ABSTRACT
The literature regarding COVID-19-associated pulmonary aspergillosis (CAPA) has shown conflicting observations, including survival of CAPA patients not receiving antifungal therapy and discrepancy between CAPA diagnosis and autopsy findings. To gain insight into the pathophysiology of CAPA, we performed a case-control study in which we compared Aspergillus test profiles in CAPA patients and controls in relation to intensive care unit (ICU) mortality. This was a multinational case-control study in which Aspergillus test results, use of antifungal therapy, and mortality were collected from critically ill COVID-19 patients. Patients were classified using the 2020 European Confederation for Medical Mycology and the International Society for Human and Animal Mycology (ECMM/ISHAM) consensus case definitions. We analyzed 219 critically ill COVID-19 cases, including 1 proven, 38 probable, 19 possible CAPA cases, 21 Aspergillus-colonized patients, 7 patients only positive for serum (1,3)-β-d-glucan (BDG), and 133 cases with no evidence of CAPA. Mortality was 53.8% in CAPA patients compared to 24.1% in patients without CAPA (P = 0.001). Positive serum galactomannan (GM) and BDG were associated with increased mortality compared to serum biomarker-negative CAPA patients (87.5% versus 41.7%, P = 0.046; 90.0% versus 42.1%, P = 0.029, respectively). For each point increase in GM or 10-point BDG serum concentration, the odds of death increased (GM, odds ratio [OR] 10.208, 95% confidence interval [CI], 1.621 to 64.291, P = 0.013; BDG, OR, 1.247, 95% CI, 1.029 to 1.511, P = 0.024). CAPA is a complex disease, probably involving a continuum of respiratory colonization, tissue invasion, and angioinvasion. Serum biomarkers are useful for staging CAPA disease progression and, if positive, indicate angioinvasion and a high probability of mortality. There is need for a biomarker that distinguishes between respiratory tract colonization and tissue-invasive CAPA disease.
KEYWORDS: COVID-19, critically ill, invasive pulmonary aspergillosis, mortality, mycology
INTRODUCTION
Over the past decade, high frequencies of invasive pulmonary aspergillosis (IPA) have been reported in critically ill influenza patients, with a mortality rate exceeding 50% (1, 2). Over the past year, numerous cohort studies have reported IPA secondary to another viral pneumonia, coronavirus disease 2019 (COVID-19), with frequencies ranging between 3% to 33% in critically ill patients (3–7). These studies have used various COVID-19-associated pulmonary aspergillosis (CAPA) case definitions, which complicates interstudy comparisons. Recently, the European Confederation for Medical Mycology and the International Society for Human and Animal Mycology (ECMM/ISHAM) published a consensus case definition which aims to standardize clinical studies and case registries (8).
The literature regarding CAPA has been confusing due to conflicting observations, including difficulty in diagnosing CAPA (9) due to low sensitivity of serum biomarkers and the reluctance to perform bronchoscopy and bronchoalveolar lavage (BAL) during the first wave (10). Consequently, other respiratory specimens and procedures have been used to diagnose CAPA, including nonbronchoscopic lavage (NBL) and bronchial aspirate (BA) (11). However, it remains unclear if detection of Aspergillus in these specimens corresponds with IPA or reflects upper respiratory tract colonization. Although several studies show higher mortality in patients with CAPA than critically ill COVID-19 patients without CAPA (3, 4), there are numerous CAPA cases reported that survived without receiving antifungal therapy (4, 5, 12). Furthermore, autopsy studies have failed to document IPA in patients who were classified as probable CAPA (11).
To gain more insight into the pathophysiology of CAPA, we performed a case-control study in which we collected data on various Aspergillus tests and fungal biomarkers, antifungal therapy, and intensive care unit (ICU) mortality and compared the Aspergillus test profiles in CAPA patients and controls in relation to ICU mortality. As ICU mortality may not have been attributable to CAPA, we also analyzed the effect of antifungal therapy on patient outcome.
MATERIALS AND METHODS
Study design.
A prospective multinational observational case-control study was performed involving six centers, Amsterdam UMC (Amsterdam, the Netherlands), Amphia Hospital (Breda, the Netherlands), Radboud University Medical Centre (Nijmegen, the Netherlands), University Hospitals Leuven (Leuven, Belgium), Hôpital Saint-Louis (Paris, France), and University Hospital of Wales (Cardiff, United Kingdom), the latter providing screening for seven regional critical care units across Wales. Centers were invited to submit cases suspected of CAPA in whom respiratory specimens were positive for Aspergillus culture, GM, or Aspergillus PCR test or in whom serum was positive for GM or Aspergillus PCR, and controls in whom no evidence of CAPA was found. CAPA was classified according to the ECMM/ISHAM 2020 consensus case definitions (8), and colonization of the airway was considered when Aspergillus culture was only positive in upper respiratory tract specimens (e.g., sputum or BA). This case-control study had been reviewed and approved by the Research Ethics Committee of the Radboud University Medical Center and conformed to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting of case-control studies (Table S3 in the supplemental material).
Participants.
Patients with severe COVID-19 were included as cases if they were ≥18 years, admitted to the ICU, and diagnosed with proven/probable CAPA. Controls with severe COVID-19 were included if they were ≥18 years, admitted to the ICU, and where no evidence for CAPA was found. For each proven/probable CAPA case, three to four critically ill COVID-19 controls consecutively admitted to the ICU were included, in whom there was no evidence for CAPA. Patients classified as possible CAPA, colonized with Aspergillus, and patients only positive for serum BDG were excluded from primary analysis and analyzed as separate groups.
Diagnostic material and tests.
BAL fluid was obtained by bronchoscopy when there was a suspicion of pulmonary aspergillosis; when bronchoscopy was not feasible, NBL enabled sampling of the lower respiratory tract but without visualization of the airways. BA was obtained by suction of respiratory material from the upper respiratory tract, while sputum was respiratory material coughed up by the patient. BA and sputum did not contribute to CAPA classification and were used as a screening tool or to follow up on antifungal therapy effect.
Diagnostic tests involved microscopy and culture of respiratory samples, GM, and Aspergillus PCR and serum GM, Aspergillus PCR, and (1,3)-β-d-glucan (BDG). These tests were performed at the participating institutes when available; otherwise, these tests were performed in one of the other participating centers. Cultured Aspergillus species were identified using colony morphology, microscopic morphology, and ability to grow at 48°C. GM was detected using Platelia Aspergillus (Bio-Rad) using a GM index of >0.5 as cutoff for serum and ≥1.0 for BAL fluid. Serum BDG was detected using the Fungitell assay (Associates of Cape Cod) with 80 pg/ml as cutoff for positivity. Aspergillus DNA was detected by PCR using the commercial AsperGenius test (Pathofinder) or an in-house PCR following international methodological recommendations (13, 14). All tests were performed following the manufacturer’s instructions, including appropriate quality controls.
