Background.
Kidney transplantation and dialysis are two major risk factors for severe forms of coronavirus disease 2019 (COVID-19). The dynamics of the immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in this population remain largely unknown.
Methods.
We report here the analysis of anti–SARS-CoV-2 antibody– and T cell–mediated immune responses in 26 kidney transplant recipients (KTRs) and 11 dialyzed patients (DPs) who recovered from COVID-19.
Results.
After a mean time of 83 ± 26 d post–symptom onset for KTRs and 97 ± 31 d for DPs, 20 KTRs (76.9%) and 10 DPs (90.9%) displayed anti-S1 immunoglobulin G SARS-CoV-2 antibodies (P = 0.34), at similar titers in both groups. SARS-CoV-2–specific interferon-γ–producing T cells were evidenced in 26 KTRs (100%) and 10 DPs (90.9%). Total numbers of SARS-CoV-2–reactive T cells were high and not statistically different between the 2 groups. No correlation between the severity of the disease and the number of reactive T cells was found in KTRs. In 5 KTRs, also evaluated 10 mo after COVID-19, weak or absent antibody response was observed, whereas specific memory T-cell response was detected in all cases.
Conclusion.
T-cell response persisted up to 3 mo post–symptom onset, even in KTRs in whom full immunosuppressive regimen was reinstated at recovery, and seems to be present up to 10 mo after infection. Our findings have implications in the understanding of the natural course of the disease in transplant patients and DPs.
INTRODUCTION
The coronavirus pandemic has significantly impacted kidney transplantation1 and kidney transplant recipients (KTRs) seem to be at higher risk of a severe form of coronavirus disease 2019 (COVID-19) because of chronic immunosuppressive treatment and other medical comorbidities.2,3 Patients with end-stage renal disease display the same overall risk.4
Antibody- and T cell–mediated responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are both required for viral clearance and resolution of the infection, as well as for protection against a second SARS-CoV-2 infection.5 In patients at risk for severe COVID-19, less emphasis has been put on anti–SARS-CoV-2 T-cell immunity than on specific antibodies as parameters of immune protection. In fact, as described for SARS-CoV-1 and Middle East respiratory syndrome-coronavirus infections,6-8 the anti-SARS-CoV-2 humoral response declines over time, whereas SARS-CoV-2–specific T-cell immunity seems to last longer, even among seronegative convalescent patients.9 There are some recently published reports that suggest a decline and loss of anti–SARS-CoV-2 antibodies in KTRs recovered from COVID-1910-12 and data are very scarce in dialyzed patients (DPs).13 Regarding post–COVID-19 T-cell immunity, little is known in both populations. We previously showed in a small cohort of KTRs and DPs that patients with severe COVID-19 were able to mount vigorous T-cell and antibody responses to SARS-CoV-2,14 which was recently confirmed in other studies.12,15 Here, we assessed both humoral and cellular SARS-CoV-2–specific immunities in a cohort of 37 KTRs and DPs on average 3 mo after COVID-19 infection as well as in 5 KTRs with longer follow-up.
MATERIAL AND METHODS
Patients
This study included 40 KTRs and 14 patients on hemodialysis (HD) or on peritoneal dialysis diagnosed with SARS-CoV-2 reverse transcription polymerase chain reaction (PCR)–confirmed COVID-19 in Rouen University Hospital, Rouen, France, between March 14, 2020, and December 28, 2020. Blood samplings were performed after recovery during their usual clinical follow-up. This study was approved by the local ethics board for noninvasive health research (Comite´ d’Ethique pour la Recherche Non Interventionnelle No. E2021-37, for the Centre de Protection des Personnes Nord-Ouest-I, Rouen University Hospital, Rouen, France) that waived the need for informed consent in this retrospective analysis.
SARS-CoV-2 Serology
An in-house–developed multiplex addressable laser bead immunoassay was used for the detection of immunoglobulin (Ig)G targeting the S1 subunit of S protein as well as IgG specific for the N protein. Sensitivity of these assays was, respectively, >97% and 100%, at >13 d post–symptom onset.16 Specificity was ≥98% for 2 parameters. Positivity threshold was 7.29 UA/mL for S1 IgG and 20.98 UA/mL for N IgG.
