ABSTRACT
Clinical isolates of Enterobacterales other than Escherichia coli (EOTEC), nonfermenting Gram-negative bacilli, and Gram-positive cocci were tested for susceptibility to fosfomycin using Etest and reference agar dilution. Applying EUCAST (v. 11.0, 2021) intravenous fosfomycin breakpoints, Etest MICs for EOTEC showed essential agreement (EA), categorical agreement (CA), major error (ME), and very major error (VME) rates of 70.4%, 88.4%, 4.1%, and 32.1%, respectively. No species of EOTEC tested with acceptable rates for all of EA (≥90%), CA (≥90%), ME (≤3%), and VME (≤3%). Etest MICs for Enterococcus faecalis, interpreted using CLSI oral/urine criteria (M100, 2021) showed EA, CA, minor error, ME, and VME rates of 98.5%, 81.2%, 18.8%, 0%, and 0%. Against Staphylococcus aureus, EA, CA, and ME rates were 84.1%, 98.7%, and 1.3% (EUCAST intravenous criteria). S. aureus isolates with fosfomycin MICs of >32 μg/ml (resistant) were not identified by agar dilution. We conclude that performing fosfomycin Etest on isolates of S. aureus will reliably identify fosfomycin-susceptible isolates with low, acceptable rates of MEs and VMEs. Testing of urinary isolates of E. faecalis by Etest is associated with an unacceptably high rate of minor errors (18.8%) but low, acceptable rates of MEs and VMEs when results are interpreted using CLSI criteria. Isolates of EOTEC tested by Etest with resulting MICs interpreted by EUCAST criteria were associated with an unacceptably high VME rate (32.1%). In vitro testing of clinical isolates beyond E. coli, E. faecalis, and S. aureus to determine susceptibility to fosfomycin is problematic with current methods and breakpoints.
KEYWORDS: Enterobacterales, MIC methods, antimicrobial activity, susceptiblity
INTRODUCTION
Fosfomycin tromethamine is currently approved for use in both the United States and Canada as a single 3-g oral dose to treat uncomplicated acute cystitis in women caused by susceptible isolates of Escherichia coli and Enterococcus faecalis. Intravenous fosfomycin sodium was approved by Canada’s federal regulatory agency (Health Canada) in 2019 for multiple clinical indications and is intended to be prescribed in combination with other antimicrobial agents (1); intravenous fosfomycin sodium is pending approval in the United States (Clinical Trials registration no. NCT02753946). Intravenous and oral fosfomycin are also marketed in European countries, Japan, and elsewhere (2).
Both CLSI and EUCAST publish in vitro susceptibility testing methods for fosfomycin (3, 4) (see Table S1 in the supplemental material). However, disk diffusion endpoint reading and MIC and zone diameter interpretative criteria for fosfomycin differ between the documents published by the two standards organizations, and breakpoints are limited by both method and the few, disparate genera/species to which they apply. Currently, CLSI provides MIC and zone diameter interpretative criteria for E. coli and E. faecalis isolated from urine and tested by agar dilution or disk diffusion (3). EUCAST defines MIC breakpoints for Enterobacterales and staphylococci tested against fosfomycin by agar dilution for both systemic and urine isolates (4). EUCAST also publishes disk diffusion zone diameter breakpoints for fosfomycin for Enterobacterales (4). In 2021, EUCAST added specific MIC (susceptible, ≤8 μg/ml; resistant, >8 μg/ml) and zone diameter (ZD; susceptible, ≥24 mm; resistant, <24 mm) criteria for E. coli (uncomplicated urinary tract infection only) treated with oral fosfomycin and revised the zone diameter breakpoints for intravenous fosfomycin for Enterobacterales from ≥24 mm (susceptible) and <24 mm (resistant) to ≥21 mm (susceptible) and <21 mm (resistant) (4).
Given the increasing prevalence of multidrug-resistant (MDR), expanded-spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant Gram-negative bacilli, vancomycin-resistant enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA) coupled with the expanding availability and use of intravenous fosfomycin, one may anticipate an increasing demand for fosfomycin susceptibility testing to help guide its clinical use. Given the workflow challenges associated with a clinical microbiology laboratory offering routine agar dilution testing, the inability to test fosfomycin by reference or automated broth microdilution, the absence of EUCAST disk diffusion criteria for intravenous fosfomycin (4), and the limited approved applications for CLSI disk diffusion (urine isolates of E. coli and E. faecalis) (3), gradient diffusion strips such as Etest (bioMérieux, Durham, North Carolina) may offer an option for laboratory testing. The current study intended to assess the ability of Etest compared to agar dilution and disk diffusion to generate equivalent in vitro activities for fosfomycin when testing recent clinical isolates of Enterobacterales other than E. coli, nonfermenting Gram-negative bacilli, and Gram-positive cocci.
MATERIALS AND METHODS
Bacterial isolates.
A collection of clinical isolates of Enterobacterales other than E. coli (n = 199), nonfermenting Gram-negative bacilli (n = 90), Enterococcus spp. (n = 90), and S. aureus (n = 156), submitted by Canadian clinical microbiology laboratories to the CANWARD surveillance study in 2018 (5), were tested for in vitro susceptibility to fosfomycin. Clinical laboratories submit isolates to CANWARD nonselectively in order to obtain a representative sample of organisms recovered in that laboratory during routine diagnostic work. Isolates were limited to one per patient. The CANWARD coordinating laboratory (Winnipeg Health Sciences Centre, Winnipeg, Canada) confirmed the identities of all isolates using MALDI-TOF spectrometry (Bruker Daltonics, Billerica, MA, USA).
Antimicrobial susceptibility testing.
Fosfomycin susceptibilities were determined by both the CLSI agar dilution (3, 6) and disk diffusion (3, 7) reference methods as well as by Etest (8). Agar dilution testing used fosfomycin disodium powder (Sigma-Aldrich, St. Louis, MO) and Mueller-Hinton agar (MHA) (supplemented with 25 μg/ml glucose-6-phosphate; Oxoid/Thermo Fisher Scientific, Nepean, Canada). Disk diffusion testing used 200-μg fosfomycin disks containing 50 μg of glucose-6-phosphate (Becton, Dickinson and Company) and MHA. MHA was also used for testing with fosfomycin Etest. Quality control testing was performed each day clinical isolates were tested as specified by CLSI using E. coli ATCC 25922 and S. aureus ATCC 29213 (3, 17, 18).
