Abstract
Background:
An increase in awareness of plant-based diets has brought forth numerous studies on bone mineral density (BMD). The present systematic review and meta-analysis was designed to compare the effect between plant-based diets and omnivores on female BMD.
Methods:
We searched the Cochrane Library, PubMed, EMBASE, and Web of Science and up to July 1, 2020. Mean difference (MD) with its 95% confidence interval (CI) was estimated to compare the outcomes of the groups. We compared BMD at the lumbar spine, femoral neck and whole body respectively between plant-based diets and omnivores. In addition, we performed subgroup analyses according to different clinical characteristics for further exploration. Two reviewers assessed trial quality and extracted data independently. All statistical analyses were performed using standard statistical procedures provided in Review Manager 5.2.
Results:
A total of 17 cross-sectional studies including 13,888 patients were identified for the present meta-analysis. Our pooled result indicated that population with plant-based diets had lower BMD than omnivores at the lumbar spine (MD −0.03; 95% CI −0.04 to −0.02; P < .0001), femoral neck (MD −0.04; 95% CI −0.05 to −0.03; P < .00001) and whole body (MD −0.04; 95% CI −0.06 to −0.01; P = .01), respectively. Further exploration indicated that especially females with plant-based diets experienced significantly lower BMD at lumbar spine (MD −0.03; 95% CI −0.04 to −0.02; 3173 pts), femoral neck (MD −0.04; 95% CI −0.05 to −0.03; 10,656 pts) and whole body (MD −0.05; 95% CI −0.10 to −0.00; P = .04). In addition, we performed subgroup analyses and found lower BMD at lumbar spine and femoral neck in both vegetarians and vegans.
Conclusions:
The present meta-analysis indicated that plant-based diets may be correlated with lower BMD of women when compared with omnivore population. However, this does not diminish the fact that a plant-based diet can be a harmful option to the overall bone health of population and more prospective researches are needed to clear the impact of plant-based diets on bone health.
Keywords: bone health, bone mineral density, meta-analysis, plant-based diets
1. Introduction
Vegetarian diets continue to gain popularity given the widespread concerns about environmental sustainability and the belief that a healthier diet prevents chronic disease development.[1–3] Also, large populations adhere to vegetarian or vegan diets for cultural or religious reasons.[4] Many studies on the effects of such diets on bone health have appeared.[5–7] In the USA, osteoporosis was associated with low bone mineral density (BMD) and was evident in 5% of men and 25% of women aged >65 years.[8,9] Osteoporosis is responsible for >8.9 million fractures annually worldwide, increasing morbidity and mortality and imposing large economic burdens.[8,9] Therefore, identifying and addressing factors associated with poor bone health is a public health imperative. Meta-analyses have suggested that vegetarians and vegans exhibit lower BMDs and a higher risk of fractures than omnivores.[10,11]
Consistent with other reports,[10,11] adult respondents to the 2007 to 2010 NHANES surveys who self-identified as vegetarians (including vegans) exhibited significantly lower BMDs than nonvegetarians.[12] However, further analysis indicated that the lower BMD at the hip (the femur and femoral neck) was attributable to a smaller body size when anthropometric (body mass index [BMI] and waist circumference) corrections were applied.[13] This was not the case for the vegetarian lumbar spine BMD, which remained significantly different from that of nonvegetarians after adjustment for anthropometric variables. Also, and importantly, the small lumbar spine BMD difference was of minimal clinical significance, being only <0.05 g/cm2 (< 3%).[13,14]
In addition, several reviews have examined the current literatures regarding the evidence of the association between plant-based diets and low BMD.[15–17] However, their conclusions still remained inconsistency. Hsu (2020)[15] considered that insufficient calcium and/or vitamin D intake was detrimental to bone metabolism and bone health and, with planning and a balanced diet, vegans can obtain healthy levels of calcium and vitamin. In contrast, Iguacel (2019)[11] indicated that vegetarian and vegan diets should be planned to avoid negative consequences on bone health, because compared with omnivores, vegetarians, and vegans had lower BMD at the femoral neck and lumbar spine and vegans also had higher fracture rates. Further, the influence of plant-based diets in different sites like lumbar spine, femoral neck, and whole body are still unclear. Thus, we designed this systematic review and meta-analysis focused on the effect of plant-based diets on different sites of body BMD comprehensively. In addition, we conducted subgroup analysis in order to explore the factors of BMD including diets, age, detection instruments, gender, and population ethnicity.
2. Methods and materials
2.1. Criteria for considering studies
We included studies if they meet the following criteria: an observational study (prospective or retrospective) comparing plant-based and omnivorous diets; a human study; and, BMD measurement via imaging. The exclusion criteria were: inclusion of subjects with bone metabolic diseases; an experimental trial involving animals, or any other nonhuman study; an abstract, letter, editorial, expert opinion, review, or case report; and, a study lacking sufficient data or that did not meet any inclusion criterion.
The present study was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou University of Chinese Medicine.
2.2. Search strategy
We searched the Cochrane Library, PubMed, EMBASE, and Web of Science up to July 1, 2020. Our strategy was based on combinations of medical subject headings and the keywords:“plant”, “Vegan”, “Vegans”, “Vegetarians”, “Vegetarian”, “Bone”, “bone mineral density”, and “Osteoporosis”. Two assessors independently screened the titles and abstracts of each study. Once relevant studies became certain, the full texts were obtained for further evaluation. Other related references we read were also searched online for full texts and assessment, once any of them meet our including criteria, they will also be included in this meta-analysis.
2.3. Quality assessment and data extraction
Two reviewers assessed the quality of each included study using the previously validated 11-item checklist which was recommended by Agency for Healthcare Research and Quality (AHRQ).[18] An item would be scored “0” if it was answered “NO” or “UNCLEAR”; if it was answered “YES”, then the item scored “1”. Article quality was assessed as follows: low quality = 0 to 3; moderate quality = 4 to 7; high quality = 8 to 11. In addition, the risk of bias for each study and the risk of bias across all studies were evaluated and shown with figures generated by RevMan 5.2 software (Copenhagen, Denmark).[19]
Data for the comparative analysis of plant-based diets versus omnivores for BMD were extracted independently by 2 reviewers, and disagreement was resolved through discussion. The extracted contents, including first authors, published years, country, sample size, interventions, baseline, and age of each study, were displayed using a standardized form. Data collected were input into RevMan 5.2 software for analysis.[19]
2.4. Statistical analysis
The data of comparable outcomes between plant-based diets and omnivores were combined-analyzed, using the standard statistical procedures provided in RevMan 5.2.[19] We compared BMD at the lumbar spine, femoral neck, and whole body respectively between plant-based diets and omnivores. In addition, we performed subgroup analyses according to different clinical characteristics for further exploration. Mean difference (MD) with its 95% confidence interval (CI) was estimated to compare the outcomes of the groups. The heterogeneity between studies was evaluated by the chi-square-based Q statistical test,[20] with Ph value, and I2 statistic, ranging from 0% to 100%, to quantify the effect of heterogeneity. Ph ≤ 0.10 was deemed to represent significant heterogeneity,[21] and pooled estimates were estimated using a random-effect model (the DerSimonian and Laird[22] method). On the contrary, if statistical study heterogeneity was not observed (Ph > 0.10), a fixed effects model (the Mantel–Haenszel[23] method) was used. The effects of outcome measures were considered to be statistically significant if pooled MDs with 95% CI did not overlap with 0.
