Abstract
Although differentiating benign and malignant thymic epithelial lesions is important to avoid unnecessary treatment and predict prognosis, it is challenging because of overlaps in the chest computed tomography (CT) findings. In this study, we investigated whether the diameter of the thymic vein and other CT findings could differentiate between benign (thymoma and thymic cysts) and malignant (thymic carcinoma, [TCa]) lesions.
We conducted a retrospective study across two tertiary referral hospitals in Japan between November 2009 and June 2018. We included 12 patients with TCa, 34 patients with thymomas, and 17 patients with thymic cysts. We analyzed the receiver operating characteristic (ROC) curve to determine the best cut-off values and performed univariate and multivariate analyses of CT findings to distinguish TCa from other benign lesions. Post-hoc analysis was performed for the maximum short axis of the thymic vein using the Mann–Whitney U test, and the number of the maximum short axis of the thymic vein ≥ the cutoff was determined using the Fisher exact test with a family-wise error-correction using Bonferroni's method.
ROC analysis showed that a maximum short axis of the thymic vein ≥2 mm was considerably more frequent in TCa than in the other lesions (P < .001 for both), with 83% sensitivity and 86% specificity. Univariate and multivariate analyses revealed the association with TCa of the number of the maximum short axis of the thymic vein ≥2 mm (P = .005, multivariate generalized linear model analysis), ill-defined margin (P = .001), and mediastinal lymphadenopathy (P < .001). Thymic vein diameter was in descendimg order of TCa > thymoma > thymic cysts with statistically significant differences between the groups (Ps < .05).
Thymic vein diameter was significantly longer in TCa than in thymoma and thymic cysts. Measurement of the maximum short axis of the thymic vein could be a powerful diagnostic tool to differentiate TCa from thymoma and thymic cysts.
Keywords: computed tomography, thymic carcinoma, thymic cysts, thymic neoplasms, thymic vein, thymoma
1. Introduction
Thymic epithelial lesions are the most common neoplasms of the anterior mediastinum and are found in 0.45% of chest computed tomography (CT) images.[1] It is challenging to differentiate between the most common thymic epithelial lesions, namely, thymic carcinoma (TCa), thymoma, and thymic cysts. According to Ackman et al, 24.3% of the patients who underwent thymectomy for a suspected thymic epithelial tumor were diagnosed with thymic cysts, which do not require treatment.[2] Considering the serious complications of thymectomy, such as great vessel injuries (reported in 1.5% of patients), and that the chemotherapy regimen differs between thymoma and TCa,[3] a precise preoperative diagnosis is necessary to avoid unnecessary thymectomy, determine the optimal regimen of chemotherapy, and predict prognosis.
Regarding diagnostic modality, chest CT is the most accessible for evaluating mediastinal lesions. Previous studies have attempted to use CT for thymic epithelial tumors.[4–6] In contrast, the diagnostic utility of ultrasonography in mediastinal tumors is limited. Although Chen et al revealed the specific patterns of lymphoma on color Doppler sonography,[7] the method for differentiating thymic epithelial tumors has not been established. The peak time of the intensity curve in dynamic contrast-enhanced study and the apparent diffusion coefficient value were reported to be useful in differentiating TCa from other thymic neoplasms, especially thymoma.[8,9] However, magnetic resonance imaging is not widely accessible compared to CT. Fluorodeoxyglucose-positron emission tomography CT is useful for differentiating TCa from other thymic epithelial tumors,[10,11] but for the same reason, CT is preferentially used.
The overlaps of the CT findings among the three pathologies make preoperative radiological diagnosis challenging,[5] leading to the low preoperative correct diagnoses of 37.5%, 77.8%, and 61.4% cases of TCa, thymomas, and thymic cysts, respectively.[12] To overcome this limitation, a better CT finding for differentiating these thymic epithelial lesions is warranted.
To our knowledge, no previous study has focused on the diagnostic performance of the diameter of the thymic vein for these lesions. We intended to compare this diameter in patients with different thymic epithelial lesions. In addition, we aimed to discuss the usefulness of the findings in differentiating these lesions.
2. Methods
This multi-center retrospective study was approved by the institutional review boards. The requirement for written informed consent was waived because of the retrospective study design. However, we protected the privacy of all patients.