Agar dilution MIC endpoints were read as the lowest concentration that completely inhibited growth (no visible colonies, excluding a single colony or inoculum haze) (6). CLSI disk diffusion zone diameter endpoints were read with no colonies within the zone of inhibition (3, 7). EUCAST disk diffusion zone diameter endpoints were read by following the guidelines provided in the EUCAST breakpoint tables for interpretation of MICs and zone diameters (4), which recommends ignoring colonies within the zone of inhibition. Etest endpoints were read by following the manufacturer’s package insert, which permits ≤5 colonies within the ellipse to be ignored (8).
Data analysis.
Percent essential agreement, percent categorical agreement, and error rate calculations used agar dilution as the reference method. Disk diffusion served as the reference method when it was compared to Etest using CLSI breakpoints for E. faecalis. Essential agreement, categorical agreement, and minor, major, and very major error rates were calculated using a standard method (9). Acceptable performance rates for categorical agreement and essential agreement are each ≥90% (9). Major error and very major error rates should each be ≤3.0% (9).
RESULTS
EUCAST MIC breakpoints (4) for intravenous fosfomycin were used to interpret MICs generated by agar dilution and Etest for Enterobacterales other than E. coli (Table 1). Fosfomycin MIC50 values generated by agar dilution and Etest were within ±1 doubling dilution of each other for all Enterobacterales other than E. coli (Morganella morganii not assessable), Acinetobacter baumannii, and Pseudomonas aeruginosa. The Etest MIC50 for Stenotrophomonas maltophilia was >2 doubling dilutions higher for Etest than for agar dilution. Fosfomycin MIC90 values generated by agar dilution and Etest were within ±1 doubling dilution of each other for most Enterobacterales other than E. coli (except E. cloacae, for which the Etest MIC90 was 2 doubling dilutions higher than by agar dilution, and M. morganii, for which the data were not assessable). A. baumannii, P. aeruginosa, and S. maltophilia MIC90 values were >2 doubling dilutions higher for Etest than for agar dilution. Of the species of Enterobacterales tested, Serratia marcescens (96.7 to 100% susceptible) had the highest percent susceptible followed by Klebsiella oxytoca/Raoultella spp. (93.3 to 96.7%) and Klebsiella aerogenes (81.0 to 90.5%). All isolates of M. morganii were resistant to fosfomycin. Percent susceptible rates determined by agar dilution and Etest were similar (≤5% difference) for all Enterobacterales other than E. coli (except K. aerogenes and K. pneumoniae, for which Etest percent susceptible rates were 10 to 20% higher than agar dilution). Etest essential agreement with agar dilution was ≥90% for only one species of Enterobacterales (S. marcescens). Etest categorical agreement with agar dilution was ≥90% for K. aerogenes, K. oxytoca/Raoultella spp., M. morganii, P. mirabilis, and S. marcescens. Etest major errors were >3% for E. cloacae, K. pneumoniae, and S. marcescens. Etest very major errors were >3% for E. cloacae (18.2%), K. aerogenes (50.0%), K. oxytoca/Raoultella spp. (50.0%), and K. pneumoniae (75.0%). Only 18.0% (9/50) of isolates of P. aeruginosa had agar dilution fosfomycin MICs of ≤32 μg/ml. Figure 1 shows a scattergram comparing fosfomycin agar dilution MIC values to fosfomycin Etest MIC values for the 199 isolates of Enterobacterales other than E. coli isolates with EUCAST MIC breakpoints (≤32 μg/ml [susceptible], >32 μg/ml [resistant]) (4) applied to the data set.
TABLE 1.
Agar dilution and Etest fosfomycin MICsg
| Pathogen (no. of isolates) and MIC test method | MIC (μg/ml) |
MIC interpretationa (%) |
Test method agreement and error rates (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | Susceptible | Resistant | EAb (n) | CAc (n) | MEd (n) | VMEe (n) | |
| Enterobacterales (199) | |||||||||
| Agar dilution | 16 | 512 | ≤1–>512 | 73.4 | 26.6 | ||||
| Etest | 16 | 512 | ≤1–>512 | 78.9 | 21.1 | 70.4 (126/179) | 88.4 (176/199) | 4.1 (6/146) | 32.1 (17/53) |
| Enterobacter cloacae (32) | |||||||||
| Agar dilution | 16 | 128 | ≤1–512 | 65.6 | 34.4 | ||||
| Etest | 32 | 512 | ≤1–>512 | 62.5 | 37.5 | 71.4 (20/28) | 84.4 (27/32) | 14.3 (3/21) | 18.2 (2/11) |
| Klebsiella aerogenes (21) | |||||||||
| Agar dilution | 16 | 64 | 1–512 | 81.0 | 19.0 | ||||
| Etest | 16 | 32 | ≤1–>512 | 90.5 | 9.5 | 85.0 (17/20) | 90.5 (19/21) | 0 (0/17) | 50.0 (2/4) |
| Klebsiella oxytoca/Raoultella spp. (30) | |||||||||
| Agar dilution | 16 | 32 | 2–256 | 93.3 | 6.7 | ||||
| Etest | 8 | 16 | ≤1–64 | 96.7 | 3.3 | 56.7 (17/30) | 96.7 (29/30) | 0 (0/28) | 50.0 (1/2) |
| Klebsiella pneumoniae (51) | |||||||||
| Agar dilution | 32 | 128 | 16–>512 | 68.6 | 31.4 | ||||
| Etest | 16 | 64 | 4–>512 | 88.2 | 11.8 | 60.0 (30/50) | 72.5 (37/51) | 5.7 (2/35) | 75.0 (12/16) |
| Morganella morganii (15) | |||||||||
| Agar dilution | 512 | >512 | 128–>512 | 0 | 100 | ||||
| Etest | >512 | >512 | 256–>512 | 0 | 100 | 25.0 (1/4) | 100 (15/15) | NAf (0/0) | 0 (0/15) |
| Proteus mirabilis (20) | |||||||||
| Agar dilution | 4 | 128 | ≤1–>512 | 75.0 | 25.0 | ||||
| Etest | 2 | 256 | ≤1–>512 | 75.0 | 25.0 | 76.5 (13/17) | 100 (20/20) | 0 (0/15) | 0 (0/5) |
| Serratia marcescens (30) | |||||||||
| Agar dilution | 16 | 16 | 4–32 | 100 | 0 | ||||
| Etest | 16 | 32 | 4–128 | 96.7 | 3.3 | 93.3 (28/30) | 96.7 (29/30) | 3.3 (1/30) | NA (0/0) |
| Acinetobacter baumannii (15) | |||||||||
| Agar dilution | 128 | 256 | 128 −512 | ||||||
| Etest | 256 | >512 | 264–>512 | 64.3 (9/14) | |||||
| Pseudomonas aeruginosa (50) | |||||||||
| Agar dilution | 128 | 256 | 4–>512 | ||||||
| Etest | 128 | >512 | 4–>512 | 77.6 (38/49) | |||||
| Stenotrophomonas maltophilia (25) | |||||||||
| Agar dilution | 128 | 256 | 64 −256 | ||||||
| Etest | >512 | >512 | 64–>512 | 32.0 (8/25) | |||||
EUCAST (4) MIC breakpoints (susceptible, ≤32 μg/ml; resistant, >32 μg/ml) apply to isolates of Enterobacterales and intravenous use of fosfomycin.