In addition to diagnostic test information, the administration of antifungal therapy and the ICU mortality was collected using the Castor database. The analysis focused on the relationship between CAPA classification and mortality and Aspergillus test profiles and mortality. In addition, the effect of antifungal therapy on mortality was investigated.
Statistical analysis.
Continuous variables are shown as median (interquartile range [IQR]) and categorical variables as counts and percentages. Student's t test and Mann-Whitney U test, as appropriate, and Fisher’s exact test were used to compare differences in baseline characteristics and clinical outcomes. To evaluate and compare the 30-day ICU mortality, univariable binary logistic regression was used. Baseline characteristics found to be significant in the univariable binary logistic regression analysis were tested in multivariable binary logistic regression analysis, with Aspergillus diagnostic test outcomes alternatively included to assess their effect on mortality. Estimated 30-day ICU mortality was expressed graphically using the Kaplan-Meier method for illustrational purposes; the statistical difference between groups was assessed by the log-rank test. All statistical analyses were performed using SPSS software version 26 (IBM, Chicago, IL, USA) and supervised by a biostatistician. P values of <0.05 were considered statistically significant.
RESULTS
CAPA classification and ICU mortality.
Between 8 February 2020 and 22 May 2020, 219 critically ill COVID-19 patients admitted to the ICU were included from the first COVID-19 wave (Fig. S1 in the supplemental material). Classification of these patients resulted in 1 proven CAPA case, 38 (17.4%) probable cases, and 19 (8.7%) possible cases. Twenty-one Aspergillus-positive (9.6%) patients did not fulfill the CAPA case definition criteria and were considered colonized with Aspergillus. One probable CAPA patient was diagnosed with tracheobronchitis. In 133 (60.7%) patients, there was no evidence for CAPA, while 7 (3.2%) patients were serum BDG positive without any other indication for CAPA. A comparison of baseline characteristics and clinical outcomes is shown in Table 1, and an overview of samples obtained and diagnostic tests performed is given in Table 2. An overview of Aspergillus test profiles is shown in Table 3.
TABLE 1.
Overview of patient baseline characteristics and clinical outcomes
| Characteristic | Data for patients with: |
||||
|---|---|---|---|---|---|
| Proven/probable CAPA (n = 39) | No evidence of CAPA (n = 133) | Possible CAPA (n = 19) | Aspergillus colonized/only serum BDG positive (n = 28) | P valuea,c | |
| Baseline characteristics | |||||
| Age (median [IQR] [yrs]) | 65 (58–75) | 60 (52–69) | 68 (58–73) | 66 (58–71) | 0.003 |
| Male (no. [%]) | 28 (71.8) | 98 (73.7) | 13 (68.4) | 19 (67.9) | 0.838 |
| Hematological malignancy (no. [%]) | 1 (2.6) | 8 (6.0) | 0 | 1 (3.6) | 0.686 |
| Solid organ malignancy (no. [%]) | 4 (10.3) | 8 (6.0) | 0 | 4 14.3) | 0.472 |
| Stem cell transplant (no. [%]) | 1 (2.6) | 2 (1.5) | 0 | 0 | 0.540 |
| Solid organ transplant (no. [%]) | 1 (2.6) | 2 (1.5) | 0 | 0 | 0.540 |
| Pulmonary disease (no. [%]) | 9 (23.1) | 21 (15.8) | 6 (31.6) | 9 (32.1) | 0.338 |
| Cardiovascular disease (no. [%]) | 16 (41.0) | 53 (39.8) | 7 (36.8) | 11 (39.3) | 0.999 |
| Diabetes mellitus (no. [%]) | 10 (25.6) | 31 (23.3) | 7 (36.8) | 3 (10.7) | 0.831 |
| Chronic kidney disease (no. [%]) | 1 (2.6) | 3 (2.3) | 0 | 2 (7.1) | 0.999 |
| Autoimmune disease (no. [%]) | 2 (5.1) | 6 (4.5) | 2 (10.5) | 2 (7.1) | 0.999 |
| EORTC/MSGERCd host factors (no. [%]) | 5 (12.8) | 11 (8.3) | 1 (5.3) | 4 (14.3) | 0.364 |
| COVID-19 treatment (treated) (no. [%]) | 26 (66.7) | 80 (60.2) | 12 (63.2) | 20 (71.4) | 0.575 |
| Hydroxychloroquine | 21 (53.8) | 74 (55.6) | 8 (42.1) | 16 (57.1) | 0.857 |
| Remdesivir | 1 (2.6) | 15 (11.3) | 0 | 2 (7.1) | 0.124 |
| Lopinavir/ritonavir | 7 (17.9) | 3 (2.3) | 1 (5.3) | 3 (10.7) | 0.001 |
| Anakinra | 2 (5.1) | 17 (12.8) | 0 | 1 (3.6) | 0.250 |
| Azithromycin | 8 (20.5) | 7 (5.3) | 0 | 2 (7.1) | 0.007 |
| Corticosteroids | 10 (25.6) | 12 (9.0) | 9 (47.4) | 6 (21.4) | 0.012 |
| Clinical outcomes | |||||
| Antifungal treatment (treated) (no. [%]) | 28 (71.8) | 16 (12.0) | 33 (70.2) | 77 (35.2)b | 0.000 |
| Voriconazole | 6 (21.4) | 5 (31.3) | 13 (39.4) | 24 (31.2) | 0.492 |
| Caspofungin | 1 (3.6) | 5 (31.3) | 1 (3.0) | 7 (9.1) | 0.018 |
| Amphotericin B | 3 (10.7) | 1 (6.3) | 6 (14.0) | 10 (13.0) | 0.638 |
| Voriconazole and anidulafungin | 17 (60.7) | 5 (31.3) | 11 (33.3) | 33 (42.9) | 0.116 |
| Voriconazole and caspofungin | 0 | 0 | 1 (3.0) | 1 (1.3) | NA |
| Voriconazole and amphotericin B | 1 (3.6) | 0 | 0 | 1 (1.3) | 0.999 |
| Time to diagnose CAPA (median [IQR] [days]) | 7 (4–15) | NA | 5 (2–15) | NA | NA |
| Length of stay in ICU (median [IQR] [days]) | 18 (13–30) | 21 (13–31) | 24 (13–42) | 21 (13–34) | 0.391 |
P value was calculated comparing proven/probable CAPA cases with controls where no evidence of CAPA was found and was considered significant when the P value was <0.05.
For one patient only positive for serum BDG, it was unknown which antifungal treatment was given.
Boldface type in the last column indicates statistical significance.
EORTC/MSGERC, European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium.
TABLE 2.