2.1. Subjects
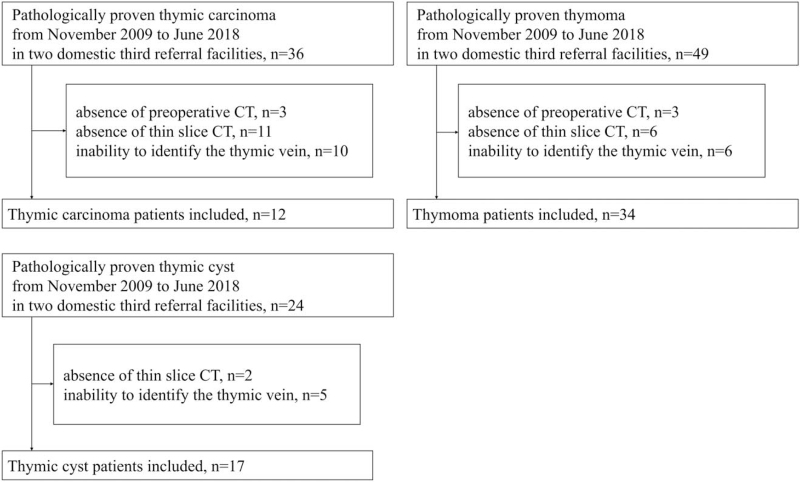
We searched the pathology databases of 2 tertiary referral hospitals in Japan between November 2009 and June 2018 and found 36 pathologically proven TCa, 49 thymomas, and 24 thymic cysts. We excluded 24 TCa, 15 thymomas, and 7 thymic cysts based on the following exclusion criteria.
-
1.
absence of a preoperative CT.
-
2.
absence of a thin-slice CT (≤3 mm slice thickness).
-
3.
inability to identify the thymic vein.
Consequently, we enrolled 12 patients with TCa, 34 with thymomas, and 17 with thymic cysts (Fig. 1).
Figure 1.
Flow chart showing the inclusion and exclusion criteria.
2.2. Computed tomography acquisition
We performed chest CT using the 4–320-row multi-detector CT unit (Aquilion 64, Aquilion PRIME or Aquilion ONE, Canon Medical Systems, Otawara, Japan; Lightspeed Plus or Ultra VCT, Discovery STE or CT750 HD CT scanner, GE Healthcare, Waukesha, Wisconsin, USA) with the following parameters: tube voltage, 120 kVp; effective current, 120 to 330 mA; helical pitch, 0.813; field of view, 30 to 45 cm; and matrix size, 512 × 512 for all CT units. We used the auto exposure control to monitor the radiation exposure while maintaining a sufficient resolution for the chest CT evaluation. CT images were acquired during a single inspiratory breath-hold to minimize motion artifacts. Reconstruction was performed with 0.625 to 3.0 mm slice thickness. Fifty patients underwent contrast-enhanced CT, 75 to 90 seconds after intravenous administration of an iodine contrast agent. The remaining thirteen patients underwent plain CT.
2.3. Computed tomography interpretation
Two chest radiologists with 3 and 6 years of experience (the latter was a board-certified diagnostic radiologist) independently interpreted all chest CT images. They were blinded to the clinical information. They measured the maximum short axis of the thymic vein and the maximum diameter of the tumor in an axial image for each case. The final values were an average of the values obtained by the radiologists. Figure 2A, B, and C outline the measurement of the thymic vein. They also assessed the CT images for lobulation of shape (lobular or not), irregularity of contour (irregular or not), definiteness of margin (well-or ill-defined), focal low-attenuation area (presence or absence), involvement of great vessels (presence or absence), and mediastinal lymphadenopathy (presence or absence). Involvement of great vessels was considered positive when abutment of ≥50% circumference or oppression, deformation, or occlusion of the great vessels was observed. Mediastinal lymphadenopathy was considered positive when the maximum short axis of the lymph node was >10 mm. If two radiologists disagreed on these findings, another chest radiologist with 14 years of experience made the final decision.
Figure 2.
Examples of the thymic vein and thymic lesion. (A) A computed tomography (CT) of a 70-year-old man. The maximum short axis of the thymic vein is 3.4 mm. The major axis of the mediastinal mass is 43 mm. Following an extended thymectomy, the patient was diagnosed with thymic carcinoma. (B) A CT of a 70-year-old woman. The maximum short axis of the thymic vein is 1.7 mm. The major axis of the mediastinal mass is 59 mm. Following a thymectomy, she was diagnosed with thymoma. (C) A CT of a 70-year-old man. The maximum short axis of the thymic vein is 1.1 mm. The major axis of the mediastinal mass is 70 mm. Following a thymectomy, he was diagnosed with thymic cyst.