Interferon-γ Enzyme-linked Immunospot Assay
Peripheral blood mononuclear cells were isolated by density gradient centrifugation of blood samples and used immediately. Peripheral blood mononuclear cells (in concentrations adjusted to 2 × 105 CD3+ T cells per well) were plated in anti-interferon (IFN)-γ–coated enzyme-linked immunospot (ELISPOT) 96-well plate in the presence of overlapping 15-mer peptide pools spanning the sequence of SARS-CoV-2 structural and nonstructural accessory proteins: S (pool S1 spanning the N-terminal part of the protein including the S1-subunit, and pool S2 spanning the C-terminal part), N, M, E, NS3A, NS7A, NS8, and NS9B (JPT, Strassberg, Germany). Negative and positive control stimulations, medium only, and CEFX peptide pool (JPT, Strassberg, Germany), respectively, were included in the assay. After overnight culture, the cells were washed and captured IFN-γ was revealed using a colorimetric assay (UCytech, Utrecht, The Netherlands). Spots were counted with an automated ELISPOT reader (AID, Strassberg, Germany). For each stimulation condition, the average spot number observed in wells without antigen was subtracted. Results were expressed as spot-forming cells (SFC) per 106 CD3+ T cells. For each assay, a specific response was considered positive if SFC number was superior to 3 SDs of the mean of spot numbers observed in wells without antigens (negative control) (ranging between 9 and 20 SFC/106 CD3+ T cells).
Statistical Methods
Quantitative data were presented as mean (SD) or median (range) when data were not normally distributed. Qualitative data were presented as percentages. The nonparametric Mann-Whitney test (quantitative data) and the chi-square test (qualitative data) were used to compare characteristics between the 2 groups. All analyses were performed using StatView version 5.0 (SAS Institute, Cary, NC), and graphs were generated using GraphPad Prism version 8.0 software (GraphPad Software, San Diego, CA).
RESULTS
General Characteristics of Patients and COVID-19
Between February and December 2020, 40 KTRs and 14 DPs (12 on HD and 2 on peritoneal dialysis, all for end-stage renal disease) were diagnosed with COVID-19 based on a positive SARS-CoV-2 reverse transcription–PCR on a nasopharyngeal swab. During follow-up, 8 KTRs (20%) died after a median time of 12.5 d (range, 1–17 d) as well as 3 DPs (21.4%) after a median time of 10 d (range, 3–45 d). We assessed the immune response to SARS-CoV-2 in 26 KTRs (26/32: 81.2%) and in 11 DPs (11/11: 100%) who recovered from SARS-CoV-2 infection, after a mean time of 83 ± 26 and 97 ± 31 d post–symptom onset in KTRs and DPs, respectively.
Among the 26 KTRs explored (Table 1), 19 (73%) were hospitalized, and among them, 3 (3/26: 11.5%) experienced a critical course of the disease, requiring intensive care. None of the KTRs received convalescent plasma of monoclonal antibodies during their COVID-19 episode, and 5 of 26 (19.2%) received a high dose of steroids. Immunosuppressive therapy was reduced in 10 cases (38.5%), with withdrawal of calcineurin inhibitor and mycophenolate mofetil in 3 cases, withdrawal of mycophenolate acid alone in 6 cases, and withdrawal of belatacept in 1 case. In the other 16 patients, immunosuppressive therapy was maintained as usual. Among the 19 KTRs hospitalized, patients recovered and were discharged from the hospital at a median of 9 d (range, 2–68 d) after symptom onset, with reintroduction of the previous immunosuppressive regimens in all but 5 patients, in whom the immunosuppressive regimen was still reduced. All 7 patients maintained at home were mildly symptomatic, and they did not require oxygen therapy: the most common symptoms were cough (4/7), fever (4/7), and diarrhea (4/7); all recovered without complication.
TABLE 1.
