PURPOSE
COSMIC-021 is evaluating cabozantinib plus atezolizumab in patients with solid tumors. We report results from patients with advanced clear cell (cc) and non–clear cell (ncc) renal cell carcinoma (RCC).
METHODS
This phase Ib study (NCT03170960) enrolled patients age ≥ 18 years with advanced RCC. A dose-escalation stage was followed by expansion cohorts. For cohort expansion, prior systemic therapy was not permitted for ccRCC but allowed for nccRCC. Patients received oral cabozantinib 40 mg once a day (ccRCC and nccRCC) or 60 mg once a day (ccRCC only) plus atezolizumab (1,200 mg intravenously, once every 3 weeks). The primary end point was investigator-assessed objective response rate (ORR) per RECIST v1.1; the secondary end point was safety.
RESULTS
A total of 102 patients were enrolled. Median follow-up was 25.8, 15.3, and 13.3 months for the 40-mg ccRCC, 60-mg ccRCC, and nccRCC groups, respectively. ORR was 53% (80% CI, 41 to 65) in the 40-mg ccRCC group (n = 34) and 58% (80% CI, 46 to 70) in the 60-mg ccRCC group (n = 36), 3% and 11%, respectively, with complete response; median progression-free survival (exploratory end point) was 19.5 and 15.1 months, respectively. In nccRCC (n = 32), ORR was 31% (80% CI, 20 to 44), all partial responses; median progression-free survival was 9.5 months. Grade 3 or 4 treatment-related adverse events (TRAEs) were reported by 71% of patients in the 40-mg ccRCC group, 67% in the 60-mg ccRCC group, and 38% in the nccRCC group; TRAEs leading to discontinuation of both agents occurred in 15%, 6%, and 3% of patients, respectively. There were no grade 5 TRAEs.
CONCLUSION
The novel combination of cabozantinib plus atezolizumab demonstrated encouraging clinical activity and acceptable tolerability in patients with advanced ccRCC and nccRCC. Disease control was observed across dose levels and histologic subtypes.
INTRODUCTION
Clear cell (cc) renal cell carcinoma (RCC) accounts for approximately 75% of all RCC diagnoses, whereas non–clear cell (ncc) RCC, a heterogeneous group of histologies, accounts for approximately 25%.1,2 Treatment of advanced ccRCC has evolved in recent years with the approval of new tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs), as well as TKI-ICI combinations that have shown improved response and survival versus standard of care, sunitinib.3-6 There are no approved standard systemic therapies for nccRCC, and prospective controlled trials have been limited. Recommended treatments include vascular endothelial growth factor receptor (VEGFR) TKIs.1,2,7 Studies have identified potential targets for therapy, including MET alterations in papillary nccRCC.2 Recently, cabozantinib monotherapy showed promising results in a phase II trial in advanced papillary RCC.8 Trials with ICI monotherapies have also been reported, but prospective data of TKI-ICI combinations are lacking.9-11
CONTEXT
Key Objective
Cabozantinib and atezolizumab have demonstrated efficacy against solid tumors as single agents and in combination regimens. COSMIC-021 is evaluating the combination of cabozantinib plus atezolizumab in patients with various advanced solid tumors, including clear cell and non–clear cell renal cell carcinoma (RCC); the latter is a patient population with high unmet medical need and limited participation in clinical trials.
Knowledge Generated
Cabozantinib plus atezolizumab demonstrated encouraging clinical activity in patients with advanced RCC regardless of histology. The safety profile with the combination was tolerable with dose modification and comparable to previous reports.
Relevance
These results support further evaluation of cabozantinib plus atezolizumab in patients with advanced RCC in the phase III trial setting, including those with non–clear cell histology. Patients with non–clear cell RCC have limited treatment options, and prospective data on tyrosine kinase inhibitor plus immune checkpoint inhibitor combinations are lacking for this population.
Cabozantinib is a multitargeted TKI that inhibits receptor tyrosine kinases involved in tumor cell growth, angiogenesis, metastasis, and immune-cell function, including VEGFR, MET, and the TAM family of kinases (TYRO3, AXL, and MER).12 Cabozantinib was approved for use as a single agent in patients with advanced RCC based on improved outcomes versus standard of care in the randomized CABOSUN (phase II) and METEOR (phase III) studies in the first-line and second-line settings, respectively.13,14 In retrospective studies, cabozantinib also demonstrated clinically meaningful benefit across subtypes of nccRCC.15,16 The recent randomized, phase II PAPMET study in patients with metastatic papillary RCC demonstrated improved progression-free survival (PFS) and objective response rate (ORR) with cabozantinib versus sunitinib.8
Cabozantinib may promote an immune-permissive environment that enhances response to ICIs.17-20 In preclinical models, cabozantinib synergized with ICIs to inhibit tumor growth and mitigate immunosuppression18; and in clinical studies, cabozantinib increased the numbers of tumor-infiltrating cytotoxic T cells and reduced immunosuppressive cells.17,20 These studies provided the rationale to combine cabozantinib with immunotherapy.21 Recently, cabozantinib in combination with the programmed cell death protein-1 inhibitor nivolumab was approved as a first-line therapy for patients with advanced ccRCC based on outcomes from the phase III CheckMate 9ER study, which showed improved PFS, overall survival, and ORR with the combination therapy versus sunitinib.6
COSMIC-021 is a multinational phase Ib study evaluating cabozantinib in combination with the programmed death-ligand 1 (PD-L1) inhibitor atezolizumab in advanced solid tumors, including RCC. Atezolizumab has demonstrated single-agent activity in patients with advanced ccRCC,22 and first-line atezolizumab plus bevacizumab, an anti-VEGF antibody, demonstrated improved PFS versus sunitinib.23
Cabozantinib plus atezolizumab showed encouraging antitumor activity in patients with ccRCC in the dose-escalation stage of COSMIC-02124 and in expansion cohorts of other solid tumors.25,26 Here, we report results for all patients with ccRCC and nccRCC enrolled in the study.
METHODS
Study Design
COSMIC-021 is a multicenter, open-label, phase Ib study initiated with a dose-escalation stage followed by tumor-specific expansion cohorts. The dose-escalation stage, which enrolled 12 patients with advanced RCC, has been completed.24 The expansion stage enrolled patients with a range of advanced solid tumors (12 different tumor types), including ccRCC and nccRCC.
Patients with RCC were enrolled at 20 study sites in the United States, France, and Spain (Appendix 1, online only). Patients with advanced ccRCC or nccRCC were required to be ≥ 18 years of age, with measurable disease per RECIST v1.1, have tumor tissue available (archival or recent biopsy), an Eastern Cooperative Oncology Group performance status of ≤ 1, and adequate organ and marrow function (Appendix 1). Patients with sarcomatoid tumor component were eligible. Prior systemic therapy was allowed in the dose-escalation stage. In the expansion cohorts, prior systemic therapy for advanced disease was not permitted in the ccRCC cohort; patients with nccRCC were allowed prior therapy with one VEGFR-targeting TKI, but prior therapy with TKIs targeting MET or with ICIs was not permitted.
The study Protocol (online only) was reviewed and approved by the institutional review board or ethics committee at participating sites. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines, the principles of the Declaration of Helsinki, and any local regulations. All patients provided written informed consent. This study is registered with ClinicalTrials.gov (NCT03170960).
Procedures and Assessments
In the dose-escalation stage, cabozantinib doses of 40 and 60 mg once daily (orally) were explored in combination with atezolizumab (1,200 mg once every 3 weeks, intravenously).24 The recommended dose for expansion cohorts was cabozantinib 40 mg based on clinical activity and tolerability; however, both the 40-mg and 60-mg regimens had acceptable safety profiles, with no dose-limiting toxicities. Following evaluation of the 40-mg cabozantinib starting dose in an expansion cohort (N = 30), the protocol allowed an option to enroll additional patients in the cohort at either the 40-mg or 60-mg starting dose. Both cabozantinib doses were evaluated in ccRCC as recommended by the study oversight committee to explore efficacy and safety at the higher dose in ccRCC, whereas only the 40-mg dose was evaluated in nccRCC. Based on tolerability after 4 weeks of treatment, patients receiving 40 mg could have their dose escalated to 60 mg at the discretion of the investigator and with sponsor approval.
Cabozantinib and atezolizumab were initiated on the same day. Patients received study treatment until progressive disease or unacceptable toxicity. Treatment beyond progression was allowed at the investigator's discretion. For managing adverse events (AEs), dose interruptions or delays were allowed for cabozantinib and atezolizumab; dose reductions were allowed for cabozantinib only (60-40 mg daily, 40-20 mg daily, and 20 mg daily to 20 mg every other day). Patients could discontinue one study drug and continue the other.
Tumor assessments by using computed tomography or magnetic resonance imaging were performed at screening, every 6 weeks for the first 12 months, and every 12 weeks thereafter. Tumor response was assessed by investigators using RECIST v1.1. Baseline PD-L1 expression was analyzed using SP142 anti–PD-L1 immunohistochemistry assay (Ventana, Roche Diagnostics); positive expression was a combined positive score ≥ 1%, with combined positive score defined as the ratio of PD-L1–expressing tumor cells, lymphocytes, and macrophages to total number of viable tumor cells. Safety was assessed routinely with a post-treatment follow-up visit 30 days after discontinuation of both study drugs. AE severity was graded by investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
End Points
The primary end point was ORR per RECIST v1.1, defined as the proportion of patients with confirmed complete response (CR) or partial response (PR). The secondary end point was safety, evaluated by the incidence and severity of AEs, including potential immune-related adverse events of special interest (AESI). Key exploratory end points included duration of response (DOR) and PFS per RECIST v1.1.
