Abstract
Introduction:
The extracellular matrix (ECM) is vital for cell and tissue development. Given its importance, extensive work has been conducted to develop biomaterials and drug delivery vehicles that capture features of ECM structure and function.
Areas covered:
This review highlights recent developments of ECM-inspired nanocarriers and their exploration for drug and gene delivery applications. Nanocarriers that are inspired by or created from primary components of ECM (e.g., elastin, collagen, hyaluronic acid, or combinations of these) are explicitly covered. An update on current clinical trials employing elastin-like proteins is also included.
Expert opinion:
Novel ECM-inspired nanoscale structures and conjugates continue to be of great interest in the materials science and bioengineering communities. Hyaluronic acid nanocarrier systems in particular are widely employed due to the functional activity of HA in mediating a large number of disease states. In contrast, collagen-like peptide nanocarriers are an emerging drug delivery design with potential relevance to a myriad of ECM-related diseases, making their continued study most pertinent. Elastin-like peptide nanocarriers have a well-established tolerability and efficacy track record in preclinical analyses that has motivated their recent advancement into the clinical arena.
Keywords: Arthritis, cancer, collagen-like peptides, drug delivery, elastin-like peptides, extracellular matrix, gene delivery, hyaluronic acid, nanocarriers, osteoarthritis, rheumatoid arthritis, wound healing
1. Introduction
The mesh-like network of macromolecules that broadly forms the non-cellular component of tissues in both vertebrates and invertebrates is known as the extracellular matrix (ECM) [1,2]. The ECM is widely regarded as essential for metazoan life and its importance is demonstrated both by the mutations in ECM genes that cause embryonic death [1], and by the high degree of conservation of certain proteins (e.g., fibrillar collagens) from simple sponges up to complex vertebrates [2,3]. Although it is commonly described as a singular entity, the quantity and composition of the ECM, or ECMs, can vary greatly from tissue to tissue [4]. However, generalizations can be made, and the core matrisome (~300 proteins in mammals) consists mainly of fibrous structural proteins including elastin, collagen, and laminin; glycosaminoglycans (GAGs), such as hyaluronic acid (HA); proteoglycans, like aggrecan; and glycoproteins, such as fibronectin (Figure 1) [4–7]. These macromolecular components have long been recognized for their integral role in imbuing connective tissues with stability, elasticity, and resistivity in response to mechanical stress/relaxation [4]. However, the structural role of the ECM is arguably matched by its more dynamic and active role of maintaining cell adhesion, proliferation, differentiation, migration, apoptosis, and overall tissue homeostasis [1,4]. Cells perform some of these functions by receiving signaling cues through their interactions with the matrix via cell-bound receptors such as integrins, or, for instance, the HA receptor CD44 [8]. Additionally, the ECM is constantly remodeled by matrix-embedded cells, via the secretion of proteases, chemokines, growth factors, and cytokines, which are dynamically deposited or liberated, and in turn, allow cells to react to their environment and modify it as needed (see ECM proteases in Figure 1) [1,7–9]. It is through these mechanisms that homeostasis is regulated in an exquisitely controlled manner [1]. Moreover, it is through the dysregulation of these ECM structures and functions that pathologies arise and cause a myriad of diseases, defects, and abnormalities [1,7,10].
Figure 1:
Schematic representation of specific macromolecular ECM components and their interaction and proximity to cells. Cellular functions in the ECM are also highlighted numerically. Proteins such as fibrillar collagen, elastin, and ECM proteases are dipicted alongside proteoglycans and the GAG, HA. Of pertinent interest to this text, the collagen higher order assembly and foundational triple helical structure is illustrated.Note that although this illustration is that of arterial extracellular space, the ECM components and functions are the same as the ECM found in other tissues. Reprinted from Journal of the American College of Cardiology, 75(17), Barallobre-Barreiro, J., et al. Extracellular Matrix in Vascular Disease, Part 2/4: JACC Focus Seminar, 2189–203, Copyright (2020), with permission from Elsevier.
In humans, such diseases include but are not limited to: osteoarthritis, Marfan syndrome, Ehlers-Danlos syndrome, osteogenesis imperfecta, and Alport syndrome [7,11,12]. Current knowledge of the etiologies for most of these diseases has primarily been centered around understanding of aberrations in the collagen protein family [11], with well over 1000 mutations being characterized for just 12 of the 28 collagen sub-types [13]. However, other ECM-related diseases can attribute at least part of their etiology to the upregulation or downregulation of cytokines, proteases, or protease inhibitors within the ECM microenvironment [14,15]. For instance, the upregulation of matrix metalloproteinases (MMPs) is implicated in tumor invasion and metastasis in cancer [15]. Likewise, osteoarthritis (OA) and rheumatoid arthritis (RA) are also characterized by the upregulation of MMPs that degrade ECM collagens, most notably in joint tissues [16,17]. Similarly, MMPs are dysregulated during the extended proinflammatory phase that is often present in chronic wounds, a phenomenon which ultimately prolongs/halts the healing process [18]. Conversely, increased levels of tissue inhibitors of metalloproteinases (TIMPs) in the ECM can lead to increased fibrosis and scarring during the wound healing cascade [14,19].
Regardless of whether a disease is directly related to alterations in specific ECM structural proteins (e.g., osteogenesis imperfecta) or merely a secondary effect of other cellular aspects of a given pathology (e.g., OA), it is evident that the ECM and its properties are highly correlated with, and affected by, disease. Thus, drug delivery approaches that target the ECM and/or modulate its dynamic bioactive processes have been at the forefront of disease therapies. Rationally, such approaches employ biomaterials that are made of or mimic ECM components to achieve this targeting or functional modulation. The study and development of ECM-inspired biomaterials includes investigations of synthetic hydrogels, animal-derived decellularized matrices, and ECM-based therapeutic nanocarriers that are either composed of or modulated with ECM macromolecules/moieties [6,20].
It is the latter of these that is the focus of this review. Nanocarriers loaded with drug or gene therapeutics are attractive in drug delivery owing to their ability to increase drug bioavailability and/or protect the drug from premature degradation [21]. Additionally, the surfaces of nanocarriers are frequently modified with stealth or bioactive ligands to further prolong systemic circulation and actively target specific tissues or disease targets, respectively [21,22]. Herein, we highlight and describe recent advances and developments in the field of ECM-based nanocarriers for drug and gene delivery. Systems that are based on elastin, collagen, hyaluronic acid, and combinations of these molecules are the focus of the review due to their significant clinical potential, as well as their diverse functions for enhancing drug delivery.
2. Elastin-like polypeptides (ELPs)
2.1. Introduction to ELPs
Elastin is a highly hydrophobic ECM protein that contains a substantial number of non-polar residues such as valine and proline [4,23]. These residues usually appear in the form of hydrophobic domains in elastin, often within sequence-specific consecutive repeats such as the galline dodecapentapeptide, (PGVGV)12 [23]. Similar sequences are also found in the elastin precursor molecule, tropoelastin, which ultimately self-assembles and crosslinks into the elastic fibers that provide the ECM with the ability to recoil from transient stretching [4]. The self-assembly of tropoelastin in vivo derives largely from a phase separation process known as coacervation, although other ECM proteins likely play a role in this natural self-assembly process [23].
Some of the earliest ELPs studied were crosslinked synthetic polymers of the common tropoelastin residue repeat motifs such as poly(VPGVG) [24], which were produced to better understand the thermodynamics of the self-assembly/coacervation phenomena of tropoelastin, and also evaluate tropoelastin’s structure [24]. With the advent of modern molecular biology techniques, specific sequences of ELPs with precise chain lengths could be easily synthesized with well-defined chemical and thermodynamic properties [25]. These recombinant ELPs typically comprise a generalized pentapeptide repeat (VPGXAAG)n, where XAA can be any amino acid with the exception of proline, and n can range from tens to hundreds of repeats [25]. Both chemically synthesized polymeric ELPs and recombinantly engineered ELPs exhibit a hallmark thermoresponsive phase separation that is characterized by an inverse transition temperature (Tt) phenomenon [24,25]. In aqueous media with a temperature below that of the Tt, ELPs exist in a solubilized state. However, at temperatures above the Tt, the ELP solutions undergo a coacervation transition, yielding ELP-rich and ELP-poor phases [25]. The value of the Tt can depend on a number of factors including guest residue hydrophobicity, concentration, and ions present in the solution, among many other factors [26]. In general, increased hydrophobicity, increased ELP concentration, and increased ion concentration will all decrease the Tt, and conversely, decreases in these variables lead to increased Tt values [26]. ELPs have been widely used in protein and nanoparticle purification techniques by increasing temperatures above the Tt as a mechanism to precipitate proteins or nanoparticles that are fused or bound with ELPs [25,27]. Exploitation of this temperature responsive coacervation has also been widely used to produce self-assembled structures deployed as novel drug delivery vehicles. Hydrophobic ELPs are generally modified with a hydrophilic moiety (typically in the form of a recombinant protein fusion) to yield amphiphilic molecules that self-assemble above the Tt of the hydrophobic ELP domain, typically into micelles that can either be spherical or cylindrical in morphology [28]. Such structures have been investigated widely by a number of groups as nanocarriers for small-molecule therapeutics or biologics [28].
2.2. ELP micelles
Micelles are just one of the many types of nanocarriers that have commonly been studied in modern drug delivery strategies. Generally, micelles are self-assembled aggregates that are comprised of amphiphilic molecules such as lipids or synthetic polymers, as well as appropriately designed ELPs. The most common strategy for making ELP-based micelles is to increase the hydrophobicity of the guest residue of the common (VPGXAAG)n repeat in one portion of the ELP (e.g., toward the N-terminus), while encoding more hydrophilic guest residues in the other portion of the ELP (e.g., toward the C-terminus), to yield a single amphiphilic molecule (i.e., diblock-ELP polymers). Figure 2 illustrates schematic representations of such ELP micelles as individual molecules of diblock-ELPs (Figure 2a) and self-assembled micelles (Figure 2, a and b) [29,30]. Each block of the diblock-ELP has its own Tt, and the micelle self-assembly process typically requires the hydrophobic block of the diblock-ELP to selectively collapse and self-aggregate via heating to temperatures above the Tt of the hydrophobic block while still maintaining a temperature that is less than the Tt of the hydrophilic block [31], so that the hydrophobic block forms the core and the hydrophilic block forms the corona of the micelle. The lowest Tt value at which micellization occurs is known as the critical micelle temperature (CMT). [31].
Figure 2:
Schematic representation of ELP micelles. a) The amphiphilic diblock-ELP (VPGVG)40-(AGVPGGGVPG)30 with a proapoptotic fusion on the N-terminus and a cell penetrating peptide (CPP) fusion on the C-terminus is shown both in its dissassembled monomeric state below the CMT and in its assembled micllar state above the CMT (i.e., the Tt of the hydrophobic (VPGVG)40 block). b) The self-assembled form of two separate (but very similar) amphiphilic diblock-ELPs. The diblock-ELP (VPGIG)48-(VPGSG)48, bears a cell binding RGD motif that is fused onto the C-terminus and the diblock-ELP, (VPGSG)48-(VPGIG)48, bears the rapamycin binding motif (FKBP12, depicted with bound rapamycin) on the N-terminus. c) A chimeric polypeptide ELP micelle that consists of a albumin binding domain on the N-terminus, an ELP monoblock (VPGAG)160 in the middle of the polypeptide, and a glycine-glycine-cysteine repeat (8 repeats) on the C-terminus. Figure 2a) was reproduced with permission from MacEwan SR, Chilkoti A. Controlled Apoptosis by a Thermally Toggled Nanoscale Amplifier of Cellular Uptake. Nano Letters. 2014 Apr;14(4):2058–2064, https://pubs.acs.org/doi/abs/10.1021/nl5002313. Further permissions/reuse related to this figure/material should be directed to the ACS. Figure 2b) was reprinted (adapted) with permission from (Peddi S, Roberts SK, MacKay JA. Nanotoxicology of an Elastin-like Polypeptide Rapamycin Formulation for Breast Cancer. Biomacromolecules. 2020 Mar;21(3):1091–1102). Copyright (2020) American Chemical Society. Figure 2c) was reprinted (adapted) with permission from (Yousefpour P, McDaniel JR, Prasad V, et al. Genetically Encoding Albumin Binding into Chemotherapeutic-loaded Polypeptide Nanoparticles Enhances Their Antitumor Efficacy. Nano Letters. 2018 Dec;18(12):7784–7793). Copyright (2018) American Chemical Society.
