PURPOSE
This phase III, multicenter, randomized, open-label study investigated the efficacy and safety of nivolumab versus chemotherapy (gemcitabine [GEM] or pegylated liposomal doxorubicin [PLD]) in patients with platinum-resistant ovarian cancer.
MATERIALS AND METHODS
Eligible patients had platinum-resistant epithelial ovarian cancer, received ≤ 1 regimen after diagnosis of resistance, and had an Eastern Cooperative Oncology Group performance score of ≤ 1. Patients were randomly assigned 1:1 to nivolumab (240 mg once every 2 weeks [as one cycle]) or chemotherapy (GEM 1000 mg/m2 for 30 minutes [once on days 1, 8, and 15] followed by a week's rest [as one cycle], or PLD 50 mg/m2 once every 4 weeks [as one cycle]). The primary outcome was overall survival (OS). Secondary outcomes included progression-free survival (PFS), overall response rate, duration of response, and safety.
RESULTS
Patients (n = 316) were randomly assigned to nivolumab (n = 157) or GEM or PLD (n = 159) between October 2015 and December 2017. Median OS was 10.1 (95% CI, 8.3 to 14.1) and 12.1 (95% CI, 9.3 to 15.3) months with nivolumab and GEM or PLD, respectively (hazard ratio, 1.0; 95% CI, 0.8 to 1.3; P = .808). Median PFS was 2.0 (95% CI, 1.9 to 2.2) and 3.8 (95% CI, 3.6 to 4.2) months with nivolumab and GEM or PLD, respectively (hazard ratio, 1.5; 95% CI, 1.2 to 1.9; P = .002). There was no statistical difference in overall response rate between groups (7.6% v 13.2%; odds ratio, 0.6; 95% CI, 0.2 to 1.3; P = .191). Median duration of response was numerically longer with nivolumab than GEM or PLD (18.7 v 7.4 months). Fewer treatment-related adverse events were observed with nivolumab versus GEM or PLD (61.5% v 98.1%), with no additional or new safety risks.
CONCLUSION
Although well-tolerated, nivolumab did not improve OS and showed worse PFS compared with GEM or PLD in patients with platinum-resistant ovarian cancer.
INTRODUCTION
Ovarian cancer is the eighth most common cancer in women globally and is associated with high morbidity and mortality.1,2 Age-standardized mortality rate was 3.9 per 100,000 in 2018, which was the highest among gynecologic cancers.1 Surgery and platinum-based chemotherapy are the first-line treatments3,4; although patients usually respond to these,5 platinum resistance eventually occurs.4 Paclitaxel, pegylated liposomal doxorubicin (PLD), topotecan, and gemcitabine (GEM) are the most common treatments currently used; however, these drugs only show suboptimal responses (response rates, 10%-15%), with an overall survival (OS) of < 12 months.6,7 Therefore, patients with platinum-resistant ovarian cancer generally have a poor prognosis and short expected OS,6 and there is a high demand for novel treatments.
CONTEXT
Key Objective
To date, there have been only a few phase III trials conducted for platinum-resistant ovarian cancer. The NINJA trial was a phase III, multicenter, randomized controlled study that investigated the efficacy and safety of nivolumab monotherapy, an anti–programmed cell death-1 antibody, versus chemotherapy (gemcitabine [GEM] or pegylated liposomal doxorubicin [PLD]) in patients with platinum-resistant ovarian cancer.
Knowledge Generated
Nivolumab monotherapy did not improve overall survival or overall response rate and showed worse progression-free survival but tended to result in a longer duration of response compared with GEM or PLD. Treatment-related adverse events observed with nivolumab were consistent with previous studies and were less frequent than with GEM or PLD.
Relevance
Nivolumab monotherapy was well-tolerated but did not meet the primary end point of demonstrating superiority in overall survival compared with GEM or PLD.
Nivolumab is a human monoclonal antibody approved for the treatment of several malignancies including melanoma, non–small-cell lung cancer, and renal cancer.8 Nivolumab enhances the antitumor activity of T cells by blocking the programmed cell death-1 (PD-1) and programmed cell death ligand-1/2 (PD-L1/L2) pathway, a pathway that enables tumor cells to evade the host immune attacks and accelerate tumor growth.9-12 PD-1, an immunoinhibitory receptor expressed on activated T cells,11 is upregulated in ovarian cancer,13 and PD-L1 expressed in ovarian cancer negatively correlates with CD8+ tumor-infiltrating lymphocyte count, a known prognostic factor in ovarian cancer.9
In a phase II trial that assessed the efficacy and safety of nivolumab in 20 patients with platinum-resistant ovarian cancer, nivolumab yielded an objective response rate of 15% (95% CI, 3.2 to 37.9) and a disease control rate of 45% (95% CI, 23.1 to 68.5).14 Two patients had target lesions disappear and experienced a durable complete response (CR; > 4.5 years).15 Grade 3 or 4 treatment-related adverse events (TRAEs) were reported by 40% of patients,14 most of which were reported in a previous study of nivolumab in a variety of cancer types.12 These promising phase II results warrant further investigation of nivolumab in a large, phase III, randomized controlled trial.
The aim of this phase III study was to investigate the efficacy and safety of nivolumab versus chemotherapy (GEM or PLD) in patients with platinum-resistant ovarian cancer.
MATERIALS AND METHODS
Study Design
This was a phase III, multicenter, randomized, open-label study to investigate the efficacy and safety of nivolumab in patients with platinum-resistant (advanced or recurrent) ovarian cancer in Japan (clinicaltrials.jp; JapicCTI-153004). This study was initially phase II but was amended to phase III. The study was reviewed and approved by local ethics review boards and complied with the Declaration of Helsinki, the Japanese Pharmaceuticals and Medical Device Law, and Good Clinical Practice. Written informed consent was obtained from each patient.
Study Population
Eligible patients were females age ≥ 20 years with epithelial ovarian cancer (including fallopian tube cancer and peritoneal cancer) and were diagnosed with platinum resistance. Platinum resistance was defined as disease progression or recurrence during or ≤ 6 months after platinum-based treatment. Other main inclusion criteria were as follows: ≤ 1 regimen after platinum resistance diagnosis; an Eastern Cooperative Oncology Group performance status of ≤ 1; tumor tissue samples available for PD-L1 expression analysis; and no prior treatment with nivolumab, other anti–PD-1, anti–PD-L1, or anti–PD-L2 therapies, other drugs that regulate T cells, or GEM or PLD. The main exclusion criteria were as follows: borderline malignant tumor, current or previous severe hypersensitivity reactions to antibody products, autoimmune disease, severe cardiovascular disease, or multiple primary cancers and/or central nervous system metastases.
Treatment Protocol
Patients were randomly assigned 1:1 to nivolumab or chemotherapy (GEM or PLD) after being stratified according to histologic type (clear cell carcinoma [CCC] or non-CCC) and the number of previous chemotherapy regimens received after platinum resistance diagnosis (0 or 1). The nivolumab group received intravenous (IV) nivolumab 240 mg once every 2 weeks (as one cycle); dose reductions were not allowed, but dose omissions were. GEM or PLD was chosen as the control because these drugs have similar efficacy in platinum-resistant ovarian cancer16; for patients randomly assigned to chemotherapy, assignment to GEM or PLD was determined by the investigator at enrollment. The chemotherapy group received either GEM 1,000 mg/m2 IV for 30 minutes once on days 1, 8, and 15 followed by a week's rest (as one cycle), or PLD 50 mg/m2 IV once every 4 weeks (as one cycle); doses were adjusted according to the percent change in body weight, and dose reductions were allowed if unfavorable signs and symptoms occurred. Treatments were discontinued when patients had CR or progressive disease according to the RECIST guideline v1.1 or experienced unacceptable toxicity. However, nivolumab treatment could continue after disease progression. At the end of treatment, patients entered the 28-day follow-up phase.
Outcome Measures
The primary outcome was OS. Secondary outcomes included the following: progression-free survival (PFS), best overall response, overall response rate (ORR), duration of response (DOR), and time to response (TTR), which were all evaluated by the investigators using RECIST (see the Data Supplement, online only, for definitions); change in the sum of target lesion diameters; and quality of life (QOL), evaluated using Functional Assessment of Cancer Therapy–Ovarian (FACT-O)17 and EuroQol-5 Dimensions (EQ-5D).18 Imaging assessments (computed tomography and magnetic resonance imaging) were performed every 8 weeks up to 48 weeks, then every 12 weeks from 60 weeks during treatment.