This work has been reported in line with preferred reporting items for systematic reviews and meta-analyses[24] and Assessing the methodological quality of systematic reviews Guidelines.[25] Our research was registered in “Research registry”.
3. Results
3.1. Included studies, study characteristics, and quality assessment
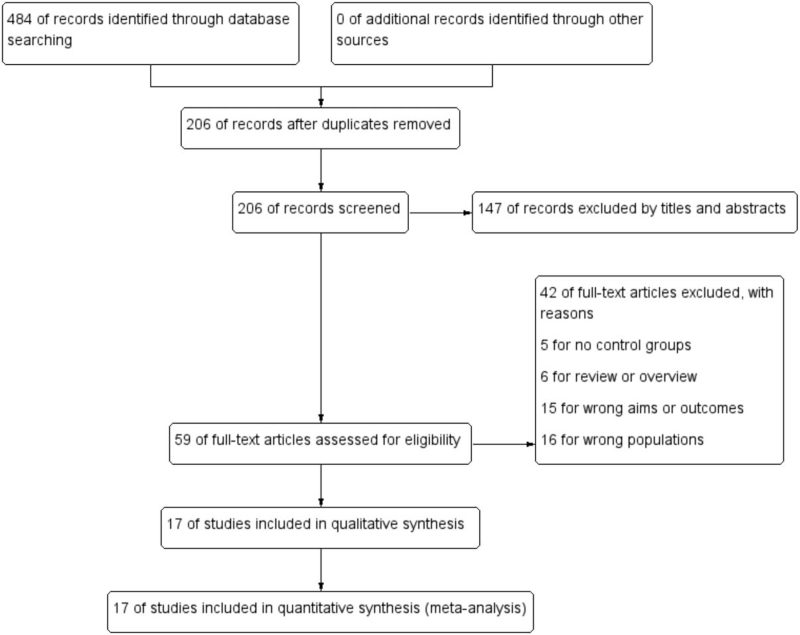
At the beginning of the search, a total of 484 records of citations were obtained; 206 of records were obtained further after duplicates were removed. Via screening the titles and abstracts, 147 studies were excluded preliminarily and then 59 studies were chosen to get full texts for further evaluation. After reading the full texts of the 59 studies, 42 studies were excluded further (16 studies for wrong populations, 6 for reviews, 5 for no controls, and 15 for wrong aims). Finally, 17 cross-sectional studies[26–41] including 13,888 subjects were reviewed and meta-analyzed. The detailed search process and summary of studies were shown in the study flow diagram (Fig. 1). Of these studies, 14 studied BMD at lumbar spine, 9 studied BMD at femoral neck, and 5 studied BMD at whole body. Five studies came from China and 4 from USA. Nine study populations were Caucasian and 7 Asian. Twelve studies measured BMD via dual-energy X-ray absorptiometry (DXA) and 4 via dual-photon absorptiometry (DPA). The other characteristics of each study were shown in Table 1.
Figure 1.
Flow diagram of literature search and selection of included studies for meta-analysis.
Table 1.
The characteristics of included studies for this meta-analysis.
| Omnivores | Plant-based diets | ||||||||||
| Study | Country | No. | Age (M ± SD) | BMD | No. | Age (M ± SD) | BMD | BMD site | Gender | Ethnicity | Instruments |
| Barr et al (1998) | Canada | 22 | 27.9 ± 5.9 | 1.216 ± 0.132 | 15 8 | 25.8 ± 4.7 28.0 ± 3.2 | 1.145 ± 0.117 1.153 ± 0.100 | LS | Female | Caucasian | DXA |
| Chiu et al (1997) | Taiwan, China | 187 | 59.5 ± 8.0 | 0.990 ± 0.170 0.750 ± 0.130 | 71 | 64.0 ± 11.5 | 0.940 ± 0.190 0.690 ± 0.110 | LS FN | Female | Asian | DPA |
| Fontana et al (2005) | USA | 7 | 53.2 ± 4.1 | 1.030 ± 0.140 | 7 | 56.5 ± 13.1 | 0.850 ± 0.080 | LS | Female | Caucasian | DXA |
| 0.750 ± 0.080 | 0.630 ± 0.100 | FN | |||||||||
| 1.100 ± 0.100 | 0.990 ± 0.006 | WB | |||||||||
| Ho-Pham et al (2009) | Vietnam | 105 | 62.0 ± 10.0 | 0.770 ± 0.140 | 105 | 62.0 ± 10.0 | 0.740 ± 0.140 | LS | Female | Asian | DXA |
| 0.630 ± 0.110 | 0.620 ± 0.110 | FN | |||||||||
| 0.900 ± 0.120 | 0.880 ± 0.110 | WB | |||||||||
| Karavasiloglou et al (2020) | Germany | 9209 | 47.0 ± 0.4 | 0.94 ± 0.110 0.80 ± 0.120 | 207 | 44.4 ± 1.3 | 0.90 ± 0.120 0.75 ± 0.130 | LS FN | Male female | Multi-race/ethnicity | DXA |
| Kim et al (2007) | Korea | 76 | 60.8 ± 6.7 | 0.809 ± 0.158 | 76 | 60.7 ± 6.9 | 0.806 ± 0.140 | LS | Female | Asian | DXA |
| 0.711 ± 0.112 | 0.684 ± 0.144 | FN | |||||||||
| Knurick et al (2015) | USA | 27 | 27.2 ± 6.7 | 1.180 ± 0.110 | 27 | 31.1 ± 9.1 | 1.120 ± 0.100 | WB | Female Male | Caucasian | DXA |
| 28 | 33.9 ± 8.6 | 1.130 ± 0.110 | |||||||||
| Krivoskova et al (2010) | Slovakia | 131 | 40.8 ± 19.8 | 1.102 ± 0.159 | 141 | 41.9 ± 19.7 | 1.085 ± 0.192 | LS | Female | Caucasian | DXA |
