Abstract
Background and Objectives
To determine the impact of endovascular therapy for large vessel occlusion stroke in patients with vs those without premorbid disability.
Methods
We performed a post hoc analysis of the TREVO Stent-Retriever Acute Stroke (TRACK) Registry, which collected data on 634 consecutive patients with stroke treated with the Trevo device as first-line endovascular thrombectomy (EVT) at 23 centers in the United States. We included patients with internal carotid or middle cerebral (M1/M2 segment) artery occlusions, and the study exposure was patient- or caregiver-reported premorbid modified Rank Scale (mRS) score ≥2 (premorbid disability [PD]) vs premorbid mRS score of 0 to 1 (no PD [NPD]). The primary outcome was no accumulated disability, defined as no increase in 90-day mRS score from the patient's premorbid mRS score.
Results
Of the 634 patients in TRACK, 407 patients were included in our cohort, of whom 53 (13.0%) had PD. The primary outcome of no accumulated disability was achieved in 37.7% (20 of 53) of patients with PD and 16.7% (59 of 354) of patients with NPD (p < 0.001), while death occurred in 39.6% (21 of 53) and 14.1% (50 of 354) (p < 0.001), respectively. The adjusted odds ratio of no accumulated disability for patients with PD was 5.2 (95% confidence interval [CI] 2.4–11.4, p < 0.001) compared to patients with NPD. However, the adjusted odds ratio for death in patients with PD was 2.90 (95% CI 1.38–6.09, p = 0.005).
Discussion
In this study of patients with anterior circulation acute ischemic stroke treated with EVT, we found that PD was associated with a higher probability of not accumulating further disability compared to patients with NPD but also with higher probability of death.
Classification of Evidence
This study provides Class II evidence that in anterior circulation acute ischemic stroke treated with EVT, patients with PD compared to those without disability were more likely not to accumulate more disability but were more likely to die.

American Heart Association/American Stroke Association guidelines recommend offering endovascular thrombectomy (EVT) to eligible patients with ischemic stroke with a premorbid modified Rankin Scale (mRS) score <2.1 Patients with premorbid disability (PD; mRS score ≥2) were largely excluded from the pivotal EVT trials.2-4 However, patients with moderate to severe PD account for up to one-third of all patients with ischemic stroke.5 Furthermore, a recent 2-center study in the United States showed that patients with premorbid mRS score ≥2 accounted for 34% of real-world EVT cases and, compared to patients with premorbid mRS score of 0 to 1, showed similar recovery to PD levels, according to the change in mRS score from prestroke to 90 days.6 A prior multicenter observational study reported similar findings but was limited to European centers.7 However, a multicenter study of patients with premorbid mRS score ≥2 treated with EVT and second-generation stentrievers at centers in the United States has not been performed. Although a prospective randomized clinical trial is needed for definitive evidence of benefit for EVT in patients with PD, to provide additional evidence for such a trial, we examined the impact of PD on outcome after EVT in a large, multicenter US registry.
Methods
Cohort
We performed a post hoc cohort analysis of the TREVO Stent-Retriever Acute Stroke (TRACK) Registry, which prospectively and retrospectively collected data on 634 consecutive patients with ischemic stroke treated with the Trevo device as first-line EVT at 23 centers in the United States.8 The TRACK registry was a postmarketing, investigator-initiated registry with the aim of producing real-world generalizable data and was funded by Stryker Neurovascular. Consecutive patients with ischemic stroke treated with first-line Trevo for EVT were included at the 23 centers between March 2013 and August 2015. The rates of successful recanalization, procedural complications, and functional outcomes in TRACK were comparable to published data from randomized clinical trials.8 Because TRACK was a real-world registry, it included patients with self- or caregiver-reported premorbid mRS score ≥2, who account for a nontrivial proportion of patients treated with EVT outside clinical trials.6 We included patients in TRACK with internal carotid or middle cerebral (M1/M2 segment) artery occlusions. We conformed with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator for purposes of replicating results.
