optimization of vindoline production at small and medium scales.
A. Effect of pH and addition of fresh YP on small‐scale vindoline production. The tested condition is notified on the top of each graphic. The curves represent the means of peak areas relative to tabersonine. Error bars: standard deviation (n = 3 biological replicates). The feedings were performed with 125 µM of tabersonine in the initial 200 µl of YPD/strain/time point.
B. Effect of growth substrate and tabersonine feeding strategies on vindoline production in fed‐batch cultivation. Growth substrates are fed by pulses (F1) or continuously (F2‐F4). Tabersonine is fed by one pulse (F1, F2), seven pulses (F3) or equivalent continuous feeding (F4) (Table S4). Bm, biomass. The replicate of F4 feeding strategy is shown in Fig. S3A.
Scaling up and engineering of bioreactor feeding to improve vindoline production
In order to scale up the production of vindoline, the Hep3 strain was next cultivated in bioreactors. In control conditions, both wild‐type and Hep3 strains showed similar maximal growth rates (0.37 and 0.36 h‐1 respectively), viability (maintained up to 98%), as well as biomass and ethanol yields on glucose. This tends to show that overexpression of the vindoline pathway in the recombinant strain was well tolerated and did not constitute any utter metabolic burden for the cells. However, the addition of tabersonine into the culture medium increased the cellular respiration on oxygen, while the maximal growth rate showed a 20% decrease (Table 1). Such phenomenon, routinely observed with the addition of weak acid (Verduyn et al., 1992), reveals an uncoupling effect of tabersonine on energy and biomass productions that did not alter cell viability in all of the experiments (up to 98% in all the cultivations) (Fig. S2).
Table 1.
Main kinetic parameters measured during vindoline production in the bioreactor.
| Strategy | Maximal growth rate (h‐1) | Max concentrations ± SD | Conversion yield (% of theoretical maximal yield) (g .g‐1) | Productivity (mg. l‐1 .h‐1) | Final fraction of vindoline on end‐products (g. g‐1) | |
|---|---|---|---|---|---|---|
| Vindoline (mg. l‐1) | Vindorosine (mg. l‐1) | |||||
| F0 | 0.36 | – | – | – | – | – |
| F1 | 0.30 | 19.1 ± 0.3 | 1.4 ± 0.1 | 0.42 (31%) | 0.32 | 0.92 |
| F2 | 0.30 | 19.2 ± 0.5 | 1.5 ± 0.1 | 0.43 (32%) | 1.7 | 0.94 |
| F3 | 0.29 | 161 ± 2 | 51 ± 2 | 0.62 (45%) | 2.1 | 0.75 |
| F4 | 0.31 | 266 ± 8 | 4.7 ± 0.4 | 1.20 ± 0.01 (88%) | 2.77 ± 0.02 | 0.98 |
Growth substrates are fed by pulses (F1) or continuously (F2‐F4). Tabersonine is fed by one pulse (F1, F2), seven pulses (F3) or equivalent continuous feeding (F4) (Table S4). Standard deviation: n = 4 independent samples, considering the duplicate experiment for F4. End products are vindoline and vindorosine.
The F1 feeding experiment in the bioreactor was based on the best production strategy previously defined, relying on 24 h‐pulsated supplementations of glucose followed by YPD addition. In these conditions, the conversion yield was found to be two times lower than in small‐scale experiments (Table 1). However, the speed of production of vindoline was two times higher, with a maximal amount of vindoline produced within only 60 h, while the competing flux leading to the formation of vindorosine remained low (Fig. 2B; Fig. S2). In addition, except for the last stage of the starting pulse, a substantial decrease in vindoline cumulated mass was observed when glucose and ethanol were depleted, thus suggesting its consumption (Fig. S2). Therefore, in order to improve vindoline production, culture conditions were further optimized. For instance, glucose depletion between pulses was avoided by continuous feeding of growth substrates (F2 cultivation). Such feeding led to a 3 times faster conversion (22 h) than in the F1 reference culture, and consequently to higher productivity (Fig. 2B; Table 1). In addition, vindoline was also continuously synthesized and not degraded any longer, even 3 days after the synthesis ended (Fig. S2). Moreover, while many biosynthetic intermediates were accumulated in the F1 condition, they were only detected in minute amounts after 22 h of bioconversion in F2, as a result of an extended MIA biosynthetic enzyme activity (Fig. 2B). Above all, this suggests that an active central primary metabolism is critical to provide sufficient amounts of cofactors required by the heterologous pathway, such as acetyl‐coA and NADPH.
