Abstract
Background:
In the past few decades, many lines of evidence implicate the importance of liver kinase B1 (LKB1) as a tumor suppressor gene in the development and progression of solid tumours. However, the prognostic and clinicopathological value of LKB1 in patients with lung cancer are controversial. This article aimed to investigate the latest evidence on this question.
Methods:
A systematic literature searched in the PubMed, Web of Science, Embase, Cochrane library, Scopus until September 20, 2020. The association between overall survival (OS), relapse-free survival (RFS), progression-free survival (PFS), clinicopathological features and LKB1 were analysed by meta-analysis.
Results:
Eleven studies including 1507 patients were included in this meta-analysis. The pooled results revealed that low LKB1 expression was significantly associated with poor overall survival (OS) (HR = 1.67, 95% CI: 1.07–2.60, P = .024) in lung cancer. However, no association was found between LKB1 expression and DFS/PFS (HR = 1.29, 95% CI: 0.70–2.39, P = .410). Pooled results showed that low LKB1 expression was associated with histological differentiation (poor vs moderate or well, OR = 4.135, 95% CI:2.524–6.774, P < .001), nodal metastasis (absent vs present, OR = 0.503, 95% CI: 0.303–0.835, P = .008) and smoking (yes vs no, OR = 1.765, 95% CI: 1.120–2.782, P = .014).
Conclusion:
These results suggest that low expression of LKB1 can be considered as a unfavorable prognostic biomarker for human lung cancer, which should be further researched.
Keywords: LKB1, lung cancer, meta-analysis, prognosis
1. Introduction
Lung cancer is the most common cause of cancer-related deaths all over the world.[1,2] About 1.8 million people are diagnosed with lung cancer every year, and 1.6 million people die because of this disease. Now several types of lung cancers can be recognized, such as small cell lung carcinomas, large cell carcinomas, adenocarcinomas, adenosquamous carcinomas and so on.[3] Despite recent rapid advances in the diagnosis, classification, and therapy, the overall survival of lung cancer is still poor and patients’ prognosis remains unfavorable.[4] Though intense research have been used to identify potential molecular prognostic markers for lung cancer, few of them are adopted into clinical use.[5,6] Therefore, new biomarkers with high accuracy for predicting the prognosis in patients with lung cancer are urgently required.
Inactivating somatic mutations of liver kinase B1 (LKB1) are frequently reported in non-small-cell lung cancer (NSCLC), malignant melanoma, and cervical carcinoma.[7–9] However, the results are controversial. LKB1 is a tumor suppressor gene encodes a serine threonine kinase with a stability role in the regulation of cellular metabolism and energy homeostasis.[10] Several studies showed that LKB1 served as a powerful biomarker of tumor functional status could guide clinical trials and patient prognosis assessment.[11] No meta-analysis has been mentioned on LKB1 and its effect on the clinicopathological parameters and prognosis of lung cancer. To address this issue, we performed meta-analysis to comprehensively evaluate the value of LKB1 in patients with lung cancer.
2. Materials and methods
2.1. Search strategy
The relevant studies were systematically searched with the language restricted to English in the PubMed, Web of Science, Embase, Cochrane library, ClinicalTrials.gov. and Scopus up to September 20, 2020. The search terms included the following keywords:
(“LKB-1” OR “liver kinase B1” OR “STK11” OR “serine-threonine kinase 11”) AND (“lung cancer” OR “lung carcinoma” OR “lung neoplasm” OR “lung tumor”). The references of the review articles and main researches were also searched in order to avoid omission.
2.2. Inclusion and exclusion criteria
Studies that were included if they met the following criteria:
-
1.
The pathological diagnosis of lung cancer must be confirmed,
-
2.
the expression of LKB1 in lung tumor tissue was measured by immunohistochemistry (IHC),
-
3.
available data about overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS) that could be accessible,
-
4.
hazard ratio (HR) and 95% confidence interval (CI) of survival data were reported or could be calculated from Kaplan–Meier survival curves,
-
5.
the study was published in English with full text.
The exclusion criteria for this literature were as follows:
-
1.
duplicate publications,
-
2.
laboratory articles, reviews, letters, meta-analysis, reviews, case reports and comments,
-
3.
no mention to LKB1 and lung cancer,
-
4.
lack of information about survival outcomes or survival curves.
2.3. Data extraction and quality assessment
The following types of data were extracted from all eligible studies: name of first author, publication year, country, number of cases, gender, smoking, tumour stage, patient's age, follow-up time, cancer histology, cancer type, cutoff value of LKB-1 positivity, detection method of LKB1 expression, survival data (OS, DFS, PFS), HRs, ICs. For some studies from which we could not extract HR and CIs directly, Engauge Digitizer software version 4.1 was used to extract survival rate from Kaplan–Meier curves.[12] Two reviewers independently assessed the quality of the eligible studies using the standard Newcastle-Ottawa Scale (NOS).[13] NOS scores of ≥7 were defined as high quality, 4 to 6 as intermediate quality and 1 to 3 as low quality. All data were cross-checked by two reviewers, and disagreements were resolved by a third researcher.