2.4. Statistical analysis
We used the intraclass correlation coefficient (2, 1) to assess the inter-rater reliability for the maximum diameter of the tumor and the maximum short axis of the thymic vein. We also evaluated the Cohen's Kappa values for the rest of the imaging findings. In addition, we compared the differences in age, maximum diameter of the tumor, and mean maximum short axis of the thymic vein between the lesions using the Kruskal–Wallis tests. Fisher exact tests were performed to compare the categorical factors.
A receiver operating characteristic (ROC) curve was generated when the maximum short axis of the thymic vein was significantly longer in TCa than in the other thymic pathologies. This facilitated determination of the cut-off value for TCa diagnosis. We calculated the sensitivity and specificity for differentiating TCa from the other pathologies (thymoma and thymic cysts) and from thymoma for the maximum short axis of the thymic vein diameter in 0.5 mm increments. Fisher exact test helped examine the differences in the number of true-positives and false-positives between the three pathologies.
We performed the univariate generalized linear model analysis to assess variance for each patient parameter and image findings. For the parameters and findings with P < .05, a multivariate generalized linear model analysis was performed. If both the maximum short axis of the thymic vein and the number of the maximum short axis of the thymic vein ≥ cut-off value based on ROC analysis were P < .05, the latter was included in the multivariate generalized linear model analysis.
We also performed a post-hoc analysis for the maximum short axis of the thymic vein using the Mann–Whitney U test, and the number of the maximum short axis of the thymic vein ≥ the cut-off using Fisher exact test. A family-wise error-corrected P value <.05 was considered statistically significant. A family-wise correction was performed using Bonferroni's method.
We performed the statistical analyses using JMP Pro, version 15.2.1 (SAS Institute, Cary, North Carolina, USA) and R software version 4.1.1 (The R foundation, Vienna, Austria).
3. Results
The mean age of the patients was 62.2 ± 11.4 years (mean ± standard deviation; range 34–85 years); 36 (57%) were men. There was no significant difference in age and sex among the three pathologies. Table 1 summarizes the patient characteristics. In line with the World Health Organization classification, thymomas were classified as follows: type A, n = 6; type AB, n = 5; type B1, n = 9 type B2, n = 6; and type B3, n = 7. One patient had a metaplastic thymoma. The types of TCa were as follows: squamous carcinoma, n = 10; adenocarcinoma, n = 1; and large cell neuroendocrine carcinoma, n = 1. Thymic cysts were surgically resected for the following reasons: inability to rule out thymoma, n = 14; increase in size, n = 2; and chest pain, n = 1.
Table 1.
Patient characteristics and CT image findings.
| All patients (n = 63) | Thymic carcinoma (n = 12) | Thymoma (n = 34) | Thymic cysts (n = 17) | P value | |
| Age∗ | 62.2 ± 11.4 (34–85) | 64.0 ± 6.7 (56–78) | 60.7 ± 12.8 (34–80) | 63.7 ± 10.5 (45–85) | .77∗∗ |
| Sex | .55∗∗∗ | ||||
| Male | 36 | 8 | 17 | 11 | |
| Female | 27 | 4 | 17 | 6 | |
| Maximum long axis of tumor (mm)∗ | 35.5 ± 18.0 (9.0–84.3) | 45.5 ± 19.9 (9.0–84.3) | 32.8 ± 16.0 (9.2–70.8) | 33.8 ± 17.7 (15.0–76.5) | .16∗∗ |
| Maximum short axis of the thymic vein (mm)∗ | 1.79 ± 0.52 (0.9–3.4) | 2.42 ± 0.46 (1.7–3.4) | 1.71 ± 0.35 (1.2–2.8) | 1.50 ± 0.51 (0.9–2.8) | <.001∗∗ |
| Lobulated shape | 7 | 16 | 6 | .052∗∗∗ | |
| Irregular contour | 2 | 1 | 0 | .15∗∗∗ | |
| Ill-defined margin | 5 | 1 | 0 | .003∗∗∗ | |
| Focal low-attenuation area | 8 | 8 | 0 | <.001∗∗∗ | |
| Involvement of great vessels | 4 | 1 | 0 | .005∗∗∗ | |
| Mediastinal lymphadenopathy | 5 | 0 | 0 | <.001∗∗∗ |
mean ± standard deviation (minimum–maximum).