Since these diblock-ELPs can be made recombinantly, they can be appended with functional and/or therapeutic proteins or peptides at either the N-terminus or the C-terminus (or both) depending on the desired application (Figure 2, a and b) [29,30]. For instance, MacEwan et al. engineered a library of recombinant, diblock-ELPs bearing a proapoptotic peptide on the N-terminus, and a cell penetrating peptide (CPP) on the C-terminus, with the diblock-ELP (VGVPG)40-(AGVPGGGVPG)30 fused in between the two (Figure 2a) [29]. Heating the diblock-ELP above the Tt/CMT of the hydrophobic (VGVPG)40 block (between 38°C and 41°C) resulted in micellization with the proapoptotic peptide sequestered in the core and the CPP presented on the surface of the micelle’s hydrophilic ELP corona ( the (AGVPGGGVPG)30 block (Tt > 41°C), Figure 2a) [29]. The CMT was designed to be greater than physiological temperature (37°C) so that the diblock-ELP would form micelles in vivo only after application of an external hyperthermal stimulus at a tumor site, resulting in CPP clustering and selective intracellular uptake in cancer cells [29]. Indeed, enhanced cell uptake and apoptosis was observed in vitro for the proapoptotic peptide-fused diblock-ELP at 42°C compared to 37°C.
In a more recent example, Peddi et al. also combined two active components in a diblock-ELP micelle system [30]. An RGD sequence (to mediate cell adhesion through integrin binding) was fused to the C-terminus of the diblock-ELP (VPGIG)48-(VPGSG)48 (termed ISR), and a rapamycin-binding motif (FKBP12) was fused to the N-terminus of a separate but similar diblock-ELP (VPGSG)48-(VPGIG)48 (termed FSI). These two ELPs with two different bioactive functionalities were mixed together resulting in co-assembly into a multivalent ELP micelle containing both RGD and FKBP12 motifs on the surface of the corona (Figure 2b) [30]. The authors found that rapamycin (an antiproliferative drug) could be bound to the FKBP12 corona motif and so termed the drug laden ELP micelle formulation ISR-FSI-Rapa. These ISR-FSI-Rapa micelles were found to be capable of inhibiting breast cancer cell proliferation in a concentration dependent manner in vitro. The authors went on to perform a multiday treatment of breast cancer xenografted mice with ISR-FSI-Rapa or a phosphate buffered saline (PBS) control. The effectiveness of the treatment was assessed by monitoring and measuring the apparent tumor volume (for both the treatment and control groups) over the course of one month. Tumor volumes of mice that were treated with the ISR-FSI-Rapa formulation did not increase substantially relative to the PBS control, with the final measurement of the tumor volumes being statistically significant by the end of the study (Figure 3, a and b, respectively). Additionally, the body weight of the mice in both the treatment and the control groups did not change significantly over the course of the study indicating that the treatment was well tolerated by the mice (Figure 3c). To determine the functionality and effect of ISR-FSI-Rapa treatment, western blotting analysis of excised tumors was performed. Specifically, western blotting detection of the substrate S6 ribosomal protein (S6RP) was performed relative to a GAPDH loading control. S6RP is a phosphorylated product of a S6K1 kinase. The phosphorylation of S6K1 to S6RP is mediated by the protein complex mTORC1, which is sensitive to rapamycin. The lack of detected S6RP in the ISR-FSI-Rapa treatment groups indicated that the delivered rapamycin was active (Figure 3d) and capable of limiting tumor growth and development [30].
Figure 3:
Multi-day treatment of BT-474 xenografted mice with rapamycin-RGD-diblock-ELP micelles (1 mg/kg rapamycin per dose). a) Tumor volume of phosphate buffered saline (PBS) control treated and rapamycin-RGD-diblock-ELP treated mice which indicates the delivered rapamycin was succssful in limiting tumor growth. b) Comparison of tumor volumes between the PBS treatment and the rapamycin-ELP micelle treatment on the last day of the treatment/study (from a)). c) Mean body weight change of the mice over the course of the study indicating the treatment was well tolerated. d) Western blotting detection of substrate S6 ribosomal protein (S6RP) in both the rapamycin-ELP micelle treatment group as well as the PBS control group. The correlation of the loss of S6RP with rapamycin delivery indicates the drugs effectiveness in its antiproliferative cell signal transduction pathway. Reprinted (adapted) with permission from (Peddi S, Roberts SK, MacKay JA. Nanotoxicology of an Elastin-like Polypeptide Rapamycin Formulation for Breast Cancer. Biomacromolecules. 2020 Mar;21(3):1091–1102). Copyright (2020) American Chemical Society.
This work by Peddi et al. exemplifies the utilization of a dually functional diblock-ELP nanocarrier that is capable of cell adhesion and drug-specific loading. Additional diblock-ELP nanocarriers with unique, intrinsic functionalities are currently being pursued as new nanomedicines [28,32]. The Van Hest group recently described diblock-ELP nanocarriers that were capable of assembling and disassembling via either a pH stimulus or a temperature stimulus [33]. A diblock-ELP with the sequence of (VPGXAAG)60-(VPGYAAG)60, where XAA is either I or H in a ratio of 1:4, and YAA is either A or G in a ratio of 3:2, was capable of assembling into micelles triggered by an increase of pH and addition of metal ions; conversely, this micelle reversibly disassembled by decreasing the pH and metal ion content. The Van Hest group also evaluated mixtures of the above diblock ELP with a similar diblock-ELP that was identical except that XAA was only I (with no H content). Heating the mixed diblock-ELPs stimulated micellization above the Tt of the I60 containing diblock-ELP [33]. Although the work did not demonstrate the utilization of these diblock-ELPs for drug delivery, it highlighted the versatile ability of diblock-ELPs to respond in a stimuli-responsive manner to either endogenous cues (e.g., pH) or exogenous cues (e.g., temperature). Such multifunctional (or in this case multi-stimuli-responsive) ELP nanoconstructs offer great promise as next generation ELP nanocarriers.
In additional examples, Gonzalez-Valdivieso et al. developed a trifunctional diblock-ELP micelle system that was capable of lysosomal escape, lysosomal-mediated cleavage of part of the diblock-ELP, and cellular apoptosis [34]. The motifs that enabled these functions were included within a single diblock-ELP construct with the amino acid sequence LAEL (for lysosomal/endosomal escape) near the N-terminus, followed by the amphiphilic diblock-ELP, [(VPGVG)2(VPGEG) (VPGVG)2]10-[VGIPG]60, a cathepsin D (lysosomal protease) cleavage site, a second, histidine-rich lysosomal escape sequence, and finally, an Akt kinase inhibitor near the C-terminus. Both of the lysosomal escape mechanisms were enabled by structural changes in the micelles that occurred at the lower pH of the lysosomal compartment. Two lysosomal escape mechanisms ensured that the kinase inhibitor could efficiently access the cytosol where Akt kinase is typically overexpressed [34]. With this construct, the authors demonstrated that the inclusion of the lysosomal escape mechanisms and lysosomal enzymatic cleavage were critical in inducing cell death in cancerous cells where Akt kinase is overexpressed, but not in healthy non-cancerous cells [34].
Other diblock-ELP micelle systems have been developed for cancer treatment. Pille et al. devised a diblock-ELP comprised of a hydrophilic block with the sequence (VPGAG)2(VPGGG)2[(VPGAG)3(VPGGG)2]11 and a hydrophobic block with the sequence (VPGIG)60 [35]. A fraction of these diblock-ELPs were fused with a heavy chain antibody fragment (in this case, 7D12) specific to the epidermal growth factor receptor (EGFR) that is overexpressed in many cancerous sub-types, including non-small cell lung cancer, inflammatory breast cancer, brain cancer, and ovarian cancer [36–38]. A photosensitizer molecule that can induce cell death upon illumination with light was also conjugated to a fraction of the diblock-ELPs. Micelles were formed by mixing the functionalized and nonfunctionalized diblock-ELPs at various ratios and then heating the mixture to 37°C. By adjusting the mixture ratio of functionalized vs. nonfunctionalized diblock-ELPs, the authors showed that the maximum possible 7D12 functional incorporation was ~50–60%, as additional 7D12 incorporation into the mixed micelle system led to the formation of larger indiscrete aggregates. The authors demonstrated that the ELP micelles actively targeted epidermoid carcinoma cancer cells and elicited light-triggered cell death via delivery of the photosensitizer drug [35]. In related work, Costa et al. devised a diblock-ELP (VPGVG)80-(VPGSG)60 that incorporated an unnatural amino acid (p-acetylphenylalanine) that was utilized for the biorthogonal conjugation of the chemotherapeutic drug doxorubicin (DOX) to the N-terminus, thereby enabling loading of DOX into the core of the assembled micelle [39]. Additionally, a EgA1 nanobody (an antibody fragment that was also capable of binding to EGFR) was fused to the C-terminus of the diblock-ELP micelle. The drug laden diblock-ELP with the EgA1 nanobody outperformed its non-targeting counterpart in inducing death in epidermoid carcinoma cells [39].
Another way to fabricate ELP micelles is to recombinantly engineer relatively hydrophilic ELP monoblocks (ELPs with a single Tt) that are either (a) capable of being chemically conjugated with a hydrophobic small-molecule drug or (b) fused with a hydrophobic protein. In these instances, the micellization process is driven by the hydrophobicity of the therapeutic molecule or fused protein, rather than by the ELP, which in these materials serves as the hydrophilic solvated micellar corona [31]. The drug-mediated micellization of ELPs was first reported by the Chilkoti research group in 2009 [40]. These ‘chimeric polypeptides’ (CPs) typically employ a cysteine-rich conjugation domain for anchoring drugs (typically chemotherapeutics), and since their initial report, CPs have been thoroughly investigated as novel chemotherapeutic nanocarriers. An interesting and unique example of a functional CP was shown by Yousefpour, et al., in which an albumin-binding domain was fused to the N-terminus of the monoblock ELP (VPGAG)160 that also bore the cysteine-rich sequence (GGC)8 on its C-terminus. Upon conjugation with DOX, micellization spontaneously occurred such that the N-terminal albumin-binding domain (ABD) was on the corona surface, while the conjugated DOX comprised the core (Figure 2c). The purpose of the ABD was to bind to albumin, such that opsonization and complement activation would be limited after systemic administration of the ABD CPs [41]. As demonstrated through in vivo experimentation with a murine colon carcinoma model, these ABD CPs were found to have less non-specific uptake from clearance organs (such as the liver, spleen, and kidneys), and they also provided a wider therapeutic window for DOX (relative to CPs without the ABD). These benefits translated to lower required doses of the drug to achieve a reduction in tumor volume as well as increased survival [41].
Other ELP-based nanocarrier systems have also used the conjugation of hydrophobic chemotherapeutic drugs for inducing micelle formation. Bhattacharyya and colleagues used the sequence SKGPG-(XGVPG)160-WPC(GGC)7 (in which the guest residue X was V:G:A in a 1:7:8 ratio) and conjugated paclitaxel to this ELP monoblock [42]. This CP nanocarrier was found to have twice the tumor uptake relative to Abraxane, an FDA-approved chemotherapeutic nanocarrier that also contains paclitaxel. Moreover, the paclitaxel CP nanocarrier significantly reduced tumor volume in murine models for both breast and prostate cancer [42]. The same CP ELP nanocarrier has also been investigated for delivery of DOX, with potential for treating poorly immunogenic 4T1 mammary murine carcinoma both via the cytotoxic action of the drug and through the corresponding stimulation of infiltrating leukocytes into the tumor to enhance the immune response [43]. In a separate report, this DOX CP formulation was also found to have a similar effect in a soft tissue sarcoma murine model derived from a malignant peripheral nerve sheath tumor (MPNST) [44]. In both of these reports, the CPs were observed to stimulate a CD8+ immune response; however, it was unclear whether the CD8+ immune response was required for the DOX CP to be efficacious given that pretreatment in the MPNST model with anti-CD8+ antibodies did not affect the efficacy of the DOX CP treatment [43,44]. Nonetheless, these works demonstrate the potential of hydrophobic drug induced micellization of ELP nanocarriers for small-molecule drug delivery and in particular chemotherapeutic drug delivery.