An additional secondary outcome was safety, assessed by type, grade, and frequency of treatment-emergent adverse events (TEAEs), TRAEs, and immune-related adverse events (irAEs), occurring from day 1 to 28 days after the last treatment administration or early in the posttreatment period. TEAEs were evaluated using the Japanese translation of the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. TRAEs were any adverse event (AE) in which treatment could not be excluded as the cause. irAEs were any AE with potential immune-related causes. Eastern Cooperative Oncology Group performance status, vital signs, electrocardiogram, and blood tests were collected at regular intervals.
Statistical Analysis
The planned sample size was 316 patients, with random assignment of 158 patients to each group, stratified by histologic type and the number of previous chemotherapy regimens received after platinum resistance diagnosis. The sample size was calculated by a log-rank test at a two-sided significance level of 5% to give approximately 90% power to detect a difference between the groups, with the aim of verifying the superiority of nivolumab over GEM or PLD in OS (Data Supplement). OS, PFS, and QOL analyses were performed on the intention-to-treat (ITT) population, defined as all randomized patients. Other efficacy analyses were performed on the response-evaluable set (RES), defined as patients with measurable disease at baseline and ≥ 1 evaluable imaging data available after random assignment. Median OS and PFS (two-sided 95% CI) were estimated by Kaplan-Meier method for each treatment group and were compared using the stratified log-rank test, with the allocation factors (histologic type and the number of previous chemotherapy regimens received after platinum resistance diagnosis) as stratification factors. Hazard ratios (HRs; two-sided 95% CI) between the groups were estimated using the stratified Cox proportional hazards model.19 Prespecified subgroup analysis was also performed for OS using patient demographics and characteristics. The ORR (two-sided 95% CI) was estimated for each treatment group using the Clopper-Pearson method,20 and odds ratios were estimated by the Cochran-Mantel-Haenszel method.21 Median DOR (two-sided 95% CI) and TTR for each treatment group were calculated using the Kaplan-Meier method.
Safety analyses were performed on the safety analysis set (SAF), defined as patients who received ≥ 1 dose of the study drugs. Incidences of TEAEs, TRAEs, and irAEs were summarized. Data cutoff was October 18, 2019. Statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Disposition
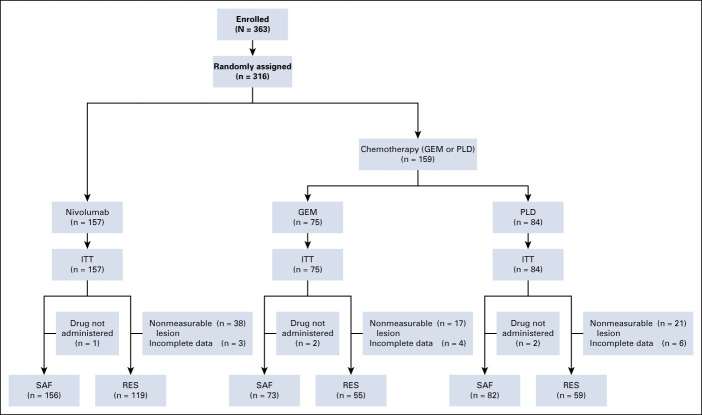
Between October 7, 2015 and July 4, 2016, and March 8, 2017 and December 21, 2017, a total of 363 patients were enrolled across 35 centers; 316 patients were randomly assigned into two groups (nivolumab: n = 157; GEM or PLD: n = 159) and were included in the ITT analyses (Fig 1). Of those, 233 patients (nivolumab: n = 119; GEM: n = 55; PLD: n = 59) and 311 patients (nivolumab: n = 156; GEM: n = 73; PLD: n = 82) were included in the RES and SAF analyses, respectively. The main reason for exclusion was that the lesions could not be measured (RES analyses) and patients did not receive the study drug (SAF analyses).
FIG 1.
CONSORT diagram of patient disposition. NOTE. Patients may have had more than one reason for exclusion. GEM, gemcitabine; ITT, intention-to-treat; PLD, pegylated liposomal doxorubicin; RES, response-evaluable set; SAF, safety analysis set.
Demographic and Baseline Clinical Characteristics
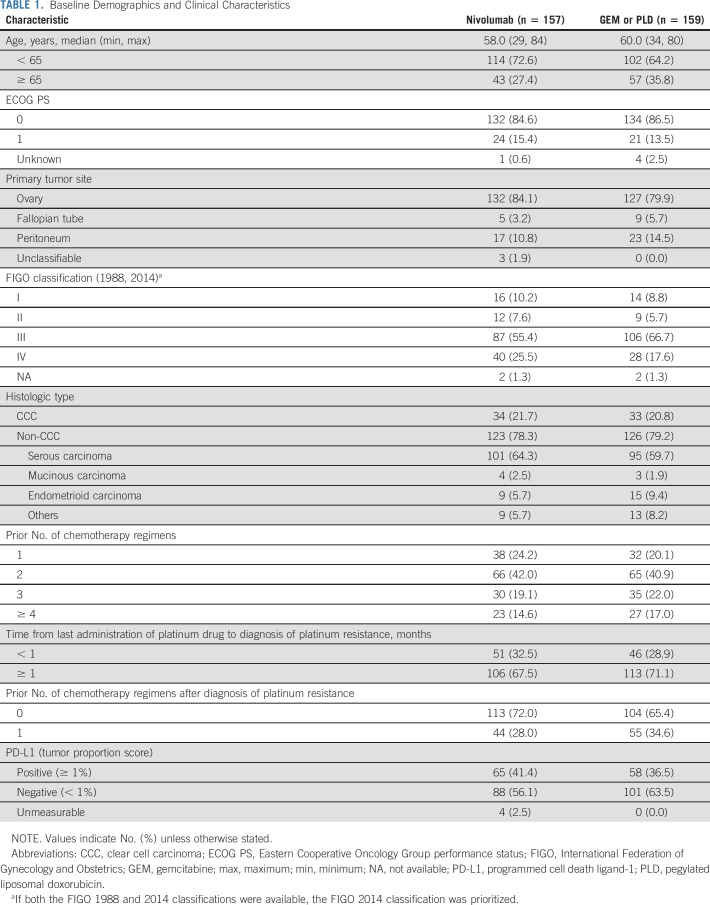
Demographic and baseline clinical characteristics were generally similar between the nivolumab and GEM or PLD groups (Table 1). In both groups, most patients were age < 65 years, and > 55% of patients had stage III disease per the International Federation of Gynecology and Obstetrics classification. The most common histologic type was serous carcinoma.
TABLE 1.
