Supplemental Digital Content is available in the text.
Background.
Severe acute respiratory syndrome coronavirus 2–specific cell-mediated immunity (SARS-CoV-2-CMI) elicited by mRNA-based vaccines in solid organ transplant (SOT) recipients and its correlation with antibody responses remain poorly characterized.
Methods.
We included 44 (28 kidney, 14 liver, and 2 double organ) recipients who received the full series of the mRNA-1273 vaccine. SARS-CoV-2-CMI was evaluated at baseline, before the second dose, and at 2 wk after completion of vaccination by an ELISpot-based interferon-γ FluoroSpot assay using overlapping peptides covering the S1 domain. SARS-CoV-2 immunoglobulin G seroconversion and serum neutralizing activity against the spike protein were assessed at the same points by commercial ELISA and an angiotensin-converting enzyme-2/spike antibody inhibition method, respectively. Postvaccination SARS-CoV-2-CMI was compared with 28 healthcare workers who received the BNT162b2 vaccine.
Results.
Positive SARS-CoV-2-CMI increased from 6.8% at baseline to 23.3% after the first mRNA-1273 dose and 59.5% after the completion of vaccination (P < 0.0001). Lower rates were observed for immunoglobulin G seroconversion (2.3%, 18.6%, and 57.1%, respectively) and neutralizing activity (2.3%, 11.6%, and 31.0%). There was a modest correlation between neutralizing titers and the magnitude of SARS-CoV-2-CMI (Spearman’s rho: 0.375; P = 0.015). Fifteen recipients (35.7%) mounted SARS-CoV-2-CMI without detectable neutralizing activity, whereas 3 (7.1%) did the opposite, yielding poor categorical agreement (Kappa statistic: 0.201). Rates of positive SARS-CoV-2-CMI among SOT recipients were significantly decreased compared with nontransplant controls (82.1% and 100.0% after the first dose and completion of vaccination, respectively; P < 0.0001). Kidney transplantation, the use of tacrolimus and prednisone, and the number of immunosuppressive agents were associated with lower cell-mediated responses. Results remained unchanged when 3 recipients with prevaccination SARS-CoV-2-CMI were excluded.
Conclusions.
Two-thirds of SOT recipients mounted SARS-CoV-2-CMI following vaccination with mRNA-1273. Notable discordance was observed between vaccine-induced cell-mediated and neutralizing humoral immunities. Future studies should determine whether these patients with incomplete responses are effectively protected.
INTRODUCTION
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been revealed as the most effective measure to control the coronavirus disease 2019 (COVID-19) pandemic. Randomized trials1,2 and observational studies3,4 have shown excellent rates of seroconversion and clinical effectiveness for mRNA-based vaccines in the general population. Increasing evidence, however, confirms that immunogenicity after solid organ transplantation (SOT) is severely compromised.5-18 As a consequence, SOT recipients may still develop COVID-19 requiring hospital admission despite the completion of vaccination series.19,20 With few exceptions,8,10,16-18 most previous studies have only assessed the induction of humoral immunity, either as total anti–SARS-CoV-2 immunoglobulin (Ig)G titers or neutralizing antibodies targeting the spike (S) glycoprotein. Vaccine-mediated immunity relies not only on long-term antibody responses but also on memory T cells.21 Therefore, immunogenicity may have been underestimated in studies not evaluating the contribution of the cellular arm to vaccine-mediated protection. In addition, the concordance between cell-mediated cellular and humoral responses elicited by vaccination in the setting of long-term immunosuppression remains largely unknown.
On the other hand, the study population in reported experiences was mostly composed of kidney transplant (KT) recipients.6-12,17 It is to be expected that vaccine-induced responses would vary across transplant types because liver transplant (LT) recipients require less intense immunosuppression than other SOT groups. The assessment of the relative impact of different agents on the ability to mount effective responses (such as antimetabolites5,15) is hampered by the fact that the majority of patients in previous cohorts were on triple therapy containing corticosteroids and a calcineurin inhibitor8,12,13,16 or belatacept.9,10
With these knowledge gaps in mind, we have comprehensively assessed the response to the mRNA-1273 vaccine in a cohort of KT and LT recipients. We analyzed the development of SARS-CoV-2–specific cell-mediated immunity with a fluorescent ELISpot for cytokine secretion (interferon [IFN]-γ FluoroSpot), the seroconversion to SARS-CoV-2 IgG antibodies by means of a commercial ELISA, and the neutralizing activity of postvaccination sera against the S protein assessed by a human angiotensin-converting enzyme (hACE)-2/spike antibody inhibition ELISA-based method. Particular focus was put on quantifying the discordance between cell-mediated and humoral responses.
MATERIALS AND METHODS
Study Design and Setting
This prospective study was performed at the University Hospital “12 de Octubre” (Madrid, Spain). All KT and LT recipients with a functioning graft and regular follow-up at our institution who received the first dose of mRNA-1273 (Moderna Biotech)—a lipid nanoparticle-encapsulated mRNA vaccine expressing the prefusion-stabilized SARS-CoV-2 S protein1—between April 15 and April 27, 2021, were potentially eligible. Recipients with a prior history of suspected or documented COVID-19 were also included, provided that they were asymptomatic for >72 h and transmission-based precautions had been discontinued. Forty-five randomly selected patients who fulfilled these criteria were offered to participate.
All participants were evaluated for SARS-CoV-2–specific immunity at 3 time points: baseline (ie, immediately before the first dose of mRNA-1273), at a 4-wk interval (ie, immediately before the second dose), and 2 wk after the completion of the full vaccine series. Peripheral blood lymphocyte subpopulations (CD3+, CD4+, and CD8+ T cells, B cells, and CD56+ CD16+ natural killer cells) and serum immunoglobulin levels were also measured at baseline. Demographics, comorbidities, immunosuppressive regimen, and trough serum levels at the time of vaccination, laboratory values, and vaccine-related adverse events (AEs) were prospectively recorded using a standardized case report form. Patients were followed up on until June 25, 2021.
The study was performed in accordance with the ethical standards as laid down in the Declarations of Helsinki and Istanbul. The study protocol was approved by the institutional research ethics committee, and all participants provided written informed consent.
SARS-CoV-2–specific Cell-mediated Immunity
Details of the IFN-γ FluoroSpot assay have been described elsewhere.22 In brief, whole blood specimens were processed within 24 h from sampling. Peripheral blood mononuclear cells (PBMCs) were freshly isolated by density-gradient centrifugation using Ficoll-Paque and seeded at 300 000 cells/well onto IFN-γ FluoroSpot plates (MabTech, Nacka Strand, Sweden) with cell culture medium containing Roswell Park Memorial Institute medium, 1% l-glutamine, 1% penicillin/streptomycin, 10% fetal bovine serum, and anti-CD28 monoclonal antibody (1 µg/mL). Test wells were performed in duplicate and supplemented with 15-mer overlapping peptides covering the S1 domain of the S protein (166 individual peptides) (SARS-CoV-2 S1 scanning pool, MabTech) at a final concentration of 1 µg/mL. To assess the presence of SARS-CoV-2–specific responses indicative of natural immunity before vaccination, baseline samples were also stimulated with the viral nucleoprotein (N protein)—102 peptides—(EMPS SARS-CoV-2 NCAP-1, JPT Peptide Technologies GmbH, Berlin, Germany) and the membrane (M) protein—53 peptides (EMPS SARS-CoV-2 VME1, JPT). Negative control wells lacked peptides, and positive control wells included anti-CD3 antibodies (MabTech). Assays were incubated for 16–18 h at 37 °C. Spots were counted using an automated IRIS FluoroSpot reader system (MabTech). To quantify specific cell-mediated responses, spots of the negative control wells were subtracted from the mean spots test wells, and the results were expressed as IFN-γ–producing spot-forming units (SFUs)/106 PBMCs. Results were excluded if negative control wells had >80 SFUs/106 PBMCs or positive control wells had <400 SFUs/106 PBMCs.