| 0.941 ± 0.136 | 0.918 ± 0.142 | FN | |||||||||
| Kaur (2013) | India | 46 | 45.0 ± 80.0 | 0.888 ± 0.140 | 204 | 45.0 ± 80.0 | 0.872 ± 0.118 | LS | Female | Caucasian | DXA |
| Lloyd et al (1991) | USA | 36 | 36.1 ± 0.4 | 1.006 ± 0.120 | 23 | 35 ± 0.7 | 1.020 ± 0.096 | LS | Female | Caucasian | DPA |
| Lau et al (1998) | Hong Kong, China | 109 | 77.0 ± 3.8 | 0.720 ± 0.140 | 40 | 79.9 ± 5.4 78.2 ± 4.9 | 0.680 ± 0.110 0.720 ± 0.150 | LS | Female | Asian | DPA |
| 0.530 ± 0.082 | 36 | 0.480 ± 0.080 0.500 ± 0.080 | FN | ||||||||
| Outila et al (2000) | Finland | 16 | 34.0 ± 7.0 | 1.177 ± 0.099 | 6 | 33.0 ± 9.0 | 1.138 ± 0.060 1.034 ± 0.174 | LS | Female | Caucasian | DXA |
| 0.999 ± 0.138 | 37.0 ± 7.0 | 0.961 ± 0.059 0.843 ± 0.116 | FN | ||||||||
| Siani et al (2003) | Italy | 10 | 38.4 ± 7.8 | 1.190 ± 0.110 | 20 | 34.8 ± 15.1 | 1.190 ± 0.070 | WB | Female Male | Caucasian | DXA |
| Tesar et al (1992) | USA | 28 | 62.9 ± 5.6 | 1.066 ± 0.155 | 28 | 62.9 ± 5.1 | 1.079 ± 0.203 | LS | Female | Caucasian | DPA |
| Wang et al (2008) | Taiwan, China | 529 | 21.0 ± 89.0 | 0.968 ± 0.183 | 489 | 21.0 ± 89.0 | 0.953 ± 0.179 | LS | Female | Asian | DXA |
| 463 | 0.829 ± 0.142 | 383 | 0.813 ± 0.127 | FN | Male | ||||||
| Xie et al (2019) | China | 246 | 32.1 ± 6.5 | 1.519 ± 0.331 | 246 | 32.7 ± 6.5 | 1.519 ± 0.310 | WB | Female Male | Asian | DPA |
| Ying-Ming and Liu (2010) | China | 302 | 50.0 ± 70.0 | 0.837 ± 0.140 | 173 | 50.0 ± 70.0 | 0.795 ± 0.140 | LS | Female | Asian | DXA |
BMD = bone mineral density, DPA = dual-photon absorptiometry, DXA = dual-energy X-ray absorptiometry, FN = femoral neck, LS = lumbar spine, M = mean, NR = not report, SD = standard deviation, WB = whole body.
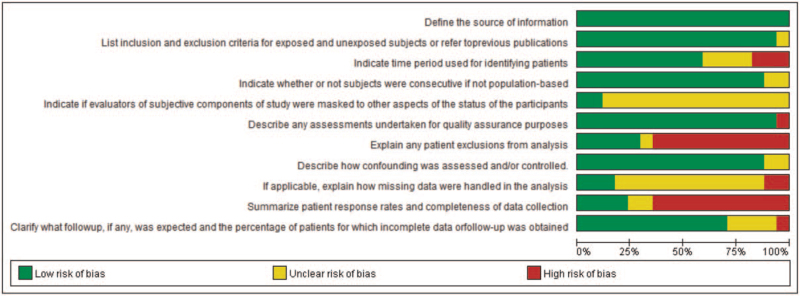
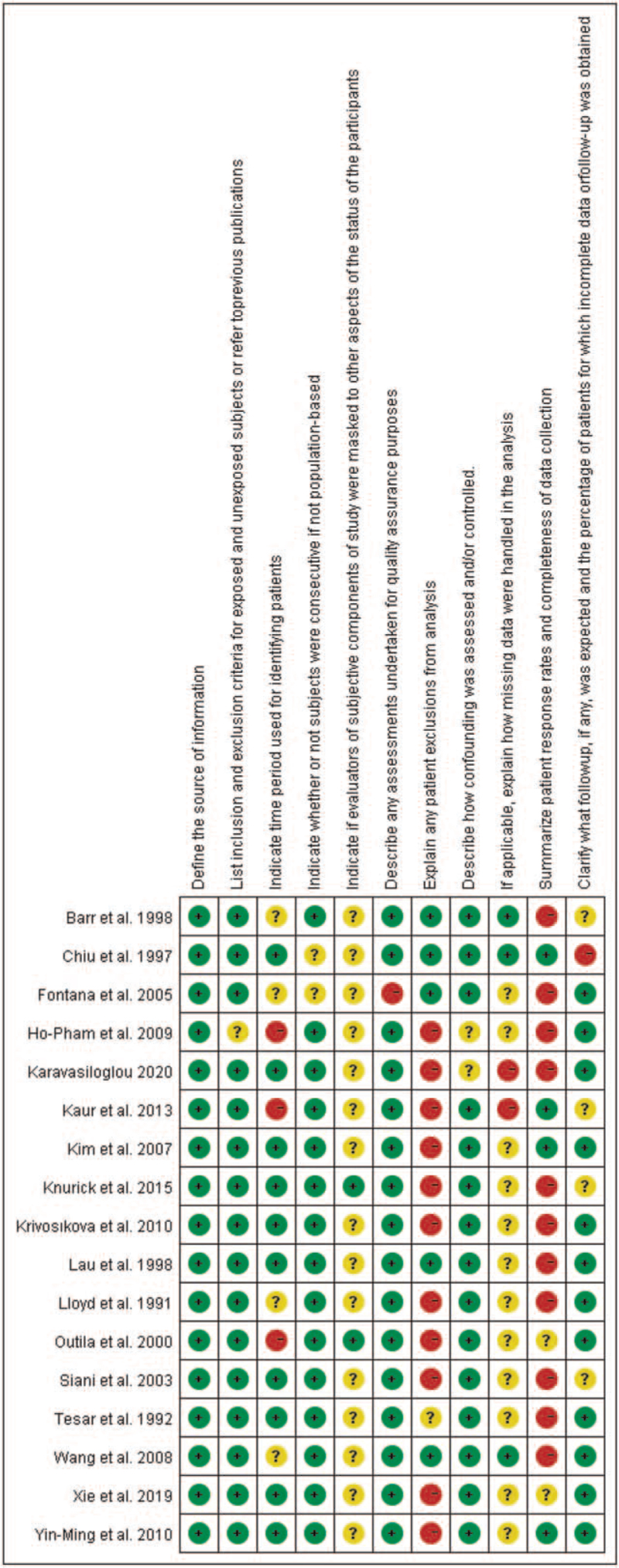
According to our definitions, there was no low quality studies included in this analysis. Eleven studies were assessed as moderate quality (64.7%) and 6 studies were evaluated as high quality. Additionally, risk-of-bias graphs were generated to further identify the risk of bias of the including studies. The risk of bias for each RCT was presented as percentages across all included studies, and the risk-of-bias item for each included study was displayed (Figs. 2 and 3). The risk-of-bias graphs indicated generally low risk of bias in the AHRQ item of “Define the source of information (survey, record review)”, “List inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications”, “Indicate whether or not subjects were consecutive if not population-based”, “Describe any assessments undertaken for quality assurance purposes”, and “Describe how confounding was assessed and/or controlled”. High risk of bias was mainly observed in item of “Explain any patient exclusions from analysis” and “Summarize patient response rates and completeness of data collection”. An unclear risk of bias was mainly observed in “Indicate if evaluators of subjective components of study were masked to other aspects of the status of the participants” and “If applicable, explain how missing data were handled in the analysis”. Table S1, Supplemental Digital Content.