Baseline Demographics and Clinical Characteristics
Efficacy Outcomes
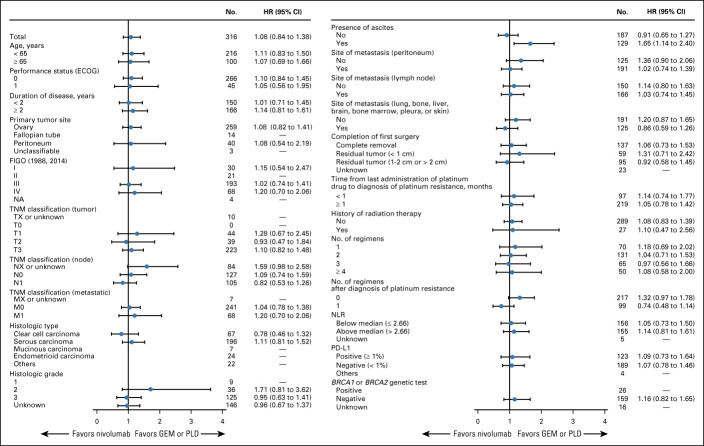
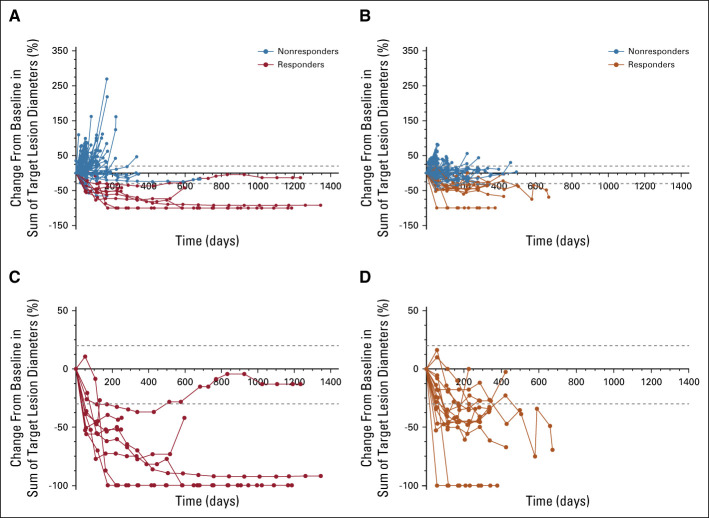
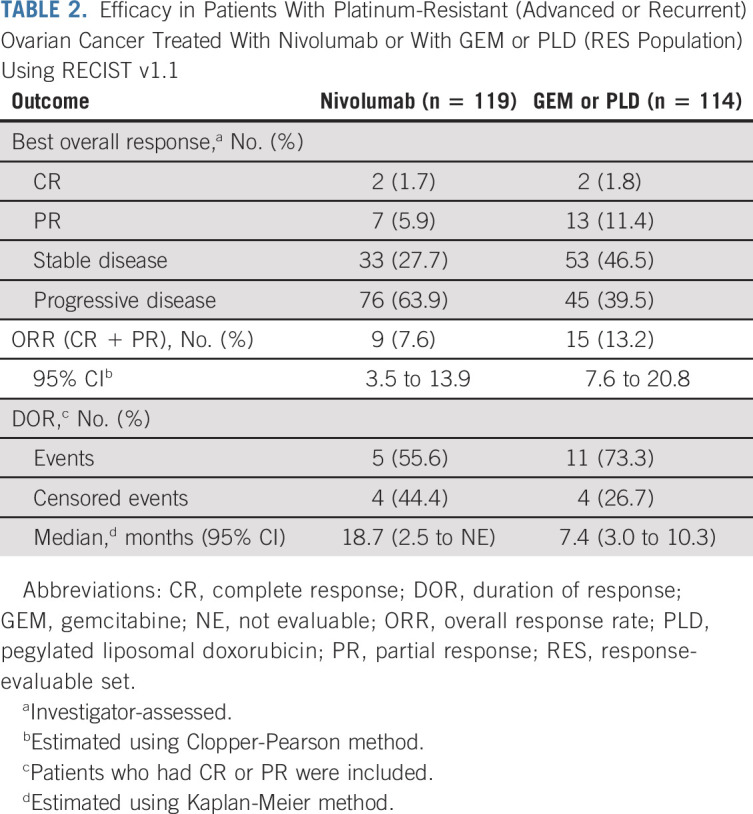
At data cutoff, 131 patients receiving nivolumab and 125 patients receiving GEM or PLD had died, 26 and 34 patients, respectively, were censored, and 23 and 32 patients, respectively, were still being followed up. There was no statistically significant difference in OS between the nivolumab and GEM or PLD groups (Fig 2A). Median OS was 10.1 months (95% CI, 8.3 to 14.1; events: n = 131, censored: n = 26) in the nivolumab group compared with 12.1 months (95% CI, 9.3 to 15.3; events: n = 125, censored: n = 34) in the GEM or PLD group (HR, 1.0; 95% CI, 0.8 to 1.3; P = .808). There was also no difference in OS between the groups for most subgroups (Fig 3), including patients who were PD-L1 (tumor proportion score) positive (≥ 1%; HR, 1.09; 95% CI, 0.73 to 1.64). However, OS was numerically longer with nivolumab compared with GEM or PLD among patients who had CCC (HR, 0.78; 95% CI, 0.46 to 1.32) and who received one chemotherapy regimen after platinum resistance diagnosis (HR, 0.74; 95% CI, 0.48 to 1.14). Median PFS was 2.0 (95% CI, 1.9 to 2.2) and 3.8 (95% CI, 3.6 to 4.2) months in the nivolumab and GEM or PLD groups, respectively (HR, 1.5; 95% CI, 1.2 to 1.9; P = .002; Fig 2B). There was no statistical difference in ORR between the groups (7.6% v 13.2%; odds ratio, 0.6; 95% CI, 0.2 to 1.3; P = .191). In the nivolumab group, nine patients experienced CR or partial response with ≥ 30% reduction in tumor size compared with 15 patients in the GEM or PLD group (Table 2; Appendix Fig A1, online only). Median DOR was 18.7 (95% CI, 2.5 to not evaluable) and 7.4 (95% CI, 3.0 to 10.3) months with nivolumab and GEM or PLD, respectively (Table 2). At the individual patient level, DOR was usually longer and TTR was similar or shorter with nivolumab than with GEM or PLD (Appendix Fig A2, online only). Mean FACT-O total score, EQ-5D index, and visual analog scale scores were stable from baseline in both groups until around week 100 and were similar between the groups at most time points (Appendix Fig A3, online only).
FIG 2.
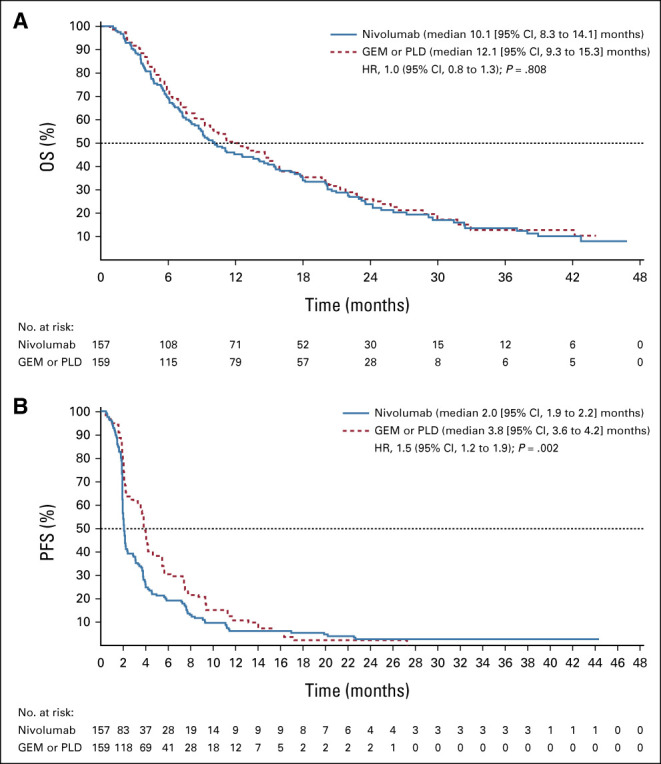
Kaplan-Meier curve of (A) OS and (B) PFS according to RECIST v1.1 (ITT population). GEM, gemcitabine; HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin.
FIG 3.
Forest plot of OS by subgroup (ITT population). Note: The HR was not calculated for subgroups where the number of patients in either the nivolumab group or the GEM or PLD group was < 10 patients. HRs (95% CI) were estimated using the Cox proportional hazards model. The UICC TNM Classification version 7 was used. BRCA1, breast cancer susceptibility gene 1; BRCA2, breast cancer susceptibility gene 2; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Obstetrics and Gynecology; GEM, gemcitabine; HR, hazard ratio; ITT, intention-to-treat; NA, not applicable; NLR, neutrophil to lymphocyte ratio; OS, overall survival; PD-L1, programmed cell death ligand-1; PLD, pegylated liposomal doxorubicin; TNM, TNM Classification of Malignant Tumors; UICC, Union for International Cancer Control.
TABLE 2.
