ABSTRACT
Group A streptococcus (GAS) causes significant morbidity and mortality in New Zealand and is responsible for invasive disease and immune sequelae, including acute rheumatic fever (ARF). Early treatment of GAS pharyngitis reduces the risk of ARF. In settings with a high burden of GAS disease, a rapid GAS pharyngitis diagnostic test with a strong negative predictive value is needed to enable prompt and accurate treatment. This prospective study compares the Xpert Xpress Strep A molecular test (Cepheid) to throat culture and a second molecular method, the BioGX group A streptococcus-open system reagent (OSR) for BD Max for the diagnosis of GAS pharyngitis. Throat swabs were collected from the emergency department and wards of Middlemore Hospital, New Zealand. The BioGX group A streptococcus OSR for BD Max contributes to the composite gold standard of throat culture or both molecular methods positive. Basic demographic, clinical, and laboratory data were collected. Two hundred five out of two hundred fourteen swabs were suitable for analysis. Of those, 28/205 (13.7%) were GAS culture positive, 45/205 (22%) Xpert Xpress Strep A positive, and 38/205 (18.5%) BioGX positive. Compared to culture, the sensitivity, specificity, and positive and negative predictive values of the Xpert Xpress Strep A molecular test were 100%, 90.4%, 62.2%, and 100%, respectively. Compared to the composite gold standard, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 100%, 95.8%, 84.4%, and 100%, respectively. Seventeen samples were Xpert Xpress positive but culture negative; 6 of these 17 swabs represent true positives with evidence of recent GAS infection. Ten samples were culture negative but both Xpert Xpress and BioGX positive. The Xpert Xpress Strep A molecular test is highly sensitive with a strong negative predictive value and rapid turnaround time. It can be safely introduced as a first-line test for throat swabs in a high-incidence ARF population.
KEYWORDS: group A Streptococcus, pharyngitis, molecular test
INTRODUCTION
Group A streptococcus (GAS) is the most common cause of bacterial pharyngitis and is associated with postinfectious complications, including acute rheumatic fever (ARF), poststreptococcal glomerulonephritis (PSGN), and scarlet fever (1–3). ARF and PSGN continue to occur at unacceptably high rates in Aotearoa, New Zealand, and almost exclusively affect Māori and Pacific children (4). The New Zealand 2018 ARF incidence was 28.6/100,000 for Māori children aged 5 to 14years, 83.2/100,000 for Pacific children, and <1/100,000 for European/other (5). Although ARF and PSGN are now uncommon in most developed countries, GAS pharyngitis and its postinfectious sequelae remain significant global problems. Rheumatic heart disease (RHD), a chronic ARF sequelae, is responsible for an estimated 300,000 premature and preventable deaths per year (6). Since 2009, outbreaks of scarlet fever have been reported in China, Hong Kong, Singapore, and South Korea (7–10). The United Kingdom also reported an unprecedented increase in scarlet fever in 2014 followed by an increase in invasive GAS infections in 2016 (7).
Early antibiotic treatment has been shown to reduce the severity, duration, and transmission of GAS pharyngitis and, in high-risk settings, decreases the risk of developing ARF (4, 11, 12). Unfortunately, GAS pharyngitis is not reliably distinguished from viral pharyngitis on examination, and throat culture remains the gold standard method for diagnosis (1, 2, 13). Although 90 to 95% sensitive for the detection of GAS, throat culture requires incubation for up to 48 h on blood agar plates (2). Consequently, rapid antigen detection tests (RADTs) are often used in low-incidence ARF populations where they have potential antimicrobial stewardship benefits (1–3). A recent Cochrane review found that RADTs reduced antibiotic prescription rates by an absolute risk difference of 25% (95% confidence interval [CI], −31 to −18%) (3). Unfortunately, RADTs have a lower sensitivity than throat culture (86 to 91% compared to 90 to 95%) (1, 14). In New Zealand, given the significant morbidity and mortality attributable to GAS, the potential consequences of missed treatment are high, and RADTs are not currently recommended without backup throat culture (15, 16). High-incidence ARF settings such as New Zealand require rapid GAS pharyngitis diagnostic tests with strong negative predictive values that enable prompt and accurate treatment decisions.
The Xpert Xpress Strep A molecular test (Cepheid) is a qualitative real-time PCR that has a higher sensitivity than RADTs when used to diagnose GAS pharyngitis (17). It targets the speB gene of GAS, encoding streptococcal pyrogenic exotoxin, a virulence factor found on all GAS (17). The Xpert Xpress Strep A test is automated, has a laboratory hands-on time of <1 min, can be processed on demand, and produces a result in approximately 25 min (17). Product information for the Xpert Xpress Strep A test from 577 samples in the United States reports a sensitivity of 100%, specificity of 94.1%, positive predictive value (PPV) of 84.1%, and a negative predictive value (NPV) of 100% (17). The wider use of GAS molecular testing, including the Xpert Xpress Strep A, has recently been supported by a detailed review article; however, to date, there is limited information about its utility in high-ARF burden settings (18). One study in the Northern Territory of Australia, a high-ARF incidence setting, compared Xpert Xpress Strep A to throat culture for 145 swabs (14). Ralph et al. report a sensitivity of 100%, specificity of 79.3%, PPV of 48.8%, and an NPV of 100% (14). They also describe increased detection of GAS in patients with ARF and PSGN, demonstrating that in high-burden GAS settings, rapid molecular tests can be used to improve antibiotic decision-making and may aid diagnosis of postinfectious sequelae (14).
The purpose of this study was to evaluate the performance of the Xpert Xpress Strep A molecular test compared to throat culture in a hospital laboratory that serves an urban New Zealand population with a high burden of GAS infection and postinfectious complications. In addition, all throat swabs underwent testing with a second PCR, the BioGX group A streptococcus-open system reagent (OSR) for BD Max, to evaluate the Xpert Xpress Strep A molecular test against a composite gold standard: throat culture or both molecular methods positive.
MATERIALS AND METHODS
Population and data collection.