Figure 2.
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
3.2. Effect of plant-based diets on BMD at the lumbar spine
A total of 14 studies with 17 sets of data compared the effect of plant-based diets and omnivores on lumbar spine BMD. Our results showed that plant-based diets population experienced lower BMD than omnivores at the lumbar spine, with a pooled MD of −0.04 (95% CI −0.05 to −0.03; P < .00001) (Fig. 4). The pooled analysis was performed using a fixed-effect model because no significant in-study heterogeneity was observed (P < .18 and I2 = 24%). Subgroup analysis found significantly lower BMD in both vegetarians (MD −0.02; 95% CI −0.04 to −0.01; 2490 pts) and vegans patients (MD −0.04; 95% CI −0.05 to −0.03; 10099 pts), respectively (Fig. 4).
Figure 4.
Forest plot of effect of vegetarian diets on bone mineral density at the lumbar spine. CI = confidence interval, SD = standard deviation.
In addition, we further conducted subgroup analysis with the following categories: year, age, study quality, instruments, ethnicity, sample of size, and gender. As showing in Table 2, significant reduction of BMD at the lumbar spine was found in the following subgroups: before 2000 (MD −0.03; 95% CI −0.05 to −0.00; P = .02) and after 2000 (MD −0.03; 95% CI −0.04 to −0.02; P < .0001); mean age ≥ 50 year (MD −0.03; 95% CI −0.04 to −0.02; P < .0001); high study quality (MD −0.04; 95% CI −0.05 to −0.02; P < .0001); DXA (MD −0.03; 95% CI −0.04 to −0.02; P < .0001); Caucasian (MD −0.03; 95% CI −0.05 to −0.01; P = .01) and Asian (MD −0.03; 95% CI −0.04 to −0.01; P < .0001); sample size ≤ 100 pts (MD −0.05; 95% CI −0.01 to 0.00; P = .03) and >100 pts (MD −0.03; 95% CI −0.04 to −0.02; P < .0001); female (MD −0.03; 95% CI −0.04 to −0.02; P < .0001), and male/female (MD −0.04; 95% CI −0.06 to −0.02; P < .0001). However, no significant difference was found in subgroup of mean age <50 year (MD −0.03; 95% CI −0.06 to 0.00; P = .08), moderate quality studies (MD −0.02; 95% CI −0.04 to 0.00; P = .08), and DPA (MD −0.02; 95% CI −0.04 to 0.00; P = .07), respectively (Table 2).
Table 2.
Subgroup analyses of the effect of plant-based diets on bone mineral density at the lumbar spine.
| Pooled results | Heterogeneity | |||||
| Subgroups | No. of study/pts | MD | 95% CI | P-value | I 2 | Analytical effect model |
| Year | ||||||
| Before 2000 | 7/738 | −0.03 | −0.05 to −0.00 | .02 | 10% | Fixed effect model |
| After 2000 | 10/11,851 | −0.03 | −0.04 to −0.02 | <.0001 | 37% | Fixed effect model |
| Mean age | ||||||
| <50 yr | 6/185 | −0.03 | −0.06 to 0.00 | .08 | 29% | Fixed effect model |
| ≥50 yr | 11/12,147 | −0.03 | −0.04 to −0.02 | <.0001 | 31% | Fixed effect model |
| Quality | ||||||
| Moderate | 7/2016 | −0.02 | −0.04 to 0.00 | .08 | 51% | Random-effect model |
| High | 10/10,085 | −0.04 | −0.05 to −0.02 | <.0001 | 0% | Fixed effect model |
| Instruments | ||||||
| DXA | 11/11,646 | −0.03 | −0.04 to −0.02 | <.0001 | 34% | Fixed effect model |
| DPA | 6/943 | −0.02 | −0.04 to 0.00 | .07 | 0% | Fixed effect model |
| Ethnicity | ||||||
| Caucasian | 9/762 | −0.03 | −0.05 to −0.01 | .01 | 42% | Fixed effect model |
| Asian | 7/2411 | −0.03 | −0.04 to −0.01 | <.0001 | 0% | Fixed effect model |
| Sample size | ||||||
| ≤100 pts | 7/240 | −0.05 | −0.01 to 0.00 | .03 | 53% | Random-effect model |
| >100 pts | 10/12,349 | −0.03 | −0.04 to −0.02 | <.0001 | 0% | Fixed effect model |
| Gender | ||||||
| Female | 9/3173 | −0.03 | −0.04 to −0.02 | <.0001 | 22% | Fixed effect model |
| Male/female | 1/9416 | −0.04 | −0.06 to −0.02 | <.0001 | – | – |
CI = confidence intervals, DPA = dual-photon absorptiometry, DXA = dual-energy X-ray absorptiometry, MD = mean difference.
3.3. Effect of plant-based diets on BMD at the femoral neck
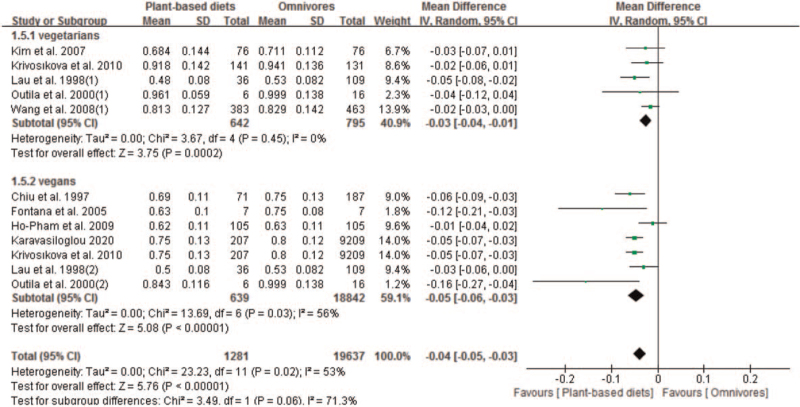
We included 10 studies with 12 sets of data that compared the effect of plant-based diets and omnivores on femoral neck BMD. Our results showed that plant-based diets population experienced lower BMD than omnivores at the femoral neck, with a pooled MD of −0.04 (95% CI −0.05 to −0.03; 20,918 pts). The pooled analysis was performed using a random-effect model because a significant in-study heterogeneity was observed (P = .02 and I2 = 53%) (Fig. 5). Subgroup analysis found significantly lower BMD in both vegetarians (MD −0.03; 95% CI −0.04 to −0.01; P = .0002) and vegans patients (MD −0.05; 95% CI −0.06 to −0.03; P < .00001), respectively (Fig. 5).