Overview of Aspergillus tests performed and test results
| Test | No. (%) of patients tested (n = 219) | No. (%) of CAPA cases tested (n = 39) | No. (%) of controls tested (n = 133) | Median (IQR) no. of samples | No. (%) of positive patients | No. (%) of negative patients |
|---|---|---|---|---|---|---|
| BAL Aspergillus culture | 77 (35) | 32 (82) | 37 (28) | 1 (1–1) | 17 (22) | 63 (82) |
| BAL GM index ≥ 1.0 | 71 (32) | 32 (82) | 31 (23) | 1 (1–1) | 22 (31) | 53 (75) |
| BAL Aspergillus PCR | 44 (20) | 15 (38) | 28 (21) | 1 (1–1) | 7 (16) | 38 (86) |
| NBL Aspergillus culture | 42 (19) | 7 (18) | 13 (10) | 2 (1–3) | 20 (48) | 33 (79) |
| NBL GM index ≥ 4.5 | 39 (18) | 6 (15) | 12 (9) | 1 (1–2) | 17 (44) | 31 (79) |
| NBL Aspergillus PCR | 26 (12) | 4 (10) | 9 (7) | 1 (0–2) | 16 (62) | 14 (54) |
| BA Aspergillus culture | 95 (43) | 6 (15) | 70 (53) | 4 (2–6) | 21 (22) | 84 (88) |
| Sputum Aspergillus culture | 72 (33) | 2 (5) | 53 (40) | 1 (1–2) | 13 (18) | 63 (88) |
| serum GM index > 0.5 | 188 (86) | 32 (82) | 113 (85) | 1 (1–4) | 8 (4) | 181 (96) |
| Serum BDG ≥ 80 pg/ml | 146 (67) | 29 (74) | 77 (58) | 1 (1–2) | 28 (19) | 124 (85) |
| Serum Aspergillus PCR | 48 (22) | 16 (41) | 20 (15) | 1 (1–1) | 6 (15) | 44 (92) |
TABLE 3.
Overview of positive Aspergillus tests and biomarkers according to CAPA classificationc
| BAL Aspergillus culture | BAL GM index ≥ 1.0 | BAL Aspergillus PCR | NBL Aspergillus culture | NBL GM index ≥ 4.5 | NBL GM index ≥ 1.2 (≥2) | NBL Aspergillus PCR | BA Aspergillus culture | Sputum Aspergillus culture | Serum GM index > 0.5 | Serum BDG ≥ 80 pg/ml | Serum Aspergillus PCR | No. (%) with proven CAPA (n = 1) | No. (%) with probable CAPA (n = 38) | No. (%) with possible CAPA (n = 19) | No. (%) Aspergillus colonized/only serum BDG positive (n = 28) | No. (%) with no evidence of CAPA (n = 133) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ○ | ○ | ○ | 1 (100)a | |||||||||||||
| ● | 4 (11) | |||||||||||||||
| ● | 12 (32) | |||||||||||||||
| ● | ● | 4 (11) | ||||||||||||||
| ● | ● | 1 (3)b | ||||||||||||||
| ● | ● | 1 (3) | ||||||||||||||
| ● | ● | ○ | 1 (3) | |||||||||||||
| ● | ● | ○ | ○ | ○ | 1 (3) | |||||||||||
| ● | ● | ● | ○ | ○ | ○ | 1 (3) | ||||||||||
| ● | ○ | 1 (3) | ||||||||||||||
| ● | ● | ● | ○ | 1 (3) | ||||||||||||
| ● | ● | ○ | 2 (5) | |||||||||||||
| ● | ● | ● | ○ | 1 (3) | ||||||||||||
| ● | 1 (3) | |||||||||||||||
| ● | ○ | 2 (5) | ||||||||||||||
| ○ | ● | ○ | 1 (3) | |||||||||||||
| ○ | ● | ○ | ○ | 2 (3) | ||||||||||||
| ○ | ○ | ○ | ○ | ● | ○ | ○ | 1 (3) | |||||||||
| ○ | ○ | ○ | ○ | ● | ○ | ● | 1 (3) | |||||||||
| ● | 4 (16) | |||||||||||||||
| ● | ● | 1 (5) | ||||||||||||||
| ● | ● | 2 (11) | ||||||||||||||
| ● | ● | ● | 1 (5) | |||||||||||||
| ● | ● | ● | 2 (11) | |||||||||||||
| ● | ○ | 1 (5) | ||||||||||||||
| ● | ● | ● | ● | ○ | 1 (5) | |||||||||||
| ● | ● | ● | ○ | ○ | 1 (5) | |||||||||||
| ● | ● | ● | ● | ○ | ○ | 1 (5) | ||||||||||
| ● | ● | ○ | 1 (5) | |||||||||||||
| ● | ● | ○ | 1 (5) | |||||||||||||
| ● | ● | ● | ○ | 1 (5) | ||||||||||||
| ● | ○ | 2 (11) | ||||||||||||||
| ● | 10 (48) | |||||||||||||||
| ● | 4 (19) | |||||||||||||||
| ● | ● | 3 (14) | ||||||||||||||
| ● | ○ | 2 (10) | ||||||||||||||
| ● | ○ | 1 (5) | ||||||||||||||
| ● | ● | ○ | 1 (5) | |||||||||||||
| ○ | 7 (5) |
Proven CAPA case diagnosed with positive Aspergillus culture performed on lung tissue obtained through biopsy.
Probable CAPA case also diagnosed with tracheobronchitis, as seen on bronchoscopy.
●, positive test that contributes to patient classification; ○, positive test that does not contribute to patient classification.
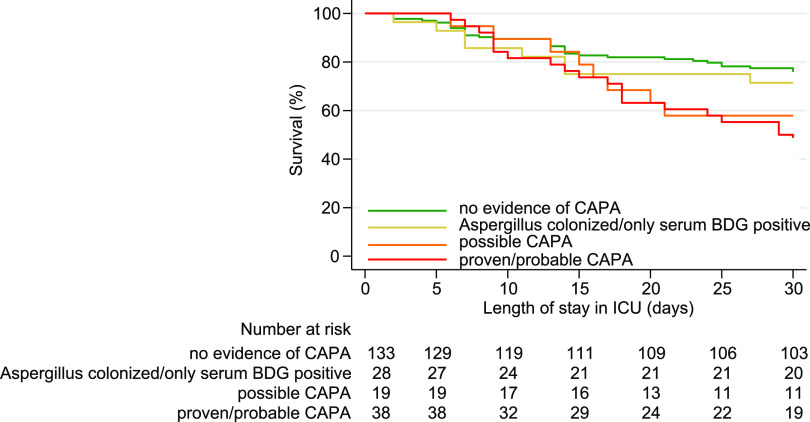
The 30-day ICU mortality in proven/probable CAPA cases compared to controls without any evidence of CAPA was higher (53.8% versus 24.1%, P = 0.001) and remained higher after adding Aspergillus-colonized patients or only serum BDG-positive patients to the control group (53.8% versus 24.8%, P = 0.001). Similarly, the 30-day ICU mortality remained higher when possible CAPA patients were included in the CAPA group (50.0% versus 24.1%, P = 0.001) and after adding Aspergillus-colonized patients or serum BDG-positive patients to the control group (50.0% versus 24.8%, P = 0.001). Kaplan-Meier analysis (Fig. 1) showed that the estimated 30-day ICU mortality was highest in patients with proven/probable CAPA (53.8%), followed by patients with possible CAPA (42.1%), Aspergillus-colonized patients, or those only positive for serum BDG (28.6%), and lowest in patients without evidence for CAPA (24.1%). A univariable comparison of the 30-day ICU mortality rates and odds ratios for the various patient baseline characteristics and Aspergillus diagnostic test outcomes are shown in Table 4. Patient age and hematological malignancies as underlying conditions were statistically significant baseline characteristics for 30-day ICU mortality and were therefore used in multivariable logistic regression analysis comparing subgroups of proven/probable CAPA cases.