Standard Protocol Approvals, Registrations, and Patient Consents
The TRACK database contained deidentified information that was deemed not to require patient consent by the Institutional Review Board (IRB) at the individual sites or at Mercy Health–St. Vincent Hospital (Toledo, OH), which served as the coordinating center for TRACK.8 The current analysis of data from the deidentified TRACK database did not require IRB approval according to the IRB policy at the University of Utah, where the analysis was conducted.
Exposure and Outcome
The study exposure was premorbid mRS score ≥2 (PD) vs premorbid mRS score 0 to 1 (no PD [NPD]). In TRACK, the premorbid mRS score was collected in a case report form by trained study personnel during patient hospitalization and reflects a patient- or caregiver-reported value for before the index stroke. The primary outcome of the study was no accumulated disability, defined as no increase in 90-day mRS score from the premorbid mRS score.6
The secondary outcomes were 90-day mortality and utility-weighted mRS (UW-mRS) score.9 The UW-mRS incorporates patient, caregiver, and societal perspectives on permanent neurologic deficits.4 The UW-mRS inverts and transforms the standard 7 ordinal mRS categories (0, 1, 2, 3, 4, 5, 6) to a continuous scale in which higher values signify better outcome: 10, 9.1, 7.6, 6.5, 3.3, 0, and 0, respectively.
Statistical Analysis
We compared patients with PD and those with NPD using the χ2 test and logistic regression models adjusted as follows: model 1 adjusted a priori for age, sex, race, baseline NIH Stroke Scale score, IV alteplase, time from stroke onset to groin puncture, postprocedural Thrombolysis in Cerebral Infarction score, and symptomatic intracranial hemorrhage within 36 hours; and model 2 adjusted on the basis of least absolute shrinkage and selection operator (LASSO) methodology10 and adjusted for age, atrial fibrillation, baseline NIH Stroke Scale score, balloon guide catheter, use of additional rescue therapy beyond Trevo, and Thrombolysis in Cerebral Infarction score. LASSO covariate selection for regression models is a methodology that is preferred to stepwise approaches because of its use of a tuning parameter to penalize the number of covariates in the model.11
We confirmed that our models had acceptable multicollinearity, defined as a variance inflation factor <10, and calculated the E value for the exposure of PD vs NPD in the models fitted to our primary outcome.12 We used marginal effects to derive predicted probabilities from logistic regression and investigated interaction terms between PD and the covariates in model 1. We also performed a sensitivity analysis in the subgroup of patients who had not died by follow-up. All statistical analysis was conducted in Stata 17.0 (StataCorp, College Station, TX), and significance was defined as a 2-tailed value of p < 0.05.
Results
Of the 634 patients in TRACK, 104 were excluded for having occlusion sites other than the internal carotid artery or M1/2 middle cerebral artery, 80 patients did not have a 90-day mRS score, and 43 patients did not have a premorbid mRS score. There were no significant imbalances in baseline characteristics between the 407 included patients and the 123 patients excluded for a missing premorbid or 90-day mRS score (Table 1). Of the 407 included patients, 53 of 407 (13.0%) had PD and 354 of 407 (87.0%) had NPD. Patient demographics are shown in Table 2. Patients with PD compared to those with NPD were older (73.3 vs 65.2 years, p < 0.001), were less likely to be male (34.0% vs 54.0%, p = 0.007), had a higher NIH Stroke Scale score (18 vs 17, p = 0.034), were less likely to receive tissue plasminogen activator (tPA) (34.0% vs 56.2%, p < 0.001), and were more likely to have atrial fibrillation (69.8% vs 40.1%, p < 0.001).
Table 1.
Baseline Demographics in Patients Included in the Final Cohort of 407 Patients vs the 123 Excluded for a Missing Premorbid or 90-Day mRS Score

Table 2.