Figure 5.
Forest plot of effect of vegetarian diets on bone mineral density at the femoral neck. CI = confidence interval, SD = standard deviation.
We also conducted subgroup analysis for the effect of plant-based diets on BMD at the femoral neck. As showing in Table 3, significant reduction of BMD at the femoral neck was found in both before 2000 (MD −0.05; 95% CI −0.06 to −0.03; P < .0001) and after 2000 (MD −0.03; 95% CI −0.05 to −0.01; P = .0003). In subgroup analysis of population ethnicity, significant reduction of femoral neck BMD was observed in both Caucasians (MD −0.05; 95% CI −0.06 to −0.03), and Asians (MD −0.03; 95% CI −0.05 to −0.01). In subgroup analysis of gender, significant reduction of femoral neck BMD was observed in female population (MD −0.04; 95% CI −0.05 to −0.03), but not male population (MD −0.02; 95% CI −0.03 to 0.00). Significant reduction was also found in both high AHRQ quality (MD −0.04; 95% CI −0.05 to −0.03) and moderate AHRQ quality (MD −0.02; 95% CI −0.04 to −0.00). In subgroup analysis of sample size, significant reduction was observed in both sample size <100 patients (MD −0.09; 95% CI −0.15 to −0.04) and sample size ≥100 patients (MD −0.03; 95% CI −0.04 to −0.02). In subgroup analysis of mean age, significant reduction was observed in both age <50 years (MD −0.07; 95% CI −0.13 to −0.01; P = .02) and ≥50 year (MD −0.03; 95% CI −0.05 to −0.02; P < .0001); in subgroup analysis of instruments, significant reduction was observed in both DXA (MD −0.03; 95% CI −0.05 to −0.01; P = .0009) and DPA (MD −0.05; 95% CI −0.06 to −0.03; P < .0001) (Table 3).
Table 3.
Subgroup analyses of the effect of plant-based diets on bone mineral density at the femoral neck.
| Pooled results | Heterogeneity | |||||
| Subgroups | No. of study/pts | MD | 95% CI | P-value | I 2 | Analytical effect model |
| Year | ||||||
| Before 2000 | 3/548 | −0.05 | −0.06 to −0.03 | <.0001 | 0% | Fixed effect model |
| After 2000 | 8/10,954 | −0.03 | −0.05 to −0.01 | .0003 | 59% | Random-effect model |
| Mean age | ||||||
| <50 yr | 4/330 | −0.07 | −0.13 to −0.01 | .02 | 61% | Random-effect model |
| ≥50 yr | 7/11,172 | −0.03 | −0.05 to −0.02 | <.0001 | 55% | Random-effect model |
| Instruments | ||||||
| DXA | 8/10,954 | −0.03 | −0.05 to −0.01 | .0009 | 59% | Random-effect model |
| DPA | 3/548 | −0.05 | −0.06 to −0.03 | <.0001 | 0% | Fixed effect model |
| Ethnicity | ||||||
| Caucasians | 5/9746 | −0.05 | −0.06 to −0.03 | <.0001 | 49% | Fixed effect model |
| Asians | 5/777 | −0.03 | −0.05 to −0.01 | <.0001 | 45% | Fixed effect model |
| Gender | ||||||
| Female | 10/10,656 | −0.04 | −0.05 to −0.03 | <.0001 | 42% | Fixed effect model |
| Male | 1/846 | −0.02 | −0.03 to 0.00 | .08 | – | – |
| Male / female | 1/9416 | −0.05 | −0.07 to −0.03 | <.0001 | – | – |
| Quality | ||||||
| Moderate | 4/1284 | −0.02 | −0.04 to −0.01 | .004 | 34% | Fixed effect model |
| High | 7/10,218 | −0.04 | −0.05 to −0.03 | <.0001 | 46% | Fixed effect model |
| Sample size | ||||||
| ≤100 pts | 3/58 | −0.09 | −0.15 to −0.04 | .001 | 37% | Fixed effect model |
| >100 pts | 8/11,444 | −0.03 | −0.04 to −0.02 | <.0001 | 46% | Fixed effect model |
CI = confidence intervals, MD = mean difference.
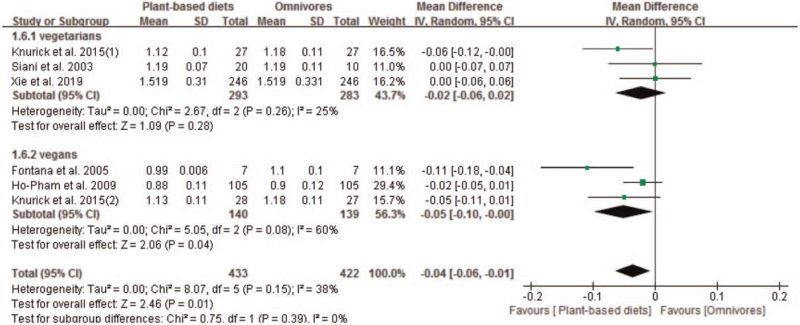
3.4. Effect of plant-based diets on BMD at the whole body
We identified 5 studies with 6 sets of data that compared the effect of plant-based diets and omnivores on BMD at the whole body. The BMD at the whole body in plant-based diets group was lower than omnivores group, with a MD of −0.04 (95% CI −0.06 to −0.01; P = .01). The pooled analysis was performed using a random effect model because significant heterogeneity was observed (P = .08 and I2 = 60%) in subgroup (Fig. 6). Subgroup analysis indicated significant reduction of BMD at the whole body in vegans (MD −0.05; 95% CI −0.10 to −0.00; P = .04). However, no significant reduction of BMD at the whole body in vegetarians was observed, with a pooled MD of −0.02 (95% CI −0.06 to 0.02; P = .28) (Fig. 6).
Figure 6.
Forest plot of effect of vegetarian diets on bone mineral density at the whole body. CI = confidence interval, SD = standard deviation.