Kruskal–Wallis test.
Fisher exact test.
3.1. Diameter of the thymic vein
Table 1 also summarizes the CT imaging findings. The maximum short axis of the thymic vein was 2.42 ± 0.46 mm (range 1.7–3.4 mm) for TCa, 1.71 ± 0.35 mm (range 1.2–2.8 mm) for thymoma, and 1.50 ± 0.51 mm (range 0.9–2.8 mm) for thymic cysts. The maximum long axis of the tumor was 45.5 ± 19.9 mm (range 9.0–84.3 mm) for TCa, 32.8 ± 16.0 mm (range 9.2–70.8 mm) for thymoma, and 33.8 ± 17.7 mm (range 15.0–76.5 mm) for thymic cysts. The Kruskal–Wallis test revealed a significant difference in the maximum short axis of the thymic vein among the 3 pathologies (P < .001), while no significant difference was found in the maximum long axis of the tumor (P = .16). Among the other imaging findings, Fisher exact test revealed statistically significant differences in ill-defined margin (P = .003), focal low-attenuation area (P < .001), involvement of great vessels (P = .005), and mediastinal lymphadenopathy (P < .001).
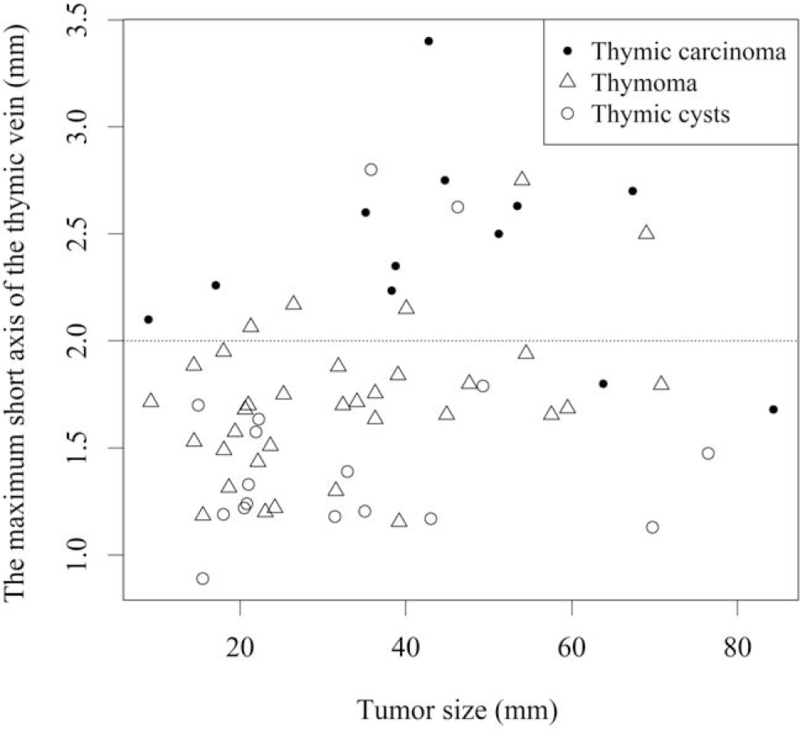
ROC curve analysis revealed that a 2 mm maximum short axis of the thymic vein was preferable as the cut-off value for distinguishing TCa from thymoma and benign lesions. The maximum short axis of the thymic vein was ≥2 mm in 10/12 (83%), 5/34 (15%), and 2/17 (12%) patients with TCa, thymoma, and thymic cysts, respectively (P < .001, Table 2). This cut-off value could differentiate TCa from the other two pathologies and thymoma with 83% sensitivity and 86% specificity, and 83% sensitivity and 85% specificity, respectively. Figure 3 demonstrates the relationship between the maximum short axis of the thymic vein and the diameter of the 3 pathologies. The maximum short axis of the thymic vein of the TCa is frequently greater than the cutoff (2 mm) compared to the other two pathologies. Table 2 summarizes the aforementioned results.
Table 2.