This prospective study was carried out in the Middlemore Hospital Microbiology Laboratory, Counties Manukau, New Zealand. Middlemore is an 800-bed secondary hospital that includes the Kidz First Children’s Hospital. Its catchment population is younger, with a higher proportion of patients living in socioeconomic deprivation than the national average. It has a high percentage of indigenous Māori (15.7%) and Pacific Island peoples (21.1%) (https://www.health.govt.nz/new-zealand-health-system/my-dhb/counties-manukau-dhb/population-counties-manukau-dhb). The Counties Manukau region has the highest rate of ARF in New Zealand, 9.4/100,000 between 2019 and 2020 (https://www.health.govt.nz/our-work/diseases-and-conditions/rheumatic-fever/reducing-rheumatic-fever).
Throat swabs were collected for clinical purposes from the emergency department and wards using an ESwab (Copan Diagnostics), a nylon-flocked swab in 1 ml of liquid Amies transport medium. Throat swabs were included if they arrived in the laboratory with a request for culture on Sunday to Wednesday of each week. This convenience sample allowed for double-reading of culture plates at 48 h. Swabs were excluded if the sample received was not an ESwab, the swab was unlabeled, clinical details were not available, or <400 μl of Amies transport medium was received per swab.
Basic demographic, clinical, and laboratory data were collected via the hospital’s electronic records and stored deidentified in a password-protected Excel spreadsheet. This included age, ethnicity, clinical indication for swab, antibiotics dispensed <14 days prior, throat swab culture result, Xpert Xpress result and cycle threshold (CT) value, and BD result and CT. If collected for clinical purposes, the following data were recorded: streptococcal serology (anti-streptolysin O titer [ASOT]/anti-DNAse B titer), inflammatory markers (erythrocyte sedimentation rate [ESR]/C-reactive protein [CRP]), and throat swabs collected in the preceding 14 days.
Microbiological methods.
Samples were received at Middlemore Hospital Laboratory, and routine throat culture was performed prior to molecular tests. This laboratory uses a Walk Away specimen processor (WASP; bioMérieux) to inoculate throat culture plates. A 10-μl loop of liquid Amies transport medium was streaked onto two plates, Columbia horse blood agar, and tryptic soy sheep blood agar (TSA) with 3% salt (Fort Richard Laboratories). Both plates were incubated for 48 h in CO2 at 37°C and assessed for β-hemolytic colonies at 24 and 48 h by medical laboratory scientists. β-Hemolytic colonies were further identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), (Vitek MS; bioMérieux) serogrouping using latex agglutination (Oxoid streptococcal grouping kit; Thermo Scientific) and further characterized Streptococcus dysgalactiae as group C (GCS) or G (GGS). Final culture reports indicate either the presence of group A, B, C, or G streptococci or the absence of β-hemolytic streptococci. Cultures positive for β-hemolytic streptococci are given a semiquantitative grade of few colonies, 1+ (light), 2+ (moderate), or 3+ (heavy growth). For the purposes of this study, throat culture plates were double-read by a laboratory scientist and study investigator at 48 h.
The Xpert Xpress Strep A molecular test was processed in accordance with Cepheid product instructions. Samples were vortexed for 5 s, and 300 μl of Amies transport medium was transferred into the Xpert Xpress Strep A cartridge using the provided pipette. The cartridge was inserted into the GeneXpert system (Cepheid; GXIV-4-L) Each lot number of Xpert Xpress Strep A tests underwent quality control (QC) with a positive (Streptococcus pyogenes strain) and negative control (Escherichia coli strain). QC isolates were cultured as above and an ESwab used to obtain three colonies. We transferred 300 μl of Amies liquid from each control swab into the Xpert Xpress Strep A cartridges as described above. Each Xpert Xpress Strep A result was reported as positive or negative and a CT value given. The Xpert Xpress allows a maximum of 43 PCR cycles and has an “early assay termination” function, meaning it will provide a positive result when the target DNA signal reaches a predetermined threshold before the full 43 cycles are complete. Samples with a high concentration of GAS target DNA require less cycles for detection.
Following the Xpert Xpress Strep A test, all samples were frozen at −20°C for 2 to 35 days so that the additional molecular test, BioGX group A streptococcus-OSR for BD Max, could be processed on all samples in batches of 24. The BioGX assay also targets the speB gene of GAS. It allows a maximum of 40 PCR cycles to determine a positive result. QC was performed as described above. Samples were processed as per BioGX manufacturer instructions. After being defrosted, samples were vortexed for 10 s, and 50 μl of Amies transport medium was pipetted into the sample buffer tube. BD Max ExK DNA-4 snap extraction kits were loaded into the extraction tray and snapped into position. One reagent tube of BioGX sample-ready lyophilized PCR master mix was snapped into position 2 of each extraction strip, and one BioGX rehydration buffer tube was snapped into position 3. The samples and extraction trays were loaded into the BD Max system as per operating instructions and the run commenced. Samples were reported as positive or negative and a CT value given. The BioGX group A streptococcus-OSR for BD Max total run time is approximately 2.25 h.
GAS culture-negative/Xpert Xpress Strep A-positive samples.
Culture-negative/Xpert Xpress Strep A-positive plates were reviewed by a senior microbiology scientist and/or consultant clinical microbiologist. Any morphologically suspected β-hemolytic colonies were purity plated and identified using MALDI-TOF MS and latex agglutination. If colonies were identified as GCS or GGS, an ESwab was used to sweep the purity plate, and this colony suspension underwent an Xpert Xpress Strep A test. Samples that were both culture and BioGX negative but Xpert Xpress PCR positive had a second, repeat BioGX test processed from the initial E swab liquid 24 h following the first BioGX run. The sample was refrozen at −20°C between tests.
Serological methods.
Streptococcal serology was performed on selected patients at the treating teams’ request. ASOT was performed using Roche diagnostics reagent on the Cobas 502 analyzer. Anti-DNase was performed using Beckman Coulter reagent on the Cobas 502 analyzer. Results were interpreted according to the 2014 New Zealand guidelines for diagnosis of rheumatic fever (https://assets.heartfoundation.org.nz/documents/shop/marketing/non-stock-resources/diagnosis-management-rheumatic-fever-guideline.pdf?1634870023).
Analysis.