In the next step, we investigated the intensification of the vindoline production by numbering‐up the pulses of tabersonine (F3 cultivation). Interestingly, growth and production were maintained all over the culture, and a circa 50% increase in the conversion yield was observed compared with F2 culture (Fig. 2B; Table 1). This marked increase probably resulted from cell environment management, reducing the impact of tabersonine uncoupling effect that induces huge pH and pO2 drops. Nevertheless, the tabersonine flux towards vindorosine highly increased at the expanse of the vindoline pathway resulting in a 3:1 vindoline/vindorosine ratio (Table 1; Fig. 2B; Fig. S2). In addition, a higher accumulation of biosynthetic intermediates was also monitored during the whole experiment. To prevent the formation of undesired products and reduce heterologous pathway bottlenecks, we combined an uninterrupted active central metabolism (continuous glucose feeding under the Crabtree effect’s critical uptake rate, avoiding ethanol production) with a restrained consumption rate of tabersonine (Fig. S2). Accordingly, we limited the specific uptake rate of tabersonine under its maximal value (1.03 ± 0.04 mg g‐1 h‐1) through a continuous feeding in the F4 cultivation (Fig. S2; Fig. S3B). This strategy prevented tabersonine accumulation in the medium after 10 h and led to a 88% tabersonine bioconversion into vindoline, which was equivalently replicated (Fig. S3A). As a consequence, very low levels of vindorosine and vindoline biosynthetic intermediates were observed. (Table 1; Fig. 2B; Fig. S2; Fig. S3A). Thus, the control of central and heterologous metabolic fluxes by rational process design appeared as a relevant strategy to further improve vindoline production. In these conditions, vindoline reached a final titre of 266 mg l‐1 after 96 h of culture, and thus, 20‐fold higher productivity than the original biosynthesis reported by Qu et al. (2015) with an episomal strain. To date, it constitutes the highest vindoline production ever reported, in terms of yield, titre and productivity.
Conclusions
Through a fine‐tuned integration of heterologous biosynthetic genes combined with the optimization of culture conditions, we generated a yeast strain synthesizing high amounts of vindoline via the bioconversion of tabersonine (88% yield). Based on the quantity of tabersonine accumulated in the seeds of V. Africana and annual exportation of 1600 tons of seeds, the total production of 22.5 tons of vindoline can be expected using this newly developed yeast strain (Kikura‐Hanajiri et al., 2009; Koroch et al., 2009). This advance thus opens new perspectives towards the development of a potential alternative supply of vindoline to avoid vinblastine and vincristine shortage in the future.
Funding information
We acknowledge funding from the ARD2020 Biopharmaceutical program of the Région Centre Val de Loire (BioPROPHARM, CatharSIS and ETOPOCentre projects), Le Studium Institute for a Research Consortium Grant and the EU Horizon 2020 research and innovation program (MIAMi project‐grantagreement N◦814645).
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author contribution
NK, GG, AO and JM contributed to conceptualization, methodology, investigation, data curation, writing – original draft and writing – review and editing. CM, PLC, IC and NP contributed to investigation and writing – review and editing. JDC VH, KK, MU and AL contributed to investigation. NI, BStP, MC and NGG contributed to formal analysis and investigation. SB and VC contributed to supervision, conceptualization, methodology, investigation, data curation, writing – original draft and writing – review and editing.
Supporting information
Fig. S1. (A) Extracellular and intracellular metabolite content in T3110 and Hep3 small‐scale cultures 120 h post feeding. Supernatants (referred to as extracellular) were prepared as described in Experimental procedures of Appendix S1. Pellets were washed twice in 500 µl of saline solution (50 mM Tris, 100 mM NaCl), resuspended in 200 µl of MeOH, lysed by 10 min sonication, and the supernatants (referred to as intracellular) were further prepared as extracellular supernatants. The histograms represent the means of peak areas. Error bars: standard deviation (n = 3 biological replicates). (B) The effect of peptone on vindoline production and intermediate accumulation in Hep3 small‐scale cultures. Hep3 feedings were set up in YPD media prepared with different peptones (Table S3). The feedings were performed with 125 µM of tabersonine in the initial 200 µl of YPD/strain/time point. The curves represent the means of peak areas relative to tabersonine. Error bars: standard deviation (n = 3 biological replicates). (C) The effect of pH on extracellular metabolite content in Hep3 small‐scale cultures. Hep3 feedings with tabersonine were set up in YPD medium buffered to pH 4, pH 5 or pH 6. The curves represent the means of peak areas relative to tabersonine. Error bars: standard deviation (n = 3 biological replicates). The feedings were performed with 125 µM of tabersonine in the initial 200 µL of YPD/strain/time point.