EA, essential agreement. The EA calculation excluded one or two indeterminate off-scale MIC readings (e.g., <1 or >512 μg/ml) where a determination of EA could not be made. The number of isolates included in calculation appears in parentheses after the percentage.
CA, categorical agreement. The number of isolates included in calculation appears in parentheses after the percentage.
ME, major errors. The number of isolates included in calculation appears in parentheses after the percentage.
VME, very major errors. The number of isolates included in calculation appears in parentheses after the percentage.
NA, not applicable.
Agar dilution and Etest fosfomycin MICs were interpreted by EUCAST fosfomycin intravenous breakpoints for 199 isolates of Enterobacterales other than E. coli and 90 isolates of nonfermenting Gram-negative bacilli.
FIG 1.
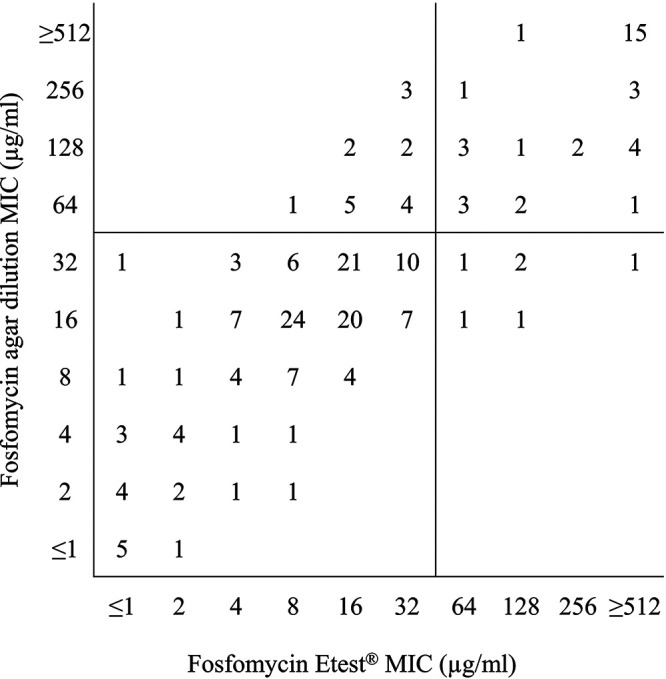
Scattergram comparing fosfomycin agar dilution MIC values to fosfomycin Etest MIC values for 199 Enterobacterales other than E. coli isolates. EUCAST MIC breakpoints (≤32 μg/ml [susceptible], >32 μg/ml [resistant] [4]) were applied to the data set.
Table 2 summarizes fosfomycin disk diffusion testing of the 199 isolates of Enterobacterales other than E. coli and 90 isolates of nonfermenting Gram-negative bacilli. Reading disk diffusion zone diameters by EUCAST criteria produced larger zones for all species of Enterobacterales other than E. coli and nonfermenting Gram-negative bacilli compared to those determined following CLSI criteria. Disk diffusion categorical agreement with agar dilution was ≥90% for K. oxytoca/Raoultella spp. (93.3%), M. morganii (100%), P. mirabilis (100%), and S. marcescens (96.7%). Disk diffusion major errors and very major errors were >3% for the majority of species of Enterobacterales other than E. coli (exceptions: P. mirabilis and M. morganii). A proportion of 32.0% (16/50) of isolates of P. aeruginosa and 8.0% (2/25) of isolates of S. maltophilia read using EUCAST disk diffusion criteria had fosfomycin zone diameters of ≥21 mm.
TABLE 2.