Responses were considered to be positive if the results were at least 3 times higher than the mean of the negative control wells and above the following antigen-specific cutoff values: >25 SFUs/106 PBMCs for the S protein, >14 SFUs/106 PBMCs for the N protein, and >21 SFUs/106 PBMCs for the SARS-CoV-2 M protein. These thresholds were established on the basis of a control group of 30 unvaccinated healthcare workers (HCWs) with no clinical or microbiological diagnosis of COVID-19, a negative SARS-CoV-2 IgG serology, and no prior known contact with COVID-19 cases recruited by July 2020. We also used a cohort of 234 patients recovered from COVID-19 who had been hospitalized during the acute phase because of moderate or severe infection (World Health Organization ordinal scale 3–7). Samples in this latter group were obtained at a mean of 6 mo (range, 3–7) from symptom onset. The area under receiver operating characteristics curve analysis allowed us to determine optimal cutoff values to discriminate between uninfected HCWs and patients recovered from COVID-19, revealing excellent diagnostic accuracy (SDC methods, SDC, http://links.lww.com/TXD/A377).
We also assessed polyfunctional Th1-biased cell-mediated responses expressing both IFN-γ and interleukin-2. Because of the exploratory nature of this analysis, no cutoff values for assay positivity were established, and data were treated in a continuous manner only.
SARS-CoV-2 Serology
Serum IgG antibodies targeting the S1 protein were detected with the EUROIMMUN Anti-SARS-CoV-2 ELISA (Euroimmun AG, Lübeck, Germany) according to the manufacturer’s instructions. Optical density (OD) values were measured at 450 nm using the PR 3100 microplate reader (Bio-Rad Life Science, Marnes-La-Coquette, France). Results were semiquantitatively evaluated by calculating the ratio of the OD value of the sample over the OD value of the calibrator (relative OD) with the following cutoff values: <0.8, negative; ≥0.8 to <1.1, borderline; and ≥1.1, positive.
Serum Neutralizing Activity Against the S Protein
The neutralizing activity elicited by the mRNA-1273 vaccine was analyzed with an in-house hACE-2/spike antibody inhibition ELISA-based method. Microtiter 96-well plates were coated overnight with a chimeric version of a monoclonal anti-Foldon antibody23 at 8 ng/µL in PBS. Plates were next blocked with PBS supplemented with BSA fraction V (Sigma-Aldrich, Munich, Germany) at 1% (PBS-BSA 1%). Then, purified HexaPro-derived construct24 containing the D614G substitution was captured by incubation at 1 ng/µL in PBS-BSA 1% at room temperature for 45 min. Following protein incubation, plates were washed with PBS, and successive incubations of sera dilutions and hACE-2 monomeric-StreTag receptor (20 ng/µL) complexed with StrepTactin-horseradish peroxidase (Bio-Rad, Hercules, CA) at 1:5000 were performed. Sera incubation was prolonged for 45 min, and after a 15-min incubation of receptor–StrepTactin-horseradish peroxidase complexes, receptor binding to captured S protein was revealed with the O-phenylenediamine dihydrochloride substrate (Sigma-Aldrich) and measured in a spectrophotometer at 493–620 nm. The assay background was determined in parallel plates with the S protein locked in the close conformation, which is unable to bind the hACE-2 receptor. For this purpose, a purified HexaPro-derived construct including a double cysteine substitution (S383C, D985C) was used. A pool of sera from healthy individuals with negative SARS-CoV-2 IgG antibodies and no prior known contact with COVID-19 cases and hACE-2 monomeric untagged receptor were used as negative and positive controls, respectively. After background subtraction, the percentage of neutralization was calculated as (1 – [OD495–620 test serum/OD495-620 negative control] × 100). The incubation of untagged hACE-2 receptor at 200 ng/µL achieved a neutralization rate >85% in all the experiments. The neutralizing antibody titer for a given patient was established as the last dilution leading to 50% inhibition of the OD value of the negative control. Because 1/10 was the lowest dilution for which this criterion was met, we used this threshold as pragmatic cutoff value.
Nontransplant Control Group
To better characterize the impact of posttransplant immunosuppression on the ability of vaccination to elicit SARS-CoV-2–specific cell-mediated immunity, we used a control group of 28 nonimmunocompromised HCWs who had received the full series of the BNT162b2 mRNA vaccine (Comirnaty, Pfizer-BioNTech) at our institution between January 15 and February 15, 2021. The IFN-γ FluoroSpot assay was performed following a schedule that mirrored that of SOT recipients: at baseline, at a 3-wk interval (ie, immediately before the second dose of BNT162b2), and at 2 wk after the completion of vaccination.
Statistical Analysis
Quantitative data were reported as the mean ± SD or the median with interquartile range (IQR). Qualitative variables were expressed as absolute and relative frequencies. Categorical variables were compared using the χ2 test. The Student t test or the Mann-Whitney U test was used for continuous variables. Repeated measures were compared with the Wilcoxon signed-rank test or the McNemar test, as appropriate. Correlations between continuous variables were evaluated with Pearson’s correlation coefficient or Spearman’s rho. The categorical agreement between the results of the IFN-γ FluoroSpot assay and the SARS-CoV-2 IgG serology and serum neutralizing activity was evaluated by the Kappa statistic. Statistical analysis was performed using SPSS version 20.0 (IBM Corp, Armonk, NY).
RESULTS
Study Population
One out of 45 approached SOT recipients refused to participate. Therefore, 44 patients (28 KT recipients, 14 LT recipients, and 2 double organ [kidney-pancreas and liver-kidney] recipients) were included (Table 1). One patient (2.3%) had a history of prior COVID-19 diagnosed 6 mo ago. All but 2 participants (95.5%) received the full mRNA-1273 vaccine series. One patient refused to receive the second dose. One further patient—a 39-y-old LT recipient that was receiving tacrolimus, mofetil mycophenolate (MMF), and prednisone—was hospitalized because of SARS-CoV-2 infection without pneumonia or oxygen therapy requirement 3 wk after the receipt of the first vaccine dose, and the second dose had to be postponed until the resolution of symptoms.
TABLE 1.