2.4. Statistical analysis
This article was performed using Stata version 12.0 (STATA Corp, College Station, TX) for statistical analysis. Correlation between LKB1 expression and prognosis (PFS, DFS and OS) of patients with lung cancer was evaluated in terms of HRs and 95% CIs. The ORs and 95% CIs were used to evaluate the association between LKB1 expression and clinicopathological characteristics of lung cancer. When it come out a result of Q-test (I2 > 50% or P < .05) indicated heterogeneity between the studies, the random effects model was used for the meta-analysis. Otherwise, a fixed-effects model was used. Subgroup analysis were carried out to detect sources of heterogeneity. Begg's (rank correlation) and Egger's (regression asymmetry) tests were performed for assessing potential publication bias. Sensitivity analysis was also performed to evaluate the stability of this meta-analysis. The P < .05 was regarded as statistically significant.
3. Results
3.1. Study selection and study characteristics
A total of 1232 potentially relevant studies were identified in literature searches. After screening titles and abstracts, a total of 11 studies[14–24] with 1507 patients were included in the meta-analysis, 1221 of which were excluded for reasons are shown in Figure 1. The main characteristics of the eligible studies are summed up in Table 1. Six articles[15–17,19,22,23] originated from China, two are from Korea[14] and Italy[20] and three are from the USA.[18,21,24] Nine articles had statistics on OS,[14–18,20–23] 2 studies had data on DFS,[23,24] and one had data on PFS.[20] The NOS score of included articles ranged from 6 to 8, which suggested that all possessed high methodological quality (Table 2).
Figure 1.
Flow diagram of literature retrieval strategy.
Table 1.
Main characteristics of the eligible studies.
| Author | Year | Country | Number | Gender (M/F) | Smoking (never/ever) | Stage | Age (years)/ Medium (range) | Follow-up (months) Medium (range) | Design | Cancer type | Cancer histology | Cutoff value of positive LKB-1 | Outcomes | Test method |
| Ki EH[14] | 2008 | Korea | 77 | 63/14 | 22/54 | I–IV | 63 (35–79) | 60 | Retrospective | NSCLC | ADA, SqCC, Large cell carcinoma, Bronchioloalveolar carcinoma, Small cell carcinoma. | Staining intensity in the tumor > 30% | OS | IHC |
| Liu SL[15] | 2013 | China | 173 | 91/82 | NA | I–II/IIIa–IIIb | N.A. | 80 | Retrospective | NSCLC | ADA, SqCC | The staining intensity in the tumors matched or exceeded the staining intensity of the normal airway | OS | IHC |
| Jiang LL[16] | 2014 | China | 142 | 82/60 | NA | I–II/III–IV | 58.2 (31–84) | 31 (3–71) | Retrospective | NSCLC | N.A. | Score ≥ 5 | OS | IHC |
| Tsai LH [17] | 2014 | China | 115 | 66/49 | 73/42 | I–III | N.A. | >120 | Retrospective | Lung adenocarcinoma | ADA | Score > 100 | OS | IHC |
| Calles A[18] | 2015 | USA | 126 | 39/87 | 21/105 | I–IV | N.A. | 60 | Retrospective | NSCLC | Non-SqCC | Any degree of LKB1staining by IHC was considered positive. | OS | IHC |
| Li Y[19] | 2016 | China | 74 | 51/23 | NA | I–IV | 31–83 | N.A. | Retrospective | NSCLC | ADA, SqCC | Score ≥ 2 | N.A. | IHC |
| Bonanno L[20] | 2017 | Italy | 98 | 30/68 | 16/82 | N.A. | 64 (56–70) | 35.4 (6–64.9) | Retrospective | NSCLC | ADA, SqCC, Large cell carcinoma, and Others | Score ≥ 2 | OS, PFS | IHC |
| Muge G[21] | 2017 | USA | 305 | NA | NA | I–IV | N.A. | 100 | Retrospective | Lung adenocarcinoma | ADA | N.A. | OS | IHC |
| Liu MJ[22] | 2018 | China | 190 | 103/87 | NA | I–II/III–IV | N.A. | 72 | Retrospective | Lung adenocarcinoma | ADA | Score ≥ 2 | OS | IHC |
| Qin ZH[23] | 2019 | China | 103 | 76/27 | 51/52 | I–IIIA | N.A. | 51.5 (1–64) | Retrospective | NSCLC | N.A. | Score ≥ 4 | OS, DFS | IHC |
| Kyle G[24] | 2020 | USA | 104 | 53/51 | 15/89 | I–III | 64 (56.5–73) | 89.7 (34.4–135.8) | Retrospective | Lung adenocarcinoma | ADA | Median score | DFS | IHC |
ADA = Adenocarcinoma, DFS = disease-free survival, F = female, IHC = immunohistochemistry, LKB1 = liver kinase B1, M = male, N.A. = not available, N = Number of patient, NSCLC = non-small cell lung cancer, OS = overall survival, PFS = progression-free survival, SqCC = squamous cell carcinoma.