Fosfomycin disk diffusion testing of 199 isolates of Enterobacterales other than E. coli and 90 isolates of nonfermenting Gram-negative bacilli
| Pathogen (no. of isolates) | EUCAST ZD, mm |
ZD interpretationa (%) |
Test method agreement and error rates (%) |
CLSI ZD, mm |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZD50b | ZD90c | Ranged | Susceptible | Resistant | CAe (n) | MEf (n) | VMEg (n) | ZD50 | ZD90 | Range | |
| Enterobacterales (199) | 23 | 15 | 6–37 | 80.4 | 19.6 | 87.9 (175/199) | 3.4 (5/146) | 35.8 (19/53) | 20 | 7 | 6–37 |
| Enterobacter cloacae (32) | 25 | 20 | 19–35 | 84.4 | 15.6 | 75.0 (24/32) | 4.8 (1/21) | 63.6 (7/11) | 19 | 9 | 6–30 |
| Klebsiella aerogenes (21) | 23 | 20 | 6–30 | 85.7 | 14.3 | 85.7 (18/21) | 5.9 (1/17) | 50.0 (2/4) | 20 | 16 | 6–29 |
| Klebsiella oxytoca/Raoultella spp. (30) | 24 | 22 | 22–32 | 100 | 0 | 93.3 (28/30) | 0 (0/28) | 100 (2/2) | 22 | 17 | 12–28 |
| Klebsiella pneumoniae (51) | 22 | 18 | 12–29 | 80.4 | 19.6 | 82.4 (42/51) | 5.7 (2/35) | 56.3 (9/16) | 18 | 14 | 9–28 |
| Morganella morganii (15) | 6 | 6 | 6–13 | 0 | 100 | 100 (15/15) | NAh | 0 (0/15) | 6 | 6 | 6–11 |
| Proteus mirabilis (20) | 34 | 15 | 6–37 | 75.0 | 25.0 | 100 (20/20) | 0 (0/15) | 0 (0/5) | 28 | 8 | 6–37 |
| Serratia marcescens (30) | 27 | 25 | 20–33 | 96.7 | 3.3 | 96.7 (29/30) | 3.3 (1/30) | NA | 24 | 18 | 12–32 |
| Acinetobacter baumannii (15) | 15 | 13 | 13–20 | 8 | 6 | 6–15 | |||||
| Pseudomonas aeruginosa (50) | 18 | 6 | 6–38 | 16 | 6 | 6–35 | |||||
| Stenotrophomonas maltophilia (25) | 15 | 12 | 6–22 | 11 | 6 | 6–20 | |||||
EUCAST zone diameter breakpoints (susceptible, ≥21 mm; resistant, <21 mm [4]) apply to isolates of Enterobacterales and intravenous use of fosfomycin.
ZD50, zone diameter at which 50% of isolates were inhibited.
ZD90, zone diameter at which 90% of isolates were inhibited.
Additional information regarding EUCAST zone diameter measurements; 97.8% (180/184) of all isolates of Enterobacterales tested that produced a zone of inhibition had ≥1 colony within the zone of inhibition, and 83.2% (153/184) of all isolates tested that produced a zone of inhibition had ≥10 colonies within the zone of inhibition; 100% (15/15) of all isolates of Acinetobacter baumannii tested had ≥1 colony within the zone of inhibition; 93.3% (14/15) of all isolates tested had ≥10 colonies within the zone of inhibition; 54.5% (24/44) of all isolates of Pseudomonas aeruginosa tested that produced a zone of inhibition had ≥1 colony within the zone of inhibition; 29.5% (13/44) of all isolates tested that produced a zone of inhibition had ≥10 colonies within the zone of inhibition; 95.8% (23/24) of all isolates of Stenotrophomonas maltophilia tested that produced a zone of inhibition had ≥1 colony within the zone of inhibition; 87.5% (21/24) of all isolates tested that produced a zone of inhibition had ≥10 colonies within the zone of inhibition.
CA, categorical agreement. The number of isolates included in calculation appears in brackets after the percentage. CA was calculated using agar dilution as the reference method.
ME, major errors. The number of isolates included in calculation appears in brackets after the percentage. The ME rate was calculated using agar dilution as the reference method.
VME, very major errors. The number of isolates included in calculation appears in brackets after the percentage. The VME rate was calculated using agar dilution as the reference method.
NA, not applicable.
CLSI MIC and zone diameter breakpoints (3) for oral fosfomycin were used to interpret MICs generated by agar dilution, disk diffusion, and Etest for E. faecalis (Table 3). Percent susceptible rates were highest for agar dilution (88.4%) and lowest (10% lower) for Etest (78.3%). Nonetheless, the essential agreement between agar dilution and Etest was high (98.5%) (Table 4). The categorical agreement among the three methods varied slightly from 81.2 to 85.5%, and all errors were minor (14.5 to 18.8% minor errors). No major or very major errors were observed. Figure 2 shows a scattergram comparing fosfomycin agar dilution MIC values to fosfomycin Etest MIC values for the 69 Enterococcus faecalis isolates with CLSI MIC breakpoints (≤64 [susceptible], 128 [intermediate], and ≥256 [resistant] μg/ml) (3) applied to the data set. The MIC50 and MIC90 values generated by Etest for E. faecalis and Enterococcus faecium were the same as, or within ±1 doubling dilution of, those generated by agar dilution.
TABLE 3.
Summary of antimicrobial susceptibility testing results using three methods (agar dilution, disk diffusion, and Etest) for clinical isolates of Enterococcus faecalis, Enterococcus faecium, and Staphylococcus aureus
| Pathogen (no.) and test method | MIC, μg/ml |
CLSI zone diam, mm |
CLSI MIC/zone diam interpretationa (%) |
EUCAST zone diam, mm |
EUCAST MIC interpretationb (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | ZD50c | ZD90d | Range | Se | If | Rg | ZD50 | ZD90 | Rangeh | S | R | |
| Enterococcus faecalisi (69) | ||||||||||||||
| Agar dilution | 64 | 128 | 8–512 | 88.4 | 8.7 | 2.9 | ||||||||
| Disk diffusion | 19 | 15 | 6–37 | 85.5 | 8.7 | 5.8 | 22 | 19 | 6–37 | |||||
| Etest | 64 | 128 | 8–>512 | 78.3 | 15.9 | 5.8 | ||||||||
| Enterococcus faeciumj (21) | ||||||||||||||
| Agar dilution | 64 | 128 | 32–128 | |||||||||||
| Disk diffusion | 12 | 10 | 6–17 | 17 | 13 | 10–25 | ||||||||
| Etest | 128 | 256 | 32–>512 | |||||||||||
| Staphylococcus aureus (156) | ||||||||||||||
| Agar dilution | 4 | 8 | ≤1–32 | 100 | 0 | |||||||||
| Disk diffusion | 27 | 23 | 15–34 | 27 | 24 | 18–34 | ||||||||
| Etest | 8 | 16 | ≤1–>512 | 98.7 | 1.3 | |||||||||
| MSSAk (56) | ||||||||||||||
| Agar dilution | 4 | 8 | ≤1–32 | 100 | 0 | |||||||||
| Disk diffusion | 26 | 22 | 21–34 | 26 | 22 | 21–34 | ||||||||
| Etest | 8 | 16 | ≤1–16 | 100 | 0 | |||||||||
| MRSAl (100) | ||||||||||||||
| Agar dilution | 8 | 16 | ≤1–32 | 100 | 0 | |||||||||
| Disk diffusion | 27 | 24 | 15–34 | 27 | 24 | 18–34 | ||||||||
| Etest | 8 | 16 | 2–>512 | 98.0 | 2.0 | |||||||||
MIC results were interpreted as susceptible (≤64 μg/ml), intermediate (128 μg/ml), and resistant (≥256 μg/ml) according breakpoints published in the CLSI M100 standard (3) for Escherichia coli and Enterococcus faecalis urinary tract isolates. Zone diameter results were interpreted as susceptible (≥16 μg/ml), intermediate (13 to 15 μg/ml), and resistant (≤12 μg/ml) according to breakpoints published in the CLSI M100 standard (3) for Escherichia coli and Enterococcus faecalis urinary tract isolates.