FIG 1.
Kaplan-Meier survival curve and table of number at risk per CAPA classification. Comparing proven/probable CAPA cases with controls where no evidence of CAPA was found was significant (P = 0.000) and nonsignificant in all other comparisons. BDG, (1,3)-β-d-glucan; CAPA, COVID-19-associated pulmonary aspergillosis.
TABLE 4.
Group comparisons and univariable logistic regression analysis for 30-day ICU mortality in proven/probable CAPA cases and controls where no evidence of CAPA was found
| Characteristic | Group comparison (n = 172) | OR (95% CI) | P valuea,b |
|---|---|---|---|
| Baseline characteristics | |||
| Age (yrs) | 1.054 (1.021–1.089) | 0.001 | |
| Gender (male vs female) (no. [%]) | 39 (31.0) vs 14 (30.4) | 1.025 (0.492–2.132) | 0.948 |
| Hematological malignancy (yes vs no) (no. [%]) | 6 (66.7) vs 47 (28.8) | 4.936 (1.185–20.560) | 0.028 |
| Solid organ malignancy (yes vs no) (no. [%]) | 2 (16.7) vs 51 (31.9) | 0.427 (0.090–2.022) | 0.284 |
| Stem cell transplant (yes vs no) (no. [%]) | 3 (100) vs 50 (29.6) | 0.999 | |
| Solid organ transplant (yes vs no) (no. [%]) | 1 (33.3) vs 52 (30.8) | 1.125 (0.100–12.684) | 0.924 |
| Pulmonary disease (yes vs no) (no. [%]) | 9 (30.0) vs 44 (31.0) | 0.955 (0.405–2.251) | 0.915 |
| Cardiovascular disease (yes vs no) (no. [%]) | 22 (31.9) vs 31 (30.1) | 1.087 (0.563–2.100) | 0.804 |
| Diabetes mellitus (yes vs no) (no. [%]) | 15 (36.6) vs 38 (29.0) | 1.412 (0.674–2.957) | 0.360 |
| Chronic kidney disease (yes vs no) (no. [%]) | 3 (75.0) vs 50 (29.8) | 7.080 (0.719–69.720) | 0.093 |
| Autoimmune disease (yes vs no) (no. [%]) | 2 (25.0) vs 51 (31.1) | 0.739 (0.144–3.785) | 0.716 |
| EORTC/MSGERC host factors (yes vs no) (no. [%]) | 9 (47.4) vs 44 (28.8) | 2.230 (0.848–5.859) | 0.104 |
| COVID-19 treatment (treated vs not treated) (no. [%]) | 29 (27.1) vs 24 (36.9) | 0.635 (0.328–1.229) | 0.178 |
| Hydroxychloroquine | 25 (26.3) vs 28 (36.4) | 0.625 (0.326–1.199) | 0.157 |
| Remdesivir | 3 (18.8) vs 50 (32.1) | 0.489 (0.133–1.794) | 0.281 |
| Lopinavir and ritonavir | 5 (50.0) vs 48 (29.6) | 2.375 (0.657–8.582) | 0.187 |
| Anakinra | 3 (15.8) vs 50 (32.7) | 0.386 (0.108–1.387) | 0.145 |
| Azithromycin | 5 (33.3) vs 48 (30.6) | 1.135 (0.368–3.501) | 0.825 |
| Corticosteroids | 7 (31.8) vs 46 (30.7) | 1.055 (0.403–2.761) | 0.913 |
| Medical center (site) | 0.855 (0.713–1.027) | 0.093 | |
| Diagnostic test outcomes | |||
| BAL Aspergillus culture (positive vs negative) (no. [%]) | 9 (52.9) vs 16 (30.8) | 2.531 (0.826–7.756) | 0.104 |
| BAL GM index ≥ 1.0 (positive vs negative) (no. [%]) | 10 (45.5) vs 15 (36.6) | 1.444 (0.504–4.139) | 0.494 |
| BAL Aspergillus PCR (positive vs negative) (no. [%]) | 4 (57.1)/11 (30.6) | 3.030 (0.578–15.880) | 0.190 |
| Serum GM index > 0.5 (positive vs negative) (no. [%]) | 7 (87.5) vs 38 (27.7) | 18.237 (2.171–153.217) | 0.008 |
| Serum BDG ≥ 80 pg/ml (positive vs negative) (no. [%]) | 9 (90.0) vs 29 (30.2) | 20.793 (2.517–171.750) | 0.005 |
| Serum Aspergillus PCR (positive vs negative) (no. [%]) | 4 (80.0) vs 12 (38.7) | 6.333 (0.630–63.639) | 0.117 |
| CAPA diagnosis | |||
| CAPA (case vs control) (no. [%]) | 21 (53.8) vs 32 (24.1) | 3.682 (1.749–7.753) | 0.001 |
| Antifungal treatment (treated vs not treated) (no. [%]) | 25 (56.8) vs 28 (21.9) | 4.699 (2.267–9.742) | 0.000 |
P value was calculated using univariable binary logistic regression analysis.
Boldface type in the last column indicates statistical significance.
BAL fluid GM and ICU mortality.