Table 2.
The Newcastle–Ottawa scale (NOS) quality assessment of the eligible studies.
| Selection | Comparability | Outcome | Total | ||||||
| Study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | quality scores |
| Ki EH[14] | ∗ | ∗ | ∗ | – | – | ∗ | ∗ | ∗ | 6 |
| Liu SL[15] | ∗ | ∗ | ∗ | – | – | ∗ | ∗ | ∗ | 6 |
| Jiang LL[16] | ∗ | ∗ | ∗ | – | ∗∗ | ∗ | ∗ | ∗ | 8 |
| Tsai LH [17] | ∗ | ∗ | ∗ | – | ∗∗ | ∗ | ∗ | ∗ | 8 |
| Calles A[18] | ∗ | ∗ | ∗ | – | – | ∗ | ∗ | ∗ | 6 |
| Li Y[19] | ∗ | ∗ | ∗ | – | – | ∗ | ∗ | ∗ | 6 |
| Bonanno L[20] | ∗ | ∗ | ∗ | – | – | ∗ | ∗ | ∗ | 6 |
| Muge G[21] | ∗ | ∗ | ∗ | – | ∗∗ | ∗ | ∗ | ∗ | 8 |
| Liu MJ[22] | ∗ | ∗ | ∗ | – | ∗∗ | ∗ | ∗ | ∗ | 8 |
| Qin ZH[23] | ∗ | ∗ | ∗ | – | ∗∗ | ∗ | ∗ | ∗ | 8 |
| Kyle G[24] | ∗ | ∗ | ∗ | – | ∗∗ | ∗ | ∗ | ∗ | 8 |
-, zero score; ∗, one score; ∗∗, two scores; a quality score ≥ 6 was considered to be high quality.
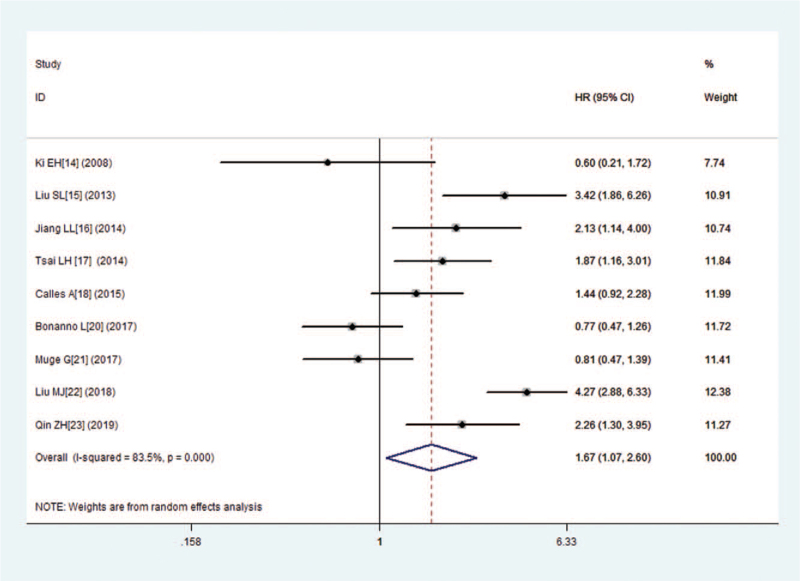
3.2. Prognostic value of LKB1 over expression for OS in lung cancer
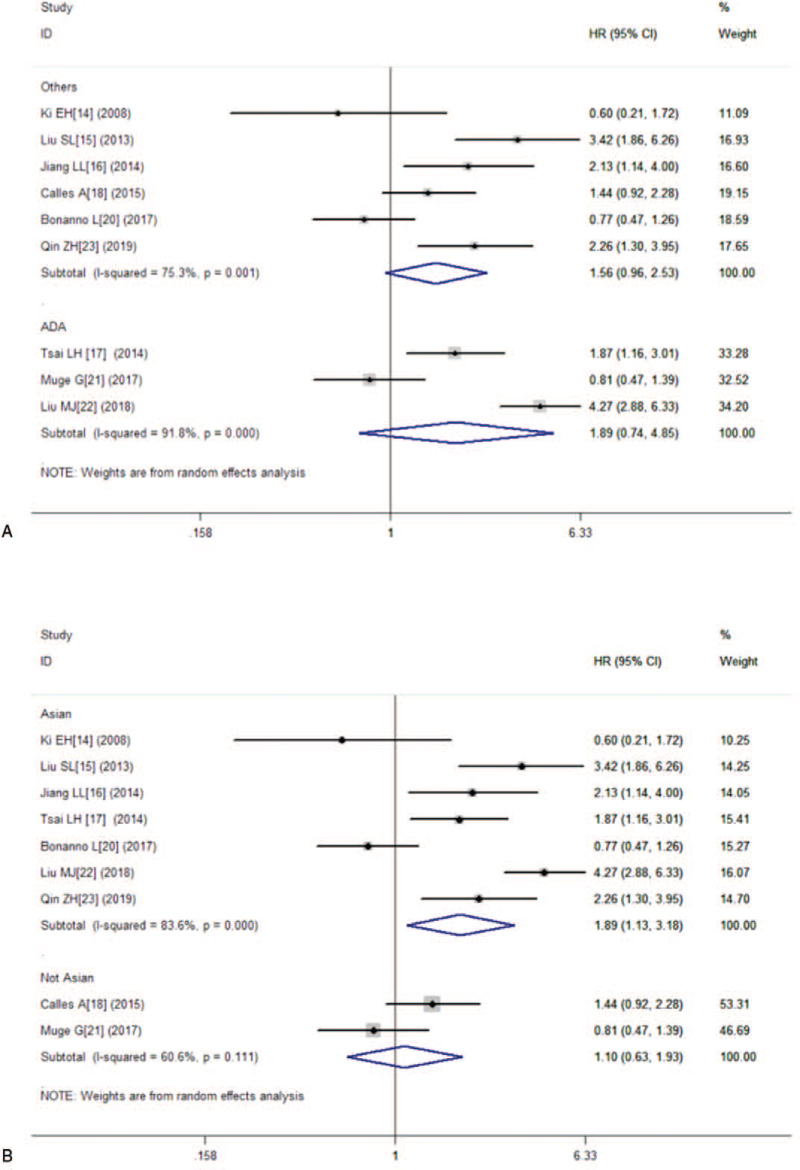
Nine studies consisting of 1329 patients reported OS. The combined HR for studies evaluating low expression of LKB1 on OS was 1.67 (95% CI:1.07–2.60, P = .024), suggesting that low expression of LKB1 was an indicator of poor prognosis for lung cancer patients (Fig. 2). Because of the significant heterogeneity (I2 = 83.5%, P = .000), this meta-analysis was calculated by using the random effects model. Furthermore, we performed subgroup analysis on country and cancer type. The results showed that no significant association was found between low expression of LKB1 and OS in lung adenocarcinoma carcinoma (HR = 1.89, 95% CI:0.74–4.85, P = .185), either in other types (HR = 1.56, 95% CI:0.96–2.53, P = .075) (Fig. 3A). The combined HRs in Asian studies and non-Asian studies were 1.89 (95% CI:1.13–3.18, P = .016) and 1.10 (95% CI:0.63–1.93, P = .732), respectively (Fig. 3B).
Figure 2.
Forest plot of the hazard ratio for the association between the LKB1 and overall survival (OS) in patients with lung cancers.
Figure 3.
Overall survival (OS) subgroup analyses. (A) OS subgroup analysis in term of different tumor types; (B) OS subgroup analysis of different regions.
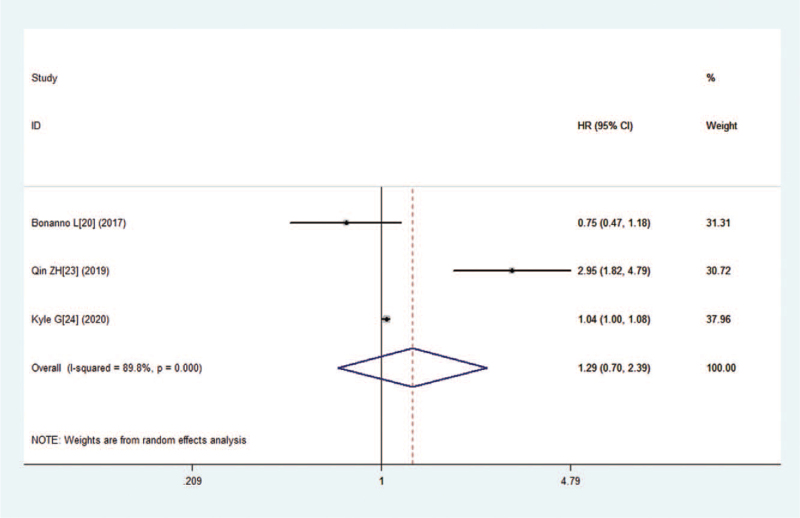
3.3. Prognostic value of LKB1 expression for PFS/DFS in lung cancer
There were two studies mentioned the data on DFS, and one had data on PFS. This meta-analysis was carried out using the random effects model on account of significant heterogeneity (I2 = 89.8%, P = .000). The combined HR for studies evaluating low expression of LKB1 on PFS/DFS was 1.29 (95% CI:0.70–2.39, P = .410), suggesting that no significant correlation was observed between low expression of LKB1 and PFS/DFS (Fig. 4).