EUCAST MIC breakpoints (≤32 [susceptible], >32 [resistant] μg/ml [4]) apply to isolates of Staphylococcus species and intravenous use of fosfomycin.
ZD50, zone diameter at which 50% of isolates are inhibited.
ZD90, zone diameter at which 90% of isolates are inhibited.
S, susceptible.
I, intermediate.
R, resistant.
Additional information regarding EUCAST zone diameter measurements. For Enterococcus faecalis, 88.4% (61/69) of all isolates tested that produced a zone of inhibition had ≥1 colony within the zone of inhibition, and 14.5% (10/69) of all isolates tested that produced a zone of inhibition had ≥10 colonies within the zone of inhibition. For Enterococcus faecium, 90.5% (19/21) of all isolates tested had ≥1 colony within the zone of inhibition, and 66.7% (14/21) of all isolates tested had ≥10 colonies within the zone of inhibition. For Staphylococcus aureus, 7.7% (12/156) of all isolates tested had ≥1 colony within the zone of inhibition, and 1.3% (2/156) of all isolates tested had ≥10 colonies within the zone of inhibition.
Zero percent (0/69) of isolates of Enterococcus faecalis were vancomycin resistant.
Aproportion of 33.3% (7/21) of isolates of Enterococcus faecium were vancomycin resistant.
MSSA, methicillin-susceptible Staphylococcus aureus.
MRSA, methicillin-resistant Staphylococcus aureus.
TABLE 4.
EA, CA, and error rates generated by in vitro testing of clinical isolates of Gram-positive cocci against fosfomycin by agar dilution, disk diffusion, and Etest
| Breakpoints used, pathogen, and method comparison | % EAa (n) | % CA (n) | % mEb (n) | % MEc (n) | % VMEd (n) |
|---|---|---|---|---|---|
| CLSI breakpointse | |||||
| Enterococcus faecalis (69) | |||||
| Agar dilution vs disk diffusion | NAf | 85.5 (59/69) | 14.5 (10/69) | 0 (0/61) | 0 (0/2) |
| Agar dilution vs Etest | 98.5 (65/66) | 81.2 (56/69) | 18.8 (13/69) | 0 (0/61) | 0 (0/2) |
| Disk diffusion vs Etest | NA | 84.1 (58/69) | 14.5 (10/69) | 0 (0/59) | 0 (0/4) |
| EUCAST breakpointsg | |||||
| Staphylococcus aureus (156) | |||||
| Agar dilution vs Etest | 84.1 (127/151) | 98.7 (154/156) | NA | 1.3 (2/156) | NA (0/0) |
| MSSA (56) | |||||
| Agar dilution vs Etest | 86.8 (46/53) | 100 (56/56) | NA | 0 (0/56) | NA (0/0) |
| MRSA (100) | |||||
| Agar dilution vs Etest | 82.7 (81/98) | 98.0 (98/100) | NA | 2.0 (2/100) | NA (0/0) |
EA, essential agreement. The EA calculation excluded indeterminate off-scale MIC readings. The number of isolates included in calculation appears in parentheses after the percentage. The EA calculation excluded isolates with agar dilution MICs of ≤1 μg/ml (lowest dilution tested) and >512 μg/ml (highest dilution tested) and Etest MICs of >512 μg/ml.
mE, minor errors. The number of isolates included in calculation appears in parentheses after the percentage.
ME, major errors. The number of isolates included in calculation appears in parentheses after the percentage.
VME, very major errors. The number of isolates included in the calculation appears in parentheses after the percentage.
MIC results were interpreted as susceptible (≤64 μg/ml), intermediate (128 μg/ml), and resistant (≥256 μg/ml) according breakpoints published in the CLSI M100 standard (3) for Escherichia coli and Enterococcus faecalis urinary tract isolates. Zone diameter results were interpreted as susceptible (≥16 mm), intermediate (13 to 15 mm), and resistant (≤12 mm) according breakpoints published in the CLSI M100 standard (3) for Escherichia coli and Enterococcus faecalis urinary tract isolates.
NA, not applicable.
EUCAST MIC breakpoints (≤32 μg/ml [susceptible], >32 μg/ml [resistant] [4]) apply to isolates of Staphylococcus species and intravenous use of fosfomycin.
FIG 2.