ROC analysis for the cut-off value of the maximum short axis of the thymic vein for distinguishing thymic carcinoma from other thymic lesions.
| Cut-off maximum short axis of the thymic vein (mm) | ||||||
| 1 | 1.5 | 2 | 2.5 | 3 | ||
| versus Thymoma + Thymic cysts | ||||||
| Area under the ROC curve | 0.89 | |||||
| Sensitivity (%) | 100 | 100 | 83 | 50 | 8 | |
| Specificity (%) | 2 | 37 | 86 | 92 | 100 | |
| True-positive (n) | 12 | 12 | 10 | 6 | 1 | |
| False-positive (n) | 50 | 32 | 7 | 4 | 0 | |
| P value [Fisher exact test] | >.99 | .012∗ | <.001∗ | .002∗ | .19 | |
| versus Thymoma | ||||||
| Area under the ROC curve | 0.89 | |||||
| Sensitivity (%) | 100 | 100 | 83 | 50 | 8 | |
| Specificity (%) | 0 | 24 | 85 | 94 | 100 | |
| True-positive (n) | 12 | 12 | 10 | 6 | 1 | |
| False-positive (n) | 34 | 26 | 5 | 2 | 0 | |
| P value [Fisher exact test] | >.99 | .090 | <.001∗ | .002∗ | .26 | |
Statistically significant.
ROC = receiver operating characteristic.
Figure 3.
Scatter diagram of the maximum diameter of the thymic vein and the diameter of the thymic lesion.
The results of the univariate and multivariate generalized linear model analyses are summarized in Table 3. The multivariate generalized linear model analysis revealed a statistically significant association between TCa and the maximum short axis of the thymic vein ≥2 mm, ill-defined margin, and mediastinal lymphadenopathy.
Table 3.
The univariate and multivariate generalized linear model analysis for patient characteristics and CT image.
| Univariate | Multivariate | |||
| Odds ratio | P value | Odds ratio | P value | |
| Sex (male) | 1.08 (0.88–1.31) | .46 | ||
| Age (yr) | 1.00 (0.99–1.01) | .54 | ||
| Maximum short axis of the thymic vein (mm) | 1.56 (1.34–1.82) | <.001∗ | ||
| Maximum short axis of the thymic vein ≥2 mm | 1.72 (1.45–2.05) | <.001∗ | 1.27 (1.08–1.49) | .005∗ |
| Maximum long axis of the tumor (mm) | 1.01 (1.00–1.01) | .032∗ | 1.00 (0.99–1.00) | .37 |
| Lobulated shape | 1.10 (0.90–1.34) | .35 | ||
| Irregular contour | 1.65 (1.06–2.57) | .032∗ | 1.03 (0.66–1.62) | .88 |
| Ill-defined margin | 2.04 (1.53–2.70) | <.001∗ | 1.75 (1.27–2.39) | .001∗ |
| Focal low-attenuation area | 1.51 (1.24–1.85) | <.001∗ | 1.10 (0.94–1.31) | .25 |
| Involvement of great vessels | 1.94 (1.40–2.68) | <.001∗ | 0.86 (0.60–1.25) | .43 |
| Mediastinal lymphadenopathy | 2.41 (1.80–3.22) | <.001∗ | 2.06 (1.44–2.95) | <.001∗ |
Statistically significant.
Post-hoc analysis revealed that the diameter was progressively larger in the order of TCa, thymoma, and thymic cysts (TCa vs thymoma, P < .001; TCa vs thymic cysts, P < .001; and thymoma vs thymic cysts, P = .011) (Table 4).
Table 4.
Comparison and post-hoc analysis of the maximum short axis of the thymic vein.
| Maximum short axis of the thymic vein (mm, mean ± SD) | ≥2 mm maximum short axis (%) | |
| Thymic carcinoma (n = 12) | 2.42 ± 0.46 | 10 (83%) |
| Thymoma (n = 34) | 1.71 ± 0.35 | 5 (15%) |
| Thymic cysts (n = 17) | 1.50 ± 0.51 | 2 (12%) |
| P value | <.001∗† | <.001∗†† |
| Post-hoc analysis | P value††† | P value†† |
| Thymic carcinoma vs thymoma | <.001∗∗ | <.001∗∗ |
| Thymic carcinoma vs thymic cysts | <.001∗∗ | <.001∗∗ |
| Thymoma vs thymic cysts | .011∗∗ | >.99 |
Statistically significant.
Statistically significant after family-wise error correction by Bonferroni's method.
†Kruskal–Wallis test.
††Fisher exact test.
†††Mann–Whitney U test.
The inter-rater reliability was almost perfect for each imaging finding (Table 5).
Table 5.