Small hydrophobic drug molecules are not the only type of therapeutic that have shown the ability to induce ELP micellization. Park et al. demonstrated that a (VPGAG)192 ELP monoblock fused with human granulocyte-macrophage colony-stimulating factor (a pro-mitotic protein) spontaneously formed biologically active micelles [45]. These micelles stimulated proliferation of TF-1 erythroblast cells and also bolstered engraftment of TF-1 cells in xenografts of mice. In a separate report, Park et al. also showed evidence of bioactive ELP micelles with a single chain antibody fragment that targeted the FMS-like tyrosine kinase 3 receptor that is relevant for treating acute myeloid leukemia [46]. With the development of new active single chain antibody fragments that target different biological receptors, their implementation onto ELPs and other nanoconstructs continues to be of primary interest to researchers and clinicians [47].
Given their innate biocompatibility and their general success in preclinical applications, some ELP systems have progressed to clinical trials. Most notably, PhaseBio Pharmaceuticals Inc. has developed a proprietary ELP-therapeutic fusion technology which has previously been applied in clinical targets such as diabetes and cardiomyopathy associated with dystrophinopathies but has more recently been tested in the treatment of pulmonary arterial hypertension (PAH). Their primary ELP based therapeutic is designated as PB1046; it is known as Pemziviptadil when formulated for the treatment of PAH, or Vasomera™ when previously formulated for application to cardiomyopathy associated with dystrophinopathies [48,49]. Pemziviptadil is a recombinantly engineered ELP that is fused with a vasoactive intestinal peptide (VIP) that binds to vasoactive intestinal active polypeptide receptors 1 and 2 [49,50]. Decreased levels of VIP have been associated with PAH, and the delivery of VIP as a means of treatment against PAH has been demonstrated previously, but only in a limited capacity, and this strategy has not been evaluated in larger scale clinical trials [51,52]. Given the limited amount of clinical data on the treatment of PAH with VIP, it is reasonable to consider that the extended-release mechanism of the ELP component of Pemziviptadil may improve the treatment of PAH [52]. In a completed phase 1 clinical trial (NCT03315507), three patients were administered subcutaneous injections of Pemziviptadil once a week for eight weeks, and in one patient, treatment was extended for up to 18 months [49,53,54]. Pemziviptadil injections did not cause any drug-related adverse events and were well tolerated. In the patient who received the extended treatment, an apparent disease-modifying effect was observed with meaningful improvement in the assessed hemodynamic parameters, and this effect was sustained for three months after the trial [49,50]. Given these results, two phase 2 studies (NCT03556020 and NCT03795428) are currently ongoing to further assess safety, tolerability and efficacy of Pemziviptadil, and Phasebio expects to have results by the second half of 2021 [49,55,56]. Notably, with the onset of the COVID-19 pandemic, Pemziviptadil was assessed in a clinical trial (NCT04433546) for its ability to treat acute respiratory distress syndrome (ARDS) that occurs in COVID-19 patients after a SARS-CoV-2 infection [57]. Although the trial was terminated, Pemziviptadil was found to be tolerable in in COVID-19 patients that took part in the trial and resulted in no adverse safety events [57,58]. Additionally, Phasebio has previously conducted clinical trials to treat type 2 diabetes mellitus with other ELP constructs that contain fusions with glucagon-like peptide 1 (designated PB1023) and long-acting basal insulin (designated PE0139), although these have not been developed for clinical use [59,60]. Regardless, ELP fusion constructs have promising safety characteristics that include acceptable tolerability and limited adverse events that support their continued clinical investigation.
Although ELP nanocarriers have consistently exhibited biocompatibility, they are not free of challenges to clinical translation. Like many other nanocarrier systems, ELP nanocarriers are readily uptaken by macrophages in the reticuloendothelial system (RES) [28]. Furthermore, only a handful of ELP nanocarriers possess active targeting ligands, and the literature suggests that a dearth of ELP nanocarriers are able to simultaneously resist RES clearance and actively bind/enter target cells. For instance, Yousefpour et al. included ABD domain on CP ELP micelles to limit clearance by the RES, but they did not incorporate an active targeting ligand for tumor-specific uptake [41]. Conversely, the DOX diblock-ELP micelle system developed by Costa et al. bears a nanobody that targets overexpressed EGFR in many cancerous sub-types (described above) but does not bear any ligands that limit opsonization or non-specific clearance [39]. As demonstrated by Peddi et al., diblock-ELP micelles that present two functionalities on micellar surface can be created (Figure 2b) so there should be no synthetic limitation in creating an ELP nanocarrier that has both active targeting functionality as well as clearance limiting functionality. Instead of synthetic challenges, such bifunctional diblock-ELP micelles would possibly possess efficacy limitations. It has been demonstrated that nanoparticles that bear antibodies or antibody fragments for active targeting tend to suffer from increased clearance due to their activation and subsequent uptake by immune cells, thus nullifying the effect of clearance limiting ligands [61]. Additionally, the presence of clearance limiting ligands can limit the ability of active targeting ligands to reach their targets [61]. Nonetheless, ELP micelles that possess both active targeting and enhanced circulation are warranted for warranted for further study and optimization.
Despite the potential challenges that they may face, it is clear that ELP micelles will continue to be a dominant ECM-based nanocarrier platform for future therapies for a wide assortment of diseases. With the advantages that these nanocarriers do possess, researchers have demonstrated their successful application in preclinical settings. In particular, ELP nanocarriers have been tested in multiple tumor models (breast, blood, brain, and ovarian cancers) in vitro and they have demonstrated superior nanocarrier-mediated cell death relative to previously approved nanoparticle formulations or free chemotherapeutic drug. More importantly, ELP nanocarriers have shown promise in in vivo breast cancer models where tumor growth volumes were either maintained, reduced, or totally abolished. ELP nanocarriers have been studied not only for more prevalent diseases such as various cancers, but also hold promise in the providing treatments for orphan diseases that have generally received less investigative attention (such as the PhaseBio Pharmaceuticals example mentioned above, investigating the clinical treatment of for PAH). The field of ELP nanocarrier therapeutics continues to be exciting, with continued and promising preclinical and clinical therapeutic efficacy data.
3. Collagen-like peptide (CLPs)
3.1. Introduction to CLPs
The development of CLPs (also known as collagen-mimetic peptides (CMPs)) shares many similarities to the development of ELPs. Like ELPs, CLPs were developed and studied due to the difficulties of studying insoluble native ECM proteins (in this case, collagen), and CLPs were thus created as a reductionist approach to studying the collagen protein family as a whole [62]. Additionally, like the tropoelastin-derived repeat sequence motif for ELPs described above, CLPs comprise a unique amino acid repeat sequence (G-XAA-YAA)n found in collagens. XAA and YAA can be any amino acid with the exception of glycine [62], although the two residues are almost exclusively the imino acids proline (P) and hydroxyproline (O), respectively, and the number of repeats within CLPs is typically 10 or less [62]. Like the protein from which it is derived, a hallmark characteristic of CLPs is their ability to fold into the unique secondary structure of a triple helix, in which three individual CLP chains, each adopting a polyproline-like type II helix, associate with and fold with two other individual chains to form a right-handed triple helix [63]. The CLP triple helices can unfold to a monomeric, single chain through heating, and can reversibly refold into a triple helix upon cooling. The midpoint of this transition is typically defined as the melting temperature (Tm), and its value depends primarily on the number of repeats (n), the total content of imino acids that comprise the XAA and YAA residues, and more specifically, the amount of hydroxyproline that is in the YAA position [62,63]. When CLPs are folded, their triple helix is remarkably compact, rigid, and linear, which makes CLPs ideal for constructing molecular scaffolds such as hydrogels [64,65], cellular adhesion materials, [66] and other supramolecular structures [67,68]. Given their unique linear and structural characteristics, as well as their ability to fold and unfold from a temperature stimulus, CLPs have promise for imbuing these functions and features into drug delivery nanocarriers. Additionally, nanocarriers that bear CLPs can be utilized to target collagens in tissues, wound sites, and collagen-based biomaterials.
3.2. Drug and gene CLP conjugates
In 2005, the Yu group discovered that CLPs in the single stranded (melted/unfolded) state could bind to collagen protein that was either partially or fully denatured, with the binding affinity increasing as temperature-mediated collagen denaturation increased [69]. Since this discovery, the group has demonstrated that collagen peptide hybridization can be carried out in vivo and be utilized as a highly sensitive diagnostic tool to detect denatured collagen in various tissues by labeling the hybridizing CLP with a fluorophore [70,71]. The unique collagen-hybridizing capacity of CLPs also triggered interest in the use of CLPs for drug and gene delivery applications, with CLPs largely being exploited to modify nanostructures and thereby impart them with collagen-binding capacity.
One of the earliest efforts in this regard was a CLP therapeutic peptide conjugate that was developed by Chattopadhyay et al. to target and treat denatured collagen in wound beds [72]. In this work, the CLP (PPG)7 was C-terminally modified with a cytoactive peptide called substance P, which had previously been demonstrated to trigger vasodilation and angiogenesis, both of which are important in the wound healing cascade [72]. The authors found that administering the CLP-substance P conjugate in a murine splinted wound model resulted in enhanced wound closure and extensive re-epithelialization relative to a substance P molecule that lacked the CLP, presumably due to the hybridization of the CLP-substance P conjugate in the wound bed. This work demonstrates the efficacy of utilizing a simple, therapeutic CLP peptide conjugate that can be readily synthesized and purified via solid-phase strategies and suggests that other moderate-sized therapeutic peptides could be employed with this (PPG)7 CLP for enhanced efficacy when delivered to pathological tissues that possess denatured collagen.
It is briefly worth mentioning that the authors claimed that the CLP (PPG)7 was incapable of forming a triple helix with itself (homotrimer) but could hybridize to denatured collagen in the wound bed in vivo (Figure 4a) [72]. This distinction is important given that other CLP therapeutics require special methodologies such as heating above the Tm of the CLP, or UV-mediated removal of steric hindering molecules (e.g., nitrobenzyl groups that prevent CLP folding), to induce the CLP to be in the single-stranded state and render it capable of hybridization [70].
Figure 4:
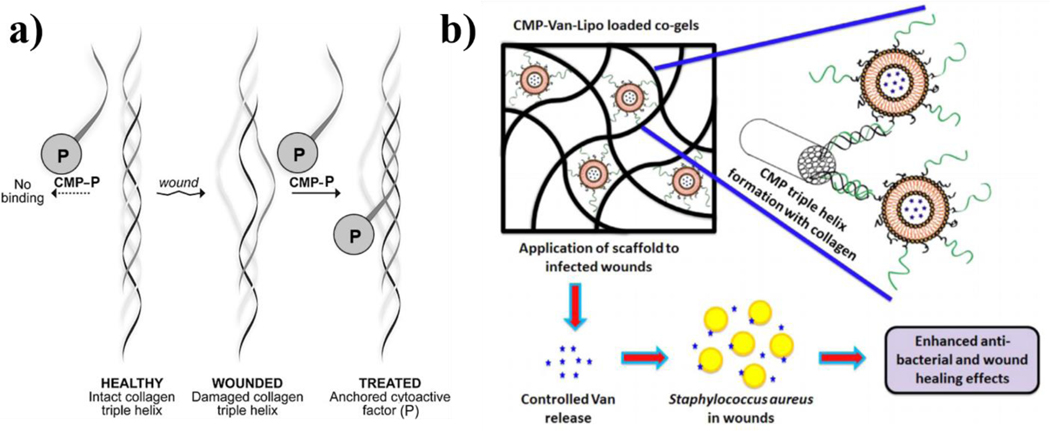
Schematic respresentations/illustrations of nanocarrier systems with CMPs (also referred to as CLPs in other literature) that are capable of hybridizing to denatured collagen protein. a) The substance P CMP (sequence (PPG)7) conjugate is shown in the single stranded state (left) and cannot bind to intact collagen protein. In contrast, collagen triple helices are damaged in certain diseases (e.g., wounds) enabling CMP hybridization/binding. b) Vancomycin-laden liposomes that are surface-modified with single-stranded CMPs are loaded into collagen-fibrin co-gels that are cabable of deliverying the antibiotic for improving healing outcomes in infected wounds. . Figure 4a) was reprinted (adapted) with permission from (Chattopadhyay S, Guthrie KM, Teixeira L, et al. Anchoring a cytoactive factor in a wound bed promotes healing. Journal of Tissue Engineering and Regenerative Medicine. 2016 Dec;10(12):1012–1020). Copyright © 2014 John Wiley & Sons, Ltd. Figure 4b) was reprinted from Acta Biomaterialia., 103, Thapa R.K., Kiick K.L., Sullivan M.O., Encapsulation of collagen mimetic peptide-tethered vancomycin liposomes in collagen-based scaffolds for infection control in wounds., 115–128., Copyright (2020), with permission from Elsevier.