Demographics and clinical characteristics of the study population (n = 44)
| Variable | |
|---|---|
| Age, y, mean ± SD | 52.4 ± 11.5 |
| Male gender, N (%) | 27 (61.4) |
| Smoking habit, N (%) | 7 (15.9) |
| Comorbidities, N (%) | |
| Hypertension | 32 (72.7) |
| Dyslipidemia | 12 (27.3) |
| Diabetes mellitus | 11 (25.0) |
| Cardiovascular disease | 14 (31.8) |
| Obesity | 6 (13.6) |
| Chronic pulmonary disease | 4 (9.1) |
| Venous thromboembolic disease | 7 (15.9) |
| Ethnicity, N (%) | |
| Caucasian | 38 (86.4) |
| Latino | 3 (6.8) |
| Other | 3 (6.8) |
| Type of transplantation, N (%) | |
| Kidney | 28 (63.6) |
| Liver | 14 (31.8) |
| Simultaneous kidney-pancreas | 1 (2.3) |
| Simultaneous liver-kidney | 1 (2.3) |
| Previous solid organ transplantation, N (%) | 5 (11.4) |
| Time interval since transplantation, y, median (IQR) | 2.3 (1.3–4.8) |
| Type of immunosuppression regimen, N (%)a | |
| Tacrolimus, MMF/MPS and prednisone | 23 (52.3) |
| Tacrolimus and MMF/MPS | 5 (11.4) |
| Tacrolimus, MMF/MPS and mTOR inhibitor | 4 (9.1) |
| Tacrolimus monotherapy | 4 (9.1) |
| MMF/MPS and mTOR inhibitor | 2 (4.5) |
| Otherb | 6 (13.6) |
| Laboratory and immunological parameters, mean ± SDa | |
| eGFR, mL/min/1.73 m2 | 57.5 ± 21.8 |
| CD3+ T-cell count, cells/μL | 926 ± 481 |
| CD4+ T-cell count, cells/μL | 444 ± 273 |
| CD8+ T-cell count, cells/μL | 446 ± 313 |
| B-cell count, cells/μL | 112 ± 85 |
| NK cell count, cells/μL | 189 ± 154 |
| Serum IgG levels, mg/dL | 1140 ± 476 |
| Serum IgA levels, mg/dL | 276 ± 140 |
| Serum IgM levels, mg/dL | 121 ± 86 |
aAt the time of the administration of the first dose of the mRNA-1273 vaccine.
bCyclosporine, MMF and prednisone (n = 1); MPA, everolimus, and prednisone (n = 1); tacrolimus, azathioprine, and prednisone (n = 1); tacrolimus and prednisone (n = 1); everolimus and prednisone (n = 1); MMF monotherapy (n = 1).
eGFR, estimated glomerular filtration rate (estimated by the Modification of Diet in Renal Disease-4 equation); IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; MMF/MPS, mycophenolate mofetil or mycophenolate sodium; mTOR, mammalian target of rapamycin; NK, natural killer; SD, standard deviation.
Induction of SARS-CoV-2–specific Cell-mediated Immunity Following Vaccination
Three patients (6.8%) exhibited positive IFN-γ–producing cell-mediated responses against the 3 SARS-CoV-2 structural proteins tested (S, N, and M) at baseline, which demonstrated the acquisition of natural immunity before vaccination. None of these patients had a previous history suggestive of COVID-19.
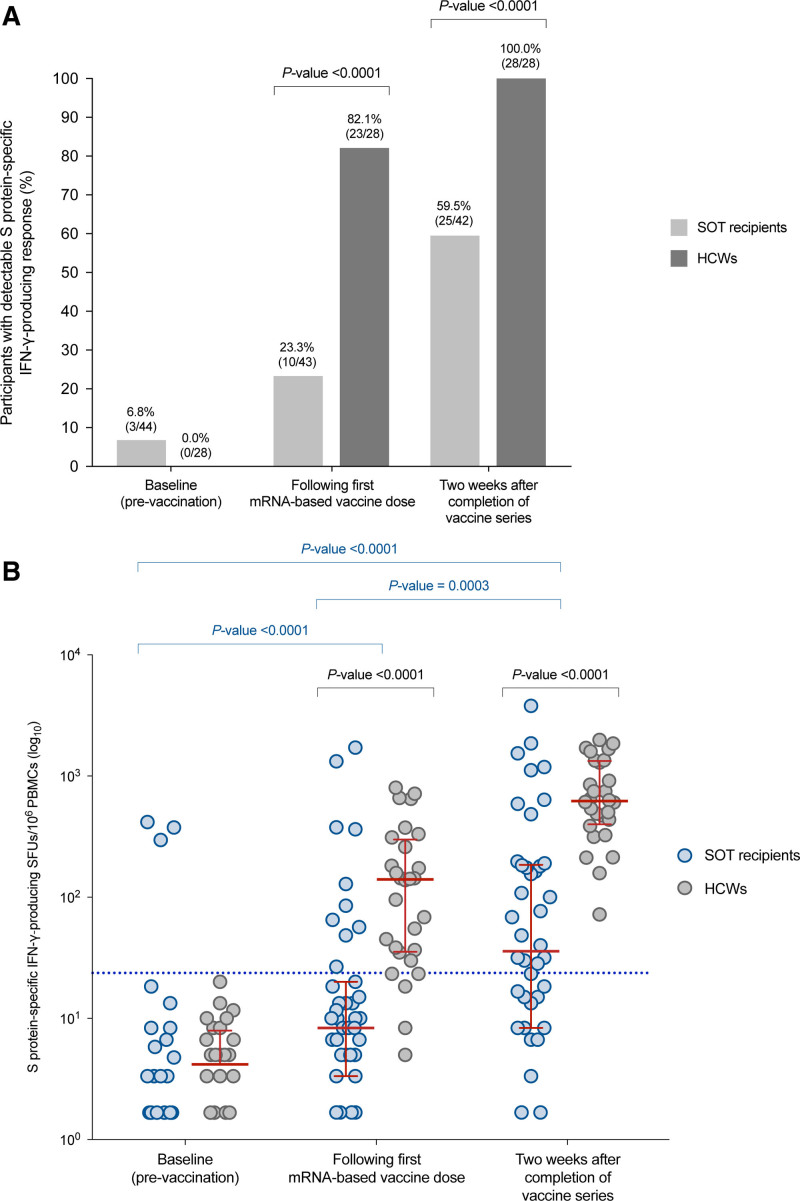
The proportion of positive S protein–specific responses (>25 SFUs/106 PBMCs) by the IFN-γ FluoroSpot assay increased to 23.3% (10 of 43) after the first dose (P = 0.039) and 59.5% (25 of 42) at 2 wk after the completion of the vaccine series (P = 0.0003) (Figure 1A).
FIGURE 1.
SARS-CoV-2–specific cell-mediated immunity in SOT recipients and HCWs who received the mRNA-1273 and BNT162bs vaccines, respectively: (A) proportion with detectable S protein–specific IFN-γ–producing response at baseline, after the first vaccine dose vaccine (ie, 4 and 3 wk apart for mRNA-1273 and BNT162b2), and at 2 wk after the completion of the vaccine series; (B) kinetics of the number of S protein–specific IFN-γ–producing SFUs at the same time points. Red bars and whiskers represent median values and interquartile ranges, respectively. The cutoff value for positivity in the IFN-γ FluoroSpot assay (>25 SFUs/106 PBMCs) is denoted by the blue dotted line. Comparisons between repeated measures were performed with the McNemar test or the Wilcoxon signed-rank test, as appropriate. HCW, healthcare worker; IFN-γ, interferon-γ; PBMC, peripheral blood mononuclear cell; S, SARS-CoV-2 spike glycoprotein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFU, spot-forming unit; SOT, solid organ transplantation.