As GM levels are associated with Aspergillus tissue burden, we investigated the association between BAL fluid GM concentration and ICU mortality. The 30-day ICU mortality in proven/probable CAPA cases with positive BAL fluid GM (GM index ≥ 1.0) was similar to that of proven/probable CAPA cases without positive BAL fluid GM (45.5% versus 50.0%; odds ratio [OR], 0.833; 95% confidence interval [CI], 0.187 to 3.723; P = 0.811) and remained nonsignificant after adjusting for age and hematological malignancies (adjusted OR, 0.602; 95% CI, 0.095 to 3.812; P = 0.590). Analyzing the initial BAL fluid GM concentration measured, for each point increase in BAL fluid GM concentration, the odds of death within 30 days of ICU admission did not increase (OR, 1.128; 95% CI, 0.833 to 1.529; P = 0.437) and remained nonsignificant after adjusting for age and hematological malignancies (adjusted OR, 1.035; 95% CI, 0.748 to 1.433; P = 0.833). Similarly, analyzing the highest BAL fluid GM concentration measured, for each point increase in BAL fluid GM concentration, the odds of death within 30 days of ICU admission did not increase (OR, 1.097; 95% CI 0.820 to 1.470; P = 0.532) (Fig. 2) and remained nonsignificant after adjusting for age and hematological malignancies (adjusted OR, 1.003; 95% CI, 0.732 to 1.374; P = 0.987).
FIG 2.
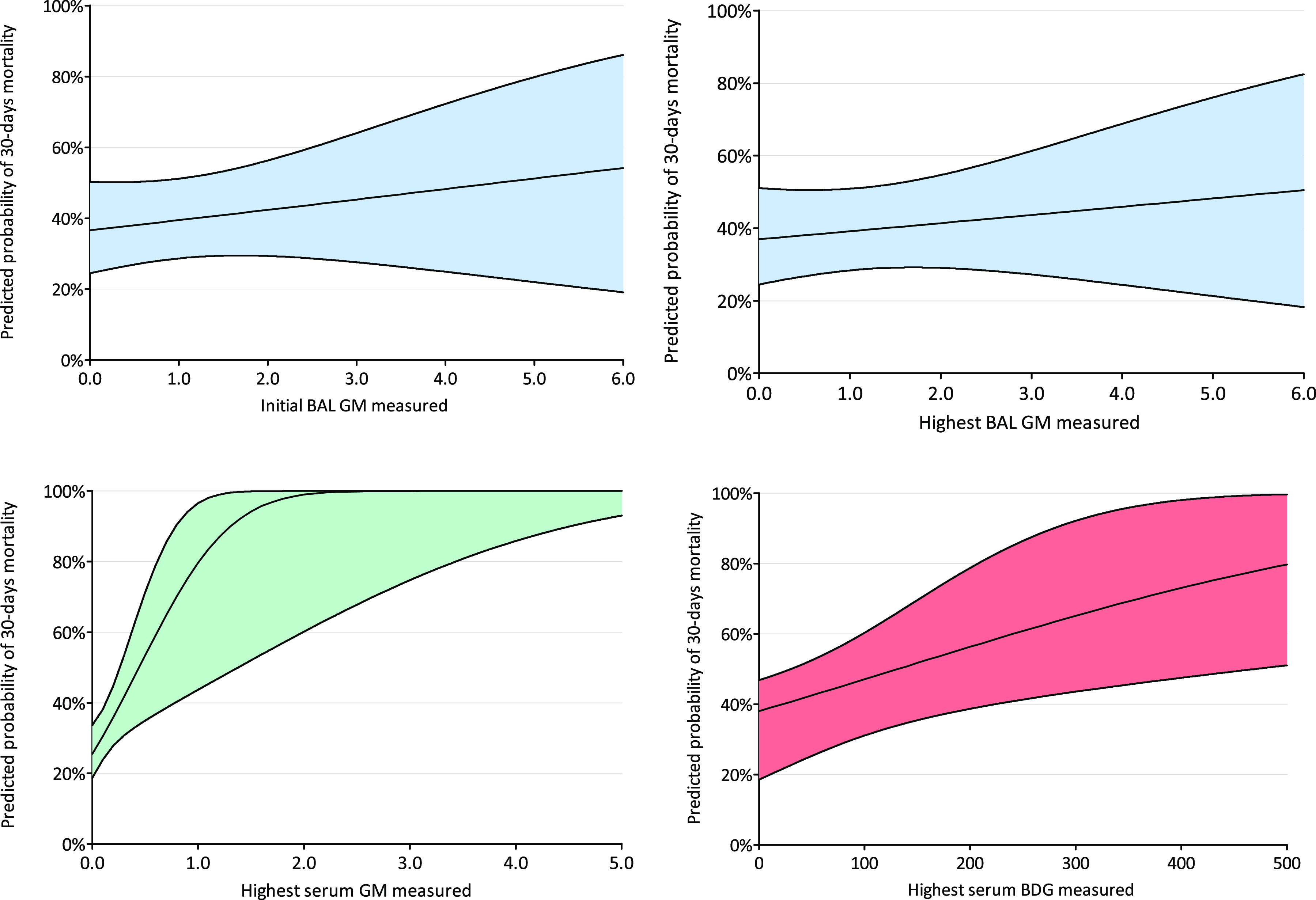
Predicted probability of 30-day mortality for initial BAL GM concentration measured (top left), highest BAL GM concentration measured (top right), highest serum GM concentration measured (bottom left), and highest serum BDG concentration measured (bottom right). BAL, bronchoalveolar lavage; BDG, (1,3)-β-d-glucan; CAPA, COVID-19-associated pulmonary aspergillosis; GM, galactomannan.
Serum fungal biomarkers and ICU mortality.
As a positive serum biomarker may indicate more advanced Aspergillus infection, we compared the mortality in patients with proven or probable CAPA, with and without positive serum biomarker. The 30-day ICU mortality in proven/probable CAPA cases with any positive serum biomarker compared to proven/probable CAPA cases without any positive serum biomarker was significantly higher (83.3% versus 35.0%; P = 0.014), remaining higher for all individual serum biomarkers, with the exception of serum Aspergillus PCR (serum GM, 87.5% versus 41.7%, OR, 9.800, 95% CI, 1.036 to 92.696, P = 0.046; serum BDG, 90.0% versus 42.1%, OR, 12.375, 95% CI, 1.294 to 118.331, P = 0.029; and serum Aspergillus PCR, 80.0% versus 63.6%, OR, 2.286, 95% CI, 0.185 to 28.186, P = 0.600). After adjusting for age and hematological malignancies, similar results were measured (serum GM, adjusted OR, 11.768, 95% CI, 1.060 to 130.631, P = 0.045; serum BDG, adjusted OR, 16.259, 95% CI, 1.339 to 197.415, P = 0.029; and serum Aspergillus, PCR-adjusted OR, 2.593, 95% CI, 0.168 to 39.991; P = 0.495). The univariable difference for serum BDG remained significant using cutoff values of 60 pg/ml and 100 pg/ml, as all proven/probable CAPA cases positive for serum BDG had a BDG value of ≥100 pg/ml, and all proven/probable CAPA cases negative for serum BDG had a BDG value of <60 pg/ml. Positive serum BDG was not only observed among proven/probable CAPA cases but also in other patient categories, including seven possible CAPA patients, four Aspergillus-colonized patients, and seven patients where no other evidence for CAPA was found (Table 3).