Fig. S2. Evolution in bioreactors of (A) cumulated masses of tabersonine, vindoline, vindorosine and fraction of vindoline on end products, and evolution of (B) cumulated masses of glucose, biomass, ethanol and viability. Consumed tabersonine (dark square), vindoline (red diamond), vindorosine (pink triangle), fraction (yellow circle), consumed glucose (blue square), biomass (brown triangle), ethanol (green diamond) and viability (brown circle). F4 duplicate is in dotted lines and empty symbols. Growth substrates are fed by pulses (F1) or continuously (F2‐F4). Tabersonine is fed by one pulse (F1, F2), seven pulses (F3) or equivalent continuous feeding (F4) (Table S4).
Fig. S3. (A) Replicate of the feeding strategy F4 presented in the Fig. 2B. Fresh medium and tabersonine are fed continuously (Table S4). (B) Evolution of the specific uptake rate of tabersonine in F4 bioreactor. Specific uptake rate (square), residual tabersonine (triangle) and biomass (circle). The replicate is in dotted line and empty symbols. The horizontal dotted line represents the maximal rate. Tabersonine is fed continuously by constant continuous feeding (Table S4).
Table S1. LC‐MS/MS characterization of tabersonine and vindoline intermediates obtained with engineered yeasts. The fragmentation was performed on molecular ions [M + H]+ using cone voltage of 30 V and collision energies of 30 eV.
Table S2. Expression cassettes integrated in yeast hotspots.
Table S3. pH measurement and vindoline quantification in small‐scale cultures.
Table S4. Strategies for bioreactor process optimization.
Table S5. List of primers used in the study.
Acknowledgements
We acknowledge funding from the ARD2020 Biopharmaceutical program of the Région Centre Val de Loire (BioPROPHARM, CatharSIS and ETOPOCentre projects), Le Studium Institute for a Research Consortium Grant and the EU Horizon 2020 research and innovation program (MIAMi project‐grantagreement N◦814645).
Microb. Biotechnol. (2021) 14(6), 2693–2699
Contributor Information
Sébastien Besseau, Email: sebastien.besseau@univ-tours.fr.
Vincent Courdavault, Email: vincent.courdavault@univ-tours.fr.
References
- Besseau, S. , Kellner, F. , Lanoue, A. , Thamm, A.M.K. , Salim, V. , Schneider, B. , et al. (2013) A pair of tabersonine 16‐hydroxylases initiates the synthesis of vindoline in an organ‐dependent manner in Catharanthus roseus . Plant Physiol 163: 1792–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courdavault, V. , O’Connor, S.E. , Oudin, A. , Besseau, S. , and Papon, N. (2020) Towards the microbial production of plant‐derived anticancer drugs. Trends in Cancer 6: 444–448. [DOI] [PubMed] [Google Scholar]
- Courdavault, V. , Papon, N. , Clastre, M. , Giglioli‐Guivarc’h, N. , St‐Pierre, B. , and Burlat, V. (2014) A look inside an alkaloid multisite plant: the Catharanthus logistics . Curr Opin Plant Biol 19: 43–50. [DOI] [PubMed] [Google Scholar]
- Guirimand, G. , Kulagina, N. , Papon, N. , Hasunuma, T. , and Courdavault, V. (2020) Innovative tools and strategies for optimizing yeast cell factories. Trends Biotechnol 39: 488–504. [DOI] [PubMed] [Google Scholar]
- Kellner, F. , Geu‐Flores, F. , Sherden, N.H. , Brown, S. , Foureau, E. , Courdavault, V. , and O’Connor, S.E. (2015) Discovery of a P450‐catalyzed step in vindoline biosynthesis: a link between the aspidosperma and eburnamine alkaloids. Chem Commun 51: 7626–7628. [DOI] [PubMed] [Google Scholar]
- Kikura‐Hanajiri, R. , Maruyama, T. , Miyashita, A. , and Goda, Y. (2009) Chemical and DNA analyses for the products of a psychoactive plant, Voacanga Africana. Yakugaku Zasshi 129: 975–982. [DOI] [PubMed] [Google Scholar]
- Koroch, A.R. , Juliani, H.R. , Kulakowski, D. , Arthur, H. , Asante‐Dartey, J. , and Simon, J.E. (2009) Voacanga africana: chemistry, quality and pharmacological activity. ACS Symp Ser 1021: 363–380. [Google Scholar]
- Mikkelsen, M.D. , Buron, L.D. , Salomonsen, B. , Olsen, C.E. , Hansen, B.G. , Mortensen, U.H. , and Halkier, B.A. (2012) Microbial production of indolylglucosinolate through engineering of a multi‐gene pathway in a versatile yeast expression platform. Metab Eng 14: 104–111. [DOI] [PubMed] [Google Scholar]
- Qu, Y. , Easson, M.L.A.E. , Froese, J. , Simionescu, R. , Hudlicky, T. , and DeLuca, V. (2015) Completion of the seven‐step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc Natl Acad Sci USA 112: 6224–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin, R.C. (2019) Faced With a Drug Shortfall, Doctors Scramble to Treat Children With Cancer. New York Times. URL https://www.nytimes.com/2019/10/14/health/cancer‐drug‐shortage.html. [Google Scholar]
- Sustainable Development Goals of The United Nations . URL https://www.un.org/sustainabledevelopment/sustainable‐development‐goals/.