Efficacy in Patients With Platinum-Resistant (Advanced or Recurrent) Ovarian Cancer Treated With Nivolumab or With GEM or PLD (RES Population) Using RECIST v1.1
Safety and Tolerability Measures
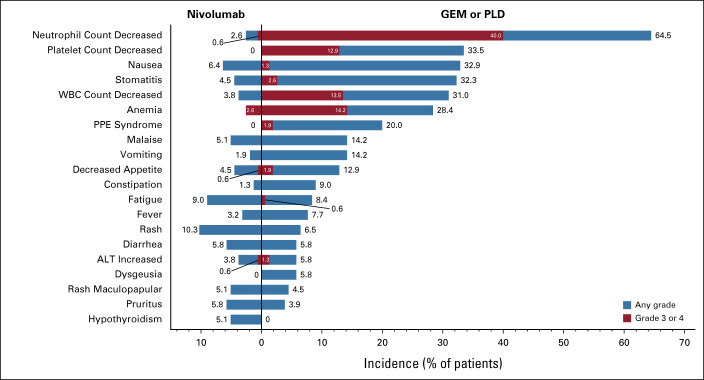
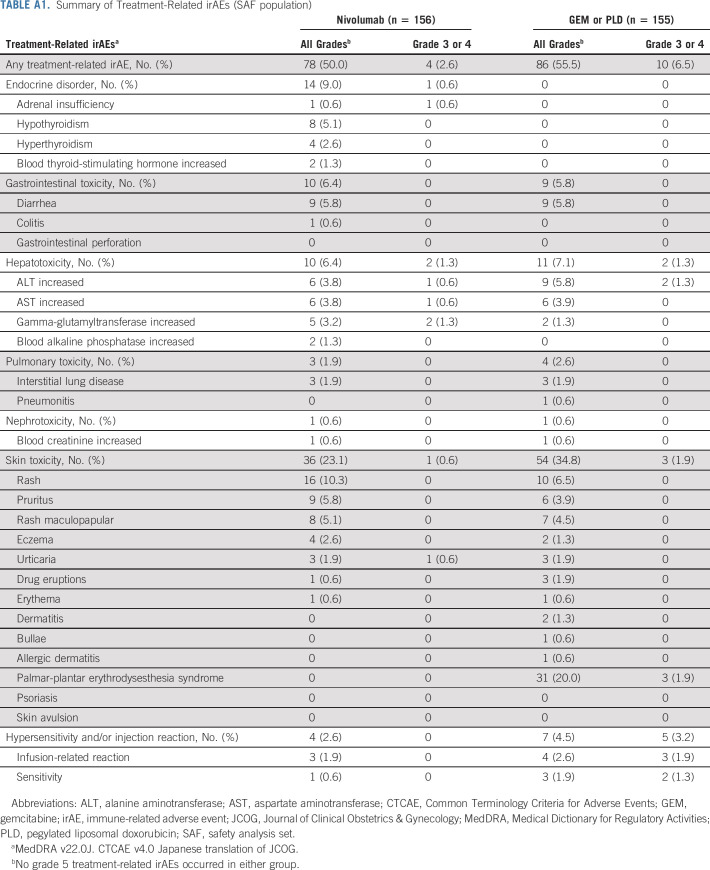
Nivolumab was well-tolerated, with a lower incidence of TRAEs (any grade) compared with GEM or PLD (61.5% v 98.1%, respectively); the incidence of TRAEs (any grade) leading to treatment discontinuation was also lower in the nivolumab group (7% v 10%, respectively). The most common TRAEs (any grade) with nivolumab were rash (10.3%), fatigue (9.0%), and nausea (6.4%) and with GEM or PLD were neutrophil count decreased (64.5%), platelet count decreased (33.5%), and nausea (32.9%; Fig 4). Fewer grade 3 or 4 TRAEs occurred in the nivolumab group compared with the GEM or PLD group (10.9% [17 of 156 patients] v 65.2% [101 of 155 patients], respectively). In the nivolumab group, anemia was the most common grade 3 or 4 TRAE, occurring in four patients (2.6%), which was still lower than with GEM or PLD (22 patients, 14.2%). In the GEM or PLD group, neutrophil count decreased was the most common grade 3 or 4 TRAE, occurring in 62 patients (40.0%), compared with one patient in the nivolumab group (0.6%). irAEs (any grade) occurred in 78 patients in the nivolumab group (50.0%) and 86 patients in the GEM or PLD group (55.5%; Appendix Table A1, online only). In the nivolumab group, most of the treatment-related irAEs were grade 1 or 2; grade 3 or 4 irAEs included adrenal insufficiency, ALT increased, AST increased, urticaria (all one patient each), and gamma-glutamyltransferase increased (two patients). In the nivolumab group, colitis occurred in one patient and there was no incidence of pneumonitis. Incidences of serious TRAEs (any grade and grade 3 or 4) were lower with nivolumab compared with GEM or PLD (any grade: 6.4% v 10.3%; grade 3 or 4: 3.8% v 9.0%). No grade 5 TRAEs or treatment-related deaths were reported in either group.
FIG 4.
TRAEs (≥ 5% in any group; SAF population). Note: All AEs were evaluated according to MedDRA v21.0J and CTCAE v4.0 Japanese translation of JCOG. No grade 5 TRAEs occurred in either group. AE, adverse event; ALT, alanine aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events; GEM, gemcitabine; JCOG, Journal of Clinical Obstetrics & Gynecology; MedDRA, Medical Dictionary for Regulatory Activities; PLD, pegylated liposomal doxorubicin; PPE, palmar-plantar erythrodysesthesia; SAF, safety analysis set; TRAE, treatment-related adverse event; WBC, white blood cell.
DISCUSSION
This phase III randomized controlled study investigated nivolumab monotherapy compared with chemotherapy in patients with platinum-resistant ovarian cancer. In this study, there was no statistical difference in OS between the groups, and ORR and PFS were worse with nivolumab versus chemotherapy. No new safety risks were observed compared with those previously reported with nivolumab monotherapy in various cancers including platinum-resistant ovarian cancer.12,14,22 Incidences of grade 3 or 4 TRAEs and serious TRAEs were numerically lower in the nivolumab group compared with the chemotherapy group. Therefore, in this study, nivolumab had a favorable safety profile but did not improve OS and showed worse PFS compared with chemotherapy in patients with platinum-resistant ovarian cancer.
To date, few phase III trials have targeted platinum-resistant ovarian cancer in a randomized trial design, and of those that have, most have evaluated biologic therapy in combination with chemotherapy; in general, survival benefit or improvement in PFS over chemotherapy has not been shown.23-26 In this study, nivolumab monotherapy was compared with single-agent, investigator-choice chemotherapy and showed no benefit in OS compared with GEM or PLD. Median PFS (2.0 months) and ORR (7.6%), which were similar to the results from the phase II KEYNOTE-100 study of an anti–PD-1 antibody pembrolizumab (median PFS: 2.1 months; ORR: 8.0%),27 were also worse with nivolumab compared with GEM or PLD. The phase III randomized controlled JAVELIN Ovarian 200 study of an anti–PD-L1 antibody avelumab alone and in combination with PLD versus PLD alone28 showed no benefit of avelumab monotherapy compared with PLD monotherapy in OS (11.8 months v 13.1 months, respectively; stratified HR, 1.14; 95% CI, 0.95 to 1.58) or PFS (1.9 months v 3.5 months, respectively; stratified HR, 1.68; 95% CI, 1.32 to 2.60).23 The phase III PENELOPE and FORWARD I studies evaluated biologic therapy (pertuzumab [a human epidermal growth factor receptor 2–targeting monoclonal antibody] added to chemotherapy, and mirvetuximab soravtansine [an antifolate receptor alpha antidrug conjugate] monotherapy, respectively) versus chemotherapy in platinum-resistant ovarian cancer.24,29 In both studies, no survival benefit or improvements in PFS were observed versus chemotherapy alone.24,25 In contrast, in the AURELIA study, bevacizumab (an anti–vascular endothelial growth factor-A) in combination with chemotherapy showed significant improvements in PFS but not OS compared with chemotherapy alone.26 Collectively, these findings suggest that ovarian cancer may be resistant to checkpoint inhibitor monotherapy.