Statistical Analyses
Thirty patients per cohort was estimated for the primary end point of ORR to ensure the lower bound of the two-sided 80% Blyth-Still-Casella CI extended ≤ 12 percentage points from the point estimate. No formal statistical comparisons to historical controls or between cohorts were planned. Categorical and continuous variables were summarized with descriptive statistics. For time-to-event end points, medians and associated 95% CIs were estimated by the Kaplan-Meier method. The efficacy population included all enrolled patients, and the safety population included all patients who received study treatment. The date of data cutoff was July 21, 2020.
RESULTS
Patients
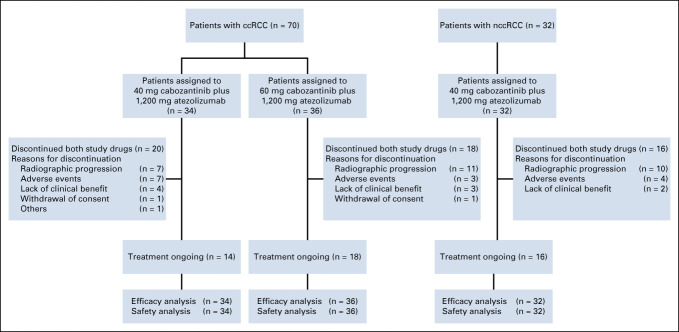
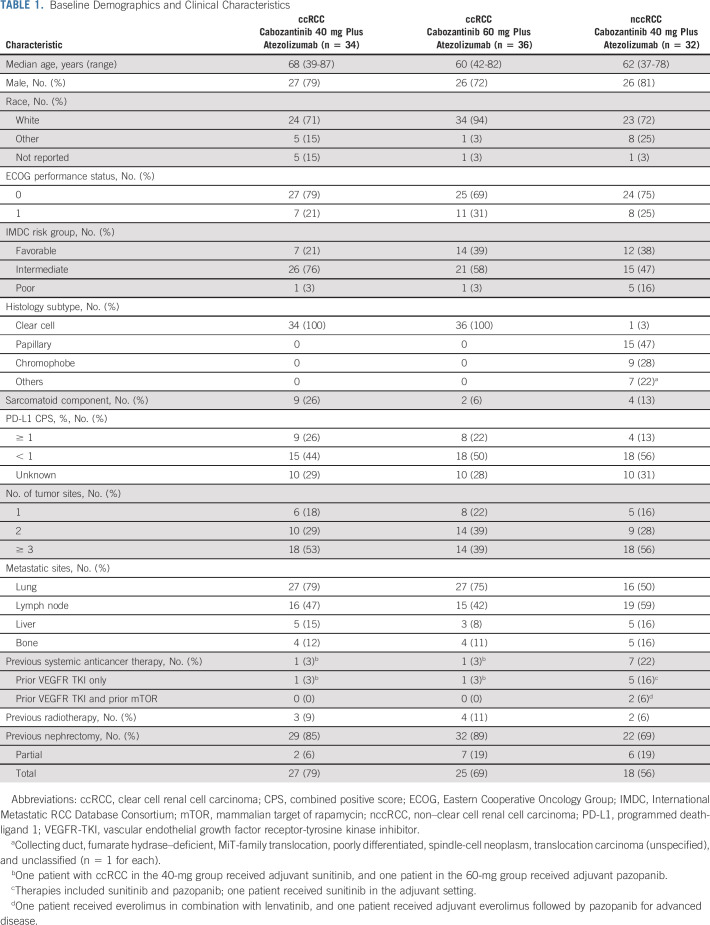
A total of 102 patients with RCC were enrolled from September 2017 to November 2019—70 patients with ccRCC (10 from dose-escalation and 60 from expansion) and 32 patients with nccRCC (two from dose-escalation and 30 from expansion). Among patients with ccRCC, 34 were enrolled in the 40-mg cabozantinib group and 36 in the 60-mg group (Fig 1). Some baseline characteristics were similar across all three groups (Table 1); however, the ccRCC 40-mg group had more patients with sarcomatoid features and IMDC intermediate-risk disease, and fewer patients with favorable-risk disease. Patients in the ccRCC cohort were treatment-naïve with respect to systemic therapy except two patients enrolled in the dose-escalation stage who had received prior adjuvant sunitinib or pazopanib. The most common histologic subtype of nccRCC was papillary (47%). Seven of 32 patients (22%) in the nccRCC cohort had received prior systemic treatment with a VEGFR TKI.
FIG 1.
Patient disposition. ccRCC, clear cell renal cell carcinoma; nccRCC, non–clear cell renal cell carcinoma.
TABLE 1.
Baseline Demographics and Clinical Characteristics
Median duration of follow-up was longer in the 40-mg ccRCC group (25.8 months; range, 20-33 months) compared with the 60-mg ccRCC group (15.3 months; range, 10-32 months) and the nccRCC cohort (13.3 months; range, 8-35 months). At data cutoff, 46% of patients in the ccRCC cohort and 50% in the nccRCC cohort were still on treatment (Fig 1). The most common reason for study discontinuation was disease progression (26% in the ccRCC cohort and 31% in the nccRCC cohort).
Efficacy
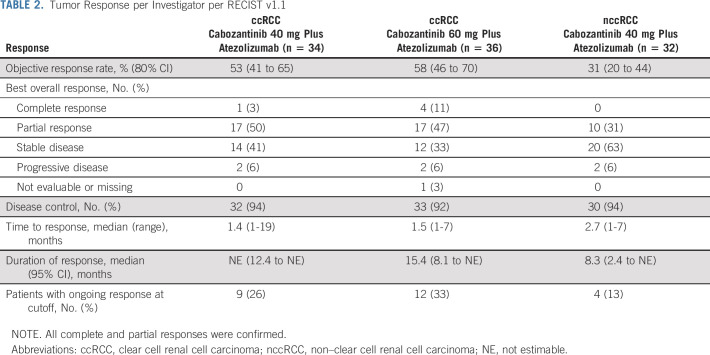
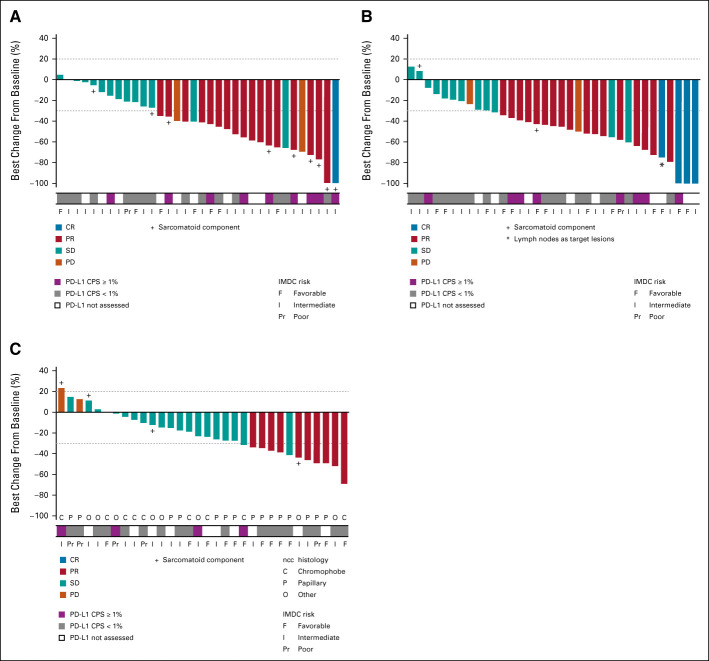
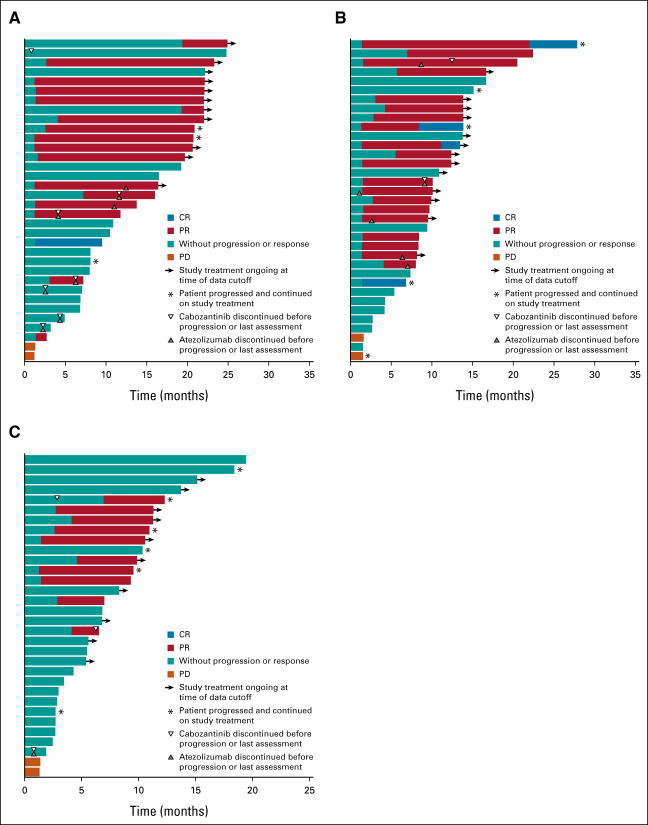
In the ccRCC cohort, ORR for confirmed responses by investigator assessment was 53% (80% CI, 41 to 65) in the 40-mg group and 58% (80% CI, 46 to 70) in the 60-mg group, with 3% and 11%, respectively, having confirmed CR (Table 2). Disease control (at least stable disease as best response) rates were 94% and 92%, respectively. Responses occurred in both PD-L1–negative and –positive patients (Fig 2). Median time to response was 1.4 months (range, 1-19 months) in the 40-mg group and 1.5 months (range, 1-7 months) in the 60-mg group (Fig 3); and median DOR was not estimable (NE; 95% CI, 12.4 to NE) and 15.4 months (95% CI 8.1 to NE), respectively, with nine and 12 patients having an ongoing response at data cutoff. Four patients in the 40-mg group had dose escalations to 60 mg with no subsequent impact on best overall response (three dose-escalated after disease progression). Among 11 patients with a tumor sarcomatoid component, one had a confirmed CR as best response, seven had confirmed PR, and three had stable disease. ORR was 62% (13 of 21) in patients with favorable risk and 53% (26 of 49) for intermediate or poor risk. Median PFS was 19.5 months (95% CI, 11.0 to NE) in the 40-mg group and 15.1 months (95% CI, 8.2 to 22.3) in the 60-mg group, with PFS rates of 67% and 58% at 1 year, respectively (Fig 4).