The magnitude of the cell-mediated response, as measured by the median number of S protein–specific IFN-γ–producing SFUs per 106 PBMCs, increased from baseline (0.0 [IQR, 0–3.3]) to the assessment following the first vaccine dose (8.3 [IQR, 3.3–20.0]) and after 2 wk from the completion of vaccination (35.8 [IQR, 8.3–185.0]) (P < 0.0001) (Figure 1B). Polyfunctional IFN-γ/interleukin-2–producing responses exhibited a similar trend, although absolute magnitudes were lower (Figure S1, SDC, http://links.lww.com/TXD/A377).
SARS-CoV-2 IgG Antibody Response
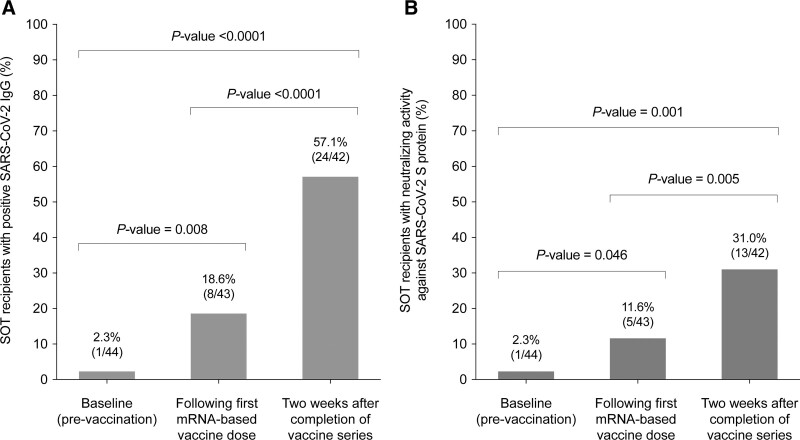
At the baseline (prevaccination) evaluation, only 1 patient (2.3%) tested positive for SARS-CoV-2 IgG antibodies. The rate of IgG seropositivity increased to 18.6% (8 of 43) after the first dose (P = 0.016) and 57.1% (24 of 42) at 2 wk after the completion of vaccination (P < 0.0001) (Figure 2A).
FIGURE 2.
SARS-CoV-2–specific humoral immunity elicited by the mRNA-1273 vaccine in SOT recipients: (A) proportion of patients with IgG antibodies targeting the SARS-CoV-2 S protein assessed by commercial ELISA at baseline, after the first vaccine dose vaccine, and at 2 wk after the completion of the vaccine series; (B) proportion with serum neutralizing activity against the S protein analyzed with an in-house hACE-2/spike antibody inhibition ELISA-based method at the same time points. Comparisons between repeated measures were performed with the Wilcoxon signed-rank test. hACE, human angiotensin-converting enzyme; IgG, immunoglobulin; S, SARS-CoV-2 spike glycoprotein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplantation.
Out of a total of 129 monitoring points, 104 (80.6%) yielded concordant results between the S protein–specific cell-mediated immunity and the SARS-CoV-2 IgG serology, with both assays testing positive in 23 points (17.8%) and negative in 81 (62.8%). In 15 points (11.6%), the IFN-γ FluoroSpot assay was positive with negative ELISA, whereas the opposite results (lack of cell-mediated immunity with positive SARS-CoV-2 IgG) were observed in 10 points (7.8%). When focused on the evaluation at 2 wk after the completion of vaccination, 8 patients (19.0%) who remained seronegative exhibited detectable cell-mediated immunity, whereas 7 patients (16.7%) with positive IgG antibodies tested below the cutoff value for the IFN-γ FluoroSpot assay (Kappa statistic, 0.266).
When the results of both assays were analyzed as continuous variables, we observed a significant—albeit weak—positive correlation between the semiquantitative results of the IgG ELISA (expressed as relative OD) and the number of S protein–specific IFN-γ–producing SFUs per 106 PBMCs following the first vaccine dose (Spearman’s rho, 0.354, P = 0.019) and after the completion of the series (Spearman’s rho, 0.472, P = 0.002) (Figure S2, SDC, http://links.lww.com/TXD/A377).
Serum Neutralizing Activity Against SARS-CoV-2 S Protein
One patient (2.3%) exhibited neutralizing activity by the hACE-2/spike antibody inhibition method at baseline. The proportion increased to 11.6% (5 of 43) after the first vaccine dose (P = 0.046) and 31.0% (13 of 42) at 2 wk after the completion of vaccination (P = 0.005) (Figure 2B).
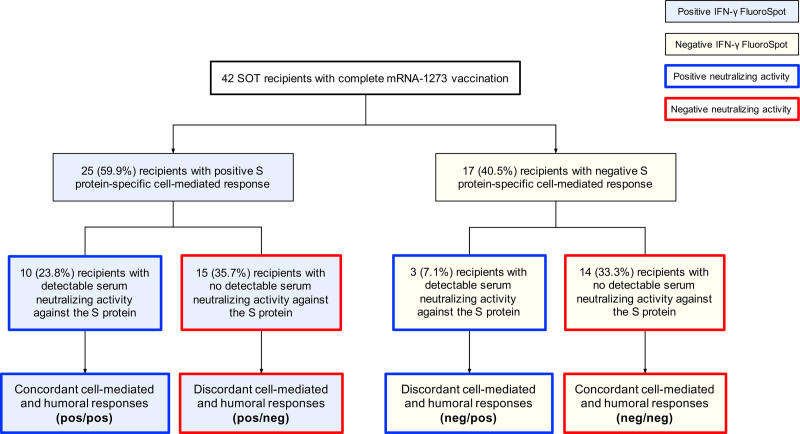
We obtained concordant results between the S protein–specific cell-mediated immunity and serum neutralizing activity in 104 out of 129 monitoring points (80.6%), with both assays concurrently testing positive and negative in 16 (12.4%) and 88 (68.2%) points, respectively. The presence of SARS-CoV-2–specific cell-mediated immunity was not accompanied by detectable neutralizing activity in 17 points (13.2%). Conversely, neutralizing activity was not associated with a positive IFN-γ FluoroSpot assay in 8 points (6.2%). By 2 wk from the administration of the second vaccine dose, 15 patients (35.7%) mounted cell-mediated responses without detectable neutralizing activity, whereas 3 of them (7.1%) did the opposite (Kappa statistic, 0.201) (Figure 3).
FIGURE 3.