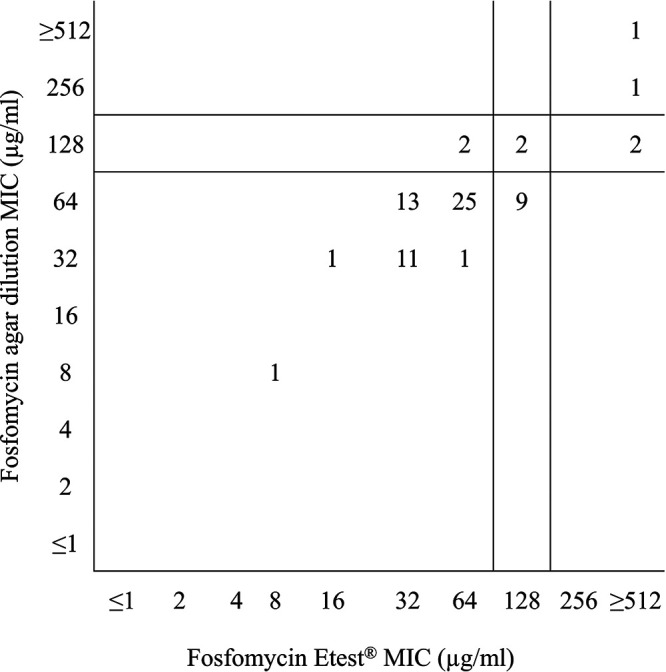
Scattergram comparing fosfomycin agar dilution MIC values to fosfomycin Etest MIC values for 69 Enterococcus faecalis isolates. CLSI MIC breakpoints (≤64 μg/ml [susceptible], 128 μg/ml [intermediate], ≥256 μg/ml [resistant] [3]) were applied to the data set.
Fosfomycin MIC50 and MIC90 values generated by agar dilution and Etest were within ±1 doubling dilution for methicillin-susceptible S. aureus (MSSA) and MRSA (Table 3). MSSA (100% susceptible) and MRSA (98% susceptible) were highly susceptible by agar dilution and Etest when MICs were interpreted by EUCAST intravenous fosfomycin breakpoints (4) (Table 3). Essential agreement between agar dilution and Etest was 86.8% for MSSA and 82.7% for MRSA, while rates of categorical agreement were higher (MSSA, 100%; MRSA, 98.0%) (Table 4). Two major errors were identified while testing MRSA, and no errors were observed for MSSA. Figure 3 shows a scattergram comparing fosfomycin agar dilution MIC values to fosfomycin Etest MIC values for the 156 S. aureus isolates with EUCAST MIC breakpoints (≤32 [susceptible], >32 [resistant] μg/ml) (4) applied to the data set.
FIG 3.
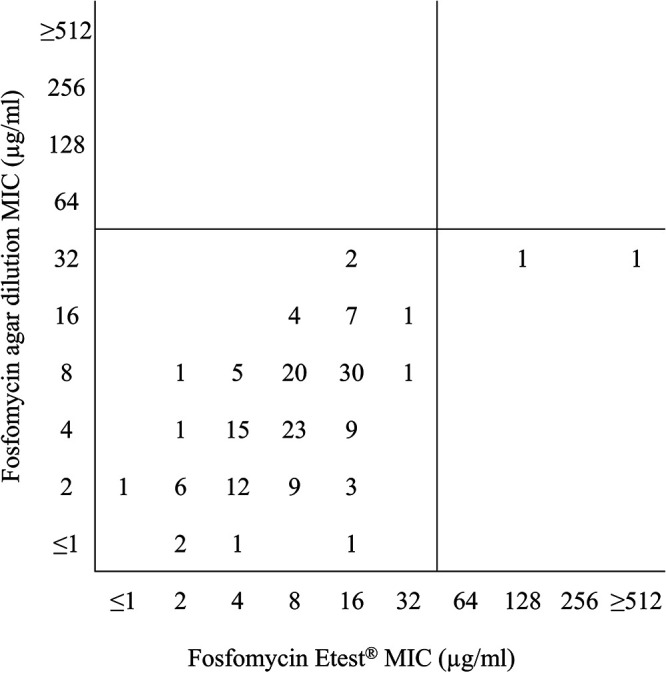
Scattergram comparing fosfomycin agar dilution MIC values to fosfomycin Etest MIC values for 156 Staphylococcus aureus isolates. EUCAST MIC breakpoints (≤32 μg/ml [susceptible], >32 μg/ml [resistant] [4]) were applied to the data set.
Reading disk diffusion zone diameters by CLSI criteria produced smaller zones for Enterococcus species but not for MSSA or MRSA than those determined following EUCAST criteria (Table 3). For E. faecalis and E. faecium, the CLSI zone diameters inhibiting 50% of isolates (ZD50) were 19 and 15 mm, and the zone diameters inhibiting 90% of isolates (ZD90) were 12 and 10 mm, respectively, compared to EUCAST ZD50 values of 22 and 17 mm and ZD90 values of 19 and 13 mm.
DISCUSSION
Fosfomycin is a broad-spectrum, bactericidal, epoxide antibacterial agent that inhibits an initial step in peptidoglycan synthesis. The current study (Tables 1 to 3) and previous studies have documented its potent in vitro activity against many species of Enterobacterales, including isolates carrying ESBLs and carbapenemases, as well as against staphylococci and enterococci (10–17). These same studies have noted that fosfomycin MICs for E. coli are lower than for Enterobacterales other than E. coli and lower for S. aureus than for other Gram-positive species.
We previously demonstrated that performing fosfomycin disk diffusion or Etest on urinary isolates of E. coli and interpreting the results using CLSI breakpoints reliably identified fosfomycin-susceptible isolates (17). In that study, we observed that disk diffusion and Etest were equivalent to agar dilution, demonstrating categorical agreement of >99% with <1% minor errors and no MEs or VMEs when CLSI interpretative criteria were applied (17). Applying EUCAST interpretative criteria (susceptible, ≤32 μg/ml; resistant, >32 μg/ml) also resulted in high categorical agreement (>99%) between agar dilution and both disk diffusion and Etest as well as no MEs but did produce unacceptable rates of VMEs (33.3 to 44.4%). If the new (2021) EUCAST MIC interpretative criteria for oral fosfomycin for E. coli isolated from uncomplicated urinary tract infections (susceptible, ≤8 μg/ml; resistant, >8 μg/ml) together with the accompanying zone diameter criteria (susceptible, ≥24 mm; resistant, <24 mm) (4) were applied to the E. coli data set in our previous study, the number of VMEs for both disk diffusion and Etest would increase (17). In our previous study of E. coli, we also found that MICs generated by agar dilution and Etest showed poor essential agreement (40.3%) due in large part to many isolates (59.3%) with MICs of 2 to 128 μg/ml by agar dilution testing two to four doubling dilutions lower by Etest (17).