The latter of these strategies was developed by the Yu group and has very recently been employed in their laboratory to design a CLP therapeutic conjugate for the treatment of rheumatoid arthritis (RA). In work by Arlotta et al., the antigen-binding portion of the anti-tumor necrosis factor alpha (TNFα) monoclonal antibody infliximab was chemically conjugated to the CLP (GPO)9, which bore a fluorophore for detection and also a nitrobenzyl functional group that prevented CLP self-trimerization [73]. TNFα is a cytokine, commonly overexpressed in RA, that upregulates expression of MMPs [74]. The authors demonstrated that UV cleavage of the nitrobenzyl group from the CLP (immediately prior to injection) enabled the CLP-anti-TNFα to bind to denatured collagen and slow disease progression and cartilage (type II collagen) degradation in a transgenic mouse model of RA, as evidenced by hematoxylin & eosin (H&E) staining and safranin-O (S-O) staining. More specifically, mice treated with the CLP-anti-TNFα conjugate possessed less synovial hyperplasia, less periarticular inflammatory cell infiltration, and limited articular cartilage degradation relative to mice treated with saline [73]. This work builds off of the concept developed by Chattopadhyay et al. and advances it by utilizing large antibody therapeutic instead of a relatively small peptide, highlighting the modularity of CLP conjugates. Given the relative nano to mesoscale dimensions of the antibody fragment, the work additionally suggests that nanocarriers could also be endowed with CLPs for enhanced therapeutic outcomes through their hybridization capabilities.
To this end, the Kiick and Sullivan research groups have investigated the therapeutic utility of sequestering drug-laden nanoparticles in collagen/fibrin co-gels via CLP-modified nanoparticles [75]. They demonstrated that after heating, the CLP (GPP)3-GPRGEKGERGPR-(GPP)3-GPCCG peptide (termed GEKGER) was capable of hybridization to denatured collagen protein, and that it also could be chemically conjugated to polymers (highlighted in more detail below) through cysteine-maleimide chemistry. It is briefly worth noting that a similar chemistry was utilized by Arlotta et al., which further indicates the considerable modularity of CLPs. Using this chemistry, GEKGER CLP-linked liposomes were synthesized and loaded with the antimicrobial drug vancomycin, and the liposomes were subsequently hybridized to collagen/fibrin co-gels via interactions with denatured collagen protein in the co-gel (Figure 4b). The CLP liposome co-gel formulation was found to resist a methicillin-resistant staphylococcus aureus (MRSA) challenge in vivo for up to 9 days, whereas non-CLP containing vancomycin liposome co-gels resisted infection for only up to 2 days [75]. These results demonstrate the important role of CLPs as non-covalent tethers that enhance nanocarrier retention and localization in collagenous matrices, ultimately improving the therapeutic index of complex drug delivery systems.
The Kiick and Sullivan research groups have also demonstrated the use of the GEKGER CLP in gene delivery applications. In 2014, Urello et al. first demonstrated that DNA-polymer electrostatic complexes (polyplexes) could be chemically modified with the GEKGER CLP and embedded and retained within collagen films and gels post CLP hybridization via heating [76]. In this study, polyplexes modified with GEKGER were found to be retained in collagen for a duration 5 times longer than polyplexes without the CLP. Additionally, the polyplexes were found to retain robust gene transfer activity over several weeks, as determined by analysis of the reporter protein Gaussia luciferase that was encoded by the DNA in the polyplex. Furthermore, gene transfer was shown to be MMP-dependent, with enhanced gene delivery driven by endocytic collagen turnover [76]. This work was subsequently successfully translated to an in vivo model in which luciferase expression was detected up to 25 days post subcutaneous injection, with the expression kinetics dependent upon the number of GEKGER CLP modifications in each polyplex [77].
These studies highlighted the exciting potential for CLP-mediated gene delivery in regenerative medicine applications. Further efforts by the Kiick and Sullivan groups investigated the therapeutic capacity of CLP-tethered polyplexes in collagen gels. In a 2016 work, Urello et al. investigated the in vitro delivery and resulting outcomes of CLP-tethered polyplexes that encoded for platelet-derived growth factor-BB (PDGF), hypothesizing that it could promote healing in wound models [78]. Indeed, it was found that the inclusion of the GEKGER CLP into PDGF encoding polyplexes enhanced cellular functions that would be desired in wound healing such as increased proliferation, migration, and gel contraction that indicated ECM remodeling by cells [78]. Most notably, in an in vitro wound model, “closure” of the wound with the CLP-modified polyplexes was similar to that achieved with a bolus administration of PDGF while using an order of magnitude less protein due to the localized protein production after gene transfer [78]. The promise of these approaches was also demonstrated in a preclinical murine wound model, in which PDGF-encoding polyplexes with different CLP functionalization densities were hybridized to fibrin/collagen gels and administered to excisional wounds in mice [79]. Images of the wound healing progression over the course of 14 days for three different CLP-polyplex gels with different degrees of CLP composition (0, 20, and 50 mol % (relative to polyplex polymer)) and three controls (saline, gels without bolus PDGF, and gels with bolus PDGF) are illustrated in Figure 5a [79]. The percent area of wound closure calculated from the images for each time point is provided in Figure 5b. The inset of Figure 5b highlights the specific differences between all testing conditions at day 9. The data indicate that not only did these CLP-polyplex gels result in faster wound healing (relative to controls), but they did so with 2 orders of magnitude less growth factor in the wound sites relative to recombinant PDGF-loaded gels [79].
Figure 5:
Treatment of excisional wounds in a murine in vivo model with 3 different control conditions (saline, collagen-fibrin co-gel, and PDGF-loaded collagen-fibrin co-gel) as well as 3 different experimental treatment conditions of PDGF-encoding, CMP-modified polyplexes hybridized into collagen-fibrin co-gels, with each treatment condition varied by the degree of CMP modification (0, 20, and 50 mol % (relative to polyplex polymer)). a) Representative wound images of the 6 different treatment conditions at the day 0, 4, 9, and 14 timepoints. b) Quantification of percent area of wound closure as determined from the images in a). The inset of b) shows the differences between all testing conditions at day 9. Error bars are standard deviations with n = 3 separate data points for each condition. Reprinted (adapted) with permission from (Thapa RK, Margolis DJ, Kiick KL, et al. Enhanced Wound Healing via Collagen-Turnover-Driven Transfer of PDGF-BB Gene in a Murine Wound Model. ACS Applied Bio Materials. 2020 Jun 15;3(6):3500–3517). Copyright (2020) American Chemical Society.
The utilization of CLPs as tethering anchors in drug and gene delivery approaches remains in its infancy. Yet, despite the technology being less than two decades old [69], several preclinical studies have demonstrated the therapeutic efficacy of CLPs in vivo [72,73,75,79]. The majority of these studies have focused on wound healing applications. This includes the use of growth factor encoding genes that are incorporated into CLP functionalized polyplexes, which have demonstrated significant healing rates and full closure in in vitro and in vivo wound models. Furthermore, CLP functionalized liposomes have been used to deliver antibiotics to wound-like environments where infection can be controlled over a longer period of time (due to extended antibiotic release) relative to free antibiotic. Simple cytoactive factor peptide-CLP conjugates have also demonstrated promising wound healing capabilities. Although not applied to wound healing, similar CLP-antibody conjugates have shown promise in arthritis therapies.
In all of these cases, the CLPs were employed as pseudo-active targeting ligands that were used to enhance treatment efficacy by specifically tethering the therapeutic moiety to denatured collagen (via the CLP hybridization / trimeric folding phenomena). The major limitation with such hybridizing conjugates/nanocarriers is that the CLP must first be rendered to the single-stranded state prior to physical application in the target tissue or biomaterial. This can be challenging since CLPs have the natural tendency to form homotrimers. Thus, to yield single strands, the trimeric CLPs must first be thermally unfolded by heating the CLP therapeutic solution to temperatures that could affect the therapeutic itself or possibly be harmful to tissue. The Yu group has cleverly mitigated this limitation by modifying CLPs with a nitrobenzyl group that can be cleaved upon irradiation with ultraviolet light, although this strategy adds additional synthetic complexity.
Interestingly, the work of Chattopadhyay et al. indicates that proper selection of the CLP sequence can eliminate these self-trimerization issues, particularly with the (PPG)7 CLP. Further investigation and application of this sequence could prove invaluable to researchers employing CLPs to drug or gene nanocarriers and allow for more facile translation to the clinic. Future work to improve upon these findings will likely include modification and optimization of the collagen hybridizing sequence, incorporation of CLPs into other types of nanocarriers, and application to additional disease targets. Although ECM diseases are ubiquitous, the focus thus far has been limited largely to wound healing and rheumatoid arthritis applications, and while CLP-mediated drug delivery is not as extensively developed as other ECM-based approaches, the few works published to date highlight its immense therapeutic potential.
4. Hyaluronic Acid (HA)
4.1. Introduction to HA
Despite its large molecular weight of one to several millions of Daltons, the HA molecule is regarded as the simplest glycosaminoglycan [80], given that its molecular backbone consists only of repeating units of D-glucuronic acid linked to N-acetyl-D-glucosamine [80]. Although the structure of HA is considered a linear random coil, it is a relatively stiff molecule, and HA solutions are highly viscous due to the highly hydrated volume of HA [80,81]. Like other constituents of the ECM, the role of HA extends beyond that of a structural substrate, and HA participates in numerous cellular processes such as proliferation, migration, and survival [82,83]. As a component of many varieties of drug delivery systems, its notable attributes include its hydrophilicity, biocompatibility, biodegradability, and lack of immunogenicity [81,82,84]. Perhaps HA’s most important feature in modern drug delivery investigative work is its ability to bind to CD44, a glycoprotein that is commonly overexpressed in various cancers as well as in synovial lymphocytes, macrophages, and fibroblasts within inflamed joints of RA and OA patients [83–86]. Thus, HA has routinely been utilized for active targeting in treating tumors and inflamed joints [84,87].
4.2. HA in drug delivery
Given its inherent ability to “bind” water molecules, HA has been routinely investigated within hydrogels, either as a central component or as an active additive, and its use in this role has been reviewed elsewhere [88]. Of greater interest here is the utilization of HA as a primary component, or as a bioactive ligand/moiety, in nanocarriers. Given the abundance of literature on the subject, we focus on the most recent publications that highlight applications in diseases of the ECM. The reader is referred to excellent reviews that discuss other HA nanoparticles/nanoformulations [84,89].
HA has been implemented in nanocarriers in various ways, including nanoparticle surface conjugation [90,91], electrostatic coating at the surface of positively charged nanoparticles [85,92], or coacervation to form HA-based nanoparticles through complexation of HA with positively charged polymers [86]. In an example of conjugating HA to nanoparticles, Y. Zhou et al. demonstrated procedures for the conjugation of HA to nanoparticles [90]; hollow mesoporous silica nanoparticles (HMSNs) were functionalized with phenyl boronic acid while HA was separately functionalized with dopamine. The dopamine functional handles on the HA reacted with the phenyl boronic acid groups on the HMSNs to form pH-sensitive boronate esters (the HA functionalized HMSNs is termed HMSN-B-HA). These nanoparticles were loaded with both DOX and indocyanine green ((ICG), a known photosensitizer agent that releases cytotoxic reactive oxygen species (ROS)) into the HMSNs, and the particles were functionalized with acid-labile HA handles such that the particles could be internalized into cells via binding with CD44 receptors. After cellular internalization, the HA was cleaved from the HMSNs through natural lysosomal acidification, which led to increased drug release of both the DOX and ICG. In contrast, drug release was hindered prior to the acid cleavage due to the HA-limited diffusion of the drugs from the core of the HMSNs. Additionally, HMSN-B-HAs exhibited enhanced uptake into 4T1 breast cancer cells due to the overexpression of the CD44 receptor on the 4T1 cells relative to non-cancerous 293T kidney cells. The unloaded HMSN-B-HAs (without ICG or DOX) were not cytotoxic to 4T1 breast cancer cells, either without illumination or with illumination. Cytotoxicity was then evaluated for 4T1 breast cancer cells for a range of DOX concentrations either in the dark or under illumination (with illumination hypothesized to increase cytotoxicity via the generation of ROS via ICG). More specifically the cytotoxicity was studied for the following test conditions: free ICG, ICG loaded particles (ICG@HMSN-B-HAs), free DOX, DOX loaded particle (DOX@HMSN-B-HAs), free DOX and ICG, and ICG and DOX loaded particles (ID@HMSN-B-HAs). For conditions in which DOX was present, a concentration dependent cytotoxicity from DOX was observed under both dark and illuminated conditions as determined via an MTT assay. Studies showed that ICG was tolerated by the cells under dark conditions but induced cell cytotoxicity under illuminated conditions due to the ICG photo-induced generation of ROS. Under both dark and illuminated conditions, the greatest cytotoxicity was observed for the maximal DOX concentration, with ICG also in the particles, i.e., the ID@HMSN-B-HAs. In addition, the authors observed that the half-maximal inhibitory concentration (IC50) in 4T1 cells of ID@HMSN-B-HAs under illuminated conditions was one-third of the IC50 value for dark conditions. Therefore, this work demonstrates the utility of pairing an HA conjugate for cancer specific targeting with existing previously characterized nanocarriers, leading to enhanced therapeutic outcomes [90].