In platinum-resistant ovarian cancer, various immunosuppressive factors—including PD-1/PD-L1, regulatory T cells, M2 macrophages, and myeloid-derived suppressor cells—interplay to create an immunosuppressive microenvironment and cause tumor immune escape.30-32 Additionally, high levels of tumor mutational burden and T cell–inflamed gene expression profile have been shown to be associated with improved PFS and objective response rate in patients of several tumor types treated with pembrolizumab; however, tumor mutational burden is generally low in ovarian cancer.33 As development of primary resistance to immune checkpoint inhibitors depends on the host's immune status,34 these characteristics of ovarian cancer may explain why nivolumab showed poor PFS in this study, further indicating that nivolumab monotherapy may be insufficient to fully restore the host's antitumor functions. A recent phase II study of recurrent and persistent ovarian cancer showed superiority of nivolumab in combination with ipilimumab, an anti–cytotoxic T lymphocyte–4 antibody, over nivolumab alone in response rate (31.4% v 12.2%) and PFS (3.9 months v 2.0 months),35 suggesting that combination therapy may be effective. The ATHENA study, a large phase III randomized study that includes a comparison between nivolumab in combination with rucaparib, a poly (ADP-ribose) polymerase inhibitor, versus rucaparib monotherapy in ovarian cancer, is currently ongoing.36 Given that promising results have previously been observed with a poly (ADP-ribose) polymerase inhibitor and anti-PD-1/PD-L1 combination,37,38 the results from the ATHENA study are eagerly anticipated.
Patients who received nivolumab seemed to have shorter TTR and longer DOR than those who received chemotherapy, suggesting that nivolumab may potentially provide a faster and more durable antitumor effect than GEM or PLD. Additional analyses to determine underlying baseline characteristics, including biomarkers, of these responding patients would be of interest; however, the number of responding patients was small, and such exploratory analyses are unlikely to provide meaningful results.
The safety profile of nivolumab in this study was similar to that reported previously in a range of cancer types,12 and no additional or new safety risks with platinum-resistant ovarian cancer were observed. TRAEs were those expected of nivolumab, including rash, fatigue, nausea, and diarrhea, and were of low severity. Grade 3 or 4 TRAEs occurred in only 10.9% of patients receiving nivolumab, notably lower than with chemotherapy (65.2%); anemia was the most common TRAE with nivolumab (2.6%), which was lower than that observed with chemotherapy (14.2%). Grade 3 or 4 hematologic events including neutrophil count decreased (40.0%), white blood cell count decreased (13.5%), and platelet count decreased (12.9%) were particularly common with chemotherapy but were rare with nivolumab (0.6%, 0.0%, and 0.0%, respectively). Interestingly, the incidences of treatment-related irAEs, which were of special interest for nivolumab, were similar between the groups. Serious TRAEs with nivolumab were less frequent than with GEM or PLD and less frequent than those reported in previous studies of nivolumab.22 In a small number of patients, QOL results generally showed a more sustained improvement in FACT-O and EQ-5D scores with nivolumab than with GEM or PLD. In combination, the safety and QOL results suggest that nivolumab may be a future treatment option in critically ill patients with platinum resistance for whom QOL is an especially important consideration.
The main strength of this study was that we used a randomized controlled study design to evaluate nivolumab monotherapy against two of the most common chemotherapies, GEM or PLD.7 This study also had a sufficiently large sample size that was powered to show significance of nivolumab versus chemotherapy. Furthermore, although assignment to GEM or PLD was determined by the study site investigators, the numbers of patients treated with GEM and PLD were similar (73 v 82), confirming that neither treatment is favored in practice. A limitation of this study was that it was not powered to detect treatment differences between subgroups.
In conclusion, although nivolumab was well-tolerated, with fewer AEs than with GEM or PLD, nivolumab monotherapy did not demonstrate superiority over GEM or PLD in OS and showed worse PFS in patients with platinum-resistant ovarian cancer.
ACKNOWLEDGMENT
We would like to thank the patients and families for their participation in the NINJA trial, and the investigators and clinical study teams who made this study possible. We thank Toshiya Oizaki, Takashi Nakada, and Toshihiro Yoshikawa for their involvement in this study. We would also like to thank the Japanese Gynecologic Oncology Group (JGOG) for their support in this trial. Medical writing assistance was provided by Prudence Stanford, PhD, and Hana Nomura, BPharm (Hons), of ProScribe—Envision Pharma Group. ProScribe's services complied with international guidelines for Good Publication Practice (GPP3).
APPENDIX
FIG A1.
Percentage change in target tumor diameter from baseline over time for the RES population: (A) Nivolumab and (B) GEM or PLD; and for the responders: (C) Nivolumab and (D) GEM or PLD. GEM, gemcitabine; PLD, pegylated liposomal doxorubicin; RES, response-evaluable set.
FIG A2.
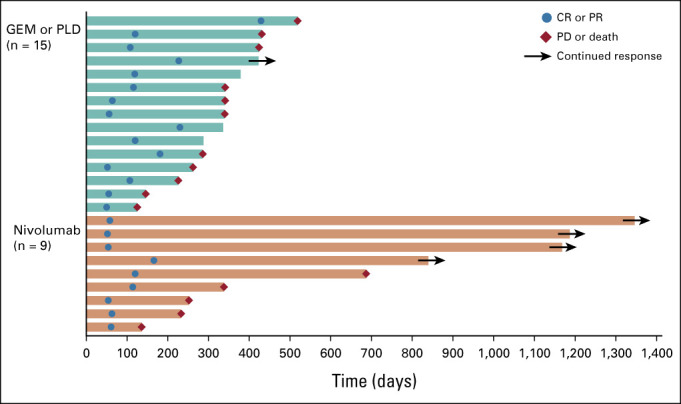
TTR and DOR of responders according to RECIST v1.1 (RES population). TTR was defined as the time from randomization to first CR or PR. DOR was defined as the time from first CR or PR to first PD or death (from any cause), whichever occurred first. Each bar represents the time from randomization to PD or death, or last data collection, in one responding patient. Patients who did not have PD or death and had not received poststudy treatment were considered as having continued response. For patients without PD or death who received poststudy treatment, the date of last diagnostic imaging performed before the start of poststudy treatment was used as the last data collection date. CR, complete response; DOR, duration of response; GEM, gemcitabine; PD, progressive disease; PLD, pegylated liposomal doxorubicin; PR, partial response; RES, response-evaluable set; TTR, time to response.
FIG A3.
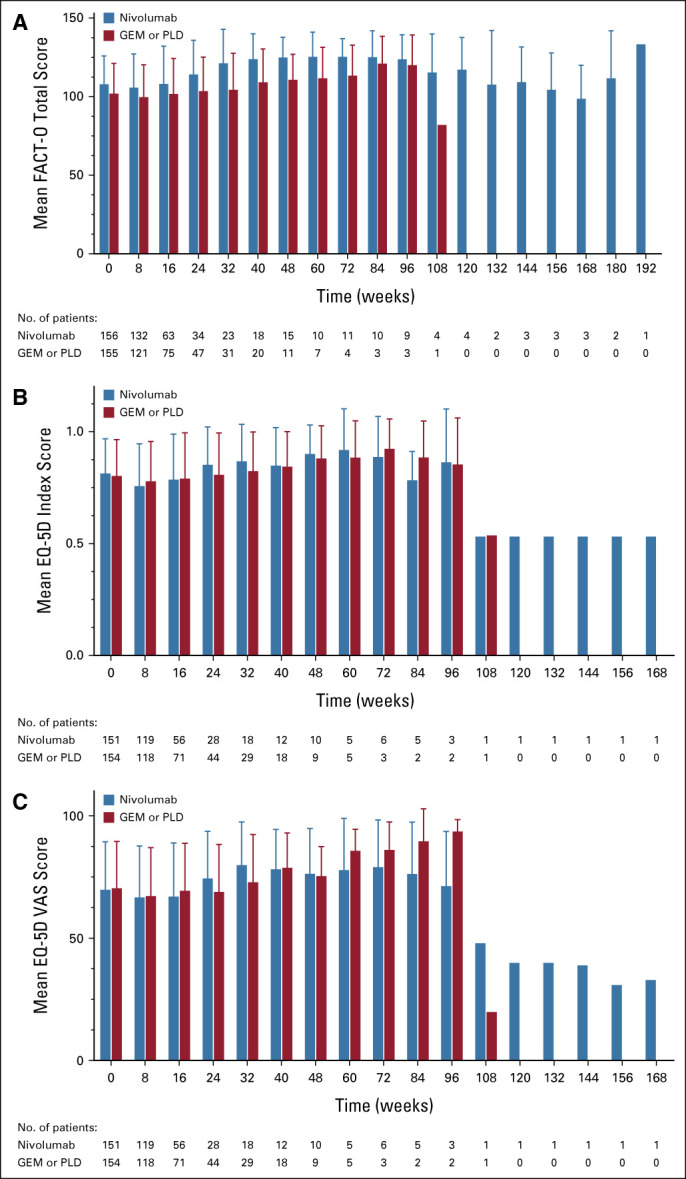
Mean change in QOL over time: (A) FACT-O total score, (B) EQ-5D index score, (C) EQ-5D VAS score (ITT population). Error bars indicate standard deviation. EQ-5D, EuroQol-5 Dimensions; FACT-O, Functional Assessment of Cancer Therapy–Ovarian; GEM, gemcitabine; ITT, intention-to-treat; PLD, pegylated liposomal doxorubicin; QOL, quality of life; VAS, visual analog scale.