Analysis was conducted using SPSS (version 26.0; IBM) software and Microsoft Excel. Mann-Whitney U tests were used to compare nonparametric continuous data and chi-square tests for categorical data. Sensitivity, specificity, PPV, and NPV were calculated using throat culture as the gold standard with 95% confidence intervals calculated using the Clopper-Pearson exact method. Test performance was reevaluated using a composite gold standard, where a true-positive result was either positive culture or both BioGX and Xpert Xpress Strep A positive. A true-negative sample was negative on culture and both molecular methods. Test performance calculations were repeated with stratification by ethnicity (Pacific and New Zealand Māori versus New Zealand European/other) (Table 1). Regression analysis was used to confirm the relationship between the Xpert Xpress Strep A CT and GAS culture grade.
TABLE 1.
Patient characteristics, clinical indication for swab, and microbiology (n = 205)
| Characteristic, indication, or result | Total (n = 205) | New Zealand Māori/Pacific (n = 143 [69.8%]) | New Zealand European/other (n = 62 [30.2%]) | P value |
|---|---|---|---|---|
| Characteristics | ||||
| Median age (IQR [yrs]) | 14 (7–26) | 14 (7.5–24) | 14 (5–34) | 0.357 |
| No. (%) female | 112 (54.6) | 80 (55.9) | 32 (51.6) | 0.567 |
| Clinical indication for swab (no. [%])a | ||||
| Pharyngotonsillitis | 125 (61.0) | 88 (61.5) | 37 (59.7) | 0.802 |
| ARF under investigation | 16 (7.8) | 13 (9.1) | 3 (4.8) | 0.297 |
| PSGN under investigation | 7 (3.4) | 6 (4.2) | 1 (1.6) | 0.35 |
| Scarlet fever | 1 (0.5) | 1 (0.7) | 0 | |
| All GAS postinfectious sequelae | 24 (11.7) | 20 (14.0) | 4 (6.5) | 0.123 |
| URTI | 23 (11.2) | 16 (11.2) | 7 (11.3) | 0.983 |
| Other infectionb | 22 (10.7) | 11 (7.7) | 11 (17.7) | 0.033 |
| Noninfective diagnosisc | 2 (1.0) | 1 (0.7) | 1 (1.6) | 0.541 |
| Unknownd | 12 (5.9) | 7 (4.9) | 5 (8.1) | 0.375 |
| History of rheumatic fever/RHD documented (no. [%]) | 5 (2.4) | 5 (3.5) | 0 | |
| Streptococcal serology during visite (no. [%]) | 31 (15.1) | 25 (17.5) | 6 (9.7) | 0.152 |
| Positive | 7 (22.6) | 7 (28) | 0 | |
| Negative | 24 (77.4) | 18 (72) | 6 (100) | |
| Throat swab <14 days prior (no. [%]) | 18 (8.8) | 15 (10.5) | 3 (4.7) | 0.189 |
| GAS positive | 5 (27.8) | 5 (33.3) | 0 | |
| GAS negative | 13 (72.2) | 10 (66.7) | 3 (100) | |
| Group C streptococcus positive (other β-hemolytic strep reported) (no. [%]) | 1 (5.6) | 1 (6.7) | 0 | |
| Antibiotics dispensed <14 days prior (no. [%]) | 26 (12.7) | 18 (12.6) | 8 (12.9) | 0.95 |
| Throat culture result (no. [%]) | ||||
| GAS positive | 28 (13.7) | 22 (15.4) | 6 (9.7) | 0.274 |
| Growth of other β-hemolytic streptococcif (no. [%]) | ||||
| S. agalactiae | 7 (3.4) | 6 (4.2) | 1 (1.6) | 0.35 |
| S. dysgalactiae (group C) | 4 (2.0) | 2 (1.4) | 2 (3.2) | 0.385 |
| S. dysgalactiae (group G) | 7 (3.4) | 6 (4.2) | 1 (1.6) | 0.35 |
| Xpert Xpress Strep A molecular test result (no. [%]) | ||||
| Positive | 45 (22) | 38 (26.6) | 7 (11.3) | 0.015 |
| BioGX molecular test result (no. [%]) | ||||
| Positive | 38 (18.5) | 32 (22.4) | 6 (9.7) | 0.032 |
Patients had more than one indication, i.e., pharyngitis and ARF/PSGN.
Other infection includes measles, mumps, enterovirus meningitis, adenovirus, oral candidiasis, meningococcal disease, tuberculosis, lymphadenitis, viral-induced wheeze, or asthma exacerbation.
Noninfective diagnosis includes Kawasaki's disease, cardiomyopathy, neutropenic postrenal transplant, or asymptomatic sibling screened for carriage for in PSGN study.
Indicates no indication on laboratory form or online record.
Streptococcal serology interpreted using New Zealand RHD Guidelines 2014.
One swab isolated both S. agalactiae and group C streptococcus.
Ethics.
This study was approved by the Human Disciplinary Ethics Commission (HDEC) of New Zealand, 19/STH/209. The need for consent was waived, as throat swabs were collected for clinical purposes as part of a patient’s routine care, and no additional samples or procedures were required for study inclusion.
RESULTS
Demographics.
During the study period, 214 consecutive swabs were considered for inclusion. One swab had no clinical details available, and 1 was a duplicate. The first 7 swabs that arrived during the study period were discarded in error prior to the second molecular test. We therefore included 205/214 swabs (95.8%) for analysis.
The median age of the cohort was 14 years (interquartile range [IQR], 7 to 26). In the cohort, 111/205 (54.1%) were of Pacific ethnicity, 32 (15.6%) were New Zealand Māori, and 62 (30.2%) New Zealand European/other (Table 1). One hundred twelve (54.6%) of the total cohort were female. The most common clinical indication for throat swab was pharyngotonsillitis (125/205, 61.0%). This was followed by upper respiratory tract infection (URTI) (23, 11.2%) and “other infection” (22, 10.7%).