Overall disposition of SOT recipients according to the results of the IFN-γ FluoroSpot assay and the hACE-2/spike antibody inhibition ELISA-based method at 2 wk after the completion of the full mRNA-1273 vaccine series. hACE, human angiotensin-converting enzyme; IFN-γ, interferon-γ; S, SARS-CoV-2 spike glycoprotein; SOT, solid organ transplantation
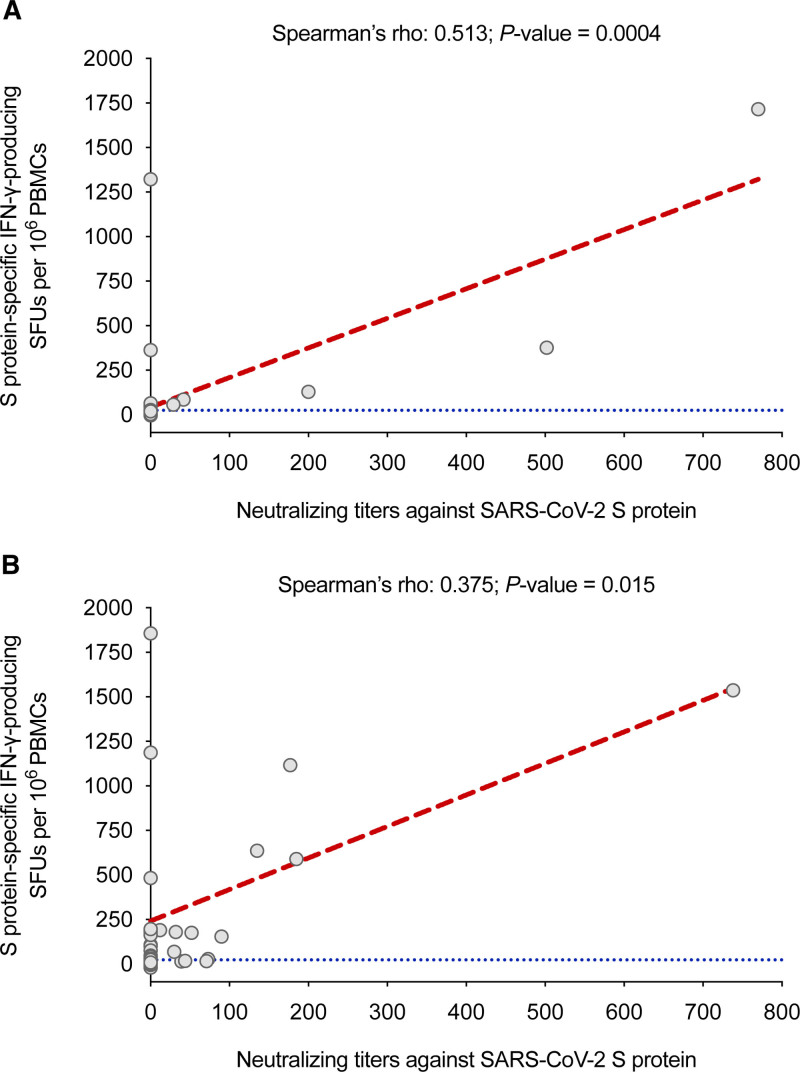
There was a significant correlation between neutralizing titers against the S protein and the number of S protein–specific IFN-γ–producing SFUs per 106 PBMCs after the first vaccine dose (Spearman’s rho, 0.513; P = 0.0004) and at 2 wk from the second dose (Spearman’s rho, 0.375; P = 0.015) (Figure 4).
FIGURE 4.
Correlation between the number of S protein–specific IFN-γ–producing SFUs per 106 PBMCs assessed by the IFN-γ FluoroSpot assay and the serum neutralizing activity against the S protein determined with an hACE-2/spike antibody inhibition ELISA-based method: (A) after the first dose of the mRNA-1273 vaccine; (B) at 2 wk after the completion of the full vaccine series. The cutoff value for positivity in the IFN-γ FluoroSpot assay (>25 SFUs/106 PBMCs) is denoted by the blue dotted line. hACE, human angiotensin-converting enzyme; IFN-γ, interferon-γ; PMBC, peripheral blood mononuclear cell; S, SARS-CoV-2 spike glycoprotein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFU, spot-forming unit.
Comparison With Nontransplant Controls
We compared SARS-CoV-2–specific cell-mediated immunity elicited by mRNA-based vaccines between SOT recipients and a nontransplant control group of 28 HCWs (21 females, mean age, 43.1 ± 15.1 y [range, 22.9–64.1]) who received the BNT162b2 vaccine. None of these HCWs had any relevant comorbidity potentially impacting vaccine immunogenicity or showed immunological or serological evidence of natural immunity against SARS-CoV-2 at baseline. The rate of positive S protein–specific responses was significantly higher in HCWs than in SOT recipients either after the first vaccine dose (82.1% [23 of 28] versus 23.3% [10 of 43]; P < 0.0001) or at 2 wk after the administration of the full series (100.0% [28 of 28] versus 59.5% [25 of 42]; P < 0.0001) (Figure 1A). The median number of S protein–specific SFUs per 106 PBMCs was also significantly higher among HCWs at both points (140.0 [IQR, 35.4–298.6] versus 8.3 [IQR, 3.3–20.0]; 621.0 [IQR, 399.5–1328.0] versus 35.8 [IQR, 8.3–185.0], respectively; P < 0.0001 for both comparisons) (Figure 1B).
Factors Associated With the Mounting of SARS-CoV-2–specific Immunity After Vaccination
Recipients with positive S protein–specific cell-mediated responses at 2 wk after the completion of vaccination had significantly higher CD4+ T-cell counts and lower IgM levels at baseline than those without them. In addition, the time interval since transplantation was longer in this group. On the other hand, the presence of serum neutralizing activity was associated with a higher estimated glomerular filtration rate and CD8+ T-cell counts. Although not achieving statistical significance, LT recipients were more likely than KT recipients to exhibit neutralizing activity elicited by vaccination (Table 2).
TABLE 2.