TABLE 2.
Tumor Response per Investigator per RECIST v1.1
FIG 2.
Best change from baseline in sum of tumor lesions in (A) patients with ccRCC receiving cabozantinib 40 mg plus atezolizumab (n = 34); (B) patients with ccRCC receiving cabozantinib 60 mg plus atezolizumab (n = 35); and (C) patients with nccRCC receiving cabozantinib 40 mg plus atezolizumab (n = 32). All complete and partial responses were confirmed. One patient in the 60-mg ccRCC group had no postbaseline tumor assessment. ccRCC, clear cell renal cell carcinoma; CPS, combined positive score; CR, complete response; IMDC, International Metastatic RCC Database Consortium; nccRCC, non–clear cell renal cell carcinoma; PD, progressive disease; PD-L1, programmed death-ligand 1; PR, partial response; RCC, renal cell carcinoma; SD, stable disease.
FIG 3.
Duration of treatment and responses in (A) patients with ccRCC receiving cabozantinib 40 mg plus atezolizumab (n = 34); (B) patients with ccRCC receiving cabozantinib 60 mg plus atezolizumab (n = 35); and (C) patients with nccRCC receiving cabozantinib 40 mg plus atezolizumab (n = 32). All complete and partial responses were confirmed. One patient in the 60-mg ccRCC group had no postbaseline tumor assessment. ccRCC, clear cell renal cell carcinoma; CR, complete response; nccRCC, non–clear cell renal cell carcinoma; PD, progressive disease; PR, partial response; RCC, renal cell carcinoma.
FIG 4.
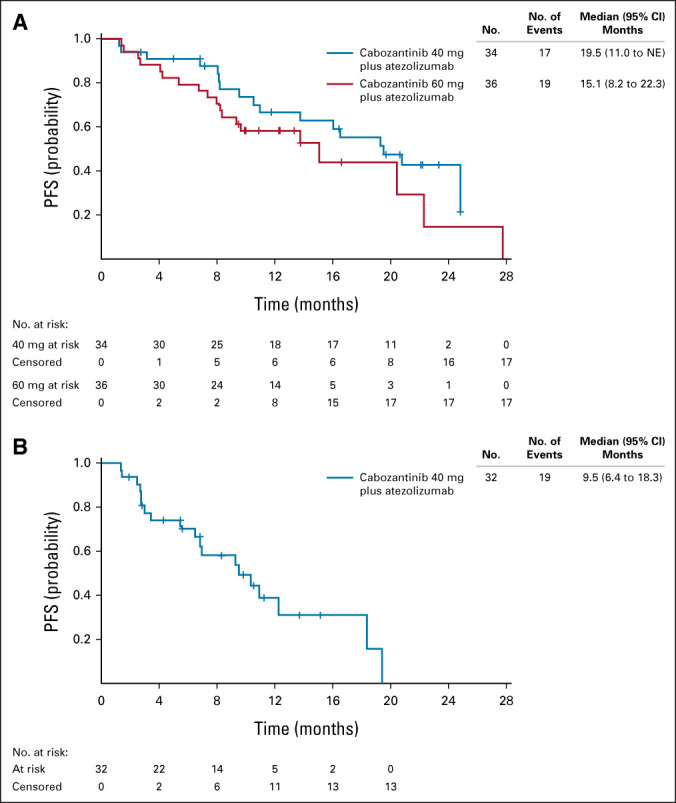
PFS in patients with (A) clear-cell renal cell carcinoma and (B) patients with non–clear cell renal cell carcinoma. NE, not estimable; PFS, progression-free survival.
In the nccRCC cohort, the ORR was 31% (80% CI, 20 to 44), all confirmed PRs. The disease control rate was 94%. Responses were observed across subtypes of nccRCC and irrespective of PD-L1 status (Fig 2). The highest ORR was among patients with papillary RCC at 47% (7 of 15). ORR was 11% (1 of 9) in patients with chromophobe histology and 25% (2 of 8) for other histologic subtypes. ORR was 42% (5 of 12) for patients with favorable risk and 25% (5 of 20) for intermediate or poor risk. Median time to response was 2.7 months, and median DOR was 8.3 months (95% CI, 2.4 to NE; Fig 3). At data cutoff, four patients had an ongoing response. Median PFS was 9.5 months (95% CI, 6.4 to 18.3; Fig 4), with a 1-year PFS rate of 39%.
Treatment Exposure and Safety
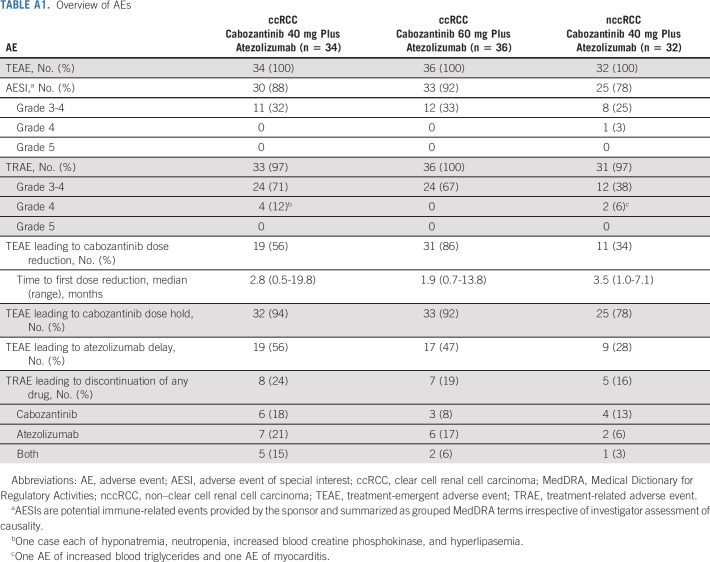
In the ccRCC cohort, median duration of treatment was 20.4 months (range, 1-33 months) in the 40-mg group and 13.0 months (range, 1-29 months) in the 60-mg group, consistent with the longer median follow-up for the 40-mg group. Cabozantinib dose reductions because of treatment-emergent AEs were required by 56% in the 40-mg group and 86% in the 60-mg group (Appendix Table A1, online only), with a median average daily cabozantinib dose of 28.7 mg (range, 10-53 mg) and 37.7 mg (range, 14-60 mg), respectively. Atezolizumab dose delays were required by 56% and 47% in the 40-mg and 60-mg groups, respectively. Treatment-related adverse events (TRAEs) leading to discontinuation of either study drug, cabozantinib, atezolizumab, or both (not necessarily at the same time) occurred in, respectively, 24%, 18%, 21%, and 15% of patients in the 40-mg group, with corresponding values of 19%, 8%, 17%, and 6% in the 60-mg group.
In the nccRCC cohort, median duration of treatment was 10.7 months (range, 1-23 months). Treatment-emergent AEs leading to cabozantinib dose reduction and atezolizumab dose delay occurred in 34% and 28% of patients, respectively. The median average daily cabozantinib dose was 31.0 mg (range, 7-40 mg). TRAEs leading to discontinuation of either study drug, cabozantinib, atezolizumab, or both, occurred in 16%, 13%, 6%, and 3% of patients, respectively.