Figure 4.
Forest plot of the hazard ratio for the association between the LKB1 and disease-free survival (DFS) and progression-free survival (PFS) in patients with lung cancer.
3.4. High LKB1 expression and clinicopathological characteristics in lung cancer
To systematically analyzed the role of LKB1 expression as a biomarker in lung cancer, we explored the correlation between low expression of LKB1 and clinicopathological characteristics. A total of 7 studies described the association between LKB1 expression and clinicopathological factors, including age, gender, histological differentiation, nodal metastasis, smoking, tumor stage, histopathological stage (Table 3). Low expression of LKB1 was association with histological differentiation (poor vs. moderate or well, OR = 4.135, 95% CI:2.524–6.774, P = .000), nodal metastasis (absent vs present, OR = 0.503, 95% CI:0.303–0.835, P = .008), smoking (yes vs no, OR = 1.765, 95% CI:1.120–2.782, P = .014). However, LKB1 expression had no significant association with age (<60 vs ≥60, OR = 1.073, 95% CI:0.639–1.800, P = .790), gender (male vs. female, OR = 0.997, 95% CI: 0.756–1.314, P = .981), histopathological stage (I-II vs III-IV, OR = 0.814, 95% CI: 0.596–1.112, P = .196), tumor stage (T1-T2 vs T3-T4, OR = 0.729, 95% CI: 0.262–2.029, P = .545) (Table 3).
Table 3.
Meta-analysis of reported clinicopathological characteristics in the included studies.
| Test for heterogeneity | ||||||
| Parameters | Number of studies | OR (95%CI) | P value | I2(%) | P | Statistic model |
| Age (<60 vs ≥60) | 4 | 1.073 (0.639–1.800) | .790 | 50.20 | .110 | Random |
| Gender (male vs female) | 8 | 0.997 (0.756–1.314) | .981 | 33.40 | .161 | Fixed |
| Smoking (yes vs no) | 4 | 1.765 (1.120–2.782) | .014 | 49.70 | .113 | Fixed |
| Histological differentiation (poor vs moderate or well) | 3 | 0.814 (0.596–1.112) | .196 | 0.00 | .712 | Fixed |
| Nodal metastasis (absent vs present) | 7 | 0.503 (0.303–0.835) | .008 | 63.00 | .013 | Random |
| Histopathological stage (I-II vs III-IV) | 7 | 0.814 (0.596–1.112) | .196 | 47.40 | .007 | Random |
| Tumor stage (T1-T2 vs T3-T4) | 4 | 0.729 (0.262–2.029) | .545 | 76.80 | .005 | Random |
3.5. Sensitivity analysis
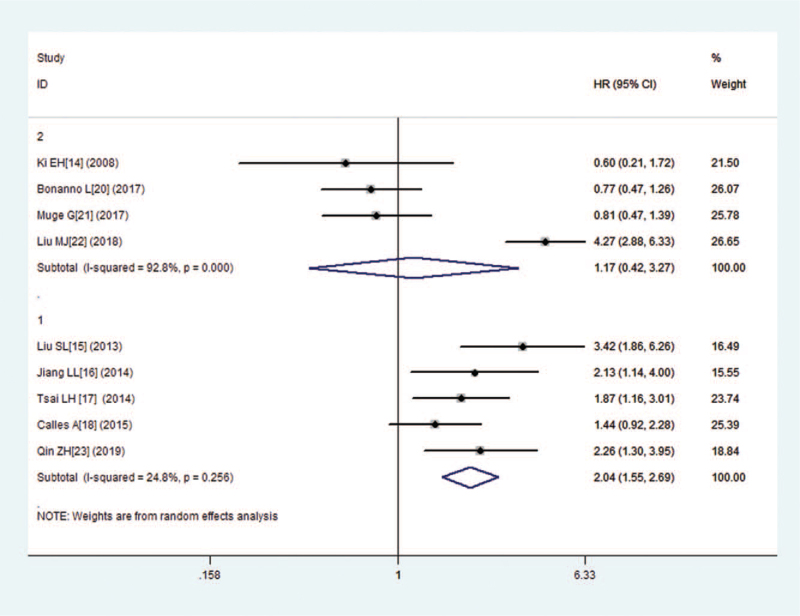
Sensitivity analysis was used to explore the potential heterogeneity within the eligible studies of OS analysis (Fig. 5). Each of the articles were successively excluded to judge the robustness of the pooled results. However, the results shown that were significant heterogeneity. According to the OS analysis, the heterogeneity test found no significant heterogeneity after excluding four studies[14,20–22] (I2 = 24.8%, P = .256). The pooled HR for OS in patients with high versus low expression of LKB1was 2.044 (95% CI: 1.551–2.694, P = .000), suggesting a poor prognostic role of LKB1 expression. Therefore, we must be careful in drawing a conclusion regarding with OS.