Baseline characteristics of patients
| KTR (n = 26) | DP (n = 11) | |
|---|---|---|
| M/F ratio | 25/16 = 1.6 | 9/5 = 1.8 |
| Age, mean ± SD, y | 58.6 ± 14.3 | 61.4 ± 17.7 |
| eGFR, mean ± SD, mL/min/1.73 m2 | 41.9 ± 9.2 | – |
| Heart failure | 6 (23) | 7 (63.6) |
| Diabetes | 6 (23) | 5 (45.4) |
| Cancer | 1 (3.8) | 0 (0) |
| Respiratory disease | 3 (11.5) | 1 (9) |
| Hypertension | 20 (76.9) | 8 (72.7) |
| BMI, kg/m2, median (IQR) | 27 (22–41) | 26 (23–38) |
| PD/HD, n | – | 2/9 |
| Time from KT, mean ± SD, y | 6.3 ± 5.4 | – |
| <1 y, n (%) | 4 (15.4) | – |
| DD, n (%) | 22 (84.6) | – |
| LKD, n (%) | 4 (15.4) | – |
| Induction at KTR, n (%) | ||
| ATG | 10 (38.5) | – |
| BAS | 16 (61.5) | – |
| IT at time of infection, n (%) | ||
| CNI + MMF | 20 (76.9) | – |
| CNI + AZA | 1 (3.8) | – |
| CNI + mTor-I | 1 (3.8) | – |
| Bela + MMF | 2 (7.7) | – |
| Bela monotherapy | 1 (3.8) | – |
| Steroids | 14 (53.8) | – |
| Hospitalization for COVID-19 infection, n (%) | 19 (73.1) | 9 (81.8) |
| ICU, n (%) | 3 (11.5) | 2 (18.2) |
| SARS-CoV-2 pneumonia, n (%) | 16 (61.5) | 6 (54.5) |
| % of pulmonary involvement, n | ||
| <25% | 8 | 4 |
| ≥25% | 8 | 2 |
ATG, antithymocyte globulins; AZA, azathioprine; BAS, basiliximab; bela, belatacept; BMI, body mass index; CNI, calcineurin inhibitor; COVID-19, coronavirus disease 2019; DD, deceased donor; DP, dialyzed patients; eGFR, estimated glomerular filtration rate; F, female; HD, hemodialysis; IT, immunosuppressive therapy; ICU, intensive care unit; IQR, interquartile range; KTR, kidney transplant recipient; LKD, living kidney donor; M, male; MMF, mycophenolate mofetil; mTor, mammalian target of rapamycin; mTor-I, mTor inhibitor; PD, peritoneal dialysis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Among the 11 DPs explored (Table 1), 7 (63.6%) were hospitalized, but none of them required intensive care. None of them received specific treatment for COVID-19. All 7 recovered and were discharged from hospital after a median of 12 d (range, 2–53 d) following symptom onset. The 4 patients maintained at home recovered rapidly. None of the patients included were vaccinated against the SARS- CoV-2.
Immune Response to SARS-CoV-2 in KTRs and DPs
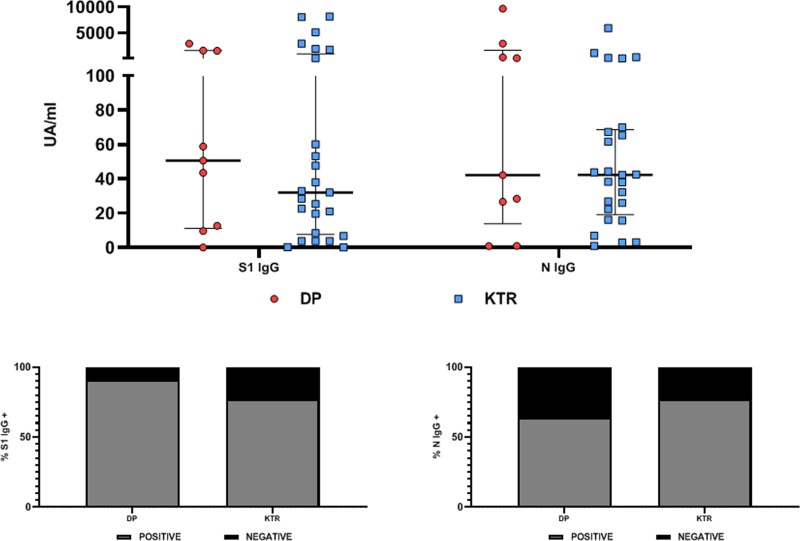
Using a multiplex addressable laser bead immunoassay serological assay, we found that at month 3 following symptom onset (83 ± 26 d for KTRs and 97 ± 31 d for DPs), 20 KTRs (76.9%) and 10 DPs (90.9%) displayed anti-S1 IgG SARS-CoV-2 antibodies (P = 0.34) (Figure 1) and that 20 KTRs (76.9%) and 7 DPs (63.6%) displayed anti-N IgG SARS-CoV-2 antibodies (P = 0.84). Seventeen KTRs (65.4%) and 8 DPs (72.7%) were positive for both anti-S1 IgG and anti-N IgG. Three KTRs (11.5%) and 1 DP (9.1%) were negative for both antibodies. Titers of S1 IgG and N IgG (Figure 1) were not statistically different between KTRs and DPs (median S1 IgG: 31.99 versus 50.5 UA/mL, P = 0.71; median N IgG: 42.26 versus 42.16, P = 0.71).