In E. coli, when fosfomycin resistance is observed, it is often associated with chromosomal mutation in cell membrane transport systems (GlpT and UhpT) that impair active transport of fosfomycin into cells (1, 2, 15). Fosfomycin resistance may also be acquired as plasmid- or transposon-borne fosfomycin-modifying enzymes that inactivate fosfomycin by binding it to glutathione (FosA), l-cysteine, bacillithiol (FosB), or water (FosX) or by cleavage of the carbon-phosphorus bond in fosfomycin by acquired kinases (FomA and FomB) (1, 2, 15). Many Enterobacterales other than E. coli naturally carry a chromosomal fosA gene that contributes to the frequent appearance of colonies within the zone of inhibition of fosfomycin diffusion tests (disk diffusion and Etest) (15). The EUCAST and Etest recommendations to ignore colonies within inhibition zones are based primarily on observations with E. coli where such colonies are rare and are associated with a fitness cost (15, 18). Choosing to ignore colonies within the zone of inhibition (4) or not (3) can have a major impact on zone diameters measured and their interpretation (Table 2).
Previously published studies comparing disk diffusion and/or Etest results with agar dilution results have frequently shown greater concordance for E. coli than for other species of Enterobacterales, particularly when CLSI breakpoints were used (11–16, 19, 20). Some studies have reported that Etest generates MICs ≥2 doubling dilutions lower than agar dilution, low essential and categorical agreement with agar dilution, and unacceptable rates of VMEs for K. pneumoniae and other Enterobacterales other than E. coli (11–13), while other studies report that Etest generates equivalent or higher MICs than agar dilution (14, 15). In the current study, we observed that testing of isolates of Enterobacterales other than E. coli by Etest with results interpreted by EUCAST criteria for intravenous fosfomycin was associated with an unacceptably high VME rate (32.1%). Several previous studies have also concluded that Etest may not be a reliable alternative to agar dilution for testing Enterobacterales other than E. coli (11–15).
MIC50, MIC90, and susceptibility results from the current study for S. aureus, E. faecalis, and E. faecium (Table 3) are in general agreement with previous in vitro susceptibility data for these pathogens (10, 14, 16). In the current study, 10% fewer E. faecalis isolates were susceptible to fosfomycin when tested by Etest than by agar dilution, even though the essential agreement between the two methods was 98.5% (Table 4). A previous North American study of E. faecalis from urine (all agar dilution fosfomycin MICs of ≤64 μg/ml) reported that all isolates tested within one doubling dilution by agar dilution and Etest; the same study compared agar dilution MICs to disk diffusion zone diameters and reported 0.1% minor errors and no MEs or VMEs (16).
Based on the results of the current study, clinical laboratories routinely performing fosfomycin testing with Etest for isolates of S. aureus, and interpreting the results using EUCAST intravenous fosfomycin MIC breakpoints (4), would appear to provide reliable results compared to agar dilution. Testing of urinary isolates of E. faecalis by Etest may be associated with an unacceptably high rate of minor errors (18.8%) but low, acceptable rates of MEs and VMEs when results are interpreted using CLSI criteria. Testing of isolates of Enterobacterales other than E. coli by Etest with results interpreted by EUCAST criteria was associated with an unacceptably high VME rate (32.1%). Poor essential agreement (70.4%) was also observed and suggests that reporting MIC values alone (without interpretation) from Etest for Enterobacterales other than E. coli is also unacceptable practice. Fosfomycin MICs generated by Etest for isolates of Enterobacterales other than E. coli must be interpreted with caution and frequently do not agree with those generated by the CLSI agar dilution reference method. Clinical outcomes using these interpretative criteria also have not been studied. S. marcescens was the only species of Enterobacterales other than E. coli where essential agreement and categorical agreement both were >90%.
The current study has limitations that require acknowledgment. First, for some species, only limited numbers of isolates were tested, and limited numbers of susceptible or resistant isolates may skew the percentages of VME identified. For example, for K. oxytoca only two fosfomycin-resistant isolates were identified by agar dilution testing (reference method), and one of those isolates was called resistant by Etest. Similarly, no fosfomycin-resistant S. aureus and only two fosfomycin-resistant E. faecalis organisms were identified by agar dilution testing. Second, the study does not provide clinical data for the isolates tested. Third, the study was not designed to determine the prevalence of resistance mechanisms that may explain the results of in vitro antimicrobial susceptibility testing.
Fosfomycin disk diffusion results for Enterobacterales other than E. coli frequently correlated with agar dilution results (categorical agreement for individual species of Enterobacterales other than E. coli ranged from 75.0% to 100%) when MICs were interpreted by EUCAST criteria; however, the rate of VMEs was again unacceptably high (35.8%). In vitro testing of fosfomycin beyond E. coli, E. faecalis, and S. aureus in patients with serious infection requires reliable antimicrobial susceptibility testing methods and interpretative criteria and is problematic with current methods and breakpoints.
ACKNOWLEDGMENTS
We thank all participating investigators and laboratories who provided isolates for the CANWARD Surveillance Study.
The current study was funded by Verity Pharmaceuticals (Mississauga, Canada). The sponsor approved the overall study design, but the collection and testing of isolates, data analysis, and manuscript preparation were independently performed by the authors of the study. G.G.Z. is a member of the Scientific Advisory Board for Verity Pharmaceuticals. None of the authors have any financial interests in Verity Pharmaceuticals.
Footnotes
Supplemental material is available online only.
Contributor Information
George G. Zhanel, Email: ggzhanel@pcsinternet.ca.