Figure 5.
Sensitivity analysis of the association between LKB1and overall survival.
3.6. Publication bias
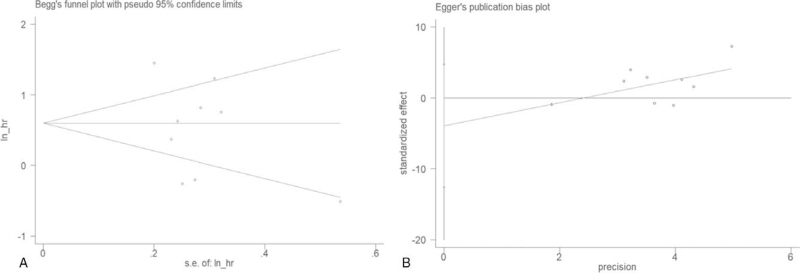
A potential publication bias was detected by Begg's test and Egger's test. Our findings with Begg's test (p = 0.917) and Egger's test (p = 0.318) implied no publication bias (Fig. 6).
Figure 6.
Funnel plots for detecting publication bias in terms of survival data. (A) Begg's funnel plot using data of overall survival to detect publication bias; (B) Egger's funnel plot using data of overall survival to detect publication bias.
4. Discussion
The cancer suppressor LKB1 is an essential serine/threonine kinase, which induces multifarious cellular processes such as cell metabolism, cell proliferation and cell migration.[25] Somatic mutations or loss-of-function alterations of LKB1 were found in different tumor types, such as cervical carcinoma, breast cancer, pancreatic cancer and non-small-cell lung cancer (NSCLC).[26–30] What's more, LKB1 is the most commonly mutated genes in NSCLC and approximately 30–35% of lung adenocarcinomas loss of the function occurring.[31] Though emergence of chemotherapy immunotherapy and targeted therapy are developing, lung cancer still a huge threat for human health due to the drug resistance and metastasis. A previous study has provided that LKB1 loss triggers complex changes in tumor microenvironment, suggesting a potential role in the response to anti-angiogenic treatment.[32] A number of articles have reported the prognostic value of LKB1 expression in tumors among patients with lung cancer and the results remain controversial. Thus, it is urgent to seek available biomarkers for early tumors detection and prognosis evaluation.
Recently, more and more attention is focused on immunotherapy targets in treating lung cancer, which have showed promising outcomes. LKB1 was deemed to be a new biomarkers in immunological therapy with the growing recognition of LKB1and its metabolic pathways.[33] The researches have showed that LKB1 directly phosphorylates and activates AMPK, which works as a master sensor of cellular growth and proliferation.[34,35] A novel set of findings were presented which remind that not only oncogene driver mutations but also tumor-suppressor gene mutations can modify the immune microenvironment in lung cancer.[36] Furthermore, the data have indicated that LKB1 mutation in NSCLC conferred enhanced radio sensitization in combination with trametinib, suggesting LKB1 mutation as a biomarker for patient's trametinib and radiotherapy combination therapy.[37] A previous study has provided certain information regarding the prognostic value of LKB1 in patients with solid tumours.[38,39] However, no meta-analysis have been performed to evaluate the prognostic value of LKB1 expression in lung cancer. This meta-analysis is aimed to investigate the effect of LKB1 expression on the prognosis and clinicopathological characteristics in lung cancer.
This meta-analysis included 11 eligible articles with a total of 1507 patients. We found that low expression of LKB1 may be an indicator of poor prognosis for lung cancer patients. Furthermore, we performed subgroup analysis on country and cancer type. The results showed that no significant association was found between low expression of LKB1 expression and OS in lung adenocarcinoma carcinoma and other types, in Asian studies and non-Asian studies. Our results showed that there were no association between low expression of LKB1 and DFS/PFS. Concerning clinicopathologic factors, low expression of LKB1 was associated with histological differentiation, nodal metastasis, and smoking. However, LKB1 expression had no significant association with age, gender, histopathological stage, and tumor stage.