An alternative HA-nanoparticle surface conjugation approach was demonstrated by Liang and coworkers in which HA was modified with aldehyde chemical functional groups for reaction with free amine groups, such as those in found in chitosan (CS), a positively charged polysaccharide [91]. The authors found that the aldehyde-containing HA was easily conjugated to the surface of siRNA-loaded chitosan nanoparticles, and these nanoparticles were readily uptaken via the CD44 receptor that is overexpressed in bladder cancer cells. They further demonstrated that by delivering siRNA encoded for silencing Bcl-2 (a protein that inhibits apoptosis and thereby permits oncogenesis), tumor growth could be limited (relative to controls) for up to 35 days in an murine tumor xenograft model in vivo [91]. Taken together, the work demonstrates the continued promise of HA-based specific targeting to cancerous cells via the CD44 receptor-mediated internalization mechanism, and it also demonstrates the ability of HA nanocarriers to be used for gene silencing.
As opposed to conjugating HA to the surfaces of nanocarriers, the substantial negative charge of HA can also be utilized to form electrostatic complexes/coacervates with positively charged macromolecules such as CS. In this vein, P. Zhou et al. used HA and CS to form electrostatic coacervates, and demonstrated that negatively charged plasmid DNA that encoded for cytokine response modifier A (CRMA-pDNA) could also be incorporated into the formulations electrostatically [86]. CRMA is a protease inhibitor that can bind to interleukin 1-beta (IL-1β). Like TNFα, IL-1β is a cytokine that can induce MMP production, and in turn, can bring about damage to collagen in the ECM. Therefore, the authors used these CRMA-pDNA laden HA-CS coacervates to treat an OA rat model, and they found that the coacervate nanoparticles inhibited synovial inflammation and cartilage damage as assessed by H&E and S-O histological staining. More specifically, they correlated the lack of collagen type II degradation with a down regulation of IL-1β, MMP-3, and MMP13 stemming from the expression of CRMA, which binds/blocks IL-1β, and in turn, limits its ability to stimulate MMP production [86]. This work again demonstrates the utility of HA has an effective gene delivery vehicle and also illustrates the ability of HA to be used not just as a CD44-targeting mechanism but also as a complexing agent.
In a similar fashion, Zhong et al. electrostatically complexed HA with the branched positively charged polymer polyethyleneimine (PEI). Although PEI has traditionally been utilized as a transfection reagent through complexation with pDNA [93], in this work, the PEI was chemically appended with the immunosuppressant drug methotrexate (MTX). The goal of the work was to form nanoparticle complexes that were drug-laden but also could be targeted to CD44 through the inclusion of HA, which is present to a greater extent in RA-affected tissues relative to healthy tissues [85]. In an in vivo mouse model for RA, these HA-PEI-MTX nanoparticles reduced a series of RA pathologies including synovial hyperplasia, pannus formation, and cartilage destruction [92].
While its negative charge can be useful for electrostatic complexation of polymers and genes into nanocarriers, the overall hydrophilicity of HA can make it amenable for simple amphiphilic nanoparticle self-assembly when it is conjugated to a relatively hydrophobic polymer. Such an approach was utilized in work by Yuan et al. in which they chemically conjugated HA to the polymer oligo(thiophene ethynylene) (OTE) and the resulting OTE-HA conjugates were found to self-assemble into nanoparticles after a solvent/anti-solvent exchange [94]. The authors had previously observed that the OTE polymer possessed broad spectrum bactericidal effects, yet it also was moderately cytotoxic to mammalian cells; thus, they speculated that the conjugation of the polymer to HA would limit cytotoxicity given that the OTE would form the core of nanoparticles (based on its hydrophobicity) and thus limit its exposure to cells (Figure 6). They further hypothesized that the bactericidal OTE polymer would selectively be released in the presence of the bacteria through the degradation of HA by the endogenous hyaluronidase, particularly given the overabundance of hyaluronidase in gram positive bacteria (Figure 6). Indeed, upon incubation with the OTE-HA nanoparticles, MRSA colony forming units were substantially reduced and bacterial cell integrity was found to be compromised. This work showcases the usefulness of HA in enabling self-assembly and altering drug delivery behavior, beyond its more traditional role as a receptor-binding ligand or as an electrostatic complexing agent.
Figure 6:
Self-assembly of OTE-HA polymer conjugates into nanoparticles with OTE comprising the core and HA comprising the surface of the nanoparticles. Live bacteria such as MRSA secrete hyaluronidase (HAase) which cleaves and hydrolyzes HA found on the surface of the nanoparticles and in turn releases the bactericidal OTE and induces bacteria cell death. Reprinted (adapted) with permission from (Yuan Q, Zhao Y, Zhang Z, et al. On-Demand Antimicrobial Agent Release from Functionalized Conjugated Oligomer-Hyaluronic Acid Nanoparticles for Tackling Antimicrobial Resistance. ACS Applied Materials & Interfaces. 2021 Jan 13;13(1):257–265.). Copyright (2020) American Chemical Society.
Given the abundant literature that indicates the value of HA in nanoparticle formulations and bioactivity, it is clear that that the inclusion of HA as a component in therapeutic nanocarrier systems will continue. HA can be incorporated into established nanocarriers such as mesoporous nanoparticles or polymeric systems such as PEI, and more advanced nanocarriers are likely to be developed with expansions in bio-orthogonal chemistries and the understanding of disease etiologies. Given HA’s unmatched functionality, physiochemical properties and its relevance to multiple diseases, HA will continue to be a major staple in nanocarrier drug delivery formulations.
5. Hybrid ECM nanocarriers
In addition to ECM materials inspired by a single ECM component, hybrid ECM nanocarriers have also been developed to leverage simultaneously the functionality of multiple ECM components. While increasing the number of ECM modalities within a single system can increase its complexity with regard to synthesis and characterization, these materials offer unique opportunities to expand the versatility and potential of the materials towards drug delivery applications.
5.1. ELP-CLP vesicles
With ELPs possessing thermoresponsive self-assembly capabilities that occur through heating and CLPs possessing hybridization capabilities that occur upon cooling, the Kiick research group hypothesized that these two ECM-inspired materials could be conjugated to form unique self-assembling structures that would also be capable of binding to collagens. An investigation to test this hypothesis was conducted in 2015 by Luo and Kiick, in which the ELP (VPGFG)6G’, where G’ designates propargyl glycine that bears an alkyne group, and the CLP N3-(GPO)4GFOGER(GPO)4GG, where N3 denotes an N-terminal azide, were synthesized via solid-phase peptide synthesis and chemically conjugated using copper catalyzed azide-alkyne click chemistry [95]. Upon heating the conjugate to a relatively high temperature (80°C) no coacervation or assembly was observed. However, CLP triple helix formation occurred upon cooling below the Tm of the CLP domain, and this resulted in ELP coacervation and assembly of the ELP-CLP trimers into vesicular nanostructures. This outcome indicated that CLP triple helix formation was a prerequisite for enabling the Tt transition of these short ELP domains. Moreover, it was observed that the ELP-CLP self-assembled structures remained assembled upon cooling to 4°C, which indicated that CLP triple helix formation substantially reduced the Tt of the ELP domain by over 75°C (e.g., from > 70°C to < 4°C). The dependence of the ELP Tt on triple helix formation is consistent with the known impact of concentration on the Tt, with three ELP chains (per trimer) localized in a very small volume when the CLP triple helix is intact [26]. In additional studies, the key impact of the CLP Tm on tuning the Tt of the ELP was confirmed. Dunshee et al. found that different CLP domains yielded different Tt values for the ELP-CLP conjugates, despite the fact that the ELP domain was identical for each conjugate [96]. By alterations in the CLP domain, Dunshee et al. were able to determine that (VPGFG)6-(GPO)7GG yielded conjugates with two observable thermoresponsive transitions: a Tm of ca. 50°C and a Tt of ca. 15°C [96], providing two distinct mechanisms for tuning drug release.
Additional investigations have also shown that subtle changes to the ELP sequence of ELP-CLP conjugates (particularly substitutions of the ELP guest residue with other aromatic amino acids) can lead to substantial changes in both Tt and structural morphology. The inclusion of tryptophan in the ELP domain yielded ELP-CLPs with low Tt values, and simulations suggested that this phenomenon was a result of the increased stiffness of tryptophan and its increased propensity for adopting turn structures [97]. In a similar fashion, the location of tyrosine substitutions, towards either the N-terminus or the C-terminus of the ELP, affected the Tt, with N-terminal substitutions yielding more dramatic changes, likely due to increased chain flexibility facilitating π-π stacking [98]. Changes to chain length and tryptophan content were also found to affect not just the Tt of these structures, but also their morphology, with transitions between nanovesicles and nanoscale “platelets” observed [99,100].
Since the initial discovery of the tunable behavior of the ELP-CLP conjugates, these conjugates have been assessed in terms of their utility as novel drug delivery nanocarriers. In 2017, Luo et al. reported that the ELP-CLP (VPGFG)6-(GPO)4GFOGER(GPO)4GG was biocompatible and could be successfully loaded with fluorescein as a model cargo [101]. It was further demonstrated that at 37°C, fluorescein could be released over the course of a few days. Moreover, heating the cargo-loaded ELP-CLP nanovesicles to 80°C (Tm = 57°C) resulted in a burst release, suggesting that the nanovesicles could be utilized for thermally mediated, stimuli-responsive drug release. Lastly, it was found that drug loaded ELP-CLP nanovesicles were capable of hybridizing to denatured collagen type II protein in vitro, and able to sequester fluorescent cargo until collagen was denatured at elevated temperatures; these data suggest the utility of these carriers for localizing hydrophobic cargo to collagen-containing tissues/matrices. Taken together, the work of Luo and colleagues showed that ELP-CLP nanovesicles were: 1) biocompatible, 2) capable of being loaded with cargo without perturbing morphology, 3) thermally responsive, resulting in enhanced drug release, and 4) able to hybridize to denatured collagen protein to localize cargo-loaded vesicles. The results exemplify the combinatorial potential of ECM-inspired hybrid materials.
6. Application of ECM-inspired nanocarriers in the treatment of OA
Given their biocompatibility, lack of immunogenicity, and diverse ECM specific functionality (for CLPs and HA), it is rational to consider ECM-inspired nanocarriers as potential therapeutic vehicles for the treatment of ECM-related diseases. As described previously, there are many different ECM-related diseases, but perhaps the disease that comes to mind most readily when considering the ECM is arthritis which on average (between 2013 and 2015) was diagnosed for 23 % of the United States adult population [102]. Of the different forms of arthroses, OA is the most common [103]. Inflammation and degradation of cartilage ECM in the joint are the primary characteristics of the disease, and these characteristics lead to joint pain and stiffness as well as a reduced quality of life [104,105]. In contrast to RA, which can be treated by a number of disease-modifying anti-rheumatic drugs (DMARDs), there are currently no clinically approved disease-modifying osteoarthritic drugs (DMOADs) [106,107]. For example, despite the fact that TNFα is implicated in both RA and OA pathogenesis [16,17], current biologic DMARDs, such as adalimumab, have not demonstrated efficacy in the treatment of OA [108]. Therefore, the therapeutic efficacy of potential small-molecule hydrophobic DMOADs could be enhanced via their implementation with nanocarriers that enhance drug solubility, limit premature protease degradation, and extend circulation/joint retention times [21,74]. ECM-inspired nanocarriers, in particular could aid DMOAD delivery by providing additional retention/targeting mechanisms via either physical retention or bioactive ligand binding. Additionally, ECM-inspired nanocarriers may be cleared less rapidly from the joint given their similarity to ECM molecules that are innately present in the synovial fluid of the joint.