Intraclass correlation coefficients and Kappa value of each measurement and finding between the 2 readers.
| Intraclass correlation coefficients (2,1) | |
| Maximum short axis of the thymic vein | 0.98 |
| Maximum long axis of the tumor | 0.99 |
| Kappa value | |
| Shape | 0.82 |
| Contour | 1.00 |
| Margin | 0.84 |
| Focal low-attenuation area | 0.91 |
| Involvement of large vessel | 1.00 |
| Mediastinal lymphadenopathy | 0.88 |
4. Discussion
To our knowledge, this is the first study to demonstrate the difference in the maximum short axis of the thymic vein for differentiating between TCa, thymoma, and thymic cysts. The maximum short axis of the thymic vein was significantly longer in patients with TCa than in those with thymoma and thymic cysts and in patients with thymoma than in those with a thymic cysts. The frequency of patients with a maximum short axis ≥2 mm was significantly more in TCa than in the other 2 pathologies. Thus, measurement of the maximum short axis of the thymic vein could be a useful diagnostic tool to differentiate the three most common thymic epithelial lesions into malignant and benign lesions.
Some authors have reported other methods for differentiating between the three pathologies.[2,4,6,13] Tomiyama et al differentiated them based on the presence of irregular contours (75%) in TCa. Furthermore, thymoma was associated with smooth contours (100%) and round shapes (88%).[6] According to Jung et al, TCa is characterized by an invasion of the great vessels, lymph node enlargement, phrenic nerve palsy, and extrathymic metastases.[4] However, Li et al could accurately diagnose TCa preoperatively in only 37.5% of cases based on those CT findings. This can be attributed to the overlap of the imaging findings.[12] In addition to thymic vein diameter, our univariate and multivariate analyses revealed the usefulness of enlarged lymph nodes and ill-defined margin, consistent with previous studies.[4,6] It is speculated that the addition of thymic vein diameter measurements to these findings might allow a more accurate diagnosis of TCa.
Preoperative diagnosis of thymic cysts can be challenging, particularly in cases where contrast agents cannot be used because of renal dysfunction or allergy. A well-defined, round or oval, water-attenuated mass was reported as the typical CT finding of thymic cysts.[14] However, thymic cysts occasionally show high attenuation due to infection or hemorrhage, leading to unnecessary thymectomy.[2] Additionally, thymic cysts can grow over time. Kim et al reported on the growth of 23.2% (n = 13/56) of thymic cysts during the median 193-day follow-up (interquartile range, 98–763 days). Moreover, the frequency of increase in size was not significantly different between thymic cysts and other thymic epithelial tumors.[13]
The difference in the maximum short axis of the thymic vein can be attributed to the advanced angiogenesis and metabolic changes in the thymic epithelial lesions.[15,16] The increased blood flow in TCa might lead to a greater enlargement of the thymic vein as a drainage vein compared to that seen in thymoma and thymic cysts. From the results of the present study, TCa should be considered when the maximum short axis of the thymic vein is ≥2 mm.
Our study had some limitations, including its retrospective design and relatively small sample size. We had to exclude some cases of thymoma and thymic cysts because the thymic vein was undetectable due to its small size. Thus, we might have underestimated the usefulness of the maximum short axis of the thymic vein in this differentiation.
In conclusion, thymic vein diameter was larger in the order of TCa, thymoma, and thymic cysts. The 2 mm maximum short axis of the thymic vein could be a useful cut-off parameter for distinguishing TCa from other pathologies.
Acknowledgments
We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Author contributions
Conceptualization: Takeyuki Watadani, Moto Nakaya, Shinichi Cho, Nana Fujita, Satoru Kamio, Hiroaki Koyama, Satoshi Suzuki, Wataru Gonoi.
Data curation: Naoya Sakamoto, Ryo Kurokawa.
Formal analysis: Naoya Sakamoto, Ryo Kurokawa.
Investigation: Naoya Sakamoto, Ryo Kurokawa.
Methodology: Ryo Kurokawa, Takeyuki Watadani, Wataru Gonoi.
Project administration: Naoya Sakamoto.
Resources: Teppei Morikawa, Haruyasu Yamada.
Supervision: Ryo Kurokawa, Osamu Abe.
Validation: Naoya Sakamoto, Ryo Kurokawa, Wataru Gonoi.
Visualization: Naoya Sakamoto, Ryo Kurokawa.
Writing – original draft: Naoya Sakamoto.