The results of our meta-analysis should be interpreted with caution given several limitations. First, all included studies were published in the English language which may lead to publication bias. Secondly, although the Begg's test and Egger's tests revealed no publication bias, most eligible articles were from Asia, which may lead to publication bias. Thirdly, sensitivity analyses revealed that the correlation between LKB1 over expression and OS was unstable, which might be explained by the small sample sizes. Therefore, we must be careful in drawing a conclusion regarding the prognostic significance of LKB1 in lung cancer.
5. Conclusions
In conclusion, this meta-analysis suggested that low expression of LKB1 may predict unfavorable prognosis, worse histological differentiation and earlier nodal metastasis of lung cancer. Furthermore, high quality and multicenter studies should be carried out to clarify the effect of LKB1 expression in lung cancer.
Author contributions
Conceptualization: Chenggong Wei.
Data curation: Chunxuan Lin, Xiaochun Lin, Kunpeng Lin, Jialiang Tan.
Formal analysis: Chunxuan Lin, Xiaochun Lin, Kunpeng Lin.
Investigation: Chunxuan Lin, Xiaochun Lin, Kunpeng Lin.
Methodology: Jialiang Tan, Taisheng Liu.
Project administration: Taisheng Liu, Chenggong Wei.
Software: Chunxuan Lin.
Supervision: Taisheng Liu.
Writing – original draft: Chunxuan Lin, Xiaochun Lin, Kunpeng Lin.
Writing – review & editing: Taisheng Liu, Chenggong Wei.
Footnotes
Abbreviations: ADA = adenocarcinoma, F = female, HR = hazard ratio, IHC = immunohistochemistry, LKB1 = liver kinase B1, M = male, N = Number of patient, N.A. = not available, NSCLC = non-small cell lung cancer, OS = overall survival, PFS = progression-free survival, RFS = relapse-free survival, SqCC = squamous cell carcinoma.
How to cite this article: Lin C, Lin X, Lin K, Tan J, Liu T, Wei C, Liu T. LKB1 expression and the prognosis of lung cancer: A meta-analysis. Medicine. 2021;100:46(e27841).
CL, XL and KL contributed equally to this work.
The authors have no funding to disclose.
The authors have no conflicts of interest to disclose.
Ethics: Ethical approval is not required, because there is no patient recruitment or personal information collection, and the included data in our study was extracted from published literatures.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299–311. [DOI] [PubMed] [Google Scholar]
- [2].Steven A, Fisher SA, Robinson BW. Immunotherapy for lung cancer. Respirology 2016;21:821–33. [DOI] [PubMed] [Google Scholar]
- [3].Hoffman RM, Sanchez R. Lung cancer screening. The medical clinics of North America 2017;101:769–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Sousa VML, Carvalho L. Heterogeneity in lung cancer. J Immunopathol 2018;85:96–107. [DOI] [PubMed] [Google Scholar]
- [5].Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res 2016;170:47–75. [DOI] [PubMed] [Google Scholar]
- [6].Bianco A, Perrotta F, Barra G, et al. Prognostic factors and biomarkers of responses to immune checkpoint inhibitors in lung cancer. Int J Mol Sci 2019;20:4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ciccarese F, Zulato E, Indraccolo S. LKB1/AMPK pathway and drug response in cancer: a therapeutic perspective. Longevity OMAC 2019;2019:8730816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hollstein PE, Eichner LJ, Brun SN, et al. The AMPK-related kinases SIK1 and SIK3 mediate key tumor-suppressive effects of LKB1 in NSCLC. Cancer Discov 2019;9:1606–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang W, Li X, Song G, et al. Prognostic significance of LKB1 promoter methylation in cutaneous malignant melanoma. Oncol Lett 2017;14:2075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 2018;8:822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen Z, Li JL, Lin S, et al. cAMP/CREB-regulated LINC00473 marks LKB1-inactivated lung cancer and mediates tumor growth. JClin Invest 2016;126:2267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [14].Hwang K, Jo H-J, Lee K, et al. The clinical and histopathologic features according to loss of LKB1 protein expression on primary lung cancer. Tuberc Respir Dis 2008;64. [Google Scholar]
- [15].Liu S, Miao Y, Fan C, et al. Clinicopathologic correlations of liver kinase B1, E-cadherin, and N-cadherin expression in non-small cell lung cancer. Appl Immunohistochem Mol Morphol 2013;21:334–40. [DOI] [PubMed] [Google Scholar]
- [16].Jiang L, Liang X, Liu M, et al. Reduced expression of liver kinase B1 and Beclin1 is associated with the poor survival of patients with non-small cell lung cancer. Oncol Rep 2014;32:1931–8. [DOI] [PubMed] [Google Scholar]