Of eight serum GM-positive patients, only one survived. This patient had a single positive serum GM but negative BAL fluid GM and culture, negative BA culture, and negative subsequent serum GM tests. Although this patient was classified as probable CAPA, it is likely that the serum GM was falsely positive.
Analyzing the highest serum GM and BDG concentration measured, for each point increase in serum GM concentration, the odds of death within 30 days of ICU admission increased (OR, 10.208; 95% CI, 1.621 to 64.291; P = 0.013) (Fig. 2), and for each 10-point increase in serum BDG concentration, the odds of death within 30 days of ICU admission increased (OR, 1.247; 95% CI, 1.029 to 1.511; P = 0.024) (Fig. 2). After adjusting for age and hematological malignancies, the odds of death within 30 days of ICU admission remained increasing significantly for each point increase in serum GM concentration (OR, 8.966; 95% CI, 1.463 to 54.939; P = 0.018) and for each 10-point increase in serum BDG (OR, 1.293; 95% CI, 1.032 to 1.620; P = 0.025). An overview of Aspergillus tests performed and test results for proven/probable CAPA cases positive for any serum biomarker are provided in Table S1.
Antifungal treatment and ICU mortality.
Antifungal treatment was started in 28 (71.8%) proven/probable CAPA cases, in 19 (100%) patients with possible CAPA, in 11 (52.4%) patients colonized with Aspergillus, in 3 (42.9%) patients only positive for serum BDG, and in 16 (12.0%) controls where no evidence of CAPA was found.
As higher mortality in CAPA patients not treated with antifungal therapy can be expected, we compared the mortality in proven/probable CAPA patients treated and not treated with antifungal therapy. The differences in 30-day ICU mortality in proven/probable CAPA patients treated with antifungal therapy was nonsignificant (57.1% versus 45.5%, P = 0.511) and remained nonsignificant after adjusting for age and hematological malignancies (OR, 1.530; 95% CI, 0.309 to 7.579; P = 0.602). An overview of Aspergillus tests profiles for proven/probable CAPA patients not treated with antifungal therapy is provided in Table S2.
DISCUSSION
Our study showed a significantly higher ICU mortality in COVID-19 patients with proven/probable CAPA (54%) than those without evidence of CAPA (24%), similar to mortality rates reported in previous studies. A recent cohort showed a mortality rate of 52.2%, of which 17.2% was attributed to Aspergillus infection (15). When we investigated mortality in patients with various Aspergillus test profiles, the presence of a positive serum biomarker was associated with a significantly higher mortality than serum biomarker negative CAPA cases. Mortality rates in serum-positive CAPA patients (80% to 90%) are very high compared to mortality rates in any other host group at risk to develop IPA. Positive serum biomarkers are indicative of angioinvasion, which is a hallmark pathological feature of IPA. Indeed, an animal study comparing cytarabine-induced neutropenic rabbits with animals with cyclosporine-methylprednisolone-induced immunosuppression showed a correlation between angioinvasion and the levels of serum GM and BDG (16). We also found a significant association between GM and BDG serum concentration and 30-day ICU mortality, which suggests a relationship between high Aspergillus tissue burden and poor outcome. A correlation between serum GM concentration and all-cause mortality was previously reported for allogeneic stem cell transplant recipients (17). Furthermore, a correlation between serum BDG level and mortality was previously found for critically ill patients with candidemia, where an initial BDG of >287 pg/ml was a significant predictor of 28-day mortality (18). However, BDG is not specific for IPA, and some studies indicate only a modest contribution of BDG to the diagnosis of invasive fungal infection in critically ill patients due to low sensitivity and positive predictive value (19). Indeed, we observed circulating BDG in various patient groups, including those with no (other) diagnostic evidence for CAPA. Thus, positive serum BDG should be interpreted with caution and supported by other diagnostic Aspergillus tests to confirm CAPA diagnosis. Our findings suggest that serum GM and serum BDG provide a valuable adjunct to Aspergillus test results from respiratory specimens in patients suspected of CAPA. Serum biomarker seropositivity may be important for early recognition of CAPA patients at risk for a poor outcome.
Based on histopathology of CAPA patients with proven tracheobronchitis, we recently postulated an angioinvasion threshold model, which distinguishes between various stages of invasive growth of Aspergillus, involving a continuum of Aspergillus colonization, tissue invasion, and angioinvasion (20). Factors that determine the ability of Aspergillus to reach the angioinvasion threshold include viral lytic effects, immune dysregulation, and immune modulation treatments. Given the low percentage of CAPA patients with positive serum biomarkers (25% for serum GM and 34% for serum BDG), the angioinvasion threshold is reached in only a minority of CAPA patients. This contrasts with influenza-associated pulmonary aspergillosis where circulating GM is detected in up to 65% of patients (2). Alternatively, in serum biomarker-negative CAPA patients, the mortality was approximately 15% higher than critically ill patients without CAPA. This modest increase in ICU mortality may indicate a limited impact of CAPA on attributable mortality. However, despite this group being classified as probable CAPA, based on positive BAL fluid GM or culture, detection of Aspergillus in BAL fluid may reflect respiratory tract colonization and not IPA (9). Also, the case definitions we used are consensus based and have never been compared with the gold standard. Besides biopsy specimens, there is currently no biomarker that enables us to distinguish between respiratory tract colonization and tissue invasion. BAL fluid GM concentration is correlated with fungal burden, and a BAL fluid GM of >3.0 was shown to rule in IPA diagnosis independent of the pretest probability (21). Bartoletti and colleagues found a correlation between initial BAL fluid GM and mortality in CAPA patients, with the odds of death increasing 1.41-fold (1.10 to 1.81; P = 0.007) for each point increase in the initial BAL fluid GM index (3). In our study, however, we were unable to find a correlation between BAL fluid GM concentration and mortality, which might be due to insufficient power. Also, positive BAL fluid GM rate and 30-day ICU mortality in patients with positive BAL fluid GM were similar in both studies; however, the mortality in patients without positive BAL fluid GM was significantly higher in our study (18 [37%] patients versus 15 [19%] patients; P = 0.038). We could not find an explanation for this high mortality, as there were no differences in baseline characteristics, clinical outcomes, or diagnostic test outcomes comparing patients alive and deceased in whom BAL fluid GM was measured. The BAL fluid GM concentration could also have been affected by factors related to the procedure (e.g., sample location, volume of saline used, etc.) A biomarker that distinguishes between respiratory tract colonization and tissue-invasive disease might include BAL fluid GM concentration, a second biomarker such as Aspergillus DNA, or a host response marker associated with tissue invasion.