TABLE A1.
Summary of Treatment-Related irAEs (SAF population)
Junzo Hamanishi
Research Funding: MSD, Ono Pharmaceutical, Sumitomo Dainippon Pharma
Noriyuki Katsumata
Consulting or Advisory Role: Nippon Zoki Pharmaceutical Co, Ltd
Kimio Ushijima
Honoraria: AstraZeneca, Chugai Pharmaceutical, Takeda, Nippon Kayaku, MSD, Kaken Pharmaceutical, Kyowa Kirin International
Research Funding: AstraZeneca, Chugai Pharmaceutical, Takeda, Kaken Pharmaceutical, Nippon Kayaku, Mochida Pharmaceutical Co, Ltd, Taiho Pharmaceutical, Eisai, Ono Pharmaceutical, AbbVie, MSD
Tadashi Kimura
Honoraria: Nobelpharma Co, Ltd, GE Healthcare Japan, Mochida Pharmaceutical Co, Ltd, Chugai Pharmaceutical Co, Ltd, Bayer Pharmaceuticals, Aska Pharmaceutical
Consulting or Advisory Role: Fermata Pharma, Rohto Pharmaceutical
Research Funding: Fuji Pharma, Nippon Shinyaku, Takeda Yakuhin, Taihou Yakuhin, Mochida Pharmaceutical Co, Ltd, Nihon Seiyaku, Zeria Pharmaceutical
Koji Matsumoto
Honoraria: Chugai Pharmaceutical, AstraZeneca, Kyowa Hakko Kirin, Eisai, MSD K.K., Eli Lilly Japan K.K., Taiho Oncology, AbbVie, Pfizer
Research Funding: Ono Pharmaceutical, MSD, AstraZeneca, Novartis, Chugai Pharmaceutical, Eisai
Kimihiko Ito
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca, AbbVie, Nippon Shinyaku, Terumo
Uncompensated Relationships: Kansai Clinical Oncology Group
Hidemichi Watari
Honoraria: MSD K.K., Tsumura & Co, AstraZeneca, Aska Pharmaceutical Co, Ltd, Terumo, Bayer Yakuhin, Kaken Pharmaceutical, Mochida Pharmaceutical Co, Ltd, Daiichi Sankyo/UCB Japan, Chugai Pharmaceutical, Takeda
Consulting or Advisory Role: Decision Resources Group Japan, Kaken Pharmaceutical, Chugai Pharmaceutical
Research Funding: Hokkaido Welfare Federation of Agricultural Cooperatives, Kaken Pharmaceutical, Mochida Pharmaceutical Co, Ltd, Taiho Pharmaceutical, Chugai Pharmaceutical, Hokkaido Cancer Society, Takeda
Other Relationship: T-PEC
Nobutaka Takahashi
Honoraria: Olympus, Ethicon/Johnson & Johnson, Intuitive Surgical, Kaken Pharmaceutical
Kosei Hasegawa
Honoraria: MSD K.K., Daiichi Sankyo, Bayer Yakuhin, Bristol-Myers Squibb K.K., Chugai Pharmaceutical, AstraZeneca, Eisai
Consulting or Advisory Role: MSD K.K., Kaken Pharmaceutical
Research Funding: Ono Pharmaceutical, Daiichi Sankyo, Takeda
Kan Yonemori
Honoraria: Eisai, Pfizer, AstraZeneca, Novartis, Taiho Pharmaceutical
Consulting or Advisory Role: Chugai Pharmaceutical, Ono Pharmaceutical, Novartis, Eisai
Research Funding: Ono Pharmaceutical
Kazuhiro Takehara
Honoraria: Takeda Pharmaceutical Company, AstraZeneca, Eisai, Chugai Pharmaceutical, MSD, Mochida Pharmaceutical Co, Ltd
Research Funding: Chugai Pharmaceutical
Hitoshi Niikura
Honoraria: Takeda
Sari Nakao
Uncompensated Relationships: Japanese Gynecologic Oncology Group
Toshiaki Saito
Honoraria: Nippon Kayaku, Kyowa Hakko Kirin, Eisai, Kaken Pharmaceutical Company
Research Funding: Chugai Pharmaceutical, Taiho Pharmaceutical
Takayuki Enomoto
Honoraria: AstraZeneca, MSD, Chugai Pharmaceutical
Satoru Nagase
Honoraria: Chugai Pharmaceutical, AstraZeneca
Aikou Okamoto
Honoraria: AstraZeneca K.K., MSD, Chugai Pharmaceutical, Takeda
Consulting or Advisory Role: AstraZeneca K.K., Chugai Pharmaceutical
Speakers' Bureau: AstraZeneca K.K.
Research Funding: Kissei Pharmaceutical, Meiji Holdings, Pfizer, Fuji Pharma, Taiho Pharmaceutical, Kaken Pharmaceutical, Chugai Pharmaceutical, Tsumura & Co, Daiichi Sankyo Company, Ltd, Shinnihonseiyaku, Mochida Pharmaceutical Co, Ltd, Aska Pharmaceutical Co, Ltd, Takeda, Terumo
Yoshito Terai
Research Funding: Ethicon/Johnson & Johnson, MSD, Covidien, Terumo, Daiichi Sankyo/UCB Japan, Chugai Pharmaceutical, Taiho Pharmaceutical
Akira Takazawa
Employment: Ono Pharmaceutical
Yusuke Takahashi
Employment: Ono Pharmaceutical
Stock and Other Ownership Interests: Ono Pharmaceutical
Yoshinobu Namba
Employment: Ono Pharmaceutical
Honoraria: Boehringer Ingelheim
Daisuke Aoki
Honoraria: AstraZeneca K.K., MSD K.K., Takeda, Chugai Pharmaceutical, Myriad Genetics
Consulting or Advisory Role: Takeda, MSD K.K., AstraZeneca K.K.
Speakers' Bureau: AstraZeneca K.K., Takeda
Keiichi Fujiwara
Honoraria: Kyowa Hakko Kirin, Zeria Pharmaceutical, Nippon Kayaku, Chugai Pharmaceutical, Eisai, Taiho Pharmaceutical, Daiichi Sankyo, Ono Pharmaceutical, Takeda
Consulting or Advisory Role: AstraZeneca, MSD, Taiho Pharmaceutical, Eisai, Takeda, Genmab, AbbVie, Pfizer, NanoCarrier
Research Funding: Eisai, Kaken Pharmaceutical, Chugai Pharmaceutical, Shionogi, ImmunoGen, ONCOtherapeutics, AstraZeneca, Eli Lilly, Zeria Pharmaceutical, Ono Pharmaceutical, MSD, Genmab, Regeneron, Merck KGaA, Ono Pharmaceutical
Travel, Accommodations, Expenses: Pfizer, AbbVie, MSD
No other potential conflicts of interest were reported.
See accompanying editorial on page 3651
DISCLAIMER
Ono Pharmaceutical Co, Ltd was involved in the study design, data collection, and data analysis and supported the preparation of the manuscript. Bristol-Myers Squibb Company was involved in the study design and supported the preparation of the manuscript. The authors directed development of the manuscript and are fully responsible for all content and editorial decisions for this manuscript.
PRIOR PRESENTATION
Presented at the European Society for Medical Oncology (ESMO) Virtual Congress 2020, September 19-21, 2020.