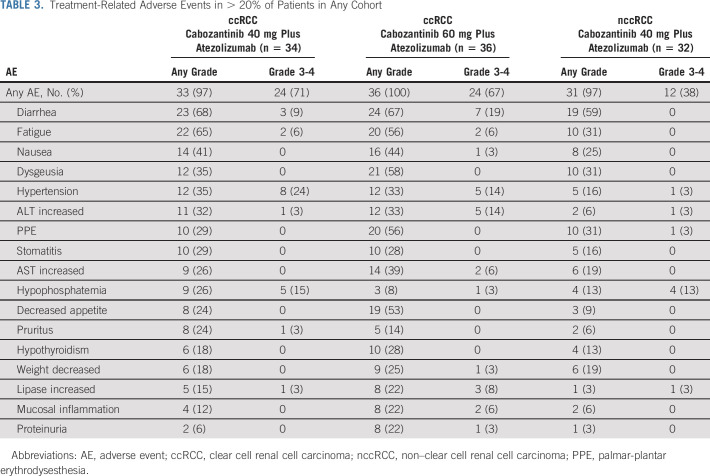
TRAEs of any grade were experienced by ≥ 97% patients in both cohorts (Table 3). In the ccRCC cohort, 71% and 67% of patients, respectively, in the 40-mg and 60-mg groups experienced grade 3 or 4 TRAEs, the most common being hypertension (24% and 14%), diarrhea (9% and 19%), hypophosphatemia (15% and 3%), and increased ALT (3% and 14%). Four patients (6%) in the ccRCC 40-mg group experienced a grade 4 TRAE, including a single case each of hyponatremia, neutropenia, increased blood creatine phosphokinase, and hyperlipasemia. There were no grade 4 TRAEs in the 60-mg group. In the nccRCC cohort, 38% of patients had a grade 3 or 4 TRAE, the most common being hypophosphatemia (13%). Two patients experienced grade 4 TRAEs (increased blood triglycerides and myocarditis). There were no grade 5 TRAEs in either RCC cohort.
TABLE 3.
Treatment-Related Adverse Events in > 20% of Patients in Any Cohort
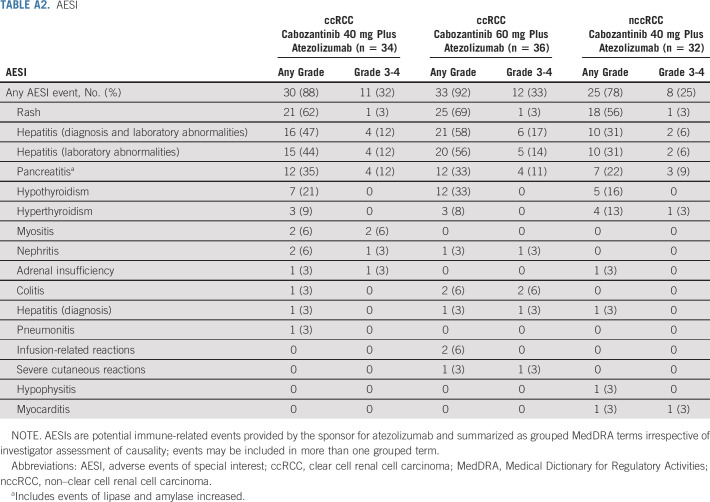
AESIs for atezolizumab of any grade and irrespective of causality were experienced by 86% of patients overall, and 30% experienced a grade 3 or 4 event (Appendix Table A2, online only). Rates were generally consistent across cohorts, and there were no grade 5 events. The most common grade 3 or 4 AESIs were hepatitis (diagnosis and laboratory abnormalities; 12%) and pancreatitis (11%). Two patients experienced grade 3 or 4 colitis, and one experienced grade 3 or 4 myocarditis. High-dose steroids (≥ 40 mg prednisone or equivalent) were required for AESIs in two patients (6%) in the 40-mg ccRCC group, 8 (22%) in the 60-mg ccRCC group, and 1 (3%) in the nccRCC cohort. Resolution of AESIs with high-dose steroids was observed for six events in five patients in the 60-mg ccRCC group. None of these AESIs recurred, but three events required discontinuation of atezolizumab. Four AESIs in four patients decreased in severity to grade 1 or 2 (one in the 40-mg ccRCC group, two in the 60-mg ccRCC group, and one in the nccRCC cohort).
DISCUSSION
In this phase Ib study, cabozantinib plus atezolizumab demonstrated encouraging clinical activity in patients with advanced RCC. Robust clinical activity was observed irrespective of histology. For the ccRCC cohort, ORR was 56% overall, with a CR rate of 3% in the 40-mg group and 11% in the 60-mg group, and ORR was 62% in patients with favorable risk and 53% in those with intermediate- or poor-risk disease. In the nccRCC cohort, responses were observed across subtypes, with an ORR of 31% overall and of 47% in patients with papillary histology. Disease control rates exceeded 90% in both the ccRCC and nccRCC cohorts.
The safety profile of cabozantinib plus atezolizumab was tolerable at both dose levels with dose modification and supportive care, including steroids for immune-related AEs. In the ccRCC cohort, dose reductions were required more frequently for 60 versus 40 mg; but rate of discontinuation because of TRAEs was lower in the 60-mg group. There were higher rates for some TRAEs in the 60-mg group (eg, palmar-plantar erythrodysesthesia), but these were generally grade 1 or 2 events, although rates of grade 3 or 4 diarrhea and liver enzymes abnormalities appeared higher with the 60-mg dose. AESIs were generally grade 1 or 2, with no grade 5 events. The small sample sizes, nonrandomized design, and differences in baseline characteristics and duration of follow-up limit interpretation.
The ORR and PFS data reported in the ccRCC cohort suggest improvements compared with first-line studies of single-agent cabozantinib or atezolizumab,14,22 with comparable outcomes to first-line TKI-ICI combinations in phase III trials3,5,6,23; however, the current study is limited by the phase Ib design, and comparisons between the studies are confounded by differences in trial designs and patient populations. Although PFS in the current study appears longer in the 40-mg cabozantinib ccRCC group than in the 60-mg ccRCC group (median 19.5 and 15.1 months, respectively), differences in baseline characteristics and duration of follow-up preclude definitive comparison.
Phase III studies in RCC have generally excluded patients with nccRCC histology,3-5 a population with a significant unmet need. The ORR of 31%, a rate of 6% for progressive disease as best response, and median PFS of 9.5 months reported here for the nccRCC cohort are clinically meaningful compared with results from retrospective and early-phase studies of single-agent TKIs, including cabozantinib.15,27-29 Similar response rates have been reported with ICI monotherapy, although the rates of progressive disease as best response were higher than in the current study.9-11,30 ORR was also comparable in a phase II study of atezolizumab plus bevacizumab in 42 patients with advanced nccRCC.31 The ORR of 47% with cabozantinib plus atezolizumab in patients with papillary RCC is encouraging and warrants further study. MET is a target of cabozantinib; and MET gene alterations occur in a modest proportion of papillary RCC cases.2 In the randomized, phase II PAPMET study, median PFS was 9.0 months for cabozantinib versus 5.6 months for sunitinib (hazard ratio, 0.60; 95% CI, 0.37 to 0.97; P = .019) in patients with advanced papillary RCC, with an ORR of 23% versus 4% (P = .01).8
Responses occurred in both PD-L1–negative and –positive patients in the ccRCC and nccRCC cohorts. PD-L1 status has not been a consistent predictor of outcome with ICIs across RCC studies, which may reflect differences in study populations or PD-L1 assays.3-5 Analyses are ongoing to further assess the relationship of outcomes with PD-L1 status and other immune-based biomarkers, including tumor-infiltrating lymphocytes and circulating immune cells.
The safety profile of cabozantinib plus atezolizumab was generally consistent with the individual agents in RCC populations14,22 and other TKI-ICI combinations.3,5,6 Grade ≥ 3 rates for individual TRAEs with cabozantinib plus atezolizumab generally fell within the ranges reported for other TKI-ICI combinations, including rates for hypertension, palmar-plantar erythrodysesthesia, fatigue, and elevated liver enzymes, as did use of high-dose steroids and rates of discontinuation because of AEs.3,5,6 Dose modification to manage AEs is common for cabozantinib and TKIs in general.32,33 Because of high interpatient variability in cabozantinib clearance, a proportion of patients with low cabozantinib clearance will require dose reduction to achieve a tolerable dose.34
In summary, we report encouraging antitumor activity with the combination of cabozantinib plus atezolizumab across ccRCC and nccRCC histologies, with a tolerable safety profile. AEs were managed with dose modifications and supportive care measures. Further evaluation of cabozantinib plus atezolizumab in RCC is ongoing in the phase III CONTACT-03 study (NCT04338269), which is evaluating cabozantinib plus atezolizumab compared with cabozantinib alone in patients with ccRCC and nccRCC who received prior ICI as a first- or second-line treatment.
ACKNOWLEDGMENT
The authors thank the patients, their families, the investigators, and site staff. The authors also thank Julie Lougheed, PhD (Exelixis Inc, Alameda, CA), for critical review of the manuscript. Editorial and writing assistance was provided by Karen O'Leary, PhD, and Michael Raffin (Fishawack Communications Inc, a part of Fishawack Health, Conshohocken, PA), which was funded by Exelixis, Inc.
APPENDIX 1.