Upregulation of the cytokine TNFα in RA and OA leads to an overabundance of MMPs that degrade collagen in the joint ECM. Therefore, CLP-based nanocarriers could be implemented to bind to denatured collagen protein found in joint cartilage and improve the retention of OA therapeutics. The only preclinical example that could be found is the CLP-based nanocarrier system developed by Arlotta et al. described above [73]. In brief, the authors created a CLP-anti-TNFα conjugate and demonstrated that delivery to a transgenic mouse model reduced collagen degradation by binding/blocking overexpressed TNFα. Such reports demonstrate the promise and potential of future applied CLP-functionalized nanocarriers that can be made to address unmet clinical challenges in treating arthroses.
ELPs have also been investigated for their ability to deliver OA/RA related biologics as ELP-therapeutic fusion constructs [74]. For example, Shamji et al. recombinantly engineered an ELP attached to a TNFα antagonist and showed that it was capable of attenuating TNFα mediated cytotoxicity in an in vitro murine L929 fibrosarcoma model [109]. In similar approach, Shamji et al. demonstrated that an ELP fused to an IL-1 receptor antagonist could decrease MMP transcription by chondrocytes [110]. In both examples, the goal of the work was to demonstrate the therapeutic activity of the respective fusion construct and to probe any effects these therapeutics had on the ELP’s Tt [109,110]. The intent of these works was to use the ELPs Tt phenomenon to induce bulk therapeutic aggregation to retain the therapeutics in disease sites longer than non-ELP conjugated therapeutics. Given these initial promising results that demonstrated full functionality of these ELP-therapeutic fusion constructs, future preclinical studies will no doubt evince favorable therapeutic outcomes in OA in vivo models.
In addition to TNFα and IL-1 being targets of choice in arthritic ECM, CD44 also is overexpressed in the inflamed joints that are commonly observed in RA and OA [85]. With this in mind, the binding/targeting properties of HA to CD44 make its utilization in ECM-based nanocarriers attractive for the treatment of OA [84,86]. Indeed, HA itself is a therapeutic for OA treatment and is FDA approved for intra-articular injections to the knee [74]. However, meta-analyses have indicated that the efficacy of these HA injections is equivocal [111], suggesting that HA alone may be insufficient. Thus, modified HA-based ECM-inspired nanocarriers are currently being investigated in preclinical models to determine if HA, in a combinatorial role with other drug molecules, could yield improved disease modifying outcomes for OA. As discussed above, Zhou et al. conceived such a combination and created HA-CS-pDNA electrostatic complex coacervates to deliver a gene that encodes for a protease inhibitor (i.e., CRMA, which binds to IL-1β) to an OA rat model and found that the IL-1β and its cell signaling products, MMP-3 and MMP-13, were downregulated resulting in more limited degradation of collagen type II in vivo [86].
Advances in the development of biologics over the last four decades paved the way for the first FDA-approved biologic medication to treat RA (Etanercept, 1998) [112]. This breakthrough ushered in a new era of DMARDs for the treatment and amelioration of RA. While recombinant technology is no longer new, the utilization of recombinant technologies to create ECM-inspired materials such as ELPs, CLP, and even HA for applied medicine still offers substantial opportunities. A new era of ECM-inspired mimics could be on the horizon and eventually lead to the discovery of the first DMOAD for the treatment of OA.
7. Conclusion
The significant role of the ECM in tissue and the structural diversity of ECM molecules suggests enormous potential for the application of ECM components in contemporary drug delivery approaches. Indeed, in the last few decades, many researchers have recognized the potential of ECM-inspired materials and have conceived myriad ways in which these materials can be configured, manipulated, modified and/or augmented. A significant benefit of the majority of ECM-inspired materials is that they are inherently biocompatible, and in some cases (such as HA), non-immunogenic. Of greater benefit is the recapitulation of the physiochemical properties and bioactive functions of these ECM-based materials. For instance, the coacervation properties of elastin polymers has been successfully leveraged in ELP-based materials to create diblock-ELPs that can self-assemble into discrete micelles rather than proceed to simple aggregation. Further manipulations to these ELP micelles resulted in the design of therapeutic protein-ELP fusions and drug-conjugated ELPs for enhancing circulation, limiting premature metabolization, and protecting nanocarriers/drugs from renal and endothelial clearance. Such therapeutic ELP nanocarriers have been applied in preclinical models for a variety of cancers both in vitro and in vivo. Additionally, ELP nanocarriers are beginning to be tested by the biopharmaceutical industry in clinical trials for orphan diseases.
Another example of ECM-inspired materials recapitulating matrix functionality is that of CLPs, which can either be therapeutic conjugates themselves or can be modified or appended to existing therapeutic nanocarriers such as polyplexes or liposomes. These CLP conjugates impart therapeutics with the ability to hijack collagen’s natural propensity for triple helix formation, enabling targeting and localization of therapeutics to denatured collagen protein that is characteristic of ECM diseases. For example, CLPs conjugated to bioactive factors, CLP-modified polyplexes, and CLP-modified liposomes have all been tested preclinically (in vitro and in vivo) for their ability to bind to denatured collagen protein in the wound microenvironment to elicit a therapeutic outcome based on delivery of substances such as a cytoactive factor, a gene encoding for a growth factor, or an antimicrobial to prevent infection. While CLP-modified therapeutics have yet to be tested in clinical trials, their unique advantages in preclinical studies suggest their future potential.
While a great deal of research has been conducted on HA-based materials, recent applications of HA in nanocarriers include their use as targeting ligands via their ability to bind to CD44 and their use as complexing reagents with positively charged polymers and proteins. Highlighted in this work is HA’s application/use in both of these roles by targeting overexpressed CD44 in OA and RA and as an electrostatic binding agent for complexes that bear both genes and drugs for delivery. Of course, inclusion of HA onto nanocarriers that are used for treating cancer remains central in preclinical investigatory work, but researchers continue to find interesting ways of demonstrating HA’s preclinical efficacy with novel materials and different applications.
Lastly, ECM-inspired hybrid nanocarriers offer a full suite of synergistic properties that can include self-assembly and drug loading, bioactive targeting, and stimuli-responsivity. A good paradigm is that of the ELP-CLP nanocarriers created by Kiick and colleagues. Such nanocarriers have so far been demonstrated to be biocompatible, capable of being loaded with a model drug, dually thermoresponsive through both the ELP domain and the CLP domain, and able to bind to denatured collagen protein through CLP mediated hybridization. These multi-functional nanocarriers highlight the distinct possibilities of combining multiple ECM-inspired components and will ideally inspire further research of other permutations and combinations of other types of ECM-inspired materials.
8. Expert opinion
Deriving inspiration from the ECM continues to be a growing trend in biomaterials laboratories, although perhaps because the ECM is, by definition, a cellular matrix, a central theme in ECM mimics and ECM-inspired materials has been their use as hydrogels. As discussed in this review, nanocarriers can be made from, or modified with, these ECM-inspired materials and have been demonstrated to recapitulate their ECM-derived functional properties for therapeutic applications. This has been observed in the Tt self-assembling properties of ELPs, the ability of CLPs to hybridize to denatured collagen proteins, as well as HA’s ability to bind to CD44. Although the focus of this review was limited to some of the most familiar components of the ECM, there are hundreds of other ECM proteins, proteoglycans, and glycoproteins with useful attributes that could be used to enhance existing nanocarrier platforms.
For instance, the glycoprotein fibronectin could be coated or chemically linked to nanocarriers to equip them with integrin-binding motifs. Researchers commonly employ the fibronectin-derived RGD sequence into various systems but inclusion of native fibronectin would provide additional motifs that can bind to other ECM molecules such as collagen, heparin, and fibrin. With these different binding partners, fibronectin has important and similar roles to HA and mediates cell-cell and cell-matrix interactions.
Perhaps more relevant to the treatment of RA and/or OA are the proteoglycans aggrecan and lubricin. Aggrecan is commonly known for being a major component of intraarticular cartilage as a biomechanical support macromolecule. It can interact with HA and form distinct aggregates with it. As such, aggrecan could be incorporated in nanocarriers in the same manner HA already is routinely used and potentially as a co-complexation agent. In contrast to aggrecan, lubricin is known as a lubricating proteoglycan because of its ability to limit protein and cell interactions. Therefore, it could be envisaged as a biomechanical supplement for intraarticular HA nanocarriers or perhaps as an anti-opsonization coating for nanocarriers that are delivered systemically (and either passively delivered or targeted), such as in nanoparticle-based cancer treatments.
The development of non-HA ECM-derived glycan-based macromolecules as nanocarriers could increase the capabilities of nanocarrier systems. Expanding investigations to include other macromolecules such as proteoglycans would at the very least alter the breadth of functional nanocarrier components. The continued application of modern recombinant synthetic techniques could enable cost-effective glycoprotein/proteoglycan production, with the additional benefit of including therapeutic fusion constructs into the molecular design, as exemplified by the ELP-based nanocarrier work described above. Due to its ease of production and molecular simplicity, HA will likely continue to be a dominant component of future ECM-inspired nanocarriers.
The majority of the progress in polypeptide-based nanocarriers, such as those based on ELP, has been in their applications toward cancer remediation. However, other works have demonstrated that they could be functionally efficacious for OA and RA treatments as well. Despite their progress, some of the most recent advances still rely on more traditional, small-molecule chemotherapeutics. Given the continued expansion of biologics, it may be worthwhile for future ELP systems to be developed for the delivery of biologics. This of course leads to additional clinical approval challenges, but this could be mitigated by including already approved biologics in ELP nanocarrier systems.
In contrast to HA and ELPs, the utilization of CLPs as therapeutic conjugates or bioactive targeting ligands in preclinical in vivo studies is extremely limited. Indeed, studies of CLPs have been expanding with regard to the analysis of their kinetic and thermoresponsive properties to provide new insights into their importance with regard to materials assembly applications. Nevertheless, current applications remain primarily focused on the use of CLPs as a diagnostic tool for detecting denatured collagen protein through fluorescent labeling. There are significant opportunities for future ECM-inspired research for both fundamental study of novel ECM mimics and also the application of ECM materials that are well understood. It is our opinion that expanding the research of ECM materials to include more of the matrisome would lead to other novel discoveries that could ultimately have major impacts in the clinic.
Article highlights.
Summary of the structural and functional features of the extracellular matrix and its importance and relevance to a variety of diseases (e.g., cancer and arthritis).
Recent developments with elastin-like peptide micellar nanocarriers for drug delivery in preclinical settings
An up-to-date overview of elastin-like peptides currently in clinical trials
Current applications of collagen-like peptides in drug and gene delivery in preclinical settings
Review of the most recent nanocarriers that incorporate hyaluronic acid for drug delivery to cancerous and arthritic diseases
Suggested future directions for extracellular matrix inspired materials for nanocarrier drug and gene delivery
Acknowledgments
Funding
This paper was funded by National Science Foundation (# EF1935049) and National Institutes of Health (# R01AR067247A).