Table 1 shows demographic data and results comparing populations at high risk of postinfectious GAS complications (Pacific and New Zealand Māori) with those at lower risk (New Zealand European/other). There were no significant differences between Pacific/New Zealand Māori and New Zealand European/other groups except for the clinical indication for swab “other infection” being higher in the New Zealand European/other group, 11/143 (7.7%) versus 11/62 (17.7%) (P = 0.033).
Test performance.
In total, 28/205 (13.7%) throat swabs were culture positive for GAS: 45/205 (22%) were Xpert Xpress Strep A molecular test positive (Table 2), and 38/205 (18.5%) were BioGX positive. All samples that were Xpert Xpress Strep A test negative were both culture and BioGX negative. The sensitivity, specificity, NPV, and PPV for the Xpert Xpress Strep A test compared to throat culture were 100%, 90.4%, 100%, and 62.2%, respectively (Table 3). Compared to the composite outcome (culture or both molecular tests positive) (Table 4), the sensitivity, specificity, NPV, and PPV were 100%, 95.8%, 100%, and 84.4%, respectively (Table 5).
TABLE 2.
Performance of Xpert Xpress Strep A molecular test and throat culture for detection of GAS in throat swabs
| Results of Xpert Xpress Strep A | Results of throat culture |
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 28 | 17 | 45 |
| Negative | 0 | 160 | 160 |
| Total | 28 | 177 | 205 |
TABLE 3.
Sensitivity, specificity, and predictive values for Xpert Xpress Strep A compared to culturea
| Statistical measure | Result for Xpert Xpress Strep A molecular test (% [95% CI]) |
|---|---|
| Sensitivity | 100 (87.6–100) |
| Specificity | 90.4 (85.1–94.3) |
| Negative predictive value | 100 |
| Positive predictive value | 62.2 (51.2–72.1) |
Clopper-Pearson exact method was used.
TABLE 4.
Performance of Xpert Xpress Strep A molecular test using composite gold standard (culture positive or two molecular tests positive)
| Results of Xpert Xpress Strep A | Results of composite gold standard |
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 38 | 7 | 45 |
| Negative | 0 | 160 | 160 |
| Total | 38 | 167 | 205 |
TABLE 5.
Sensitivity, specificity, and predictive values for Xpert Xpress Strep A compared to composite gold standarda
| Statistical measure | Result for Xpert Xpress Strep A molecular test (% [95% CI]) |
|---|---|
| Sensitivity | 100 (90.8–100) |
| Specificity | 95.81 (91.6–98.3) |
| Negative predictive value | 100 |
| Positive predictive value | 84.4 (72.4–91.8) |
Clopper-Pearson exact method was used.
Although there was a trend toward more culture-positive GAS swabs in the Pacific/Māori group compared with New Zealand European/other, 22/143 (15.4%) versus 6/62 (9.7%), this was not statistically significant (P = 0.274). There were, however, significantly more positive samples in the Pacific/New Zealand Māori group on both molecular methods compared to New Zealand European/other group as follows: 32/143 (22.4%) versus 6/62 (9.7%) (P = 0.032) on BioGX and 38/143 (26.6%) versus 7/62 (11.3%) (P = 0.015) on Xpert Xpress Strep A.
In total, 120/205 (58.5%) patients were of Māori or Pacific ethnicity and aged 3 to 35 years and therefore met the New Zealand Heart Foundation (NZHF) high-risk criteria for ARF (16). This guideline recommends high-risk patients with pharyngitis have a throat swab and receive empirical antibiotics. Of this high-risk cohort, 21/120 (17.5%) were culture positive for GAS, and 34/120 (28.3%) were Xpert Xpress Strep A test positive. Additionally, 24/205 (11.7%) swabs were from patients thought to have poststreptococcal infectious sequelae (ARF, PSGN, or scarlet fever). Of these swabs, 20/24 (83.3%) were from patients of Pacific or New Zealand Māori ethnicity. Ten out of 24 (41.7%) tested positive for GAS by Xpert Xpress Strep A compared to 9/24 (37.5%) positive by BioGX and 6/24 (25.0%) positive on throat culture.
Serology.
Streptococcal serology was performed in 31/205 (15.1%) of patients during their admission. According to the NZHF diagnostic criteria for ARF, 7/31 (22.6%) patients had positive streptococcal antibody titers (https://assets.heartfoundation.org.nz/documents/shop/marketing/non-stock-resources/diagnosis-management-rheumatic-fever-guideline.pdf?1634870023). Of those with positive streptococcal serology, 7/7 patients were of Pacific or New Zealand Māori ethnicity, and 4/7 had PSGN/ARF documented as the indication for throat swab. Two out of seven patients with positive serology had positive GAS throat cultures in the community within the last 14 days. Although no patient with positive streptococcal serology had a GAS culture-positive study swab, 2/7 (28.6%) were BioGX test positive, and 3/7 (42.9%) were Xpert Xpress Strep A positive.
GAS culture-negative/Xpert Xpress Strep A-positive results.
In total, 17/205 swabs (8.3%) were GAS culture negative/Xpert Xpress Strep A test positive. Of these swabs, 10/17 were culture negative and both BioGX and Xpert Xpress Strep A positive (Table 6). Five out of ten patients had clear evidence of recent GAS infection and are thought to represent true-positive PCR results, 4/10 had positive GAS throat swabs in the community 1 to 3 days prior, and 2/10 had positive streptococcal serology. Additionally, 1/10 patient was diagnosed with PSGN, 1/10 with scarlet fever, and 1/10 with ARF. Antibiotics were dispensed prior to sample collection (2 to 4 days) in 4/10 culture-negative/both BioGX and Xpert Xpress Strep A-positive swabs.
TABLE 6.