Subgroup analysis indicated significant reduction of BMD at the whole body in plant-based diets group in subgroups of publish year after 2010 (MD −0.04; 95% CI −0.07 to −0.00; P = .03), Mean age ≥ 50 years (MD −0.03; 95% CI −0.05 to −0.01; P = .02), high AHRQ quality (MD −0.03; 95% CI −0.05 to −0.01; P = .02), DXA (MD −0.04; 95% CI −0.06 to −0.01; P = .001), Caucasians (MD −0.06; 95% CI −0.09 to −0.02; P = .0007), sample size ≤100 pts (MD −0.06; 95% CI −0.09 to −0.02; P = .0007) and female (MD −0.05; 95% CI −0.10 to −0.00; P = .04). No significant difference of BMD at the whole body between plant-based diets and omnivores was observed in subgroups of publish year before 2010, mean age <50 years, DPA, Asians, moderate AHRQ quality, male/female and sample size >100 pts, respectively (Table 4).
Table 4.
Subgroup analyses of the effect of plant-based diets on bone mineral density at the whole body.
| Pooled results | Heterogeneity | |||||
| Subgroups | No. of study/pts | MD | 95% CI | P-value | I 2 | Analytical effect model |
| Year | ||||||
| Before 2010 | 3/254 | −0.04 | −0.10 to 0.02 | . 17 | 63% | Random-effect model |
| After 2010 | 3/601 | −0.04 | −0.07 to −0.00 | .03 | 19% | Fixed effect model |
| Mean age | ||||||
| <50 yr | 2/44 | −0.06 | −0.16 to 0.05 | .32 | 76% | Random-effect model |
| ≥50 yr | 4/811 | −0.03 | −0.05 to −0.01 | .02 | 0% | Fixed effect model |
| Quality | ||||||
| Moderate | 2/44 | −0.06 | −0.16 to 0.05 | .32 | 76% | Random-effect model |
| High | 4/811 | −0.03 | −0.05 to −0.01 | .02 | 0% | Fixed effect model |
| Instruments | ||||||
| DXA | 5/363 | −0.04 | −0.06 to −0.01 | .001 | 40% | Fixed effect model |
| DPA | 1/492 | 0.00 | −0.06 to 0.06 | 1.00 | – | – |
| Ethnicity | ||||||
| Caucasians | 4/153 | −0.06 | −0.09 to −0.02 | .0007 | 29% | Fixed effect model |
| Asians | 2/702 | −0.02 | −0.04 to 0.01 | .27 | 0% | Fixed effect model |
| Sample size | ||||||
| ≤100 pts | 4/153 | −0.06 | −0.09 to −0.02 | .0007 | 29% | Fixed effect model |
| >100 pts | 2/702 | −0.02 | −0.04 to 0.01 | .27 | 0% | Fixed effect model |
| Gender | ||||||
| Female | 3/279 | −0.05 | −0.10 to −0.00 | .04 | 60% | Random-effect model |
| Male/female | 3/576 | −0.02 | −0.06 to 0.01 | .19 | 25% | Fixed effect model |
CI = confidence intervals, MD = mean difference.
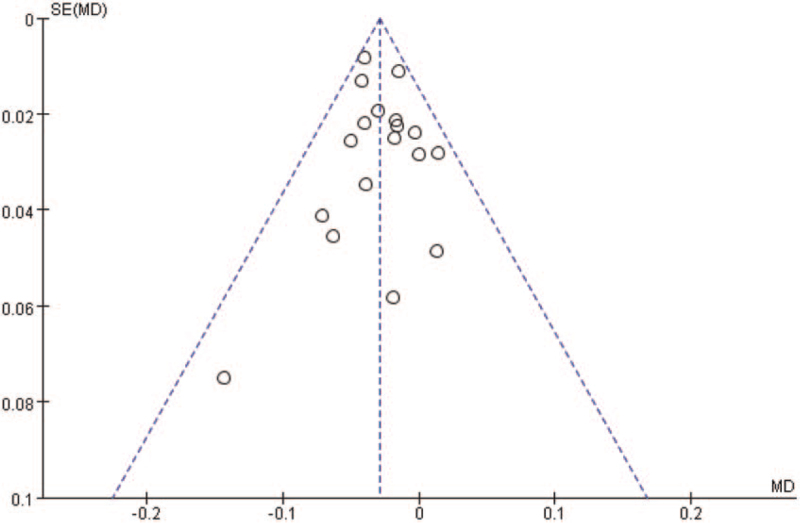
3.5. Publication bias
Begg funnel plot was generated to assess publication bias in the included studies. As shown in Figure 7, the plots displayed no obvious asymmetry and showed no clear evidence of publication.
Figure 7.
Begg funnel plot for detecting publication bias. MD = mean difference.
4. Discussion
Nutrition and food intake have long been key components of health. Diets abstaining from animal products have existed for millennia for a diversity of reasons ranging from religious to ethical concerns.[15] In recent times, plant-based diets have dramatically risen in public awareness, particularly as meat and dairy products are linked to higher environmental impact and drivers of human-induced climate change.[42]
Current research indicates a possible link between decreased calcium/vitamin D levels in the body and low BMD. Low BMD is used as one of the main diagnostic criteria for osteoporosis.[43] The possible connection between a vegan lifestyle, low BMD and the development of osteoporosis has important societal as well as medical implications. Low BMD and the development of osteoporosis often lead to fractures – hip, spine and wrist fractures being the most common.[44] Incidence of hip fractures results in costly medical procedures that tax the health-care system and increased morbidity and mortality rates.[44]
However, because the reason for osteoporosis and fracture was multifactorial, many current researches failed to clear the effect of plant-based diets on bone health. Low BMD has been demonstrated correlated to osteoporosis and fractures. Thus, we conducted this meta-analysis and systematic review aiming to comprehensively compare the effect between plant-based diets and omnivores on BMD. We found that population with plant-based diets had lower BMD than omnivores at the lumbar spine (MD −0.04; 95% CI −0.06 to −0.02), femoral neck (MD −0.04; 95% CI −0.05 to −0.02) and whole body (MD −0.03; 95% CI −0.06 to −0.01), respectively. In addition, we performed subgroup analyses and found lower BMD at lumbar spine, femoral neck, and whole body in both vegetarians and vegans. Previous studies have suggested that vegetarians may have higher BMD and bone mineral content than omnivores.[34,45–47] However, recent studies have not found any positive impact of vegetarian diets on bone health and some of them have found a negative impact.[28,32,33,35] The present results were in line with a previous meta-analysis and found lower BMD at the lumbar spine and the femoral neck for vegetarians compared with omnivores.[10,11] Ho-Pham et al[10] (2009) concluded that vegetarians and vegans had approximately 4% lower BMD at the lumbar spine and femoral neck than omnivores. In addition, our subgroup analysis found both vegetarians and vegans lead to lower BMD at the lumbar spine, the femoral neck, and whole body. Both Caucasians and Asians population were found having lower BMD at the lumbar spine and femoral neck. The lower BMD at the lumbar spine was found in both mean age <50 year and ≥ 50 year. For BMD at the femoral neck, we found that the significant reduction was observed in female participants but not in male participants.