Baseline Patient Demographics, Procedural Outcomes, and 90-Day Outcomes

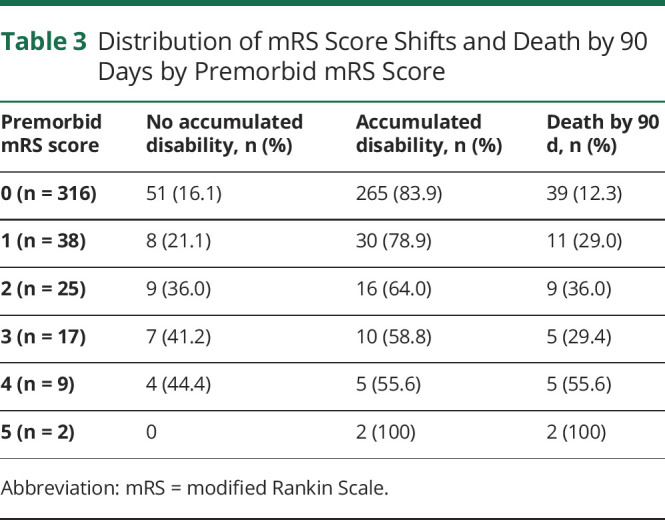
The primary outcome of no accumulated disability was achieved in 37.7% (20 of 53) of patients with PD and 16.7% (59 of 354) of patients with NPD (p < 0.001), while death occurred in 39.6% (21 of 53) and 14.1% (50 of 354) (p < 0.001), respectively. The change in UW-mRS score was significantly less in those with PD vs those with NPD (−0.25 ± 0.38 vs −0.37 ± 0.35, p < 0.001) (Table 1). The proportions of no accumulated disability and 90-day death by the individual premorbid mRS scores are shown in Table 3. For premorbid mRS scores of 0, 1, 2, 3, and 4, the primary outcome of no accumulated disability was seen in 16.1%, 21.1%, 36.0%, 41.2%, and 44.4%, respectively.
Table 3.
Distribution of mRS Score Shifts and Death by 90 Days by Premorbid mRS Score
The odds ratio of no accumulated disability for patients with PD in model 1 was 7.7 (95% confidence interval [CI] 3.3–18.3, p < 0.001) and in model 2 was 5.2 (95% CI 2.4–11.4, p < 0.001) (Table 3). The E values for these models were 14.8 and 9.9, respectively. Thus, the observed odds ratios of 7.7 and 5.2 could be explained away by an unmeasured confounder that was associated with both the treatment and outcome by a risk ratio of 14.8-fold or 9.9-fold above and beyond the measured confounders. In model 1, the predicted probability of no accumulated disability was 50.1% (95% CI 35.7%–64.5%) for patients with PD vs 15.8% (95% CI 12.2%–19.3%) for patients with NPD (p < 0.001). In model 1, the odds ratio for death in patients with PD was 2.73 (95% CI 1.22–6.14, p = 0.015) and in model 2 was 2.90 (95% CI 1.38–6.09, p = 0.005) (Table 4). In model 1, the predicted probability of death was 28.4% (95% CI 16.6–40.2) for patients with PD vs 14.8% (95% CI 11.2–18.4) for patients with NPD (p < 0.001). We investigated interactions between PD and the covariates in model 1 and did not find them to be significant (Table 5).
Table 4.
Odds Ratios for No Accumulated Disability and Death by 90 Days Shown for Patients With Premorbid Disability Compared to Those With No Premorbid Disability

Table 5.
Significance Level of Interaction Terms Between PD and Covariates

In the sensitivity analysis of patients who had not died by the end of follow-up, we had a cohort of 336 patients, of whom 20 of 32 (62.5%) with PD had no accumulated disability vs 59 of 304 (19.4%) of patients with NPD (p < 0.001). The odds ratio of no accumulated disability for patients with PD in model 1 was 13.7 (95% CI 5.0–37.0, p < 0.001) and in model 2 was 9.3 (95% CI 3.7–23.4, p < 0.001).
Classification of Evidence
This study provides Class II evidence that in anterior circulation acute ischemic stroke treated with EVT, patients with PD compared to those without disability were more likely not to accumulate more disability but were more likely to die.
Discussion
In this study of patients with anterior circulation acute ischemic stroke treated with EVT, we found that PD was associated with a higher probability of not accumulating further disability compared to patients with NPD but also with a higher probability of death. These results expand on a previous analysis6 and show that, in a real-world cohort of consecutive patients treated with the Trevo stentriever from 23 centers in the United States, EVT in patients with PD yields a 50% predicted probability of retaining the premorbid level of impairment.