FIGURE 1.
SARS-CoV-2 antibody response in SARS-CoV-2 RT-PCR–positive KTRs (n = 26) and DPs (n = 11) 3 mo after infection. Upper panel, titers of S1 IgG, N IgG, and S1 IgM. Lower panel, proportions of DPs and KTRs with detectable S1 (left panel) or N IgG (right panel). DP, dialyzed patient; Ig, immunoglobulin; KTR, kidney transplant recipient; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
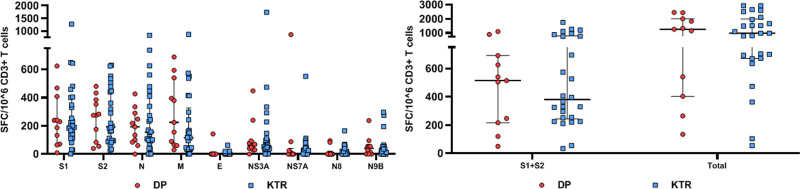
We then evaluated in the same patients the T-cell response to 9 structural and nonstructural accessory SARS-CoV-2 proteins using an IFN-γ ELISPOT assay. Among the 26 KTRs explored, all displayed IFN-γ–producing T cells reactive to at least 6 of the 9 peptide pools used. Among the 11 DPs explored, 10 displayed IFN-γ–producing T cells reactive to at least 6 of the 9 peptide pools. Nevertheless, 1 DP (9.1%) had a very weak response, and no S1 or N IgG could be detected. Total numbers of SARS-CoV-2–reactive T cells were high both in KTRs and HD patients and not statistically different between the 2 groups (1291 ± 877 SFC/106 CD3+ T cells in KTRs and 1255 ± 849 SFC/106 CD3+ T cells in DPs; P = 0.65). Responses to S1 and S2, N, and M were dominant and similar in magnitude in the 2 populations (P > 0.05). T cells reactive to structural protein E and accessory proteins ORF3A, ORF7A, ORF8, and ORF9B were less numerous (Figure 2). SARS-CoV-2–reactive T cells represented on average 0.125% ± 0.08% of total CD3+ T cells in DPs and 0.13% ± 0.08% in KTRs (P = 0.8). Taking into account CD3+ T-cell counts, median number of SARS-CoV-2–reactive T cells was 1.11 (IQR, 0.39–2.16) in DPs and 1.15/mm3 (IQR, 0.4–1.9) in KTRs (P = 0.6).
FIGURE 2.
SARS-CoV-2–reactive IFN-γ–producing T cells in SARS-CoV-2 RT-PCR–positive patients 3 mo after infection. Numbers of T cells (expressed as SFC/106 CD3+ T cells) reactive to 9 overlapping peptide pools spanning SARS-CoV-2 structural proteins S (pool S1 and S2), N, M, E, and accessory proteins ORF-3A, -7A, -8, and -9B in transplanted patients (KTRs) and patients on dialysis (DPs) with SARS-CoV-2–positive RT-PCR. DP, dialyzed patient; IFN-γ, interferon γ; KTR, kidney transplant recipient; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFC, spot-forming cell.