Results for throat culture-negative/Xpert Xpress Strep A-positive samples (n = 17)d
| Test result | Age (yrs) | Gender | Ethnicity | Indication | Pretreated antibiotics <14 days | Throat swab <14 days | Streptococcal serology | Type of growth on throat culture (gradee) | Xpert CT value | BioGX CT value |
|---|---|---|---|---|---|---|---|---|---|---|
| Positive by BioGX (n = 10)a | 2 | Male | Pacific | Pharyngitis | No | No | Not done | MOF (2) | 33.7 | 33.2 |
| 2.9 | Male | Pacific | Tonsillitis, scarlet fever | Yes , co-trimoxazole for 3 days prior, 1 day of i.v. penicillin prior | Yes, GAS (3 days prior) | Not done | MOF (2) | 31.3 | 31.9 | |
| 5 | Male | New Zealand Māori | Tonsillitis | No | No | Not done | MOF (2) | 33.4 | 31.1 | |
| 6 | Male | Pacific | Pharyngitis | No | Yes, GAS (1 day prior) | Not done | SAGA (1), GCS (FC), MOF (2) | 32.6 | 31 | |
| 9 | Female | Pacific | Recurrent pharyngitis | No | No | Not done | MOF (2), Haemophilus parahaemolyticus (2) | 35.2 | 34.1 | |
| 10 | Female | Pacific | PSGN | Yes , amoxicillin for 4 days prior | Yes, GAS (3 days prior) | Positive | MOF (2) | 35.7 | 39.3 | |
| 10 | Female | Pacific | Possible ARF: chorea, acute on chronic carditis | No | No | Positive | MOF (2), H. parahaemolyticus | 29.2 | 30.5 | |
| 16 | Female | Pacific | Pharyngitis | Yes , erythromycin for 2 days prior | Yes, GAS (1 day prior) | Negative | MOF (1), Corynebacterium urealyticum (2) | 23.2 | 25 | |
| 29 | Female | Pacific | Tonsillitis | Yes , i.m. penicillin given 2 days prior | No | Not done | MOF (2) | 26.1 | 25.5 | |
| 33 | Female | New Zealand Māori | Pharyngitis | No | No | Not done | MOF (2), H. parahaemolyticus (FC) | 32.1 | 33.5 | |
| Total | 9.5 (5.25–14.5)c | 6/10 (60%) female | 8/10 (80%) Pacific | 1/10 ARF, 1/10 PSGN, 1/10 scarlet fever | 4/10 (40%) | 4/10 (40%) positive GAS | 2/10 (20%) positive | 1/10 GCS/SAGA | 31.25 | 31.51 |
| Negative twice by BioGX (n = 7)b | 3.4 | Female | Pacific | Pharyngitis | No | No | Not done | MOF (1) | 39.4 | |
| 3.9 | Male | Pacific | Pharyngitis | No | No | Not done | GGS (FC), MOF (1) | 39.3 | ||
| 6 | Male | Pacific | PSGN | Yes (2/7 prior cefuroxime) | No | Positive | MOF (2), Neisseria flava/Neisseria perflava/Neisseria subflava (2) | 38.2 | ||
| 6 | Male | New Zealand European | Pharyngitis | No | No | Not done | MOF (2) | 38.5 | ||
| 9 | Male | Pacific | Pharyngitis | No | No | Not done | MOF (3), Kingella kingae (FC) | 35.3 | ||
| 13 | Female | New Zealand Māori | Pharyngitis | No | No | Not done | MOF (1), N. subflava (FC) | 36 | ||
| 22 | Female | Pacific | Pharyngitis | No | No | Not done | MOF (1) | 39.5 | ||
| Total | 6 (3.78–10)c | 3/7 (42.9%) Female | 5/7 (71.4%) Pacific | 1/7 PSGN | 1/7 (14.3%) | 0/7 | 1/7 (14.3%) positive | 1/7 GGS | 38.0 |
There were 5/10 true positives according to clinical data.
There was 1/7 true positive according to clinical data.
Data represent median (IQR).
Abbreviations: PSGN, poststreptococcal glomerulonephritis; ARF, acute rheumatic fever; GAS, group A streptococcus; CT, cycle threshold; MOF, mixed oropharyngeal flora; SAGA, S. agalactiae; GCS, group C streptococcus; GGS group G streptococcus; i.m., intramuscular; i.v., intravenous.
FC, few colonies; 1, light; 2, moderate; 3, heavy.
The remaining 7/17 swabs were culture negative and BioGX negative on two separate testing runs, but Xpert Xpress Strep A positive (Table 6). One patient had a clinical history that suggested this was a true-positive PCR result: PSGN with raised streptococcal titers and pretreatment with 2 days of cephalosporin prior to this study throat swab.
In total, 2/17 of swabs were culture positive for other β-hemolytic streptococci (Table 6). One culture-negative/Xpert Xpress Strep A and BioGX-positive swab had growth of both S. agalactiae and GCS. This patient had a positive GAS community swab 1 day prior, although no electronic record of antibiotics being dispensed. Repeat Xpert Xpress Strep A tests from the S. agalactiae and GCS purity plates were negative. One culture and BioGX negative but Xpert Xpress Strep A-positive swab cultured GGS. This sample had three repeat purity plates of β-hemolytic colonies, and each was again identified on MALDI-TOF MS and latex agglutination as GGS. A repeat Xpert Xpress Strep A test was conducted from this GGS purity plate and was Xpert Xpress Strep A test negative.
Xpert Xpress Strep A cycle thresholds by culture and BioGX result.
The Xpert Xpress Strep A CT values of GAS culture-positive samples were significantly lower than culture-negative samples, with a median CT of 20.1 (IQR, 18.3 to 25.1) versus 35.2 (IQR, 31.7 to 38.4) (P < 0.001). The CT was inversely associated with the grade of GAS growth (R2 = 0.586; P < 0.001) (Fig. 1). Of GAS culture-negative/Xpert Xpress Strep A-positive samples, there was a significantly lower Xpert Xpress CT in those with positive BioGX (32.4; IQR, 29.2 to 33.7) than those with a negative BioGX (38.5; IQR, 36.0 to 39.4) (P < 0.001).
FIG 1.