Study Sites and Investigators
France: Yohann Loriot, Center Hospitalier Universitaire Institut Gustave Roussy, Villejuif; Stephane Culine, Hôpital Saint Louis, Paris
Spain: Cristina Suárez, Hospital Universitari Vall d'Hebrón, Barcelona; Daniel Castellano, Hospital Universitario 12 de Octubre, Madrid
USA: Bradley McGregor, Dana Farber Cancer Institute and Massachusetts General Hospital, Boston, MA; Che-Kai Tsao, Icahn School of Medicine at Mount Sinai, New York, NY; William Kelly, Thomas Jefferson University Hospital, Philadelphia, PA; Kristen Spencer, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ; Robert Dreicer, University of Virginia, Charlottesville, VA; Lance Pagliaro, Mayo Clinic, Rochester, MN; Ira Winer, Karmanos Cancer Institute, Detroit, MI; Thomas Hutson, Texas Oncology - Baylor Charles A. Sammons Cancer Center, Dallas, TX; Isaac Tafur, Joe Arrington Cancer Research and Treatment Center, Lubbock, TX; Sandy Srinivas, Stanford University Medical Center, Stanford, CA; Sumanta Pal, City of Hope Comprehensive Cancer Center, Duarte, CA; Neeraj Agarwal, Huntsman Cancer Institute, Salt Lake City, UT; Rana R. McKay, University of California San Diego, San Diego, CA; Jerome Goldschmidt, Blue Ridge Cancer Care, Blacksburg, VA; Polina Khrizman, MD Anderson Cancer Center at Cooper, Camden, NJ
Eligibility Criteria
General inclusion criteria for solid tumor cohorts
Cytologically or histologically and radiologically confirmed solid tumor that is inoperable locally advanced, metastatic, or recurrent.
Measurable disease per Response Evaluation Criteria in Solid Tumors 1.1 as determined by the investigator. Measurable disease must be outside the radiation field if prior radiation therapy was administered.
Tumor tissue material available (archival or recent tumor biopsy).
Recovery to baseline or ≤ Grade 1 Common Terminology Criteria for Adverse Events v4 from toxicities related to any prior treatments, unless adverse events are clinically nonsignificant and/or stable on supportive therapy.
Age 18 years or older on the day of consent.
Eastern Cooperative Oncology Group performance status of 0 or 1.
-
Adequate organ and marrow function based upon meeting all the following laboratory criteria within 14 days before first dose of study treatment:
○ Absolute neutrophil count ≥ 1,500/μL (≥ 1.5 × 109/L) without granulocyte colony-stimulating factor support within 2 weeks before screening laboratory sample collection
○ White blood cell count ≥ 2,500/μL (≥ 2.5 × 109/L)
○ Platelets ≥ 100,000/μL (≥ 100 × 109/L) without transfusion within 2 weeks before screening laboratory sample collection
○ Hemoglobin ≥ 9 g/dL (≥ 90 g/L) without transfusion within 2 weeks before screening laboratory sample collection
○ ALT, AST, and alkaline phosphatase ≤ 3 × upper limit of normal (ULN); alkaline phosphatase ≤ 5 × ULN with documented bone metastases
○ Total bilirubin ≤ 1.5 × ULN (for subjects with Gilbert's disease ≤ 3 × ULN)
○ Serum creatinine ≤ 1.5 × ULN or calculated creatinine clearance ≥ 40 mL/min (≥ 0.67 mL/s) using the Cockcroft-Gault equation
○ Urine protein/creatinine ratio ≤ 1 mg/mg (≤ 113.2 mg/mmol).
Capable of understanding and complying with the protocol requirements and must have signed the informed consent document.
Sexually active fertile patients and their partners must agree to use highly effective methods of contraception that alone or in combination result in a failure rate of < 1% per year when used consistently and correctly during the course of the study and for five months after the last dose of study treatment.
Female patients of childbearing potential must not be pregnant at screening.
-
Patients with the following conditions are eligible for the study:
○ A history of autoimmune-related hypothyroidism and on thyroid replacement hormone therapy. Patients with prior history of thyroiditis are allowed if they have undergone subtotal, near-total, or total thyroidectomy
○ Controlled type 1 diabetes mellitus and on an insulin regimen
○ Asthma
○ Eczema, psoriasis, lichen simplex chronicus, or vitiligo with dermatologic manifestations only provided all of following are true: rash covers < 10% of body surface area; disease is well controlled at baseline and requires only low-potency topical corticosteroids; no occurrence of acute exacerbations of the underlying condition requiring psoralen plus ultraviolet A radiation, methotrexate, retinoids, biologic agents, oral calcineurin inhibitors, or high-potency or oral corticosteroids within the previous 12 months.
General exclusion criteria for solid tumor cohorts
Prior treatment with cabozantinib or immune checkpoint inhibitors including anticytotoxic T-cell lymphocyte-4, anti–programmed cell death protein-1, anti–programmed death-ligand 1, anti–programmed death-ligand 2, anti–OX-40, and anti-CD137 therapy.
Receipt of any type of small molecule kinase inhibitor (including investigational kinase inhibitor) within 2 weeks before first dose of study treatment.
Receipt of any type of anticancer antibody (including investigational antibody) or systemic chemotherapy within 4 weeks before first dose of study treatment.
Radiation therapy for bone metastasis within 2 weeks, or any other local radiation therapy within 4 weeks before first dose of study treatment. Patients who have received systemic treatment with radionuclides within 6 weeks before the first dose of study treatment are not eligible. Patients with clinically relevant ongoing complications from prior radiation therapy are not eligible. Known brain metastases or cranial epidural disease unless adequately treated with radiotherapy and/or surgery (including radiosurgery) and stable for at least 4 weeks before first dose of study treatment. Eligible patients must be neurologically asymptomatic and without corticosteroid treatment at the time of first dose of study treatment.
Concomitant anticoagulation with or plan to use oral anticoagulants (eg, warfarin, direct thrombin, and Factor Xa inhibitors) or platelet inhibitors (eg, clopidogrel). Low-dose aspirin for cardioprotection (per local applicable guidelines) and low-dose (prophylactic), low-molecular-weight heparins are permitted.
Diagnosis of immunodeficiency or is receiving systemic steroid therapy (> 10 mg daily prednisone equivalent) or any other form of immunosuppressive therapy within 2 weeks before first dose of study treatment. Inhaled, intranasal, intra-articular, and topical corticosteroids and mineralocorticoids are allowed.
Administration of a live, attenuated vaccine within 30 days before the first dose of study treatment.
-
The patient has uncontrolled, significant intercurrent, or recent illness including:
○ Congestive heart failure New York Heart Association Class 3 or 4, unstable angina pectoris, serious cardiac arrhythmias
○ Uncontrolled hypertension defined as sustained blood pressure > 140 mm Hg systolic or > 90 mm Hg diastolic despite optimal antihypertensive treatment
○ Stroke (including transient ischemic attack), myocardial infarction, or other ischemic event, or thromboembolic event (eg, deep venous thrombosis or pulmonary embolism) within 6 months before first dose. Upon sponsor approval, patients with a diagnosis of incidental, subsegmental pulmonary embolism or deep venous thrombosis within 6 months are allowed if stable, asymptomatic, and treated with low-molecular-weight heparins for at least 2 weeks before first dose. Iatrogenic arterial embolization procedures such as tumor arterial embolization or splenic artery embolization are allowed.
-
GI disorders including those associated with a high risk of perforation or fistula formation:
○ Tumors invading the GI tract, active peptic ulcer disease, inflammatory bowel disease, diverticulitis, cholecystitis, symptomatic cholangitis or appendicitis, acute pancreatitis or acute obstruction of the pancreatic or biliary duct, or gastric outlet obstruction. Presence of primary GI tumor is not excluded
○ Abdominal fistula, GI perforation, bowel obstruction, or intra-abdominal abscess within 6 months before first dose. Complete healing of an intra-abdominal abscess must be confirmed before first dose
○ Gastric or esophageal varices that are untreated or incompletely treated with bleeding or high risk for bleeding. Patients treated with adequate endoscopic therapy (according to institutional standards) without any episodes of recurrent GI bleeding requiring transfusion or hospitalization for at least 6 months before study entry are eligible.
Clinically significant hematuria, hematemesis, or hemoptysis of > 0.5 teaspoon (2.5 mL) of red blood, or other history of significant bleeding (eg, pulmonary hemorrhage) within 12 weeks before first dose.
Cavitating pulmonary lesion(s) or known endobronchial disease manifestation.
Lesion invading a major blood vessel including, but not limited to, inferior vena cava, pulmonary artery, or aorta.
-
Other clinically significant disorders such as:
○ Active or history of autoimmune disease or immune deficiency including, but not limited to, myasthenia gravis, myositis, autoimmune hepatitis, systemic lupus erythematosus, rheumatoid arthritis, psoriatic arthritis, inflammatory bowel disease, antiphospholipid antibody syndrome, Wegener granulomatosis, Sjögren's syndrome, Guillain-Barré syndrome, or multiple sclerosis
○ Active infection requiring systemic treatment, infection with human immunodeficiency virus or acquired immunodeficiency syndrome–related illness, acute or chronic hepatitis B or C infection, or known positive test for tuberculosis infection if supported by clinical or radiographic evidence of disease
○ History of idiopathic pulmonary fibrosis, organizing pneumonia (eg, bronchiolitis obliterans), drug-induced pneumonitis, idiopathic pneumonitis, or evidence of active pneumonitis on screening chest computed tomography scan
○ Serious nonhealing wound or ulcer or bone fracture
○ Malabsorption syndrome
○ Free thyroxine (FT4) outside the laboratory normal reference range. Asymptomatic subjects with FT4 abnormalities can be eligible after sponsor approval
○ Moderate to severe hepatic impairment for subjects with chronic liver disease (Child-Pugh B or C)
○ Requirement for hemodialysis or peritoneal dialysis
○ History of solid organ or allogenic stem-cell transplant.