We were unable to find a correlation between antifungal therapy and mortality. Such a correlation has been found previously and supports a role of CAPA in patient mortality (3, 4). However, other studies have failed to find a benefit of antifungal therapy and report CAPA cases surviving without receiving antifungal therapy (4, 5, 12). It is likely that the survival outcome of CAPA does not solely depend on the administration of antifungal therapy, and other treatment interventions may be beneficial to preclude or even reverse Aspergillus tissue invasion. For instance, the administration of corticosteroids to limit the detrimental effects caused by cytokine storm syndrome may increase the host ability to prevent further tissue invasion by Aspergillus hyphae (20). At the same time, corticosteroids are known to impair fungal killing by monocytes and macrophages, depending on treatment duration and dose (22). Dexamethasone has been deemed effective as a treatment against COVID-19 and was more frequently used during the second wave, which might influence the incidence of CAPA (23). We are performing a multicenter cohort study (CAPA Plus 2.0) comparing the frequency of CAPA during the second COVID-19 wave with the first COVID-19 wave.
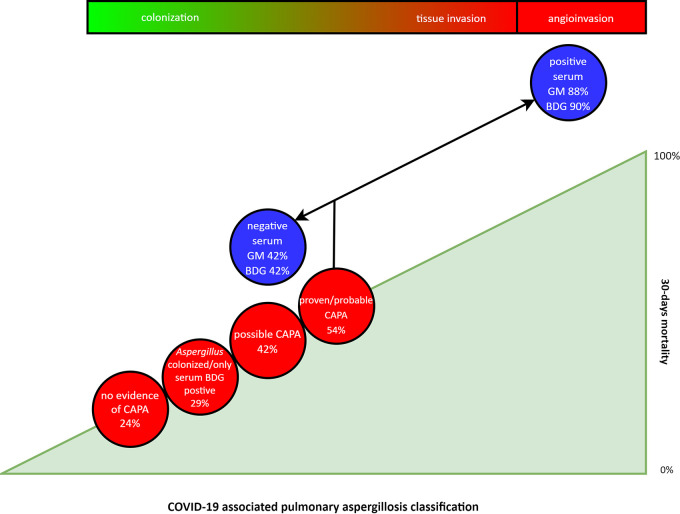
The presence and classification of CAPA and infection stage show incremental mortality (Fig. 3), indicating that CAPA is a complex disease involving various stages. Further studies are needed to develop a stage-specific diagnostic strategy. Staging of CAPA patients will also improve clinical trial design. As the risk of CAPA depends on various determinants, including lytic effects of the viral infection, immune dysregulation, and predisposing host factors, interventions aimed at diminishing or rebalancing each of these effects may alter the ability of the Aspergillus infection to progress. The effects of these multifactorial interventions in addition to antifungal therapy will be better monitored if we succeed in identifying the disease stages in CAPA more accurately.
FIG 3.
Schematic representation of 30-day mortality, CAPA classification, and possible role of Aspergillus tissue and angioinvasion. BDG, (1,3)-β-d-glucan; CAPA, COVID-19-associated pulmonary aspergillosis; GM, galactomannan.
ACKNOWLEDGMENTS
The authors are indebted to Ton de Haan (Department for Health Evidence, Radboud University Medical Centre, Nijmegen, Netherlands) for supervising as biostatistician and all colleagues in the various centers for submitting their data and maintaining the database.
All of the individual participant data that underlie the results reported in this article will be made available, after deidentification, together with the study protocol, to researchers who provide a methodologically sound proposal to achieve aims in the approved proposal, immediately following publication and ending 5 years thereafter. Proposals should be directed to the corresponding author; data requestors will need to sign a data access agreement.
No external funding was received for this study.
R.J.M.B. reports grants and other from Pfizer, MSD, and Gilead, and other from Mundipharma, Astellas, F2G, Amplyx, and Cidara outside the submitted work. A.A. reports personal fees from Gilead and Pfizer and nonfinancial support from Astellas outside the submitted work. K.L. reports personal fees from SMB Laboratoires, Gilead, FUJIFILM Wako, and Thermo Fisher Scientific and nonfinancial support from Pfizer outside the submitted work. J.B.B. reports grants from Gilead Sciences, F2G Ltd, and Thermo Fisher Scientific outside the submitted work. J.W. reports research grants and speakers’ fees from Pfizer, MSD, and Gilead outside the submitted work. P.L.W. reports personal fees from Gilead, Pfizer, F2G, IMMY, and MSD and other from Bruker, Launch, and Associates of Cape Cod outside the submitted work. F.L.V.D.V. reports personal fees from Gilead and Sobi and grants from VIDI outside the submitted work. P.E.V. reports research grants from Gilead Sciences, Astellas, MSD, F2G, and Bio-Rad; he is a speaker for Gilead Sciences and MSD and is on the advisory boards for Pfizer, MundiPharma, Cidara, MSD, and F2G. M.E., S.D., A.V.A., R.G.B., T.R., S.V.D.S.-V.D.B., N.A.F.J., K.V.D., W.J.G.M., M.H.E.R., J.A.S., A.C., and S.S. declare no conflicting interests.
Footnotes
Supplemental material is available online only.
Contributor Information
Paul E. Verweij, Email: Paul.Verweij@radboudumc.nl.