- Verduyn, C. , Postma, E. , Scheffers, W.A. , and Van Dijken, J.P. (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous‐culture study on the regulation of respiration and alcoholic fermentation. Yeast 8: 501–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. (A) Extracellular and intracellular metabolite content in T3110 and Hep3 small‐scale cultures 120 h post feeding. Supernatants (referred to as extracellular) were prepared as described in Experimental procedures of Appendix S1. Pellets were washed twice in 500 µl of saline solution (50 mM Tris, 100 mM NaCl), resuspended in 200 µl of MeOH, lysed by 10 min sonication, and the supernatants (referred to as intracellular) were further prepared as extracellular supernatants. The histograms represent the means of peak areas. Error bars: standard deviation (n = 3 biological replicates). (B) The effect of peptone on vindoline production and intermediate accumulation in Hep3 small‐scale cultures. Hep3 feedings were set up in YPD media prepared with different peptones (Table S3). The feedings were performed with 125 µM of tabersonine in the initial 200 µl of YPD/strain/time point. The curves represent the means of peak areas relative to tabersonine. Error bars: standard deviation (n = 3 biological replicates). (C) The effect of pH on extracellular metabolite content in Hep3 small‐scale cultures. Hep3 feedings with tabersonine were set up in YPD medium buffered to pH 4, pH 5 or pH 6. The curves represent the means of peak areas relative to tabersonine. Error bars: standard deviation (n = 3 biological replicates). The feedings were performed with 125 µM of tabersonine in the initial 200 µL of YPD/strain/time point.
Fig. S2. Evolution in bioreactors of (A) cumulated masses of tabersonine, vindoline, vindorosine and fraction of vindoline on end products, and evolution of (B) cumulated masses of glucose, biomass, ethanol and viability. Consumed tabersonine (dark square), vindoline (red diamond), vindorosine (pink triangle), fraction (yellow circle), consumed glucose (blue square), biomass (brown triangle), ethanol (green diamond) and viability (brown circle). F4 duplicate is in dotted lines and empty symbols. Growth substrates are fed by pulses (F1) or continuously (F2‐F4). Tabersonine is fed by one pulse (F1, F2), seven pulses (F3) or equivalent continuous feeding (F4) (Table S4).
Fig. S3. (A) Replicate of the feeding strategy F4 presented in the Fig. 2B. Fresh medium and tabersonine are fed continuously (Table S4). (B) Evolution of the specific uptake rate of tabersonine in F4 bioreactor. Specific uptake rate (square), residual tabersonine (triangle) and biomass (circle). The replicate is in dotted line and empty symbols. The horizontal dotted line represents the maximal rate. Tabersonine is fed continuously by constant continuous feeding (Table S4).
Table S1. LC‐MS/MS characterization of tabersonine and vindoline intermediates obtained with engineered yeasts. The fragmentation was performed on molecular ions [M + H]+ using cone voltage of 30 V and collision energies of 30 eV.
Table S2. Expression cassettes integrated in yeast hotspots.
Table S3. pH measurement and vindoline quantification in small‐scale cultures.
Table S4. Strategies for bioreactor process optimization.
Table S5. List of primers used in the study.