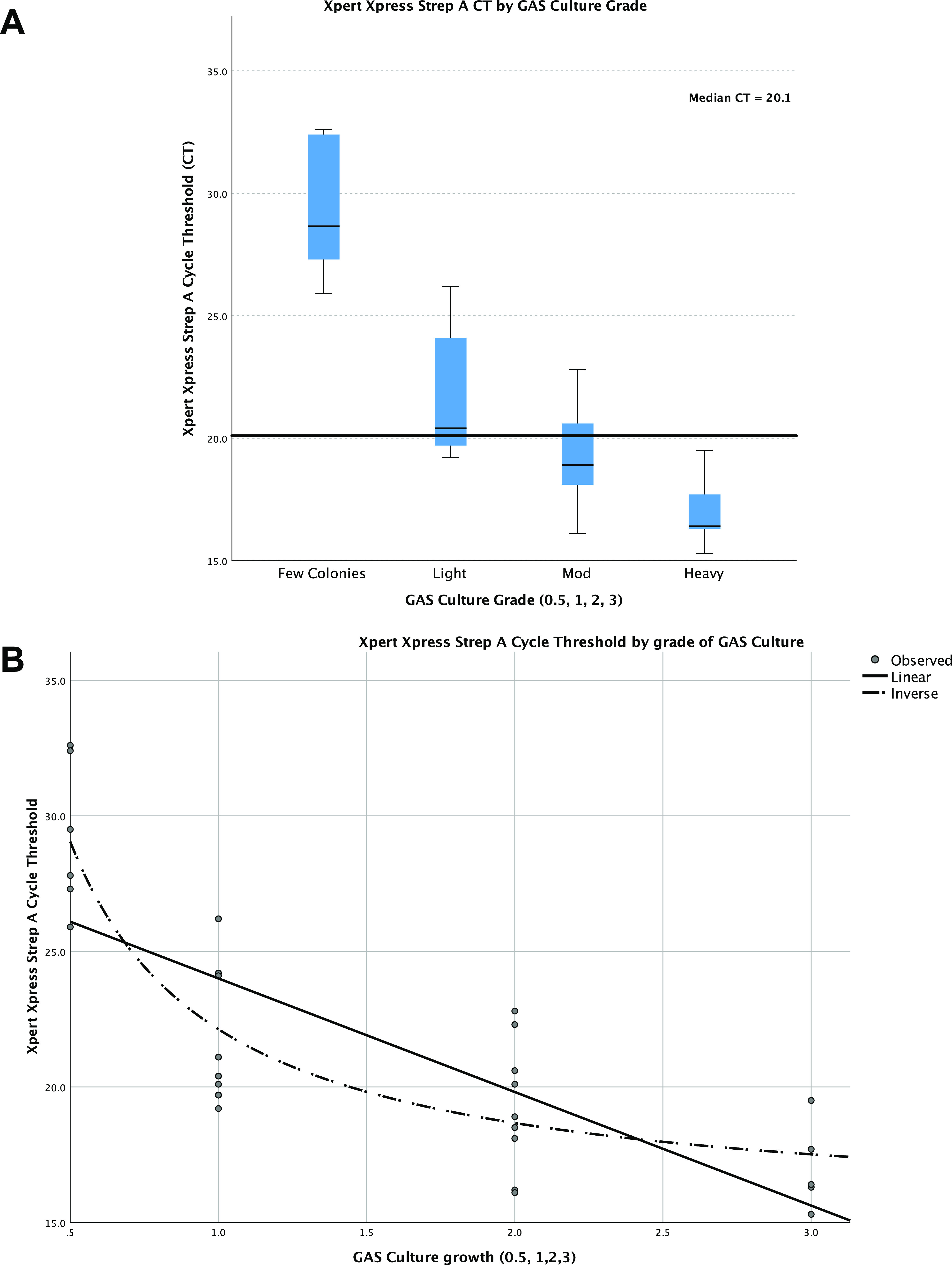
(A) Cycle threshold (CT) values for Xpert Xpress molecular test by grade of group A streptococcus (GAS). (B) CT and GAS culture grade were inversely associated on regression analysis. 0.5, few colonies; 1, mild growth; 2, moderate; 3, heavy growth.
DISCUSSION
This study evaluated the performance of the Xpert Xpress Strep A molecular test in a population with a high incidence of GAS disease and found it to be 100% sensitive compared to throat culture. With an NPV of 100%, the Xpert Xpress Strep A molecular test can be safely introduced as a first-line test for throat swabs in our setting, reserving culture for swabs with a positive Xpert Xpress Strep A result. To our knowledge, this is the largest peer-reviewed study to date comparing the Xpert Xpress Strep A to throat culture as well as a second, alternate molecular test. This is also the first published evaluation of the BioGX group A streptococcus-OSR for BD Max.
In a high-burden GAS population such as Counties Manukau, New Zealand, this study suggests there could be a net antimicrobial stewardship benefit by using the Xpert Xpress Strep A test first instead of culture for pharyngitis. Previous studies have demonstrated that it is not possible to reliably distinguish bacterial from viral pharyngitis in children (19). Although, internationally, there are a number of clinical prediction tools to assess a child’s risk of having bacterial pharyngitis, these have not been validated in the New Zealand setting (16). The 2019 New Zealand sore throat guidelines state that due to this lack of validation, clinical prediction rules should not be used to manage GAS in New Zealand high-risk settings such as Counties Manukau (16). Instead, New Zealand guidelines recommend that patients with pharyngitis who meet high-risk criteria for rheumatic fever have a throat swab sent for culture and receive empirical antibiotics. In this setting, the definition of high-risk includes personal/family risk of rheumatic fever or two or more of Māori or Pacific ethnicity, aged 3 to 35years, and living in crowded circumstances or a low socioeconomic area (16). In our cohort, 59% (120/205) of the study population met high-risk criteria (16). Of these high-risk patients, 72% (86/120) were both culture and Xpert Xpress Strep A negative and would have been offered empirical antibiotics unnecessarily. Of the culture-negative/Xpert Xpress PCR-positive swabs, 6/17 clearly represented true-positive PCR results, including patients with recent positive GAS throat culture, positive streptococcal serology, or new diagnoses of poststreptococcal conditions. Therefore, in our cohort, if Xpert Xpress Strep A was used prior to throat culture, a rapid negative result would allow doctors to confidently avoid unnecessary antibiotics for 86 patients. Allowing for treatment of 11 possible false-positive PCR results, use of the Xpert Xpress Strep A test as an initial test instead of culture would result in 75 fewer unnecessary prescriptions (one-third of this cohort).