It is worth noting that lifestyle factors may have influenced the associations between diet and BMD. Vegetarians and vegans tend to show healthier behaviors, such as higher levels of physical activity, lower smoking rates, and lower alcohol and caffeine intakes, than omnivores.[48] Particularly, in the studies included in this systematic review and meta-analyses, vegans and vegetarians generally reported higher levels of physical activity, lower smoking rates, lower alcohol and caffeine intakes, lower BMI, and lower energy and calcium intakes than omnivores, although some of these group differences were not statistically significant in several studies.[33,36]
In the relationship between vegetarian/vegan diets and bone health, it is important to consider the possible effect that overall dietary quality can have. Among the studies included in the present systematic review and meta-analysis, only 1 study considered overall dietary quality.[31] In this study, diet quality was superior for individuals adhering to a vegan diet as compared with the other diet groups, and there were no differences in BMD among vegans, vegetarians, and omnivores, which suggested that a high-quality vegan/vegetarian diets would look similar to that of an omnivore in relation to bone health. Our results indicated significantly lower BMD in female with plant-based diets, which may be mainly related to hormonal changes in postmenopausal women. Long-term female vegetarians may need effective nutritional supplements (particularly calcium and Vitamin D) to increase BMD levels and reduce the risk of osteoporosis.[49]
The present study had several limitations. First, most of the studies in the present meta-analysis included only women, and hence results are mainly applicable to this population. Second, investigations included a very heterogeneous population (ie, some of the studies focused on Buddhist nuns or religious followers of Buddhism, whereas others focused on young adult vegetarians with very different characteristics). Third, some factors associated with BMD, such as the time that vegetarians and vegans had been following the diet, daily energy intake, number of hours engaged in physical activity, BMI, use of hormone replacement therapy, sunlight exposure, consumption of alcohol, and smoking behavior, could not be evaluated because this information was not reported for most of the studies. Another limitation is the reliance on self-reported measurements, which are prone to errors, for such data as BMI, physical activity, and fracture rates. The lack of dietary quality information for most studies could be considered another limitation. Furthermore, whether individuals had a low bone mass or osteoporosis prior to starting a vegetarian or vegan diet, which could influence the results, was not reported for any of the included studies. Moreover, for some of the studies, it was reported that the participants were mostly vegetarians or vegans or long-term vegetarians without specifying the duration of the diet, which make definitions sometimes ambiguous for interpretation. Finally, our study focused on the effect of plant-based diets on BMD. We grouped plant-based diets as vegetarians and vegans. Thus, behavioral omnivore diet patterns were against vegetarians and vegans. There was no definition of behavioral omnivore diet patterns in our included studies, which anecdotally lead to confusion for the readers.
5. Conclusions
The present meta-analysis indicated that plant-based diets may be correlated with lower BMD of women when compared with omnivore population. However, this does not diminish the fact that a plant-based diet can be a harmful option to the overall bone health of population and more prospective researches are needed to clear the impact of plant-based diets on bone health.
Author contributions
Conceptualization: Huashan Pan.
Data curation: Mingjun Hu, Lijuan Zou.
Formal analysis: Huashan Pan, Shengcai He.
Methodology: Huashan Pan, Shengcai He.
Software: Huashan Pan, Shengcai He, Xingfa Ma, Haoyin Tan.
Writing – original draft: Huashan Pan, Shengcai He, Xingfa Ma, Haoyin Tan.
Writing – review & editing: Huashan Pan, Shengcai He, Xingfa Ma, Haoyin Tan.
Supplementary Material
Footnotes
Abbreviations: AHRQ = Agency for Healthcare Research and Quality, BMD = bone mineral density, BMI = body mass index, CI = confidence interval, DPA = dual-photon absorptiometry, DXA = dual-energy X-ray absorptiometry, MD = mean difference.
How to cite this article: Ma X, Tan H, Hu M, He S, Zou L, Pan H. The impact of plant-based diets on female bone mineral density: Evidence based on seventeen studies. Medicine. 2021;100:46(e27480).
Both XM and HT contributed equally to this work.
The authors have no conflicts of interest to disclose.
Funding: Sports Intervention and Chronic Disease Intelligent Health Promotion Service Center (No. 510S03X0101).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Fresán U, S. J. Vegetarian diets: planetary health and its alignment with human health. Adv Nutr 2019;10: (Suppl 4): S380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gardner CD, H. M. Food revolution. Am J Lifestyle Med 2017;11:387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lusk JL. Consumer research with big data: applications from the food demand survey (FooDS). Am J Agric Econ 2017;99:303–20. [Google Scholar]
- [4].Leitzmann C. Vegetarian nutrition: past, present, future. Am J Clin Nutr 2014;100: (Suppl 1): 496S–502S. [DOI] [PubMed] [Google Scholar]
- [5].Burckhardt P. The role of low acid load in vegetarian diet on bone health: a narrative review. Swiss Med Wkly 2016;146:w14277. [DOI] [PubMed] [Google Scholar]
- [6].Fleseriu M. Recombinant growth hormone treatment, osteoporosis and fractures, more complicated than it seems!. Endocrine 2018;59:463–5. [DOI] [PubMed] [Google Scholar]
- [7].Veronese N, Reginster JY. The effects of calorie restriction, intermittent fasting and vegetarian diets on bone health. Aging Clin Exp Res 2019;31:753–8. [DOI] [PubMed] [Google Scholar]
- [8]. International Osteoporosis Foundation. Facts and statistics. [Internet]. Available from: www.iofbonehealth.org. Accessed March 2020. [Google Scholar]
- [9]. Center for Disease Control. Percentage of adults aged 65 and over with osteoporosis or low bone mass at the femur neck or lumbar spine: United States, 2005–2010. [Internet]. Available from: https://www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.htm. Accessed March 2020. [Google Scholar]
- [10].Ho-Pham LT, NguyenF ND, NguyenF TV. Effect of vegetarian diets on bone mineral density: a Bayesian meta-analysis. Am J Clin Nutr 2009;90:943–50. [DOI] [PubMed] [Google Scholar]