Footnotes
Declaration of interest
LC Dunshee is supported by the National Science Foundation and the National Institutes of Health. KL Kiick works in the laboratories supported by grants from the National Science Foundation and the National Institutes of Health. MO Sullivan works in the laboratories supported by grants from the National Science Foundation and the National Institutes of Health. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease [Review]. Nature Reviews Molecular Cell Biology. 2014. December;15(12):786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harel R, Tanzer ML. EXTRACELLULAR-MATRIX.3. EVOLUTION OF THE EXTRACELLULAR-MATRIX IN INVERTEBRATES. Faseb Journal. 1993. September;7(12):1115–1123. [DOI] [PubMed] [Google Scholar]
- 3.Exposito JY, Cluzel C, Garrone R, et al. Evolution of collagens [Review]. Anatomical Record. 2002. November;268(3):302–316. [DOI] [PubMed] [Google Scholar]
- 4. Alberts B, Johnson A, Lewis J, et al. Molecular Biology of the Cell, Sixth Edition [Book]. Molecular Biology of the Cell, Sixth Edition. 2015:1–1342. • An exellent book that is recommended for all things cellular biology
- 5.Hynes RO, Naba A. Overview of the Matrisome-An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harbor Perspectives in Biology. 2012. January;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aamodt JM, Grainger DW. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials. 2016. April;86:68–82. • This review has a good illustration of the extracellular matrix
- 7.Barallobre-Barreiro J, Loeys B, Mayr M, et al. Extracellular Matrix in Vascular Disease, Part 2/4 JACC Focus Seminar. Journal of the American College of Cardiology. 2020. May 5;75(17):2189–2203. [DOI] [PubMed] [Google Scholar]
- 8.Theocharis AD, Skandalis SS, Gialeli C, et al. Extracellular matrix structure [Review]. Advanced Drug Delivery Reviews. 2016. February;97:4–27. [DOI] [PubMed] [Google Scholar]
- 9.Clause KC, Barker TH. Extracellular matrix signaling in morphogenesis and repair. Current Opinion in Biotechnology. 2013. October;24(5):830–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu PF, Takai K, Weaver VM, et al. Extracellular Matrix Degradation and Remodeling in Development and Disease [Article]. Cold Spring Harbor Perspectives in Biology. 2011. December;3(12):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateman JF, Boot-Handford RP, Lamande SR. Genetic diseases of connective tissues: cellular and extracellular effects of ECM mutations. Nature Reviews Genetics. 2009. March;10(3):173–183. [DOI] [PubMed] [Google Scholar]
- 12.Iozzo RV, Gubbiotti MA. Extracellular matrix: The driving force of mammalian diseases. Matrix Biology. 2018. October;71–72:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases [Review]. Annals of Medicine. 2001. February;33(1):7–21. [DOI] [PubMed] [Google Scholar]
- 14. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance [Article]. Journal of Cell Science. 2010. December;123(24):4195–4200. • This article has a good alternative illustration of the extracellular matrix and what it looks like under different disease conditions
- 15.Rao JS. Molecular mechanisms of glioma invasiveness: The role of proteases [Review]. Nature Reviews Cancer. 2003. July;3(7):489–501. [DOI] [PubMed] [Google Scholar]
- 16.Jarvelainen H, Sainio A, Koulu M, et al. Extracellular Matrix Molecules: Potential Targets in Pharmacotherapy [Review]. Pharmacological Reviews. 2009. June;61(2):198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs [Review]. Cardiovascular Research. 2006. February;69(3):562–573. [DOI] [PubMed] [Google Scholar]
- 18.Frykberg RG, Banks J. Challenges in the Treatment of Chronic Wounds. Advances in Wound Care. 2015. Sep 1;4(9):560–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue ML, Jackson CJ. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring [Review]. Advances in Wound Care. 2015. March;4(3):119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaefer L, Reinhardt DP. Special issue: Extracellular matrix: Therapeutic tools and targets in cancer treatment. Advanced Drug Delivery Reviews. 2016. February 1;97:1–3. [DOI] [PubMed] [Google Scholar]
- 21.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications [Review]. Nature Reviews Drug Discovery. 2010. August;9(8):615–627. [DOI] [PubMed] [Google Scholar]
- 22.Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream [Article]. Science. 2004. March;303(5665):1818–1822. [DOI] [PubMed] [Google Scholar]
- 23.Chung MIS, Miao M, Stahl RJ, et al. Sequences and domain structures of mammalian, avian, amphibian and teleost tropoelastins: Clues to the evolutionary history of elastins. Matrix Biology. 2006. October;25(8):492–504. [DOI] [PubMed] [Google Scholar]
- 24.Urry DW, Okamoto K, Harris RD, et al. SYNTHETIC, CROSS-LINKED POLYPENTAPEPTIDE OF TROPOELASTIN - ANISOTROPIC, FIBRILLAR ELASTOMER. Biochemistry. 1976 1976;15(18):4083–4089. [DOI] [PubMed] [Google Scholar]
- 25.McDaniel JR, Callahan DJ, Chilkoti A. Drug delivery to solid tumors by elastin-like polypeptides. Advanced Drug Delivery Reviews. 2010. December 30;62(15):1456–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers [Review]. Journal of Physical Chemistry B. 1997. December;101(51):11007–11028. [Google Scholar]
- 27.Hartzell EJ, Lieser RM, Sullivan MO, et al. Modular Hepatitis B Virus-like Particle Platform for Biosensing and Drug Delivery. Acs Nano. 2020. Oct 27;14(10):12642–12651. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins IC, Milligan JJ, Chilkoti A. Genetically Encoded Elastin-Like Polypeptides for Drug Delivery. Advanced Healthcare Materials. 2021. July;10(13). [DOI] [PubMed] [Google Scholar]
- 29.MacEwan SR, Chilkoti A. Controlled Apoptosis by a Thermally Toggled Nanoscale Amplifier of Cellular Uptake. Nano Letters. 2014. April;14(4):2058–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peddi S, Roberts SK, MacKay JA. Nanotoxicology of an Elastin-like Polypeptide Rapamycin Formulation for Breast Cancer. Biomacromolecules. 2020. March;21(3):1091–1102. • This review is an excellent review of some of the earlier work that was done with ELPs in drug delivery
- 31.MacEwan SR, Chilkoti A. Applications of elastin-like polypeptides in drug delivery. Journal of Controlled Release. 2014. September;190:314–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley EG, Albert JNL, Sullivan MO, et al. Stimuli-responsive copolymer solution and surface assemblies for biomedical applications. Chemical Society Reviews. 2013;42(17):7057–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdelghani M, Shao J, Le DHT, et al. Self-Assembly or Coassembly of Multiresponsive Histidine-Containing Elastin-Like Polypeptide Block Copolymers. Macromolecular Bioscience. 2021. June;21(6). [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Valdivieso J, Girotti A, Munoz R, et al. Self-Assembling ELR-Based Nanoparticles as Smart Drug-Delivery Systems Modulating Cellular Growth via Akt. Biomacromolecules. 2019. May;20(5):1996–2007. [DOI] [PubMed] [Google Scholar]
- 35.Pille J, van Lith SAM, van Hest JCM, et al. Self-Assembling VHH-Elastin-Like Peptides for Photodynamic Nanomedicine. Biomacromolecules. 2017. April;18(4):1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieser RM, Chen W, Sullivan MO. Controlled Epidermal Growth Factor Receptor Ligand Display on Cancer Suicide Enzymes via Unnatural Amino Acid Engineering for Enhanced Intracellular Delivery in Breast Cancer Cells. Bioconjugate Chemistry. 2019. February;30(2):432–442. [DOI] [PubMed] [Google Scholar]
- 37.Xu N, Fang W, Mu L, et al. Overexpression of wildtype EGFR is tumorigenic and denotes a therapeutic target in non-small cell lung cancer. Oncotarget. 2016. January 26;7(4):3884–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nature Reviews Cancer. 2012. August;12(8):553–563. [DOI] [PubMed] [Google Scholar]
- 39.Costa SA, Mozhdehi D, Dzuricky MJ, et al. Active Targeting of Cancer Cells by Nanobody Decorated Polypeptide Micelle with Bio-orthogonally Conjugated Drug. Nano Letters. 2019. January;19(1):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacKay JA, Chen MN, McDaniel JR, et al. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection [Article]. Nature Materials. 2009. December;8(12):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yousefpour P, McDaniel JR, Prasad V, et al. Genetically Encoding Albumin Binding into Chemotherapeutic-loaded Polypeptide Nanoparticles Enhances Their Antitumor Efficacy. Nano Letters. 2018. December;18(12):7784–7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya J, Bellucci JJ, Weitzhandler I, et al. A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models. Nature Communications. 2015. August;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mastria EM, Cai LY, Kan MJ, et al. Nanoparticle formulation improves doxorubicin efficacy by enhancing host antitumor immunity. Journal of Controlled Release. 2018. January 10;269:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodd RD, Scherer A, Huang W, et al. Tumor Subtype Determines Therapeutic Response to Chimeric Polypeptide Nanoparticle-based Chemotherapy in Pten-deleted Mouse Models of Sarcoma. Clinical Cancer Research. 2020. September 15;26(18):5036–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park M, Vaikari VP, Dhandhukia JP, et al. Human Granulocyte-Macrophage Colony-Stimulating Factor Fused to Elastin-Like Polypeptides Assembles Biologically-Active Nanoparticles. Bioconjugate Chemistry. 2020. May;31(5):1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park M, Vaikari VP, Lam AT, et al. Anti-FLT3 nanoparticles for acute myeloid leukemia: Preclinical pharmacology and pharmacokinetics. Journal of Controlled Release. 2020. August 10;324:317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee C, Choi M, MacKay JA. Live long and active: Polypeptide-mediated assembly of antibody variable fragments. Advanced Drug Delivery Reviews. 2020. December;167:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.PhaseBio Pharmaceuticals I. PhaseBio Receives FDA Orphan Drug Designation for Vasomera (PB1046) for the Treatment of Cardiomyopathy Associated with Dystrophinopathies [Web Page]. Online: PhaseBio Pharmaceuticals, Inc,; 2015. [cited 2021 May, 26]. Available from: https://investors.phasebio.com/news-releases/news-release-details/phasebio-receives-fda-orphan-drug-designation-vasomera-pb1046
- 49.PhaseBio Pharmaceuticals I. PhaseBio Presents Data from Phase 1b/2a Trial of Pemziviptadil for the Treatment of Pulmonary Arterial Hypertension at 15th Pulmonary Vascular Research Institute Virtual World Congress [Web Page]. Online: PhaseBio Pharmaceuticals, Inc; 2021. [cited 2021 May, 26]. Available from: https://investors.phasebio.com/news-releases/news-release-details/phasebio-presents-data-phase-1b2a-trial-pemziviptadil-treatment
- 50.Benza RL, Paul S, Chakinala M, et al. , editors. Case study of long-term safety, tolerability, and hemodynamic response of Pb1046, a sustained-release analogue for vasoactive intestinal peptide (VIP), in an adult subject with pulmonary arterial hypertension (PAH). 2020 Annual World Congress of the Pulmonary Vascular Research Institute; 2020. March 26, 2021: Pulmonary Circulation. [Google Scholar]
- 51.Farber HW, Loscalzo J. Mechanisms of disease: Pulmonary arterial hypertension [Review]. New England Journal of Medicine. 2004. October;351(16):1655–1665. [DOI] [PubMed] [Google Scholar]
- 52.Woodcock CSC, Chan SY. The Search for Disease-Modifying Therapies in Pulmonary Hypertension [Review]. Journal of Cardiovascular Pharmacology and Therapeutics. 2019. July;24(4):334–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.A Study to Assess the Safety, Tolerability, and Hemodynamic Response of PB1046 in Subjects With PAH [Internet]. Online: clinicaltrials.gov 2017. [cited May 27, 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03315507?term=phasebio&draw=2&rank=8.
- 54.Inc. PP. A Study to Assess the Safety, Tolerability, and Hemodynamic Response of PB1046 in Subjects With PAH [Web Page]. Online: clincialtrials.gov; 2017. [updated October, 8th 2019; cited 2021 May, 27]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT03315507?term=phasebio&draw=2&rank=8
- 55.Phase 2 Study to Assess Safety, Tolerability and Efficacy of Once Weekly SC Pemziviptadil (PB1046) in Subjects With Symptomatic PAH (VIP) [Internet]. Online: clinicaltrials.gov. 2018. [cited May 27, 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03556020?term=phasebio&draw=2&rank=6.
- 56.Long-Term, Open Label Extension Study of Pemziviptadil (PB1046) in PAH Subjects Following Completion of Study PB1046-PT-CL-0004 (VIP Extend) [Internet]. Online: clinicaltrials.gov. 2019. [cited May 27, 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT03795428?term=phasebio&draw=2&rank=5.
- 57.Pemziviptadil (PB1046), a Long-acting, Sustained Release Human VIP Analogue, Intended to Provide Clinical Improvement to Hospitalized COVID-19 Patients at High Risk for Rapid Clinical Deterioration and Acute Respiratory Distress Syndrome (ARDS). (VANGARD) [Internet]. Online: clinicaltrials.gov. 2020. [cited May 27, 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT04433546?term=phasebio&draw=2&rank=1.