Correlation Between Humoral and Cellular Responses and Characteristics of the Course of COVID-19 in KTRs
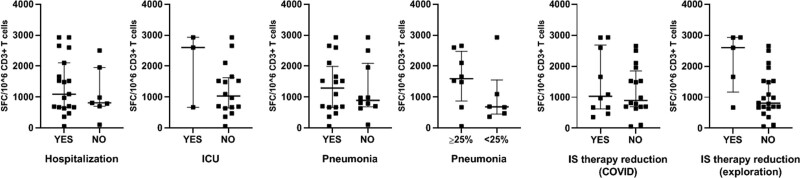
We evaluated if the course and the severity of the infection had an impact on the T-cell immune response to SARS-CoV-2 in KTRs (Figure 3). Total numbers of SARS-CoV-2–reactive T cells were not statistically different between KTRs hospitalized and maintained at home (P = 0.42), or in patients hospitalized in intensive care unit (P = 0.24). Diagnosis of SARS-CoV-2 pneumonia did not correlate with a higher magnitude of the T-cell response (P = 0.95), even when considering involvement of pulmonary parenchyma over 25% (P = 0.25).
FIGURE 3.
SARS-CoV-2–reactive IFN-γ–producing T cells in KTRs according to the severity of the disease 3 mo after infection. T-cell numbers (expressed as SFC/106 CD3+ T cells) reactive to 9 overlapping peptide pools spanning SARS-CoV-2 structural proteins S (pool S1 and S2), N, M, E, and accessory proteins ORF3-A, -7A, -8, and -9B in transplanted patients (KTRs) and patients on dialysis (DPs) with SARS-CoV-2–positive RT-PCR. IS therapy reduction (COVID-19): immunosuppressive therapy reduced at time of infection but reintroduced at discharge. IS therapy reduction (exploration): immunosuppressive therapy reduced at time of infection and not reintroduced at discharge. COVID-19, coronavirus disease 2019; DP, dialyzed patient; IFN-γ, interferon γ; ICU, intensive care unit; IS, immunosuppressive; KTR, kidney transplant recipient; pneumonia < or >25%, % of pulmonary involvement; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFC, spot-forming cell.
Reduction of immunosuppressive therapy at the time of COVID-19 diagnosis did not impact SARS-CoV-2–specific T-cell numbers (P = 0.75). No correlation between the time since symptom onset and specific T-cell numbers reactive was observed in KTRs. These numbers were similar in KTRs with or without S1 IgG antibodies (P = 0.85). All 6 KTRs with no detectable S1 IgG antibodies displayed significant numbers of SARS-CoV-2–specific T cells.
Regarding humoral response, 68.4% (13/19) of the KTRs hospitalized and 100% (7/7) of those maintained at home displayed anti-S1 IgG SARS-CoV-2 antibodies (P = 0.09). Diagnosis of SARS-CoV-2 pneumonia did not correlate with the presence of anti-S1 IgG SARS-CoV-2 (75% [12/16] in the KTRs with pneumonia and 80% [8/10] in KTRs without, P = 0.9). Reduction of immunosuppressive therapy at the time of COVID-19 diagnosis did not impact anti-S1 IgG SARS-CoV-2 positivity: 80% (8/10) when immunosuppression was reduced and 75% (12/16) when not reduced (P = 0.9).