In this study, the Xpert Xpress Strep A increased the detection of GAS in patients with possible ARF/PSGN/scarlet fever from 6/24 (25%) to 10/24 (41.7%). Increased detection of GAS was also noted in Ralph et al., from 3 (12%) culture positives to 8 (32%) molecular positives (14). Given that evidence of antecedent GAS infection is a diagnostic criterion for ARF and PSGN, improved and rapid detection of GAS will support prompt diagnosis of poststreptococcal sequelae.
The Xpert Xpress Strep A molecular test is significantly faster and less resource intensive than both throat culture and the alternate molecular test used in this study, BioGX. Although the BioGX was as sensitive as the Xpert Xpress Strep A, with an NPV of 100%, it requires more laboratory hands-on time than the Xpert Xpress Strep A, is most efficiently performed in batches of 12 or 24, and has a significantly longer processing time. The Middlemore laboratory typically receives less than 10 throat swabs per day, and, with its rapid, on-demand processing, the Xpert Xpress Strep A test is better suited to our population.
When comparing any molecular test to culture as the gold-standard, culture-negative/PCR-positive results can be difficult to interpret for a number of reasons illustrated in this study. Primarily, the Xpert Xpress Strep A requires detection of the speB gene rather than growth of GAS (17). A positive molecular result/negative culture could therefore reflect the presence of nonviable GAS, recent infection, pretreatment, or low-burden pharyngeal carriage (14, 20, 21). In this study, the CT of Xpert Xpress Strep A culture-positive swabs was inversely correlated with the number of GAS colonies found on culture. Culture-negative/Xpert Xpress Strep A-positive samples had a significantly higher CT value than culture-positive samples. This illustrates that the Xpert Xpress Strep A detects the presence of GAS at a lower threshold than achievable by culture and is more sensitive. Although the speB gene is specific to GAS, there is evidence of horizontal genetic material transfer between β-hemolytic streptococci as well as reports of speB expression by GCS and GGS (14, 22–24). This could theoretically cause false positives, and possible cross-reactivity was noted in the Ralph et al. paper with 2 GCS and 2 GGS isolates, each of which had high CT values approaching the upper limit of detection (14). In our cohort, 2/17 culture-negative/Xpert Xpress Strep A-positive swabs were culture positive for other β-hemolytic streptococci (GCS, GBS, GGS); one of which had a community swab culture positive for GAS 1 day prior. Although these two swabs may represent cross-reactivity, we were unable to replicate positive Xpert Xpress Strep A results using a pure suspension of these isolates, and the remainder of the 16 nongroup A β-hemolytic streptococci were Xpert Xpress Strep A negative.
Following the interim analysis of this study and the demands of SARS-CoV-2 PCR on medical laboratory scientist time, the Xpert Xpress Strep A test has been introduced as the initial test for all throat swabs arriving in the Middlemore Hospital Laboratory. Negative Xpert Xpress Strep A swabs do not proceed to culture, and a negative molecular result is released to the clinical team. The introduction of the Xpert Xpress Strep A in our setting does not replace the need for clinical assessment, nor the decision to treat. We recognize that occasionally the diagnosis of non-GAS pathogens such as Fusobacteria or Archanobacteria are important, and thus, electronic communication of a negative result includes a statement that the swab will be stored for 7 days in case additional tests are required. Positive Xpert Xpress swabs have an interim positive molecular result released and go on to have confirmatory culture. This is particularly important in our context where ARF is a notifiable public health condition and GAS isolates from patients with rheumatic fever are sent to the national reference laboratory for emm typing. Although cross-reaction with other β-hemolytic strep was not demonstrated in our study, culture of molecular positive isolates ensures this possibility is monitored.
A limitation of this study is that data collection was limited to electronic records without access to detailed clinical notes or patient interviews; therefore, clinical details such as antibiotic dispensing information may be underestimated. In Counties Manukau, the majority of primary and intermediate schools are enrolled in Mana Kidz, a school-based sore throat diagnosis and treatment program where antibiotic dispensing information is not always captured electronically. Also, although larger than the Australian cohort, the small sample makes it difficult to interpret subgroup analyses (14). During the study, consecutive throat swabs were included, and thus, laboratory scientists were not blinded to swab study involvement. Anecdotally, during the study period, more purity plates, MALDI-TOF MS, and latex agglutination of possible β-hemolytic colonies were performed, and this may have increased the sensitivity of throat culture during the study period. Lastly, we note the Xpert Xpress Strep A requires a significantly larger sample volume (300 μl) than culture (20 μl) and BioGX PCR (50 μl). It is possible the larger sample volume contributed to the increased sensitivity of the Xpert Xpress compared to both throat culture and BioGX. In future analyses, it would be helpful to culture a larger volume for comparison. It is also important to note that because the BioGX PCR is most efficiently performed in batches of 12 or 24, all samples were frozen and thawed prior to having this molecular method processed. It is possible that freezing the samples may have altered the BioGX sensitivity and therefore affected the specificity of the Xpert Xpress Strep A test compared to the composite gold standard.
Conclusion. In high-burden GAS settings such as Counties Manukau, New Zealand, the Xpert Xpress Strep A molecular test is significantly faster than culture, has an excellent NPV, and allows for rapid and reliable identification of patients with GAS pharyngitis. A negative Xpert Xpress molecular test allows clinicians to confidently withhold antibiotics and is likely to result in a net antimicrobial stewardship benefit in populations with a high burden of GAS-related disease.
ACKNOWLEDGMENTS
We thank Middlemore Microbiology scientists for their assistance in this project.
The initial 200 Xpert Xpress molecular test kits were provided at a reduced cost by Cepheid. Cepheid had no involvement in the planning, design, analysis, or write-up of this study.
Contributor Information
Amanda Taylor, Email: ATaylor@adhb.govt.nz.