The implication of our study and prior studies6,7 is that additional research is needed to understand the impact of EVT in patients with stroke with PD. The consistent finding that EVT is associated with a high probability of retaining the premorbid level of disability suggests that stronger evidence than the existing post hoc analyses could change management and guidelines for acute stroke care. However, this finding could also reflect a ceiling effect of the mRS, namely that the scale is insensitive to the shifts between higher ordinal values, particularly between mRS scores of 3 and 4 and scores of 4 and 5.13 Nonetheless, if acute interventions are withheld in patients with stroke with PD, the resulting increase in their level of disability can have devastating consequences. For example, in a prior study.5 of the Oxford Vascular Study, patients with stroke with a premorbid mRS score of 3 had an adjusted hazard ratio of 3.20 (95% CI 1.85–5.54) for 5-year mortality/institutionalization with every 1-point-higher shift in poststroke mRS score. If a randomized clinical trial showed that EVT could reduce that risk for patients with PD, then more informed discussion with patients and caregivers could guide often difficult treatment decisions in this patient population.
There were significant baseline imbalances in our cohort between patients with PD and those with NPD. Patients with PD were older (73.3 vs 65.2 years), less likely to be male (34.0% vs 54.0%), more likely to have atrial fibrillation (69.8% vs 40.1%), and less likely to receive tPA (34.0% vs 56.2%). While we controlled for these imbalances in our regression model, they warrant mention. The first 3 imbalances (age, sex, atrial fibrillation) reflect the fact that patients with PD have survived a prior stroke or other medical event that yielded disability, which makes them more likely to be older, to be female (longer lifespan), and to have atrial fibrillation (associated with advanced age).14,15 The reason for the final imbalance, that patients with PD were less likely to receive tPA, cannot be fully explored with this dataset. Potential explanations include that older patients are less likely to receive tPA, that the PD may have masked symptoms and delayed presentation, that there were other contraindications to tPA that were not captured in TRACK, or that the lack of an evidence base for tPA in patients with PD led to withholding of therapy.16,17
Limitations of our study include its post hoc design and that we did not have a more granular outcome measure than mRS such as the Barthel Index.18 Another major limitation is that we do not have data on patients with large vessel occlusion who did not have EVT, which would have permitted an estimate of the effect of EVT in patients with PD. This would also be valuable to understand the decision-making regarding treatment for patients with large vessel occlusion with PD and reduce confounding from the possibility that patients with PD who had EVT were generally healthier and thus offered intervention. Another limitation is that we excluded 80 patients who did not have a 90-day mRS score and 43 patients who did not have a premorbid mRS score, which may have biased our results, although the baseline demographics were not significantly different in the excluded patients. The current study also only included patients treated with the Trevo stentriever as first-line therapy and could be biased as a result of homogeneity in treatment approach. Finally, because premorbid mRS score was a patient- or caregiver-reported variable, there could be measurement bias, but the measurement of premorbid mRS is inherently subjected to this bias.
Compared to patients with anterior circulation large vessel occlusion stroke without PD treated with EVT, those with PD were more likely to maintain their premorbid functional status at follow-up but also more likely to die as a result of their stroke. As the indications for EVT expand, these preliminary results are hypothesis generating and suggest that dedicated prospective research is needed to compare EVT to medical therapy in patients with stroke with PD.
Glossary
- CI
confidence interval
- EVT
endovascular thrombectomy
- IRB
Institutional Review Board
- LASSO
least absolute shrinkage and selection operator
- mRS
modified Rankin Scale
- NPD
no PD
- PD
premorbid disability
- tPA
tissue plasminogen activator
- TRACK
TREVO Stent-Retriever Acute Stroke
- UW-mRS
utility-weighted mRS
Appendix. Authors

Footnotes
Infographic: http://links.lww.com/WNL/B648
Class of Evidence: NPub.org/coe
Study Funding
Dr. de Havenon is supported by NIH-NINDS K23NS105924.