Major surgery (eg, GI surgery and removal or biopsy of brain metastasis) within 4 weeks or minor surgery (eg, simple excision or tooth extraction) within 10 days before first dose of study treatment
Corrected QT interval calculated by the Fridericia formula > 500 ms per electrocardiogram within 14 days before first dose of study treatment.
Pregnant or lactating females.
Inability to swallow tablets.
Previously identified allergy or hypersensitivity to components of the study treatment formulations.
Diagnosis of another malignancy within 2 years before first dose of study treatment, except for superficial skin cancers or localized, low-grade tumors deemed cured and not treated with systemic therapy.
Eligibility criteria for patients with renal cell carcinoma enrolled in the dose-escalation stage and expansion cohorts
-
Dose escalation
Patients with renal cell carcinoma (RCC; clear cell and non–clear cell histologies) with or without prior systemic anticancer therapy
-
Dose expansion
○ Patients with RCC with clear cell histology (including those with mixed sarcomatoid component) and without prior systemic anticancer therapy for inoperable locally advanced or metastatic disease
-
○ Patients with RCC with non–clear cell histology (including those with sarcomatoid component)
• Prior therapy with up to one vascular endothelial growth factor receptor-tyrosine kinase inhibitor (eg, sunitinib and pazopanib) is allowed for inoperable locally advanced, recurrent, or metastatic disease
• Tyrosine kinase inhibitors targeting MET or prior therapy with immune checkpoint inhibitors is not allowed.
Maintenance anticancer therapy after the initial anticancer therapy does not count toward the limit of prior systemic therapies, provided there is no tumor progression between the initial anticancer therapy and the start of maintenance anticancer therapy. In addition, radiosensitization chemotherapy and retreatment with the same anticancer agent do not count toward the limit of prior systemic therapies.
TABLE A1.
Overview of AEs
TABLE A2.
AESI
Sumanta K. Pal
Consulting or Advisory Role: F. Hoffmann LaRoche
Research Funding: Eisai, Genentech, Roche, Exelixis, Pfizer
Travel, Accommodations, Expenses: Genentech, Seattle Genetics
Bradley McGregor
Consulting or Advisory Role: Bayer, Seattle Genetics/Astellas, Exelixis, AstraZeneca, Astellas Pharma, Genentech/Roche, Nextar, Janssen Oncology, Pfizer, EMD Serono, Eisai, Dendreon, Bristol Myers Squibb
Research Funding: Bristol Myers Squibb, Exelixis, Calithera Biosciences, Seattle Genetics/Astellas
Cristina Suárez
Consulting or Advisory Role: Bristol Myers Squibb, Ipsen, Sanofi, Pfizer, EUSA Pharma, Astellas Pharma, Novartis, Merck Sharp & Dohme, Eisai
Speakers' Bureau: Bristol Myers Squibb, Ipsen, Pfizer, Roche/Genentech, AstraZeneca, Merck Sharp & Dohme
Research Funding: Astellas Pharma, Roche/Genentech, Exelixis, AstraZeneca, Bristol Myers Squibb, Pfizer, Novartis, Janssen Oncology, Calithera Biosciences, AB Science, Arog, AVEO, Bayer, SFJ Pharmaceuticals Group, Blueprint Medicines, Clovis Oncology, Boehringer Ingelheim, Cougar Biotechnology, Deciphera, GlaxoSmithKline, Incyte, Karyopharm Therapeutics, MedImmune, Nanobiotix, Millennium, Puma Biotechnology, Teva
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche, Ipsen
Che-Kai Tsao
Consulting or Advisory Role: Eisai, Clovis Oncology, Merck
Research Funding: Exelixis
William Kelly
Honoraria: Janssen Oncology, Bayer
Consulting or Advisory Role: Merck Sharp & Dohme
Research Funding: Sanofi, Novartis, Janssen Oncology, Bayer, Exelixis, Seattle Genetics, Endocyte, Amgen, BioClin Therapeutics, Sarah Cannon Research Institute, Roche
Travel, Accommodations, Expenses: Janssen Oncology, Merck Sharp & Dohme
Ulka Vaishampayan
Consulting or Advisory Role: Pfizer, Exelixis, Bayer, Bristol Myers Squibb/Medarex, Merck Serono, Advanced Accelerator Applications, Alkermes, Helsinn Therapeutics
Speakers' Bureau: Pfizer, Bayer, Exelixis
Research Funding: Astellas Pharma, Exelixis, Bristol Myers Squibb, Merck KGaA
Lance Pagliaro
Research Funding: Pfizer, Genentech/Roche, Exelixis, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck
Benjamin L. Maughan
Consulting or Advisory Role: Janssen Oncology, Exelixis, Tempus, Bristol Myers Squibb, Astellas Medivation, Bayer, AVEO, Clovis Oncology, Merck, Peloton Therapeutics
Research Funding: Clovis Oncology, Bristol Myers Squibb, Bavarian Nordic, Exelixis
Travel, Accommodations, Expenses: Exelixis
Yohann Loriot
Honoraria: Sanofi, Pfizer
Consulting or Advisory Role: Janssen, Astellas Pharma, Roche, AstraZeneca, MSD Oncology, Seattle Genetics, Bristol Myers Squibb, Immunomedics, Taiho Pharmaceutical
Research Funding: Sanofi, Janssen Oncology, MSD Oncology, AstraZeneca, Clovis Oncology, Exelixis, Boehringer Ingelheim, Incyte, Pfizer, Oncogenex, Medivation, CureVac, Nektar
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Roche, MSD Oncology, AstraZeneca, Seattle Genetics
Daniel Castellano
Consulting or Advisory Role: Janssen Oncology, Roche/Genentech, Astellas Pharma, AstraZeneca, Pfizer, Novartis, Ipsen, Bristol Myers Squibb, MSD Oncology, Bayer, Lilly, Sanofi, Pierre Fabre, Boehringer Ingelheim
Research Funding: Janssen Oncology
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol Myers Squibb, AstraZeneca Spain
Sandy Srinivas
Consulting or Advisory Role: Eisai, Bayer, Bristol Myers Squibb, Merck, Exelixis, AstraZeneca, Seattle Genetics
Research Funding: Bristol Myers Squibb, Genentech, Merck, Exelixis, Eisai, Bayer, AstraZeneca, Seattle Genetics/Astellas
Other Relationship: Pfizer
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca
Research Funding: Pfizer, Bayer, Tempus
Robert Dreicer
Consulting or Advisory Role: Astellas Pharma, Pfizer, Eisai, Merck, EMD Serono, Propella Therapeutics, Myovant Sciences, Bayer, Tavanta Therapeutics, Veru, Infinity Pharmaceuticals, Pfizer/Astellas
Research Funding: Seattle Genetics, Bristol Myers Squibb, Exelixis, Novartis
Thomas Hutson
Employment: Texas Oncology
Honoraria: Pfizer, Astellas Pharma, Bristol Myers Squibb, Exelixis, Eisai, Novartis, Johnson & Johnson, Bayer/Onyx
Consulting or Advisory Role: Bayer/Onyx, Pfizer, Novartis, Astellas Pharma, Johnson & Johnson, Bristol Myers Squibb, Eisai, Exelixis
Speakers' Bureau: Pfizer, Johnson & Johnson, Eisai, Exelixis, Astellas Pharma, Bristol Myers Squibb
Research Funding: Pfizer, Johnson & Johnson, Exelixis, Eisai, Bristol Myers Squibb
Sarita Dubey
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Scott Werneke
Employment: Exelixis
Stock and Other Ownership Interests: Exelixis
Ashok Panneerselvam
Employment: Exelixis
Stock and Other Ownership Interests: Exelixis
Dominic Curran
Employment: Exelixis, Syneos Health (I)
Stock and Other Ownership Interests: Exelixis
Travel, Accommodations, Expenses: Exelixis
Christian Scheffold
Employment: Exelixis
Stock and Other Ownership Interests: Exelixis
Patents, Royalties, Other Intellectual Property: Patent
Toni K. Choueiri
Employment: Dana Farber Cancer Hospital
Leadership: Dana Farber Cancer Hospital, NCCN, KidneyCan, ASCO
Stock and Other Ownership Interests: Pionyr, Tempest Therapeutics
Honoraria: NCCN, UpToDate, Michael J. Hennessy Associates, ASCO, Harborside Press, Analysis Group, AstraZeneca, Alexion Pharmaceuticals, Sanofi/Aventis, Bayer, Bristol Myers Squibb, Genentech/Roche, GlaxoSmithKline, Merck, Novartis, Peloton Therapeutics, Pfizer, Corvus Pharmaceuticals, Ipsen, Foundation Medicine, Eisai, PlatformQ Health, Clinical Care Options, Navinata Health, Kidney Cancer Association, Exelixis, Prometheus, Lpath, The New England Journal of Medicine, Lancet Oncology, Cerulean Pharma, Alligent, EMD Serono, HERON, Lilly, Janssen Oncology, IQvia, Aveo, NCI GU Steering Committee
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Infinity Pharmaceuticals, Aravive
Research Funding: Pfizer, Novartis, Merck, Exelixis, TRACON Pharma, GlaxoSmithKline, Bristol Myers Squibb, AstraZeneca, Peloton Therapeutics, Roche/Genentech, Celldex, Agensys, Eisai, Takeda, Prometheus, Ipsen, Corvus Pharmaceuticals, Cerulean Pharma, Seattle Genetics/Astellas, Bayer, Foundation Medicine, Roche, Calithera Biosciences, Analysis Group, NCI, Gateway for Cancer Research, Congressionally Directed Medical Research Programs (DOD)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430, titled Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy, International Patent Application No. PCT/US2018/12209, titled PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
Neeraj Agarwal
Consulting or Advisory Role: Pfizer, Medivation/Astellas, Bristol Myers Squibb, AstraZeneca, Nektar, Lilly, Bayer, Pharmacyclics, Foundation Medicine, Astellas Pharma, Exelixis, Merck, Novartis, Eisai, Seattle Genetics, EMD Serono, Janssen Oncology, AVEO, Calithera Biosciences, MEI Pharma, Genentech, Gilead Sciences
Research Funding: Bayer, Bristol Myers Squibb, Takeda, Pfizer, Exelixis, Amgen, AstraZeneca, Calithera Biosciences, Celldex, Eisai, Genentech, Immunomedics, Janssen, Merck, Lilly, Nektar, ORIC Pharmaceuticals, CRISPR Therapeutics, Arvinas
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2020 Virtual Congress of the European Society of Medical Oncology, September 19-21, 2020.