Evaluation of the Immune Response to SARS-CoV-2 in KTRs Recovered From COVID-19 With Longer Follow-Up
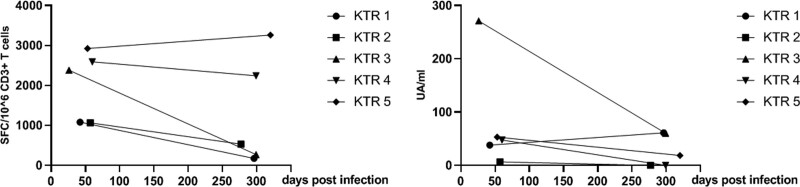
Five KTRs were evaluated both early (43 ± 13 d) and late (298 ± 15 d) after recovery from SARS-CoV-2 infection (Figure 4). Among them, 4 had a severe form of COVID-19 requiring hospitalization in an intensive care unit. S1 IgG antibody titers decreased at month 10 in all but 1 KTR (KTR 1) and were no longer detected in 2 KTRs (KTR 2 and 4). All were negative for anti-N IgG. SARS-CoV-2–reactive T-cell numbers between the 2 measurements remained stable in 3 patients (KTR 2, 4, and 5) and decreased, but were still detectable, in the 2 others (KTR 1 and 3), reaching values ranging from 210 to 530 SFC/106 CD3+ T cells.
FIGURE 4.
Early and late SARS-CoV-2–reactive IFN-γ–producing T cells and S1 IgG anti-SARS-CoV-2 antibodies in KTRs recovered from COVID-19. S1 IgG antibody titers decreased were no longer detected in 2 KTRs (KTR 2 and 4). SARS-CoV-2–reactive T-cell numbers between the 2 measurements decreased in KTR 1 and 3 but were still detectable. COVID-19, coronavirus disease 2019; IFN-γ, interferon γ; KTR, kidney transplant recipient; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
We report here the first assessment of the humoral and cellular immune responses to SARS-CoV-2 in KTRs and DPs recovering from severe and nonsevere forms of COVID-19, approximately 3 mo after infection onset. The morbidity and mortality due to SARS-CoV-2 infection are dramatically high in these 2 populations.3,4 Documentation of the immunity conferred by the infection is highly important to better evaluate the risk of reinfection and the strategy of vaccination after recovery from COVID-19, although the immune correlates of protection from COVID-19 are still unknown. In the general population, SARS-CoV-2–specific T-cell immunity conferred by polyfunctional, mainly IFN-γ–producing CD4+ T cells remains stable throughout convalescence, whereas humoral responses wane.9,17,18 All KTRs in our study were able to mount a vigorous specific T cell–mediated immunity after severe or nonsevere infection, similarly to DPs. This T-cell response persisted up to 3 mo post–symptom onset, even in KTRs in whom full immunosuppressive regimen was reinstated at recovery, and seems to be present up to 10 mo after infection in KTRs. However, regarding humoral immunity, detectable antibody titers waned over time in KTRs.
Benotmane et al10 described in 29 KTRs the 6-mo evolution of SARS-CoV-2 antibodies. Although 20.7% of the study patients became seronegative, 72.4% still displayed anti–SARS-CoV-2 antibodies up to 6 mo after COVID-19 despite a decrease of antibody titers in all patients. Waning of humoral immunity was also confirmed in a recent letter published by Chavarot et al11 about 42 COVID-19 KTRs. Most KTRs developed SARS-CoV-2–specific antibodies. Antibody titers rapidly decreased in all patients, and >60% of them showed negative or equivocal IgG results at month 6. Interestingly, antibody detection also turned negative or equivocal in patients with severe disease. Very recently, Burack et al19 also reported that among 70 solid organ transplanted (SOT) recipients with COVID-19, only 51% of them had detectable N IgG antibodies at a median of 47.5 d after COVID-19 diagnosis. Transplant-related variables, including the level and nature of immunosuppression, were important predictors of antibodies detection. These findings raise the concern that SOT recipients with COVID-19 may be less likely to form SARS-CoV-2–specific antibodies. There are very scarce data in DPs. La Milia et al20 reported in a very small cohort of HD patients that symptomatic COVID-19 confers higher and durable (up to 6 mo) anti-SARS-CoV-2 spikes IgG titers than asymptomatic disease in chronic HD patients. Antibody titers in symptomatic patients were not lower than in COVID-19 symptomatic healthcare staff members. Sakhi et al13 described the 6-mo kinetics of IgG antibody response in 83 patients on HD recovered from SARS-CoV-2 infection. A lack of seroconversion assessed at month 2 postdiagnosis was observed in only 10% of the patients and associated with an immunocompromised status (previous transplant or immunosuppressive therapy). In addition, as previously observed in the general population, a faster decay of S IgG antibodies was observed in patients with nonsevere infection. Forbes et al21 confirmed in a large series of 122 patients that DPs mounted a robust and sustained antibody response after COVID-19. In the same vein, Clarke et al22,23 recently showed in immunocompromised patients on HD antibody responses persisting for up to 6 mo, even in patients with mild or asymptomatic infection. Interestingly, they provided data suggesting that the humoral immune response induced by SARS-CoV-2 was associated with a reduced risk of subsequent PCR + infection. Overall, as suggested by our results, the humoral response to SARS-CoV-2 seems to be weaker in KTRs compared with DPs, particularly beyond the acute phase of infection. Nevertheless, this difference was not statistically different, possibly because of the low number of patients. However, despite disappearance of binding antibodies, which does not correlate with that of neutralizing antibodies,24 long-lasting B cell–24 or T cell–specific memory25,26 might still provide some level of protection against reexposure to SARS-CoV-2.