Writing – review & editing: Ryo Kurokawa, Teppei Morikawa, Haruyasu Yamada, Wataru Gonoi.
Footnotes
Abbreviations: CT = computed tomography, ROC = receiver operating characteristic, TCa = thymic carcinoma.
How to cite this article: Sakamoto N, Kurokawa R, Watadani T, Morikawa T, Nakaya M, Cho S, Fujita N, Kamio S, Koyama H, Suzuki S, Yamada H, Abe O, Gonoi W. Differential diagnosis of thymic epithelial neoplasms on computed tomography using the diameter of the thymic vein. Medicine. 2021;100:46(e27942).
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Henschke CI, Lee IJ, Wu N, et al. CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology 2006;239:586–90. [DOI] [PubMed] [Google Scholar]
- [2].Ackman JB, Verzosa S, Kovach AE, et al. High rate of unnecessary thymectomy and its cause. Can computed tomography distinguish thymoma, lymphoma, thymic hyperplasia, and thymic cysts? Eur J Radiol 2015;84:524–33. [DOI] [PubMed] [Google Scholar]
- [3].Özkan B, Toker A. Catastrophes during video-assisted thoracoscopic thymus surgery for myasthenia gravis. Interact Cardiovasc Thorac Surg 2016;23:450–3. [DOI] [PubMed] [Google Scholar]
- [4].Jung KJ, Lee KS, Ha J, Kim J, Kim TS, Kim EA. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol 2001;176:433–9. [DOI] [PubMed] [Google Scholar]
- [5].Sadohara J, Fujimoto K, Müller NL, et al. Thymic epithelial tumors: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and TCs. Eur J Radiol 2006;60:70–9. [DOI] [PubMed] [Google Scholar]
- [6].Tomiyama N, Johkoh T, Mihara N, et al. Using the World Health Organization classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol 2002;179:881–6. [DOI] [PubMed] [Google Scholar]
- [7].Chen HJ, Liao WC, Liang SJ, Li CH, Tu CY, Hsu WH. Diagnostic impact of color doppler ultrasound-guided core biopsy on fine-needle aspiration of anterior mediastinal masses. Ultrasound Med Biol 2014;40:2768–76. [DOI] [PubMed] [Google Scholar]
- [8].Sakai S, Murayama S, Soeda H, Matsuo Y, Ono M, Masuda K. Differential diagnosis between thymoma and non-thymoma by dynamic MR imaging. Acta Radiol 2002;43:262–8. [DOI] [PubMed] [Google Scholar]
- [9].Abdel Razek AA, Khairy M, Nada N. Diffusion-weighted MR imaging in thymic epithelial tumors: correlation with World Health Organization classification and clinical staging. Radiology 2014;273:268–75. [DOI] [PubMed] [Google Scholar]
- [10].Fukumoto K, Taniguchi T, Ishikawa Y, et al. The utility of [18F]-fluorodeoxyglucose positron emission tomography-computed tomography in thymic epithelial tumours. Eur J Cardiothorac Surg 2012;42:e152–6. [DOI] [PubMed] [Google Scholar]
- [11].Treglia G, Sadeghi R, Giovanella L, Cafarotti S, Filosso P, Lococo F. Is (18)F-FDG PET useful in predicting the WHO grade of malignancy in thymic epithelial tumors? a meta-analysis. Lung Cancer 2014;86:05–13. [DOI] [PubMed] [Google Scholar]
- [12].Li HR, Gao J, Jin C, Jiang JH, Ding JY. Comparison between CT and MRI in the diagnostic accuracy of thymic masses. J Cancer 2019;10:3208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim H, Yoon SH, Kim J, et al. Growth of thymic epithelial tumors and thymic cysts: differential radiologica points. Thorac Cancer 2019;10:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jeung MY, Gasser B, Gangi A, et al. Imaging of cystic masses of the mediastinum. Radiographics 2002;22:S79–93. [DOI] [PubMed] [Google Scholar]
- [15].Neufeld G, Kessler O. Pro-angiogenic cytokines and their role in tumor angiogenesis. Cancer Metastasis Rev 2006;25:373–85. [DOI] [PubMed] [Google Scholar]
- [16].Mérida I, Ávila-Flores A. Tumor metabolism: new opportunities for cancer therapy. Clin Transl Oncol 2006;8:711–6. [DOI] [PubMed] [Google Scholar]