SUPPORT
Supported by Ono Pharmaceutical Co, Ltd and Bristol-Myers Squibb Company, manufacturer/licensee of nivolumab.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Qualified researchers may request Ono Pharmaceutical to disclose individual patient-level data from clinical studies through the following website: https://www.clinicalstudydatarequest.com/. For more information on Ono Pharmaceutical's Policy for the Disclosure of Clinical Study Data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.
AUTHOR CONTRIBUTIONS
Conception and design: Junzo Hamanishi, Ikuo Konishi, Toru Sugiyama, Keiichi Fujiwara, Daisuke Aoki, Noriyuki Katsumata, Yusuke Takahashi, Yoshinobu Namba, Akira Takazawa
Administrative support: Yusuke Takahashi, Ikuo Konishi
Provision of study materials or patients: Junzo Hamanishi, Nobuhiro Takeshima, Noriyuki Katsumata, Kimio Ushijima, Tadashi Kimura, Satoshi Takeuchi, Koji Matsumoto, Kimihiko Ito, Masaki Mandai, Hidekatsu Nakai, Noriaki Sakuragi, Hidemichi Watari, Nobutaka Takahashi, Hidenori Kato, Kosei Hasegawa, Kan Yonemori, Mika Mizuno, Kazuhiro Takehara, Hitoshi Niikura, Takashi Sawasaki, Sari Nakao, Toshiaki Saito, Takayuki Enomoto, Satoru Nagase, Nao Suzuki, Takashi Matsumoto, Eiji Kondo, Kenzo Sonoda, Satomi Aihara, Youichi Aoki, Aikou Okamoto, Hirokuni Takano, Hiroshi Kobayashi, Hisamori Kato, Yoshito Terai, Daisuke Aoki, Keiichi Fujiwara, Toru Sugiyama, Ikuo Konishi
Collection and assembly of data: Junzo Hamanishi, Nobuhiro Takeshima, Noriyuki Katsumata, Kimio Ushijima, Tadashi Kimura, Satoshi Takeuchi, Koji Matsumoto, Kimihiko Ito, Masaki Mandai, Hidekatsu Nakai, Noriaki Sakuragi, Hidemichi Watari, Nobutaka Takahashi, Hidenori Kato, Kosei Hasegawa, Kan Yonemori, Mika Mizuno, Kazuhiro Takehara, Hitoshi Niikura, Takashi Sawasaki, Sari Nakao, Toshiaki Saito, Takayuki Enomoto, Satoru Nagase, Nao Suzuki, Takashi Matsumoto, Eiji Kondo, Kenzo Sonoda, Satomi Aihara, Youichi Aoki, Aikou Okamoto, Hirokuni Takano, Hiroshi Kobayashi, Hisamori Kato, Yoshito Terai, Daisuke Aoki, Keiichi Fujiwara, Toru Sugiyama, Ikuo Konishi
Data analysis and interpretation: Junzo Hamanishi, Ikuo Konishi, Toru Sugiyama, Keiichi Fujiwara, Daisuke Aoki, Noriyuki Katsumata, Akira Takazawa, Yusuke Takahashi
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab Versus Gemcitabine or Pegylated Liposomal Doxorubicin for Patients With Platinum-Resistant Ovarian Cancer: Open-Label, Randomized Trial in Japan (NINJA)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Junzo Hamanishi
Research Funding: MSD, Ono Pharmaceutical, Sumitomo Dainippon Pharma
Noriyuki Katsumata
Consulting or Advisory Role: Nippon Zoki Pharmaceutical Co, Ltd
Kimio Ushijima
Honoraria: AstraZeneca, Chugai Pharmaceutical, Takeda, Nippon Kayaku, MSD, Kaken Pharmaceutical, Kyowa Kirin International
Research Funding: AstraZeneca, Chugai Pharmaceutical, Takeda, Kaken Pharmaceutical, Nippon Kayaku, Mochida Pharmaceutical Co, Ltd, Taiho Pharmaceutical, Eisai, Ono Pharmaceutical, AbbVie, MSD
Tadashi Kimura
Honoraria: Nobelpharma Co, Ltd, GE Healthcare Japan, Mochida Pharmaceutical Co, Ltd, Chugai Pharmaceutical Co, Ltd, Bayer Pharmaceuticals, Aska Pharmaceutical
Consulting or Advisory Role: Fermata Pharma, Rohto Pharmaceutical
Research Funding: Fuji Pharma, Nippon Shinyaku, Takeda Yakuhin, Taihou Yakuhin, Mochida Pharmaceutical Co, Ltd, Nihon Seiyaku, Zeria Pharmaceutical
Koji Matsumoto
Honoraria: Chugai Pharmaceutical, AstraZeneca, Kyowa Hakko Kirin, Eisai, MSD K.K., Eli Lilly Japan K.K., Taiho Oncology, AbbVie, Pfizer
Research Funding: Ono Pharmaceutical, MSD, AstraZeneca, Novartis, Chugai Pharmaceutical, Eisai
Kimihiko Ito
Honoraria: AstraZeneca
Consulting or Advisory Role: AstraZeneca, AbbVie, Nippon Shinyaku, Terumo
Uncompensated Relationships: Kansai Clinical Oncology Group
Hidemichi Watari
Honoraria: MSD K.K., Tsumura & Co, AstraZeneca, Aska Pharmaceutical Co, Ltd, Terumo, Bayer Yakuhin, Kaken Pharmaceutical, Mochida Pharmaceutical Co, Ltd, Daiichi Sankyo/UCB Japan, Chugai Pharmaceutical, Takeda
Consulting or Advisory Role: Decision Resources Group Japan, Kaken Pharmaceutical, Chugai Pharmaceutical
Research Funding: Hokkaido Welfare Federation of Agricultural Cooperatives, Kaken Pharmaceutical, Mochida Pharmaceutical Co, Ltd, Taiho Pharmaceutical, Chugai Pharmaceutical, Hokkaido Cancer Society, Takeda
Other Relationship: T-PEC
Nobutaka Takahashi
Honoraria: Olympus, Ethicon/Johnson & Johnson, Intuitive Surgical, Kaken Pharmaceutical
Kosei Hasegawa
Honoraria: MSD K.K., Daiichi Sankyo, Bayer Yakuhin, Bristol-Myers Squibb K.K., Chugai Pharmaceutical, AstraZeneca, Eisai
Consulting or Advisory Role: MSD K.K., Kaken Pharmaceutical
Research Funding: Ono Pharmaceutical, Daiichi Sankyo, Takeda
Kan Yonemori
Honoraria: Eisai, Pfizer, AstraZeneca, Novartis, Taiho Pharmaceutical
Consulting or Advisory Role: Chugai Pharmaceutical, Ono Pharmaceutical, Novartis, Eisai
Research Funding: Ono Pharmaceutical
Kazuhiro Takehara
Honoraria: Takeda Pharmaceutical Company, AstraZeneca, Eisai, Chugai Pharmaceutical, MSD, Mochida Pharmaceutical Co, Ltd
Research Funding: Chugai Pharmaceutical
Hitoshi Niikura
Honoraria: Takeda
Sari Nakao
Uncompensated Relationships: Japanese Gynecologic Oncology Group
Toshiaki Saito
Honoraria: Nippon Kayaku, Kyowa Hakko Kirin, Eisai, Kaken Pharmaceutical Company
Research Funding: Chugai Pharmaceutical, Taiho Pharmaceutical
Takayuki Enomoto
Honoraria: AstraZeneca, MSD, Chugai Pharmaceutical
Satoru Nagase
Honoraria: Chugai Pharmaceutical, AstraZeneca
Aikou Okamoto
Honoraria: AstraZeneca K.K., MSD, Chugai Pharmaceutical, Takeda
Consulting or Advisory Role: AstraZeneca K.K., Chugai Pharmaceutical
Speakers' Bureau: AstraZeneca K.K.