Disclosure
Dr. de Havenon's department has received funding from AMAG and Regeneron pharmaceuticals for investigator-initiated research, and Dr. de Havenon receives royalties from UpToDate, Inc. Dr. Zaidat serves as a consultant for Neuravi/Cerenovus, Stryker, Penumbra, and Medtronic. Dr. Mistry, Dr. Castonguay, Dr. English, and Dr. Veznedaroglu report no disclosures. Dr. Saver is an employee of the University of California, which holds a patent on retriever devices for stroke. The University of California, Regents receives funding for Dr. Saver's services as a scientific consultant regarding trial design and conduct to Covidien/Medtronic and Stryker. Dr. Saver serves as a consultant for Modest, Abbott, Medtronic, Stryker, and Neuravi/Cerenovus. Dr. Saver also has contracted stock options for Modest and Rapid Medical. Dr. Nguyen receives research support from Medtronic. Dr. Satti serves as a consultant for Balt, Cerenovus, Cordis, Penumbra, Medtronic, Stryker, and Terumo. Dr. Nogueira reports consulting fees for advisory roles with Anaconda, Biogen, Cerenovus, Genentech, Imperative Care, Medtronic, Phenox, Prolong Pharmaceuticals, and Stryker Neurovascular and stock options for advisory roles with Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, Vesalio, Viz.a.i., and Perfuze. Dr. Khatri's department has received funding from Nervive (NIH grant), Cerenovus (grant), Diamedica (scientific advisory board), and Lumosa (consultant), and she receives funding from Bayer (trial national leader) and UpToDate, Inc (royalties). Dr. Mocco has stock/options in Cerebrotech, Imperative Care, Q'Apell, Perflow, CVAid, RIST, and Viz.ai. Dr. Mocco receives research support from Stryker Neurovascular, Microvention, Penumbra, and Medtronic. Go to Neurology.org/N for full disclosures.
References
- 1.Powers WJ, Rabinstein AS, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. [DOI] [PubMed] [Google Scholar]
- 5.Ganesh A, Luengo-Fernandez R, Pendlebury ST., Rothwell PM. Long-term consequences of worsened poststroke status in patients with premorbid disability. Stroke. 2018;49(10):2430-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salwi S, Cutting S, Salgado AD, et al. Mechanical thrombectomy in patients with ischemic stroke with prestroke disability. Stroke. 2020;51(5):1539-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldhoorn R-JB, Merel V, Dippel DWJ, et al. Safety and outcome of endovascular treatment in prestroke-dependent patients. Stroke. 2018;49(10):2406-2414. [DOI] [PubMed] [Google Scholar]
- 8.Zaidat OO, Castonguay AC, Nogueira RG, et al. TREVO stent-retriever mechanical thrombectomy for acute ischemic stroke secondary to large vessel occlusion registry. J Neurointerventional Surg. 2018;10(6):516-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkland SA, Voormolen DC, Esmee V, et al. Utility-weighted modified Rankin scale as primary outcome in stroke trials. Stroke. 2018;49(4):965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribbing J, Nyberg J, Caster O, Jonsson EN. The LASSO: a novel method for predictive covariate model building in nonlinear mixed effects models. J Pharmacokinet Pharmacodyn. 2007;34(4):485-517. [DOI] [PubMed] [Google Scholar]
- 11.Desboulets LDD. A review on variable selection in regression analysis. Econometrics. 2018;6:1-27. [Google Scholar]
- 12.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. [DOI] [PubMed] [Google Scholar]
- 13.Harrison JK, McArthur KS, Quinn TJ. Assessment scales in stroke: clinimetric and clinical considerations. Clin Interv Aging. 2013;8:201-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasson I. Trends in life expectancy and lifespan variation by educational attainment: United States, 1990-2010. Demography. 2016;53(2):269-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N-9N. [DOI] [PubMed] [Google Scholar]
- 16.Messé SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? Neurology. 2016;87(15):1565-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlino G, Corazza E, Lorenzut S, Gigli GL, Cargnelutti D, Valente M. Efficacy and safety of intravenous thrombolysis in patients with acute ischemic stroke and pre-existing disability. J Clin Med. 2019;8(1):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn TJ, Langhorne P, Stott DJ. Barthel Index for stroke trials. Stroke. 2011;42(4):1146-1151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator for purposes of replicating results.