SUPPORT
Supported by Exelixis Inc.
CLINICAL TRIAL INFORMATION
T.K.C. and N.A. contributed equally to this work.
DATA SHARING STATEMENT
Currently, the sponsor does not make data publicly available, although data are shared with certain external parties upon request.
AUTHOR CONTRIBUTIONS
Conception and design: Sumanta K. Pal, Bradley McGregor, Ulka Vaishampayan, Thomas Hutson, Ashok Panneerselvam, Christian Scheffold, Toni K. Choueiri, Neeraj Agarwal
Administrative support: Neeraj Agarwal
Provision of study materials or patients: Sumanta K. Pal, Bradley McGregor, Cristina Suárez, Che-Kai Tsao, William Kelly, Ulka Vaishampayan, Lance Pagliaro, Benjamin L. Maughan, Yohann Loriot, Daniel Castellano, Sandy Srinivas, Rana R. McKay, Robert Dreicer, Thomas Hutson, Toni K. Choueiri, Neeraj Agarwal
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cabozantinib in Combination With Atezolizumab for Advanced Renal Cell Carcinoma: Results From the COSMIC-021 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sumanta K. Pal
Consulting or Advisory Role: F. Hoffmann LaRoche
Research Funding: Eisai, Genentech, Roche, Exelixis, Pfizer
Travel, Accommodations, Expenses: Genentech, Seattle Genetics
Bradley McGregor
Consulting or Advisory Role: Bayer, Seattle Genetics/Astellas, Exelixis, AstraZeneca, Astellas Pharma, Genentech/Roche, Nextar, Janssen Oncology, Pfizer, EMD Serono, Eisai, Dendreon, Bristol Myers Squibb
Research Funding: Bristol Myers Squibb, Exelixis, Calithera Biosciences, Seattle Genetics/Astellas
Cristina Suárez
Consulting or Advisory Role: Bristol Myers Squibb, Ipsen, Sanofi, Pfizer, EUSA Pharma, Astellas Pharma, Novartis, Merck Sharp & Dohme, Eisai
Speakers' Bureau: Bristol Myers Squibb, Ipsen, Pfizer, Roche/Genentech, AstraZeneca, Merck Sharp & Dohme
Research Funding: Astellas Pharma, Roche/Genentech, Exelixis, AstraZeneca, Bristol Myers Squibb, Pfizer, Novartis, Janssen Oncology, Calithera Biosciences, AB Science, Arog, AVEO, Bayer, SFJ Pharmaceuticals Group, Blueprint Medicines, Clovis Oncology, Boehringer Ingelheim, Cougar Biotechnology, Deciphera, GlaxoSmithKline, Incyte, Karyopharm Therapeutics, MedImmune, Nanobiotix, Millennium, Puma Biotechnology, Teva
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche, Ipsen
Che-Kai Tsao
Consulting or Advisory Role: Eisai, Clovis Oncology, Merck
Research Funding: Exelixis
William Kelly
Honoraria: Janssen Oncology, Bayer
Consulting or Advisory Role: Merck Sharp & Dohme
Research Funding: Sanofi, Novartis, Janssen Oncology, Bayer, Exelixis, Seattle Genetics, Endocyte, Amgen, BioClin Therapeutics, Sarah Cannon Research Institute, Roche
Travel, Accommodations, Expenses: Janssen Oncology, Merck Sharp & Dohme
Ulka Vaishampayan
Consulting or Advisory Role: Pfizer, Exelixis, Bayer, Bristol Myers Squibb/Medarex, Merck Serono, Advanced Accelerator Applications, Alkermes, Helsinn Therapeutics
Speakers' Bureau: Pfizer, Bayer, Exelixis
Research Funding: Astellas Pharma, Exelixis, Bristol Myers Squibb, Merck KGaA
Lance Pagliaro
Research Funding: Pfizer, Genentech/Roche, Exelixis, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck
Benjamin L. Maughan
Consulting or Advisory Role: Janssen Oncology, Exelixis, Tempus, Bristol Myers Squibb, Astellas Medivation, Bayer, AVEO, Clovis Oncology, Merck, Peloton Therapeutics
Research Funding: Clovis Oncology, Bristol Myers Squibb, Bavarian Nordic, Exelixis
Travel, Accommodations, Expenses: Exelixis
Yohann Loriot
Honoraria: Sanofi, Pfizer
Consulting or Advisory Role: Janssen, Astellas Pharma, Roche, AstraZeneca, MSD Oncology, Seattle Genetics, Bristol Myers Squibb, Immunomedics, Taiho Pharmaceutical
Research Funding: Sanofi, Janssen Oncology, MSD Oncology, AstraZeneca, Clovis Oncology, Exelixis, Boehringer Ingelheim, Incyte, Pfizer, Oncogenex, Medivation, CureVac, Nektar
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology, Roche, MSD Oncology, AstraZeneca, Seattle Genetics
Daniel Castellano
Consulting or Advisory Role: Janssen Oncology, Roche/Genentech, Astellas Pharma, AstraZeneca, Pfizer, Novartis, Ipsen, Bristol Myers Squibb, MSD Oncology, Bayer, Lilly, Sanofi, Pierre Fabre, Boehringer Ingelheim
Research Funding: Janssen Oncology
Travel, Accommodations, Expenses: Pfizer, Roche, Bristol Myers Squibb, AstraZeneca Spain
Sandy Srinivas
Consulting or Advisory Role: Eisai, Bayer, Bristol Myers Squibb, Merck, Exelixis, AstraZeneca, Seattle Genetics
Research Funding: Bristol Myers Squibb, Genentech, Merck, Exelixis, Eisai, Bayer, AstraZeneca, Seattle Genetics/Astellas
Other Relationship: Pfizer
Rana R. McKay
Consulting or Advisory Role: Janssen, Novartis, Tempus, Exelixis, Pfizer, Bristol Myers Squibb, Astellas Medivation, Dendreon, Bayer, Sanofi, Merck, Vividion Therapeutics, Calithera Biosciences, AstraZeneca
Research Funding: Pfizer, Bayer, Tempus
Robert Dreicer
Consulting or Advisory Role: Astellas Pharma, Pfizer, Eisai, Merck, EMD Serono, Propella Therapeutics, Myovant Sciences, Bayer, Tavanta Therapeutics, Veru, Infinity Pharmaceuticals, Pfizer/Astellas
Research Funding: Seattle Genetics, Bristol Myers Squibb, Exelixis, Novartis
Thomas Hutson
Employment: Texas Oncology
Honoraria: Pfizer, Astellas Pharma, Bristol Myers Squibb, Exelixis, Eisai, Novartis, Johnson & Johnson, Bayer/Onyx
Consulting or Advisory Role: Bayer/Onyx, Pfizer, Novartis, Astellas Pharma, Johnson & Johnson, Bristol Myers Squibb, Eisai, Exelixis
Speakers' Bureau: Pfizer, Johnson & Johnson, Eisai, Exelixis, Astellas Pharma, Bristol Myers Squibb
Research Funding: Pfizer, Johnson & Johnson, Exelixis, Eisai, Bristol Myers Squibb
Sarita Dubey
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Scott Werneke
Employment: Exelixis
Stock and Other Ownership Interests: Exelixis
Ashok Panneerselvam
Employment: Exelixis
Stock and Other Ownership Interests: Exelixis
Dominic Curran
Employment: Exelixis, Syneos Health (I)
Stock and Other Ownership Interests: Exelixis
Travel, Accommodations, Expenses: Exelixis
Christian Scheffold
Employment: Exelixis
Stock and Other Ownership Interests: Exelixis
Patents, Royalties, Other Intellectual Property: Patent
Toni K. Choueiri
Employment: Dana Farber Cancer Hospital
Leadership: Dana Farber Cancer Hospital, NCCN, KidneyCan, ASCO
Stock and Other Ownership Interests: Pionyr, Tempest Therapeutics
Honoraria: NCCN, UpToDate, Michael J. Hennessy Associates, ASCO, Harborside Press, Analysis Group, AstraZeneca, Alexion Pharmaceuticals, Sanofi/Aventis, Bayer, Bristol Myers Squibb, Genentech/Roche, GlaxoSmithKline, Merck, Novartis, Peloton Therapeutics, Pfizer, Corvus Pharmaceuticals, Ipsen, Foundation Medicine, Eisai, PlatformQ Health, Clinical Care Options, Navinata Health, Kidney Cancer Association, Exelixis, Prometheus, Lpath, The New England Journal of Medicine, Lancet Oncology, Cerulean Pharma, Alligent, EMD Serono, HERON, Lilly, Janssen Oncology, IQvia, Aveo, NCI GU Steering Committee
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, Peloton Therapeutics, UpToDate, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Navinata Health, Harborside Press, ASCO, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO, NiKang Therapeutics, Kanaph Therapeutics, Infinity Pharmaceuticals, Aravive
Research Funding: Pfizer, Novartis, Merck, Exelixis, TRACON Pharma, GlaxoSmithKline, Bristol Myers Squibb, AstraZeneca, Peloton Therapeutics, Roche/Genentech, Celldex, Agensys, Eisai, Takeda, Prometheus, Ipsen, Corvus Pharmaceuticals, Cerulean Pharma, Seattle Genetics/Astellas, Bayer, Foundation Medicine, Roche, Calithera Biosciences, Analysis Group, NCI, Gateway for Cancer Research, Congressionally Directed Medical Research Programs (DOD)
Patents, Royalties, Other Intellectual Property: International Patent Application No. PCT/US2018/058430, titled Biomarkers of Clinical Response and Benefit to Immune Checkpoint Inhibitor Therapy, International Patent Application No. PCT/US2018/12209, titled PBRM1 Biomarkers Predictive of Anti-Immune Checkpoint Response