Research Funding: Kissei Pharmaceutical, Meiji Holdings, Pfizer, Fuji Pharma, Taiho Pharmaceutical, Kaken Pharmaceutical, Chugai Pharmaceutical, Tsumura & Co, Daiichi Sankyo Company, Ltd, Shinnihonseiyaku, Mochida Pharmaceutical Co, Ltd, Aska Pharmaceutical Co, Ltd, Takeda, Terumo
Yoshito Terai
Research Funding: Ethicon/Johnson & Johnson, MSD, Covidien, Terumo, Daiichi Sankyo/UCB Japan, Chugai Pharmaceutical, Taiho Pharmaceutical
Akira Takazawa
Employment: Ono Pharmaceutical
Yusuke Takahashi
Employment: Ono Pharmaceutical
Stock and Other Ownership Interests: Ono Pharmaceutical
Yoshinobu Namba
Employment: Ono Pharmaceutical
Honoraria: Boehringer Ingelheim
Daisuke Aoki
Honoraria: AstraZeneca K.K., MSD K.K., Takeda, Chugai Pharmaceutical, Myriad Genetics
Consulting or Advisory Role: Takeda, MSD K.K., AstraZeneca K.K.
Speakers' Bureau: AstraZeneca K.K., Takeda
Keiichi Fujiwara
Honoraria: Kyowa Hakko Kirin, Zeria Pharmaceutical, Nippon Kayaku, Chugai Pharmaceutical, Eisai, Taiho Pharmaceutical, Daiichi Sankyo, Ono Pharmaceutical, Takeda
Consulting or Advisory Role: AstraZeneca, MSD, Taiho Pharmaceutical, Eisai, Takeda, Genmab, AbbVie, Pfizer, NanoCarrier
Research Funding: Eisai, Kaken Pharmaceutical, Chugai Pharmaceutical, Shionogi, ImmunoGen, ONCOtherapeutics, AstraZeneca, Eli Lilly, Zeria Pharmaceutical, Ono Pharmaceutical, MSD, Genmab, Regeneron, Merck KGaA, Ono Pharmaceutical
Travel, Accommodations, Expenses: Pfizer, AbbVie, MSD
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Yamagami W, Nagase S, Takahashi F, et al. : Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol 28:e32, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ottevanger PB: Ovarian cancer stem cells more questions than answers. Semin Cancer Biol 44:67-71, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Matulonis UA, Sood AK, Fallowfield L, et al. : Ovarian cancer. Nat Rev Dis Primers 2:16061, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja FA, Chopra N, Ledermann JA: Optimal first-line treatment in ovarian cancer. Ann Oncol 23:x118-127, 2012. (suppl 10) [DOI] [PubMed] [Google Scholar]
- 6.Ledermann JA, Raja FA, Fotopoulou C, et al. : Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:vi24-32, 2013. (suppl 6) [DOI] [PubMed] [Google Scholar]
- 7.Luvero D, Milani A, Ledermann JA: Treatment options in recurrent ovarian cancer: Latest evidence and clinical potential. Ther Adv Med Oncol 6:229-239, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pharmaceuticals and Medical Devices Agency : Review Report: Opdivo (Nivolumab) Intravenous Infusion, 2017. https://www.pmda.go.jp/files/000223201.pdf [Google Scholar]
- 9.Hamanishi J, Mandai M, Iwasaki M, et al. : Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 104:3360-3365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwai Y, Ishida M, Tanaka Y, et al. : Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99:12293-12297, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida Y, Agata Y, Shibahara K, et al. : Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11:3887-3895, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443-2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homicsko K, Coukos G: Targeting programmed cell death 1 in ovarian cancer. J Clin Oncol 33:3987-3989, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Hamanishi J, Mandai M, Ikeda T, et al. : Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 33:4015-4022, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Hamanishi J: Efficacy of immuno-checkpoint inhibitors in ovarian cancer (abstr S065.3). Int J Gynaecol Obstet 143:43-157, 2018. (suppl 3) [Google Scholar]
- 16.Mutch DG, Orlando M, Goss T, et al. : Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol 25:2811-2818, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. : Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol 19:1809-1817, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Pickard AS, Wilke CT, Lin H-W, et al. : Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics 25:365-384, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Klein JP, Moeschberger ML: Survival Analysis: Techniques for Censored and Truncated Data. New York, NY, Springer, 1997 [Google Scholar]
- 20.Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404-413, 1934 [Google Scholar]
- 21.Agresti A: Categorical Data Analysis (ed 3). Hoboken, NJ, John Wiley & Sons, 2012 [Google Scholar]
- 22.Zhao B, Zhao H, Zhao J: Serious adverse events and fatal adverse events associated with nivolumab treatment in cancer patients: Nivolumab-related serious/fatal adverse events. J Immunother Cancer 6:101, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. : Avelumab alone or in combination with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin alone in platinum-resistant or refractory epithelial ovarian cancer: Primary and biomarker analysis of the phase III JAVELIN Ovarian 200 trial (abstr 1). Gynecol Oncol 154:21-22, 2019. (suppl 1) [Google Scholar]
- 24.Kurzeder C, Bover I, Marmé F, et al. : Double-blind, placebo-controlled, randomized phase III trial evaluating pertuzumab combined with chemotherapy for low tumor human epidermal growth factor receptor 3 mRNA-expressing platinum-resistant ovarian cancer (PENELOPE). J Clin Oncol 34:2516-2525, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Moore K, Oza A, Colombo N, et al. : FORWARD I (GOG 3011): A phase III study of mirvetuximab soravtansine, a folate receptor alpha (FRa)-targeting antibody-drug conjugate (ADC), versus chemotherapy in patients (pts) with platinum-resistant ovarian cancer (PROC) (abstr 992O). Ann Oncol 30:v403, 2019. (suppl 5) [Google Scholar]
- 26.Pujade-Lauraine E, Hilpert F, Weber B, et al. : Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 32:1302-1308, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Matulonis UA, Shapira-Frommer R, Santin AD, et al. : Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann Oncol 30:1080-1087, 2019 [DOI] [PubMed] [Google Scholar]
- 28.Pujade-Lauraine E, Fujiwara K, Dychter SS, et al. : Avelumab (anti-PD-L1) in platinum-resistant/refractory ovarian cancer: JAVELIN Ovarian 200 phase III study design. Future Oncol 14:2103-2113, 2018 [DOI] [PubMed] [Google Scholar]
- 29.Moore KN, Vergote I, Oaknin A, et al. : FORWARD I: A phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Future Oncol 14:1669-1678, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Yang C, Xia B-R, Zhang Z-C, et al. : Immunotherapy for ovarian cancer: Adjuvant, combination, and neoadjuvant. Front Immunol 11:577869, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan Q, Zhang H, Zheng J, et al. : Turning cold into hot: Firing up the tumor microenvironment. Trends Cancer 6:605-618, 2020 [DOI] [PubMed] [Google Scholar]
- 32.Le Saux O, Ray-Coquard I, Labidi-Galy SI: Challenges for immunotherapy for the treatment of platinum resistant ovarian cancer. Semin Cancer Biol 10.1016/j.semcancer.2020.08.017 [epub ahead of print on September 12, 2020] [DOI] [PubMed] [Google Scholar]
- 33.Cristescu R, Mogg R, Ayers M, et al. : Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362:eaar3593, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hegde PS, Karanikas V, Evers S: The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res 22:1865-1874, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Zamarin D, Burger RA, Sill MW, et al. : Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: An NRG oncology study. J Clin Oncol 38:1814-1823, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US National Library of Medicine : ClinicalTrials.gov Identifier: NCT03522246. A Study in Ovarian Cancer Patients Evaluating Rucaparib and Nivolumab as Maintenance Treatment Following Response to Front-Line Platinum-Based Chemotherapy (ATHENA), 2018. https://clinicaltrials.gov/ct2/show/NCT03522246 [Google Scholar]
- 37.Konstantinopoulos PA, Waggoner SE, Vidal GA, et al. : TOPACIO/Keynote-162 (NCT02657889): A phase 1/2 study of niraparib + pembrolizumab in patients (pts) with advanced triple-negative breast cancer or recurrent ovarian cancer (ROC)—Results from ROC cohort. J Clin Oncol 36, 2018. (suppl; abstr 106) [Google Scholar]
- 38.Lampert EJ, Zimmer A, Padget M, et al. : Combination of PARP inhibitor olaparib, and PD-L1 inhibitor durvalumab, in recurrent ovarian cancer: A proof-of-concept phase II study. Clin Cancer Res 26:4268-4279, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request Ono Pharmaceutical to disclose individual patient-level data from clinical studies through the following website: https://www.clinicalstudydatarequest.com/. For more information on Ono Pharmaceutical's Policy for the Disclosure of Clinical Study Data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.