Nathan A. Ledeboer, Medical College of Wisconsin
REFERENCES
- 1.Verity Pharmaceuticals, Inc. 2019. IVOZFO. Fosfomycin for injection product monograph. Verity Pharmaceuticals, Inc, Mississauga, Canada. [Google Scholar]
- 2.Zhanel GG, Zhanel MA, Karlowsky JA. 2018. Intravenous fosfomycin: an assessment of its potential for use in the treatment of systemic infections in Canada. Can J Infect Dis Med Microbiol 2018:8912039. doi: 10.1155/2018/8912039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing, 31st ed. M100. CLSI, Wayne, PA. [Google Scholar]
- 4.European Committee on Antimicrobial Susceptibility Testing. 2021. Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0, 2021. http://www.eucast.org. Accessed 11 June 2021.
- 5.Zhanel GG, Adam HJ, Baxter MR, Fuller J, Nichol KA, Denisuik AJ, Golden AR, Hink R, Lagacé-Wiens PRS, Walkty A, Mulvey MR, Schweizer F, Bay D, Hoban DJ, Karlowsky JA, Canadian Antimicrobial Resistance Alliance (CARA) and CANWARD . 2019. 42936 pathogens from Canadian hospitals: 10 years of results (2007–16) from the CANWARD surveillance study. J Antimicrob Chemother 74:iv5–iv21. doi: 10.1093/jac/dkz283. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M07-A11, approved standard, 11th ed. CLSI, Wayne, PA. [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2018. Performance standards for antimicrobial disk susceptibility tests. M02, 13th ed. CLSI, Wayne, PA. [Google Scholar]
- 8.bioMérieux. 2012. ETEST fosfomycin FM 1024 US. Customer information sheet. CIS007. bioMérieux, Durham, NC. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2015. Verification of commercial microbial identification and antimicrobial susceptibility testing systems. M52, 1st ed. CLSI, Wayne, PA. [Google Scholar]
- 10.Flamm RK, Rhomberg PR, Watters AA, Sweeney K, Ellis-Grosse EJ, Shortridge D. 2019. Activity of fosfomycin when tested against US contemporary bacterial isolates. Diagn Microbiol Infect Dis 93:143–146. doi: 10.1016/j.diagmicrobio.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Camarlinghi G, Parisio EM, Antonelli A, Nardone M, Coppi M, Giani T, Mattei R, Rossolini GM. 2019. Discrepancies in fosfomycin susceptibility testing of KPC-producing Klebsiella pneumoniae with various commercial methods. Diagn Microbiol Infect Dis 93:74–76. doi: 10.1016/j.diagmicrobio.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 12.van den Bijllaardt W, Schijffelens MJ, Bosboom RW, Stuart JC, Diederen B, Kamping G, Le T-H, Kampinga G, Le T-N, Overdevest I, Stals F, Voorn P, Waar K, Mouton JW, Muller AE. 2018. Susceptibility of ESBL Escherichia coli and Klebsiella pneumoniae to fosfomycin in the Netherlands and comparison of several testing methods including Etest, MIC test strip, Vitek2, Phoenix and disc diffusion. J Antimicrob Chemother 73:2380–2387. doi: 10.1093/jac/dky214. [DOI] [PubMed] [Google Scholar]
- 13.van Mens SP, ten Doesschate T, Kluytmans-van den Bergh MFQ, Mouton JW, Rossen JWA, Verhulst C, Bonten MJM, Kluytmans JAJW. 2018. Fosfomycin Etest for Enterobacteriaceae: interobserver and interlaboratory agreement. Int J Antimicrob Agents 52:678–681. doi: 10.1016/j.ijantimicag.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch EB, Raux BR, Zucchi PC, Kim Y, McCoy C, Kirby JE, Wright SB, Eliopoulos GM. 2015. Activity of fosfomycin and comparison of several susceptibility testing methods against contemporary urine isolates. Int J Antimicrob Agents 46:642–647. doi: 10.1016/j.ijantimicag.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Elliott ZS, Barry KE, Cox HL, Stoesser N, Carroll J, Vegesana K, Kotay S, Sheppard AE, Wailan A, Crook DW, Parikh H, Mathers AJ. 2019. The role of fosA in challenges with fosfomycin susceptibility testing of multispecies Klebsiella pneumoniae carbapenemase-producing clinical isolates. J Clin Microbiol 57:e00634-19. doi: 10.1128/JCM.00634-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs PC, Barry AL, Brown SD. 1999. Fosfomycin tromethamine susceptibility of outpatient urine isolates of Escherichia coli and Enterococcus faecalis from ten North American medical centres by three methods. J Antimicrob Chemother 43:137–140. doi: 10.1093/jac/43.1.137. [DOI] [PubMed] [Google Scholar]
- 17.Karlowsky JA, Lagacé-Wiens PRS, Laing NM, Baxter MR, Adam HJ, Zhanel GG. 2020. Susceptibility of clinical isolates of Escherichia coli to fosfomycin as measured by four in vitro testing methods. J Clin Microbiol 58:e01306-20. doi: 10.1128/JCM.01306-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas AE, Ito R, Mustapha MM, McElheny CL, Mettus RT, Bowler SL, Kantz SF, Pacey MP, Pasculle AW, Cooper VS, Doi Y. 2018. Frequency and mechanisms of spontaneous fosfomycin nonsusceptibility observed upon disk diffusion testing of Escherichia coli. J Clin Microbiol 56:e01368-17. doi: 10.1128/JCM.01368-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbott IJ, Dekker J, van Gorp E, Wijma RA, Raaphorst MN, Klaassen CHW, Meletiadis J, Mouton JW, Peleg AY. 2020. Impact of bacterial species and baseline resistance on fosfomycin efficacy in urinary tract infections. J Antimicrob Chemother 75:988–996. doi: 10.1093/jac/dkz519. [DOI] [PubMed] [Google Scholar]
- 20.Perdigão-Neto LV, Oliveira MS, Rizek CF, Carrilho CMDM, Costa SF, Levin AS. 2014. Susceptibility of multiresistant Gram-negative bacteria to fosfomycin and performance of different susceptibility testing methods. Antimicrob Agents Chemother 58:1763–1767. doi: 10.1128/AAC.02048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jcm.01635-21-s0001.pdf, PDF file, 0.07 MB (72.2KB, pdf)