Travel, Accommodations, Expenses: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol Myers Squibb, Roche/Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Exelixis, Prometheus, Alligent, Ipsen, Corvus Pharmaceuticals, Lpath, Alexion Pharmaceuticals, Sanofi/Aventis, UpToDate, Peloton Therapeutics, NCCN, Michael J. Hennessy Associates, Analysis Group, Kidney Cancer Association, Clinical Care Options, PlatformQ Health, Harborside Press, Navinata Health, The New England Journal of Medicine, Lancet Oncology, EMD Serono, HERON, Lilly, ESMO
Other Relationship: Medical writing and editorial assistance support may have been funded by Communications companies funded by pharmaceutical companies such as ClinicalThinking, Health Interactions, Envision Pharma Group, Fishawack Group of Companies, Parexel
Neeraj Agarwal
Consulting or Advisory Role: Pfizer, Medivation/Astellas, Bristol Myers Squibb, AstraZeneca, Nektar, Lilly, Bayer, Pharmacyclics, Foundation Medicine, Astellas Pharma, Exelixis, Merck, Novartis, Eisai, Seattle Genetics, EMD Serono, Janssen Oncology, AVEO, Calithera Biosciences, MEI Pharma, Genentech, Gilead Sciences
Research Funding: Bayer, Bristol Myers Squibb, Takeda, Pfizer, Exelixis, Amgen, AstraZeneca, Calithera Biosciences, Celldex, Eisai, Genentech, Immunomedics, Janssen, Merck, Lilly, Nektar, ORIC Pharmaceuticals, CRISPR Therapeutics, Arvinas
No other potential conflicts of interest were reported.
REFERENCES
- 1.Choueiri TK, Motzer RJ: Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 376:354-366, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Albiges L, Flippot R, Rioux-Leclercq N, et al. : Non-clear cell renal cell carcinomas: From shadow to light. J Clin Oncol 36:3624-3631, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Penkov K, Haanen J, et al. : Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103-1115, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rini BI, Plimack ER, Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Powles T, Burotto M, et al. : Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829-841, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Kindey Cancer Version 1.2021, 2020. https://www.nccn.org/ [Google Scholar]
- 8.Pal SK, Tangen C, Thompson IM, Jr., et al. : A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet 397:695-703, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKay RR, Bossé D, Xie W, et al. : The clinical activity of PD-1/PD-L1 inhibitors in metastatic non-clear cell renal cell carcinoma. Cancer Immunol Res 6:758-765, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogelzang NJ, Olsen MR, McFarlane JJ, et al. : Safety and efficacy of nivolumab in patients with advanced non-clear cell renal cell carcinoma: Results from the phase IIIb/IV CheckMate 374 study. Clin Genitourin Cancer 18:461-468, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Lee J-L, Ziobro M, Suarez C, et al. : First-line pembrolizumab (pembro) monotherapy in advanced non-clear cell renal cell carcinoma (nccRCC): Updated follow-up for KEYNOTE-427 cohort B. J Clin Oncol 38, 2020. (suppl; abstr 5034) [Google Scholar]
- 12.Yakes FM, Chen J, Tan J, et al. : Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10:2298-2308, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1814-1823, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choueiri TK, Halabi S, Sanford BL, et al. : Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol 35:591-597, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez Chanza N, Xie W, Asim Bilen M, et al. : Cabozantinib in advanced non-clear-cell renal cell carcinoma: A multicentre, retrospective, cohort study. Lancet Oncol 20:581-590, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell MT, Bilen MA, Shah AY, et al. : Cabozantinib for the treatment of patients with metastatic non-clear cell renal cell carcinoma: A retrospective analysis. Eur J Cancer 104:188-194, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Apolo AB, Nadal R, Tomita Y, et al. : Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: An open-label, single-centre, phase 2 trial. Lancet Oncol 21:1099-1109, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X, Horner JW, Paul E, et al. : Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543:728-732, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patnaik A, Swanson KD, Csizmadia E, et al. : Cabozantinib eradicates advanced murine prostate cancer by activating anti-tumor innate immunity. Cancer Discov 7:750-765, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolaney SM, Ziehr DR, Guo H, et al. : Phase II and biomarker study of cabozantinib in metastatic triple-negative breast cancer patients. Oncologist 22:25-32, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choueiri TK, Kaelin WG, Jr: Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med 26:1519-1530, 2020 [DOI] [PubMed] [Google Scholar]
- 22.McDermott DF, Huseni MA, Atkins MB, et al. : Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rini BI, Powles T, Atkins MB, et al. : Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 393:2404-2415, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Agarwal N, Vaishampayan U, Green M, et al. : Phase Ib study (COSMIC-021) of cabozantinib in combination with atezolizumab: Results of the dose escalation stage in patients (pts) with treatment-naïve advanced renal cell carcinoma (RCC). Ann Oncol 29, 2018. (abstr 872P) [Google Scholar]
- 25.Agarwal N, Loriot Y, McGregor BA, et al. : Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: Results of cohort 6 of the COSMIC-021 study. J Clin Oncol 38, 2020. (suppl; abstr 5564) [Google Scholar]
- 26.Neal JW, Lim FL, Felip E, et al. : Cabozantinib in combination with atezolizumab in non-small cell lung cancer (NSCLC) patients previously treated with an immune checkpoint inhibitor: Results from cohort 7 of the COSMIC-021 study. J Clin Oncol 38, 2020. (suppl; abstr 9610) [Google Scholar]
- 27.Buti S, Bersanelli M, Maines F, et al. : First-Line PAzopanib in NOn-clear-cell renal cArcinoMA: The Italian retrospective multicenter PANORAMA study. Clin Genitourin Cancer 15:e609-e614, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Armstrong AJ, Halabi S, Eisen T, et al. : Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicentre, open-label, randomised phase 2 trial. Lancet Oncol 17:378-388, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park I, Lee SH, Lee JL: A multicenter phase II trial of axitinib in patients with recurrent or metastatic non-clear-cell renal cell carcinoma who had failed prior treatment with temsirolimus. Clin Genitourin Cancer 16:e997-e1002, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Koshkin VS, Barata PC, Zhang T, et al. : Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer 6:9, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGregor BA, McKay RR, Braun DA, et al. : Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol 38:63-70, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerendash BS, Creel PA: Practical management of adverse events associated with cabozantinib treatment in patients with renal-cell carcinoma. Onco Targets Ther 10:5053-5064, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruiz JN, Belum VR, Creel P, et al. : Current practices in the management of adverse events associated with targeted therapies for advanced renal cell carcinoma: A national survey of oncologists. Clin Genitourin Cancer 12:341-347, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacy SA, Miles DR, Nguyen LT: Clinical pharmacokinetics and pharmacodynamics of cabozantinib. Clin Pharmacokinet 56:477-491, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Currently, the sponsor does not make data publicly available, although data are shared with certain external parties upon request.