Sandra S. Richter, Mayo Clinic
REFERENCES
- 1.Luo R, Sickler J, Vahidnia F, Lee YC, Frogner B, Thompson M. 2019. Diagnosis and management of group a streptococcal pharyngitis in the United States, 2011–2015. BMC Infect Dis 19:193. 10.1186/s12879-019-3835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanz RR, Zheng XT, Carter DM, Steele MC, Shulman ST. 2018. Caution needed: molecular diagnosis of pediatric group A streptococcal pharyngitis. J Pediatric Infect Dis Soc 7:e145–e147. 10.1093/jpids/pix086. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JF, Pauchard JY, Hjelm N, Cohen R, Chalumeau M. 2020. Efficacy and safety of rapid tests to guide antibiotic prescriptions for sore throat. Cochrane Database Syst Rev 6:CD012431. 10.1002/14651858.CD012431.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennon D, Anderson P, Kerdemilidis M, Farrell E, Crengle Mahi S, Percival T, Jansen D, Stewart J. 2017. First presentation acute rheumatic fever is preventable in a community setting: a school based intervention. Pediatr Infect Dis J 36:1113–1118. 10.1097/INF.0000000000001581. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Environmental Science and Research Limited. 2019. Rheumatic fever report: January 2018 to December 2018. https://surv.esr.cri.nz/PDF_surveillance/RheumaticFever/Rheumaticfeverbi-annualreportJan-Dec2018.pdf.
- 6.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA. 2017. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 377:713–722. 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 7.Lynskey NN, Jauneikaite E, Li HK, Zhi X, Turner CE, Mosavie M, Pearson M, Asai M, Lobkowicz L, Chow JY, Parkhill J, Lamagni T, Chalker VJ, Sriskandan S. 2019. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis 19:1209–1218. 10.1016/S1473-3099(19)30446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamagni T, Guy R, Chand M, Henderson KL, Chalker V, Lewis J, Saliba V, Elliot AJ, Smith GE, Rushton S, Sheridan EA, Ramsay M, Johnson AP. 2018. Resurgence of scarlet fever in England, 2014–16: a population-based surveillance study. Lancet Infect Dis 18:180–187. 10.1016/S1473-3099(17)30693-X. [DOI] [PubMed] [Google Scholar]
- 9.Yung CF, Thoon KC. 2018. A 12 year outbreak of scarlet fever in Singapore. Lancet Infect Dis 18:942. 10.1016/S1473-3099(18)30464-X. [DOI] [PubMed] [Google Scholar]
- 10.Moreland NJ, Webb RH. 2019. Against the trend: a decrease in scarlet fever in New Zealand. Lancet Infect Dis 19:1285–1286. 10.1016/S1473-3099(19)30617-6. [DOI] [PubMed] [Google Scholar]
- 11.Dunne EM, Marshall JL, Baker CA, Manning J, Gonis G, Danchin MH, Smeesters PR, Satzke C, Steer AC. 2013. Detection of group A streptococcal pharyngitis by quantitative PCR. BMC Infect Dis 13:312. 10.1186/1471-2334-13-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinks A, Glasziou PP, Del Mar CB. 2013. Antibiotics for sore throat. Cochrane Database Syst Rev CD000023. 10.1002/14651858.CD000023.pub4. [DOI] [PubMed] [Google Scholar]
- 13.Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, Martin JM, Van Beneden C. 2012. Executive summary: clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 55:1279–1282. 10.1093/cid/cis847. [DOI] [PubMed] [Google Scholar]
- 14.Ralph AP, Holt DC, Islam S, Osowicki J, Carroll DE, Tong SYC, Bowen AC. 2019. Potential for molecular testing for group A streptococcus to improve diagnosis and management in a high-risk population: a prospective study. Open Forum Infect Dis 6:ofz097. 10.1093/ofid/ofz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Upton A, Lowe C, Stewart J, Taylor S, Lennon D. 2014. In vitro comparison of four rapid antigen tests for group A streptococcus detection. N Z Med J 127:77–83. [PubMed] [Google Scholar]
- 16.National Heart Foundation New Zealand. 2019. Group A streptococcal sore throat management guideline: 2019 update. National Heart Foundation of New Zealand, Auckland, New Zealand. https://assets.heartfoundation.org.nz/documents/shop/heart-healthcare/non-stock-resources/gas-sore-throat-rheumatic-fever-guideline.pdf?1615455698. [Google Scholar]
- 17.Cepheid. 2017. Xpert Xpress Strep A XPRSTREPA-CE-10 package insert. Cepheid, Sunnyvale, CA. [Google Scholar]
- 18.Thompson TZ, McMullen AR. 2020. Group A streptococcus testing in pediatrics: the move to point-of-care molecular testing. J Clin Microbiol 58:e01494-19. 10.1128/JCM.01494-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaikh N, Leonard E, Martin JM. 2010. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 126:e557-64–e564. 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez MD, McElvania E. 2018. New developments in rapid diagnostic testing for children. Infect Dis Clin North Am 32:19–34. 10.1016/j.idc.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanz RR, Ranniger EJ, Rippe JL, Dietz RL, Oktem CL, Lowmiller CL, Shulman ST. 2019. Highly sensitive molecular assay for group A streptococci over-identifies carriers and may impact outpatient antimicrobial stewardship. Pediatr Infect Dis J 38:769–774. 10.1097/INF.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 22.Behera B, Mathur P, Bhardwaj N, Jain N, Misra MC, Kapil A, Singh S. 2014. Antibiotic susceptibilities, streptococcal pyrogenic exotoxin gene profiles among clinical isolates of group C or G Streptococcus dysgalactiae subsp. equisimilis & of group G S. anginosus group at a tertiary care centre. Indian J Med Res 139:438–445. [PMC free article] [PubMed] [Google Scholar]
- 23.Davies MR, Tran TN, McMillan DJ, Gardiner DL, Currie BJ, Sriprakash KS. 2005. Inter-species genetic movement may blur the epidemiology of streptococcal diseases in endemic regions. Microbes Infect 7:1128–1138. 10.1016/j.micinf.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Upton A, Bissessor L, Farrell E, Shulman ST, Zheng X, Lennon D. 2016. Comparison of illumigene group A streptococcus assay with culture of throat swabs from children with sore throats in the New Zealand school-based rheumatic fever prevention program. J Clin Microbiol 54:153–156. 10.1128/JCM.02440-15. [DOI] [PMC free article] [PubMed] [Google Scholar]