Comparison of clinical characteristics between SOT recipients who did or did not develop SARS-CoV-2–specific cell-mediated immunity (>25 S protein–specific IFN-γ–producing SFUs/106 PBMCs) or detectable serum neutralizing activity against the S protein (hACE-2/spike antibody inhibition ELISA-based method) at 2 wk after the completion of the full mRNA-1273 vaccine series
| Variable | SARS-CoV-2–specific cell-mediated immunity | Serum neutralizing activity against SARS-CoV-2 S protein | ||||
|---|---|---|---|---|---|---|
| Vaccine response (n = 25) | No vaccine response (n = 17) | P | Vaccine response (n = 13) | No vaccine response (n = 29) | P | |
| Age of recipient, y, mean ± SD | 53.0 ± 12.4 | 52.0 ± 10.6 | 0.789 | 52.0 ± 12.2 | 52.8 ± 11.5 | 0.836 |
| Gender of recipient (male), N (%) | 17 (68.0) | 10 (58.8) | 0.744 | 8 (61.5) | 19 (65.5) | 1.000 |
| Smoking habit, N (%) | 5 (20.0) | 2 (11.8) | 0.681 | 2 (15.4) | 5 (17.2) | 1.000 |
| Comorbidities, N (%) | ||||||
| Hypertension | 17 (68.0) | 14 (82.4) | 0.477 | 8 (61.5) | 23 (79.3) | 0.270 |
| Dyslipidemia | 6 (24.0) | 5 (29.4) | 0.733 | 4 (30.8) | 7 (24.1) | 0.713 |
| Diabetes mellitus | 5 (20.0) | 6 (35.3) | 0.305 | 4 (30.8) | 7 (24.1) | 0.713 |
| Cardiovascular disease | 10 (40.0) | 4 (23.5) | 0.266 | 6 (46.2) | 8 (27.6) | 0.298 |
| Obesity | 3 (12.0) | 3 (17.6) | 0.672 | 2 (15.4) | 4 (13.8) | 1.000 |
| Chronic pulmonary disease | 4 (16.0) | 0 (0.0) | 0.134 | 3 (23.1) | 1 (3.4) | 0.080 |
| Venous thromboembolic disease | 4 (16.0) | 3 (17.6) | 1.000 | 1 (7.7) | 6 (20.7) | 0.405 |
| Ethnicity other than Caucasian, N (%) | 3 (12.0) | 3 (17.6) | 0.672 | 13 (100.0) | 23 (79.3) | 0.153 |
| Type of transplantation, N (%) | 0.179 | 0.068 | ||||
| Kidney (including double organ) | 15 (60.0) | 14 (82.4) | 6 (46.2) | 23 (79.3) | ||
| Liver | 10 (40.0) | 3 (17.6) | 7 (53.8) | 6 (20.7) | ||
| Previous solid organ transplantation, N (%) | 2 (8.0) | 3 (17.6) | 0.379 | 3 (23.1) | 2 (6.9) | 0.162 |
| Time interval since transplantation, y, median (IQR) | 2.9 (1.5–5.1) | 1.9 (0.8–3.2) | 0.053 | 2.9 (1.9–4.1) | 1.9 (0.8–5.1) | 0.318 |
| Immunosuppression regimen containing, N (%)a | ||||||
| Tacrolimus | 20 (80.0) | 16 (94.1) | 0.374 | 10 (76.9) | 26 (89.7) | 0.353 |
| Trough serum levels, ng/mL, mean ± SD | 8.0 ± 3.9 | 9.1 ± 2.7 | 0.377 | 8.2 ± 4.5 | 8.6 ± 2.9 | 0.749 |
| MMF/MPS | 20 (80.0) | 11 (64.7) | 0.305 | 8 (61.5) | 23 (79.3) | 0.270 |
| Trough serum levels, ng/mL, mean ± SD | 3.8 ± 2.2 | 3.9 ± 1.9 | 0.938 | 2.9 ± 1.8 | 4.2 ± 2.1 | 0.392 |
| mTOR inhibitor | 5 (20.0) | 3 (17.6) | 1.000 | 4 (30.8) | 4 (13.8) | 0.226 |
| Trough serum levels, ng/mL, mean ± SD | 5.4 ± 2.8 | 6.6 ± 4.4 | 0.656 | 5.5 ± 3.1 | 6.1 ± 3.7 | 0.807 |
| Prednisone | 15 (60.0) | 15 (88.2) | 0.081 | 6 (46.2) | 24 (82.8) | 0.026 |
| Laboratory and immunological parameters, mean ± SDa | ||||||
| eGFR, mL/min/1.73 m2 | 59.5 ± 20.7 | 54.6 ± 20.7 | 0.457 | 69.4 ± 21.7 | 52.2 ± 18.0 | 0.011 |
| CD3+ T-cell count, cells/μL | 1010 ± 483 | 738 ± 382 | 0.074 | 1067 ± 490 | 836 ± 440 | 0.165 |
| CD4+ T-cell count, cells/μL | 520 ± 300 | 334 ± 208 | 0.044 | 471 ± 278 | 436 ± 285 | 0.733 |
| CD8+ T-cell count, cells/μL | 456 ± 283 | 368 ± 233 | 0.327 | 560 ± 320 | 365 ± 221 | 0.038 |
| B-cell count, cells/μL | 118 ± 85 | 108 ± 90 | 0.733 | 135 ± 113 | 105 ± 74 | 0.350 |
| NK cell count, cells/μL | 165 ± 133 | 224 ± 187 | 0.258 | 188 ± 110 | 188 ± 174 | 0.991 |
| Serum IgG levels, mg/dL | 1032 ± 259 | 1109 ± 303 | 0.393 | 1064 ± 217 | 1062 ± 302 | 0.990 |
| Serum IgA levels, mg/dL | 238 ± 123 | 302 ± 140 | 0.139 | 245 ± 129 | 272 ± 135 | 0.552 |
| Serum IgM levels, mg/dL | 92 ± 67 | 146 ± 87 | 0.036 | 89 ± 41 | 124 ± 90 | 0.213 |
aAt the time of the administration of the first dose of the mRNA-1273 vaccine.
eGFR, estimated glomerular filtration rate (Modification of Diet in Renal Disease-4 equation); hACE, human angiotensin-converting enzyme; IFN-γ, interferon-γ; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; MMF/MPS, mycophenolate mofetil or mycophenolate sodium; mTOR, mammalian target of rapamycin; NK, natural killer; PBMC, peripheral blood mononuclear cell; S, SARS-CoV-2 spike glycoprotein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; SFU, spot-forming unit; SOT, solid organ transplantation.
We also analyzed the magnitude of SARS-CoV-2–specific cell-mediated immunity and serum neutralizing activity as continuous variables. The number of S protein–specific IFN-γ–producing SFUs were significantly lower among KT recipients (compared with LT recipients), as well as in patients receiving tacrolimus, prednisone, and a triple immunosuppression regimen (as compared with 1- or 2-drug regimens) (Figure S3, SDC, http://links.lww.com/TXD/A377). In addition, there was a significant positive correlation with baseline CD3+ and CD4+ T-cell counts (Table S1, SDC, http://links.lww.com/TP/C311). In contrast, neutralizing titers against the S protein were also significantly lower in KT recipients and those receiving prednisone (Figure S4, SDC, http://links.lww.com/TXD/A377), whereas titers positively correlated with the CD8+ T-cell count and estimated glomerular filtration rate at baseline (Table S1, SDC, http://links.lww.com/TP/C311).
Sensitivity Analysis Excluding Patients With Natural Immunity
In view of the well-known impact of preexisting natural immunity on vaccine immunogenicity in SOT recipients,8 we performed a sensitivity analysis in which the 3 patients with positive IFN-γ–producing cell-mediated responses against S, N, and M proteins or serum neutralizing activity at baseline were excluded. In comparison with the overall cohort, no meaningful differences in the kinetics of SARS-CoV-2–specific cell-mediated (Figure S5, SDC, http://links.lww.com/TXD/A377) or humoral immunity after vaccination (Figure S6, SDC, http://links.lww.com/TXD/A377) were observed. Likewise, clinical factors found to be associated with the development and magnitude of vaccine-induced immune responses remain unchanged (Tables S2 and S3, SDC, http://links.lww.com/TXD/A377).
AEs
There were no serious vaccine-related AEs. At least 1 solicited or unsolicited event of local or systemic reactogenicity was reported by 12 recipients (27.3%): pain at the injection site (n = 6), headache (n = 3), fatigue (n = 2), fever (n = 1), tachycardia (n = 1), and nausea (n = 1). None of the patients experienced graft rejection during vaccination or in the subsequent follow-up. There were no differences in the occurrence of AEs according to the presence of SARS-CoV-2–specific cell-mediated immunity at baseline (0.0% [0 of 3] versus 29.3% [12 of 41] for those with or without prevaccine response, respectively; P = 0.551).