- [17].Tsai LH, Chen PM, Cheng YW, et al. LKB1 loss by alteration of the NKX2-1/p53 pathway promotes tumor malignancy and predicts poor survival and relapse in lung adenocarcinomas. Oncogene 2014;33:3851–60. [DOI] [PubMed] [Google Scholar]
- [18].Calles A, Sholl LM, Rodig SJ, et al. Immunohistochemical loss of LKB1 is a biomarker for more aggressive biology in KRAS-mutant lung adenocarcinoma. Clin Cancer Res 2015;21:2851–60. [DOI] [PubMed] [Google Scholar]
- [19].Li Y, Li Y, Yang H, et al. Expressions and significance of PTEN and LKB1 in non-small cell lung cancer. Sichuan da xue xue bao 2016;47:507–11. [PubMed] [Google Scholar]
- [20].Bonanno L, De Paoli A, Zulato E, et al. LKB1 expression correlates with increased survival in patients with advanced non-small cell lung cancer treated with chemotherapy and bevacizumab. Clin Can Res 2017;23:3316–24. [DOI] [PubMed] [Google Scholar]
- [21].Çeliktas M, Tanaka I, Tripathi SC, et al. Role of CPS1 in cell growth. Metabolism and prognosis in LKB1-inactivated lung adenocarcinoma. J Natl Cancer Inst 2017;109:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu M, Jiang L, Fu X, et al. Cytoplasmic liver kinase B1 promotes the growth of human lung adenocarcinoma by enhancing autophagy. Cancer Sci 2018;109:3055–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Qin Z, Xie R, Zhang W, et al. Liver kinase B1 correlates with prognosis and epithelial-mesenchymal transition of resectable early stage non-small cell lung cancer. Transl Cancer Res 2020;9:639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mitchell KG, Parra ER, Zhang J, et al. LKB1/STK11 expression in lung adenocarcinoma and associations with patterns of recurrence. Ann Thorac Surg 2020;110:1131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kullmann L, Krahn MP. Controlling the master-upstream regulation of the tumor suppressor LKB1. Oncogene 2018;37:3045–57. [DOI] [PubMed] [Google Scholar]
- [26].Cui X, Wang X, Zhou X, et al. miR-106a regulates cell proliferation and autophagy by targeting LKB1 in HPV-16-associated cervical cancer. Mol Cancer Res 2020;18:1129–41. [DOI] [PubMed] [Google Scholar]
- [27].Tanwar PS, Mohapatra G, Chiang S, et al. Loss of LKB1 and PTEN tumor suppressor genes in the ovarian surface epithelium induces papillary serous ovarian cancer. Carcinogenesis 2014;35:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morton JP, Jamieson NB, Karim SA, et al. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology 2010;139:586–97. 597 e581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sengupta S, Nagalingam A, Muniraj N, et al. Activation of tumor suppressor LKB1 by honokiol abrogates cancer stem-like phenotype in breast cancer via inhibition of oncogenic Stat3. Oncogene 2017;36:5709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lattouf H, Kassem L, Jacquemetton J, et al. LKB1 regulates PRMT5 activity in breast cancer. Int J Cancer 2019;144:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kaufman JM, Amann JM, Park K, et al. LKB1 Loss induces characteristic patterns of gene expression in human tumors associated with NRF2 activation and attenuation of PI3K-AKT. J Thorac Oncol 2014;9:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bonanno L, Zulato E, Pavan A, et al. LKB1 and tumor metabolism: the interplay of immune and angiogenic microenvironment in lung cancer. Int J Mol Sci 2019;20:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Santarpia M, Aguilar A, Chaib I, et al. Non-small-cell lung cancer signaling pathways, metabolism, and PD-1/PD-L1 antibodies. Cancers 2020;12:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Galan-Cobo A, Sitthideatphaiboon P, Qu X, et al. LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS-mutant lung adenocarcinoma. Cancer Res 2019;79:3251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rodón L, Svensson RU, Wiater E, et al. The CREB coactivator CRTC2 promotes oncogenesis in LKB1-mutant non-small cell lung cancer. Science Adv 2019;5:eaaw6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res 2016;76:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Y, Li N, Jiang W, et al. Mutant LKB1 confers enhanced radiosensitization in combination with trametinib in KRAS-mutant non-small cell lung cancer. Clinical Cancer Res 2018;24:5744–56. [DOI] [PubMed] [Google Scholar]
- [38].Ren YH, Zhao FJ, Mo YH, et al. Association between LKB1 expression and prognosis of patients with solid tumours: an updated systematic review and meta-analysis. BMJ Open 2019;9:e027185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xiao J, Zou Y, Chen X, et al. The prognostic value of decreased LKB1 in solid tumors: a meta-analysis. PLoS One 2016;11:e0152674. [DOI] [PMC free article] [PubMed] [Google Scholar]