Regarding SARS-CoV-2–specific T-cell immunity in KTRs and DPs, little is still known about its duration after recovery from disease and the conferred protection from reinfection. We previously reported in a small cohort of KTRs that following reduction of immunosuppressive therapy, kidney-transplanted patients with severe COVID-19 were able to mount vigorous T-cell responses to SARS-CoV-2.14 This was confirmed by Thieme et al27 who showed in 10 transplant recipients during the acute phase of infection (3 to 4 d after diagnosis) the effective generation of a neutralizing antibody and T-cell response similar to a control group of nonimmunocompromised patients. Very recently, Favà et al12 reported that SARS-CoV-2 elicited robust adaptive immune responses in 28 SOT recipients, both at the cellular and humoral levels, although with a certain delay as compared with immunocompetent individuals. Fernández-Ruiz et al15 suggested that detectable SARS-CoV-2–specific T cell–mediated immunity (S1/M-reactive IFN-γ–producing CD4+ and CD8+ T cells) persisted for at least 6 mo in 21 KT recipients recovered from moderate to severe COVID-19. We provide here, in a larger cohort of KTRs and DPs, evidence that functional SARS-CoV-2–reactive T cells are detected after a median time of 85 d from symptom onset. Importantly, the numbers and reactivity profile of specific T cells induced in KTRs were similar to those observed in DPs. Moreover, in KTRs, disease severity, reduction of immunosuppressive therapy at the acute phase of the infection, or its reintroduction after recovery apparently did not influence the magnitude of the T-cell response detected on average 3 mo after symptom onset. Somewhat reassuringly, a memory T-cell response could still be detected, although with variable levels of contraction of the response, in a few KTRs 10 mo after COVID-19. Overall, with longer follow-up in the long term, the humoral immune response to SARS-CoV-2 waned in our small cohort of KTRs, whereas specific T-cell immunity remained present and probably protective (none of the patients of the cohort experienced reinfection).
There are some limitations to this study; first, the limited sample size does not allow us to draw solid conclusions about the humoral and cellular immune responses to natural infection in this population. Second, the variability of the timing from symptom onset to the immune response assessment could have introduced a bias in interpretation of the results.
In conclusion, we reported here specific humoral and cellular immunities in KTRs and DPs 3 mo from symptom onset. SARS-CoV-2–specific T-cell immunity remained present in a large majority of patients, similar in severe and nonsevere forms of the disease. Our findings have implications in the understanding of the natural course of the disease in transplant and DPs. These results raise the question of the protection conferred by SARS-CoV-2 infection in this population in which weak anti-SARS-CoV-2 antibody responses were very recently reported after a first dose28 and a second dose29 of an mRNA COVID-19 vaccine in naive patients.
ACKNOWLEDGMENTS
The authors wish to thank the healthcare professionals of the University hospital of Rouen who were involved in the care of the patients and the nurses from the kidney transplant unit.
Footnotes
Published online 17 November, 2021.
D.B. and S.C. participated in research design and data analysis. D.B., S.C., and D.G. participated in the writing of the article. All participants participated in the validation of the article writing and performance of the research. S.C., J.L., and L.D. contributed new reagents or analytic tools.