- [11].Iguacel I, Miguel-Berges ML, Gómez-Bruton A, Moreno LA, Julián C. Veganism, vegetarianism, bone mineral density, and fracture risk: a systematic review and meta-analysis. Nutr Rev 2019;77:01–18. [DOI] [PubMed] [Google Scholar]
- [12].Juan WY, Y. S, Britten P. Food intake patterns of self-identified vegetarians among the U.S. population, 2007–2010. Proc Food Sci 2015;4:86–93. [Google Scholar]
- [13].Karavasiloglou N, Selinger. E, Gojda J, Rohrmann S, Kühn T. Differences in bone mineral density between adult vegetarians and non-vegetarians become marginal when accounting for differences in anthropometric factors. J Nutr 2020;150:1266–71. [DOI] [PubMed] [Google Scholar]
- [14].Lodder MC, Lems WF, Ader HJ, et al. Reproducibility of bone mineral density measurement in daily practice. Ann Rheum Dis 2004;63:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hsu E. Plant-based diets and bone health: sorting through the evidence. Curr Opin Endocrinol Diabetes Obes 2020;27:248–52. [DOI] [PubMed] [Google Scholar]
- [16].Shapses SA. Do we need to be concerned about bone mineral density in vegetarians and vegans? J Nutr 2020;150:983–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smith AM. Veganism and osteoporosis: a review of the current literature. Int J Nurs Pract 2006;12:302–6. [DOI] [PubMed] [Google Scholar]
- [18].Reynolds P. Agency for Healthcare Research and Quality. Am J Phys Med Rehabil 2005;84:1034–5. [Google Scholar]
- [19]. Review Manager (RevMan) [Computer Program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012. [Google Scholar]
- [20].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [21].University of York Centre for Reviews, Dissemination. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. York: CRD, University of York; 2009. [Google Scholar]
- [22].DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [24].Moher D, Liberati A, Tetzlaff1 J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shea BJ, Reeves BC, Hamel C, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Barr SI, Prior JC, Janelle KC, Lentle BC. Spinal bone mineral density in premenopausal vegetarian and nonvegetarian women: cross-sectional and prospective comparisons. J Am Diet Assoc 1998;98:760–5. [DOI] [PubMed] [Google Scholar]
- [27].Chiu JF, LanF SJ, Yang CY, et al. Long-term vegetarian diet and bone mineral density in postmenopausal Taiwanese women. Calcif Tissue Int 1997;60:245–9. [DOI] [PubMed] [Google Scholar]
- [28].Fontana L, Shew JL, Holloszy JO, Villareal DT. Low bone mass in subjects on a long-term raw vegetarian diet. Arch Intern Med 2005;165:684–9. [DOI] [PubMed] [Google Scholar]
- [29].Ho-Pham LT, Nguyen PLT, Le TTT, et al. Veganism, bone mineral density, and body composition: a study in Buddhist nuns. Osteoporos Int 2009;20:2087–93. [DOI] [PubMed] [Google Scholar]
- [30].Kim M-H, Choi M-K, Sung C-J. Bone mineral density of Korean postmenopausal women is similar between vegetarians and nonvegetarians. Nutr Res 2007;27:612–7. [Google Scholar]
- [31].Knurick JR, Johnston CS, Wherry SJ, Aguayo I. Comparison of correlates of bone mineral density in individuals adhering to lacto-ovo, vegan, or omnivore diets: a cross-sectional investigation. Nutrients 2015;7:3416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Krivosíková Z, Krajcovicová-Kudlácková M, Spustová V, et al. The association between high plasma homocysteine levels and lower bone mineral density in Slovak women: the impact of vegetarian diet. Eur J Nutr 2010;49:147–53. [DOI] [PubMed] [Google Scholar]
- [33].Lau EM, Kwok T, Woo J, Ho SC. Bone mineral density in Chinese elderly female vegetarians, vegans, lacto-vegetarians and omnivores. Eur J Clin Nutr 1998;52:60–4. [DOI] [PubMed] [Google Scholar]
- [34].Lloyd T, Schaeffer JM, Walker MA, Demers LM. Urinary hormonal concentrations and spinal bone densities of premenopausal vegetarian and nonvegetarian women. Am J Clin Nutr 1991;54:1005–10. [DOI] [PubMed] [Google Scholar]
- [35].Kaur M. Prevalence and associated risk factors of osteoporosis in post-menopausal women in North India. Mal J Nutr 2013;19:285–92. [Google Scholar]
- [36].Outila TA, Kärkkäinen MU, Seppänen RH, Lamberg-Allardt CJ. Dietary intake of vitamin D in premenopausal, healthy vegans was insufficient to maintain concentrations of serum 25-hydroxyvitamin D and intact parathyroid hormone within normal ranges during the winter in Finland. J Am Diet Assoc 2000;100:434–41. [DOI] [PubMed] [Google Scholar]
- [37].Siani V, Mohamed EI, Maiolo C, et al. Body composition analysis for healthy Italian vegetarians. Acta Diabetol 2003;40: (Suppl 1): S297–8. [DOI] [PubMed] [Google Scholar]
- [38].Tesar R, Notelovitz M, Shim E, Kauwell G, Brown J. Axial and peripheral bone density and nutrient intakes of postmenopausal vegetarian and omnivorous women. Am J Clin Nutr 1992;56:699–704. [DOI] [PubMed] [Google Scholar]
- [39].Wang YF, Chiu JS, Chuang MH, Chiu JE, Lin CL. Bone mineral density of vegetarian and non-vegetarian adults in Taiwan. Asia Pac J Clin Nutr 2008;17:101–6. [PubMed] [Google Scholar]
- [40].Xie L, Wang B, Cui X, Tang Q, Cai W, Shen X. Young adult vegetarians in Shanghai have comparable bone health to omnivores despite lower serum 25(OH) vitamin D in vegans: a cross-sectional study. Asia Pac J Clin Nutr 2019;28:383–8. [DOI] [PubMed] [Google Scholar]
- [41].Ying-Ming L, Liu S-H. Estimation of the 10-year probability of osteoporotic fracture in postmenopausal Taiwanese women with FRAX. Tzu Chi Med J 2010;22:29–35. [Google Scholar]
- [42].Rosi A, Mena P, Pellegrini N, et al. Environmental impact of omnivorous, ovolacto-vegetarian, and vegan diet. Sci Rep 2017;7:6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Anonymous. Consensus development conference: prophylaxis and treatment of osteoporosis. Am J Med 1991;90:107–10. [DOI] [PubMed] [Google Scholar]
- [44].Gueldner SH, Burke MS, Smiciklas-Wright H. Springer, Preventing and Managing Osteoporosis. New York: 2000. [Google Scholar]
- [45].Tylavsky FA, Anderson JJ. Dietary factors in bone health of elderly lactoovovegetarian and omnivorous women. Am J Clin Nutr 1988;48:842–9. [DOI] [PubMed] [Google Scholar]
- [46].Ellis FR, Holesh S, Ellis JW. Incidence of osteoporosis in vegetarians and omnivores. AmJ Clin Nutr 1972;25:555–8. [DOI] [PubMed] [Google Scholar]
- [47].Marsh AG, Sanchez TV, Michelsen O, et al. Vegetarian lifestyle and bone mineral density. Am J Clin Nutr 1988;48:837–41. [DOI] [PubMed] [Google Scholar]
- [48].Davey GK, Spencer EA, Appleby PN, et al. EPIC-Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr 2003;6:259–69. [DOI] [PubMed] [Google Scholar]
- [49].Chuang TL, Lin CH, Wang YF. Effects of vegetarian diet on bone mineral density. Tzu Chi Med J 2021;33:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.