- 58.PhaseBio Pharmaceuticals I. PhaseBio Provides Pemziviptadil (PB1046) Program Update [Web Page]. Online: PhaseBio Pharmaceuticals, Inc; 2020. [cited 2021 May, 27]. Available from: https://investors.phasebio.com/news-releases/news-release-details/phasebio-provides-pemziviptadil-pb1046-program-update
- 59.PhaseBio Pharmaceuticals I. PhaseBio Pharmaceuticals Announces Positive Data for Type 2 Diabetes Treatments PE0139 and PB1023 [Web Page]. Online: PhaseBio Pharmaceuticals, Inc; 2015. [cited 2021 May, 27]. Available from: https://investors.phasebio.com/news-releases/news-release-details/phasebio-pharmaceuticals-announces-positive-data-type-2-diabetes
- 60.PhaseBio Pharmaceuticals I. Pipeline [Web Page]. Online: PhaseBio Pharmaceuticals, Inc; 2021. [cited 2021 May, 27]. Available from: https://phasebio.com/pipeline/#tab-id-2
- 61.Bertrand N, Wu J, Xu XY, et al. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology [Review]. Advanced Drug Delivery Reviews. 2014. February;66:2–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shoulders MD, Raines RT. Collagen Structure and Stability. Annual Review of Biochemistry. Annual Review of Biochemistry. Vol. 78. Palo Alto: Annual Reviews; 2009. p. 929–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Persikov AV, Ramshaw JAM, Brodsky B. Collagen model peptides: Sequence dependence of triple-helix stability. Biopolymers. 2000;55(6):436–450. [DOI] [PubMed] [Google Scholar]
- 64.Parmar PA, St-Pierre JP, Chow LW, et al. Enhanced articular cartilage by human mesenchymal stem cells in enzymatically mediated transiently RGDS-functionalized collagen mimetic hydrogels. Acta Biomaterialia. 2017. March;51:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Islam MM, Ravichandran R, Olsen D, et al. Self-assembled collagen-like-peptide implants as alternatives to human donor corneal transplantation. Rsc Advances. 2016 2016;6(61):55745–55749. [Google Scholar]
- 66.Krishna OD, Jha AK, Jia XQ, et al. Integrin-mediated adhesion and proliferation of human MSCs elicited by a hydroxyproline-lacking, collagen-like peptide [Article]. Biomaterials. 2011. Sep;32(27):6412–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanrikulu IC, Forticaux A, Jin S, et al. Peptide tessellation yields micrometre-scale collagen triple helices. Nature Chemistry. 2016. November;8(11):1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarkar B, O’Leary LER, Hartgerink JD. Self-Assembly of Fiber-Forming Collagen Mimetic Peptides Controlled by Triple-Helical Nucleation. Journal of the American Chemical Society. 2014. October;136(41):14417–14424. [DOI] [PubMed] [Google Scholar]
- 69. Wang AY, Mo X, Chen CS, et al. Facile modification of collagen directed by collagen mimetic peptides [Article]. Journal of the American Chemical Society. 2005. March;127(12):4130–4131. • First demonstration of CLPs (also CMPs) ability to hybridize to denatured collagen protein
- 70.Li Y, Foss CA, Summerfield DD, et al. Targeting collagen strands by photo-triggered triple-helix hybridization [Article]. Proceedings of the National Academy of Sciences of the United States of America. 2012. September;109(37):14767–14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bennink LL, Li Y, Kim B, et al. Visualizing collagen proteolysis by peptide hybridization: From 3D cell culture to in vivo imaging [Article]. Biomaterials. 2018. November;183:67–76. [DOI] [PubMed] [Google Scholar]
- 72.Chattopadhyay S, Guthrie KM, Teixeira L, et al. Anchoring a cytoactive factor in a wound bed promotes healing [Article]. Journal of Tissue Engineering and Regenerative Medicine. 2016. December;10(12):1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arlotta KJ, San BH, Mu H-H, et al. Localization of Therapeutic Fab-CHP Conjugates to Sites of Denatured Collagen for the Treatment of Rheumatoid Arthritis. Bioconjugate Chemistry. 2020. August;31(8):1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy [Review]. Nature Reviews Rheumatology. 2014. January;10(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thapa RK, Kiick KL, Sullivan MO. Encapsulation of collagen mimetic peptide-tethered vancomycin liposomes in collagen-based scaffolds for infection control in wounds. Acta Biomaterialia. 2020. Feb;103:115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Urello MA, Kiick KL, Sullivan MO. A CMP-based method for tunable, cell-mediated gene delivery from collagen scaffolds [Article]. Journal of Materials Chemistry B. 2014;2(46):8174–8185. [DOI] [PubMed] [Google Scholar]
- 77.Urello MA, Kiick KL, Sullivan MO. ECM turnover-stimulated gene delivery through collagen-mimetic peptide-plasmid integration in collagen [Article]. Acta Biomaterialia. 2017. October;62:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urello MA, Kiick KL, Sullivan MO. Integration of growth factor gene delivery with collagen-triggered wound repair cascades using collagen-mimetic peptides [Article]. Bioengineering & Translational Medicine. 2016. June;1(2):207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thapa RK, Margolis DJ, Kiick KL, et al. Enhanced Wound Healing via Collagen-Turnover-Driven Transfer of PDGF-BB Gene in a Murine Wound Model. Acs Applied Bio Materials. 2020. June 15;3(6):3500–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bastow ER, Byers S, Golub SB, et al. Hyaluronan synthesis and degradation in cartilage and bone. Cellular and Molecular Life Sciences. 2008. February;65(3):395–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tiwari S, Bahadur P. Modified hyaluronic acid based materials for biomedical applications. International Journal of Biological Macromolecules. 2019. January;121:556–571. [DOI] [PubMed] [Google Scholar]
- 82.Dosio F, Arpicco S, Stella B, et al. Hyaluronic acid for anticancer drug and nucleic acid delivery. Advanced Drug Delivery Reviews. 2016. February 1;97:204–236. [DOI] [PubMed] [Google Scholar]
- 83.Toole BP. Hyaluronan: From extracellular glue to pericellular cue. Nature Reviews Cancer. 2004. July;4(7):528–539. [DOI] [PubMed] [Google Scholar]
- 84. Choi KY, Han HS, Lee ES, et al. Hyaluronic Acid-Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Advanced Materials. 2019. August;31(34). •• An excellent review of HA nanoparticles for drug delivery as well as dianostic applications
- 85.Zhou ML, Hou JR, Zhong ZR, et al. Targeted delivery of hyaluronic acid-coated solid lipid nanoparticles for rheumatoid arthritis therapy [Article]. Drug Delivery. 2018. Mar;25(1):716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou P-h, Qiu B, Deng R-h, et al. Chondroprotective Effects of Hyaluronic Acid-Chitosan Nanoparticles Containing Plasmid DNA Encoding Cytokine Response Modifier A in a Rat Knee Osteoarthritis Model. Cellular Physiology and Biochemistry. 2018 2018;47(3):1207–1216. [DOI] [PubMed] [Google Scholar]
- 87.Ferrari M, Onuoha SC, Pitzalis C. Trojan horses and guided missiles: targeted therapies in the war on arthritis. Nature Reviews Rheumatology. 2015. June;11(6):328–337. [DOI] [PubMed] [Google Scholar]
- 88. Lam J, Truong NF, Segura T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds [Review]. Acta Biomaterialia. 2014. Apr;10(4):1571–1580. •• A review of HA based hydrogels, another major application of the HA molecule for drug delivery purposes
- 89. Wickens JM, Alsaab HO, Kesharwani P, et al. Recent advances in hyluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discovery Today. 2017. April;22(4):665–680. •• Review of HA modified nanocarriers for drug delivery. Sepcific relevance to cancer treatments
- 90.Zhou Y, Chang C, Liu Z, et al. Hyaluronic Acid-Functionalized Hollow Mesoporous Silica Nanoparticles as pH-Sensitive Nanocarriers for Cancer Chemo-Photodynamic Therapy. Langmuir. 2021. March 2;37(8):2619–2628. [DOI] [PubMed] [Google Scholar]
- 91.Liang Y, Wang Y, Wang L, et al. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioactive Materials. 2021. February;6(2):433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhong S, Liu P, Ding J, et al. Hyaluronic Acid-Coated MTX-PEI Nanoparticles for Targeted Rheumatoid Arthritis Therapy. Crystals. 2021. April;11(4). [Google Scholar]
- 93.Boussif O, Lezoualch F, Zanta MA, et al. A VERSATILE VECTOR FOR GENE AND OLIGONUCLEOTIDE TRANSFER INTO CELLS IN CULTURE AND IN-VIVO - POLYETHYLENIMINE. Proceedings of the National Academy of Sciences of the United States of America. 1995. August 1;92(16):7297–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan Q, Zhao Y, Zhang Z, et al. On-Demand Antimicrobial Agent Release from Functionalized Conjugated Oligomer-Hyaluronic Acid Nanoparticles for Tackling Antimicrobial Resistance. Acs Applied Materials & Interfaces. 2021. Jan 13;13(1):257–265. [DOI] [PubMed] [Google Scholar]
- 95.Luo TZ, Kiick KL. Noncovalent Modulation of the Inverse Temperature Transition and Self-Assembly of Elastin-b-Collagen-like Peptide Bioconjugates [Article]. Journal of the American Chemical Society. 2015. December;137(49):15362–15365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dunshee LC, Sullivan MO, Kiick KL. Manipulation of the dually thermoresponsive behavior of peptide-based vesicles through modification of collagen-like peptide domains [Article]. Bioengineering & Translational Medicine. 2020. Jan;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prhashanna A, Taylor PA, Qin J, et al. Effect of Peptide Sequence on the LCST-Like Transition of Elastin-Like Peptides and Elastin-Like Peptide-Collagen-Like Peptide Conjugates: Simulations and Experiments. Biomacromolecules. 2019. March;20(3):1178–1189. [DOI] [PubMed] [Google Scholar]
- 98.Taylor PA, Huang H, Kiick KL, et al. Placement of tyrosine residues as a design element for tuning the phase transition of elastin-peptide-containing conjugates: experiments and simulations. Molecular Systems Design & Engineering. 2020. Aug 1;5(7):1239–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qin JY, Luo TZ, Kiick KL. Self-Assembly of Stable Nanoscale Platelets from Designed Elastin-like Peptide-Collagen-like Peptide Bioconjugates [Article]. Biomacromolecules. 2019. Apr;20(4):1514–1521. [DOI] [PubMed] [Google Scholar]
- 100.Qin J, Sloppy JD, Kiick KL. Fine structural tuning of the assembly of ECM peptide conjugates via slight sequence modifications. Science Advances. 2020. October;6(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo TZ, David MA, Dunshee LC, et al. Thermoresponsive Elastin-b-Collagen-Like Peptide Bioconjugate Nanovesicles for Targeted Drug Delivery to Collagen-Containing Matrices [Article]. Biomacromolecules. 2017. Aug;18(8):2539–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barbour KE, Helmick CG, Boring M, et al. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation - United States, 2013–2015. Mmwr-Morbidity and Mortality Weekly Report. 2017. Mar 10;66(9):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis [Article]. Best Practice & Research in Clinical Rheumatology. 2014. Feb;28(1):5–15. [DOI] [PubMed] [Google Scholar]
- 104.Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. Journal of Musculoskeletal & Neuronal Interactions. 2006. Oct-Dec;6(4):376–378. [PubMed] [Google Scholar]
- 105.Breedveld FC. Osteoarthritis - the impact of a serious disease [Article; Proceedings Paper]. Rheumatology. 2004. Feb;43:I4–I8. [DOI] [PubMed] [Google Scholar]
- 106.Chaudhari K, Rizvi S, Syed BA. Rheumatoid arthritis: current and future trends. Nature Reviews Drug Discovery. 2016. May;15(5):305–306. [DOI] [PubMed] [Google Scholar]
- 107.Cai X, Yuan S, Zeng Y, et al. New Trends in Pharmacological Treatments for Osteoarthritis. Frontiers in Pharmacology. 2021. April 15;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ghouri A, Conaghan PG. Update on novel pharmacological therapies for osteoarthritis. Therapeutic Advances in Musculoskeletal Disease. 2019. July 23;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shamji MF, Chen J, Friedman AH, et al. Synthesis and characterization of a thermally-responsive tumor necrosis factor antagonist [Article]. Journal of Controlled Release. 2008. August;129(3):179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shamji MF, Betre H, Kraus VB, et al. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist - Sustained release of a local antiinflammatory therapeutic [Article]. Arthritis and Rheumatism. 2007. November;56(11):3650–3661. [DOI] [PubMed] [Google Scholar]
- 111.Oo WM, Liu X, Hunter DJ. Pharmacodynamics, efficacy, safety and administration of intra-articular therapies for knee osteoarthritis. Expert Opinion on Drug Metabolism & Toxicology. 2019. [DOI] [PubMed] [Google Scholar]
- 112.Curtis JR, Singh JA. Use of Biologics in Rheumatoid Arthritis: Current and Emerging Paradigms of Care. Clinical Therapeutics. 2011. Jun;33(6):679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]