DISCUSSION
In contrast to most previous studies,5-7,9,11-15 we have performed a comprehensive evaluation of vaccine immunogenicity that comprised different methodological approaches to both SARS-CoV-2–specific cell-mediated and humoral immunities. Less than two-thirds of the SOT recipients that received the 2-dose regimen of the mRNA-1273 vaccine mounted positive cellular responses. This response rate was significantly lower than that obtained in the nontransplant control group and appeared to be influenced by the type of transplantation and the amount of immunosuppression. A considerable proportion of recipients exhibiting S protein–specific IFN-γ–producing SFUs above the threshold established for positivity in the IFN-γ FluoroSpot assay had no detectable vaccine-induced humoral immunity analyzed either by SARS-CoV-2 IgG serology or serum neutralizing activity (19.0% and 35.7%, respectively). Moreover, discordance was also observed in the opposite direction, resulting in a less than moderate categorical agreement between cell-mediated and antibody responses.
A few recent studies have investigated SARS-CoV-2–specific cell-mediated responses among vaccinated SOT recipients.8,10,16,17 Cucchiari et al found that 54.7% of KT recipients had a positive S protein–reactive ELISpot assay after the second dose of mRNA-1273. Although this figure was close to that of our experience, it should be noted that one-quarter of the patients in that study were also reactive to the N protein, raising the possibility of acquired natural—rather than vaccine-induced—immunity or cross-reactivity with seasonal coronaviruses.8 In a cohort of 101 KT recipients treated with belatacept, the rates of S1-specific cell-mediated immunity by ELISpot after the first and second dose of the BNT162b2 vaccine were 5.0% and 30.4%, respectively.10 The deleterious impact of the costimulation blocker belatacept on mRNA vaccine immunogenicity has also been reported for IgG antibody responses.9 In a mixed population mostly consisting of KT recipients, Schmidt et al reported that heterologous boosting with the mRNA-based vaccine after priming with the adenovirus-vector vaccine ChAdOx1 nCoV-19 led to the most pronounced induction of SARS-CoV-2–specific CD4+ T cells.17
To our knowledge, only 1 previous study has assessed cell-mediated immunity elicited by SARS-CoV-2 vaccination in the LT population.17 Although not achieving statistical significance, LT recipients were more likely than KT recipients to mount S protein–specific responses (76.9% versus 51.7%, respectively). In addition, the number of IFN-γ–producing SFUs per 106 PBMCs was significantly higher among LT recipients. This difference across transplant types was concordant with the results we have observed for neutralizing activity and with other studies assessing IgG seroconversion.5 Herrera et al17 also found more robust cell-mediated responses for LT recipients compared with heart transplant recipients. Ours, however, is the first study to date to compare immunogenicity across different groups of abdominal SOT recipients.
One of the most relevant findings was the notable discordance observed between SARS-CoV-2–specific cell-mediated and humoral responses, including neutralizing activity against the S protein. Although pointed out in some previous studies,8,16,18 this issue had not been assessed in detail. Cucchiari et al8 analyzed by Luminex the presence of IgG antibodies against the receptor-binding domain and found that half of the KT recipients who remained seronegative after the completion of vaccination exhibited a positive S-reactive ELISpot. In the same line, 46.2% of SOT recipients with negative IgG antibody in a recent study still had a positive CD4+ T-cell response.16 In contrast, only 1.9% of 205 hemodialysis patients who received either the BNT162b2 or mRNA-1273 vaccine mounted a cell-mediated response without IgG antibodies detectable by the chemiluminescent immunoassay.25 A single dose of the mRNA-1273 vaccine elicited robust neutralizing activity and Th1-biased responses in a murine model.26 Moreover, all the participants in the phase-1 trial who received two 100-μg mRNA-1273 doses generated consistent antibody and CD4+ T-cell responses.27 Therefore, it may be hypothesized that long-term immunosuppressive therapy contributes to discordant cellular and humoral vaccine-induced responses after SOT. A recent study in nontransplant patients treated with rituximab reported that B-cell depletion affects seroconversion but does not necessarily abrogate T-cell–mediated responses following vaccination.28 Because the generation of long-lived antibody-secreting plasma cells requires the collaboration between CD4+ subsets (such as follicular helper T cells) and activated B cells, it remains to be assessed whether IgG seronegative recipients with a positive IFN-γ FluoroSpot assay or those who seroconverted but remained below the threshold established for IFN-γ–producing SFUs—which accounted for 19.0% and 16.7% of our cohort, respectively—are effectively protected against SARS-CoV-2 infection.29 In addition to the type of SOT, we found that a lower prevaccination CD4+ T-cell count, a shorter interval since transplantation, a maintenance immunosuppression regimen including tacrolimus or prednisone, and the number of immunosuppressive drugs received at the time of vaccination (triple therapy versus 1- or 2-drug regimens) predicted the development and magnitude of SARS-CoV-2–specific cell-mediated immunity. These associations remain essentially unchanged when the 3 patients exhibiting natural immunity at baseline were excluded. Taken together, these findings point to the overall amount of immunosuppression as a major determinant of vaccine immunogenicity, as supported by the differences observed with HCWs in the control group. A previous study also identified lymphopenia as a risk factor for defective cellular response.8 Our study further shows that this association is mainly mediated by the CD4+ T-cell subset. We have previously reported the deleterious impact of tacrolimus on the amount of cell-mediated immunity elicited by natural SARS-CoV-2 infection in SOT recipients recovered from COVID-19.19,30 In the same line, calcineurin inhibitors have been shown to decrease polyfunctional T-cell responses upon in vitro stimulation with cytomegalovirus antigens.31 In contrast, no apparent effect on vaccine responsiveness was observed for the use of antimetabolites, which is in contrast with other studies focused on antibody responses.5,15 Interestingly, a high MMF dose—but not tacrolimus levels—is associated with a lower likelihood of seroconversion after influenza vaccination in KT recipients.32 These findings should be borne in mind if a strategy based on MMF dose reduction—with a compensatory increase in tacrolimus exposure—is attempted to improve vaccine immunogenicity because calcineurin inhibitors, rather than antimetabolites, seem to act as major determinants of the capacity to mount protective SARS-CoV-2–specific cell-mediated immunity.
A number of limitations should be acknowledged. First, because of the limited sample size, no multivariate analysis could be performed, and the associations found between clinical characteristics and vaccine-induced immunity remain exploratory. Second, the cutoff value applied to define positive responses by the IFN-γ FluoroSpot assay has not been clinically validated, and different protective thresholds may be applicable to the transplant setting. Nevertheless, these thresholds were established on the basis of a reference group of patients who recovered from severe COVID-19 with naturally acquired immunity. This approach mirrors what followed to determine positive—and presumably protective—cell-mediated responses against other viruses (ie, cytomegalovirus) in commercial ELISA- and ELISpot-based assays, which also used the area under receiver operating characteristics curve analysis to discriminate between IgG seropositive and seronegative individuals.33-35 Third, because of logistical reasons, the HCWs within the control group received the BNT162b2 vaccine rather than the mRNA-1273 administered to SOT recipients. Increasing evidence reveals that mRNA-1273 is able to elicit higher antibody titers than BNT162b2,5,36 which is likely due to the higher mRNA content and the longer interval between priming and boosting. Data for cell-mediated immunity are much more limited, although no significant differences between both vaccine products in the magnitude of responses quantified by ELISpot have been reported for pregnant and lactating women,37 rituximab-treated patients,28 or KT recipients.38 Nevertheless, because SOT recipients in our cohort received the mRNA-1273 vaccine (which is presumably associated with higher immunogenicity), the difference observed in cell-mediated responses with regard to the nontransplant control group may have been actually underestimated. Fourth, the dispersion of vaccine-induced responses among HCWs was relatively large, which was likely due to their wide age range. Finally, because we assessed SARS-CoV-2–specific responses at 1 single point after the completion of the vaccination course, the medium- and long-term decay of postvaccination immunity were not investigated.