The authors declare no funding.
L.D. and O.B. are designated as inventors for the European Patent application EP 20315157.6 filed on April 14, 2020, in the names of Inserm, Université de Rouen, and CHU de Rouen and entitled “Methods for detecting the presence of coronavirus-specific antibodies in a subject.” The other authors declare no conflicts of interest.
All relevant data are within the article.
REFERENCES
- 1.Danziger-Isakov L, Blumberg EA, Manuel O, et al. Impact of COVID-19 in solid organ transplant recipients. Am J Transplant. 2021;21:925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caillard S, Anglicheau D, Matignon M, et al. ; French SOT COVID Registry. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98:1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caillard S, Chavarot N, Francois H, et al. ; French SOT COVID Registry. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98:1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vabret N, Britton GJ, Gruber C, et al. ; Sinai Immunology Review Project. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu WJ, Zhao M, Liu K, et al. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. ; Karolinska COVID-19 Study Group. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benotmane I, Gautier Vargas G, Velay A, et al. Persistence of SARS-CoV-2 antibodies in kidney transplant recipients. Am J Transplant. 2021;21:2307–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavarot N, Leruez-Ville M, Scemla A, et al. Decline and loss of anti-SARS-CoV-2 antibodies in kidney transplant recipients in the 6 months following SARS-CoV-2 infection. Kidney Int. 2021;99:486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Favà A, Donadeu L, Sabé N, et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021;21:2749–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakhi H, Dahmane D, Attias P, et al. ; Mondor NephroCov Study Group. Kinetics of anti-SARS-CoV-2 IgG antibodies in hemodialysis patients six months after infection. J Am Soc Nephrol. 2021;32:1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candon S, Guerrot D, Drouot L, et al. T cell and antibody responses to SARS-CoV-2: experience from a French transplantation and hemodialysis center during the COVID-19 pandemic. Am J Transplant. 2021;21:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Ruiz M, Olea B, Giménez E, et al. SARS-CoV-2-specific cell-mediated immunity in kidney transplant recipients recovered from COVID-19. Transplantation. 2021;105:1372–1380. [DOI] [PubMed] [Google Scholar]
- 16.Drouot L, Hantz S, Jouen F, et al. Evaluation of humoral immunity to SARS-CoV-2: diagnostic value of a new multiplex addressable laser bead immunoassay. Front Microbiol. 2020;11:603931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonifacius A, Tischer-Zimmermann S, Dragon AC, et al. COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity. 2021;54:340–354. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. [DOI] [PubMed] [Google Scholar]
- 19.Burack D, Pereira MR, Tsapepas DS, et al. Prevalence and predictors of SARS-CoV-2 antibodies among solid organ transplant recipients with confirmed infection. Am J Transplant. 2021;21:2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Milia V, Tonolo S, Luzzaro F, et al. The humoral immune response to SARS-CoV-2 mounts and is durable in symptomatic haemodialysis patients. Nephrol Dial Transplant. 2021;36:1132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forbes S, Davari M, Gnanasampanthan S, et al. Persistence of antibody response to SARS-CoV-2 in a cohort of haemodialysis patients with COVID-19. Nephrol Dial Transplant. 2021;36:1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke C, Prendecki M, Dhutia A, et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol. 2020;31:1969–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke CL, Prendecki M, Dhutia A, et al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int. 2021;99:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokal A, Chappert P, Barba-Spaeth G, et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell. 2021;184:1201–1213. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansari A, Arya R, Sachan S, et al. Immune memory in mild COVID-19 Patients and unexposed donors reveals persistent T cell responses after SARS-CoV-2 infection. Front Immunol. 2021;12:636768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang CK, Kim M, Lee S, et al. Longitudinal analysis of human memory T-cell response according to the severity of illness up to 8 months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis. 2021;224:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thieme CJ, Anft M, Paniskaki K, et al. The magnitude and functionality of SARS-CoV-2 reactive cellular and humoral immunity in transplant population is similar to the general population despite immunosuppression. Transplantation. 2021;105:2156–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21:2719–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]