Kimberly E. Hanson, University of Utah
REFERENCES
- 1.van de Veerdonk FL, Kolwijck E, Lestrade PP, Hodiamont CJ, Rijnders BJ, van Paassen J, Haas PJ, Oliveira Dos Santos C, Kampinga GA, Bergmans DC, van Dijk K, de Haan AF, van Dissel J, van der Hoeven HG, Verweij PE, Dutch Mycoses Study Group . 2017. Influenza-associated aspergillosis in critically ill patients. Am J Respir Crit Care Med 196:524–527. 10.1164/rccm.201612-2540LE. [DOI] [PubMed] [Google Scholar]
- 2.Schauwvlieghe A, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, Lagrou K, Verweij PE, Van de Veerdonk FL, Gommers D, Spronk P, Bergmans D, Hoedemaekers A, Andrinopoulou ER, van den Berg C, Juffermans NP, Hodiamont CJ, Vonk AG, Depuydt P, Boelens J, Wauters J, Dutch-Belgian Mycosis Study Group . 2018. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 6:782–792. 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 3.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, Fornaro G, Tonetti T, Pizzilli G, Francalanci E, Giuntoli L, Rubin A, Moroni A, Ambretti S, Trapani F, Vatamanu O, Ranieri VM, Castelli A, Baiocchi M, Lewis R, Giannella M, Viale P, PREDICO study group . 2020. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study. Clin Infect Dis 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, Pandey M, Whitaker H, May A, Morgan M, Wise MP, Healy B, Blyth I, Price JS, Vale L, Posso R, Kronda J, Blackwood A, Rafferty H, Moffitt A, Tsitsopoulou A, Gaur S, Holmes T, Backx M. 2020. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alanio A, Delliere S, Fodil S, Bretagne S, Megarbane B. 2020. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med 8:e48–e49. 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutsaert L, Steinfort N, Van Hunsel T, Bomans P, Naesens R, Mertes H, Dits H, Van Regenmortel N. 2020. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care 10:71. 10.1186/s13613-020-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. 2020. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med 202:132–135. 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, Klimko N, Lass-Florl C, Oladele RO, Vinh DC, Zhu LP, Boll B, Bruggemann R, Gangneux JP, Perfect JR, Patterson TF, Persigehl T, Meis JF, Ostrosky-Zeichner L, White PL, Verweij PE, Cornely OA, European Confederation of Medical Mycology, International Society for Human Animal Mycology, Asia Fungal Working Group, INFOCUS LATAM/ISHAM Working Group, ISHAM Pan Africa Mycology Working Group, European Society for Clinical Microbiology, Infectious Diseases Fungal Infection Study Group, ESCMID Study Group for Infections in Critically Ill Patients, Interregional Association of Clinical Microbiology and Antimicrobial Chemotherapy, Medical Mycology Society of Nigeria, Medical Mycology Society of China Medicine Education Association, Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology, Association of Medical Microbiology, Infectious Disease Canada . 2020. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis 10.1016/S1473-3099(20)30847-1. [DOI] [Google Scholar]
- 9.Lamoth F, Lewis RE, Walsh TJ, Kontoyiannis DP. 2021. Navigating the uncertainties of COVID-19 associated aspergillosis (CAPA): a comparison with influenza associated aspergillosis (IAPA). J Infect Dis 10.1093/infdis/jiab163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchett MA, Oberg CL, Belanger A, De Cardenas J, Cheng G, Nacheli GC, Franco-Paredes C, Singh J, Toth J, Zgoda M, Folch E. 2020. Society for Advanced Bronchoscopy Consensus Statement and Guidelines for bronchoscopy and airway management amid the COVID-19 pandemic. J Thorac Dis 12:1781–1798. 10.21037/jtd.2020.04.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flikweert AW, Grootenboers M, Yick DCY, Du Mee AWF, van der Meer NJM, Rettig TCD, Kant MKM. 2020. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care 59:149–155. 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Permpalung N, Chiang TP, Massie AB, Zhang SX, Avery RK, Nematollahi S, Ostrander D, Segev DL, Marr KA. 2021. COVID-19 associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infect Dis 10.1093/cid/ciab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alanio A, Menotti J, Gits-Muselli M, Hamane S, Denis B, Rafoux E, Peffault de la Tour R, Touratier S, Bergeron A, Guigue N, Bretagne S. 2017. Circulating Aspergillus fumigatus DNA is quantitatively correlated to galactomannan in serum. Front Microbiol 8:2040. 10.3389/fmicb.2017.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White PL, Mengoli C, Bretagne S, Cuenca-Estrella M, Finnstrom N, Klingspor L, Melchers WJ, McCulloch E, Barnes RA, Donnelly JP, Loeffler J, European Aspergillus PCR Initiative . 2011. Evaluation of Aspergillus PCR protocols for testing serum specimens. J Clin Microbiol 49:3842–3848. 10.1128/JCM.05316-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmanton-Garcia J, Sprute R, Stemler J, Bartoletti M, Dupont D, Valerio M, Garcia-Vidal C, Falces-Romero I, Machado M, de la Villa S, Schroeder M, Hoyo I, Hanses F, Ferreira-Paim K, Giacobbe DR, Meis JF, Gangneux JP, Rodriguez-Guardado A, Antinori S, Sal E, Malaj X, Seidel D, Cornely OA, Koehler P, FungiScope European Confederation of Medical Mycology/The International Society for Human and Animal Mycology Working Group . 2021. COVID-19-associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis 27:1077–1086. 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petraitiene R, Petraitis V, Bacher JD, Finkelman MA, Walsh TJ. 2015. Effects of host response and antifungal therapy on serum and BAL levels of galactomannan and (1–3)-beta-D-glucan in experimental invasive pulmonary aspergillosis. Med Myco 53:558–568. 10.1093/mmy/myv034. [DOI] [PubMed] [Google Scholar]
- 17.Fisher CE, Stevens AM, Leisenring W, Pergam SA, Boeckh M, Hohl TM. 2013. The serum galactomannan index predicts mortality in hematopoietic stem cell transplant recipients with invasive aspergillosis. Clin Infect Dis 57:1001–1004. 10.1093/cid/cit393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacobbe DR, Esteves P, Bruzzi P, Mikulska M, Furfaro E, Mesini A, Tatarelli P, Grignolo S, Viscoli C, Colombo AL, Del Bono V. 2015. Initial serum (1,3)-beta-D-glucan as a predictor of mortality in proven candidaemia: findings from a retrospective study in two teaching hospitals in Italy and Brazil. Clin Microbiol Infect 21:954.e9–954.e17. 10.1016/j.cmi.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Azoulay E, Guigue N, Darmon M, Mokart D, Lemiale V, Kouatchet A, Mayaux J, Vincent F, Nyunga M, Bruneel F, Rabbat A, Bretagne S, Lebert C, Meert AP, Benoit D, Pene F. 2016. (1, 3)-Beta-D-glucan assay for diagnosing invasive fungal infections in critically ill patients with hematological malignancies. Oncotarget 7:21484–21495. 10.18632/oncotarget.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Veerdonk FL, Brüggemann RJM, Vos S, de Hertogh G, Wauters J, Rm H, Netea MG, Schouten JA, Verweij PE. 2021. COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defense and fungal invasion. Lancet Respir Med 9:P795–P802. 10.1016/S2213-2600(21)00138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Haese J, Theunissen K, Vermeulen E, Schoemans H, De Vlieger G, Lammertijn L, Meersseman P, Meersseman W, Lagrou K, Maertens J. 2012. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J Clin Microbiol 50:1258–1263. 10.1128/JCM.06423-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyrmizi I, Gresnigt MS, Akoumianaki T, Samonis G, Sidiropoulos P, Boumpas D, Netea MG, van de Veerdonk FL, Kontoyiannis DP, Chamilos G. 2013. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. J Immunol 191:1287–1299. 10.4049/jimmunol.1300132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. 2021. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384:693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jcm.01229-21-s0001.pdf, PDF file, 0.04 MB (37.7KB, pdf)
Table S2. Download jcm.01229-21-s0002.pdf, PDF file, 0.04 MB (36.1KB, pdf)
Table S3. Download jcm.01229-21-s0003.pdf, PDF file, 0.04 MB (36.2KB, pdf)
Fig. S1. Download jcm.01229-21-s0004.pdf, PDF file, 0.2 MB (197.2KB, pdf)