In conclusion, 59.5% of SOT recipients mounted S protein–specific cell-mediated immunity by 2 wk after the second dose of the mRNA-1273 SARS-CoV-2 vaccine. This response rate was higher than that observed for serum neutralizing activity against the S protein (31.0%) but markedly decreased as compared with nontransplant healthy controls. The agreement between cellular and humoral immunities—particularly neutralizing antibodies—was poor. Our research adds to the emerging, albeit limited, evidence suggesting that KT and LT recipients may benefit from SARS-CoV-2 vaccination even in the absence of seroconversion to IgG antibodies because detectable cell-mediated responses can still be mounted. Future studies, however, must determine whether discordant responses effectively confer protection against COVID-19, as well as the impact on SARS-CoV-2–specific cell-mediated immunity of novel strategies such as heterologous vaccination17 or the administration of a third vaccine dose.39,40
Supplementary Material
Footnotes
Published online 17 November, 2021.
The authors declare no conflicts of interest.
This work was supported by the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (COVID-19 Research Call COV20/00181) and cofinanced by the European Development Regional Fund “A way to achieve Europe.” M.F.R. holds a research contract “Miguel Servet” (CP18/00073) and R.L.G. a research contract “Rio Hortega” (CM19/00120), both from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation.
M.F.R., P.P.R., E.P.A., and J.M.A. designed the research. P.A.V., O.C., T.R.M., R.L.G., E.G.R., V.M., and M.M.B. performed immunological assays. M.F.R., T.R.M., R.S.J., F.L.M., C.L., and A.A. performed clinical evaluation and patient follow-up. M.F.R. and O.C. performed clinical data collection. M.F.R. performed statistical analysis and wrote the article. R.L.G., R.S.J., F.L.M., C.L., A.A., P.P.R., E.P.A., and J.M.A. critically reviewed the final draft of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kho MML, Reinders MEJ, Baan CC, et al. ; RECOVAC Collaborators. The RECOVAC IR study: the immune response and safety of the mRNA-1273 COVID-19 vaccine in patients with chronic kidney disease, on dialysis or living with a kidney transplant—a prospective, controlled, multicenter observational cohort by the REnal patients COVID-19 VACcination (RECOVAC) consortium COVID-19 VACcination (RECOVAC) consortium. Nephrol Dial Transplant. 2021;36:1761–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech). Viruses. 2021;13:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21:2727–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou MT, Boyarsky BJ, Chiang TPY, et al. Immunogenicity and reactogenicity after SARS-CoV-2 mRNA vaccination in kidney transplant recipients taking belatacept. Transplantation. 2021;105:2119–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavarot N, Ouedrani A, Marion O, et al. Poor anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105:e94–e95. [DOI] [PubMed] [Google Scholar]
- 11.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99:1498–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupper A, Katchman H. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus: not alarming, but should be taken gravely. Am J Transplant. 2021;21:2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benotmane I, Gautier-Vargas G, Cognard N, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99:1487–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. [Epub ahead of print. August 4, 2021]. doi:10.1111/ajt.16766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. [Epub ahead of print. July 22, 2021]. doi:10.1111/ajt.16768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt T, Klemis V, Schub D, et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant. [Epub ahead of print. August 28, 2021]. doi:10.1111/ajt.16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali NM, Alnazari N, Mehta SA, et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation. 2021;105:e104–e106. [DOI] [PubMed] [Google Scholar]
- 20.Malinis M, Cohen E, Azar MM. Effectiveness of SARS-CoV-2 vaccination in fully-vaccinated solid organ transplant recipients. Am J Transplant. 2021;21:2916–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amanna IJ, Slifka MK. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 2011;411:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Ruiz M, Olea B, Almendro-Vázquez P, et al. T cell–mediated response to SARS-CoV-2 in liver transplant recipients with prior COVID-19. Am J Transplant. 2021;21:2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battles MB, Más V, Olmedillas E, et al. Structure and immunogenicity of pre-fusion-stabilized human metapneumovirus F glycoprotein. Nat Commun. 2017;8:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh CL, Goldsmith JA, Schaub JM, et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369:1501–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broseta JJ, Rodríguez-Espinosa D, Rodríguez N, et al. Humoral and cellular responses to mRNA-1273 and BNT162b2 SARS-CoV-2 vaccines administered to hemodialysis patients. Am J Kidney Dis. 2021;78:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. 2021;80:1345–1350. [DOI] [PubMed] [Google Scholar]
- 29.Heeger PS, Larsen CP, Segev DL. Implications of defective immune responses in SARS-CoV-2 vaccinated organ transplant recipients. Sci Immunol. 2021;6:eabj6513. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Ruiz M, Olea B, Giménez E, et al. SARS-CoV-2-specific cell-mediated immunity in kidney transplant recipients recovered from COVID-19. Transplantation. 2021;105:1372–1380. [DOI] [PubMed] [Google Scholar]
- 31.Fuhrmann S, Lachmann R, Streitz M, et al. Cyclosporin A and tacrolimus reduce T-cell polyfunctionality but not interferon-γ responses directed at cytomegalovirus. Immunology. 2012;136:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar D, Campbell P, Hoschler K, et al. Randomized controlled trial of adjuvanted versus nonadjuvanted influenza vaccine in kidney transplant recipients. Transplantation. 2016;100:662–669. [DOI] [PubMed] [Google Scholar]
- 33.QuantiFERON-CMV. ELISA assay (Qiagen GmbH) package insert. Available at https://www.quantiferon.com/wp-content/uploads/2021/03/L1075110-R06-QF-CMV-ELISA-IFU-CE-Final.pdf. Accessed September 1, 2021.
- 34.T-SPOT.CMV. ELISpot assay (Oxford Immunotec Ltd) package insert. Available at http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/PI-CMV-IVD-UK-V2.pdf. Accessed September 1, 2021.
- 35.Barabas S, Spindler T, Kiener R, et al. An optimized IFN-γ ELISpot assay for the sensitive and standardized monitoring of CMV protein-reactive effector cells of cell-mediated immunity. BMC Immunol. 2017;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steensels D, Pierlet N, Penders J, et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collier AY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021;325:2370–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021;9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385:661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stumpf J, Tonnus W, Paliege A, et al. Cellular and humoral immune responses after 3 doses of BNT162b2 mRNA SARS-CoV-2 vaccine in kidney transplant. Transplantation. [Epub ahead of print. July 22, 2021]. doi:10.1097/TP.0000000000003903 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.